Abstract
Objective
To investigate the involvement of interleukin (IL)-26 in neuroinflammatory processes in multiple sclerosis (MS), in particular in blood-brain barrier (BBB) integrity.
Methods
Expression of IL-26 was measured in serum, CSF, in vitro differentiated T helper (TH) cell subsets, and postmortem brain tissue of patients with MS and controls by ELISA, quantitative PCR, and immunohistochemistry. Primary human and mouse BBB endothelial cells (ECs) were treated with IL-26 in vitro and assessed for BBB integrity. RNA sequencing was performed on IL-26–treated human BBB ECs. Myelin oligodendrocyte glycoprotein35–55 experimental autoimmune encephalomyelitis (EAE) mice were injected IP with IL-26. BBB leakage and immune cell infiltration were assessed in the CNS of these mice using immunohistochemistry and flow cytometry.
Results
IL-26 expression was induced in TH lymphocytes by TH17-inducing cytokines and was upregulated in the blood and CSF of patients with MS. CD4+IL-26+ T lymphocytes were found in perivascular infiltrates in MS brain lesions, and both receptor chains for IL-26 (IL-10R2 and IL-20R1) were detected on BBB ECs in vitro and in situ. In contrast to IL-17 and IL-22, IL-26 promoted integrity and reduced permeability of BBB ECs in vitro and in vivo. In EAE, IL-26 reduced disease severity and proinflammatory lymphocyte infiltration into the CNS, while increasing infiltration of Tregs.
Conclusions
Our study demonstrates that although IL-26 is preferentially expressed by TH17 lymphocytes, it promotes BBB integrity in vitro and in vivo and is protective in chronic EAE, highlighting the functional diversity of cytokines produced by TH17 lymphocytes.
The abundance of immune cells and their products in CNS lesions and in the CSF of patients with multiple sclerosis (MS), together with the genetic risk conferred by immune gene variants, supports the concept that MS is an autoimmune disorder.1–5 It is strongly believed that myelin-reactive CD4+ T helper (TH) lymphocytes are activated by an environmental trigger, after which they cross the blood-brain barrier (BBB), invade the CNS, and initiate a chronic, relapsing inflammatory cascade leading to tissue damage.3,6–8 These TH lymphocytes produce cytokines such as interleukin (IL)-17, IL-22, and granulocyte macrophage colony-stimulating factor (GM-CSF); the former 2 being involved in BBB breakdown8 and the latter stimulating CCR2+ monocytes, which promote tissue damage.9
IL-26 was first discovered as an inducible gene in Herpesvirus saimiri–transformed human T lymphocytes,10 but has since been described as a TH17-associated cytokine that is regulated by IL-1β, IL-23, and RORγt.5,11,12 IL-26 is part of the IL-10 cytokine family and binds to a heterodimeric receptor composed of the IL-10R2 and IL-20R1 chains, which results in activation of STAT1 and STAT3.13,14 Although the IL26 gene is absent in rodents, Ohnuma et al15 have recently demonstrated that recombinant human (rh) IL-26 can bind to and function through the murine receptor complex. In recent years, studies regarding the role of IL-26 in various immunologic diseases have started to emerge.15–20 In chronic inflammatory diseases including Crohn disease, rheumatoid arthritis, chronic hepatitis C infection, and chronic graft-versus-host disease, IL-26 appears to play a proinflammatory role, acting on epithelial cells, monocytes, natural killer cells, and fibroblasts, respectively.15–17,19 It was also recently found to be involved in antibacterial host defense.18,20–22 However, the role of IL-26 in MS, where TH17 lymphocytes are believed to play a major role, has not yet been investigated.
In the current study, we hypothesized that IL-26 has a proinflammatory role and is involved in BBB disruption. We report that the concentration of IL-26 is elevated in the serum and CSF of untreated patients with MS compared with healthy individuals and controls, respectively. We further demonstrate that IL-26–expressing TH lymphocytes are found within perivascular infiltrates in the brain of patients with MS. In contrast to our hypothesis, we demonstrate that the administration of IL-26 in mice with experimental autoimmune encephalomyelitis (EAE) reduces disease severity compared with control-treated mice. This is associated with a barrier-promoting effect of IL-26 through upregulation of tight junction molecules. Overall, our results demonstrate that IL-26, preferentially produced by TH17 lymphocytes, has a beneficial effect on barrier function during neuroinflammation and reduces clinical and pathologic disease burden in sterile autoimmune CNS inflammation.
Methods
Ethics
Blood was obtained from patients with MS and healthy donors (HDs) after written informed consent and ethical approval (Centre Hospitalier de l'Université de Montréal research ethic committee approval number BH07.001). Patients were classified according to the 2010 McDonald's revised criteria for MS diagnosis (Polman et al., 2011), as previously described (Larochelle et al., 2012). Other neurologic disease controls (OND) consisted of migraine, glioblastoma, and subjective neurologic symptoms with normal neurologic examination, normal MRI, and normal CSF analysis. With informed consent and ethical approval (Centre Hospitalier de l'Université de Montréal research ethic committee approval number BH07.001), human temporal lobe material was obtained from patients who underwent surgical treatment for intractable temporal lobe epilepsy and used for isolation of primary BBB endothelial cells (ECs). Human fetal brain tissue was obtained with ethical approval (Centre Hospitalier de l'Université de Montréal research ethic committee approval number HD07.002) for isolation of astrocytes.
All animal procedures were approved by the CHUM Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care (approval number N15035APs).
Data availability
Data not provided in the article because of space limitations can be shared at the request of other investigators for purposes of replicating procedures and results.
Results
IL-26 is preferentially expressed by TH17 lymphocytes
To address the phenotype of TH lymphocytes expressing IL-26, we cultured human naive (CD45RA+) TH lymphocytes from HDs in TH1, TH2, or TH17 differentiating conditions. We found that TH17 lymphocytes expressed the highest level of IL26 messenger RNA (mRNA) after 3 days in culture, which correlated with expression of IL17 (figure e-1, A and B, links.lww.com/NXI/A295). TH1 lymphocytes also expressed IL26 mRNA, but only after 6 days in culture. To identify the cytokine (or combination of cytokines) that induces expression of IL26 mRNA in naive TH lymphocytes, we treated naive TH lymphocytes from HDs with TH17-inducing cytokines daily for 6 days. We found that the combination of IL-1β, IL-23, and transforming growth factor (TGF)-β1 induced the highest level of IL26 mRNA expression in these lymphocytes (figure e-1C) and that IL-6, alone or in combination with other cytokines, did not significantly affect IL26 gene expression. To address whether IL26 is also expressed in memory TH lymphocytes, we cultured CD45RO+ TH lymphocytes from HDs in TH1- or TH17-polarizing conditions. IL-26 mRNA (figure e-1D) and protein (figure e-1E) expression was highest in TH17 lymphocytes, generated from memory CD4+ lymphocytes after 6 days in culture, when compared with TH1 lymphocytes or ex vivo memory TH lymphocytes. Because intracellular flow cytometry with commercially available antibodies does not work in our hands, we used immunocytofluorescent microscopy to analyze coexpression of IL-26 with IL-17 and RORγt in TH17 differentiated lymphocytes of HDs (figure e-1F). Quantification revealed a greater enrichment of IL-26+ cells in IL-17–expressing TH lymphocytes compared with interferon (IFN)-γ–expressing TH lymphocytes (figure e-1G). In addition, levels of IL26 mRNA significantly correlated with mRNA levels of TH17 markers IL17, IL22, RORC, and MCAM, but not with Csf2 (figure e-1H). To investigate whether IL-26 is primarily associated with TH17 lymphocytes and not with other immune cells, we measured its levels via qPCR in different ex vivo or activated/differentiated immune cell subsets collected from HDs. The highest IL26 mRNA levels were detected in TH17 lymphocytes polarized from memory TH lymphocytes (figure e-2A), whereas lower IL26 mRNA levels were detected in CD8+ T cytotoxic 17 (TC17) lymphocytes and TCR-stimulated memory TH lymphocytes without addition of polarizing cytokines. B lymphocytes, monocytes, M1 and M2 macrophages, and immature and mature dendritic cells (DCs) did not have detectable IL26 mRNA levels.
IL-26 is elevated in the serum and CSF of untreated patients with MS
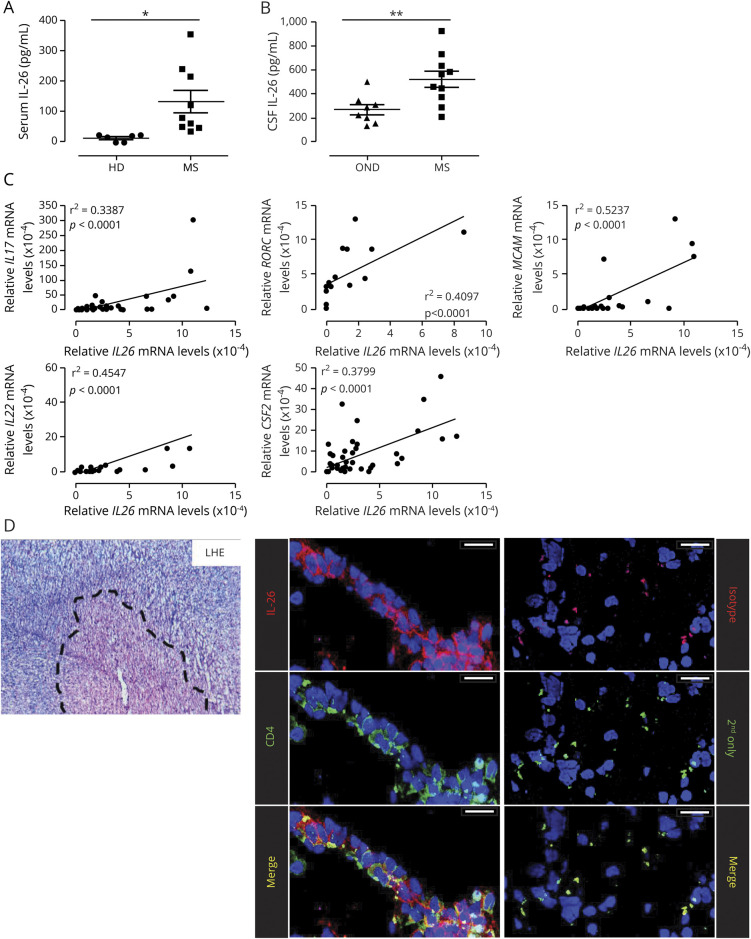
To determine whether IL-26 is augmented in relevant biological compartments during MS disease, we measured the concentration of IL-26 in the serum and CSF of controls and untreated patients with MS. IL-26 was significantly elevated in the serum of untreated patients with relapsing-remitting MS compared with HDs (figure 1A). In addition, IL-26 was approximately twofold higher in the CSF of untreated patients with MS compared with OND (figure 1B). Using MS patient–derived TH17 differentiated lymphocytes, we also found that IL26 mRNA significantly correlated with expression of IL17, IL22, RORC, and MCAM (figure 1C), as previously demonstrated using the blood of HDs. In addition, expression of IL26 mRNA also correlated with expression of Csf2 mRNA in TH17 lymphocytes of patients with MS (figure 1C). Finally, immunohistofluorescence imaging of active MS brain lesions (18 lesions, 6 distinct patients) revealed innumerable CD4+ T lymphocytes expressing IL-26 in perivascular infiltrates (figure 1D).
Figure 1. IL-26 is increased in the serum and CSF of untreated patients with MS and is expressed by CNS-infiltrating lymphocytes.
(A) IL-26 protein levels in the serum of HDs (n = 6) and untreated patients with RRMS (MS, n = 9). (B) IL-26 protein levels in the CSF of persons with OND (n = 8) and untreated patients with MS (n = 10). (C) Relative expression of IL26 mRNA plotted against the relative expression of other T helper (TH)17-associated genes (IL17, IL22, Csf2, RORC, and MCAM) in ex vivo CD4+CD45RO+ T lymphocytes, TH1- and TH17-polarized lymphocytes (after 6 days in culture) from 5 to 18 patients with MS. mRNA expression is relative to 18S mRNA and was assessed by qPCR. (D) Autopsy-derived MS CNS material was stained with Luxol Fast Blue/H&E (LHE, left panel) to identify lesions (dashed line). Colocalization of IL-26 (red) with CD4+ cells (green) and TOPRO-3 (blue, nuclei) in MS lesions is shown (left panel). As a control, CNS material was stained with an isotype control and secondary antibody (red) or secondary antibody alone (green, right panel). Images shown are representative of immunostainings on CNS samples from 6 patients with MS (3 tissue blocks per patient). Scale bars: 25 μm. Data are presented as mean ± SEM (A and B). *p < 0.05; **p < 0.01. Statistical tests: Student 2-tailed t test (A and B) and Pearson correlation (C). HDs = healthy donors; IL = interleukin; mRNA = messenger RNA; OND = other neurologic disease controls; RRMS = relapsing-remitting MS.
IL-26 increases BBB tightness through upregulation of tight junction molecules
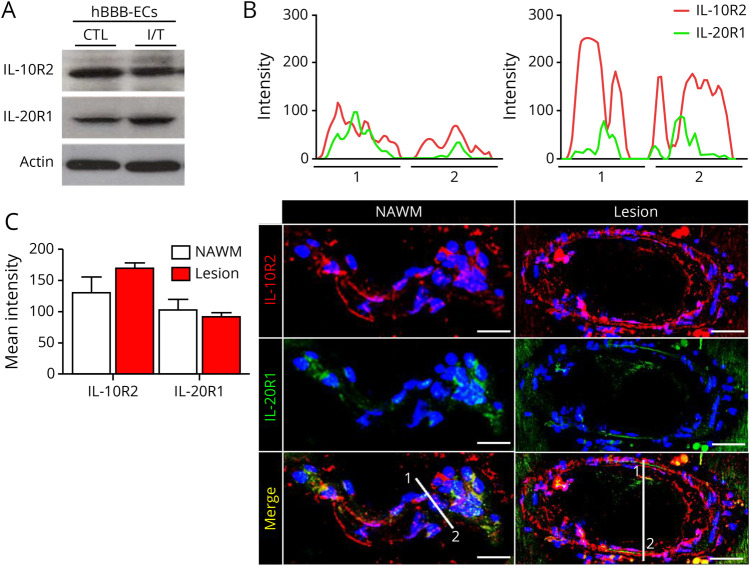
Our group previously demonstrated that the TH17-associated cytokines, IL-17A and IL-22, increase BBB permeability and facilitate TH lymphocyte migration across the BBB.8 Because IL-26 is preferentially expressed by TH17 lymphocytes, we hypothesized that it has a similar effect on the BBB. To investigate whether BBB ECs can respond to IL-26 stimulation, we first assessed expression of the IL-10R2 and IL-20R1 chains. Indeed, we detected both IL-26 receptor chains on primary cultures of human BBB ECs in vitro (figure 2A) as well as on CNS-ECs in situ (figure 2, B and C). Inflammatory conditions did not significantly affect the expression of either chain (figure 2A). Further investigation of the 2 receptor chains demonstrated their spatial colocalization on ECs in situ both in normal-appearing white matter and in MS lesions (figure 2, B and C), suggesting that these cells can respond to IL-26.
Figure 2. Presence of IL-10R2 and IL-20R1 on BBB ECs.
(A) IL-26 receptor expression on resting (CTL) and inflamed (IFNγ and TNFα, I/T) human BBB ECs by Western blot. Representative image of n = 4 experiments is shown. (B) Autopsy-derived MS CNS material stained for IL-10R1 (red) and IL-20R2 (green) and with TOPRO-3 (blue, nuclei). Representative plots of IL-20R1 and IL-10R2 signal intensity and colocalization of both receptor chain (upper graphs) in areas 1 and 2 are shown for NAWM (left) or MS lesions (right). (C) Semiquantitative analysis of the signal intensity from 10 µm z-stack reconstructions along the line markers in the images shown in (B), for IL-20R1 and IL-10R2, in NAWM and MS lesion. Mean fluorescence intensity was averaged from 3 distinct measurements on each vessel (n = 33 blood vessels). Images shown are representative of immunostainings on brain lesions of 6 patients with MS (1–2 tissue blocks per patient). Scale bars: 50 μm for lesion and 30 μm for NAWM. Data are represented as mean ± SEM (C). Statistical tests: Student 2-tailed t test (C). BBB ECs = blood-brain barrier endothelial cells; CTL = control; IL = interleukin; NAWM = normal-appearing white matter.
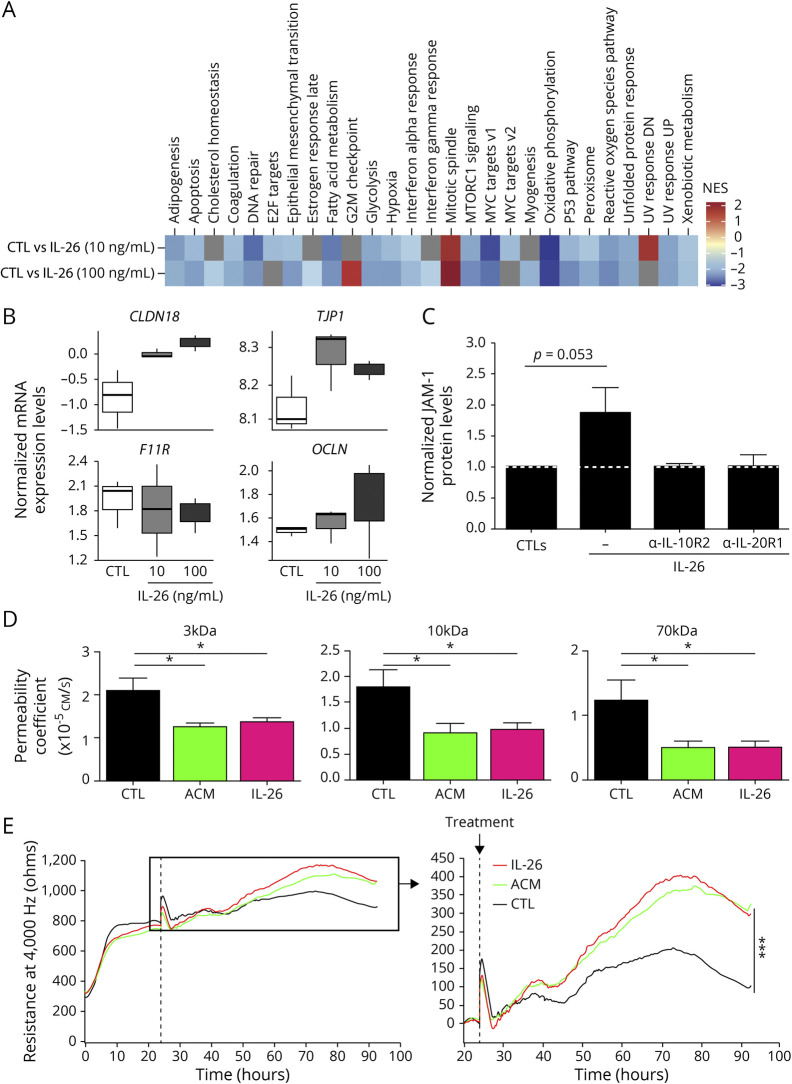
To assess the effect of IL-26 on human BBB ECs, we investigated the transcriptome of resting (control [CTL]) vs rhIL-26 treated BBB ECs. In total, 459 differentially expressed genes (DEGs; false discovery rate < 0.1 & |logFC| > 0.25) were identified, all in the 100 ng/mL condition (figure e-3, A and B, links.lww.com/NXI/A295). To understand what functional pathways might be associated with these transcriptional changes, a ranked gene-set enrichment analysis (GSEA) approach was used to alleviate the limitations of setting predefined numerical thresholds of significance to form discrete gene sets (See Methods section). Our data demonstrate that rhIL-26 treatment resulted mostly in a general downregulation of pathways, notably those involved in the signaling of proinflammatory cytokines tumor necrosis factor-α, IFN-γ, IFN-α, and IL-6, as well as in apoptosis and adipogenesis (figure 3A, figure e-3C). The most significant enrichment was associated with a downregulation of oxidative phosphorylation and was consistent in both conditions (figure e-3C). This suggests that IL-26 could mediate cellular metabolism through decrease in energy production. Of interest, using the ranked approach for GSEA shows that although there are no DEGs in the 10 ng/mL condition according to predefined criteria, the pathways associated with the transcriptomic variation is consistent with the 100 ng/mL condition, indicating that the response in both conditions is correlated. We indeed observe correlation between the fold changes calculated in each condition (R = 0.62, p < 2.2 × 10−16). Because BBB integrity is highly dependent on the expression of tight junction (TJ) molecules (e.g., junctional adhesion molecules [JAM], claudins [CLDN], occludin [OCLN], and zonula occludens [ZO]),23 we hypothesized that some of these molecules would also be downregulated on IL-26 stimulation. Therefore, we decided to focus on the expression profiles of specific BBB-associated TJ molecules within the same transcriptome data. This allowed us to observe that stimulation with IL-26 upregulates the mRNA levels of TJP1 (ZO-1), OCLN, and CLDN18, while also downregulating F11R (JAM-1) levels (figure 3B). CLDN5 mRNA was below the detection limit. Collectively, these data indicate that unlike IL-17, IL-26 has a unique effect on BBB ECs where it downregulates proinflammatory pathways and upregulates TJ molecules.
Figure 3. IL-26 enhances BBB function in vitro.
(A and B) Human BBB ECs derived from 1 donor were treated with 0, 10, or 100 ng/mL recombinant human (rh)IL-26 in triplicate for 24 hours. RNA sequencing was performed on messenger RNA (mRNA) isolated from these samples. (A) A heatmap of the enrichment scores for pathways found in the hallmark curated database is shown. Only the pathways that are significant in at least 1 condition are shown (adjusted p value < 0.25). Gray tiles are nonsignificant, and the enrichment is not shown for clarity purposes. (B) The boxplots show the distributions of the voom-normalized expression of claudin 18 (CLDN18), ZO-1 (TJP1), JAM-1 (F11R), and occludin (OCLN) mRNA levels using the same samples as for (A). Normalization was performed with limma/voom. (C) JAM-1 protein expression by human BBB ECs pretreated with or without 5 μg/mL anti–IL-10R2 or 5 μg/mL anti–IL-20R1. After 1 hour, 100 ng/mL rhIL-26 or control was added for 24 hours. Samples were analyzed by Western blot (n = 3). Protein expression, relative to beta-actin, was calculated and normalized to the respective controls (upper panels, white dotted line). (D) In vitro permeability was investigated using a modified Boyden chamber assay. The permeability coefficients of 3-kDa, 10-kDa, and 70-kDa fluorescently labeled dextran through untreated (CTL), 100 ng/mL IL-26, or 40% ACM (positive control) treated monolayer of human BBB ECs (n = 4 experiments). Each condition was performed in triplicate. (E) Transendothelial electrical resistance of human BBB EC monolayers was measured continuously for 92 hours. ACM (positive control) or rhIL-26 was added 24 hours after BBB EC plating (dotted line, left panel and arrow right panel). The right panel is an enlarged representation of the insert box in the left panel. Representative graph of n = 4 experiments is shown. All conditions were performed in triplicate. Data are represented as mean ± SEM (C and D). *p < 0.05; ***p < 0.001. Statistical tests: Student 2-tailed t test (C), a 1-way analysis of variance (ANOVA) (D), and repeated-measures 1-way ANOVA followed by the Dunnett multiple comparison test (E). ACM = astrocyte-conditioned medium; BBB ECs = blood-brain barrier endothelial cells; CTL = control; IL = interleukin; SEM = standard error of the mean.
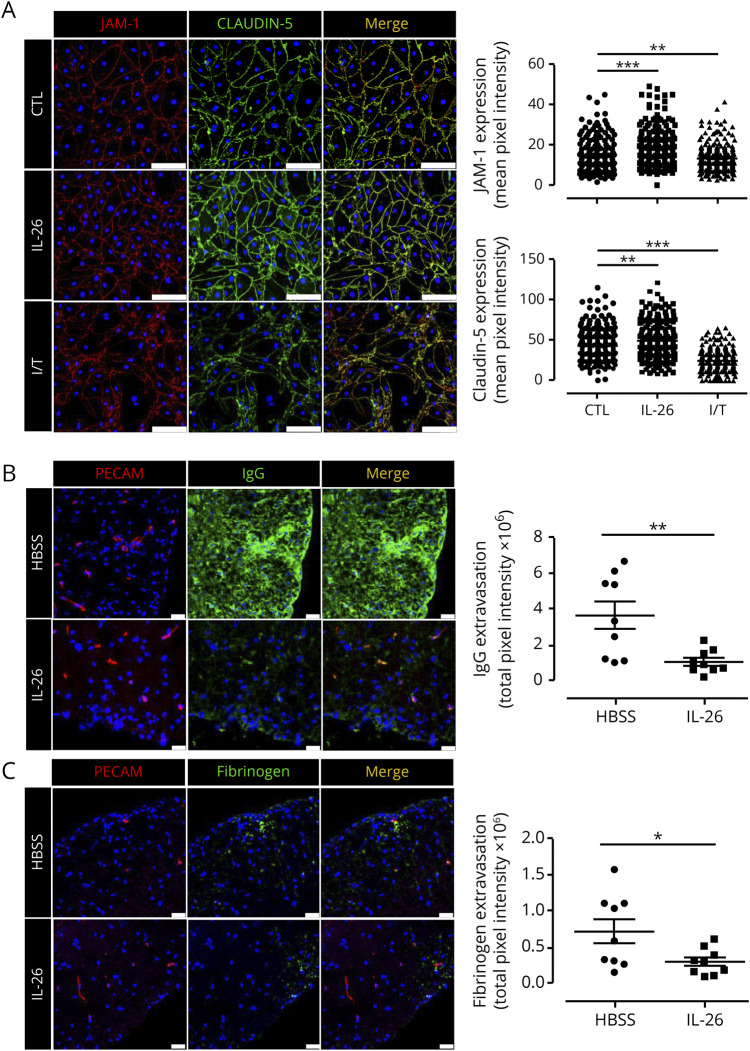
To confirm our observations at the protein level, we performed Western blot analysis on IL-26–treated BBB ECs and observed an increase in JAM-1 protein levels compared with CTL BBB ECs (figure 3C and figure e-4A, links.lww.com/NXI/A295, p = 0.053). The ZO-1 protein level did not change (figure e-4B, p = 0.431). To confirm that JAM-1 upregulation is due to IL-26 signaling via its heterodimeric receptor, we pretreated BBB ECs with blocking antibodies against IL-10R2 or IL-20R1 before rhIL-26 was added. The IL-26–induced upregulation in the JAM-1 level in BBB ECs was prevented by pretreatments with anti–IL-10R2 or anti–IL-20R1 (figure 3C and figure e-4A). To investigate whether IL-26 has a functional effect on BBB integrity, we performed an in vitro permeability assay using modified Boyden chambers. Briefly, we treated primary human BBB ECs, grown to confluency on inserts, with rhIL-26, and measured their permeability to 3-, 10- and 70-kDa dextrans via spectroscopy. Of interest, rhIL-26–treated human BBB ECs acquired a significantly lower permeability coefficient to each of the dextrans compared with untreated BBB ECs (figure 3D), suggesting that IL-26 indeed has a positive effect on BBB integrity compared with IL-17 and IL-22. Consistent with these observations, the transendothelial electrical resistance (TEER) of human BBB ECs monolayers was also significantly and sustainably increased by rhIL-26 treatment to the same extent as the positive control (astrocyte-conditioned medium; figure 3E).
IL-26 therapy reduces BBB permeability during neuroinflammation
Although mice do not express IL-26, they do express a functional receptor, which responds to rhIL-26.15 Previous studies on IL-26 have shown that results obtained from mouse models using rhIL-26 provide information, which is supportive of human in vitro or in situ studies.15,24,25 Before investigating the contribution of IL-26 on BBB permeability in vivo, we first sought to confirm whether rhIL-26 affects mouse BBB ECs in vitro. Mouse brain ECs were isolated and treated with 100 ng/mL rhIL-26, and subsequent expression of TJ molecules JAM-1, ZO-1, and CLDN5 was assessed. In line with our aforementioned observations, we found that rhIL-26–treated mouse BBB ECs significantly upregulated the expression of both JAM-1 and CLDN5 (figure 4A), whereas it had no effect on ZO-1 (not shown). In addition, rhIL-26 enhanced the organizational structure of the intercellular TJ strands between mouse BBB ECs (figure 4A). To study the impact of IL-26 on the BBB in vivo, we analyzed its effect on BBB permeability in EAE mice immunized with myelin oligodendrocyte glycoprotein (MOG)35–55 and treated with 200 ng rhIL-26 daily IP from day 5–24 postimmunization. In situ analysis of endogenous serum proteins extravasation into the CNS revealed that rhIL-26 treatment significantly reduced extravasation of both IgG (figure 4B) and fibrinogen (figure 4C) in spinal cords of EAE animals compared with Hanks balanced salt solution (control)-treated mice.
Figure 4. IL-26 treatment reduces fibrinogen and IgG leakage in EAE mice.
(A) Monolayers of mouse BBB ECs immunostained for tight junction molecules JAM-A (red) and claudin-5 (green) and nuclei (blue) after treatment with IFN-γ and TNF-α (I/T), 100 ng/mL IL-26, or left untreated (24 hours treatment). Representative images of n = 4 experiments are shown. JAM-A and Claudin-5 mean fluorescent intensity across the mouse BBB EC membrane was quantified at 30 different places per image. Three fields of view were randomly analyzed and quantified for each condition and for each experiment (n = 240 measurements per condition). Scale bars: 100 μm. (B and C) MOG35–55 immunized C57BL/6 mice were injected IP with either HBSS or 200 ng recombinant human IL-26 in HBSS daily from day 5 to day 24. Immunohistofluorescence of IgG (green, B, central panel), fibrinogen (green, C, central panel), and PECAM-1 (red, left panel) was performed on spinal cords of EAE mice at day 27 postinduction. Fluorescence of IgG (B, right panel) and fibrinogen (C, right panel) extravasation was quantified (n = 3 animals per group and 3 sections per mouse). Scale bars: 25 μm. Data are represented as mean ± SEM (A–C). *p < 0.05; **p < 0.01; ***p < 0.001. Statistical tests: 1-way analysis of variance followed by the Dunnett multiple comparison test (A) and Student 2-tailed t test (B and C). BBB ECs = blood-brain barrier endothelial cells; EAE = experimental autoimmune encephalomyelitis; HBSS = Hanks balanced salt solution; IL = interleukin; MOG = myelin oligodendrocyte glycoprotein; SEM = standard error of the mean.
IL-26 therapy reduces EAE severity and limits infiltration of pathogenic T lymphocytes
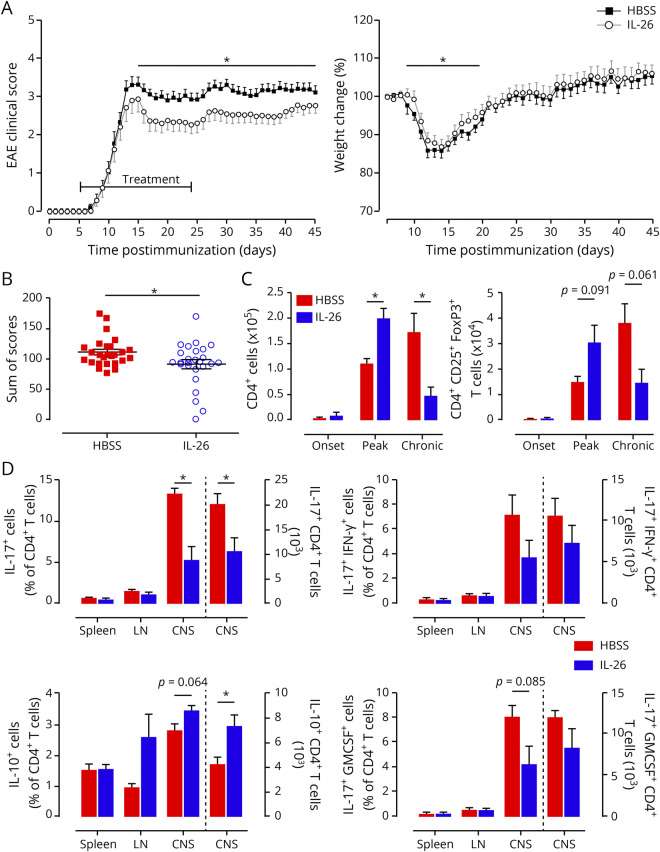
To evaluate whether the protective effects of IL-26 on the BBB in EAE translate into a clinically detectable benefit, we monitored the symptoms of EAE mice treated with IL-26 daily for 45 days. We found that rhIL-26–treated EAE mice experienced a less severe clinical disease course compared with control-treated mice, particularly during the chronic phase of the disease (figure 5, A and B), but also at the peak of the disease. Surprisingly, this beneficial outcome was associated with an increased number of CNS-infiltrating TH lymphocytes at peak of disease, and a decreased number during the chronic phase, compared with control-treated mice (figure 5C). A deeper analysis of these lymphocytes revealed that the administration of IL-26 resulted in an infiltration of both conventional and regulatory (CD4+Foxp3+CD25+) T lymphocytes (Treg) at peak of disease (figure e-5A, links.lww.com/NXI/A295 and figure 5C). When analyzing cytokine production by different TH subsets, we found that the proportion and number of IL-17–expressing TH lymphocytes were significantly reduced in the CNS of rhIL-26–treated animals, whereas the number and proportion of IL-10–expressing TH lymphocytes were increased compared with control animals (figure 5D). No clear effect was observed for IFN-γ+IL-17+ TH lymphocytes, but a tendency toward a lower proportion of GM-CSF+IL-17+ TH lymphocytes was observed (figure 5D). In the myeloid compartment within the CNS at peak of disease, no significant differences were found for the number of inflammatory (Ly6C+) or patrolling (Ly6C−) monocytes between IL-26–treated and control animals (figure e-5, B and C). In addition, no differences were observed for the numbers of Ly6Cint neutrophils (figure e-5D). Of interest, the number of microglia was decreased in the chronic phase of IL-26–treated mice compared with control mice (figure e-5E).
Figure 5. IL-26 treatment ameliorates clinical symptoms of EAE.
(A) MOG35–55 immunized C57BL/6 mice injected IP with either 200 μL HBSS (black squares) or 200 μL containing 200 ng recombinant human IL-26 in HBSS (gray open circles) were scored (left panel) and weighed (normalized to day 0; right panel) daily. IL-26 treatment was started at day 5 after disease induction and was given daily until day 24. (B) Distribution and sum of the clinical scores for the 2 different treatment groups. Each dot is 1 animal. (C) The CNS of perfused mice was collected, and infiltrating immune cells were isolated, counted, and analyzed by flow cytometry at onset (day 7), peak (day 15), and chronic (day 27) phases of disease. (D) The CNS, spleen, and LN of perfused mice were collected, and immune cells were isolated, counted, and analyzed by flow cytometry for intracellular cytokine staining at peak (day 15) of disease. Bars to the left of the dotted line represent cell percentages (left y-axis), and bars to the right of the dotted line represents total number of cells (right y-axis). Data are represented as mean ± SEM (A–D). *p < 0.05. Statistical analyses performed: Student 2-tailed t test of the area under the curve (A); Student 2-tailed t test (B–D). Data shown are representative of 6 independent EAE experiments with 9–30 animals per group (A and B). n = 3 animals per group (C and D). EAE = experimental autoimmune encephalomyelitis; GM-CSF = granulocyte macrophage colony-stimulating factor; HBSS = Hanks balanced salt solution; IL = interleukin; LN = lymph nodes; MOG = myelin oligodendrocyte glycoprotein; SEM = standard error of the mean.
Discussion
Although IL-26 is suspected to be proinflammatory and antimicrobial,15–17,19,20 the exact role of this cytokine in the context of neuroinflammatory disorders has not been identified. Here, we demonstrated that IL-26, in contrast to IL-17 and IL-22, produced by TH17 lymphocytes, reduced BBB permeability through upregulation of TJ molecules, thereby reducing clinical severity in EAE mice treated with rhIL-26 in vivo.
Our data demonstrate that IL26 mRNA expression was induced in naive TH lymphocytes by a combination of the TH17-polarizing cytokines IL-1β, IL-23, and TGF-β1, whereas IL-6, a nonredundant cytokine for TH17 differentiation, seems to have barely an effect on IL-26 levels. In addition, IL26 transcript expression correlated strongly with TH17 markers. Overall, these data support its preferential association with the TH17 lineage, although broader regulation across TH lineages cannot be ruled out, especially because our postmortem MS brain analyses show that the majority of TH lymphocytes in perivascular cuffs express IL-26. Together with our data showing nondetectable mRNA levels in B cells, monocytes, macrophages, and DCs, this suggests that TH lymphocytes (especially TH17 lymphocytes) are the most relevant source of IL-26 in MS.
Because both IL-17 and IL-22 have been shown to be involved in BBB disruption,8 we wanted to determine whether the same held true for IL-26. Surprisingly, IL-26 enhanced BBB integrity by reducing permeability of BBB ECs both in vitro and in vivo. This seemed to be a direct effect because both IL-26 receptor chains were found to be expressed on BBB ECs. We further demonstrated that this reduction in leakage was due to an increased expression of TJ molecules, especially JAM-1 and CLDN5. Although our pathway analysis indicated an increase in TJP1, this was not seen on the protein level. A possible explanation could be that the effect on protein levels might take longer than 24 hours. Of interest, F11R levels seemed to be decreased, whereas protein levels were increased after 24 hours of IL-26 treatment. This could also be related to the time point of protein detection. However, it is also possible that the translation rate is increased on IL-26 treatment. Protein levels are, however, the most important readout for TJ molecules and hold the most biological relevance for BBB integrity. These results in combination with the observed increase in IL-26 levels in MS suggest a compensatory mechanism to restore BBB integrity and reduce BBB leakage.
A barrier-promoting effect was already shown for IL-10,26–28 which shares one of the receptor chains of the IL-26 receptor, suggesting that the barrier-promoting effect of IL-26 is established through signaling via the IL-10R2 subunit. We have confirmed that both receptor units, IL-10R2 and IL-20R1, are critically involved in this barrier-enhancing effect. Because it has been shown that activation of both receptor units signals via the STAT1/STAT3 pathway,14 the barrier-strengthening effect of IL-26 seems to also be mediated via this pathway. Recently, Itoh et al.29 showed a possible opposite effect of IL-26 on vasculature in a psoriasis model. This difference might either be due to a secondary inflammatory effect via the epidermal keratinocytes and dermal fibroblast or a direct effect. To study this, they used human umbilical vein ECs, which were able to respond to IL-26 stimulation. However, these cells did not express the IL-20R1, which was confirmed by the lack of p-STAT3 upregulation. Our data show that human primary BBB ECs do express the IL-20R1 and that IL-26 signals via this receptor. The fact that both cell types react differently to IL-26 might not be very surprising because numerous studies have already reported differences with regard to permeability, TEER, migration, and chemokine production.30,31
A previous study has shown that IL-26 is not expressed in mice, although they do express a functional IL-26 receptor.15 Therefore, murine cell lines or mouse models have been used to further unravel the mechanisms of IL-26 and to study the effect of IL-26 in vivo.15,24,25 Moreover, the results of these studies were in line with findings obtained in human in vitro experiments. To assess the role of IL-26 in vivo in the context of neuroinflammation, we administered rhIL-26 to EAE mice immunized with MOG35–55. Mice treated with IL-26 displayed a milder disease course and a reduced BBB permeability. Most surprisingly, there were a higher number of IL-10–producing CNS-infiltrating TH lymphocytes in the IL-26–treated group. In parallel, we also observed a reduction in CNS-infiltrating pathogenic TH lymphocytes in IL-26–treated mice (IL-17+IFN-γ+). Whether these findings are the result of (1) suppression of pathogenic TH lymphocytes by infiltrating Tregs, (2) skewing of pathogenic TH lymphocytes toward Tregs by modification in the cytokine environment induced by IL-26, (3) a direct effect of IL-26 on polarization of TH lymphocytes, or finally (4) an effect of IL-26 on the expression of CAMs by BBB ECs, has yet to be investigated. However, it is unlikely that IL-26 directly skews TH lymphocytes toward a regulatory phenotype because these cells do not express the IL-20R1. This is supported by our data showing that rhIL-26 does not influence TH17 lymphocyte polarization (figure e-6A, links.lww.com/NXI/A295). In addition, a study by Oral et al.32 also shows that IL-26 did not affect naive TH lymphocyte differentiation, proliferation, or viability. An indirect effect on TH lymphocytes, via antigen-presenting cells, can not be excluded. Recent studies show that IL-26 can bind to genomic and mitochondrial self-DNA and bacterial DNA and activate the Toll-like receptor 9 or directly activate stimulator of interferon genes in myeloid cells.20,33 Nevertheless, this effect of IL-26 induces IFN-α, IL-6, and IL-1β, which trigger a proinflammatory, rather than an anti-inflammatory, response. Alternatively, IL-26 could also act on other immune or CNS cells, which would, in turn, skew TH lymphocytes toward an IL-10–producing phenotype. Finally, as Tregs have been reported to migrate faster across BBB ECs than non-Tregs,34 we therefore speculate that IL-26, produced by CNS-infiltrating TH lymphocytes in the context of neuroinflammation, could affect the recruitment of IL-10–producing TH lymphocytes across the CNS barriers by a yet unknown mechanism. In conclusion, our data reveal that IL-26 is a protective cytokine, which is preferentially produced by TH17 lymphocytes on inflammation. During neuroinflammation, the administration of IL-26 promotes BBB functions through upregulation of TJ molecules, increases the number of IL-10–producing CNS-infiltrating TH lymphocytes, and reduces the number of CNS-infiltrating pathogenic TH lymphocytes. These findings might have important therapeutic implications for patients with MS, which will be validated in future studies.
Acknowledgment
The authors are grateful to Dr. D. Gauchat (Flow Cytometry Core Facility, CRCHUM, Montreal, QC, Canada) for her technical support with flow cytometry and Dr. A. Cleret-Buhot (Cell Imaging Core Facility, CRCHUM, Montreal, QC, Canada) for her technical support with confocal microscopy. They thank Dr. E. Haddad (Pediatric Immunologist, CHU Sainte-Justine Research Center) for providing human fetal CNS material.
Glossary
- BBB
blood-brain barrier
- CLDN
claudins
- DEG
differentially expressed gene
- EAE
experimental autoimmune encephalomyelitis
- EC
endothelial cell
- GM-CSF
granulocyte macrophage colony-stimulating factor
- GSEA
gene set enrichment analysis
- HDs
healthy donors
- IL
interleukin
- JAM
junctional adhesion molecule
- MOG
myelin oligodendrocyte glycoprotein
- mRNA
messenger RNA
- OCLN
occludin
- OND
other neurologic disease controls
- rh
recombinant human
- TEER
transendothelial electrical resistance
- TH
T helper
- TJ
tight junction
- ZO
zonula occludens
Appendix. Authors
Study funding
This work was funded by a grant from the Canadian Institutes of Health Research (MOP136980). A. Prat holds a senior Canada Research Chair in Multiple Sclerosis. M. Charabati held a postdoctoral studentship from the Fonds de recherche du Québec–Santé (FRQS) and is now supported by a doctoral scholarship from the Multiple Sclerosis Society of Canada (MSSC). E. Peelen and S. Zandee held a postdoctoral studentship from the FRQS/MSSC Partnership Award. B. Broux held a postdoctoral studentship from Fonds Wetenschappelijk Onderzoek–Vlaanderen. L. Hachehouche held PhD studentships from the MSSC, FRQS, and Neuroinflammation Training Program.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol 2004;61:1613–1615. [DOI] [PubMed] [Google Scholar]
- 2.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest 2012;122:1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broux B, Stinissen P, Hellings N. Which immune cells matter? The immunopathogenesis of multiple sclerosis. Crit Rev Immunol 2013;33:283–306. [DOI] [PubMed] [Google Scholar]
- 4.International Multiple Sclerosis Genetics Consortium, Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 2013;45:1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008;9:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafler DA. Multiple sclerosis. J Clin Invest 2004;113:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Codarri L, Greter M, Becher B. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol 2013;34:114–119. [DOI] [PubMed] [Google Scholar]
- 8.Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 2007;13:11731175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croxford AL, Lanzinger M, Hartmann FJ, et al. The cytokine GM-CSF drives the inflammatory signature of CCR2 monocytes and licenses autoimmunity. Immunity 2015;43:502–514. [DOI] [PubMed] [Google Scholar]
- 10.Knappe A, Hor S, Wittmann S, Fickenscher H. Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J Virol 2000;74:3881–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007;8:950–957. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly RP, Sheikh F, Dickensheets H, Savan R, Young HA, Walter MR. Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev 2010;21:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheikh F, Baurin VV, Lewis-Antes A, et al. Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J Immunol 2004;172:2006–2010. [DOI] [PubMed] [Google Scholar]
- 14.Hor S, Pirzer H, Dumoutier L, et al. The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J Biol Chem 2004;279:33343–33351. [DOI] [PubMed] [Google Scholar]
- 15.Ohnuma K, Hatano R, Aune TM, et al. Regulation of pulmonary graft-versus-host disease by IL-26+CD26+CD4 T lymphocytes. J Immunol 2015;194:3697–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dambacher J, Beigel F, Zitzmann K, et al. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut 2009;58:1207–1217. [DOI] [PubMed] [Google Scholar]
- 17.Corvaisier M, Delneste Y, Jeanvoine H, et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol 2012;10:e1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Che KF, Tengvall S, Levanen B, et al. Interleukin-26 in antibacterial host defense of human lungs. Effects on neutrophil mobilization. Am J Respir Crit Care Med 2014;190:10221031. [DOI] [PubMed] [Google Scholar]
- 19.Miot C, Beaumont E, Duluc D, et al. IL-26 is overexpressed in chronically HCV-infected patients and enhances TRAIL-mediated cytotoxicity and interferon production by human NK cells. Gut 2015;64:1466–1475. [DOI] [PubMed] [Google Scholar]
- 20.Meller S, Di Domizio J, Voo KS, et al. TH17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol 2015;16:970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scala E, Di Caprio R, Cacciapuoti S, et al. A new T helper 17 cytokine in hidradenitis suppurativa: antimicrobial and proinflammatory role of interleukin-26. Br J Dermatol 2019;181:1038–1045. [DOI] [PubMed] [Google Scholar]
- 22.Woetmann A, Alhede M, Dabelsteen S, et al. Interleukin-26 (IL-26) is a novel antimicrobial peptide produced by T cells in response to staphylococcal enterotoxin. Oncotarget 2018;9:19481–19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao A, Che KF, Bozinovski S, et al. Recombinant human IL-26 facilitates the innate immune response to endotoxin in the bronchoalveolar space of mice in vivo. PLoS One 2017;12:e0188909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng YJ, Wang CY, Lin YH, et al. Interleukin 26 suppresses receptor activator of nuclear factor kappaB ligand induced osteoclastogenesis via down-regulation of nuclear factor of activated T-cells, cytoplasmic 1 and nuclear factor kappaB activity. Rheumatology (Oxford) 2016;55:2074–2083. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Duan XS, Xu LJ, et al. Interleukin-10 mediates the neuroprotection of hyperbaric oxygen therapy against traumatic brain injury in mice. Neuroscience 2014;266:235–243. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Zhang P, Ma Y, et al. Lactobacillus plantarum prevents the development of colitis in IL-10-deficient mouse by reducing the intestinal permeability. Mol Biol Rep 2011;38:1353–1361. [DOI] [PubMed] [Google Scholar]
- 28.Mazzon E, Puzzolo D, Caputi AP, Cuzzocrea S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol Med 2002;8:353–366. [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh T, Hatano R, Komiya E, et al. Biological effects of IL-26 on T cell-mediated skin inflammation, including psoriasis. J Invest Dermatol 2019;139:878–889. [DOI] [PubMed] [Google Scholar]
- 30.Man S, Ubogu EE, Williams KA, Tucky B, Callahan MK, Ransohoff RM. Human brain microvascular endothelial cells and umbilical vein endothelial cells differentially facilitate leukocyte recruitment and utilize chemokines for T cell migration. Clin Dev Immunol 2008;2008:384982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukaliak JA, Dorovini-Zis K. Expression of the beta-chemokines RANTES and MIP-1 beta by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol 2000;59:339–352. [DOI] [PubMed] [Google Scholar]
- 32.Oral HB, Kotenko SV, Yilmaz M, et al. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol 2006;36:380–388. [DOI] [PubMed] [Google Scholar]
- 33.Poli C, Augusto JF, Dauve J, et al. IL-26 confers proinflammatory properties to extracellular DNA. J Immunol 2017;198:3650–3661. [DOI] [PubMed] [Google Scholar]
- 34.Schneider-Hohendorf T, Stenner MP, Weidenfeller C, et al. Regulatory T cells exhibit enhanced migratory characteristics, a feature impaired in patients with multiple sclerosis. Eur J Immunol 2010;40:3581–3590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations can be shared at the request of other investigators for purposes of replicating procedures and results.