Decades-old model of slow adaptation in sensory hair cells of the auditory and vestibular systems requires revamp.
Abstract
Hair cells detect sound and motion through a mechano-electric transduction (MET) process mediated by tip links connecting shorter stereocilia to adjacent taller stereocilia. Adaptation is a key feature of MET that regulates a cell’s dynamic range and frequency selectivity. A decades-old hypothesis proposes that slow adaptation requires myosin motors to modulate the tip-link position on taller stereocilia. This “motor model” depended on data suggesting that the receptor current decay had a time course similar to that of hair-bundle creep (a continued movement in the direction of a step-like force stimulus). Using cochlear and vestibular hair cells of mice, rats, and gerbils, we assessed how modulating adaptation affected hair-bundle creep. Our results are consistent with slow adaptation requiring myosin motors. However, the hair-bundle creep and slow adaptation were uncorrelated, challenging a critical piece of evidence upholding the motor model. Considering these data, we propose a revised model of hair cell adaptation.
INTRODUCTION
Hair cells of the auditory and vestibular systems convert mechanical information from sound and head movements into electrical signals using a specialized mechanosensitive organelle called the hair bundle. The hair bundle is composed of rows of actin-filled stereocilia arranged in increasing height, which projects from the apical surface of the cell. A filamentous tip link connects adjacent rows of stereocilia and is responsible for gating mechano-electric transduction (MET) channels. At rest, the tip link is under constant tension (1). This tension contributes substantially to the total stiffness of the hair bundle and establishes the resting open probability of the MET channel, which has a role in the high sensitivity of the auditory and vestibular systems. Mechanical input from sound or head movements produces a nanometer-scale deflection of the hair bundle. Positive stimuli, defined as toward the tallest row of stereocilia, generate greater tension in the tip link, resulting in increased open probability of MET channels. Cations pass through the MET channel and depolarize the hair cell, leading to increased synaptic release onto auditory and vestibular afferents.
Adaptation of the MET process is a key mechanism that is hypothesized to contribute to the dynamic range and sharp frequency tuning of the auditory system. Adaptation decreases the hair cell’s response to static stimuli (sustained hair-bundle displacements) by shifting the operating range of the MET process to preserve the sensitivity of the system. In this article, we focus on a component of adaptation termed slow adaptation that operates with a time constant on the order of 10 ms or more.
Slow adaptation has been studied for many decades. Experiments showed that slow adaptation requires Ca2+ entry into stereocilia, calmodulin, and myosin motor activity (2–6). These studies identified a hair-bundle creep, defined as a continuous movement of the hair bundle in the same direction as the stimulus when using step-like force stimulations delivered by a flexible fiber or a fluid jet (Fig. 1A) (7). The time constant of the receptor current decay, which is an indicator of the slow adaptation process, correlated with the time constant of hair-bundle creep. In addition, stimulating the hair bundle orthogonally to its axis of sensitivity, which does not activate MET channels, notably diminished the creep. These data were used to argue for a causal relationship between slow adaptation and the creep.
Fig. 1. Intracellular Ca2+ buffering modulates slow adaptation magnitude.

(A) With a step-like force stimulus (M), the hair-bundle displacement (X) exhibits a creep. The creep appears to correlate with the slow adaptation receptor current decay (I). This correlation was presumed to be causal and led to the motor model of adaptation (schematized in the yellow stereocilia), with myosin motors indicated in red. The motor model predicts that lower adaptation magnitudes would lead to lower creep magnitudes (gray and light blue traces). (B) Top and side view schematic representations of the experimental setup for recording MET currents in OHCs using fluid-jet stimulation showing the patch pipette (P), the fluid-jet stimulator (FJ), and perfusion pipette (Perf). (C) Bundle displacements (X) and currents (I) recorded with stimulus waveform timing (M) at −80 mV (darker) and +80 mV (lighter) with different intracellular BAPTA concentrations. (D) Activation curves generated from cells shown in (C). (E) Summary plots of the percentage of adaptation and resting open probability (resting Popen). Data (+80 mV) have the gray shaded background. Cells shown in (C) are marked with filled symbols. Mean ± SD shown. Paired Student’s t tests were performed for the data in each internal solution between −80 and +80 holding potentials. Unpaired, unequal variance Student’s t tests were used to compare among all intracellular solutions at −80 mV and separately at +80 mV. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Number of cells (animals): 0.1 mM BAPTA, 7 (7); 1 mM BAPTA, 17 (17); 10 mM BAPTA, 10 (10); 1.4 mM Ca2+, 4 (4).
The hair-bundle creep indicates that the effective stiffness of the hair bundle decreases during the force stimulus. Because the resting tip-link tension contributes to the hair-bundle stiffness (1), it was postulated that the slow adaptation mechanism modulated tip-link tension. Thus, the presumed causal link between the creep and slow adaptation laid the foundation of the widely accepted “motor model” of slow adaptation [reviewed in (6, 8)].
In the motor model (Fig. 1A, yellow stereocilia), myosin motors (red) are attached to the tip link at its upper insertion and climb toward the top of the stereocilium to generate resting tension in the tip link. Upon hair-bundle deflection, tip-link tension increases, and MET channels open, allowing the influx of Ca2+ into stereocilia. Ca2+-bound calmodulin is hypothesized to facilitate the slipping of myosin motors down the stereocilium to release tip-link tension, thus causing channels to close and resulting in the adapted state. The release in tip-link tension would be the direct cause of the observed hair-bundle creep; thus, if adaptation magnitude is reduced, the creep magnitude would be similarly reduced (Fig. 1A). Later, data indicated that the activity of myosin Ic (Myo1c) was required for slow adaptation in mammalian vestibular hair cells (5).
Recent data call into question the validity of the motor model of slow adaptation in cochlear hair cells. First, MET channels are not located at the same end of the tip link as the putative adaptation motors (9); thus, a direct source of Ca2+ near the adaptation motors is missing to explain the Ca2+ dependence of slow adaptation for at least half of the MET channels (10). Second, the developmental onset of slow adaptation in cochlear hair cells precedes the developmental expression of Myo1c by about 2 days (11), questioning Myo1c’s role in slow adaptation of cochlear hair cells. Together, these studies called into question the decades-old model and motivated the current study.
Here, we revisited the mechanisms of slow adaptation in cochlear hair cells under the premise that methodological difficulties have contributed to the limited experiments testing the motor model hypothesis. First, we confirmed that slow adaptation is modulated by both Ca2+ and the application of adenosine triphosphatase (ATPase) inhibitors. In rat cochlear hair cells, the hair bundle exhibits a mechanical creep when stimulated with a step-like force using the fluid jet (12), similar to the creep observed in bullfrog saccular hair cells (7). We found that slow adaptation and the hair-bundle creep exhibited similar time constants, but to our surprise, manipulation of the magnitude of slow adaptation had no effect on the magnitude of creep. Using two different force stimulation methods, we found that, in mouse and gerbil vestibular hair cells, the hair-bundle creep was also uncorrelated with the magnitude of slow adaptation. These data support the unexpected conclusion that slow adaptation does not involve mechanical changes to the hair bundle, which was previously hypothesized to be the direct result of climbing and slipping of myosin motors at the upper tip-link insertion. These findings call for a major revision in the model of hair cell adaptation.
RESULTS
Slow adaptation is Ca2+ dependent but uncorrelated with hair-bundle creep
When delivering a 50-ms step-like force with a fluid jet (Fig. 1B), the outer hair cell (OHC) receptor current peaks and then decays with a time constant of ~25 ms, which is an indication of the slow adaptation process (12). We quantified the magnitude of adaptation as the ratio of peak to steady-state current. To confirm the Ca2+ dependence of slow adaptation in the current study, we manipulated the intracellular Ca2+ buffering and holding potential. Increasing the intracellular concentration of BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid], a Ca2+ chelator, decreases the slow adaptation magnitude observed in rat OHCs, which supports that slow adaptation is modulated by Ca2+ [Fig. 1C and E, with the corresponding current versus displacement curves (activation curves) in Fig. 1D]. Depolarizing the cell to positive potentials decreases Ca2+ entry into stereocilia (10) and significantly reduced the slow adaptation magnitude (Fig. 1, C and E). The current decay with fluid-jet stimuli was best fit with a single exponential equation (12). In cells that had >15% adaptation magnitude for the ~50% peak current (Imax) step, time constants were 14.8 ± 5.4 ms (n = 7), 21.3 ± 10.2 ms (n = 14), and 29.3 ± 13.3 ms (n = 3) [P = 0.09, one-way analysis of variance (ANOVA)] for 0.1, 1, and 10 mM BAPTA, respectively. All of these findings are consistent with data regarding slow adaptation in turtle and mouse auditory hair cells, as well as bullfrog saccular hair cells (2, 3, 7, 13–15). Thus, the current decay observed with the fluid jet was consistent with slow adaptation in terms of kinetics and Ca2+ dependence.
The magnitude of slow adaptation at negative potentials for 10 mM BAPTA was not significantly different from all data at positive potentials (Fig. 1, C and E; one-way ANOVA, P = 0.14), indicating that 10 mM BAPTA at negative potentials was able to buffer the Ca2+ before it was able to reach the slow adaptation effector site. We also tested an intracellular solution with 1.4 mM free Ca2+ (1.4 Ca, Fig. 1E). With high intracellular Ca2+ levels, we hypothesize that the intracellular Ca2+-binding sites would be saturated; therefore, any potential Ca2+ entering the stereocilium when channels open would have no effect. With the 1.4 mM Ca2+ intracellular solution, the cells exhibited low adaptation magnitudes, supporting our hypothesis and the Ca2+ dependence of slow adaptation.
The second hallmark of adaptation is the change in the position of the activation curve along the displacement axis during a sustained stimulus (fig. S1B), which we term activation curve shifts (2, 10, 12). To confirm adaptation’s Ca2+ dependence with activation curve shifts, we performed multi-pulse experiments to track the activation curve shifts during a sustained-force step (fig. S1, A to C). We plotted activation curves using the true displacement of the hair bundle extracted from our recently developed high-speed imaging method (12). Activation curve shifts were larger for 0.1 mM versus 10 mM BAPTA for negative potentials at 10 and 50 ms after the adapting step onset (fig. S1C; P = 0.0025 and P = 0.0019, unpaired, unequal variance Student’s t test), but activation curve shifts were comparable at positive potentials across all intracellular solutions (fig. S1C; P = 0.44 and P = 0.42, one-way ANOVA). These data were consistent with the 50-ms step data (Fig. 1, C to E) and further supported a Ca2+-dependent slow adaptation process.
To demonstrate that the observed effects were not unique to fluid-jet stimuli, we used a rigid glass probe to deliver displacement-controlled step stimuli to the hair bundle. We previously found that slow adaptation time constants on the order of 10 ms did not appear until stimulations elicited ~75% Imax (10). Slow adaptation time constants for displacement stimuli were 10.4 ± 5.7 ms (n = 16), using 50-ms step-like displacements and 0.1 mM intracellular BAPTA (fig. S2A). Similar to when using fluid-jet force steps, we found that slow adaptation is also Ca2+ dependent with step-like displacements (fig. S2). When comparing between negative and positive potentials, the magnitude of slow adaptation decreased when 0.1 mM intracellular BAPTA was used (P = 5.2 × 10−5), but with 10 mM intracellular BAPTA, the magnitude of slow adaptation was comparable (P = 0.57). These data corroborated the findings with the fluid jet (Fig. 1, C and E) and indicated that the effects on slow adaptation are independent of the stimulus method.
In bullfrog saccular hair cells, the time courses of the mechanical creep of the hair bundle and slow adaptation are correlated (7). These previous experiments used a two-electrode voltage clamp, a photodiode, and flexible fiber stimulation to measure the receptor current and assay hair-bundle mechanics simultaneously. Historically, this methodology was experimentally difficult such that both throughput and experimental manipulations were limited. With the development of patch-clamp electrophysiology and our recently developed high-speed imaging method, we have substantially increased the experimental throughput of hair-bundle mechanics experiments, allowing us to perform a broader array of manipulations to measure mechanical changes in the hair bundle while simultaneously recording the receptor current. In cochlear hair cells, we observed hair-bundle creep with a similar time course as slow adaptation when using step-like force stimuli with a fluid jet (Figs. 1C and 2A). Because the creep is hypothesized to be a manifestation of slow adaptation, we investigated whether the observed creep was correlated with the magnitude of slow adaptation. If creep and slow adaptation are causally related, we expect to observe less hair-bundle creep when there is less slow adaptation (Fig. 1A). To quantify the creep, we used the following method. First, we needed to ensure our fluid jet delivered a fast, step-like force. We previously showed that with the typical 1-kHz filtering of the fluid-jet stimulus, the fluid drag force plateaued 0.5 ms after stimulus onset (12). This allowed us to measure the changes in effective hair-bundle stiffness 0.5 ms after stimulus onset, i.e., the hair-bundle creep. In the various experimental paradigms, we fit the creep with a double exponential equation, which provided a better fit than a single exponential (12). We used the stimulation at ~50% peak current to compare the adaptation magnitude across cells because this stimulus magnitude often provided the greatest slow adaptation magnitude. If the creep was causally related to slow adaptation, then changes to the magnitude of slow adaptation should have concomitant changes in the kinetics and/or magnitude of the creep. Unexpectedly, despite the differences in the magnitude of adaptation (Fig. 1, C and E), we found no changes in the slow time constant (τ2) and no consistent changes in the magnitude of the creep that correlated with changes in the magnitude of adaptation (Fig. 1C and 2, A and B). Creep magnitude was quantified by the contribution of the slow creep component to the total creep [A2/(A1 + A2), where A1 and A2 are the magnitudes of the fast and slow creep components], the normalized magnitude of the creep [(A1 + A2)/y0, where y0 is the magnitude of the total displacement], and the normalized magnitude of the slow creep component (A2/y0). We also plotted the correlation between the normalized magnitude of the slow creep component (A2/y0) and the slow adaptation magnitude of most of the displacements in all cells (i.e., not only the steps eliciting ~50% peak current) recorded in 0.1 mM BAPTA (Fig. 2C), where the slow adaptation magnitude was high at negative potentials and low at positive potentials, and found that these two components were uncorrelated (Pearson’s r = 9.2 × 10–4, P = 0.99). Despite the magnitudes of slow adaptation and the hair-bundle creep being uncorrelated, we did see a slight but significant correlation between the time constants of the slow adaptation current decay and the hair-bundle creep (Fig. 2D; Pearson’s r = 0.24, P = 8.7 × 10–4). In addition, we analyzed the magnitude of adaptation as a shift in the activation curve with the inferred shift method used by other groups (14, 16) to determine whether there was a correlation with the slow component of the creep (A2) for the 0.1 mM BAPTA condition at positive and negative potentials (Fig. 2E). For the inferred shift, the shift correlated with the creep because as step size increased, the inferred shift increases. However, if the creep was causally related to the magnitude of adaptation, then the data at negative and positive potentials should follow the same correlation. We found two separate correlations at negative and positive potentials, indicating that slow adaptation and the creep are not causally related.
Fig. 2. Hair-bundle creep does not correlate with adaptation magnitude for different intracellular Ca2+ buffering and holding potentials.
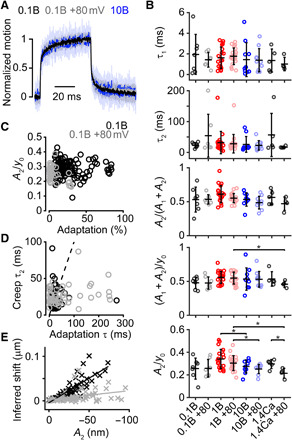
(A) Mean (solid) ± SD (shaded) of the normalized displacements eliciting a current with an amplitude of ~50% Imax from 0.1 mM BAPTA recorded at −80 mV and +80 mV and 10 mM BAPTA at −80 mV. (B) Summary plots of the bundle creep properties for all conditions in Fig. 1. From the top, the plots show the fast (τ1) and slow (τ2) time constants of the creep, the relative magnitude of the slow component of the creep [A2/(A1 + A2)], the magnitude of creep normalized to the total displacement [(A1 + A2)/y0], and the magnitude of the slow creep component normalized to the total displacement (A2/y0) for a given step. (C) No correlation (Pearson’s r = 9.2 × 10−4, P = 0.99) was found between A2/y0 and the adaptation magnitude for cells recorded with an intracellular concentration of 0.1 mM BAPTA at negative (black) and positive (gray) potentials. (D) A slight correlation (Pearson’s r = 0.24, P = 8.7 × 10−4) was found between τ2 and the slow adaptation τ in all cells recorded with an intracellular concentration of 0.1 mM BAPTA at negative (black) and positive (gray) potentials. Dashed line represents the trend for an ideal linear correlation. Correlation analysis includes all positive deflections in all cells except for the smallest deflection, where the signal-to-noise ratio was low. (E) Correlation between the inferred shift and the slow component of the creep (A2) in all cells recorded with an intracellular concentration of 0.1 mM BAPTA at negative (black) and positive (gray) potentials. The cells held at positive potentials show a decreased slope in the inferred shift versus creep magnitude, indicating that these two variables are not causally related. Same statistical details and cell (animal) numbers are shown as in Fig. 1.
Because both Ca2+ and voltage manipulations resulted in no change in the creep, we needed to ensure that our technique had the sensitivity to detect changes in the mechanical properties of the hair bundle. We altered the hair-bundle properties in two different ways: (i) Ca2+-chelator treatment and (ii) fixation of the tissue (fig. S3). The structural integrity of tip links is Ca2+ dependent. As such, Ca2+ chelators, which break tip links and ankle links (17), are often used to determine the apparent contribution of the tip links to the total stiffness of the hair bundle (1). After Ca2+-chelator treatment using BAPTA, MET is abolished, which confirms the rupture of tip links required for gating MET channels, and the hair-bundle stiffness decreased 43 ± 12% (fig. S3, A and B). This suggests that the tip links constitute a substantial portion of the total hair-bundle stiffness such that a change in the tip-link tension, as hypothesized by the motor model, would be detectable. Ca2+-chelator treatment of the hair bundle affected the characteristics of the creep by decreasing the fast time constant and reducing the contribution of the slow creep component (fig. S3D). Conversely, fixation of the tissue showed an increase in the hair-bundle stiffness (fig. S3C) and a decrease in the normalized magnitude of the creep (fig. S3D). These results indicate that changes in mechanical properties of the hair bundle are detectable with our technique.
Inhibition of ATPase activity and PMCA pumps modulates slow adaptation but not hair-bundle creep
Next, we investigated whether myosin motors are required for slow adaptation in cochlear hair cells and how inhibition of plasma membrane Ca2+-ATPases (PMCA) modulates slow adaptation. We pharmacologically inhibited the myosin motor ATPase cycle using two different drugs to reduce the possibility of our results being due to off-target effects: 60 mM intracellular cesium sulfate (Cs2SO4) and 4 mM intracellular sodium orthovanadate (Na3VO4). Intracellular sulfate anions (SO42−) can competitively inhibit the myosin ATPase cycle through binding to the nucleotide-binding pocket of myosin motors (18), but they are not potent inhibitors of PMCAs (4, 19, 20). By contrast, orthovanadate anions (VO43−) are potent inhibitors of the myosin ATPase cycle (21) and also inhibit PMCAs and other ATPases (19). We used an intracellular solution with 0.1 mM BAPTA to enhance the baseline magnitude of slow adaptation, which allowed better experimental sensitivity to our various pharmacological manipulations. Using the stimulus that elicited ~50% peak current, the slow adaptation magnitude decreased significantly with the presence of SO42− or VO43− (Fig. 3, A and B; P = 0.0003 and P = 5.0 × 10−5 for SO42− versus control and VO43− versus control, respectively; n = 8, 8, and 16 for control, SO42−, and VO43− respectively), confirming a role of myosin activity in slow adaptation. We also confirmed effects on adaptation with multi-pulse experiments (fig. S4; shift 10 ms after the adaptative step, P = 0.3361 and P = 0.0004 for VO43− versus control and SO42− versus control, respectively; shift 50 ms after the adaptative step, P = 0.0340 and P = 7.9 × 10−5 for VO43− versus control and SO42− versus control, respectively; n = 8, 8, and 16 for control, VO43−, and SO42−, respectively) and with rigid probe displacement steps (fig. S2, A and C, black to orange traces; P = 0.00038). Myosin inhibition with SO42− did not affect the resting open probability, but we noticed that cells exposed to VO43− also exhibited a slight reduction in resting open probability (Fig. 3E). To explore this further, we performed experiments using 1 mM BAPTA in the intracellular solution (Fig. 3, C and F), which increases the baseline resting open probability (Fig. 1E). Here, VO43−, but not SO42−, significantly decreased the MET resting open probability (Fig. 3F; control to VO43−, P = 2.77 × 10−5; SO42− to VO43−, P = 2.95 × 10−8). With 1 mM BAPTA in the intracellular solution, there were no significant changes observed in the slow adaptation magnitude, but here, the control conditions were already low; thus, a floor effect was likely (Fig. 3C). These results were similar to previous reports of using VO43− in cochlear hair cells (22) and also indicate a separation between setpoint regulation and slow adaptation.
Fig. 3. Inhibition of myosin ATPase activity and PMCA pumps modulates slow adaptation, but not the hair-bundle creep.
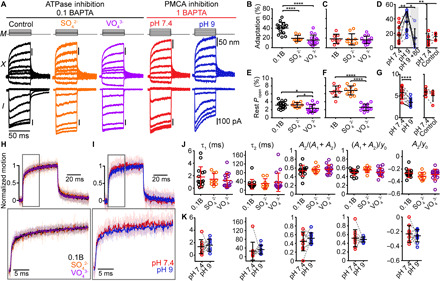
(A) Hair-bundle displacements (X) and currents (I) recorded with stimulus waveform (M) using 0.1 mM BAPTA (control, black) with 60 mM cesium sulfate (Cs2SO4, orange) or 4 mM sodium orthovanadate (Na3VO4, purple) intracellularly. Using 1 mM BAPTA intracellularly, data were taken in normal extracellular solution (pH 7.4, red) and after 10-min PMCA inhibition with pH 9 (blue) in the same cell. Inhibition of ATPase activity and PMCA pumps has opposite effects on the adaptation magnitude. (B to D) Summary plots of adaptation magnitude and (E to G) resting open probability. (B and E) Using 0.1 mM BAPTA intracellular buffer with SO42− or VO43−. (C and F) Using 1 mM BAPTA intracellular buffer with SO42− or VO43−. (D and G) Before (pH 7.4) and after PMCA inhibition (pH 9). Gray shading indicates data at +80 mV. Additional control data plots show the adaptation magnitude and resting open probability at 5 min (pH 7.4) and 20 to 25 min in the normal pH 7.4 extracellular solution (Control). The cells shown in (A) are shaded. (H and I) Mean (solid) ± SD (shaded) of normalized displacements eliciting ~50% Imax with a color legend and an onset motion expansion (gray box). Summary plots of the bundle creep properties recorded during (J) ATPase inhibition or (K) PMCA inhibition. Dashed lines connect data points for a given cell. Mean ± SD shown. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001. Number of cells (animals): 0.1 mM BAPTA, 15 (13); 0.1 mM BAPTA-Cs2SO4, 8 (7); 0.1 mM BAPTA-Na3VO4, 16 (15); 1 mM BAPTA, 8 (8); 1 mM BAPTA-Cs2SO4, 10 (10); 1 mM BAPTA-Na3VO4, 14 (13); pH 9 to 10 (10); Control, 5 (5).
PMCAs are abundant in the stereocilia of OHCs and contribute to the removal of Ca2+ from the stereocilia (19, 23). Inhibition of PMCAs results in a large increase of the stereocilia Ca2+ concentration (19), highlighting the importance of PMCAs in stereocilia Ca2+ homeostasis. PMCAs are Ca2+/H+ exchangers; lowering the extracellular H+ concentration inhibits Ca2+ efflux. To investigate the effect of PMCA inhibition, we raised the pH of the extracellular solution to 9 (23) and recorded MET currents with 1 mM BAPTA in the intracellular solution. Upon PMCA inhibition, the slow adaptation magnitude increased significantly (Fig. 3, A and D; P = 0.004, Student’s t test; n = 10) with a time constant of 18.0 ± 5.8 ms (n = 10). The increased adaptation was eliminated at positive potentials where Ca2+ was prevented from entering through the MET channel (P = 0.014, n = 6), consistent with a Ca2+-dependent slow adaptation process (Fig. 3D). Multi-pulse experiments further confirmed this finding, where activation curve shifts were significantly greater after PMCA inhibition (fig. S5; P = 0.043 and P = 0.0068, n = 10, for 10- and 50-ms adapting steps) but were absent at positive potentials (fig. S5). The inhibition of PMCAs also decreased the resting open probability (P = 8 × 10−6, Student’s t test; n = 10; Fig. 3, A and G). Inhibiting PMCAs with pH 9 reduced resting open probability and increased slow adaptation magnitude.
Together, these data are consistent with (i) myosin motors being necessary for the slow adaptation process in cochlear hair cells, similar to bullfrog hair cells, and (ii) PMCAs modulating the magnitude of slow adaptation, likely through modulating Ca2+ homeostasis. Together, the data presented in Figs. 1 to 3 demonstrate that the current decay observed with a step-like force stimulus was consistent with the Ca2+-dependent slow adaptation studied by other groups in turtle auditory and bullfrog saccular hair cells (2, 13, 15).
These manipulations resulted in decreased and increased slow adaptation magnitude, which allowed further investigation of the correlation between the creep and slow adaptation magnitude. Thus, we analyzed the creep in the hair-bundle displacement during the inhibition of myosin activity and PMCAs. With reduced adaptation magnitude when using SO42− and VO43−, the displacements of the bundle did not exhibit any significant changes in τ2, A2/(A1 + A2), (A1 + A2)/y0, or (A2/y0) (Fig. 3, H and J), similar to findings in Fig. 2. Using PMCA inhibition, we analyzed the effects of increasing adaptation magnitude on hair-bundle creep within a given cell. Again, we found no changes in the hair-bundle creep (Fig. 3, I and K). Together with the data from Figs. 1 and 2, our results support that despite the similar time courses of the creep and slow adaptation, the creep is not a consequence of slow adaptation.
Creep and slow adaptation are uncorrelated in vestibular hair cells
To determine whether the lack of correlation between slow adaptation and hair-bundle creep spanned across hair cell types, we performed experiments on mammalian vestibular hair cells. Here, we used flexible glass fibers to deliver step-like force stimulations, similar to the previous experiments in the bullfrog sacculus (Fig. 4A) (7). We patch-clamped type II hair cells from the mature gerbil utricle and measured hair-bundle displacement using high-speed imaging with 0.1 mM BAPTA intracellularly (Fig. 4B). Using a 300-ms step stimulation, the magnitude of adaptation was 66 ± 30% (n = 16) for stimulations eliciting ~50% peak current. For cells that had an adaptation magnitude of >30%, which allowed a high signal-to-noise ratio, we fit the adaptation with a double exponential equation. The resulting time constants were 4.2 ± 6.2 ms and 77 ± 42 ms (n = 13, Fig. 4D). These values were comparable to previously reported time constants for the mouse utricle (24). At positive potentials, the adaptation magnitude was 28 ± 18% (n = 16), significantly different from the magnitude at negative potentials (P = 5.4 × 10−5, paired Student’s t test; Fig. 4, C and D). In contrast to the differences in adaptation magnitude, no significant differences were found for the observed bundle creep between positive and negative potentials (Fig. 4, C to E). We fit the hair-bundle displacement with a double exponential equation, which was better than a single exponential fit by the Akaike information criterion. Although the slow time constant of the creep (Fig. 4E; τ2: 73 ± 58 ms) was similar to the slow adaptation time constant (Fig. 4D; τ2: 77 ± 42 ms; P = 0.65, paired Student’s t test), the contribution of the slow component to the creep [A2/(A1 + A2)] or the magnitude of creep relative to the total displacement of the hair bundle [(A1 + A2)/y0] was similar at negative and positive potentials. These data indicate that, also, in mammalian vestibular hair cells, slow adaptation and hair-bundle creep are uncorrelated, which further supports that the bundle creep is not a consequence of slow adaptation.
Fig. 4. The hair-bundle creep is uncorrelated with slow adaptation in vestibular hair cells when stimulated with flexible glass fibers.
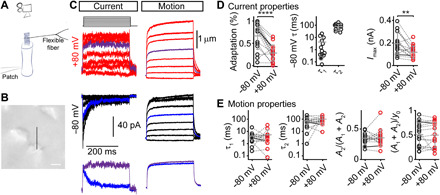
(A) Schematic representation of the experimental setup during the recording of MET currents in vestibular hair cells when stimulated with a flexible fiber. (B) Image of the vestibular hair bundle used for the recording in (C) with a black line to show where the bundle displacement was analyzed. Scale bar, 2 μm. (C) Currents and bundle displacements from the same cell in a P21 gerbil utricle when stimulated with a flexible glass fiber (0.139 mN/m stiffness) at +80 mV (red) and −80 mV (black). Marked traces (violet and blue) highlight a similar step size and are directly compared below. (D) Summary plots of the current properties for the stimulation eliciting ~50% Imax fit with a double exponential decay equation. The left plot summarizes the percentage of adaptation at −80 mV and +80 mV. Fast (τ1) and slow (τ2) adaptation time constants at −80 mV, as well as the Imax at −80 mV and +80 mV, are shown. (E) Summary plots of the bundle motion properties. Gray lines connect data points for a given cell, with cells shown in (A) marked. Bars represent mean ± SD. **P ≤ 0.01, ****P ≤ 0.0001. Number of cells (animals): 13 (13).
Myo1c inhibition does not affect bundle creep
In vestibular hair cells, Myo1c is required for slow adaptation (5, 16). In previous experiments, Holt and colleagues demonstrated that Myo1c participates in slow adaptation in utricular hair cells by using an N6(2-methylbutyl)–adenosine diphosphate analog (NMB-ADP) to inhibit the activity of a mutated Myo1c (Myo1cY61G) (5). The Y61G mutation in Myo1c increases the affinity for specific ADP analogs. When the ADP analog binds, it maintains the mutated Myo1c in a tight interaction with actin and immobilizes the motors. The ADP analog binds poorly to wild-type (WT) Myo1c and has minimal effects on its ATPase activity and motility (25). During inhibition of Myo1c activity, the mechanical properties of the hair bundle were not previously investigated. We confirmed that, in Myo1cY61G/Y61G mice, the presence of 250 μM NMB-ADP significantly reduced the slow adaptation magnitude in type II utricular hair cells (Fig. 5, A and B) without a significant change in resting open probability (WT = 3.1 ± 1.4%, n = 10, WT with NMB-ADP = 3.7 ± 1.5%, n = 7, P = 0.42; Myo1cY61G/Y61G = 2.3 ± 0.9%, n = 9, Myo1cY61G/Y61G with NMB-ADP = 3.8 ± 3.0%, n = 9, P = 0.17). The presence of NMB-ADP increased the adaptation magnitude and slightly decreased the adaptation time constant in WT cells but decreased the adaptation magnitude and slightly increased the adaptation time constant in Myo1cY61G/Y61G mice (Fig. 5B). Despite a decrease in adaptation magnitude in Myo1cY61G/Y61G mice due to the presence of NMB-ADP, there were no significant changes to the time constant or magnitude of the slow creep in the bundle displacement (Fig. 5, C and D). Myo1c is the proposed motor in the motor model of slow adaptation, but our data show that, when Myo1c activity was inhibited, the bundle creep did not decrease as expected according to the motor model. All our data across different manipulations of slow adaptation were consistent and suggested that the creep is not a manifestation of slow adaptation, questioning the validity of the motor model.
Fig. 5. In vestibular hair cells, Myo1c inhibition modulates the adaptation magnitude but not the hair-bundle creep; however, in the cochlea, Myo1c inhibition has no effect on slow adaptation.
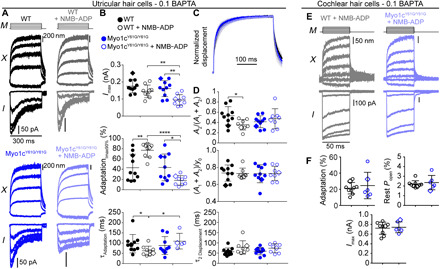
In the utricle, (A) bundle displacements (X) and currents (I) recorded with stimulus waveform (M) using 0.1 mM BAPTA intracellularly with and without 250 μM NMB-ADP in WT and Myo1cY61G/Y61G type II utricular hair cells. (B) Summary plots of Imax and current decay properties for steps eliciting ~90% Imax because slow adaptation was more prominent for larger step sizes in vestibular hair cells with fluid-jet stimulation. (C) Mean (solid) ± SD (shaded) of the normalized displacements eliciting ~90% Imax from each experimental condition. (D) Summary plots for properties of the hair-bundle creep show no effect on the mechanical properties of the bundle. In the cochlea, (E) bundle displacements (X) and currents (I) recorded with stimulus waveform (M) using 0.1 mM BAPTA intracellularly with 250 μM NMB-ADP in WT (gray) and Myo1cY61G/Y61G (light blue) OHCs. (F) Summary plots show no difference in the percentage of adaptation (P = 0.65), resting open probability (P = 0.64), and peak current (P = 0.76). Percentages of adaptation were calculated for the stimulus eliciting ~50% Imax. Cell examples are marked as diamonds. Black error bars represent mean ± SD. Unpaired, unequal variance Student’s t tests were used. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001. For utricle, numbers of cells (animals): Myo1cY61G/Y61G, 10 (6); Myo1cY61G/Y61G + NMB-ADP, 8 (6); WT, 10 (5); and WT + NMB-ADP, 8 (5). For cochlea, numbers of cells (animals): WT + NMB-ADP, 9 (9); Myo1cY61G/Y61G + NMB-ADP, 6 (5).
Myo1c is not required for slow adaptation in cochlear OHCs
In cochlear OHCs, slow adaptation requires myosin motors (Fig. 3), and in vestibular hair cells, the myosin motor involved in slow adaptation is Myo1c (5, 16) (Fig. 5, A and B). Using the previously characterized mouse model (Myo1cY61G/Y61G) and the same inhibitor (NMB-ADP) used for vestibular hair cells, we investigated the role of Myo1c in cochlear OHCs. In contrast with the data from vestibular hair cells, the presence of 250 μM NMB-ADP in the 0.1 mM BAPTA intracellular solution did not affect the slow adaptation magnitude, resting open probability, or peak MET current (Fig. 5, E and F). These data indicate that, in cochlear hair cells, Myo1c is not the myosin motor involved in slow adaptation and that mechanistic details differ between cochlear and vestibular hair cells.
DISCUSSION
The motor model of slow adaptation was hypothesized to require the activity of myosin motors to modulate the position of the upper tip-link insertion point, regulate tip-link tension, and adjust the open probability of the MET channel. This hypothesis was based on key experiments performed in nonmammalian hair cells, which demonstrated that, in response to a sustained stimulus, the decay of the MET current was temporally matched to a mechanical creep in the displacement of the hair bundle. Thus, a mechanical model of the slow adaptation process was developed, which proposed that the hair-bundle creep was causally related to the MET current decay (slow adaptation) and could be explained by a single mechanism that involved the modulation of tip-link tension by a myosin motor (6, 7). Here, we demonstrate that, in mammalian hair cells, slow adaptation is Ca2+ dependent and manifests as a current decay when stimulated with fluid-jet force steps (Fig. 1). In addition, our data are consistent with myosin motors being responsible for this adaptation. However, regardless of how we modulated the magnitude of slow adaptation, the hair-bundle creep remained the same (Figs. 1 to 5), which demonstrates that the hair-bundle creep and slow adaptation are not causally related. The lack of correlation observed in both auditory and vestibular hair cells eliminates the major support for the current motor model of slow adaptation.
Studies of hair-cell MET use a variety of stimulator types. Many experiments are done with rigid glass probes that deliver displacements to the hair bundle (10). However, these experiments are unable to provide mechanical information about the hair bundle. Force stimulations are required to assay mechanical changes in the hair bundle. Two methods to deliver force stimulations are with a flexible glass fiber, which is physically coupled to the hair bundle and delivers force steps via a piezoelectric actuator, or a fluid jet, which uses a piezoelectric disc to eject aqueous solution onto the hair bundle from the tip of a pipette positioned several micrometers away. We used both types of stimuli to show that our results are invariant of the stimulus method. To extract the mechanical properties of the hair bundle, the hair-bundle displacement must be measured during the force stimulus. Previously, measuring hair-bundle displacement with a photodiode was cumbersome (1, 3, 7). High-speed imaging made displacement measurements substantially easier (12) and facilitated testing the premise of the motor model with more experimental conditions.
Applicability to nonmammalian hair bundles
Are our findings specific to the mammalian hair cells? This is unlikely because the characteristics of the mechanical creep we observe are similar to that of bullfrog saccular hair cells. In addition, in turtle auditory papilla hair cells, one experiment showed the persistence of the mechanical creep when slow adaptation was abolished at positive potentials (3), which is consistent with our results in mammalian hair cells. Experiments modulating slow adaptation were not performed in the original experiments in bullfrog saccular hair cells, but there is no indication that a different result from ours would be observed.
Can the observed creep be an artifact?
We currently do not fully understand the cause of the hair-bundle creep; however, it is unlikely to be a stimulation artifact. Our data (fig. S3) and the data of others (1) show that the creep is modified upon Ca2+-chelator treatment of the hair bundle, but the creep persists; therefore, the Ca2+ chelator–sensitive links are not the only contributor to the creep. We previously showed that the creep is not an artifact of motion of the underlying epithelium (12). In addition, the creep is altered with tissue fixation (fig. S3) and in a mutant mouse model (26), suggesting a biological origin. The fluid-jet stimulation of W-shaped cochlear OHCs has the potential for complex fluid dynamics to contribute to the mechanical creep; however, the same creep is observed when stimulating the hair bundle with a flexible fiber (27), which does not have the same potential confounds. In addition, we observed similar phenomena when using the fluid jet and flexible fiber on vestibular hair bundles (Figs. 4 and 5), which do not have the same complex fluid dynamic patterns with their more cohesive hair bundle. Thus, our results are unlikely due to a stimulation artifact, and further studies are required to determine the cause of the creep.
Different myosin motors for different jobs
The current motor model of slow adaptation is unable to explain the persistence of the hair-bundle creep when the slow adaptation magnitude is modulated. Our data indicate a decoupling between the hair-bundle creep and slow adaptation and do not support the climbing and slipping of myosin motors at the upper tip-link insertion to mediate slow adaptation. However, this does not preclude myosin motors from generating resting tension in the tip link (28). Resting tip-link tension is still a prerequisite for the high sensitivity and speed of the auditory system. Formerly, one set of myosin motors was hypothesized to both generate resting tip-link tension and mediate slow adaptation (Fig. 6A). However, many myosin motors with different localizations are known to be important for hearing function (8). It is plausible that one myosin motor subtype is responsible for slow adaptation and, at another subcellular location, another subtype or the same subtype is responsible for generating resting tip-link tension (Fig. 6B).
Fig. 6.
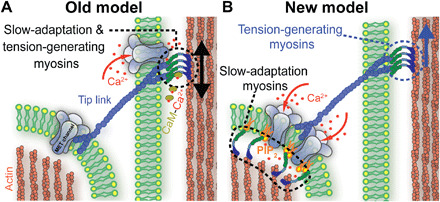
Schematic representation of the MET machinery between two adjacent rows of stereocilia comparing the old motor model and revised model of adaptation. (A) In the old model, MET channels are located at both ends of the tip link. Myosin motors are located at the upper tip-link insertion, generate resting tension, and mediate adaptation by climbing and slipping along the side of the stereocilium to regulate tip link tension. (B) In our revised model, different myosin motor populations are responsible for tension generation at the upper tip-link insertion and for mediating slow adaptation. We propose that slow adaptation does not involve the modulation of tip-link tension, requires the function of myosin motors near the MET channels, and involves PIP2.
The upper tip-link insertion is the best location to generate resting tip-link tension. Myosin VIIa is a natural candidate. It has the correct localization, and a specific myosin VIIa isoform is necessary for proper tip-link tension (26). We hypothesize that this motor is only responsible for generating resting tip-link tension, but not responsible for slow adaptation. Consistent with this hypothesis, myosin VIIa is a slow, processive, high-duty ratio motor, which optimizes it for generating and maintaining tension, but precludes it from operating at the faster dynamic rate of slow adaptation (29). However, definitive experiments are still required to test whether myosin VIIa has a role in adaptation.
Experiments with various ATPase inhibitors could be consistent with a myosin motor being responsible for the generation of tip-link tension. Both SO42− and VO43− affected slow adaptation, but only VO43− affected resting open probability, which is an assay of resting tip-link tension (Fig. 3). SO42− inhibits myosin motors when it is attached to the actin filament, whereas VO43− inhibits the motor when it is detached from the actin (18, 21). This difference in inhibition mechanism could lead to an added effect on resting open probability of VO43− in our data. If the myosin motor is detached from the actin, tip-link tension would decrease and allow channels to close, decreasing the overall stiffness of the hair bundle. Our data appear consistent with this possibility because the hair-bundle displacements due to the initial force were greater for VO43− as compared to SO42− (fig. S6). However, further experiments are required to directly test this hypothesis with tip-link tension measurements (1).
MET channels in mammalian auditory hair cells are located only at the lower end of the tip link (9), and our data suggest that slow adaptation affects channels at this location (Fig. 6B). In the original motor model of adaptation, modulating tip-link tension at the upper tip-link insertion led to the closing of MET channels. Data indicate that the resting tip-link tension contributes to about half of the total stiffness of the hair bundle based on experiments that measure hair-bundle displacement before and after treatment with Ca2+ chelators (fig. S3) (1). Thus, any reduction in tip-link tension during a sustained force would decrease the effective hair-bundle stiffness and manifest as a creep. Because MET channels are only localized to the lower end of the tip link (9), any modulation of the channel from the upper end of the tip link must travel through the tip link to modulate the force sensed by the channel. Because we did not observe any changes in the creep associated with slow adaptation, tip-link tension modulation cannot be involved in the slow adaptation mechanism. Therefore, the myosin motors responsible for slow adaptation are likely localized near the MET channels, and the mechanism of action must be independent of altering the mechanical properties of the hair bundle. A potential mechanism that fits these requirements is an intrinsic modulation of the channel.
Potential molecular mechanisms of slow adaptation
A conceivable intrinsic modulation of the channel is to alter the lipid environment immediately surrounding the MET channel to modify gating. We previously showed that the MET channel is sensitive to the lipid environment (28). In addition, phosphatidylinositol 4,5-bisphosphate (PIP2) can regulate MET and adaptation because PIP2 depletion reduces adaptation and decreases MET channel conductance (30–32). PIP2 is also a known cargo of Myo1c (33), so myosin motors may work in conjunction with PIP2 to mediate slow adaptation. One potential mechanism is that myosin motors may adjust the tension in the lipid membrane to change the energy barriers of channel gating. The lipid tension generation could be mediated through the myosin motor head attached to the actin cytoskeleton and the tail attached to PIP2. The lipid tension may be regulated by Ca2+ because PIP2 clustering and confirmation are affected by Ca2+ (34).
A viable alternative mechanism is that PIP2 could modulate the channel directly. In this scenario, myosin motors are required only to transport and concentrate PIP2 near the channels. PIP2 can bind and modulate multiple ion channels, including the Ca2+-activated chloride channel TMEM16A (35). In particular, PIP2 regulates TMEM16A activation and desensitization by directly binding to basic residues near the transmembrane to cytosolic interface of the channel. Recently, the MET channel complex component TMC1 was suggested to share a structural relationship with the TMEM16 family of ion channels (36, 37), and TMC1 also has multiple basic residues that are conserved across species that lie near the putative transmembrane to cytosolic interface. TMIE is another MET channel complex component that modulates MET conductance, and it is shown to bind and require PIP2 for proper MET channel function (32). Therefore, PIP2 could work through binding TMC1 or TMIE to mediate slow adaptation. Other potential mechanisms exist, and much more work is required to discover the exact molecular mechanism.
Unlike vestibular hair cells, cochlear hair cells do not require Myo1c activity for slow adaptation (Fig. 5). In the cochlea, Myo1c is localized throughout the stereocilium (11, 38), unlike the specific localization at the two ends of the tip link in vestibular hair cells (39). These differing localization patterns would suggest different functions for Myo1c in each cell type, consistent with our electrophysiology data (Fig. 5). In the cochlea, another myosin motor is likely responsible for slow adaptation, which could be another myosin I isozyme found in the cochlea or other myosin motor subtypes important for hearing (8, 38). This alternative myosin motor could interact with PIP2, or the cochlear hair cells may have a different mechanism altogether because PIP2 has yet to be shown to be important for slow adaptation in cochlear hair cells.
Other adaptation mechanisms
Historically, adaptation is separated into two forms based on experimental evidence: fast and slow adaptation (13). We previously proposed that fast adaptation with a time constant on the order of 1 ms or less is not driven by Ca2+ entry (10, 12). Here, we investigated a Ca2+-dependent slow adaptation that has a time constant on the order of 10 ms. However, these two adaptation mechanisms are not the only mechanisms to control the setpoint of the MET channel. We have described another potential mechanism that involves the lipid bilayer that has a time constant on the order of 100 ms (28). This third mode of channel modulation was separate from fast adaptation, but experiments testing its independence to slow adaptation have yet to be done. It is unlikely to be the same mechanism as the slow adaptation described here because the time constant of the effect is on the order of 100 ms, whereas slow adaptation has time constants on the order of 10 ms. In addition, we see that setpoint regulation can be independent of effects on slow adaptation with intracellular SO42− (Fig. 3, B and E). There are likely more mechanisms that work on even longer time scales to regulate the MET channels’ sensitivity either through biochemical modulation (40) or through Ca2+-dependent actin remodeling (41).
In summary, our findings demonstrate that the hair-bundle creep is not a manifestation of slow adaptation. These data do not support the old motor model of slow adaptation and suggests that the slow adaptation mechanism occurs near the MET channels at the lower end of the tip link. Our revised model of the MET process has one set of myosin motors necessary for slow adaptation and a second set that is responsible for tip-link tension generation.
METHODS
Tissue preparation
Animals of either sex were euthanized using methods approved by the University of Colorado Institutional Animal Care and Use Committee. Organs of Corti were dissected from postnatal (P) day 6 to 10 Sprague-Dawley rats (the large majority of experiments used P7-P8) and P8-P9 Myo1cY61G/Y61G mice (16) and C57BL/6J mice and placed in recording chambers as previously described (28). Briefly, organs of Corti were dissected out of the temporal bone. The cochlear coil was peeled off the modiolus, and a section was cut to isolate a mid-apical region of the organ of Corti. The stria vascularis was partially peeled back on both ends, and Reissner’s membrane was separated from the stria vascularis. The tectorial membrane was peeled off the tissue with fine forceps. The tissue was then placed in a recording chamber where two strands of dental floss held the tissue, one on the modiolar side and the other on the stria vascularis. Utricles were dissected from P15-P27 Mongolian gerbils and P9-P12 Myo1cY61G/Y61G and C57BL/6J mice. The utricle was dissected out by removing the overlying membrane, and much of the nerve stump. The otoconial membrane with otoconia was carefully removed with fine forceps. After dissection, utricles were placed in similar recording chambers used for the organs of Corti. Tissue was viewed using a 100× [1.0 numerical aperture (NA), Olympus] water-immersion objective with a Phantom Miro 320s camera (Vision Research, Wayne, NJ) on a SliceScope (Scientifica) with Olympus optics. For rigid probe experiments, tissue was viewed with a 60× (1.0 NA, Olympus) water-immersion objective on an FN1 (Nikon). Tissue was perfused with extracellular solution containing 140 mM NaCl, 2 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM Hepes, 2 mM creatine monohydrate, 2 mM Na-pyruvate, 2 mM ascorbic acid, 6 mM dextrose, pH 7.4, 300 to 310 mOsm. For the inhibition of PMCA pumps, the concentration of H+ ions was reduced by elevating the pH of the extracellular solution to 9. Apical perfusion with pipette tip sizes of 150 to 300 μm provided local perfusion to the hair bundles and delivered elevated pH solutions (Fig. 1A). For tissue fixation, cochleae were first dissected and mounted in the recording dish, then fixed with 4% paraformaldehyde in phosphate-buffered saline overnight at 4°C. The solution was prepared from a 16% paraformaldehyde stock (Electron Microscopy Sciences).
Electrophysiological recordings
Whole-cell patch-clamp was achieved on first- or second-row OHCs from middle to apical turns of the mammalian cochlea and on type II cells from the extrastriolar regions of the utricle using an Axon 200B amplifier or a MultiClamp 700B amplifier (Molecular Devices) for rigid probe experiments with thick-walled borosilicate patch pipettes (2 to 6 megohms). Type II cells were identified morphologically by (i) the shape of the cell body (type II cells do not have the typical flask-shaped neck of type I cells) and (ii) the absence of the nerve calyx. Patch pipettes were filled with an intracellular solution containing 125 mM CsCl, 3.5 mM MgCl2, 5 mM adenosine triphosphate (ATP), 5 mM creatine phosphate, 10 mM Hepes, 1 mM cesium BAPTA, 3 mM ascorbic acid, pH 7.2, 280 to 290 mOsm. For 0.1 and 10 mM BAPTA intracellular solutions, the BAPTA concentration was adjusted accordingly, and CsCl concentrations were adjusted to reach 280 to 290 mOsm. For the VO43− experiments, 4 mM sodium orthovanadate (Na3VO4) was added to the intracellular solution from a 200 mM stock solution. To make the stock solution, Na3VO4 powder was solubilized in water, and the solution was adjusted to pH ≈ 10 with 1 M NaOH to facilitate solubilization. After complete solubilization, the stock solution was gradually adjusted to pH 7.9 with 1 N HCl, and the yellow color that developed after adding HCl was removed with cycles of boiling and cooling. For the SO42− experiments, the intracellular solution contained 60 mM Cs2SO4, 48.8 mM CsCl, 3.5 mM MgCl2, 5 mM ATP, 5 mM creatine phosphate, 10 mM Hepes, 0.1 mM cesium BAPTA, 3 mM ascorbic acid, pH 7.2, 280 to 290 mOsm. Ca2+ intracellular solution (1.4 mM) contained 121 mM CsCl, 3.5 mM MgCl2, 3.5 mM CaCl2, 3.5 mM ATP, 5 mM creatine phosphate, 10 mM Hepes, 2 mM ascorbic acid, pH 7.2, 280 to 290 mOsm. Experiments were performed at 18° to 22°C. Whole-cell currents were filtered at 10 kHz and sampled at 0.05 to 1 MHz using USB-6356 or USB-6366 (National Instruments) controlled by jClamp (SciSoft Company). Vestibular hair cell currents were subsequently filtered offline at 1 kHz in MATLAB. All experiments used −80-mV holding potential unless otherwise noted. No correction for liquid junction potential was done. The apical perfusion was always on while attaining a gigohm seal on the hair cell.
Ca2+-chelator application
Ca2+-chelator experiments were performed using two methods. The primary method used 5 mM BAPTA dissolved into the normal extracellular solution and was loaded into a pipette. The solution was delivered using Picospritzer III (Parker Hannafin) to cochlear and vestibular hair cells, perpendicular to the axis of sensitivity of the hair bundle. An iontophoresis technique was used for a subset of OHCs (1). An iontophoresis pipette (5 to 8 megohms) filled with a solution of 200 mM EDTA in 25 mM KCl (pH 7.4) was placed near the hair bundle in normal extracellular solution. Currents were delivered using an MVCS 02 unit (npi electronic GmbH) with a holding current of +10 nA and an ejection current of −650 to −750 nA.
Hair-bundle stimulation and motion recording
For most of the electrophysiological recordings, hair bundles were stimulated with a custom three-dimensional—printed fluid jet driven by a piezoelectric disc bender (27 mm, 4.6 kHz; Murata Electronics or PUI Audio). Thin-wall borosilicate pipettes were pulled to tip diameters of 5 to 15 μm, filled with extracellular solution, and mounted in the fluid-jet stimulator. The piezoelectric disc bender was driven by waveforms generated using jClamp. Stimulus waveforms were filtered using an eight-pole Bessel filter at 1 kHz (L8L 90PF, Frequency Devices Inc.) and variably attenuated (PA5, Tucker Davis) before being sent to a high-voltage/high-current Crawford amplifier to drive the piezoelectric disc bender (12). In some experiments, a short flexible fiber (long fibers have too much viscous drag) was used to stimulate vestibular hair bundles using a co-fired piezoelectric stack actuator (AE0505D16F, Thorlabs). The fiber was positioned close to the tallest row of the hair bundle. During stimulations, videos were taken of the hair-bundle motion with high-speed imaging at 10,000 frames per second using a Phantom Miro 320s camera (Vision Research). Videos were saved for each stimulation and analyzed offline.
In some experiments, OHCs were also stimulated with a rigid glass probe. The stimulator was a custom-built piezoelectric stimulator using a co-fired piezoelectric actuator (AE0505D08F, Thorlabs). Probes were shaped using an MF-900 microforge (Narishige) to match the general size of an OHC hair bundle. The stimulus signal was filtered at 10 kHz with a model 3382 8-pole Bessel filter (Krohn-Hite).
Preparation and calibration of the flexible fiber
Flexible fibers were shaped using an MF-900 microforge (Narishige) with a 90° bent shank with a short, thin fiber that was less than ~50 μm. Straight shank reference fibers that were longer and thicker in diameter were calibrated by hanging PMMA microspheres (PMPMS-1.2 90-160 μm, Cospheric LLC). Stimulation fibers were pushed against the reference fibers to calibrate their stiffness. Some fiber experiments did not have stiffness calibrations, and the fiber stiffness was judged as appropriate based on whether hair bundles exhibited bundle creep. Measured fiber stiffness was between 0.072 and 0.586 mN/m. Reference fiber stiffness was 0.302 mN/m.
Hair-bundle motion analysis
Custom MATLAB (MathWorks) scripts were used for the analysis of the hair-bundle displacement (12). Movie frames were imported into MATLAB, and the position of the hair bundle was extracted using a Gaussian fit to a high-pass–filtered hair-bundle image for a given vertical row of pixels in the image. For most cells, the vertical displacement of the hair bundle near the center was taken as the displacement of the hair bundle.
Genotyping
Myo1c-Y61G mice used for the experiments were genotyped using a tail fragment. DNA extractions and purifications were performed using a mouse genotyping kit (KAPA Mouse Genotyping Kits, KAPA Biosystems), according to the manufacturer’s instructions. The DNA samples were then amplified by polymerase chain reaction (PCR) using the following primers: p1 (5′-gcattccagcatcctgcaacc-3′) and p2 (5′-cagtgtcagccactgcaaacc-3′). PCR products were purified with a PCR clean-up kit (NucleoSpin Gel and PCR Clean-up, Macherey-Nagel), according to the manufacturer’s instructions, and digested with Bam HI as previously described (16).
Data analysis
Activation curves were generated using the displacement values when the peak current occurred for 50-ms step traces. For multi-pulse experiments, displacement and current were taken 1 ms after stimulus onset because the steps were short (5 ms) and peak currents were not always reached during this time period. Normalized currents (I/Imax) were generated by subtracting leak current defined as the smallest remaining current during the negative steps and normalizing to the peak current. Activation curves were fit with a double Boltzmann equation
| (1) |
where Z1 and Z2 are the slope factors, and x0 is the setpoint.
For mechanical stimulus steps, adaptation time constant fits were obtained at ~50% peak current using a double exponential equation
| (2) |
where τ1 and τ2 are the decay constants, and A1 and A2 are the respective amplitudes; the hair-bundle displacement was also fit with the same equation starting at a time after the force stimulus plateaued, which was 0.5 ms after stimulus onset (12). All fits were automated in MATLAB.
Data were analyzed using jClamp, MATLAB, and Excel (Microsoft). Graphs were created using MATLAB, GraphPad Prism, and Adobe Illustrator. The resting mechanosensitive current divided by the maximum mechanosensitive current is Popen, where we assume that we can achieve a Popen of 100%. Peak MET current is the difference between current values elicited from the maximal negative and maximal positive stimulation.
Statistics
No statistical methods were used to predetermine sample sizes. Statistical analysis used Student’s two-tailed t tests or ANOVA analysis from MATLAB or Excel. Paired t tests were used when comparing data points in the same cell, and unpaired-unequal variance tests or ANOVA analyses were used to compare data between cell populations. Significance (P values) are as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are presented as mean ± SD unless otherwise noted. Ns are the number of cells recorded, and we state the number of animals used.
Supplementary Material
Acknowledgments
We would like to thank K. Beam, A. Ricci, A. Person, and J. Shin for critical feedback on this manuscript. Funding: This work was supported by R00 DC013299 and R01 DC016868 to A.W.P. and F31 DC018457 to A.A.M. Author contributions: A.W.P. conceived/designed the study, performed experiments, analyzed data, and oversaw all the work in this manuscript. G.A.C. participated in the study design, performed a large majority of the experiments and analysis, and helped write the manuscript. A.A.M. performed experiments, analyzed data, and helped revise the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/33/eabb4922/DC1
REFERENCES AND NOTES
- 1.Tobin M., Chaiyasitdhi A., Michel V., Michalski N., Martin P., Stiffness and tension gradients of the hair cell’s tip-link complex in the mammalian cochlea. eLife 8, e43473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eatock R. A., Corey D. P., Hudspeth A. J., Adaptation of mechanoelectrical transduction in hair cells of the bullfrog’s sacculus. J. Neurosci. 7, 2821–2836 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford A. C., Evans M. G., Fettiplace R., Activation and adaptation of transducer currents in turtle hair cells. J. Physiol. 419, 405–434 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamoah E. N., Gillespie P. G., Phosphate analogs block adaptation in hair cells by inhibiting adaptation-motor force production. Neuron 17, 523–533 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Holt J. R., Gillespie S. K. H., Provance D. W. Jr., Shah K., Shokat K. M., Corey D. P., Mercer J. A., Gillespie P. G., A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Hudspeth A. J., Gillespie P. G., Pulling springs to tune transduction: Adaptation by hair cells. Neuron 12, 1–9 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Howard J., Hudspeth A. J., Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog’s saccular hair cell. Proc. Natl. Acad. Sci. U.S.A. 84, 3064–3068 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng A. W., Salles F. T., Pan B., Ricci A. J., Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction. Nat. Commun. 2, 523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beurg M., Fettiplace R., Nam J.-H., Ricci A. J., Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat. Neurosci. 12, 553–558 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng A. W., Effertz T., Ricci A. J., Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80, 960–972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waguespack J., Salles F. T., Kachar B., Ricci A. J., Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J. Neurosci. 27, 13890–13902 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caprara G. A., Mecca A. A., Wang Y., Ricci A. J., Peng A. W., Hair bundle stimulation mode modifies manifestations of mechanotransduction adaptation. J. Neurosci. 39, 9098–9106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y.-C., Ricci A. J., Fettiplace R., Two components of transducer adaptation in auditory hair cells. J. Neurophysiol. 82, 2171–2181 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Stauffer E. A., Holt J. R., Sensory transduction and adaptation in inner and outer hair cells of the mouse auditory system. J. Neurophysiol. 98, 3360–3369 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assad J. A., Hacohen N., Corey D. P., Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc. Natl. Acad. Sci. U.S.A. 86, 2918–2922 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stauffer E. A., Scarborough J. D., Hirono M., Miller E. D., Shah K., Mercer J. A., Holt J. R., Gillespie P. G., Fast adaptation in vestibular hair cells requires Myosin-1c activity. Neuron 47, 541–553 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodyear R. J., Marcotti W., Kros C. J., Richardson G. P., Development and properties of stereociliary link types in hair cells of the mouse cochlea. J. Comp. Neurol. 485, 75–85 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Tesi C., Barman T., Travers F., Sulphate is a competitive inhibitor of the binding of nucleotide to myosin. A comparison with phosphate. FEBS Lett. 236, 256–260 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Yamoah E. N., Lumpkin E. A., Dumont R. A., Smith P. J. S., Hudspeth A. J., Gillespie P. G., Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J. Neurosci. 18, 610–624 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X., Inesi G., Effects of anions on the Ca2+, H+ and electrical gradients formed by the sarcoplasmic reticulum ATPase in reconstituted proteoliposomes. FEBS Lett. 328, 301–304 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Goodno C. C., Inhibition of myosin ATPase by vanadate ion. Proc. Natl. Acad. Sci. U.S.A. 76, 2620–2624 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kros C. J., Marcotti W., van Netten S. M., Self T. J., Libby R. T., Brown S. D. M., Richardson G. P., Steel K. P., Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat. Neurosci. 5, 41–47 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Beurg M., Nam J.-H., Chen Q., Fettiplace R., Calcium balance and mechanotransduction in rat cochlear hair cells. J. Neurophysiol. 104, 18–34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollrath M. A., Eatock R. A., Time course and extent of mechanotransducer adaptation in mouse utricular hair cells: Comparison with frog saccular hair cells. J. Neurophysiol. 90, 2676–2689 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Gillespie P. G., Gillespie S. K., Mercer J. A., Shah K., Shokat K. M., Engineering of the Myosin-Iβ nucleotide-binding pocket to create selective sensitivity to N6-modified ADP analogs. J. Biol. Chem. 274, 31373–31381 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Li S., Mecca A., Kim J., Caprara G. A., Wagner E. L., du T.-T., Petrov L., Xu W., Cui R., Rebustini I. T., Kachar B., Peng A. W., Shin J.-B., Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex. Nat. Commun. 11, 2066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell I. J., Kossl M., Richardson G. P., Nonlinear mechanical responses of mouse cochlear hair bundles. Proc. Biol. Sci. 250, 217–227 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Peng A. W., Gnanasambandam R., Sachs F., Ricci A. J., Adaptation independent modulation of auditory hair cell mechanotransduction channel open probability implicates a role for the lipid bilayer. J. Neurosci. 36, 2945–2956 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato O., Komatsu S., Sakai T., Tsukasaki Y., Tanaka R., Mizutani T., Watanabe T. M., Ikebe R., Ikebe M., Human myosin VIIa is a very slow processive motor protein on various cellular actin structures. J. Biol. Chem. 292, 10950–10960 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirono M., Denis C. S., Richardson G. P., Gillespie P. G., Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44, 309–320 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Effertz T., Becker L., Peng A. W., Ricci A. J., Phosphoinositol-4,5-bisphosphate regulates auditory hair-cell mechanotransduction-channel pore properties and fast adaptation. J. Neurosci. 37, 11632–11646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham C. L., Qiu X., Wu Z., Zhao B., Peng G., Kim Y.-H., Lauer A., Müller U., TMIE defines pore and gating properties of the mechanotransduction channel of mammalian cochlear hair cells. Neuron , (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hokanson D. E., Ostap E. M., Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate. Proc. Natl. Acad. Sci. U.S.A. 103, 3118–3123 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarmento M. J., Coutinho A., Fedorov A., Prieto M., Fernandes F., Ca2+ induces PI(4,5)P2 clusters on lipid bilayers at physiological PI(4,5)P2 and Ca2+ concentrations. Biochim. Biophys. Acta 1838, 822–830 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Le S. C., Jia Z., Chen J., Yang H., Molecular basis of PIP2-dependent regulation of the Ca2+-activated chloride channel TMEM16A. Nat. Commun. 10, 3769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballesteros A., Fenollar-Ferrer C., Swartz K. J., Structural relationship between the putative hair cell mechanotransduction channel TMC1 and TMEM16 proteins. eLife 7, e38433 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan B., Akyuz N., Liu X. P., Asai Y., Nist-Lund C., Kurima K., Derfler B. H., György B., Limapichat W., Walujkar S., Wimalasena L. N., Sotomayor M., Corey D. P., Holt J. R., TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99, 736–753.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumont R. A., Zhao Y.-d., Holt J. R., Bähler M., Gillespie P. G., Myosin-I isozymes in neonatal rodent auditory and vestibular epithelia. J. Assoc. Res. Otolaryngol. 3, 375–389 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steyger P. S., Gillespie P. G., Baird R. A., Myosin Iβ is located at tip link anchors in vestibular hair bundles. J. Neurosci. 18, 4603–4615 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricci A. J., Fettiplace R., The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J. Physiol. 501, 111–124 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vélez-Ortega A. C., Freeman M. J., Indzhykulian A. A., Grossheim J. M., Frolenkov G. I., Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian auditory hair cells. eLife 6, e24661 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/33/eabb4922/DC1


