Abstract
Context:
Acute postoperative pain following video-assisted thoracoscopic surgery (VATS) needs considerable attention, if untreated leads to chronic pain and postoperative lung dysfunction. Dexmedetomidine, α2 adrenoceptor agonist has shown promising results of opioid-sparing effects.
Aims:
The objectives of this study are to investigate the effect of dexmedetomidine on postoperative analgesia and pulmonary mechanics in patients undergoing VATS.
Settings and Design:
This is a randomized controlled trial.
Subjects and Methods:
We conducted a prospective, randomized, double-blind study on lung cancer patients undergoing VATS. Each patient received either dexmedetomidine or comparable volume of normal saline during the intraoperative period. In the recovery unit, postoperative visual analog scale (VAS) score, rescue analgesic requirements, arterial blood gas values, and pulmonary function tests were recorded.
Statistical Analysis Used:
Data are analyzed using unpaired t-test, Mann–Whitney U-test, and Fischer's exact test. P < 0.05 was considered statistically significant.
Results:
VAS scores were significantly lower (P < 0.05) in the dexmedetomidine group at rest, on coughing and on mobilization from supine to sitting position. The partial pressure of arterial oxygen measured in postanesthesia care unit was significantly higher in the dexmedetomidine group (88 ± 8.2 vs 78 ± 9.1 mmHg). Forced expiratory volume in 1 was significantly greater in the dexmedetomidine group compared to the control group on the first 2 postoperative days (P < 0.05). The length of hospital stay was significantly reduced by dexmedetomidine.
Conclusions:
Intraoperative dexmedetomidine administration improves the quality of analgesia and postoperative lung function in patients undergoing VATS.
Keywords: Analgesia, dexmedetomidine, pulmonary function, video-assisted thoracoscopic surgery
INTRODUCTION
Video-assisted thoracoscopic surgery (VATS) is a minimally invasive thoracic surgery with a wide range of applications, including both diagnosis and treatment of lung pathology, and has gradually replaced conventional thoracotomy. It has known benefits of lesser postoperative pain, early mobilization, faster recovery and reduced duration of hospital stay.[1,2] However, the patients undergoing VATS still experience a moderate degree of acute postoperative pain.[3,4,5] Suboptimal intraoperative management of pain has major respiratory consequences and results in persistent chronic pain, psychological distress, and adverse effects on quality of life.[6,7]
Dexmedetomidine is a selective α2 adrenoreceptor agonist showed beneficial effects on postoperative pain control.[8,9] It combines analgesic, sedative and anxiolytic properties with preservation of respiratory function by its opioid-sparing effects.[9,10,11,12,13,14]
Although the perioperative administration of dexmedetomidine has proven favorable outcomes after major abdominal surgeries,[14,15] the outcome of patients undergoing thoracic surgery remains obscure. The aims of this study are to investigate the effects of intraoperative administration of dexmedetomidine on postoperative pain and pulmonary mechanics following VATS procedures.
SUBJECTS AND METHODS
After obtaining approval of the ethical committee, this prospective, randomized, double-blinded study was carried out on 80 adult patients of an American Society of Anesthesiologists Physical Status II or III, aged >20 years, undergoing VATS. Patients were excluded from the study if they were hemodynamically unstable, presence of arrhythmias, severe bradycardia (heart rate <45 beats/min), severe functional liver and kidney diseases, and psychiatric and central nervous disturbances. Enrolled patients were randomly allocated into two groups: Group C (control) and Group D (dexmedetomidine) using a computer-generated randomized sequence.
An informed and written consent was obtained from the patients during preanesthetic checkup 1 day before the surgery. In the operating room, patients were premedicated with intravenous (i.v.) glycopyrrolate 0.2 mg and midazolam 2 mg. Routine monitoring including oxygen saturation, electrocardiogram, blood pressure, bispectral index and capnogram was applied.
In Group D, dexmedetomidine 200 μg was mixed with normal saline to achieve a total volume of 50 ml. Using an infusion pump, i.v. dexmedetomidine 1 μg.kg−1 was administered over a period of 20 min before induction and infusion of 0.5 μg.kg−1 continued until 20 min before termination of surgery. A comparable volume of normal saline was administered in the control group. The attending anesthesiologists, surgeons, anesthesia and recovery nurses, and patients were all blinded to randomization.
All patients were induced with i.v. propofol 2 mg.kg−1, fentanyl 2 μg.kg-1 and atracurium 0.5 mg.kg−1, and a tracheal tube was inserted. Anesthesia was maintained using isoflurane in the oxygen-air mixture. For the first 72 h after surgery, all patients received 1 g of i.v. paracetamol every 8 h. If additional analgesic was required, i.v. tramadol 1 mg.kg−1 was administered. Patients were given i.v. ondansetron 4 mg, if they demanded antiemetics. All patients were instructed preoperatively on the use of the visual analog scale (VAS) and to request supplementary analgesic if needed.
Pain scores were assessed at 10 min, 20 min, 60 min, 2 h, 6 h, 12 h, 24 h, and 48 h after surgery on a VAS (0 = no pain, 10 = worst pain imaginable) at rest, on coughing and on mobilization from the supine to the sitting position [Figure 1]. The number of tramadol doses was recorded. Postoperative pulmonary function was assessed using an automated flow-sensing spirometer for forced expiratory volume in 1 s (FEV1) measurement on postoperative days (POD) 1 and 2. In the postoperative recovery unit, respiratory rate measurement and arterial gas analysis were performed at room air, and the partial pressure of arterial oxygen (PaO2) and carbon dioxide (PaCO2) were assessed. Patients were discharged from postanesthesia care unit (PACU) when the modified Aldrete score was 9, and the time of discharge was recorded. Postoperative pulmonary complications including atelectasis and pneumonia, time to chest tube removal, incidence of prolonged air leakage and length of hospital stay were documented for all the patients.
Figure 1.
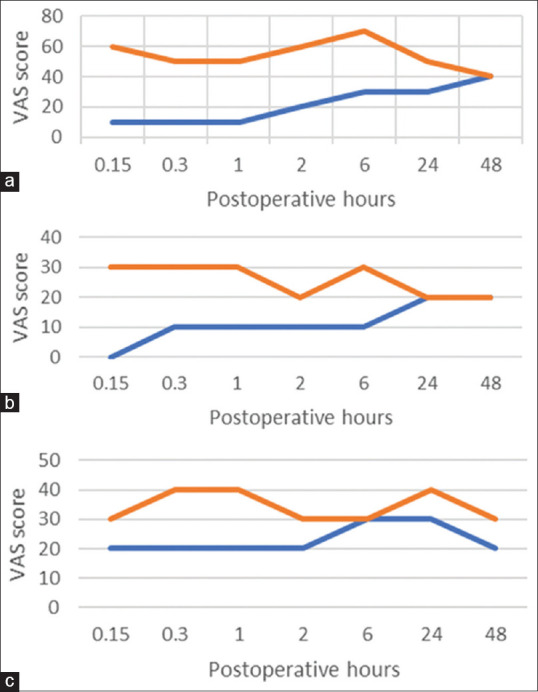
Mean visual analog scale pain scores (a) at rest, (b) on coughing and (c) during mobilization from the supine to the sitting position
Statistical analysis
All values were reported as mean ± standard deviation for continuous variables and numbers (percentages) for categorical variables. Parameters showing a nonnormal distribution are expressed as medians and interquartile ranges. Intergroup comparisons were performed and analyzed using an unpaired t-test, Mann–Whitney U-test for continuous variables and Fischer's exact test for categorical variables. P < 0.05 was considered statistically significant.
RESULTS
A total of 84 adult patients scheduled to undergo VATS were assessed for the study. Four patients were withdrawn from the study and 80 patients were randomly assigned to each group. As shown in Table 1, the demographic and clinical characteristics showed no significant differences between the groups.
Table 1.
Patient and perioperative data
| Group D | Group C | |
|---|---|---|
| Age (years) | 54±8.21 | 56±2.43 |
| Gender, male/female, n | 38/2 | 35/5 |
| Weight (kg) | 61±5.2 | 58±3.3 |
| ASA status, n(%) | ||
| II | 32 (80) | 31 (77.5) |
| III | 8 (20) | 9 (22.5) |
| Smoking, n(%) | ||
| Past/current smoker | 28 (70) | 29 (72.5) |
| Nonsmoker | 12 (30) | 11 (27.5) |
| Pulmonary function tests | ||
| FEV1, L | 2.5±0.8 | 2.6±0.6 |
| FEV1, % predicted | 95.1±11.1 | 96.3±12 |
| FVC, L | 3.1±0.4 | 2.92±0.4 |
| FVC, % predicted | 95.1±12.3 | 94.7±14 |
| FEV1/FVC (%) | 80.6±5.4 | 89.0±3.4 |
| DLCO(%) | 98.1±0.5 | 97.2±0.8 |
| Duration of surgery (min) | 156±12 | 163±16 |
| Type of surgery | ||
| Lobectomy | 21 | 17 |
| Segmentectomy | 11 | 12 |
| Wedge resection | 8 | 11 |
Data are expressed as mean±SD. FEV1=Forced expiratory volume in 1 s, FVC=Forced vital capacity, DLCO=Diffusion capacity of carbon monoxide, SD=Standard deviation, ASA=American society of anesthesiologists
Patients in the dexmedetomidine group experienced better analgesia for first 24 h following surgery as depicted by lower VAS values at rest, on coughing and on mobilization from supine to sitting position [Figure 1]. Moreover, the need for rescue analgesic was significantly reduced by dexmedetomidine. PaO2 measured in PACU was higher in Group D compared with the control group. Although the respiratory rate was lower in the dexmedetomidine group compared with the control group, PaCO2 was not different between the groups. FEV1 was significantly greater in Group D compared with the control group on POD 1 and 2 [Table 2].
Table 2.
Postoperative data
| Group D | Group C | |
|---|---|---|
| Respiratory rate, n | 16±5 | 21±3 |
| PaO2, mmHg | 88±8.2 | 78±9.1 |
| PaCO2, mmHg | 41±6.5 | 42±5.1 |
| FEV1, POD 1, L | 2.2±0.4 | 1.8±0.5 |
| FEV1, POD 2, L | 2.3±0.5 | 2.0±0.3 |
| Rescue analgesics | ||
| n(%) | 29 (72.5) | 37 (92.5) |
| Doses | 63 | 101 |
Data are presented as mean±SD or n(%). PaO2=Partial pressure of arterial oxygen, PaCO2=partial pressure of carbon dioxide, POD=Postoperative day, FEV1=Forced expiratory volume in 1 s, SD=Standard deviation
As shown in Table 3, the incidence of postoperative complications such as atelectasis, pneumonia, and prolonged air leakage was not significantly different between the groups. The length of hospital stay was significantly shorter in the dexmedetomidine group compared with the control group.
Table 3.
Postoperative complications and length of hospital stay
| Group D | Group C | |
|---|---|---|
| Complications, n | ||
| Atelectasis | 3 | 2 |
| Pneumonia | 2 | 2 |
| Time to chest tube removal, days | 2 (1-7) | 3 (1-8) |
| Prolonged air leakage, n(%) | 4 (10) | 6 (15) |
| Length of hospital stay, days | 7 (3-9) | 10 (6-13) |
Data are presented as n(%) or median (IQR). IQR=Interquartile range
DISCUSSION
VATS is generally considered to be a less invasive and painful offering several advantages, such as quicker recovery, enhanced curability and shorter duration of hospital stay. Surprisingly, VATS is associated with a prevalence of chronic pain comparable to that of open procedures, with rates of pain ranging from 23% to 63%, which is probably due to intercoastal nerve and muscle damage from trocar insertion.[16,17,18,19] Intraoperative nociception represents only a small portion of the noxious stimuli that could ultimately sensitize the central nervous system (CNS), exacerbating acute pain and initiating chronic pain.
Lung volumes after thoracic surgery may be reduced by up to 50% and aggressive analgesic therapy leads to improvement in lung mechanics and reduced rates of postoperative pulmonary complications.[20,21]
Although thoracic epidural and paravertebral analgesic regimens have been studied and recommended, systemic analgesics are alternative to more invasive techniques. Even the systemically administered opioids through a patient-controlled pumps produce a potential side effects of respiratory depression and hypoventilation.[22]
Dexmedetomidine is an imidazole compound demonstrates specific and selective α2 adrenoreceptor agonistic activity. Presynaptic activation of the α2 receptor inhibits the release of noradrenaline, terminating the propagation of pain signals. Postsynaptic activation of α2 receptor in the CNS inhibits sympathetic activity. These effects in combination produce anxiolysis, sedation and analgesia. The locus ceruleus in the CNS has highest density of α2 receptors and is also the site of origin of descending noradrenergic pathway involved in the modulation of nociceptive transmission suggesting the supraspinal action of dexmedetomidine.[9]
In one preliminary multicenter study,[23] Venn et al. demonstrated intense analgesic properties of dexmedetomidine in postoperative cardiac and general surgical patients. The study has shown that dexmedetomidine significantly reduced the requirements of rescue analgesic and sedative medications for up to 24 h. Patients were calmly and easily aroused from sleep to allow excellent communication and cooperation while intubated and ventilated.
In our study, dexmedetomidine showed significant analgesic efficacy and improved postoperative pulmonary functions in the initial 24 h. It enhances the analgesic effects of coadministered opioids. These results are in consistent with previous studies on postoperative patients. Dexmedetomidine has a relatively short elimination half-life of 2–2.5 h.[24] The reasons for long-lasting effects of dexmedetomidine are yet to be elucidated.
Belleville et al.[25] reported of irregular breathing patterns and obstructive sleep apnea in healthy young patients who received high doses of dexmedetomidine. These results were probably attributable to rapid and deep sedation as well as patient anatomical variables. This study also demonstrated marked sedation, mild hypercapnia and a decrease in minute ventilation with little change in respiratory rate and an early transient increase in oxygen consumption. In our study, dexmedetomidine significantly improved oxygenation and lung mechanics in the immediate postoperative period. Pain modulation and opioid-sparing properties of dexmedetomidine are desirable to enhance recovery after thoracic procedures.
This study has several limitations. First, this trial has not studied the effects of dexmedetomidine on intraoperative hemodynamics, as it was conducted on patients with good cardiorespiratory reserve. Although dexmedetomidine administration has been related to the incidence of bradycardia and hypotension,[9] such side effects were not encountered in our study. Therefore, additional studies are needed to investigate the effects of dexmedetomidine during one-lung ventilation. Second, the beneficial effects of dexmedetomidine on the prevention of neuropathic pain were not studied. Further investigations are required to focus and report the incidence of chronic pain after VATS.
CONCLUSIONS
In conclusion, our results suggested that intraoperative dexmedetomidine administration could improve the quality of analgesia and postoperative lung function in patients undergoing VATS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Li WW, Lee RL, Lee TW, Ng CS, Sihoe AD, Wan IY, et al. The impact of thoracic surgical access on early shoulder function: Video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg. 2003;23:390–6. doi: 10.1016/s1010-7940(02)00795-9. [DOI] [PubMed] [Google Scholar]
- 2.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–5. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 3.Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin. 2008;26:355–67, vii. doi: 10.1016/j.anclin.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: Experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–5. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 5.Landreneau RJ, Mack MJ, Hazelrigg SR, Naunheim K, Dowling RD, Ritter P, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg. 1994;107:1079–85. doi: 10.1097/00132586-199412000-00051. [DOI] [PubMed] [Google Scholar]
- 6.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: A non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899–904. doi: 10.2147/JPR.S139387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren C, Chi M, Zhang Y, Zhang Z, Qi F, Liu Z. Dexmedetomidine in postoperative analgesia in patients undergoing hysterectomy. A CONSORT-prospective, randomized controlled trial. Medicine (Baltimore) 2016;95:2854–60. doi: 10.1097/MD.0000000000001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong B, Lim C, Kang H, Eom H, Kim Y, Cho HJ, et al. Thoracic paravertebral block with adjuvant dexmedetomidine in video-assisted thoracoscopic surgery: A randomized, double-blind study. J Clin Med. 2019:8. doi: 10.3390/jcm8030352. pii: E352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Shim JK, Song JW, Kim EK, Kwak YL. Dexmedetomidine added to an opioid-based analgesic regimen for the prevention of postoperative nausea and vomiting in highly susceptible patients: A randomised controlled trial. Eur J Anaesthesiol. 2016;33:75–83. doi: 10.1097/EJA.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 12.Hoy SM, Keating GM. Dexmedetomidine: A review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011;71:1481–501. doi: 10.2165/11207190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Sairaku A, Yoshida Y, Hirayama H, Nakano Y, Ando M, Kihara Y. Procedural sedation with dexmedetomidine during ablation of atrial fibrillation: A randomized controlled trial. Europace. 2014;16:994–9. doi: 10.1093/europace/eut363. [DOI] [PubMed] [Google Scholar]
- 14.Ge DJ, Qi B, Tang G, Li JY. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal colectomy: A CONSORT-prospective, randomized, controlled clinical trial. Medicine (Baltimore) 2015;94:e1727. doi: 10.1097/MD.0000000000001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekker A, Haile M, Kline R, Didehvar S, Babu R, Martiniuk F, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol. 2013;25:16–24. doi: 10.1097/ANA.0b013e31826318af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5:89–96. doi: 10.1053/eujp.2001.0225. [DOI] [PubMed] [Google Scholar]
- 17.Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: A follow-up study. Acta Anaesthesiol Scand. 1999;43:563–7. doi: 10.1034/j.1399-6576.1999.430513.x. [DOI] [PubMed] [Google Scholar]
- 18.Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of chronic pain after thoracic surgery. Anesthesiology. 2017;126:938–51. doi: 10.1097/ALN.0000000000001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104:594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Brocki BC, Westerdahl E, Langer D, Souza DSR, Andreasen JJ. Decrease in pulmonary function and oxygenation after lung resection. ERJ Open Res. 2018;4(11) doi: 10.1183/23120541.00055-2017. pii: 00055-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebio R, Gimenez E, Garcia A, Sanesteban Y, Yanez I. Exercise capacity after video assisted thoracic surgery for lung cancer. Eur Respir J. 2014;44:P(4291). [Google Scholar]
- 22.Shapiro A, Zohar E, Zaslansky R, Hoppenstein D, Shabat S, Fredman B. The frequency and timing of respiratory depression in 1524 postoperative patients treated with systemic or neuraxial morphine. J Clin Anesth. 2005;17:537–42. doi: 10.1016/j.jclinane.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: A pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998;89:574–84. doi: 10.1097/00000542-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans I Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]


