Abstract
Purpose
Patients with relapsed and/or refractory multiple myeloma (RRMM) are living longer due in part to changing treatment patterns. It is important to understand how changing treatment patterns affect patients’ lives beyond extending survival. Research suggests that direct patient report is the best way to capture information on how patients feel and function in response to their disease and its treatment. Therefore, the purpose of this review is to summarize evidence of patients’ experience collected through patient-reported outcomes (PRO) in RRMM patients, and to explore PRO reporting quality.
Methods
We conducted a systematic search to identify manuscripts reporting PROs in RRMM and summarized available evidence. We assessed PRO reporting quality using the Consolidated Standards of Reporting Trials (CONSORT) PRO Extension checklist.
Results
Our search resulted in 30 manuscripts. Thirteen unique PRO measures were used to assess 18 distinct PRO domains. Pain, fatigue, and emotional function were commonly assessed domains though reporting formats limited our ability to understand prevalence and severity of PRO challenges in RRMM. Evaluation of PRO reporting quality revealed significant reporting deficiencies. Several reporting criteria were included in less than 25% of manuscripts.
Conclusions
Existing evidence provides a limited window for understanding the patient experience of RRMM and is further limited by suboptimal reporting quality. Observational studies are needed to describe prevalence, severity and patterns of PROs in RRMM overtime. Future studies that incorporate PROs would benefit from following existing guidelines to ensure that study evidence and conclusions can be fully assessed by readers, clinicians and policy makers.
Keywords: Patient-reported outcomes, Relapsed/refractory multiple myeloma, Multiple myeloma, CONSORT PRO, Quality of life, Systematic review
Background
In 2019 there will be an estimated 32,110 new multiple myeloma (i.e., myeloma) cases, 12,960 myeloma deaths, and 131,392 people living with myeloma in the United States [1, 2]. Median age at diagnosis is 69 and more than 75% of new diagnoses happen between the ages of 55 and 84 [2]. New cases have increased 54% over the past decade and are forecasted to continue increasing due to an aging population [3–6]. Incidence is slightly higher in men than women, and two times higher in African Americans compared to other racial/ethnic groups [2].
While still incurable, this once acutely terminal illness has become chronic for many [5, 7, 8]. Overall median survival for myeloma was about 2 years before the year 2000 [9]. Today, overall median survival is more than 5 years, and is greater than 10 years for younger patients or those with less aggressive disease [7, 10, 11]. Improvements in survival have been made possible by the rapid introduction of new therapies beginning in 2003, and resulting changes in treatment patterns [9, 12].
It is important to understand how changing treatment patterns may affect patient’s lives beyond extending survival. Research suggests that symptom burden and health-related quality of life (HRQoL) for those with myeloma are quite poor [13–16]. Effects of myeloma and its treatment may impact HRQoL domains such as physical and emotional well-being, social functioning, and financial burden. These issues are particularly important in relapsed and/or refractory multiple myeloma (RRMM).1 RRMM represents a new stage of disease and treatment for patients, in which they move from treatment to treatment in hopes of maintaining control of their disease, until all treatments ultimately fail. The exposure to many lines of therapy that RRMM patients face increases risk of cumulative toxicities and burdensome side effects [18–20]. In light of the detrimental effects of RRMM and its treatment it is important to better understand the RRMM patient’s experience so interventions can be designed to reduce suffering. Previous reviews have explored the experiences of all myeloma patients and we feel the unique circumstances of patients with RRMM deserve to be explored separately [15, 21].
Research suggests that direct patient report is the best way to capture information on how patients feel and function in response to disease and treatment [22–24]. Historically these data were assessed and reported by clinicians. We now know that collecting this information through direct patient report improves detection, is more sensitive to change and more highly correlated to overall health status than clinicians’ assessments [22, 25, 26]. Additionally, these patient-reported outcomes (PROs) capture important data that lie outside of the traditional clinical outcomes of efficacy and safety and can help us to more completely understand how a myeloma diagnosis affects a patient’s life [27, 28].
PROs have become increasingly important in evaluating new therapies, and are also being used to inform clinical and health policy decision making [28–31]. To be useful, PRO evidence must be clearly and comprehensively reported [32]. In response, the CONSORT PRO Group proposed guidelines to improve reporting of PROs in randomized clinical trials [32]. The purpose of this review is to summarize what is known about PROs in people living with RRMM, and to evaluate PRO reporting quality using the CONSORT PRO Extension guidelines.
Methods
This review was conducted following PRISMA guidelines. Review protocol was registered with PROSPERO (https://www.crd.york.ac.uk/prospero/); Registration ID: CRD42019114886.
Search strategy
We conducted a systematic search of the following databases: PubMed, Comprehensive Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, SCOPUS and EMBASE in November 2018. Search terms, outlined in Table 1, were grouped into three major domains; (1) Multiple myeloma, (2) Relapsed/refractory, and (3) Symptoms and quality of life, and searched as both keywords and subject headings, where applicable. In addition, reference lists from eligible articles and previous systematic reviews were searched to identify additional relevant manuscripts. Our full search strategy can be found in the online supplemental material Table S1.
Table 1.
Search strategy
| Concepts | Search terms |
|---|---|
| Multiple myeloma | Multiple myeloma, myeloma, myelomas, myelomatosis, myelomatoses, Kahler’s disease |
| AND | |
| Relapsed/ refractory | Recurrence, neoplasm recurrence local, retreatment, relapse, relapses, relapsed, refractory, RRMM, recurrence, recurrences |
| AND | |
| Symptoms and quality of life | Quality of life, life quality, well being, well-being, health related quality of life, health-related quality of life, health status, function, functional, surveys and questionnaires, survey, questionnaire, measure, signs and symptoms, symptoms, nausea, nauseous, vomiting, constipation, diarrhea, diarrhea, shortness of breath, dyspnea, peripheral neuropathy, peripheral neuropathies, cognition, memory, pain, insomnia, sleep disturbance, fatigue, anxiety, anxieties, anxious, nervousness, depression, depressions, depressive, depressed, distress, burden, stress psychological, stress, stresses, suffering |
Study selection
Clinical trials, and observational studies assessing patient outcomes through direct report were eligible for inclusion. Criteria for inclusion were: (1) Study population was either exclusively adults (≥ 18 years old) with RRMM or separate results were reported for adults with RRMM; (2) Results included HRQoL domains, functional status, or symptoms assessed through direct patient report; (3) Written or published in English; and, (4) Published in or after 2003, when the proteasome inhibitor bortezomib was FDA approved for use in myeloma marking the beginning of significant changes in myeloma treatment and outcomes [33, 34]. Qualitative studies, editorials, commentaries, letters, reviews, published abstracts, conference proceedings, and unpublished studies were excluded.
Identified articles were downloaded into reference management software (EndNote X8.2, Clarivate Analytics, Philadelphia PA) to identify and remove duplicates and uploaded into a systematic review management platform (Covidence, Melbourne Australia) for screening and full text review by two independent reviewers, MRL and RH. Discrepancies were resolved through discussion. If consensus could not be reached, a third reviewer, ALB, made the final decision.
Data extraction
Data were extracted by author MRL into a standardized matrix developed and refined by MRL, RH, and ALB, and then applied across all included studies using an electronic spreadsheet (Excel 16.22, Microsoft, Seattle WA) [35]. The matrix captured information on study and sample characteristics, PRO measurement strategies, and study instruments and results. Online supplemental material, primary study reports and clinicaltrials.gov records were referenced when available for additional study information.
Assessing PRO reporting quality
Clear and comprehensive reporting of study results help ensure that results can be understood, assessed and useful to guide care and policy. To this end, the Consolidated Standards of Reporting Trials (CONSORT) statement was developed to improve the quality of randomized clinical trial reporting [36]. In February 2013 an extension was published providing guidance for reporting PROs in randomized clinical trials in which PROs were primary or secondary endpoints [32]. Regardless of study design, all study reports benefit from clear and comprehensive reporting in ways that allow readers to fully assess the evidence presented. In the absence of guidelines specific to non-RCT study designs (e.g., single arm clinical trials, observational studies) we applied the CONSORT PRO guidelines across study designs. We believe the CONSORT PRO Extension criteria provide a useful way to assess PRO reporting in a variety of study designs, though it exceeds its intended purpose.
We developed a standardized rubric based on the CONSORT PRO guidelines to evaluate PRO reporting quality (online supplementary material Table S2). The rubric contains 15 criteria each scored ‘1’ if met and ‘0’ if not met. Possible scores ranged from 0 to 15. Two CONSORT PRO criteria require assessment across multiple timepoints and are less applicable to cross-sectional studies; ‘Assessment timepoints specified’ and ‘Number of PRO participants at each timepoint reported’. Several scoring strategies were considered for cross-sectional studies, including omitting these two criteria when scoring or scoring them ½ point each if met for a study’s one timepoint. Either of these strategies would lower the potential scores cross-sectional studies could achieve. We decided instead to award a full point for these criteria if met for a cross-sectional study’s one timepoint, in order to avoid reducing potential scores by default. Two of our authors, MRL and RH used this rubric to independently score PRO reporting quality. Scoring discrepancies were resolved through discussion.
Please note PRO CONSORT extension guidelines are meant to evaluate reporting quality of PRO results and not the psychometric soundness of the PRO measures (PROMs) used. Also note suboptimal reporting quality does not indicate that reported results are invalid or that studies were not conducted rigorously. Suboptimal reporting quality does however make it difficult for readers to assess a study’s evidence and conclusions.
Results
Our literature search identified 5426 manuscripts, of which 2655 duplicates were removed; 2771 manuscripts were screened for eligibility. Screening and full text review resulted in 30 manuscripts representing 23 separate studies. Results of the search strategy and screening process are documented in Fig. 1 according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [37].
Fig. 1.
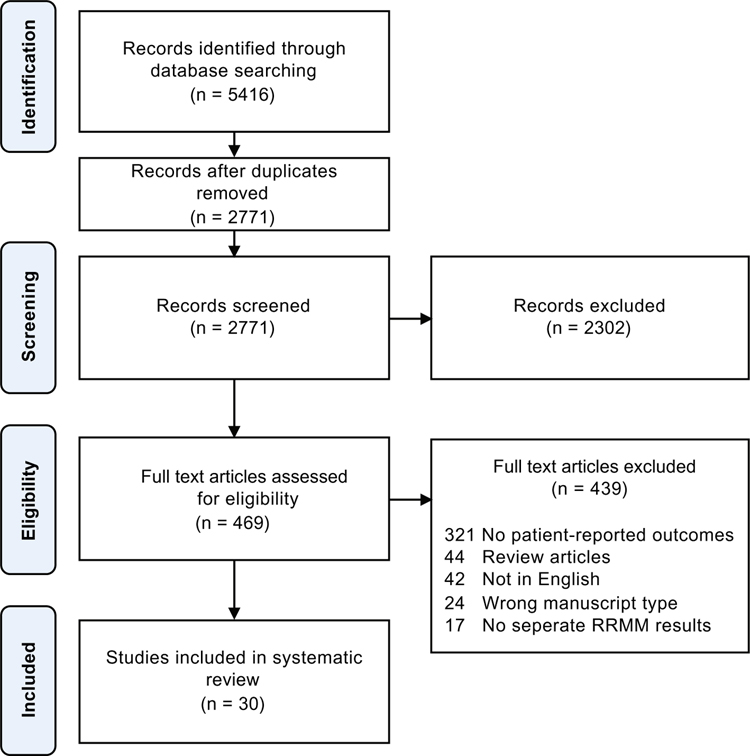
PRISMA diagram
In some cases, two or more manuscripts reporting PRO results, originated from a single clinical trial, often a primary report focused on efficacy and safety results and a secondary report focused on patient-reported outcomes. For example, 25 manuscripts included in this review were the result of 18 clinical trials. Fifteen manuscripts report primary efficacy/safety results from clinical trials and ten manuscripts represent secondary reports focused on PRO results. Additionally, five manuscripts reported results of observational studies. Eight studies were conducted in Europe, nine internationally, five in the United States of America, and one in the Middle East. Sixteen (53.3%) manuscripts were published after the CONSORT PRO guidelines were published in February, 2013 [32]. Selected study characteristics are presented in Table 2.
Table 2.
Selected study characteristics
| Clinical trials | PRO reporting qualitya | ||||
|---|---|---|---|---|---|
| Citation sample size | Study design | PRO endpoint | PRO measures | Reporting format | |
| Richardson et al. 2003 [40] n = 202 |
Phase 2 open label single arm clinical trial | Secondary | EORTC QLQ-C30b EORTC QLQ-MY24c |
Change over time | 2 |
| Dubois et al. 2006 [41] n = 202 |
Primary | FACT/GOG-NTXd FACIT Fatiguee |
8 | ||
| Jagannath et al. 2004 [55] n = 54 |
Phase 2 open label randomized clinical trial | Secondary | FACT/GOG-NTX | Change over time | 6 |
| Richardson et al. 2006 [56] n = 256 |
Primary | 10 | |||
| Waage et al. 2004 [57] n = 65 |
Phase 2 open label single arm clinical trial | Primary | EORTC QLQ-C30 | Mean PROM scores | 9 |
| Lee et al. 2008 [58] n = 598 |
Phase 3 open label randomized clinical trial | Primary | EORTC QLQ-C30 FACT/GOG-NTX |
Between group differences in change over time | 12 |
| Richardson 2009 [59] n = 296 |
Primary | 10 | |||
| Vij et al. 2009 [60] n = 96 |
Phase 2 open label single arm clinical trial |
Exploratory | BPI-SFf FACT-Gg EuroQol 5Dh |
Change over time | 6 |
| Alegre et al. 2012 [42] n = 63 |
Open label single arm clinical trial | Secondary | EORTC QLQ-C30 EORTC QLQ-MY20i |
Change over time | 8 |
| Hjorth et al. 2012 [61] n = 131 |
Phase 3 open label randomized clinical trial | Secondary | EORTC QLQ-C30 | Change over time, group differences |
10 |
| Safaee et al. 2012 [62] n = 30 |
Historical control trial | Secondary | VAS Painj | Mean PROM scores | 9 |
| Vij et al. 2012 [63] n = 35 |
Phase 2 open label single arm clinical trial | Secondary | FACT/GOG-NTX | Change over time | 8 |
| Vij et al. 2012 [64] n = 129 |
3 | ||||
| Richardson et al. 2013 [65] n = 55 |
Phase 2 open label single arm clinical trial | Secondary | FACT/GOG-NTX subscale | Change over time | 4 |
| Callander et al. 2015 [66] n = 32 |
Unspecified two arm clinical trial | Primary | FACT/GOG-NTX FACIT Fatigue |
Mean PROM Scores, Change over time | 40 |
| Lonial et al. 2015 [67] n = 646 |
Phase 3 open label randomized controlled trial | Exploratory | BPI-SF EORTC QLQ-C30 EORTC QLQ-MY20 |
Between group differences in change over time | 3 |
| Cella et al. 2018 [44] n = 646 |
Primary | 9 | |||
| Stewart et al. 2015 [68] n = 792 |
Phase 3 open label randomized controlled trial | Secondary | EORTC QLQ-C30 EORTC-MY20 |
Group differences | 6 |
| Stewart et al. 2016 [45] n = 792 |
Primary | 15 | |||
| Weisel et al. 2015 [69] n = 433 |
Phase 3 open label randomized controlled trial | Primary | EORTC QLQ-C30 EORTC-MY20 |
Between group differences in change over time | 12 |
| Moreau et al. 2016 [70] n = 722 |
Phase 3 double blind randomized placebo-controlled trial | Secondary | EORTC QLQ-C30 EORTC-MY20 |
Between group differences in change over time | 3 |
| Leleu et al. 2018 [47] n = 722 |
Secondary | 13 | |||
| Baljevic et al. 2017 [43] n = 16 |
Phase 2 open label single arm clinical trial | Exploratory | EORTC QLQ-C30 EORTC-MY20 MDASI-MMk |
Change overtime | 3 |
| Richardson et al. 2017 [46] n = 768 |
Phase 3 double blind randomized placebo-controlled trial | Secondary | EORTC QLQ-C30 GHS/QOL subscalel | Change overtime | 3 |
| Robinson et al. 2017 [71] n = 263 |
Phase 2 double blind randomized placebo-controlled clinical trial | Primary | Economic Questionnaire EORTC QLQ-C30 FACIT Fatigue |
Mean PROM scores | 10 |
| Observational studies | PRO reporting quality | ||||
| Citation sample size | Study design | Measures | Results reporting format | ||
| Basile et al. 2010 [72] n = 3 |
Retrospective review | Longitudinal | VAS Pain | Mean PROM scores | 9 |
| Briani et al. 2013 [73] n = 30 |
Prospective longitudinal cohort | Longitudinal | NRS Painm | Change overtime | 5 |
| Ramsenthaler et al. 2016 [16] n = 182 |
multi-site cross-sectional cohort | Cross-sectional | EORTC QLQ-C30 EORTC QLQ-MY20 EuroQol 5D MyPOSn |
Mean PROM scores | 15 |
| Samuelson et al. 2016 [74] n = 32 |
cross-sectional cohort | Cross-sectional | EORTC QLQ-C30 | Correlations with BNP | 7 |
| Leleu et al. 2017 [75] n = 258 |
Prospective longitudinal cohort | Longitudinal | EORTC QLQ-C30 EORTC QLQ-MY20 EORTC QLQ-CIPN20o |
Mean PROM score, change overtime | 11 |
PRO Reporting Quality Possible scores range from 0 to 15. Higher scores indicate higher quality.
EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30.
EORTC QLQ-MY24 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Myeloma 24.
FACIT Fatigue Functional Assessment of Chronic Illness Therapy Fatigue.
FACT/GOG-NTX Functional Assessment of Cancer Therapy Gynecologic Oncology Group Neurotoxicity.
BPI-SF Brief pain inventory short form.
FACT-G Functional Assessment of Cancer Therapy General.
EuroQOL 5d European Quality of Life Five Dimension.
EORTC QLQ-MY20 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Myeloma 20.
VAS Pain Visual Analog Scale Pain.
MDASI-MM MD Anderson Symptom Index Multiple Myeloma.
EORTC QLQ-C30 GHS/QOL European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 Global Health Status/Quality of Life subscale.
NRS Pain Numeric Rating Scale Pain.
MyPOS Myeloma Palliative care Outcome Scale.
EORTC QLQ-CIPN20 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy Induced Peripheral Neuropathy 20
Study participant characteristics
A total of 5635 RRMM patients were included in the studies reported here. Sample size ranged from 3 to 792 with an overall mean sample size of 235. Mean sample size for clinical trials was 270 and for observational studies 101. Median age was 63.5 years, and ages ranged from 27 to 93. Overall, samples contained more males (57.0%) mirroring the higher incidence of myeloma among men in the United States (56.0%) and globally (54.4%) [38, 39]. Of the twelve studies that reported race/ethnicity data, the combined sample was 82.2% white, 6.1% black, and 10.0% other.
Summarizing patient-reported outcomes in RRMM
Nine individual symptoms and nine PRO domains were reported across studies using 13 unique PROMs (Table 3). Individual symptoms refer to specific physical or emotional effects of RRMM or its treatment. PRO domains refers to more integrated concepts, which include collections of symptoms measured and reported together (i.e., symptom indexes), or concepts that involve symptoms, effects on function, and/or changes in behaviors together (e.g., physical well-being). Many PROs were assessed with a variety of PROMs. For example, the outcome ‘Global Health Status/QoL’ was assessed using three unique PROMs.
Table 3.
Patient-reported outcomes (PROs)
| PROs assessed | PROs reported | PRO measures | |
|---|---|---|---|
| PRO Domains | |||
| Emotional function | 26 | 11 | EORTC QLQ-C30a EORTC QLQ-MY20b FACT-Gc MyPOSd |
| Global health satus/QoL | 19 | 16 | EORTC QLQ-C30 FACT-G EuroQol 5De |
| Physical function | 16 | 14 | EORTC QLQ-C30 FACT-G |
| Social function | 16 | 10 | EORTC QLQ-C30 FACT-G |
| Financial burden | 16 | 6 | EORTC QLQ-C30 Financial burden |
| Role function | 15 | 8 | EORTC QLQ-C30 |
| Cognitive function | 15 | 8 | EORTC QLQ-C30 |
| Symptom index | 12 | 8 | EORTC QLQ-MY20 FACT-G MyPOS MDASI-MMf |
| Healthcare factors | 2 | 2 | Financial burden MyPOS |
| Individual symptoms | |||
| Pain | 20 | 15 | BPI-SFg EORTC QLQ-C30 NRS painh VAS paini |
| Fatigue | 18 | 11 | EORTC QLQ-C30 FACIT fatiguej |
| Dyspnea | 15 | 8 | EORTC QLQ-C30 |
| Constipation | 15 | 7 | EORTC QLQ-C30 |
| Nausea/vomiting | 15 | 7 | EORTC QLQ-C30 |
| Diarrhea | 15 | 6 | EORTC QLQ-C30 |
| Appetite loss | 15 | 6 | EORTC QLQ-C30 |
| Sleep disturbance | 15 | 6 | EORTC QLQ-C30 |
| Peripheral neuropathy | 8 | 8 | EORTC QLQ-CIPN20k FACT/GOG-NTXl |
EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30.
EORTC QLQ-MY20 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Myeloma 20.
FACT-G Functional Assessment of Cancer Therapy General.
MyPOS Myeloma Palliative care Outcome Scale.
EuroQOL 5d European Quality of Life Five Dimension.
MDASI-MM MD Anderson Symptom Index Multiple Myeloma.
BPI-SF Brief pain inventory short form.
NRS Pain Numeric Rating Scale Pain.
VAS Pain Visual Analog Scale Pain.
FACIT Fatigue Functional Assessment of Chronic Illness Therapy Fatigue.
EORTC QLQ-CIPN20 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy Induced Peripheral Neuropathy 20.
FACT/GOG-NTX Functional Assessment of Cancer Therapy Gynecologic Oncology Group Neurotoxicity
Individual symptoms
Pain was the most commonly assessed symptom, followed by fatigue, dyspnea, nausea/vomiting, and constipation (Table 3). PRO results for symptoms were most often reported as change from baseline scores (e.g., improved, maintained, or worsened from baseline), as group differences in change, or as between group differences, without reporting baseline prevalence or severity (Table 2). Six studies reported baseline PROM scores though with limited information on how to interpret scores. Four studies reported individual symptom results grouped by treatment response [40–44]. In each case, better treatment response was associated with better symptom outcomes.
Pro domains
‘Emotional Function’ was the most commonly assessed PRO domain, followed by ‘Global Health status/QoL’, ‘Physical Function’, and ‘Social Function’. Results for PRO domains were also most often reported as change from baseline, group differences in change or between group differences. Five studies compared PRO domains between patients grouped by disease response. As with individual symptoms, patients who experienced a complete or partial response had better outcomes than those with minimal responses or disease progression [40–42, 45–47].
Characteristics of PRO reporting in RRMM
Our analysis of PRO reporting quality occurred at the manuscript level using a grading rubric based on the CONSORT PRO Extension checklist. Scores indicate the number of CONSORT PRO Extension criteria met. Possible scores ranged from 0 to 15. Higher scores indicate more met criteria and higher reporting quality. Individual PRO reporting quality scores across 25 clinical trial and five observational study manuscripts along with selected study characteristics are presented in Table 2.
Table 4 presents reporting quality scores evaluated across several study design characteristics. Overall mean reporting quality score was 8.0 out of a possible 15, indicating that on average, manuscripts did not meet 7 CONSORT PRO criteria. Scores ranged from 2 to 15. Reporting quality scores were higher in observational studies (9.4, n = 5) than in clinical trials (7.7, n = 25). Within clinical trials, the highest scores were achieved by manuscripts for which a PRO was identified as a primary endpoint (10.5, n = 10) and for manuscripts that were secondary reports of study results focused on PROs (10.2, n = 10). The mean scores for cross-sectional studies was 11.0 (n = 2) and for longitudinal studies (clinical trials, or longitudinal observational studies) was 7.8 (n = 28). Mean scores for manuscripts published before the introduction of the CONSORT PRO guidelines were 7.9 (n = 14) and for manuscripts published after, 8.1 (n = 16).
Table 4.
PRO reporting quality score
| Study design | n | CONSORT PRO score | |
|---|---|---|---|
| m | Range | ||
| All manuscripts | 30 | 8.0 | 2–15 |
| Pre 2013 | 14 | 7.9 | 2–12 |
| Post 2013 | 16 | 8.1 | 3–15 |
| Clinical trials | 25 | 7.7 | 2–15 |
| Phase | |||
| II | 11 | 6.3 | 2–10 |
| III | 11 | 8.7 | 3–15 |
| Unspecified | 3 | 9.0 | 8–10 |
| Design | |||
| Randomized trial | 14 | 8.7 | 3–13 |
| Single arm | 9 | 5.7 | 2–9 |
| Historical control | 1 | 9.0 | ~ |
| Unspecified | 1 | 10.0 | ~ |
| PRO endpoint | |||
| Primary | 10 | 10.5 | 8–15 |
| Secondary | 12 | 6.3 | 2–13 |
| Exploratory | 3 | 4.0 | 3–6 |
| Manuscript type | |||
| Primary efficacy/safety report | 15 | 6.0 | 2–10 |
| PRO focused secondary report | 10 | 10.2 | 3–15 |
| Observational | 5 | 9.4 | 5–15 |
| Longitudinal | 3 | 8.3 | 5–11 |
| Cross-sectional | 2 | 11.0 | 7–15 |
PRO patient-reported outcome. Possible scores range from 0 to 15. Higher scores indicate higher quality
The most commonly adhered to items from the CONSORT PRO criteria checklist included ‘PRO data is interpreted in relation to clinical outcomes’ (93%) ‘Assessment timepoints specified’ (90%), ‘PRO identified in abstract as primary or secondary outcome’ (73%) and ‘PRO results for each domain presented’ (73%), (see Table 5). The least commonly adhered to items from the checklist included ‘PRO hypothesis stated’ (10%), ‘Method of questionnaire administration specified’ (17%), ‘Statistical approaches for missing data specified’ (23%) and ‘PRO specific limitations and implications for generalizability and clinical practice’
Table 5.
CONSORT PRO extension criteria
| Criteria | Manuscripts meeting criteria n = 30 (%) |
|---|---|
| 1. PRO identified in abstract as primary or secondary outcome | 22 (73.3) |
| 2. Background and rationale for PRO assessment | 18 (60.0) |
| 3. PRO hypothesis stated | 3 (10.0) |
| 4. Relevant domains identified | 21 (70.0) |
| 5. Present evidence and/or reference of reliability and validity for PRO instruments | 15 (50.0) |
| 6. Person completing PRO specified | 17 (56.7) |
| 7. Method of administration specified | 5 (16.7) |
| 8. Assessment timepoints specified | 27 (90.0) |
| 9. Statistical methods for analyzing PRO data specified | 18 (60.0) |
| 10. Statistical approaches for missing data specified | 7 (23.3) |
| 11. Number of PRO participants at each timepoint reported | 12 (40.0) |
| 12. Baseline PRO data reported | 16 (53.3) |
| 13. PRO results for all domains reported | 22 (73.3) |
| 14. PRO specific limitations and implications for generalizability and clinical practice | 8 (26.7) |
| 15. Patient-reported outcome data are interpreted in relation to clinical outcomes including survival data | 28 (93.3) |
CONSORT PRO consolidated standards of reporting trials patient-reported outcomes. patient-reported outcome,
Discussion
Our systematic review aimed to summarize evidence of patients’ experience collected through patient-reported outcomes (PRO) in RRMM patients and assess PRO reporting quality. We identified thirteen unique PROMs used to assess 18 PROs. We found it difficult to summarize patient experience given the contours of the available evidence and the format in which it is reported. Additionally, we identified suboptimal PRO reporting quality across studies as measured by the CONSORT PRO Extension checklist.
PRO results were often reported in a format that precluded readers from understanding the severity of symptoms or PRO domain problems. The majority of manuscripts report change in PRO scores without reporting baseline values and/or prevalence data. This reflects the current mix of study designs in RRMM PRO research, which is predominated by clinical trials. The practice of reporting change scores, or group differences aligns well with the purposes of clinical trials, however it limits our ability to summarize evidence or make conclusions about the burden (prevalence, severity) of symptoms and HRQoL issues in RRMM patients. Key questions about the prevalence, severity and patterns of symptoms and other patient challenges remain unanswered. Clinical trial designs have other limitations that must be considered, for example highly selected trial populations may not reflect the general population of patients [48]. A diverse mixture of study designs is needed to comprehensively capture the experiences of patients with RRMM. Observational cohort studies that focus on describing PROs (prevalence, severity, patterns over time) across the trajectory of myeloma treatment could further inform the relationship between treatment patterns and patient outcomes such as symptom burden and HRQoL issues. There is also an opportunity assess PROs not explored in studies reported here such as patient clinician communication, self-efficacy in managing chronic illness, and fear of recurrence. Additionally, non-cancer specific PROs deserve attention, especially given patients with myeloma are likely older and experiencing multiple comorbid conditions affecting their quality of life [49]. Further, in light of the limited racial/ethnic diversity in existing studies, future work should prioritize recruiting racially representative samples and begin to explore relationships between race/ethnicity and PROs.
Our review concluded that overall PRO reporting quality in RRMM studies is suboptimal, with wide variability. As may be expected, manuscripts that included PRO as the primary outcome tended to score higher, although even in this group serious deficiencies remained. Some of the common reporting deficits undermine the reader’s ability to fully assess the evidence and conclusions drawn from PRO data. For example, many manuscripts failed to report the amount of missing data in their analyses, or a statistical analysis plan to account for the potential bias missing data introduces. In addition to evaluating PRO reporting quality we identified frequently missed reporting criteria which provides a roadmap for improving the quality of PRO reporting in future RRMM research.
These findings are similar to what was found in reviews of PRO evidence across a variety of cancer diagnoses. Reviews in head and neck, ovarian, colorectal and prostate cancer similarly found suboptimal reporting quality [50–53]. In an overview of PRO reporting quality in randomized clinical trials evaluating systemic cancer therapy Bylicki et al. found ‘PRO hypothesis stated’ (26%), ‘Method of questionnaire administration specified’ (38%), ‘Statistical approaches for missing data specified’ (37%) and ‘PRO specific limitations and implications for generalizability and clinical practice’ (35%) were the least adhered to CONSORT PRO Extension criteria, matching our findings closely [54].
These common reporting deficiencies may be due to a lack of awareness of recommended guidelines, or a perceived lack of importance of PROs in relation to other outcomes like survival. Authors may also struggle to thoroughly report on all outcomes within the limited space available in journals. Somewhat paradoxically the existence of guidelines such as CONSORT PRO, may contribute to the perception that PRO measurement lacks rigor as it highlights reporting deficiencies that are sometimes unavoidable due to space limitations. Higher scores for secondary PRO focused manuscripts would support the theory that with dedicated space PRO reporting quality improves. It is important to note however, that even amongst these PRO focused secondary manuscripts, on average 1/3 of the CONSORT PRO criteria were not met. Score comparisons for manuscripts published before and after the introduction of the CONSORT PRO guidelines revealed no appreciable difference (7.9 and 8.1 respectively), suggesting that the CONSORT PRO guidelines have not had much impact on PRO reporting quality in the RRMM literature. Together this suggests that RRMM researchers have room for improvement in the reporting of PROs and that increased adherence to reporting guidelines like the CONSORT PRO will be important to improve the quality of PRO reporting and increase the usefulness of evidence generated in guiding clinical practice and policy decisions.
This systematic review has some important limitations. The CONSORT PRO guidelines were introduced in 2013 and designed to evaluate the reporting of PROs that were primary or secondary endpoints in randomized controlled trials. We developed a rubric, that was applied to manuscripts written before 2013 and applied it to study designs beyond the intended scope. Our scoring rubric had limitations and there are some important caveats to consider. Each item was given equal weight in the total score, though some items may be more important than others. Scoring posed a particular problem for cross-sectional studies (n = 2) as some criteria assumed multiple timepoints. Several possible scoring modifications were considered to address this. We decided to award a full point to cross-sectional studies if these criteria were met for their one timepoint, though it could be argued that meeting reporting criteria for one time point is easier than for multiple time points. Despite these scoring complications we believe The CONSORT PRO guidelines and our scoring rubric are useful tools for broadly understanding the quality of PRO reporting in the RRMM literature, particularly by highlighting aspects of PRO reporting that are frequently missing.
Conclusions
Our systematic review summarized available PRO evidence in RRMM and evaluated PRO reporting quality using the CONSORT PRO guidelines. We found the available PRO evidence base was predominately from clinical trials and that the format results were reported in made it difficult to describe prevalence, severity or patterns of symptoms and HRQOL issues. Observational studies are needed to describe the RRMM patient experience and should include a variety PROs including those not usually assessed in clinical trials. We also found that PRO reporting quality was suboptimal and future studies which incorporate PROs would benefit from following existing guidelines to ensure that study evidence and conclusions can be fully assessed by readers, clinicians and policy makers.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Amanda Woodward MLIS and Jamie Conklin MLIS for their assistance with this manuscript.
Ashley Leak Bryant has received research grants and speaker honoraria from Carevive Systems Inc. Thomas W. LeBlanc has received grants from American Cancer Society, AstraZeneca, Duke University, Jazz Pharmaceuticals, the National Institute of Nursing Research/National Institutes of Health, and Seattle Genetics, has received speaking honoraria from Celgene for non-branded speaking engagements and is a member of a speakers bureau for Agios, has served on advisory boards for AbbVie, Agios, Amgen, Daiichi-Sankyo, Heron, Medtronic, and Otsuka, and is currently or has been within the last 24 months a consultant for Agios, AstraZeneca, Carevive Systems, Flatiron, Helsinn, Otsuka, Pfizer, and Seattle Genetics.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11136-019-02392-6) contains supplementary material, which is available to authorized users.
Multiple myeloma relapse is defined as progressive disease following a response to treatment, and refractory disease is defined as failure to respond to treatment, or progressive disease while on treatment or within 60 days of treatment [17].
Conflict of interest Matthew R. LeBlanc, Rachel Hirschey, and Sophia K. Smith declare that they have no conflicts of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, & Jemal A (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute (2019). Cancer stat facts: Myeloma. Retrieved 5 May 2019 from https://seer.cancer.gov/statfacts/html/mulmy.html.
- 3.Rosenberg PS, Barker KA, & Anderson WF (2015). Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood, 125(2), 410–412. 10.1182/blood-2014-10-609461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith BD, Smith GL, Hurria A, Hortobagyi GN, & Buchholz TA (2009). Future of cancer incidence in the United States: Burdens upon an aging, changing nation. Journal of Clinical Oncology, 27(17), 2758–2765. 10.1200/jco.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, & Jemal A (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68(1), 7–30. 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. (2008). Cancer statistics, 2008. CA: A Cancer Journal for Clinicians, 58(2), 71–96. 10.3322/ca.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Kristinsson SY, Anderson WF, & Landgren O (2014). Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia, 28(6), 1346–1348. 10.1038/leu.2014.23. [DOI] [PubMed] [Google Scholar]
- 8.Kristinsson SY, Landgren O, Dickman PW, Rangert Derolf AR, & Björkholm M (2007). Patterns of survival in multiple myeloma: A population-based study of patients diagnosed in Sweden from 1973 to 2003. Journal of Clinical Oncology, 25(15), 1993–1999. 10.1200/jco.2006.09.0100. [DOI] [PubMed] [Google Scholar]
- 9.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. (2008). Improved survival in multiple myeloma and the impact of novel therapies. Blood, 111(5), 2516–2520. 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan GJ, & Rasche L (2017). Haematological cancer: Where are we now with the treatment of multiple myeloma? Nature Reviews Clinical Oncology, 14(8), 461 10.1038/nrclinonc.2017.82. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society (2018). Cancer Statistics Center: Myeloma. Retrieved June 9 2018 from https://cancerstatisticscenter.cancer.org/?_ga=2.121553931.226068755.1528521260-452354456.1486498770#!/cancer-site/Myeloma.
- 12.Kumar L, Verma R, & Radhakrishnan VR (2010). Recent advances in the management of multiple myeloma. National Medical Journal of India, 23(4), 210–218. [PubMed] [Google Scholar]
- 13.Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, & Hays RD (2015). Health-related quality of life in older adult survivors of selected cancers: Data from the SEERMHOS linkage. Cancer, 121(5), 758–765. 10.1002/cncr.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manitta V, Zordan R, Cole-Sinclair M, Nandurkar H, & Philip J (2011). The symptom burden of patients with hematological malignancy: A cross-sectional observational study. Journal of Pain and Symptom Management, 42(3), 432–442. 10.1016/j.jpainsymman.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Ramsenthaler C, Kane P, Gao W, Siegert RJ, Edmonds PM, Schey SA, et al. (2016). Prevalence of symptoms in patients with multiple myeloma: A systematic review and meta-analysis. European Journal of Haematology, 97(5), 416–429. 10.1111/ejh.12790. [DOI] [PubMed] [Google Scholar]
- 16.Ramsenthaler C, Osborne TR, Gao W, Siegert RJ, Edmonds PM, Schey SA, et al. (2016). The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: A multi-centre study. BMC Cancer, 16, 427 10.1186/s12885-016-2410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O, et al. (2016). Management of relapsed multiple myeloma: Recommendations of the International Myeloma Working Group. Leukemia, 30(5), 1005–1017. 10.1038/leu.2015.356. [DOI] [PubMed] [Google Scholar]
- 18.Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Castillo J, et al. (2018). NCCN guidelines insights: Multiple myeloma, version 3.2018. Journal of the National Comprehensive Cancer Network, 16(1), 11–20. 10.6004/jnccn.2018.0002. [DOI] [PubMed] [Google Scholar]
- 19.Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. (2017). Multiple myeloma. Nature Reviews Disease Primers, 3, 17046 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 20.Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV, et al. (2004). Clinical course of patients with relapsed multiple myeloma. Mayo Clinic Proceedings, 79(7), 867–874. 10.1016/s0025-6196(11)62152-6. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen LK, Jarden M, Andersen CL, Frederiksen H, & Abildgaard N (2017). A systematic review of health-related quality of life in longitudinal studies of myeloma patients. European Journal of Haematology, 99(1), 3–17. 10.1111/ejh.12882. [DOI] [PubMed] [Google Scholar]
- 22.Basch E (2010). The missing voice of patients in drug-safety reporting. New England Journal of Medicine, 362(10), 865–869. 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basch E (2014). The rationale for collecting patient-reported symptoms during routine chemotherapy. American Society of Clinical Oncology Educational Book, 34(1), 161–165. 10.14694/EdBook_AM.2014.34.161. [DOI] [PubMed] [Google Scholar]
- 24.Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, et al. (2009). Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. Journal of the National Cancer Institute, 101(23), 1624–1632. 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basch E (2018). High compliance rates with patient-reported outcomes in oncology trials submitted to the US Food and Drug Administration. Journal of the National Cancer Institute. 10.1093/jnci/djy183. [DOI] [PubMed]
- 26.Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, et al. (2012). Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. Journal of Clinical Oncology, 30(34), 4249–4255. 10.1200/jco.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 27.Wilson IB, & Cleary PD (1995). Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. JAMA, 273(1), 59–65. [PubMed] [Google Scholar]
- 28.Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. (2007). Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value in Health, 10(Suppl 2), S125–137. 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 29.Basch E (2018). Patient-reported outcomes: an essential component of oncology drug development and regulatory review. Lancet Oncology, 19(5), 595–597. 10.1016/s1470-2045(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 30.Basch E (2017). Patient-reported outcomes—harnessing patients’ voices to improve clinical care. New England Journal of Medicine, 376(2), 105–108. 10.1056/NEJMp1611252. [DOI] [PubMed] [Google Scholar]
- 31.Basch E (2016). Missing patients’ symptoms in cancer care delivery—the importance of patient-reported outcomes. JAMA Oncology, 2(4), 433–434. 10.1001/jamaoncol.2015.4719. [DOI] [PubMed] [Google Scholar]
- 32.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, & Brundage MD (2013). Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA, 309(8), 814–822. 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 33.Kane RC, Bross PF, Farrell AT, & Pazdur R (2003). Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. The Oncologist, 8(6), 508–513. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S (2006). Progress in the treatment of multiple myeloma. Lancet, 367(9513), 791–792. 10.1016/s0140-6736(06)68311-6. [DOI] [PubMed] [Google Scholar]
- 35.Garrard J (2014). Health sciences literature review made easy: The matrix method (4th ed.). Burlington, MA: Jones and Bartlett Learning. [Google Scholar]
- 36.Schulz KF, Altman DG, & Moher D (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine, 152(11), 726–732. 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Cancer Society (2019). Cancer Statistics Center. Retrieved Mar 4 2019 from https://cancerstatisticscenter.cancer.org/?_ga=2.149046513.650737073.1551729015-1327710948.1453219586#!/cancer-site/Myeloma.
- 39.Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, et al. (2018). Global burden of multiple myeloma: A systematic analysis for the global burden of disease study 2016. JAMA Oncol, 4(9), 1221–1227. 10.1001/jamaoncol.2018.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. (2003). A phase 2 study of bortezomib in relapsed, refractory myeloma. New England Journal of Medicine, 348(26), 2609–2617. 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 41.Dubois D, Dhawan R, van de Velde H, Esseltine D, Gupta S, Viala M, et al. (2006). Descriptive and prognostic value of patient-reported outcomes: The bortezomib experience in relapsed and refractory multiple myeloma. Journal of Clinical Oncology, 24(6), 976–982. 10.1200/jco.2005.04.0824. [DOI] [PubMed] [Google Scholar]
- 42.Alegre A, Oriol-Rocafiguera A, Garcia-Larana J, Mateos MV, Sureda A, Martinez-Chamorro C, et al. (2012). Efficacy, safety and quality-of-life associated with lenalidomide plus dexamethasone for the treatment of relapsed or refractory multiple myeloma: The Spanish experience. Leukemia and Lymphoma, 53(9), 1714–1721. 10.3109/10428194.2012.662643. [DOI] [PubMed] [Google Scholar]
- 43.Baljevic M, Zaman S, Baladandayuthapani V, Lin YH, de Partovi CM, Berkova Z, et al. (2017). Phase II study of the c-MET inhibitor tivantinib (ARQ 197) in patients with relapsed or relapsed/refractory multiple myeloma. Annals of Hematology, 96(6), 977–985. 10.1007/s00277-017-2980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cella D, McKendrick J, Kudlac A, Palumbo A, Oukessou A, Vij R, et al. (2018). Impact of elotuzumab treatment on pain and health-related quality of life in patients with relapsed or refractory multiple myeloma: Results from the ELOQUENT-2 study. Annals of Hematology. 10.1007/s00277-018-3469-4. [DOI] [PMC free article] [PubMed]
- 45.Stewart AK, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, et al. (2016). Health-related quality-of-life results from the open-label, randomized, phase iii aspire trial evaluating carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma. Journal of Clinical Oncology, 34(32), 3921–3930. 10.1200/JCO.2016.66.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson P, Roy A, Acharyya S, Panneerselvam A, Mendelson E, Gunther A, et al. (2017). Treatment-free interval as a metric of patient experience and a health outcome of value for advanced multiple myeloma: The case for the histone deacetylase inhibitor panobinostat, a next-generation novel agent. Expert Review of Hematology, 10(10), 933–939. 10.1080/17474086.2017.1369399. [DOI] [PubMed] [Google Scholar]
- 47.Leleu X, Masszi T, Bahlis NJ, Viterbo L, Baker B, Gimsing P, et al. (2018). Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomiblenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. American Journal of Hematology. 10.1002/ajh.25134. [DOI] [PubMed]
- 48.Costa LJ, Hari PN, & Kumar SK (2016). Differences between unselected patients and participants in multiple myeloma clinical trials in US: A threat to external validity. Leukemia and Lymphoma, 57(12), 2827–2832. 10.3109/10428194.2016.1170828. [DOI] [PubMed] [Google Scholar]
- 49.Hari P, Romanus D, Luptakova K, Blazer M, Yong C, Raju A, et al. (2018). The impact of age and comorbidities on practice patterns and outcomes in patients with relapsed/refractory multiple myeloma in the era of novel therapies. Journal of Geriatric Oncology, 9(2), 138–144. 10.1016/j.jgo.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Mercieca-Bebber R, Friedlander M, Calvert M, Stockler M, Kyte D, Kok PS, et al. (2017). A systematic evaluation of compliance and reporting of patient-reported outcome endpoints in ovarian cancer randomised controlled trials: Implications for generalisability and clinical practice. Journal of Patient-Reported Outcomes, 1(1), 5 10.1186/s41687-017-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercieca-Bebber RL, Perreca A, King M, Macann A, Whale K, Soldati S, et al. (2016). Patient-reported outcomes in head and neck and thyroid cancer randomised controlled trials: A systematic review of completeness of reporting and impact on interpretation. European Journal of Cancer, 56, 144–161. 10.1016/j.ejca.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 52.Rees JR, Whale K, Fish D, Fayers P, Cafaro V, Pusic A, et al. (2015). Patient-reported outcomes in randomised controlled trials of colorectal cancer: An analysis determining the availability of robust data to inform clinical decision-making. Journal of Cancer Research and Clinical Oncology, 141(12), 2181–2192. 10.1007/s00432-015-1970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Efficace F, Feuerstein M, Fayers P, Cafaro V, Eastham J, Pusic A, et al. (2014). Patient-reported outcomes in randomised controlled trials of prostate cancer: Methodological quality and impact on clinical decision making. European Urology, 66(3), 416–427. 10.1016/j.eururo.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bylicki O, Gan HK, Joly F, Maillet D, You B, & Peron J (2015). Poor patient-reported outcomes reporting according to CONSORT guidelines in randomized clinical trials evaluating systemic cancer therapy. Annals of Oncology, 26(1), 231–237. 10.1093/annonc/mdu489. [DOI] [PubMed] [Google Scholar]
- 55.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. (2004). A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. British Journal of Haematology, 127(2), 165–172. 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 56.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. (2006). Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. Journal of Clinical Oncology, 24(19), 3113–3120. 10.1200/jco.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 57.Waage A, Gimsing P, Juliusson G, Turesson I, Gulbrandsen N, Eriksson T, et al. (2004). Early response predicts thalidomide efficiency in patients with advanced multiple myeloma. British Journal of Haematology, 125(2), 149–155. 10.1111/j.1365-2141.2004.04879.x. [DOI] [PubMed] [Google Scholar]
- 58.Lee SJ, Richardson PG, Sonneveld P, Schuster MW, Irwin D, San Miguel JF, et al. (2008). Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. British Journal of Haematology, 143(4), 511–519. 10.1111/j.1365-2141.2008.07378.x. [DOI] [PubMed] [Google Scholar]
- 59.Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, et al. (2009). Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: Impact of a dosemodification guideline. British Journal of Haematology, 144(6), 895–903. 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 60.Vij R, Horvath N, Spencer A, Taylor K, Vadhan-Raj S, Vescio R, et al. (2009). An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. American Journal of Hematology, 84(10), 650–656. 10.1002/ajh.21509. [DOI] [PubMed] [Google Scholar]
- 61.Hjorth M, Hjertner O, Knudsen LM, Gulbrandsen N, Holmberg E, Pedersen PT, et al. (2012). Thalidomide and dexamethasone vs. bortezomib and dexamethasone for melphalan refractory myeloma: A randomized study. European Journal of Haematology, 88(6), 485–496. 10.1111/j.1600-0609.2012.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Safaee R, Ahmadzadeh A, Sharifian R, Emami A, Yekaninejad MS, Jalili MH, et al. (2012). Combination of cyclophosphamide, etoposide, carboplatin and dexamethasone as a salvage regimen for refractory multiple myeloma patients: A comparison with a historical control group. Hematology Reports, 4(3), e14 10.4081/hr.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K, et al. (2012). An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. British Journal of Haematology, 158(6), 739–748. 10.1111/j.1365-2141.2012.09232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. (2012). An open-label, single-arm, phase 2 (PX-171–004) study of single-agent carfilzomib in bortezomibnaive patients with relapsed and/or refractory multiple myeloma. Blood, 119(24), 5661–5670. 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richardson PG, Schlossman RL, Alsina M, Weber DM, Coutre SE, Gasparetto C, et al. (2013). PANORAMA 2: Panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood, 122(14), 2331–2337. 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 66.Callander N, Markovina S, Eickhoff J, Hutson P, Campbell T, Hematti P, et al. (2014). Acetyl-L-carnitine (ALCAR) for the prevention of chemotherapy-induced peripheral neuropathy in patients with relapsed or refractory multiple myeloma treated with bortezomib, doxorubicin and low-dose dexamethasone: A study from the Wisconsin Oncology Network. Cancer Chemotherapy and Pharmacology, 74(4), 875–882. 10.1007/s00280-014-2550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. (2015). Elotuzumab therapy for relapsed or refractory multiple myeloma. New England Journal of Medicine, 373(7), 621–631. 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 68.Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. (2015). Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. New England Journal of Medicine, 372(2), 142–152. 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 69.Weisel K, Dimopoulos M, Song KW, Moreau P, Palumbo A, Belch A, et al. (2015). Pomalidomide and low-dose dexamethasone improves health-related quality of life and prolongs time to worsening in relapsed/refractory patients with multiple myeloma enrolled in the MM-003 randomized phase III trial. Clinical Lymphoma, Myeloma and Leukemia, 15(9), 519–530. 10.1016/j.clml.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. (2016). Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. New England Journal of Medicine, 374(17), 1621–1634. 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 71.Robinson D Jr., Orlowski RZ, Stokes M, He J, Huse S, Chitnis A, et al. (2017). Economic burden of relapsed or refractory multiple myeloma: Results from an international trial. European Journal of Haematology, 99(2), 119–132. 10.1111/ejh.12876. [DOI] [PubMed] [Google Scholar]
- 72.Basile A, Tsetis D, Cavalli M, Fiumara P, Di Raimondo F, Coppolino F, et al. (2010). Sacroplasty for local or massive localization of multiple myeloma. Cardiovascular and Interventional Radiology, 33(6), 1270–1277. 10.1007/s00270-009-9761-x. [DOI] [PubMed] [Google Scholar]
- 73.Briani C, Torre CD, Campagnolo M, Lucchetta M, Berno T, Candiotto L, et al. (2013). Lenalidomide in patients with chemotherapy-induced polyneuropathy and relapsed or refractory multiple myeloma: Results from a single-centre prospective study. Journal of the Peripheral Nervous System, 18(1), 19–24. 10.1111/jns5.12002. [DOI] [PubMed] [Google Scholar]
- 74.Samuelson C, O’Toole L, Boland E, Greenfield D, Ezaydi Y, Ahmedzai SH, et al. (2016). High prevalence of cardiovascular and respiratory abnormalities in advanced, intensively treated (transplanted) myeloma: The case for ‘late effects’ screening and preventive strategies. Hematology, 21(5), 272–279. 10.1080/10245332.2015.1122258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leleu X, Kyriakou C, Vande Broek I, Murphy P, Bacon P, Lewis P, et al. (2017). Prospective longitudinal study on quality of life in relapsed/refractory multiple myeloma patients receiving second- or third-line lenalidomide or bortezomib treatment. Blood Cancer Journal, 7(3), e543 10.1038/bcj.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


