Abstract
The development of simple, practical and inexpensive catalysis systems using natural materials is one of the main goals of pharmaceutical chemistry as well as green chemistry. Owing to the ability of easy separation of nanocatalyst, those goals could be approached by applying heterogeneous bionanocatalyst in combination with magnetic nanoparticles. Starch is one of the most abundant natural polymers; therefore, preparing bionanocatalyst from starch is very valuable as starch is largely available and inexpensive. An ecologically benign and efficacious heterogeneous nanocatalyst was prepared based on a biopolymer, and its attributes and morphology were specified by using Fourier transform infrared spectra, scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), thermal analysis and vibrating sample magnetometer measurements; followed by studying catalytic behaviour of bionanocomposite in a multicomponent reaction to synthesize of 4H-pyran derivatives. 4H-pyran is extremely valuable in pharmaceutical chemistry, and the development of methods for synthesis of different derivatives of 4H-pyran is momentous. Revealing environmentally benign nature, mild condition, easy work-up, low cost and non-toxicity are some of the advantages of this protocol. Besides, the bionanocomposite was recovered using an external magnetic bar and could be re-used at least six times with no further decrease in its catalytic activity.
Keywords: green chemistry, bionanocomposite, starch, CuFe2O4, 4H-pyran, nanocatalyst
1. Introduction
Developing a new generation of hybrid nanostructured materials, the green nanomaterial is currently a primary objective of research in nanotechnology. The recent study on bionanocomposite has turned into an emerging field for chemists due to the quality of bionanocomposites in eliminating or minimizing wastes and implementing sustainable processes. That is, a combination of biopolymers and inorganic nanoparticles leads to the development of new novel materials with distinctive properties to be used in various applications [1,2]. The specifically large surface area of nanoparticles makes them extremely active; thus, green chemistry has focused on preparing the bionanocomposite using a biopolymer to serve as a protecting agent for nanoparticles [3]. Hence, choosing polymers from renewable resources has recently received great attention from chemists, and using biodegradable and renewable polymers for various applications has become highly desirable [4]. Starch is one of the most favourite sources of biopolymers. Owing to its valuable characteristics such as low cost, biodegradability and extensive accessibility, starch has gained much attention [5].
On the other hand, magnetic nanoparticles are widely used as parts of nanomaterials and, therefore, are being used in various applications such as serving as catalysts as well as in medical sciences. One of the most important challenges in the catalyst industry is the easy dissociation of nanocatalyst from the reaction media. The superparamagnetic nature of magnetic material causes its detachment from the reaction mixture by an external magnetic bar. This unique property removes the necessity of catalyst purification as well as a tedious and hard work-up upon reaction termination and prevents the loss of catalyst [6,7].
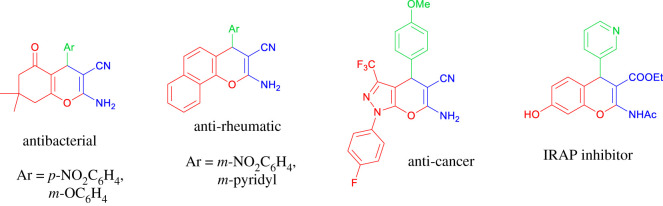
The challenging task of achieving a simple, cost-effective and green reaction procedure for medicinal chemistry is a noteworthy area of research in both academic and pharmaceutical studies. Multicomponent reactions (MCRs), which can be one of the most beneficial protocols in synthetic chemistry, start from simple materials [8,9]. On the other hand, fused benzo 4H-pyran, to be specific, the 4H-chromene derivatives, have been recognized as an important class of natural oxygen-containing heterocyclic compounds that are extensively available in fruits and vegetables. 4H-pyran offers different biological and pharmaceutical activities such as antifungal, antiviral, antioxidant, antileishmanial, antiallergenic, antibacterial, hypotensive, anticoagulant, diuretic and anti-tumor activities [10,11] (figure 1). Therefore, proceeding with the synthesis of such complex heterocyclic compounds has been considered as one of the most interesting fields by both organic and medicinal chemists. Various ways have been reported through modifying the synthesis of 4H-pyran and its derivatives using diverse catalysts and different reaction conditions. Several improved reactions have been presented which use a variety of catalysts such as PEG1000-DAIL [12], hexadecyltrimethylammonium bromide (HTMAB) [13], sodium selenate [14], tetra-methyl ammonium hydroxide [15], amberlite IRA400 (OH-) [16] and MgO [17]. Some of the previous methods have posed a number of problems such as lengthy procedures, being highly corrosive, using expensive catalysts, volatile solvent, laborious work-up and high energy consumption caused by the high-temperature reaction. Furthermore, there has been an intense demand for developing a new and green protocol for synthesizing 4H-pyran derivatives. There are several heterogeneous catalysts for the synthesis of 4H-pyran derivatives. Highly efficient triazine functionalized ordered mesoporous organosilica as a unique metal-free organocatalyst under solvent-free conditions for the three-component reaction in synthesizing 4H-pyran derivatives. Easy work-up, being waste-free, and the ability to re-use catalysts are among the advantages of this method [18]. Tungstic acid functionalized mesoporous SBA-15 is another efficient heterogeneous catalyst for one-pot synthesis of 4H-pyran in water as a solvent. This catalyst system has a high surface area and good acidity; thus, it can catalyse the reaction efficiently and with high output. Furthermore, this catalyst can be recycled multiple times without further decrease in its catalytic activity [19].
Figure 1.
Selected examples of pyran derivatives with pharmaceutical and biological activity.
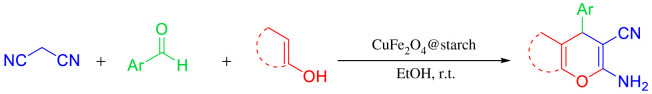
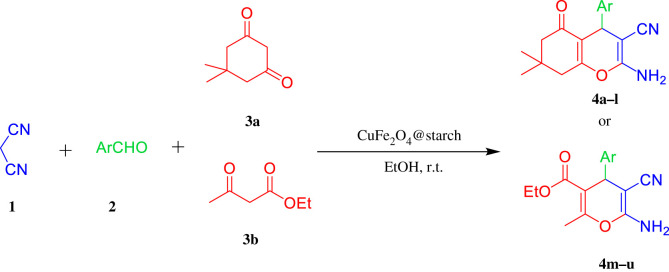
In this regard, in order to continue our previous research on magnetically recyclable nanocatalysts in MCRs [20–24], we hereby propose CuFe2O4@starch bionanocatalyst for the three-component reaction of the aromatic aldehyde, malononitrile and enolizable C-H activated acidic in ethanol as a solvent for the synthesis of 4H-pyran derivatives at room temperature (scheme 1).
Scheme 1.
One-pot three-component reaction of different enolizable C-H activated acidic compounds, aldehydes and malononitrile catalysed by CuFe2O4@starch in ethanol at room temperature.
One of the arguments in favour of the novelty of CuFe2O4@starch, in comparison with another heterogeneous catalyst, is bionanocomposite synthesized from biocompatible materials makes the synthesis of 4H-pyran derivatives more eco-friendly. In addition, due to the CuFe2O4 nanoparticles, the bionanocatalyst has a larger active surface area that generates higher activity as well as a high yield of pure product. Besides, it can be recycled and re-used frequently, and the work-up procedure and separation of catalyst from reaction media are straightforward, as it requires only an external magnet. CuFe2O4@starch is stable, resistant and is economically affordable owing to its simple synthesis procedure from commercially available materials, generating the small amounts of chemical wastes, having short reaction times and going through easy separation steps.
2. Material and methods
2.1. Chemicals and instruments
The solvents, chemicals and reagents were purchased from Merck, Fluka and Aldrich chemical companies and were used without further purification. The melting points were measured on an Electrothermal 9100 apparatus. The IR spectra were recorded on a Shimadzu IR-470 spectrometer in KBr pellets procedures. Scanning electron microscopy (SEM) images were taken with VEGA2 TESCAN, the statistical data of particle sizes from FE-SEM imaging was obtained by Digimizer software. The X-ray (EDX) analysis was recorded with a Numerix DXP–X10P. Thermal analysis was performed by Bahr-STA 504 instrument in the air atmosphere. XRD patterns of the solid powders were developed using a JEOL JDX–8030 (30 kV, 20 mA), the NMR spectra were recorded by Bruker DRX-300 Advance instrument (300 MHz for HNMR and 75.4 MHz for CNMR) while DMSO was used as a solvent. The chemical shifts are given in parts per million (ppm), and the coupling constants (J) are reported in hertz (Hz) scales. Merck starch gel GF254 plates were used for the analytical thin-layer chromatography (TLC) procedure.
2.2. Synthesis of CuFe2O4 nanoparticles
CuFe2O4 nanoparticles were prepared through thermal decomposing of Cu(NO3)2 and Fe(NO3)3 in water in the presence of sodium hydroxide. To describe it briefly, first Fe(NO3)3·9H2O (3.3 g, 8.2 mmol) and Cu(NO3)2·3H2O (1 g, 4.1 mmol) were dissolved in 75 ml of distilled water, then 3 g (75 mmol) of NaOH was dissolved in 15 ml of distilled water and added to it at room temperature over a period of 10 min, during which a reddish-black precipitate was formed. Next, the reaction mixture was put in an ultrasound device at 90°C. After 2 h, it was cooled at room temperature. An external magnetic bar was then used to separate the synthesized magnetic nanoparticles. The procedure was followed by washing the particles with distilled water several times and drying it in an air oven overnight at 80°C. Finally, the nanoparticles were ground in a mortar and pestle and were kept in a furnace at 700°C for 5 h (step-up temperature 20°C per minute). The product was then cooled at room temperature slowly. The result was procuring 820 mg of magnetic CuFe2O4 nanoparticles.
2.3. Synthesis of CuFe2O4@starch nanocomposite
About 0.1 g CuFe2O4 nanoparticles were dispersed in 5 ml distilled water by ultrasonic waves; parallel to that, 0.9 g starch was dissolved in 10 ml distilled water; these two were mixed and were stirred at room temperature for 8 h. The resulting solution was cast and dried on a glass plate for 48 h to afford starch-supported magnetic nanocomposite.
2.4. General procedure for the synthesis of 4H-pyran derivatives
The mixture of aryl aldehyde (1 mmol), enolizable C-H activated acidic compounds (3a, 3b, 5, 7) (1 mmol) and malononitrile (1.1 mmol) with the presence of CuFe2O4@starch (0.03 g) as a catalyst in 3 ml of ethanol as a solvent of the reaction stirred for an appropriate time at room temperature. The completion of the reaction was monitored by TLC (ethyl acetate/n-hexane, 1 : 3). The magnetic nanocomposite was easily removed with the help of a magnetic stirring bar and external magnetic bar as soon as the stirring was stopped, followed by filtering the reaction solution. The precipitate was then purified by washing with ethanol. The pure products were obtained from the reaction media. The CuFe2O4@starch was then cleaned up with EtOH, was air-dried and used for other reactions without a significant loss of its catalytic characteristics after frequent usage.
2.5. Spectral data for selected products
2-amino-7,7-dimethyl-4-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4b): White solid: m.p. 180–182°C, yield: 94%. 1H NMR (300 MHz, DMSO-d6): δ = 0.93 (3H, s, CH3), 1.02 (3H, s, CH3), 2.06 (2H, dd, CH2, 3J = 15 Hz), 2.22 (2H, dd, CH2, 3J = 15 Hz), 4.34 (1H, s, CH), 7.18 (2H, br s, NH2), 7.41 (2H, d, Ar, 3J = 8.1 Hz), 8.14 (2H, d, Ar, 3J = 5.4 Hz). 13C NMR (75 MHz, DMSO-d6): 27.37 (2CH3), 28.78 (C(CH3)), 32.07 (CH2), 35.95, 50.3 (CH2), 57.99 (C(CN)), 112.09, 119.89, 124.30 (CN), 129.30, 146.7, 152.81, 159.16, 163.55, 196.
2-amino-7,7-dimethyl-4-(2-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4c): White solid: m.p.: 224–226°C, yield: 91%. 1H NMR (300 MHz, DMSO-d6): δ = 0.86 (3H, s, CH3), 0.99 (3H, s, CH3), 1.96 (2H, dd, CH2, 2J = 15.9 Hz), 2.16 (2H, dd, CH2, 2J = 15 Hz), 4.91 (1H, s, CH), 7.19 (2H, br s, NH2), 7.32 (1H, d, Ar, 3J = 7.5 Hz), 7.32–7.67 (1H, m, Ar),7.78 (1H, d, Ar, 3J = 8.1 Hz). 13C NMR (75 MHz, DMSO-d6): δ = 27.21 (2CH3), 28.75 (C(CH3)), 30.72(CH), 32.31 (CH2), 50.34 (CH2), 56.72 (C(CN)), 113.1, 119.48, 124.24 (CN), 128.39, 130.59, 134.02, 139.42, 149.59, 159.74, 163.22, 196.48.
3. Results and discussion
3.1. Characterization of CuFe2O4@starch nanocomposite
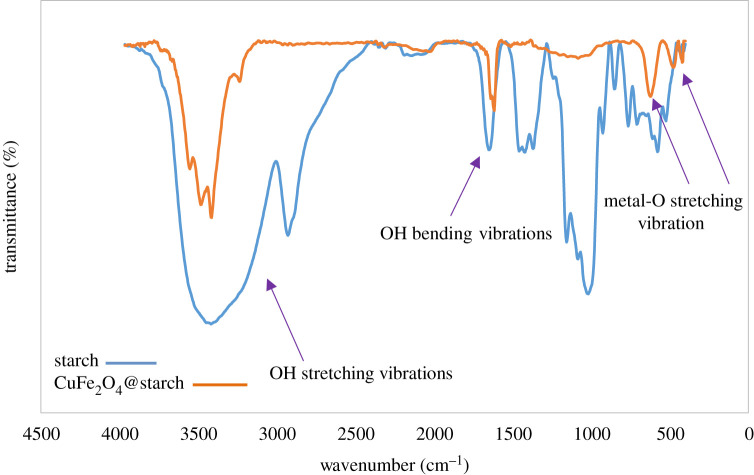
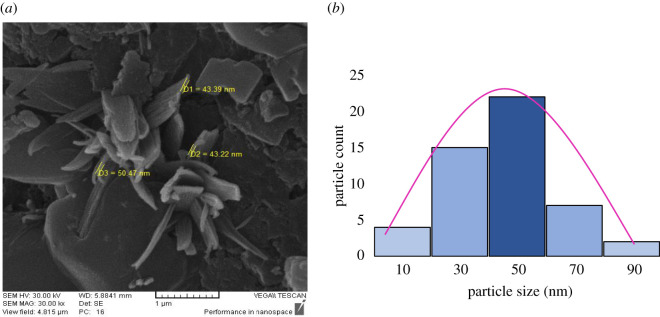
The nanocomposite containing CuFe2O4 nanoparticles was prepared and characterized by various analyses. The comparative IR spectra of CuFe2O4@starch and starch are shown in figure 2. The peaks in the 3200–3800 cm–1 range were corresponding to the OH stretching vibrations in the starch. The broadening in this region suggested the intermolecular hydrogen bonding, and the C-H stretching was observed at the wavelength of 2923 cm–1. The peak appearing at 1643 cm–1 is related to OH bending vibration, and the peaks at 1157, 1083 and 1000 cm–1 are related to C-O bond stretching. In the FT-IR spectra of CuFe2O4@starch, hydroxyl group signals, which are corresponded to starch, have been decreased. This happens because of the chelation of copper and iron atoms with the oxygen atoms of starch. The characteristic bands of Cu-O and Fe-O at 474 and 621 cm–1, respectively, represented the metal-oxygen group of CuFe2O4. The CuFe2O4@starch was characterized by SEM analysis to find its morphology and size (figure 3a). According to the SEM image, the average size of synthesizing CuFe2O4 nanoparticles is less than 50 nm, and CuFe2O4 nanoparticles were loaded on the surfaces of the starch. There was a potent interaction between the nanoparticles and the starch matrix, due to which, the dispersed CuFe2O4 nanoparticles in the composite were considered as sustained. To specify the size of nanoparticles, 50 particles were used at random. The mean particle size of nanoparticles, as analysed by Digimizer software, is about 47 nm (figure 3b).
Figure 2.
IR spectra of starch and CuFe2O4@starch bionanocomposite.
Figure 3.
(a) SEM image of CuFe2O4@starch bionanocomposite and (b) the particle size distribution diagram of the CuFe2O4@starch calculated by Digimizer software.
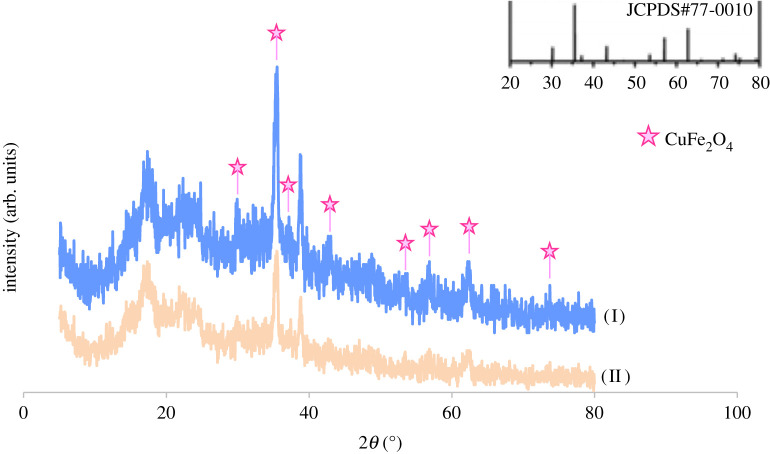
The XRD pattern was prepared and analysed to specify the structure of the inorganic nanoparticles in the bionanocomposite. As shown in figure 4, the nanocomposite exhibited the main peaks, which were consistent with the characteristic peaks of CuFe2O4 (JCPDS No. 77-0010), indicating the peaks at the dispersion angle 2θ = 30.2°, 35.4°, 37.1°, 42.9°, 53.4°, 56.8°, 62.4° and 73.7° are the main peaks of CuFe2O4. In addition, the mean size of the nanoparticles characterized by X-ray line broadening using the Scherrer equation (D = kλ/β cos θ) was about 43 nm.
Figure 4.
The XRD pattern of (I) CuFe2O4@starch bionanocomposite and (II) re-used bionanocomposite.
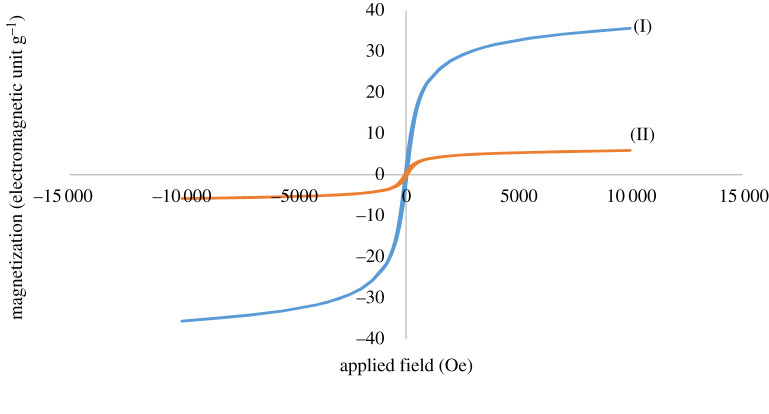
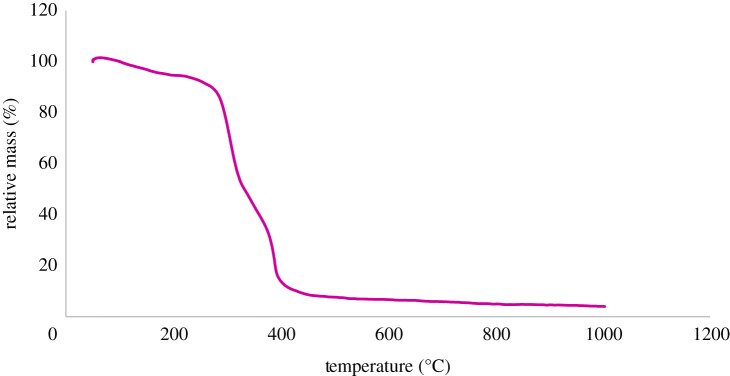
The magnetic property of CuFe2O4@starch bionanocomposite was studied by a vibrating sample magnetometer (VSM) at room temperature (figure 5). The VSM magnetization curve of CuFe2O4@starch proved that the magnetic property of bionanocomposite was sufficient to detach easily from the reaction media using an external magnet. The TGA analysis has shown the thermal stability of bionanocomposite. The procedure was carried out in the air atmosphere and underwent heating over the temperature range of 50–1000°C. CuFe2O4@starch showed desirable thermal stability. The minor increase, as seen at the beginning of the graph, is caused by adsorbing of the surrounding moisture. The loss of weight in around 100–200°C temperature is due to the removal of the surface adsorbed water and other solvents, or, because of the molecules physically adsorbed onto the surface of the bionanocomposite. The reduction in weight, as observed in around 300–400°C, is due to the organic structure of bionanocomposite. The graph shows that after 400°C, there will be no significant change in the bionanocomposite mass, being an indication of the presence of nanoparticles in the bionanocomposite (figure 6). The fact that up to 300°C there is no considerable reduction in the mass of CuFe2O4@starch indicates the synthesized bionanocomposite has good thermal stability in multicomponent reaction procedures.
Figure 5.
VSM magnetization curve of (I) CuFe2O4 (II) CuFe2O4@starch bionanocomposite.
Figure 6.
TGA curves of CuFe2O4@starch bionanocomposite.
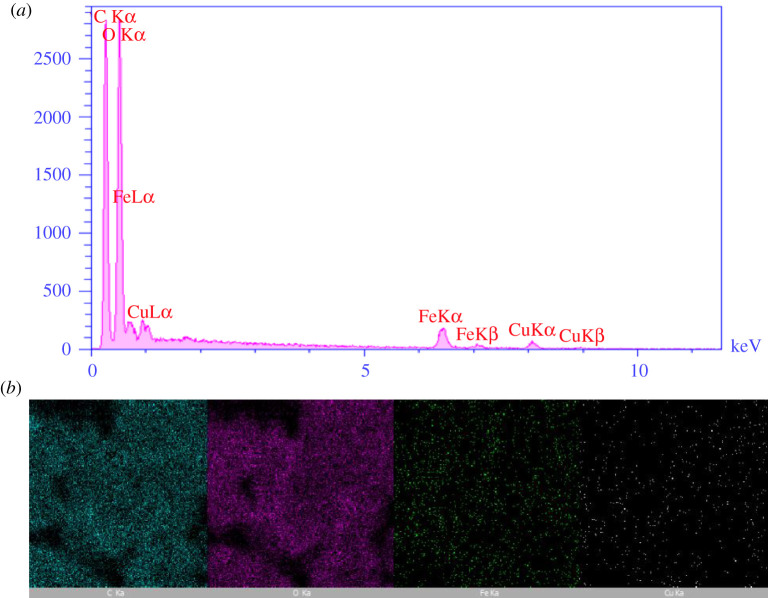
The EDX analysis shows the chemical mixture of bionanocomposite. This analysis indicates the engagement of Fe, Cu, C and O atoms elements in the present bionanocomposite (figure 7a); hence, proving CuFe2O4 nanoparticles are loaded on the surface of starch. As both organic and the inorganic parts of the prepared bionanocatalyst contain an oxygen atom, the highest percentage of weight is related to the oxygen element. Furthermore, the elemental mapping of EDX patterns shows the presence of Fe, Cu, O and C elements in the bionanocomposite (figure 7b).
Figure 7.
(a) EDX analysis of CuFe2O4@starch bionanocomposite and (b) elemental mapping of CuFe2O4@starch bionanocomposite.
3.2. The catalytic activity of CuFe2O4@starch bionanocomposite as nanocatalyst
3.2.1. Application CuFe2O4@starch for the synthesis of 4H-pyran derivatives
To demonstrate the catalytic activity of the synthesized bionanocomposite, we studied the application of CuFe2O4@starch as heterogeneous bionanocatalyst in the synthesis of 4H-pyran derivatives. The three-component reaction of 4-chlorobenzaldehyde, malononitrile and dimedone was chosen as the model reaction to optimize the reaction conditions. The outcome of applying different solvents, temperature and various amounts of CuFe2O4@starch bionanocatalyst on the reaction efficiency was studied. As it can be seen in table 1, the reaction efficiency in the absence of any catalyst, with water or ethanol at room temperature or reflux condition, was traced. The outcome of the product in H2O as a green solvent was significantly less than EtOH under similar reaction conditions. According to the observed results, the optimum amount of bionanocatalyst was 30 mg. To study the generality of this method, three different reaction categories were applied for the synthesis of 4H-pyran derivatives after optimizing the reaction conditions.
Table 1.
Optimization of the reaction conditions for preparation of 2-amino-4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile.
| entry | solvent | temp. (°C) | catalyst (mg) | yielda (%) |
|---|---|---|---|---|
| 1 | EtOH | room temperature | — | Trace |
| 2 | EtOH | reflux | — | Trace |
| 3 | H2O | room temperature | — | Trace |
| 4 | H2O | reflux | — | Trace |
| 5 | EtOH | room temperature | 20 | 65 |
| 6 | EtOH | room temperature | 30 | 96b |
| 7 | EtOH | room temperature | 40 | 96 |
| 8 | EtOH | reflux | 30 | 90 |
| 9 | H2O | room temperature | 30 | trace |
| 10 | CH3CN | room temperature | 30 | 89 |
| 11 | CH3CN | reflux | 30 | 91 |
aIsolated yield for product 4a, via coupling reaction between malononitrile (1.1 mmol) and 4-chlorobenzaldehyde (1.0 mmol), dimedone (1.0 mmol), in the presence of CuFe2O4@starch (30 mg) as a catalyst and ethanol as a solvent at room temperature.
bOptimum conditions.
To prove and expand the extent of this new effective methodology, the optimized reaction conditions were developed to cover other enolizable C-H activated acidic compounds, including dimedone and ethyl acetoacetate. As table 2 shows, aromatic aldehydes with electron-withdrawing groups react faster than aromatic aldehydes with electron-donating groups. Furthermore, the three-component reaction with ethyl acetoacetate (3b), as an acyclic 1,3-dicarbonyl, required a longer reaction period compared to dimedone (3a) under similar reaction conditions (scheme 2).
Table 2.
Three-component synthesis of different 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-benzo[b]pyrans (4a–u) via condensation of malononitrile (1), various aldehydes (2) and dimedone (3a) or ethyl acetoacetate (3b) in the presence of CuFe2O4@starch at room temperature.
| entry | aldehyde | product | time (min) | isolated yields (%) | m.p. (obsd) (°C) | m.p. (lit) (°C) |
|---|---|---|---|---|---|---|
| 1 | 4-chlorobenzaldehyde | 4a | 20 | 96 | 246 | 240–242 [25] |
| 2 | 4-nitrobenzaldehyde | 4b | 25 | 94 | 180–182 | 180–182 [11] |
| 3 | 2-nitrobenzaldehyde | 4c | 25 | 91 | 224–226 | 223–225 [26] |
| 4 | 3-bromobenzaldehyde | 4d | 30 | 92 | 211–215 | 224–226 [27] |
| 5 | 4-hydroxybenzaldhyde | 4e | 30 | 89 | 121 | 116–120 [28] |
| 6 | 4-dimethylaminobenzaldehyde | 4f | 35 | 91 | 225–228 | 220–222 [28] |
| 7 | 3,4-dimethoxybenzaldehyde | 4g | 30 | 92 | 180–183 | 179–181 [29] |
| 8 | 2-hydroxy 3-methoxybenzaldehyde | 4h | 50 | 87 | 177–180 | 181–183 [29] |
| 9 | 3-nitrobenzaldehyde | 4i | 25 | 92 | 226–228 | 229–231 [30] |
| 10 | 3-methoxybenzaldehyde | 4j | 35 | 90 | 193–196 | 195–197 [31] |
| 11 | 2,4-dichlorobenzaldehyde | 4k | 20 | 94 | 122 | 120–122 [29] |
| 12 | 4-methylbenzaldehyde | 4l | 40 | 93 | 214–216 | 212–214 [31] |
| 13 | 4-chlorobenzaldehyde | 4m | 25 | 95 | 179–181 | 173–175 [32] |
| 14 | 2-nitrobenzaldehyde | 4n | 32 | 91 | 185–187 | 183–184 [33] |
| 15 | 3-nitrobenzaldehyde | 4o | 35 | 93 | 179–182 | 183–185 [32] |
| 16 | 4-nitrobenzaldehyde | 4p | 30 | 96 | 180–183 | 183–185 [32] |
| 17 | 4-hydroxybenzaldhyde | 4q | 35 | 89 | 192–194 | 192–194 [34] |
| 18 | 4-dimethylaminobenzaldehyde | 4r | 45 | 86 | 160–162 | 163–165 [35] |
| 19 | 2-chlorobenzaldehyde | 4s | 45 | 83 | 194–197 | 193–194[33] |
| 20 | 3-chlorobenzaldehyde | 4t | 50 | 84 | 180–182 | 177–178 [33] |
| 21 | 4-methoxybenzaldehyde | 4u | 40 | 87 | 138–140 | 140–141 [30] |
Scheme 2.
One-pot three-component reaction of malononitrile (1), aldehyde (2) and dimedone (3a) or ethyl acetoacetate (3b) catalysed by CuFe2O4@starch at room temperature.
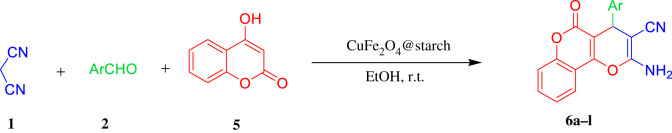
To reveal the potency of bionanocatalyst, the optimized reaction conditions were developed to include another enolizable C-H activated acidic compound, 4-hydroxycoumarin, on the same reaction conditions (table 3 and scheme 3).
Table 3.
Three-component synthesis of different 3-amino-1-5-oxo-1,5-dihydropyrano[2,3-c] chromene-2-carbonitrile (6a–l) via condensation of malononitrile (1), various aldehydes (2) and 4-hydroxycoumarin (5) in the presence of CuFe2O4@starch at room temperature.
| entry | aldehyde | product | time (min) | isolated yields (%) | m.p. (obsd) (°C) | m.p. (lit) (°C) |
|---|---|---|---|---|---|---|
| 1 | 4-chlorobenzaldehyde | 6a | 30 | 95 | 277–279 | 280–282 [36] |
| 2 | 4-nitrobenzaldehyde | 6b | 32 | 91 | 253–255 | 256 [37] |
| 3 | 2-nitrobenzaldehyde | 6c | 30 | 94 | 256 | 258–260 [36] |
| 4 | 3-nitrobenzaldehyde | 6d | 37 | 89 | 232–234 | 237 [37] |
| 5 | 4-hydroxybenzaldhyde | 6e | 40 | 80 | 268–271 | 272–274 [38] |
| 6 | 4-dimethylaminobenzaldehyde | 6f | 45 | 82 | 222–223 | 220–222 [39] |
| 7 | 3,4-dimethoxybenzaldehyde | 6g | 42 | 90 | 171–173 | 175–177 [38] |
| 8 | 4-methoxybenzaldehyde | 6h | 50 | 92 | 238–240 | 234–236 [38] |
| 9 | 4-methylbenzaldehyde | 6i | 45 | 87 | 233–235 | 231–233 [38] |
| 10 | 4-bromoenzaldehyde | 6j | 30 | 89 | 234–236 | 230–234 [36] |
| 11 | 4-fluorobenzaldehyde | 6k | 25 | 94 | 199–201 | 203 [37] |
| 12 | benzaldehyde | 6l | 40 | 93 | 250–252 | 248 [37] |
Scheme 3.
One-pot three-component reaction of 4-hydroxycoumarin (8), malononitrile (1) and aldehydes (2) catalysed by CuFe2O4@starch at room temperature.
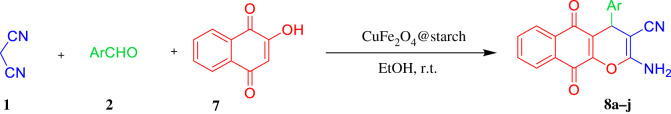
Finally, different derivatives of 4H-chromene were synthesized from the three-component reaction of malononitrile, various aldehyde and 2-hydroxy-1,4-naphthoquinone to serve as the enolizable C-H activated acidic compound in CuFe2O4@starch as a heterogeneous bionanocatalyst (table 4 and scheme 4).
Table 4.
Three-component synthesis of different 2-amino-5,10-dihydro-5,10-dioxo-4H-benzo[g]chromene-3-carbonitrile (8a–j) via condensation of malononitrile (1), various aldehydes (2) and 2-hydroxy-1,4-naphthoquinone (7) in the presence of CuFe2O4@starch at room temperature.
| entry | aldehyde | product | time (min) | isolated yields (%) | M.p. (Obsd) (°C) | M.P. (Lit) (°C) |
|---|---|---|---|---|---|---|
| 1 | 4-chlorobenzaldehyde | 8a | 25 | 97 | 245–247 | 243–244 [11] |
| 2 | 2-chlorobenzaldehyde | 8b | 30 | 90 | 240–242 | 238–240 [39] |
| 3 | 4-nitrobenzaldehyde | 8c | 28 | 89 | 229–232 | 232–234 [11] |
| 4 | 2-nitrobenzaldehyde | 8d | 33 | 87 | 246–248 | 242–244 [39] |
| 5 | 4-hydroxybenzaldhyde | 8e | 37 | 89 | 263–265 | 258–260 [11] |
| 6 | 3,4-dimethoxybenzaldehyde | 8f | 40 | 93 | 275–277 | 270–272 [39] |
| 7 | 4-methoxybenzaldehyde | 8g | 38 | 87 | 260–263 | 257–259 [40] |
| 8 | 4-methylbenzaldehyde | 8h | 45 | 88 | 259–262 | 254–257 [40] |
| 9 | 4-fluorobenzaldehyde | 8i | 28 | 91 | 290–293 | 286–288 [39] |
| 10 | 4-bromoenzaldehyde | 8j | 30 | 95 | 242–244 | 249–251 [11] |
Scheme 4.
One-pot three-component reaction of 2-hydroxy-1,4-naphthoquinone (7), malononitrile (1) and different aldehydes (2) catalysed by CuFe2O4@starch at room temperature.
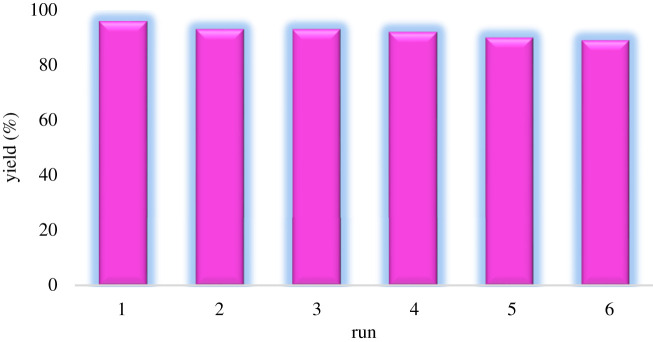
3.3. Catalyst recycling
The ability to recover and re-use bionanocatalyst as a heterogeneous catalyst is one of the most critical properties of bionanocomposite that makes it valuable, unique and beneficial. To sum up, after completion of the reaction, bionanocatalyst was magnetically separated from the reaction media. It was washed with ethanol and was dried at room temperature. The reusability of the bionanocomposite was studied on the model reaction as well. CuFe2O4@starch can be recycled and re-used six times with 96, 93, 93, 92, 90 and 89% of product yields, respectively (figure 8).
Figure 8.
Reusability of the CuFe2O4@starch bionanocomposite.
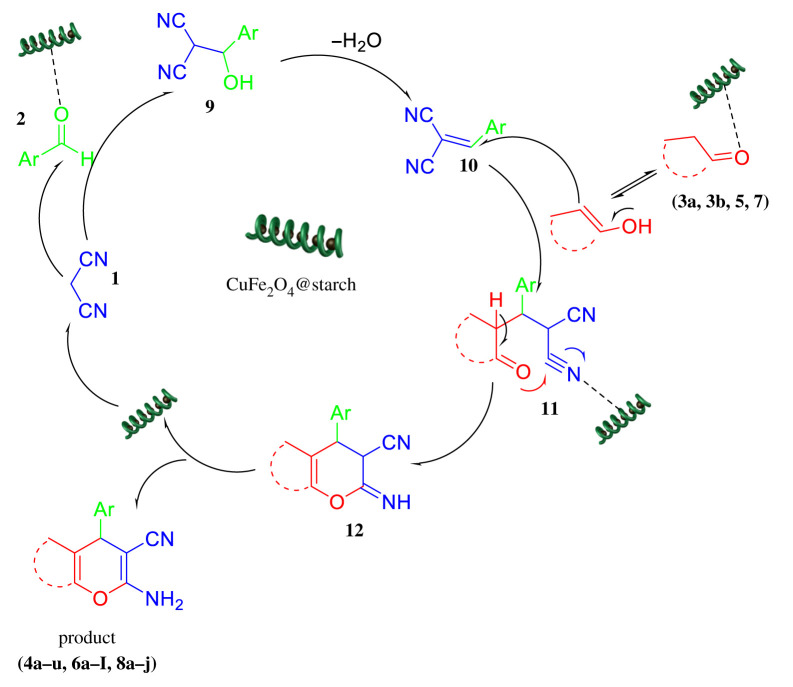
3.4. Reaction mechanism
A proposed mechanism for the synthesis of 4H-pyran derivatives catalysed by CuFe2O4@starch is shown in figure 9. As it can be seen, CuFe2O4@starch acted as Lewis acid and was also able to increase the electrophilicity of carbonyl groups. First, aldehyde and dimedon were condensed together through the Knoevenagel condensation process. Then, this intermediate reacted as a Michael acceptor; thus, the attack of enolizable C-H activated acidic compounds to this molecule led to an open-chain intermediate. Finally, an intramolecular cyclization of this intermediate gave the desired products.
Figure 9.
A plausible mechanism for the one-pot three-component reaction of different enolizable C-H activated acidic compounds (3a, 3b, 5, 7), malononitrile (1), aldehydes (2) catalysed by CuFe2O4@starch at room temperature.
4. Conclusion
In this study, we have synthesized green heterogeneous bionanocatalyst from inexpensive, readily available and natural materials with distinctive properties such as being eco-friendly, inexpensive and showing high efficiency for the synthesis of 4H-pyran derivatives. In terms of efficiency and eco-friendliness, CuFe2O4@starch usage is fundamental in the synthesis of 4H-pyran. Savings in energy consumption, low cost, using ethanol as a green solvent, short reaction time, highly excellent yields and easy work-up due to using an external magnetic bar for detaching the bionanocatalyst from the reaction mixture are some of the advantages of this method. In addition, the simplicity of operation and the smaller amount of the chemical wastes make bionanocatalyst economically affordable for the synthesis of heterocyclic compounds. Due to the presence of CuFe2O4 nanoparticles in the bionanocatalyst, it has a high active surface area, which leads to increased catalytic activity. Proceeding in three different reaction batches catalysed with CuFe2O4@starch in short reaction times and showing high efficiency are strong indicators of high performance and extensibility of the synthesized bionanocatalyst. The optimum reaction condition for those three different reactions catalysed by CuFe2O4@starch for synthesizing various derivatives of 4H-pyran is associated with the principles of green chemistry. This reaction takes place in ethanol as a solvent at room temperature without using energy, and the optimum amount of bionanocatalyst obtained in these reactions was 30 mg. Hence, at the end of the reaction, the easy purification process for the product and the possibility of re-using and recovering the catalyst without further decrease in catalytic activity is significant. Furthermore, using CuFe2O4@starch in terms of time and energy is valuable, which gives it the property of being scaled up in the pharmaceutical industry.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Imam Khomeini International University of Qazvin and the Iran University of Science and Technology.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
M.K. carried out the laboratory work, performed the synthesis experiments, prepared data analyses, participated in the design of the study and drafted the manuscript; M.B. and A.M. conceived of the study, designed the study, coordinated the study and helping in drafted the manuscript and interpretation of data. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The authors received no specific funding for this work.
References
- 1.Darder M, Aranda P, Ruiz-Hitzky E. 2007. Bionanocomposites: a new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 19, 1309–1319. ( 10.1002/adma.200602328) [DOI] [Google Scholar]
- 2.Ruiz-Hitzky E, Darder M, Aranda P. 2005. Functional biopolymer nanocomposites based on layered solids. J. Mater. Chem. 15, 3650–3662. ( 10.1039/b505640n) [DOI] [Google Scholar]
- 3.Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset J-M. 2011. Magnetically recoverable nanocatalysts. Chem. Rev. 111, 3036–3075. ( 10.1021/cr100230z) [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Dean K, Li L. 2006. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 31, 576–602. ( 10.1016/j.progpolymsci.2006.03.002) [DOI] [Google Scholar]
- 5.Mohanty AK, Misra M, Hinrichsen G. 2000. Biofibres, biodegradable polymers and biocomposites: an overview. Macromol. Mater. Eng. 276–277, 1–24. () [DOI] [Google Scholar]
- 6.Baig RBN, Varma RS. 2013. Magnetically retrievable catalysts for organic synthesis. Chem. Commun. 49, 752–770. ( 10.1039/c2cc35663e) [DOI] [PubMed] [Google Scholar]
- 7.Kumar AS, Reddy MA, Knorn M, Reiser O, Sreedhar B. 2013. Magnetically recoverable CuFe2O4 nanoparticles: catalyzed synthesis of aryl azides and 1,4-diaryl-1,2,3-triazoles from boronic acids in water. Eur. J. Org. Chem. 21, 4674–4680. ( 10.1002/ejoc.201300343) [DOI] [Google Scholar]
- 8.Toure BB, Hall DG. 2009. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 109, 4439–4486. ( 10.1021/cr800296p) [DOI] [PubMed] [Google Scholar]
- 9.Ruijter E, Scheffelaar R, Orru RVA. 2011. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 50, 6234–6246. ( 10.1002/anie.201006515) [DOI] [PubMed] [Google Scholar]
- 10.Moafi L, Ahadi S, Bazgir A. 2010. New HA 14-1 analogues: synthesis of 2-amino-4-cyano-4H-chromenes. Tetrahedron Lett. 51, 6270–6274. ( 10.1016/j.tetlet.2010.09.090) [DOI] [Google Scholar]
- 11.Dekamin MG, Eslami M, Maleki A. 2013. Potassium phthalimide-N-oxyl: a novel, efficient, and simple organocatalyst for the one-pot three-component synthesis of various 2-amino-4H-chromene derivatives in water. Tetrahedron 69, 1074–1085. ( 10.1016/j.tet.2012.11.068) [DOI] [Google Scholar]
- 12.Zhi H, Lu C, Zhang Q, Luo J. 2009. A new PEG-1000-based dicationic ionic liquid exhibiting temperature-dependent phase behavior with toluene and its application in one-pot synthesis of benzopyrans. Chem. Commun. 20, 2878–2880. ( 10.1039/b822481a) [DOI] [PubMed] [Google Scholar]
- 13.Jin T-S, Wang A-Q, Wang X, Zhang J-S, Li T-S. 2004. A clean one-pot synthesis of tetrahydrobenzo[b]pyran derivatives catalyzed by hexadecyltrimethyl ammonium bromide in aqueous media. Synlett. 5, 0871–0873. ( 10.1055/s-2004-820025) [DOI] [Google Scholar]
- 14.Hekmatshoar R, Majedi S, Bakhtiari K. 2008. Sodium selenate catalyzed simple and efficient synthesis of tetrahydro benzo[b]pyran derivatives. Catal. Commun. 9, 307–310. ( 10.1016/j.catcom.2007.06.016) [DOI] [Google Scholar]
- 15.Balalaie S, Sheikh-Ahmadi M, Bararjanian M. 2007. Tetra-methyl ammonium hydroxide: an efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media. Catal. Commun. 8, 1724–1728. ( 10.1016/j.catcom.2007.01.034) [DOI] [Google Scholar]
- 16.Khodaei MM, Bahrami K, Farrokhi A. 2010. Amberlite IRA-400 (OH−) as a catalyst in the preparation of 4h-benzo[b]pyrans in aqueous media. Synth. Commun. 40, 1492–1499. ( 10.1080/00397910903097336) [DOI] [Google Scholar]
- 17.Seifi M, Sheibani H. 2008. High surface area MgO as a highly effective heterogeneous base catalyst for three-component synthesis of tetrahydrobenzopyran and 3,4-dihydropyrano[c]chromene derivatives in aqueous media. Catal. Lett. 126, 275–279. ( 10.1007/s10562-008-9603-5) [DOI] [Google Scholar]
- 18.Mondal J, Modak A, Nandi M, Uyama H, Bhaumik A. 2012. Triazine functionalized ordered mesoporous organosilica as a novel organocatalyst for the facile one-pot synthesis of 2-amino-4H-chromenes under solvent-free conditions. RSC Adv. 2, 11 306–11 317. ( 10.1039/C2RA22291D) [DOI] [Google Scholar]
- 19.Kundu SK, Mondal J, Bhaumik A. 2013. Tungstic acid functionalized mesoporous SBA-15: a novel heterogeneous catalyst for facile one-pot synthesis of 2-amino-4H-chromenes in aqueous medium. Dalton Trans. 42, 10 515–10 524. ( 10.1039/c3dt50947h) [DOI] [PubMed] [Google Scholar]
- 20.Taheri-Ledari R, Rahimi J, Maleki A. 2019. Synergistic catalytic effect between ultrasound waves and pyrimidine-2,4-diamine-functionalized magnetic nanoparticles: applied for synthesis of 1,4-dihydropyridine pharmaceutical derivatives. Ultrason. Sonochem. 59, 104 737–104 746. ( 10.1016/j.ultsonch.2019.104737) [DOI] [PubMed] [Google Scholar]
- 21.Maleki A, Kamalzare M. 2014. Fe3O4@cellulose composite nanocatalyst: preparation, characterization and application in the synthesis of benzodiazepines. Catal. Commun. 53, 67–71. ( 10.1016/j.catcom.2014.05.004) [DOI] [Google Scholar]
- 22.Maleki A, Ghamari N, Kamalzare M. 2014. Chitosan-supported Fe3O4 nanoparticles: a magnetically recyclable heterogeneous nanocatalyst for the syntheses of multifunctional benzimidazoles and benzodiazepines. RSC Adv. 4, 9416–9423. ( 10.1039/c3ra47366j) [DOI] [Google Scholar]
- 23.Taheri-Ledari R, Hashemi SM, Maleki A. 2019. High-performance sono/nano-catalytic system: CTSN/Fe3O4–Cu nanocomposite, a promising heterogeneous catalyst for the synthesis of N-arylimidazoles. RSC Adv. 9, 40 348–40 356. ( 10.1039/c9ra08062g) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Maleki A, Kamalzare M. 2014. An efficient synthesis of benzodiazepine derivatives via a one-pot, three-component reaction accelerated by a chitosan-supported superparamagnetic iron oxide nanocomposite. Tetrahedron Lett. 55, 6931–6934. ( 10.1016/j.tetlet.2014.10.120) [DOI] [Google Scholar]
- 25.Azizi N, Dezfooli S, Khajeh M, Hashemi MM. 2013. Efficient deep eutectic solvents catalyzed synthesis of pyran and benzopyran derivatives. J. Mol. Liq. 186, 76–80. ( 10.1016/j.molliq.2013.05.011) [DOI] [Google Scholar]
- 26.Alinezhad H, Tarahomi M, Maleki B, Amiri A. 2019. SO3H-functionalized nano-MGO-D-NH2: synthesis, characterization and application for one-pot synthesis of pyrano[2,3-d ]pyrimidinone and tetrahydrobenzo[b]pyran derivatives in aqueous media. Appl. Organomet. Chem. 33, 4661–4675. ( 10.1002/aoc.4661) [DOI] [Google Scholar]
- 27.Chen L, Bao S, Yang L, Zhang X, Li B, Li Y. 2017. Cheap thiamine hydrochloride as efficient catalyst for synthesis of 4H-benzo[b]pyrans in aqueous ethanol. Res. Chem. Intermed. 43, 3883–3891. ( 10.1007/s11164-016-2843-x) [DOI] [Google Scholar]
- 28.Borhade AV, Agashe JA, Tope DR. 2017. An efficient multicomponent synthesis of tetrahydropyrans using novel recyclable nanocrystalline Y2(CO3)3 catalyst. Russ. J. Appl. Chem. 90, 1005–1014. ( 10.1134/s1070427217060234) [DOI] [Google Scholar]
- 29.Zolfigol MA, Yarie M, Baghery S. 2016. [4,4′-Bipyridine]-1,1′-diium tricyanomethanide as a nanostructured molten salt and its catalytic application in the synthesis of tetrahydrobenzo[b]pyrans, amido and aminoalkyl naphthol derivatives. J. Mol. Liq. 222, 923–932. ( 10.1016/j.molliq.2016.07.132) [DOI] [Google Scholar]
- 30.Nagaraju S, Paplal B, Sathish K, Giri S, Kashinath D. 2017. Synthesis of functionalized chromene and spirochromenes using l-proline-melamine as highly efficient and recyclable homogeneous catalyst at room temperature. Tetrahedron Lett. 58, 4200–4204. ( 10.1016/j.tetlet.2017.09.060) [DOI] [Google Scholar]
- 31.Esmaeilpour M, Javidi J, Dehghani F, Nowroozi Dodeji F. 2015. A green one-pot three-component synthesis of tetrahydrobenzo[b]pyran and 3,4-dihydropyrano[c]chromene derivatives using a Fe3O4@SiO2–imid–PMAn magnetic nanocatalyst under ultrasonic irradiation or reflux conditions. RSC Adv. 5, 26 625–26 633. ( 10.1039/C5RA01021G) [DOI] [Google Scholar]
- 32.Maleki A, Movahed H, Ravaghi P, Kari T. 2016. Facile in situ synthesis and characterization of a novel PANI/Fe3O4/Ag nanocomposite and investigation of catalytic applications. RSC Adv. 6, 98 777–98 787. ( 10.1039/c6ra18185f) [DOI] [Google Scholar]
- 33.Pham DD, Vo-Thanh G, Le TN. 2017. Efficient and green synthesis of 4H-pyran derivatives under ultrasound irradiation in the presence of K2CO3 supported on acidic montmorillonite. Synth. Commun. 47, 1684–1691. ( 10.1080/00397911.2017.1342844) [DOI] [Google Scholar]
- 34.Tiwari J, Singh S, Saquib M, Tufail F, Sharma AK, Singh S, Singh J, Singh J. 2017. Organocatalytic mediated green approach: a versatile new L-valine promoted synthesis of diverse and densely functionalized 2-amino-3-cyano-4H-pyrans. Synth. Commun. 48, 188–196. ( 10.1080/00397911.2017.1393087) [DOI] [Google Scholar]
- 35.Pagadala R, Maddila S, Jonnalagadda SB. 2015. An efficient, multicomponent, one-pot synthesis of tetra substituted pyrans in water. J. Heterocycl. Chem. 52, 1226–1229. ( 10.1002/jhet.2125) [DOI] [Google Scholar]
- 36.Patra A, Mahapatra T. 2012. Synthesis of dihydropyrano[c]chromenes from hydroxycoumarins using Aliquat®336 catalysed condensation in aqueous medium. J. Indian Chem. Soc. 89, 925–932. ( 10.1002/chin.201313122) [DOI] [Google Scholar]
- 37.Paul S, Bhattacharyya P, Das AR. 2011. One-pot synthesis of dihydropyrano[2,3-c]chromenes via a three component coupling of aromatic aldehydes, malononitrile, and 3-hydroxycoumarin catalyzed by nanostructured ZnO in water: a green protocol. Tetrahedron Lett. 52, 4636–4641. ( 10.1016/j.tetlet.2011.06.101) [DOI] [Google Scholar]
- 38.Das P, Dutta A, Bhaumik A, Mukhopadhyay C. 2014. Heterogeneous ditopic ZnFe2O4 catalyzed synthesis of 4H-pyrans: further conversion to 1,4-DHPs and report of functional group interconversion from amide to ester. Green Chem. 16, 1426–1435. ( 10.1039/c3gc42095g) [DOI] [Google Scholar]
- 39.Maleki B. 2016. One-pot synthesis of some2-amino-4H-benzo[g]chromenes. Org. Prep. Proc. Int. 48, 81–87. ( 10.1080/00304948.2016.1127104) [DOI] [Google Scholar]
- 40.Wang X-H, et al. 2009. A facile route to the synthesis of 1,4-pyranonaphthoquinone derivatives under microwave irradiation without catalyst. J. Heterocycl. Chem. 46, 832–836. ( 10.1002/jhet.153) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.