Abstract
Background and Objectives
The reported risk of nodal metastasis in hard palate and upper gingival squamous cell carcinoma (SCC) has been inconsistent with inadequate consensus regarding the utility of neck dissection in the clinically negative (cN0) neck.
Materials and Methods
Using the National Cancer Database (NCDB), cN0 patients diagnosed with SCC of the head and neck with the subsites of the hard palate and upper gingiva were identified from 2004–2014.
Results
A total of 1,830 patients were identified, and END was performed on 422 patients with cN0 tumors. Pathologically positive nodes occurred in 14% (59/422) of patients in this cohort. Higher tumor stage, academic hospital type, and large hospital volume (>28 cancer-specific cases/year) were associated with a higher likelihood of END both in univariate and multivariate analyses (P < 0.05). Patients >80 years of age were less likely to receive END on multivariate analysis (OR 0.52, 0.32–0.84). No variables, including advanced T stage, predicted occult metastases. Cox proportional hazards regression analysis showed that patients who underwent END demonstrated improved OS over an 11-year period (hazard ratio 0.75, P = 0.002). On subgroup analysis this improvement was significant in patients with both stage T1 and T4 tumors.
Conclusions
Tumor stage, hospital type, and hospital volume were associated with higher rates of END for patients with cN0 hard palate SCC and after controlling for clinical factors, END was associated with improved overall survival.
Keywords: neck dissection, hard palate, gingiva, carcinoma squamous cell head and neck, logistic models, prevalence
INTRODUCTION
Metastasis to the neck is the most important prognostic factor in head and neck cancer.[1–3] Selective neck dissection of high risk nodal groups is both diagnostic and therapeutic, providing pathologic data and determining the need for adjuvant treatment. Elective neck dissection (END) for patients with clinically negative neck nodes (cN0) remains an issue of controversy at some disease sites. When considering the morbidity versus therapeutic benefit of neck dissection, END has traditionally only been advised for patients whose risk of occult metastases is greater than 15% to 20%.[4,5] A 2016 systematic review and meta-analysis examining T1-T2 oral tongue squamous cell carcinoma (SCC) showed that END significantly reduced the risk of regional recurrence and improved disease specific survival compared with management by observation alone.[6] While even the most modern radiologic investigative tools have a sensitivity of 70–80% for detecting nodal metastases, END facilitates appropriate upstaging of patients with occult disease and optimization of adjuvant treatment for locoregional control. In oral cavity SCC, the addition of END is feasible as most are undergoing primary site surgical resection according to NCCN guidelines with studies implicating a >15% survival benefit with surgery plus adjuvant radiation vs. definitive chemoradiation. [7]
In squamous cell carcinoma (SCC) of the maxilla, older literature posits that the incidence of nodal metastases in the cN0 neck is too low to warrant END, with rates from 14.9%–17.1%.[8] However, recently published studies have cited variable rates, suggesting as many as 40% of hard palate SCCs have regional metastasis, and 11.9%–29.2% have occult disease.[9,10] Given that 5-year disease-specific survival rates are cited as low as 21% for patients with N+ disease compared to 47.4% for N0 disease, along with a 28.4% regional failure rate, there is a potential benefit of END in this population to better risk stratify and escalate adjuvant therapy as appropriate.[11,12] In addition to the increased risk of occult disease, the complication rate for END has also diminished with studies demonstrating only a 5.5% rate of marginal mandibular nerve injury and a 3.8% complication rate of neck and shoulder dysfunction.[13]
Since there is no consensus regarding the utility of END in patients with hard palate and upper gingival SCC, we used population data from the National Cancer Database (NCDB) to examine which factors were associated with performing an END. In addition, we sought to identify clinical factors associated with occult metastasis in cN0 disease, as well as to determine the impact of END on overall survival (OS) in this patient cohort.
MATERIALS AND METHODS
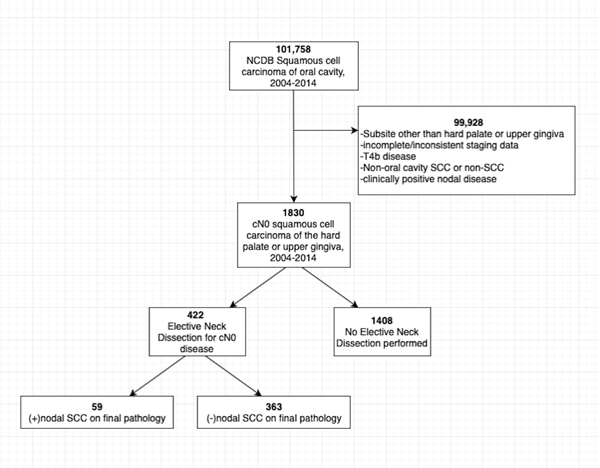
The data source for this study was the National Cancer Database (NCDB), a joint program of the American Cancer Society and the Commission on Cancer of the American College of Surgeons. The NCDB collects hospital-based registry data for patients with invasive cancer diagnoses from over 1,400 co-accredited facilities and captures approximately 70% of new cancer cases in the United States annually.[14] The data were used in accordance with NCDB Participant User Files. All patients diagnosed or first treated for oral cavity SCC restricted to the subsites of hard palate or upper gingiva at an NCDB site from 2004 to 2014 were identified. Patients were excluded from analysis for: (i) multiple cancer diagnoses or non-oral cavity SCC diagnoses; (ii) primary tumor site at other oral cavity subsites; (iii) not being treated at the reporting facility; (iv) incomplete clinical staging; (vi) unresectable disease, defined as cT4b classification; (vi) conflicting data after neck dissection if number of positive nodes did not correlate with pN stage; or (vii) untreated or unknown treatment regimen or treatment sequence (Figure 1).
Figure 1:
Flowchart outlining 101,758 NCDB patients included for analysis. 99,928 patients were excluded from analysis for: (i) multiple cancer diagnoses or non-OCSCC diagnoses; (ii) primary tumor site at other oral cavity subsites; (iii)patients not treated at the reporting facility; (iv) incomplete or inconsistent clinical staging; (vi) unresectable disease, defined as cT4b classification; (vi) patients with conflicting data after neck dissection if number of positive nodes did not correlate with pN stage; or (vii) untreated or unknown treatment regimen or treatment sequence.
Outcomes
The primary outcomes studied were neck dissection and occult cervical metastasis. Neck dissection of all types were examined, provided there was yield of at least one node on final pathology. For Cox proportional hazards regression analysis, OS over an 11-year period was used as the primary outcome after controlled adjustment for patient comorbidities, type and length of adjuvant treatment, and all other prior medical and surgical treatments.
Statistical Analysis
Chi-squared analysis was performed to examine predictors of END based on tumor and demographic variables, including cT stage, race, sex, age, hospital volume (mean annual number of cancer-specific cases from 2004–2014), hospital type (academic, integrated, or community), and insurance type (private, government, or no insurance). Univariate chi-squared analysis was performed to determine factors significantly associated with END as well as occult metastases among patients with cN0 disease, defined by pathologically confirmed positive lymph nodes after END of the cN0 neck, or regional recurrence of the untreated cN0 neck. Multivariate logistic regression analysis was used to identify possible predictors of END among cN0 patients. Cox proportional hazards regression analysis was performed to determine the effects of END on OS after controlling for patient, tumor, and treatment factors. Survival curves were generated using a Kaplan-Meier regression method, and differences between survival curves were compared at an 11-year interval. Analyses were conducted using Stata Statistical Software (Release 12.1; Stata Inc., College Station, TX). For all comparisons, a P-value (P) < 0.05 was statistically significant.
RESULTS
A total of 1,830 patients were selected based on the NCDB database algorithm (Figure 1). END was performed on 23.0% (422/1830) patients with cN0 tumors. There was a 14% (59/422) rate of pathologically positive nodes.
Upper gingival tumors were more likely to receive END than tumors of the hard palate at 27.2%(256/942) vs. 18.7%(166/888), respectively (P < 0.05). cT4 tumors and patients < 50 years of age were significantly associated with the highest rates of END, 34%(180/532) and 28.3%(28/99), respectively (P < 0.05). Patients treated at academic or integrated hospital types, as well as patients with private insurance were also more likely to receive END than patients treated in community hospitals or those with public insurance (28.6% (301/1051) vs. 11.8% (74/628) (Table I). Higher tumor stage, hospital type (academic), and hospital volume (>28 cases/year) were significantly associated with a higher odds ratio (OR) of END in multivariate analyses. There was a positive association with private insurance status noted but it was not statistically significant (OR 2.02, [0.82–4.96]). (Table II). Patients >80 years of age were significantly less likely to undergo END in multivariate analysis with an OR of 0.52 (0.32–0.84).
Table I.
Univariate analysis of effect of patient factors, tumor factors, and hospital factors on rate of neck dissection for cN0 patients with hard palate and upper gum SCC
| Group | Sub-Group | % Neck Dissection (n) | P value (x2) |
|---|---|---|---|
| TNMa stage | cTb1 | 11.3 (75) | < 0.00001 |
| cT2 | 26.9 (137) | ||
| cT3 | 23.3 (30) | ||
| cT4 | 33.8 (180) | ||
| Sub-site | Hard palate | 18.7 (166) | 0.00002 |
| Upper gingiva | 27.1 (256) | ||
| Patient age (y) | <50 | 28.3 (28) | 0.0002 |
| 50–64 | 26.6 (107) | ||
| 65–79 | 25.5 (185) | ||
| >80 | 16.9 (102) | ||
| Sex | Male | 23.9 (196) | 0.426 |
| Female | 22.3 (226) | ||
| Race | White | 22.9 (379) | 0.912 |
| Black | 23.0 (29) | ||
| Other | 25.5 (14) | ||
| Hospital type | Academic | 28.6 (301) | < 0.00001 |
| Integrated | 31.1 (47) | ||
| Community | 11.8 (74) | ||
| Hospital volume (cases/yr) | 1–28 | 9.4 (35) | < 0.0001 |
| 29–80 | 14.6 (66) | ||
| 81–190 | 30.3 (138) | ||
| 191–641 | 33.3 (183) | ||
| Patient insurance | No insurance | 15.4 (6) | 0.05 |
| Private | 26.9 (119) | ||
| Government | 22.0 (297) |
- TNM, tumor, nodal, metastasis;
- cT, clinical tumor stage.
Table II.
Multivariate analysis on factors affecting neck dissection in cNa0 patients with hard palate and upper gingival SCCb
| Group | Subgroup | Odds ratio | 95% Confidence Interval |
|---|---|---|---|
| Age | < 50 | Refc | Ref |
| 50–65 | 0.91 | 0.56–1.50 | |
| 65–79 | 0.87 | 0.54–1.38 | |
| > 80 | 0.52 | 0.32–0.84 | |
| cTd stage | cT1 | Ref | Ref |
| cT2 | 2.87 | 2.11–3.91 | |
| cT3 | 2.36 | 1.47–3.80 | |
| cT4 | 3.99 | 2.95–5.38 | |
| Hospital type | Academic | Ref | Ref |
| Integrated | 0.33 | 0.25–0.43 | |
| Community | 1.12 | 0.78–1.62 | |
| Race | White | Ref | Ref |
| Black | 1.00 | 0.65–1.54 | |
| Other | 1.14 | 0.61–2.12 | |
| Hospital volume | 1–28 | Ref | Ref |
| 29–80 | 1.66 | 1.07–2.57 | |
| 81–190 | 4.20 | 2.81–6.27 | |
| 191–641 | 4.82 | 3.26–7.13 | |
| Insurance | No insurance | Ref | Ref |
| Private | 2.02 | 0.82–4.96 | |
| Government | 1.55 | 0.64–3.74 |
- cN, clinical nodal stage
- SCC, squamous cell carcinoma
- Ref, reference
- cT, clinical tumor stage.
Regarding predictors of occult metastasis, on univariate analysis clinical, demographic, and hospital factors were queried in association with occult metastasis on END. No variable when studied showed a statistically significant association with occult metastasis (Table III).
Table III.
Univariate analysis of effect of patient factors, tumor factors, and hospital factors on rate of occult metastases after neck dissection for cNa0 patients with hard palate and upper gum SCCb
| Group | Sub-group | % Occult metastases (n) | P value (x2) |
|---|---|---|---|
| TNMc stage | cTd1 | 9.3 (7) | 0.633 |
| cT2 | 15.3 (21) | ||
| cT3 | 13.3 (4) | ||
| cT4 | 15.0 (27) | ||
| Sub-site | Hard palate | 11.4 (19) | 0.227 |
| Upper gingiva | 15.6 (40) | ||
| Patient age (y) | < 50 | 17.9 (5) | 0.652 |
| 50–65 | 14.0 (15) | ||
| 65–79 | 11.9 (22) | ||
| > 80 | 16.7 (17) | ||
| Sex | Male | 13.8 (27) | 0.910 |
| Female | 14.2 (32) | ||
| Race | White | 13.5 (51) | 0.210 |
| Black | 24.1 (7) | ||
| Other | 7.1 (1) | ||
| Hospital type | Academic | 15.0 (45) | 0.106 |
| Integrated | 19.2 (9) | ||
| Community | 6.8 (5) | ||
| Hospital volume (# beds) | 1–28 | 20.0 (7) | 0.413 |
| 29–80 | 10.6 (7) | ||
| 81–190 | 11.6 (16) | ||
| 191–641 | 15.9 (29) | ||
| Patient insurance | No insurance | 33.3 (2) | 0.386 |
| Private | 13.5 (16) | ||
| Government | 13.8 (41) |
- cN, clinical nodal stage
- SCC, squamous cell carcinoma
- TNM, tumor, nodal, metastasis
- cT, clinical tumor stage.
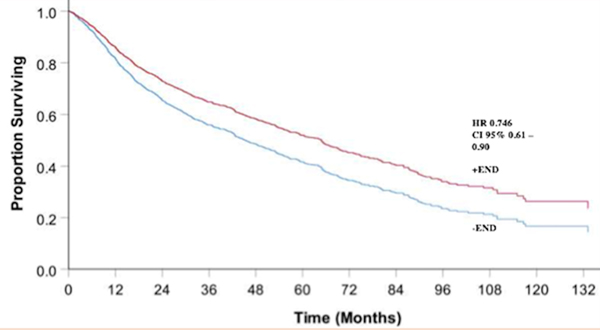
Cox proportional hazards regression analysis showed that after controlling for tumor factors (such as T stage), patient factors, and treatment factors (i.e. radiotherapy or concurrent chemoradiotherapy), patients who underwent END demonstrated improved OS over an 11-year period (hazard ratio (HR) 0.74, P = 0.002, Figure 2). On subgroup analysis, when stratified by patient T stage those patients with T1 tumors demonstrated improved OS (HR 0.56, [0.33–0.97]) as well as those with advanced T4 disease (HR 0.62, [0.47–0.81]). The patients with T2 and T3 tumors approached but did not have statistically significant improved OS following END with HR 0.81, [0.59–1.11] and HR 0.74, [0.41–1.34]).
Figure 2:
Overall survival comparing cN0 patients who receive neck dissection vs. no neck dissection over an 11-year period.
DISCUSSION
In 1994, Weiss et al.[15] utilized decision analysis to recommend END when the probability of occult cervical metastases exceeded 20%. END has since become crucial in the management of patients with clinicaly N0 oral cavity and oropharyngeal cancer due to its ability to improve regional control, diseae-specific survival,[16] as well as OS. In a 2015 study that examined T1 T2 N0 oral tongue SCC patients at Memorial Sloan Kettering Cancer Center and Princess Margaret Cancer Center from 1985 to 2005, regional recurrence-free survival was seen in 80% of patients who received END. Moreover, recurrence in the neck was assosicated with a signicantly poorer disease-specific survival vs. those who did not have neck recurrence (33% vs. 97%; P < .0001).[17] Thus, traditional management protocols have emphasized the importance of diagnosing and treating occult neck disease at the time of primary resection when the risk of nodal metastasis is >15–20%.
Management of the N0 neck in patients with palatal and upper gingival SCC has been controversial. Until recently, few studies have documented associated metastatic rates with poor regional control and survival. However, newer studies have suggested that the risk of occult cervical metastasis is also greater than 20% in hard palate and gingivall cancers, necessitating the need for END in this group of patients.[18] A 2016 retrospective review of 114 patients with hard palate carcinoma revealed a 26% rate of neck metastases, which appears consistent with other retrospective review data.[19,20] Simental et al. found that 34.6% of patients displayed either occult or clinical cervical metastasis with a five year disease-specific survival of only 59%, despite control of disease at the primary site.[8]
The NCDB data demonstrates a rate of pathologic nodal disease in clinically N0 SCC within the hard palate or upper gingiva subsites of 14%, similar to Montes et al. who noted a positive occult nodal rate of 14.4%.[21] This is considerably lower than the 27% occult positive cervical nodal rate seen in the Simental et al. study. One possible explanation for this is the early detection of disease reflected in 27.1% patients noted to have T2 tumors vs. 23.2% with T3 tumors. In Meng et al.’s series of 78 patients, they noted that although rates of positive nodal metastasis from T1 T2 tumors were lower than 15%; those from higher stage tumors were greater than 40%.[22] In a retrospective series of 20 patients, Moreno-Sanchez et al. showed that cervical metastasis was only present in pT3T4 tumors of the maxillary alveolus and hard palate.[23] This may explain the relatively lower occult metastatic rate seen in our review, given 27.1% of END performed were for T2 disease and may have lowered the occult positive rate. The improved OS among T4 tumors after END seen in this study also demonstrates the higher likelihood of regional occult metastases with advanced local disease.
Higher clinical stage (cT4) tumors of the hard palate/upper gingiva were significantly associated with an increased likelihood of END as well as OS, which complements literature supporting the utility of END among higher stage tumors in the head and neck.[24] Elsewhere in the oral cavity, cT1, cT2, and cT3 tongue cancers are associated with 30%, 50%, and 70% respective incidence of microscopic nodal metastasis.[24,25] Ogura et al. showed that maxillary bone invasion in upper gingival carcinoma was independently associated with increased cervical metastasis with an odds ratio of 19.7 (95% confidence interval 1.03–37.5) on multivariate analysis, reflective of higher tumor stage.[26]
A possible argument against END in this population is the high rate of regional control after therapeutic neck dissection when nodal metastases are detected following primary resection. In one study, salvage neck dissection after regional recurrence in a patient cohort with oral cavity SCC sucessfully eliminated disease in 71.4% of patents.[27] In a 2006 retrospective review by Simental et al., surgery for regional failure was successful in 66% of patients.[25] However, in these instances there is concern that many at risk patients may not have received disciplined and periodic surveillance, increasing the risk of undetected nodal recurrence. A 2015 retrospective cohort study examining the effects of compliance with post treatment surveillance on overall survival noted a 30% non-compliance rate over the study period and decreased overall survival when >3 appointments were missed.[28] In addition the functional postoperative outcome with salvage treatment may be increasingly morbid, with possible secondary hospitalization, longer recovery time, and more extensive surgery. A 2017 case series by van den Bovenkamp et al posited a 28% complication rate for salvage neck dissection among head and neck cancer patients, with the extent of surgery being the only significant predictor of complications.[29] This favors aggressive primary elective surgical management of the neck with END as both a prognostic and therapeutic strategy, despite relatively lower rates of occult nodal disease compared to prior studies.
Patients < 80 years of age, academic hospital type, and hospital volume were also factors associated with END in our study. There may be a bias towards younger patients for aggressive salvage and elective treatment of the neck, as these patients display improved 5 year disease-specific survival postoperatively.[21,30] Increased physical fitness and presumed tolerance for morbidity of surgery may lower the threshold for head and neck surgeons to operate in this patient population. There has also been a general trend for large volume academic centers to wholistically treat head and neck patients with more advanced disease, as high volume centers have independently been associated with improved survival.[31] Inverso et al. showed that uninsured patients and patients with Medicaid are more likely to present with metastatic disease and are less likely to be treated definitively, which may explain the trend towards a signficant difference in rates of END between public and privately insured patients in our study. [32]
Patient race was not significantly associated with END in both univariate and multivariate studies, despite prior data suggesting that minority patients may present with a higher rate of late-stage disease and have higher incidence of cancer-related mortality relative to caucasian patients.[33]The 1998 NCDB Report on Cancer of the Head and Neck showed a 10% proportionate increase in the incidence rate of head and neck cancer among African Americans and a 21.4% proportionate increased incidence in Hispanic patients (from 2.8% to 3.4%) from 1985 to 1994.[30] Additional studies have suggested that African American patients with human papillomavirus positive oropharyngeal tumors have lower survival rates than Caucasian patients.[34,35]. It is possible that a true association race and the incidence of END was not detected secondary to small sample size or inaccurate reporting within the NCDB database. Also, given the bias of this study towards early stage tumors without clinically evident nodal metastasis, traditionally disenfranchised populations who may present with locally advanced disease may have not been well represented.
Our study is the first large, database study to demonstrate an OS benefit of END for cN0 patients with SCC of the mucosal maxilla (gingiva and hard palate). While the rate of occult pathological lymph nodes was less than 15%, there was a statistically signfiicant survival benefit seen in the positive END cohort. The authors believe the rate of occult metastasis and the impact of END on patient survival are integral to the decision making process for END. In addition, considering that recent literature has demonstrated declining surgical morbiditiy of END, this may justify a more aggressive approach to the N0 neck given the improvement in OS.
This study has the general limitations of a national database review. Though the sample size was large, surgeon bias may exist for the selection of patients for END, such as a predilection for advanced T stages where END would be believed to confer increased survival due to the higher likelihood of occult metastasis. Moreover, it is possible that patients included in this data set received END soley to isolate vessels for microvascular free tissue transfer, rather than for a complete oncologic resection of at risk nodal basins. Since we were not able to isolate such cases, this may have lead to an underdiagnosis of pN+ patients in the END group and would likely lead to a underestimate of the true survival benefit of END. Finally, the NCDB records OS alone. The lack of disease-specific survival data precludes forming conclusions about the actual progression of disease in response to END in this group of patients. As younger patients were more likely to undergo elective neck dissection and multiple studies have demonstrated improved overall survival in younger patients, this may play a role demonstration of improved OS in the END. In the absence of other oncologic outcome data, it is impossible to determine the true oncologic benefit of END. Finally, as with all large, database-driven studies, data entry is potentially biased based on how operative reports and physician documentation are analyzed. Errors in this process may affect the data and conclusions drawn. Further studies should investigate the impact of elective neck dissection on outcomes in patients with palate and gingival malignancy.
CONCLUSIONS
Higher tumor stage, younger age and academic hospital type, and hospital volume were positively associated with END for patients with cN0 hard palate and upper gingival SCC. When controlling for factors such as tumor stage and patient age, END was associated with improved OS compared to patients who did not have neck dissection. This benefit was maintained in a subgroup analysis among exclusively T1 and T4 stage tumors. Further investigation should be pursued to determine the oncologic impact of END in this population.
Synopsis for Table of Contents:
The purpose of this study is to identify patient, tumor, and treatment factors predictive of elective neck dissection (END) in cN0 patients with hard palate/upper gingival SCC, and to determine the impact of END on overall survival (OS). Using the National Cancer Database (NCDB), cN0 patients diagnosed with SCC of the head and neck with the subsites of the hard palate and upper gingiva were identified from 2004–2014. Cox proportional hazards regression analysis showed that patients who underwent END demonstrated improved OS over an 11-year period.
Acknowledgments
Financial Disclosures: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Data Availability Statement: The data that support the findings of the study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Chiesa F Centenary of Crile’s operation. From radical to selective neck dissection. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2006;26(6):307–308. [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Archives of otolaryngology--head & neck surgery. 2002;128(7):751–758. [DOI] [PubMed] [Google Scholar]
- 3.Park JH, Nam W, Kim HJ, Cha IH . Is elective neck dissection needed in squamous cell carcinoma of maxilla? Journal of the Korean Association of Oral and Maxillofacial Surgeons. 2017;43(3):166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlito A, Rinaldo A, Silver CE, et al. Elective and therapeutic selective neck dissection. Oral oncology. 2006;42(1):14–25. [DOI] [PubMed] [Google Scholar]
- 5.D’Cruz AK, Vaish R, Kapre N, et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. The New England journal of medicine. 2015;373(6):521–529. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Ghanem S, Yehuda M, Carmel NN, et al. Elective Neck Dissection vs Observation in Early-Stage Squamous Cell Carcinoma of the Oral Tongue With No Clinically Apparent Lymph Node Metastasis in the Neck: A Systematic Review and Meta-analysis. JAMA otolaryngology-- head & neck surgery. 2016;142(9):857–865. [DOI] [PubMed] [Google Scholar]
- 7.Spiotto MT, Jefferson G, Wenig B, Markiewicz M, Weichselbaum RR, Koshy M. Differences in Survival With Surgery and Postoperative Radiotherapy Compared With Definitive Chemoradiotherapy for Oral Cavity Cancer: A National Cancer Database Analysis. JAMA otolaryngology-- head & neck surgery. 2017;143(7):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simental AA Jr., Johnson JT, Myers EN. Cervical metastasis from squamous cell carcinoma of the maxillary alveolus and hard palate. The Laryngoscope. 2006;116(9):1682–1684. [DOI] [PubMed] [Google Scholar]
- 9.Shah JP, Andersen PE. Evolving role of modifications in neck dissection for oral squamous carcinoma. The British journal of oral & maxillofacial surgery. 1995;33(1):3–8. [DOI] [PubMed] [Google Scholar]
- 10.Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer. 1990;66(1):109–113. [DOI] [PubMed] [Google Scholar]
- 11.Rani P, Bhardwaj Y, Dass PK, Gupta M, Malhotra D, Ghezta NK. Neck dissection for oral squamous cell carcinoma: our experience and a review of the literature. Journal of the Korean Association of Oral and Maxillofacial Surgeons. 2015;41(6):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Wu D, Liu WW, et al. Survival impact of cervical metastasis in squamous cell carcinoma of hard palate. Oral surgery, oral medicine, oral pathology and oral radiology. 2013;116(1):23–27. [DOI] [PubMed] [Google Scholar]
- 13.Dedivitis RA, Guimaraes AV, Pfuetzenreiter EG Jr., Castro MA. [Neck dissection complications]. Brazilian journal of otorhinolaryngology. 2011;77(1):65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Database NC. https://www.facs.org/qualityprograms/cancer/ncdb. Accessed September 1, 2017.
- 15.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Archives of otolaryngology--head & neck surgery. 1994;120(7):699–702. [DOI] [PubMed] [Google Scholar]
- 16.Duvvuri U, Simental AA Jr., D’Angelo G, et al. Elective neck dissection and survival in patients with squamous cell carcinoma of the oral cavity and oropharynx. The Laryngoscope. 2004;114(12):2228–2234. [DOI] [PubMed] [Google Scholar]
- 17.Ganly I, Goldstein D, Carlson DL, et al. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119(6):1168–1176. [DOI] [PubMed] [Google Scholar]
- 18.Khafif A, Lopez-Garza JR, Medina JE. Is dissection of level IV necessary in patients with T1-T3 N0 tongue cancer? The Laryngoscope. 2001;111(6):1088–1090. [DOI] [PubMed] [Google Scholar]
- 19.Os AD, Karakullukcu B, Leemans CR, et al. Management of the clinically N0 neck in squamous cell carcinoma of the maxillary alveolus and hard palate. Head & neck. 2016;38(12):1794–1798. [DOI] [PubMed] [Google Scholar]
- 20.Yorozu A, Sykes AJ, Slevin NJ. Carcinoma of the hard palate treated with radiotherapy: a retrospective review of 31 cases. Oral oncology. 2001;37(6):493–497. [DOI] [PubMed] [Google Scholar]
- 21.Montes DM, Carlson ER, Fernandes R, et al. Oral maxillary squamous carcinoma: an indication for neck dissection in the clinically negative neck. Head & neck. 2011;33(11):1581–1585. [DOI] [PubMed] [Google Scholar]
- 22.Meng FY, Ko JY, Lou PJ, et al. The determining risk factors for treatment outcomes in patients with squamous cell carcinoma of the hard palate. Annals of surgical oncology. 2012;19(6):2003–2010. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Sanchez M, Gonzalez-Garcia R, Gonzalez-Ballester D, Ruiz--Laza L, Moreno-Garcia C, Monje F. What is the role of elective neck dissection in patients with squamous cell carcinoma of the upper jaw?. Revista Española de Cirugía Oral y Maxilofacial. 2017;39(1):1–62. [Google Scholar]
- 24.Patel RS, Clark JR, Gao K, O’Brien CJ. Effectiveness of selective neck dissection in the treatment of the clinically positive neck. Head & neck. 2008;30(9):1231–1236. [DOI] [PubMed] [Google Scholar]
- 25.Pitman KT. Rationale for elective neck dissection. American journal of otolaryngology. 2000;21(1):31–37. [DOI] [PubMed] [Google Scholar]
- 26.Ogura I, Kurabayashi T, Sasaki T, Amagasa T, Okada N, Kaneda T. Maxillary bone invasion by gingival carcinoma as an indicator of cervical metastasis. Dento maxillo facial radiology. 2003;32(5):291–294. [DOI] [PubMed] [Google Scholar]
- 27.Kwon IJ, Kim SM. Importance of elective neck dissection in cN0 maxillary squamous cell carcinoma. Journal of the Korean Association of Oral and Maxillofacial Surgeons. 2018;44(1):34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Shnayder Y. The impact of compliance in posttreatment surveillance in head and neck squamous cell carcinoma. JAMA otolaryngology-- head & neck surgery. 2015;141(6):519–525. [DOI] [PubMed] [Google Scholar]
- 29.van den Bovenkamp K, Noordhuis MG, Oosting SF, et al. Clinical outcome of salvage neck dissections in head and neck cancer in relation to initial treatment, extent of surgery and patient factors. Clinical otolaryngology : official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2017;42(3):693–700. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Archives of otolaryngology--head & neck surgery. 1998;124(9):951–962. [DOI] [PubMed] [Google Scholar]
- 31.Cheung MC, Koniaris LG, Perez EA, Molina MA, Goodwin WJ, Salloum RM. Impact of hospital volume on surgical outcome for head and neck cancer. Annals of surgical oncology. 2009;16(4):1001–1009. [DOI] [PubMed] [Google Scholar]
- 32.Lassig AA, Joseph AM, Lindgren BR, et al. The effect of treating institution on outcomes in head and neck cancer. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012;147(6):1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daraei P, Moore CE. Racial Disparity Among the Head and Neck Cancer Population. Journal of cancer education : the official journal of the American Association for Cancer Education. 2015;30(3):546–551. [DOI] [PubMed] [Google Scholar]
- 34.Isayeva T, Xu J, Dai Q, et al. African Americans with oropharyngeal carcinoma have significantly poorer outcomes despite similar rates of human papillomavirus-mediated carcinogenesis. Human pathology. 2014;45(2):310–319. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute. 2013;105(3):175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]