In normal homeostasis, cancer-defense or stem cell therapy, epidermal progenitors undergo constant competition to reach an equilibrium state. In this issue of Cell Stem Cell, Mesa et al. (2018) and Murai et al. (2018) show skin epidermal progenitors maintain tissue homeostasis through competitive equilibrium under physiological self-renewal or oncogenic conditions.
Locating at the outermost of the body, skin epidermis protects the organism from environmental insults. The epidermis is composed of multi-layered keratinocytes which undergo continuous renewal. Normally proliferation is seen only in the basal layer, where the stem cells reside. Homeostasis reached by controlling cell division, differentiation and shedding results in the formation of region-specific epidermal thickness. Yet whether the stem cells decide to divide through the control of an intrinsic clock, or in response to the extrinsic interplay of local cells and their environment has not been elucidated. Here two new studies in this issue of Cell Stem Cell from the groups of Valentina Greco (Mesa et al, 2018) and Phil Jones (Murai et al., 2018),, together with other studies (Deschene et al., 2014; Lynch et al., 2017), provide insights into this question and reveal competition within the epidermis
Mesa et al., (2018) used intravital imaging to study the plantar skin of transgenic mice with membrane or nuclear labeling. They examined the proliferation or differentiation status of individual cells and tracked the subsequent behavior of neighboring cells directly surrounding these events. They observed that division and differentiation events occur in nearly equal numbers within a 7-day window. Quantitative measurements demonstrated that there is one extra cell division in cells that are located directly adjacent to cells completing differentiation, suggesting that self-renewal in the adult epidermis may be driven by the differentiation events of their neighboring cells. To test this hypothesis, they examined the order of differentiation and proliferation events between adjacent epidermal cells. By removing the terminally differentiated epidermal layer which activates both differentiation and proliferation of basal cells, they observed that cell differentiation (in 12 hr) precedes proliferation (in 24 hr) during repair. On the other hand, inhibition of cell division with inhibitors (e.g., Mitomycin C) causes decreased cell density in the basal layer but doesn’t alter cell differentiation, implying that proliferation is not required for differentiation.
The authors further analyzed how cell cycling control is affected by the differentiation of neighboring cells. Cell cycle analyses employing a Fucci-G1 reporter mouse shows that the duration of S/G2/M is a constant 12 hours, but the duration of G1 differs between cell cycles. Cells grow to their largest size and exit from G1 concurrently with the differentiation events of neighboring cells. This implies that when one epidermal progenitor cell differentiates, it provides an extra one-cell-size space, allowing the neighboring progenitor cells to expand their size. One of these cells, through stochasticity or by being more poised, takes over the space first, exits G1 and enters S/G2/M. Once the space is filled, other neighboring progenitor cells sense it and will not divide. To test whether the extra-space that temporally changes the local cell density is sufficient to drive cell division, they used sub-micron scale laser ablation, and a genetic mouse model (K14CreER combined with ROSA26-eGFP-DTA) to remove individual cells from the basal layer. Indeed, they observed the division of neighboring cells.
Together, these results show how tissue homeostasis occurs at single cell resolution in the epidermis. Coordination of extrinsic (limited space for expansion) and intrinsic factors (ability to divide) drives tissue to reach the equilibrium. When one space is released by differentiation or by laser ablation, more than one competent progenitor competes to fill the available space. These results are in line with the concept of the river of stem cells (Chuong and Widelitz, 2009). Thus, collectively, the epidermis can maintain tissue integrity robustly.
The work raises many interesting questions. One major question is how progenitor cells “sense” and respond to the activity of their neighbors. This extrinsic factor can be mediated by chemical factors released from differentiating neighboring cells or by a mechanical force elicited due to changes of cell adhesion and/or cortical tension (Miroshnikova et al., 2018). This is reminiscent of contact inhibition, a classical phenomenon related to growth control and cancer (Mendonsa et al., 2018), and we now can appreciate its implications in vivo.
Since human skin is often exposed to sun ultraviolet (UV) light, the normal appearing eyelid skin can carry 18 – 32% mutated epidermal cells. Some of these cells may contain oncogenic mutations but they don’t necessarily form skin tumors (Brown et al., 2017; Martincorena et al., 2015). How does the epidermis cope with these situations? Murai et al., (2018) used a mouse model carrying a heterozygous p53 mutation (Trp53R245W) that generates a dominant negative form of p53 to investigate the behavior of these cells and their progenies in a wild type keratinocyte background over a 9-month time course, under either normal conditions or with UV irradiation.
Lineage tracing shows that basal cells in wild-type mice have a constant proliferation and differentiation rate, whereas the single-allele p53 mutant cells (Trp53R245W) generate more progeny than the wild type cells, indicating that the mutant population has a competitive advantage over their wild type neighbors. Transmission electron microscopy and immunostaining for desmosomal proteins showed desmosome formation was disrupted in p53 mutant mice. This causes enhancement of the lateral migration of p53 mutant suprabasal cells, leading to an accumulation of cells in the first suprabasal layer. Intriguingly, this layer starts to disappear 6 months after formation. This finding implies that the mutant cells slowly adapt to the cellular environment and lose their competitive advantage, resulting in the maintenance of tissue integrity without tumor formation. To further test if one competitive advantage can be influenced or replaced by the other, the authors exposed the mutant epidermis to UV irradiation. UV exposure causes accelerated cell colonization by p53 mutant cells over the first 12 weeks, but the colonization is decreased with protracted UV exposure (e.g., 36 weeks). Ultra-deep targeted exome sequencing identified 58 mutations over 74 genes in the epidermis exposed to UV for 36 weeks but not in those exposed for 12 weeks. It is conceivable that a new competitive advantage is generated by the new mutations, which take over the basal compartment by constraining the p53 mutant clones, leading to a decreased population of p53 mutant cells.
Together, these two papers provide convincing evidence that the equilibrium achieved by phenotypic adaption and cell competition ensures that the epidermis maintains homeostasis and tissue integrity. Competitive equilibrium may be a way that tissues ensure normal development and homeostasis, and to defend against neoplasms (Figure 1), by adapting to ever-changing internal dynamics and external changes in contexts such as homeostasis, placode induction, tissue regeneration and somatic mutations. Beyond the epidermis, similar Darwinian type control may be in operation in the tissue homeostasis of other organ systems. Inspired by clonal selection of immunoglobulin producing cells, Dr. GM Edelman published the book “Neural Darwinism” in which he proposed that neural development operates as a selective system, resembling natural selection in evolution (Edelman, 1987). Here we paraphrased the title to show competitive equilibrium may be a norm underlying the robustness of tissue homeostasis.
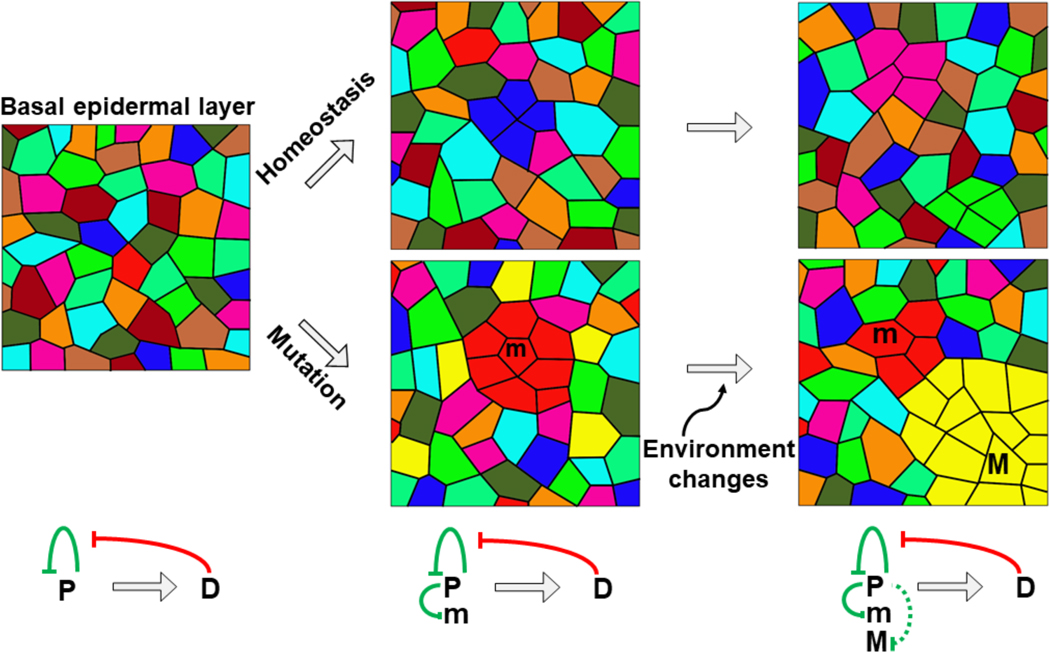
Figure 1.
Schematic illustration of epidermal cell behaviors under homeostasis or oncogenic conditions. Colors represent different progenitor cells. The top row shows that under normal condition, proliferation of progenitor cells (P) is maintained by negative feedback control exerting from differentiated cells (D). Collectively, tissue homeostasis is maintained by the competitive equilibrium among progenitor cells. The bottom row shows a single-allele mutated cells (m, dark red) can be regulated. External events such as UV irradiation can produce a clone (M, yellow) which is not regulated anymore.
However, significant questions remain. How do neighboring progenitor cells, having the same poised cell cycle state, outcompete others to fill the extra space and divide, or is it just a stochastic event? How do mutant cells (m) adapt to their cellular environment and revert to homeostasis? What is the threshold a mutant cell (M) has to surpass to break the equilibrium and form a tumor? The research direction illuminated here should shed light on the mechanisms of tissue homeostasis and pathogenesis control, thus providing new targets for the design of strategies to prevent malignant transformation.
Acknowledgement
Authors are supported by NIH grants AR 060306 in USA, and in Taiwan, MOST funding and Featured Areas Research Center Program to China Medical University / Hospital, Taiwan. We thank Dr. RB Widelitz for discussion.
References
- Brown S, Pineda CM, Xin T, Boucher J, Suozzi KC, Park S, Matte-Martone C, Gonzalez DG, Rytlewski J, Beronja S, et al. (2017). Correction of aberrant growth preserves tissue homeostasis. Nature 548, 334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, and Widelitz RB (2009). The river of stem cells. Cell Stem Cell 4, 100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschene ER, Myung P, Rompolas P, Zito G, Sun TY, Taketo MM, Saotome I, and Greco V (2014). beta-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science 343, 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM (1987). Neural Darwinism: The Theory of Neuronal Group Selection. Basic Books; New Ed edition, 371. [DOI] [PubMed] [Google Scholar]
- Lynch MD, Lynch CNS, Craythorne E, Liakath-Ali K, Mallipeddi R, Barker JN, and Watt FM (2017). Spatial constraints govern competition of mutant clones in human epidermis. Nat Commun 8, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB, Tubio JM, et al. (2015). Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonsa AM, Na TY, and Gumbiner BM (2018). E-cadherin in contact inhibition and cancer. Oncogene 37, 4769–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa KR, Kawaguchi K, Cockburn K, Gonzalez D, Boucher J, Xin T, Klein AM, and Greco V (2018). Homeostatic epidermal stem cell self-renewal is driven by local differentiation. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnikova YA, Le HQ, Schneider D, Thalheim T, Rubsam M, Bremicker N, Polleux J, Kamprad N, Tarantola M, Wang I, et al. (2018). Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat Cell Biol 20, 69–80. [DOI] [PubMed] [Google Scholar]
- Murai K, Skrupskelyte G, Piedrafita G, Hall M, Kostiou V, Ong SH, Nagy T, Cagan A, Goulding D, Klein AM, et al. (2018). Epidermal tissue adapts to restrain progenitors carrying clonal p53 mutations. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]