Abstract
Background
We assessed the safety and effectiveness of baloxavir marboxil administration in Japanese children with influenza.
Methods
This open-label study administered 1 weight-adjusted dose of baloxavir to 107 children aged 1–11 years with laboratory-confirmed, febrile influenza virus infection of ≤48 hours duration.
Results
Adverse events (AEs) were reported in 34.6% of patients, most commonly vomiting (7.5%); no serious AEs or AEs causing discontinuation occurred. The median time to alleviation of influenza illness was 44.6 hours (95% confidence interval, 38.9–62.5 hours), to resolution of fever was 21.4 hours, and to sustained cessation of infectious viral shedding was 24.0 hours. However, viruses with amino acid substitutions in the viral polymerase acidic protein at position I38 (PA/I38T/M) emerged in 18 of 77 (23.4%) patients. Emergence was associated with longer infectious virus detectability (median time, 180.0 hours) and time to illness alleviation (median, 79.6 vs 42.8 hours in patients without PA/I38T/M-substituted viruses). Among patients with PA/I38T/M-substituted virus emergence, those with baseline hemagglutinin inhibition (HAI) antibody titer <40 experienced delay in time to illness alleviation (median, 85.4 vs 56.0 hours in patients with higher baseline HAI antibody titer).
Conclusions
A single, oral dose of baloxavir marboxil was well tolerated and rapidly reduced viral titers, but the common emergence of PA/I38T/M-substituted viruses warrants consideration of alternative dosing regimens in young children.
Clinical Trials Registration
Japan Pharmaceutical Information Center Clinical Trials Information (Japic CTI-163417).
Keywords: antiviral, baloxavir marboxil, influenza, children, Japan
A single, oral dose of baloxavir marboxil was well tolerated and rapidly reduced viral titers, but the common emergence of PA/I38T/M-substituted viruses warrants consideration of alternative dosing regimens in young children.
(See the Brief Report by Behillil et al on pages 1089–91 and the Editorial Commentary by Whitley and Monto on pages 1092–4.)
Influenza is a well-recognized cause of acute respiratory illness in children. Infants and young children, especially those below the age of 2, are at highest risk of complications and hospitalizations [1, 2]. Pediatric mortality due to influenza is relatively uncommon but occurs in those with underlying conditions and those who were previously healthy [3]. One assessment estimated that 13–32 million cases of influenza-associated acute lower respiratory illness and 28 000 to 111 500 associated deaths occurred worldwide in children aged under 5 years in 2008 [4]. Children aged 5 to 17 years had the highest rate of influenza infection during the 2009 A(H1N1) pandemic [5]. In addition, school-aged children are also important in transmission of influenza viruses, including those resistant to antivirals [6], to one another and to their household contacts.
Annual immunization is the primary means for protection against influenza, and children aged 6–59 months are one of the World Health Organization priority groups for vaccine [1]. However, the effectiveness of vaccination is often suboptimal, and vaccination is often unavailable or underutilized [7]. Treatment with an anti-influenza drug, particularly oseltamivir, is recommended for children in many countries. However, oseltamivir shows less clinical benefit in children with influenza B compared with influenza A virus infections [8–10], and depending on age and virus type/subtype about 4–12% of oseltamivir-treated children with uncomplicated influenza infection have had oseltamivir-resistant variants detected [11, 12]. Highly oseltamivir-resistant seasonal A(H1N1) viruses, due to an H275Y substitution in the viral neuraminidase, circulated worldwide in 2008–2009, and community-level transmission of oseltamivir-resistant A(H1N1)pdm09 virus has been reported [13, 14]. Therefore, there remains an unmet need to develop antiviral drugs with new mechanisms of action.
Baloxavir marboxil (formerly S-033188, hereafter baloxavir) is an oral prodrug that is metabolized rapidly to baloxavir acid (BXA), an inhibitor of the polymerase acidic protein (PA) cap-dependent endonuclease [15, 16]. In a phase 3 study in adults and adolescents with uncomplicated influenza, single-dose baloxavir significantly improved time to alleviation of influenza symptoms compared with placebo and reduced viral titers compared with placebo and oseltamivir [17]. However, baloxavir treatment–emergent viruses with reduced susceptibility due to amino acid substitution of isoleucine at position 38 of PA (PA/I38X) were detected in 2.2–9.7% of baloxavir-treated patients [17] in association with prolongation of infectious virus detectability, transient rises in viral titers, and initial delay in illness alleviation [18].
The present study is the first to assess the safety, pharmacokinetics, and clinical and virologic outcomes of baloxavir treatment including the effects of PA/I38X-substituted virus emergence in children aged 1–11 years with influenza virus infection. The plasma concentrations of BXA at 24 hours postdose (C24) in this study [19] were within the range observed in influenza-infected adults with confirmed baloxavir safety and efficacy [20] and exceeded the target concentration (6.85 ng/mL) [21, 22] for efficacy estimated by nonclinical studies.
METHODS
Study Design
This was a prospective, open-label, multicenter study in Japanese pediatric outpatients during the 2016–17 season. All patients received a single oral dose of baloxavir (Shionogi & Co, Ltd) on day 1 without regard to food intake. The dose depended on the weight of the patient (Supplementary Table 1) was based on the modeling of BXA pharmacokinetics in children [19].
The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the institutional review board or ethics committee at each center. Parents/legal guardians of participating patients provided written informed consent; participating children gave oral or written informed assent according to their capabilities. The study was registered at the Japan Pharmaceutical Information Center Clinical Trials Information (Japic CTI-163417).
Study Population
Patients enrolled were aged 1 to less than 12 years, with a body weight of 5 or more kg, a diagnosis of influenza illness confirmed by fever 38°C or higher, and by a positive rapid influenza virus test and ability to swallow tablets. Patients aged 7 or more years were enrolled if they had at least 1 respiratory symptom (cough or nasal discharge/congestion) of moderate or greater severity. The time between onset of symptoms (when body temperature first exceeded 37.5°C) and screening was 48 hours or less.
Safety Assessments
Safety measures included incidence and severity of adverse events (AEs), treatment-related AEs, vital signs, and clinical laboratory tests (see Supplementary Methods). Adverse events were classified by system organ class and preferred term using the Medical Dictionary for Regulatory Activities (MedDRA version 19.1).
Clinical and Virologic Assessments
The following outcome measures were assessed by the patient or the parent/guardian and recorded in an electronic diary: axillary temperature (4 times daily until day 3 and then morning and evening, days 4 to 14), assessment of severity of cough and nasal discharge/nasal congestion (morning and evening, days 1 to 9, and then each evening, days 10 to 14) on a 4-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe).
Nasopharyngeal swab samples were collected on days 1, 2, 3, and/or 4, 6, and 9 for virologic studies (Supplementary Materials). Serum for hemagglutinin inhibition (HAI) antibody testing was obtained on days 1 and 15.
The primary clinical endpoint was the time to illness alleviation (TTIA), defined as the time from baloxavir administration until the following criteria were reached and sustained for at least 21.5 hours: cough and nasal discharge/nasal congestion, both assessed as 0 (absent) or 1 (mild), and axillary temperature less than 37.5°C.
The secondary endpoints included times to first cessation (prespecified) and sustained cessation (post hoc analysis) of infectious virus detection, time to resolution of fever, recurrence of fever (post hoc analysis), and the incidence of influenza-related complications (Supplementary Materials).
Sanger sequencing of the PA gene was conducted on paired pre- and last post-treatment swab samples to detect PA/I38X-substituted viruses (Supplementary Materials).
Statistical Analysis
The sample size (N = 100) was selected to provide sufficient numbers for an initial assessment of safety and pharmacokinetics and was not based on power.
The safety analysis population consisted of all patients who received the study drug. For analysis of clinical and virology endpoints, the intention-to-treat infected (ITTI) population consisted of all patients who received the study drug and had laboratory-confirmed influenza by reverse transcriptase–polymerase chain reaction (RT-PCR).
For the TTIA, a survival curve and the median time (with 95% confidence intervals [CIs]) was estimated by the Kaplan–Meier method as the prespecified analysis with censoring of those not experiencing illness alleviation by the last observation timepoint.
In post hoc analyses, the baseline factors associated with emergence of viruses with PA/I38T/M substitutions were identified using a logistic regression model for patients with A(H3N2) virus, because such variants were only detected in those with A(H3N2) infection. The factors assessed included sex, body weight, baseline symptom score, axillary temperature, HAI antibody titer, infectious virus titer, and time-to-treatment from illness onset. Age and influenza vaccination status were excluded because they had statistically significant associations with baseline HAI antibody titer (Supplementary Tables 2 and 3). Meal after baloxavir administration was excluded because almost all patients had eaten a meal after administration. We also evaluated the relationship of baseline HAI antibody titer to TTIA.
All analyses and tabulations were performed using SAS software version 9.2 or higher (SAS Institute, Cary, North Carolina) and WinNonlin version 6.2.1.
RESULTS
Patient Characteristics
The study enrolled 108 patients, of whom 107 received the study drug (safety population). Three patients were excluded from the ITTI population because of negative RT-PCR for influenza virus. In the ITTI population (Table 1), the median age of patients in the study was 8.0 years; 83.7% of patients had influenza A(H3N2) virus, and 84.6% of patients had a time-to-treatment from onset of influenza illness of 24 hours or less.
Table 1.
Demographics and Baseline Characteristics in the Intention-to-Treat Infected Population
| Characteristics | Baloxavir Marboxil (N = 104) |
|---|---|
| Age, years | 7.4 ± 2.6 |
| Median, years | 8.0 |
| Range, years | 1–11 |
| <6 years, n (%) | 25 (24.0) |
| ≥6 to <9 years, n (%) | 36 (34.6) |
| ≥9 to ≤12 years, n (%) | 43 (41.3) |
| Male, n (%) | 53 (51.0) |
| Body temperature, °C | 38.78 ± 0.60 |
| Time to treatment from influenza onset, n (%) | |
| ≥0 to ≤12 hours | 47 (45.2) |
| >12 to ≤24 hours | 41 (39.4) |
| >24 to ≤36 hours | 14 (13.5) |
| >36 to ≤48 hours | 2 (1.9) |
| Influenza virus type/subtype based on rapid influenza diagnostic test, n (%) | |
| A | 95 (91.3) |
| B | 9 (8.7) |
| Negative | 0 |
| Influenza virus subtype based on RT-PCR, n (%) | |
| A(H1N1)pdm | 2 (1.9) |
| A(H3N2) | 87 (83.7) |
| B | 8 (7.7) |
| Mixed infection | 4 (3.8) |
| Othera | 3 (2.9) |
| Influenza vaccination given in the last 6 months, n (%) | 28 (26.9) |
Data are presented as mean ± SD unless otherwise indicated.Abbreviations: RT-PCR, reverse transcription–polymerase chain reaction; SD, standard deviation.
aThe patients whose subtype was unknown were treated as “Other.”
Safety
There were no serious AEs or AEs leading to discontinuation reported. Forty-nine AEs were reported in 37 (34.6%) patients (Table 2). The most common category was gastrointestinal disorders (15.0%) with vomiting (all grade 1) reported in 8 patients (7.5%). Two instances occurred within 1 hour (15 and 52 minutes), 3 between 1.6 to 10.5 hours, and 2 instances over 12 hours after administration (1 case unspecified). Treatment-related AEs were reported in 4 (3.7%) patients; all were judged grade 1 and resolved spontaneously (Supplementary Table 4).
Table 2.
Type and Duration of Adverse Events
| System Organ Class Preferred Term |
Baloxavir Marboxil (N = 107) |
|||
|---|---|---|---|---|
| Any Grade | Grade 2 | |||
| n (%) | Number of Events [Duration of AE: Min, Max]a | n (%) | Number of Events [Duration of AE: Min, Max]a | |
| Patients with any AE | 37 (34.6) | 49 [1, NR] | 19 (17.8) | 22 [3, NR] |
| Infections and infestations | 14 (13.1) | 15 [3, 16] | 14 (13.1) | 15 [3, 16] |
| Pharyngitis | 3 (2.8) | 3 [9, 12] | 3 (2.8) | 3 [9, 12] |
| Bronchitis | 2 (1.9) | 2 [8, 12] | 2 (1.9) | 2 [8, 12] |
| Sinusitis | 2 (1.9) | 2 [8, 16] | 2 (1.9) | 2 [8,16] |
| Oral herpes | 2 (1.9) | 2 [6, 8] | 2 (1.9) | 2 [6, 8] |
| Gastroenteritis | 1 (0.9) | 1 [3] | 1 (0.9) | 1 [3] |
| Influenza | 1 (0.9) | 1 [7] | 1 (0.9) | 1 [7] |
| Nasopharyngitis | 1 (0.9) | 1 [6] | 1 (0.9) | 1 [6] |
| Varicella | 1 (0.9) | 1 [12] | 1 (0.9) | 1 [12] |
| Bacterial infection | 1 (0.9) | 1 [5] | 1 (0.9) | 1 [5] |
| Bacterial rhinitis | 1 (0.9) | 1 [11] | 1 (0.9) | 1 [11] |
| Metabolism and nutrition disorders | 1 (0.9) | 1 [3] | 1 (0.9) | 1 [3] |
| Dehydration | 1 (0.9) | 1 [3] | 1 (0.9) | 1 [3] |
| Psychiatric disorders | 1 (0.9) | 1 [1] | 0 | 0 |
| Nightmare | 1 (0.9) | 1 [1] | 0 | 0 |
| Nervous system disorders | 2 (1.9) | 2 [1, 2] | 0 | 0 |
| Headache | 2 (1.9) | 2 [1, 2] | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 4 (3.7) | 5 [4, 46] | 3 (2.8) | 4 [6, 46] |
| Upper respiratory tract inflammation | 2 (1.9) | 3 [6, 14] | 2 (1.9) | 3 [6, 14] |
| Epistaxis | 1 (0.9) | 1 [4] | 0 | 0 |
| Rhinitis allergic | 1 (0.9) | 1 [46] | 1 (0.9) | 1 [46] |
| Gastrointestinal disorders | 16 (15.0) | 16 [1, NR] | 0 | 0 |
| Vomiting | 8 (7.5) | 8 [1, 3] | 0 | 0 |
| Diarrhea | 3 (2.8) | 3 [1, 2] | 0 | 0 |
| Constipation | 2 (1.9) | 2 [3, 3] | 0 | 0 |
| Dental caries | 1 (0.9) | 1 [NR] | 0 | 0 |
| Acetonaemic vomiting | 1 (0.9) | 1 [1] | 0 | 0 |
| Feces, soft | 1 (0.9) | 1 [1] | 0 | 0 |
| Skin and subcutaneous tissue disorders | 2 (1.9) | 2 [3, NR] | 1 (0.9) | 1 [3] |
| Dry skin | 1 (0.9) | 1 [NR] | 0 | 0 |
| Urticaria | 1 (0.9) | 1 [3] | 1 (0.9) | 1 [3] |
| Musculoskeletal and connective tissue disorders | 2 (1.9) | 2 [2, 8] | 0 | 0 |
| Back pain | 1 (0.9) | 1 [2] | 0 | 0 |
| Myalgia | 1 (0.9) | 1 [8] | 0 | 0 |
| Investigations | 2 (1.9) | 3 [5, 11] | 0 | 0 |
| Alanine aminotransferase increased | 1 (0.9) | 1 [11] | 0 | 0 |
| Aspartate aminotransferase increased | 1 (0.9) | 1 [10] | 0 | 0 |
| Blood urine present | 1 (0.9) | 1 [5] | 0 | 0 |
| Injury, poisoning, and procedural complications | 2 (1.9) | 2 [6, NR] | 1 (0.9) | 1 [NR] |
| Ligament sprain | 2 (1.9) | 2 [6, NR] | 1 (0.9) | 1 [NR] |
All AEs were classified as grade 1 or grade 2. Most AEs (26 patients) occurred in the first 7 days, although 3 patients reported AEs on day 22 or later (nasopharyngitis, ligament sprain, oral herpes; none were considered to be study drug related).Abbreviations: AE, adverse event; Max, maximum; Min, minimum; NR, not recorded.
aDuration of AE (days) = (date of outcome) − (date of onset) + 1. If the AE did not resolve or was not resolving until the day of outcome assessment, duration of the AE is defined as NR.
Clinical Measures
The median TTIA was 44.6 hours (95% CI, 38.9–62.5 hours) (Table 3), with 81.6% of patients being alleviated by 120 hours after treatment (Supplementary Figure 1). The TTIA for patients with influenza B was similar to that in patients with influenza A(H3N2) (Table 3). The median time to resolution of fever was 21.4 hours (95% CI, 19.8–25.8 hours) (Table 3). Recurrence of fever after day 3 and day 4 in patients who had become afebrile was observed in 11.1% (11/99) and 10.7% (11/103), respectively. Two patients developed influenza-related complications: both were grade 2 bronchitis occurring on days 1–8 or 4–15 before resolving.
Table 3.
Efficacy Time-to-Event Outcomes in the Intention-to-Treat Infected Population
| Baloxavir Marboxil (N = 104) | Time-to-Event Outcomes (Hours) |
|---|---|
| Time to alleviation of influenza illness (n = 103) | 44.6 (38.9–62.5) |
| Subgroup of influenza A(H3N2) (n = 86) | 45.2 (38.2–62.5) |
| Subgroup of influenza B (n = 8) | 44.7 (18.3–133.8) |
| Time to first cessation of viral shedding by virus titer (n = 101) | 24.0 (ND–ND) |
| Subgroup of influenza A(H3N2) (n = 87) | 24.0 (ND–ND) |
| Subgroup of influenza B (n = 8) | 96.0 (24.0–168.0) |
| Time to sustained cessation of infectious virus detection (n = 101) | 24.0 (24.0–48.0) |
| Subgroup of influenza A(H3N2) (n = 87) | 24.0 (24.0–48.0) |
| Subgroup of influenza B (n = 8) | 144.0 (24.0–168.0) |
| Time to resolution of fever (n = 103) | 21.4 (19.8–25.8) |
| Time to resumption of normal activity (n = 103) | 126.3 (99.4–130.7) |
Data are presented as median (95% confidence interval). All patients whose outcome was not missing were included in this analysis.Abbreviation: ND, not able to be determined.
Virologic Measures
Infectious virus titers rapidly declined within 1 day after baloxavir treatment, and the average titer remained low thereafter (Figure 1, Table 4). The median time to sustained cessation of infectious viral detection was 24.0 hours (Table 3). The time was shorter for patients with influenza A(H3N2) (median, 24.0 hours) than influenza B (median, 144.0 hours) (Table 3).
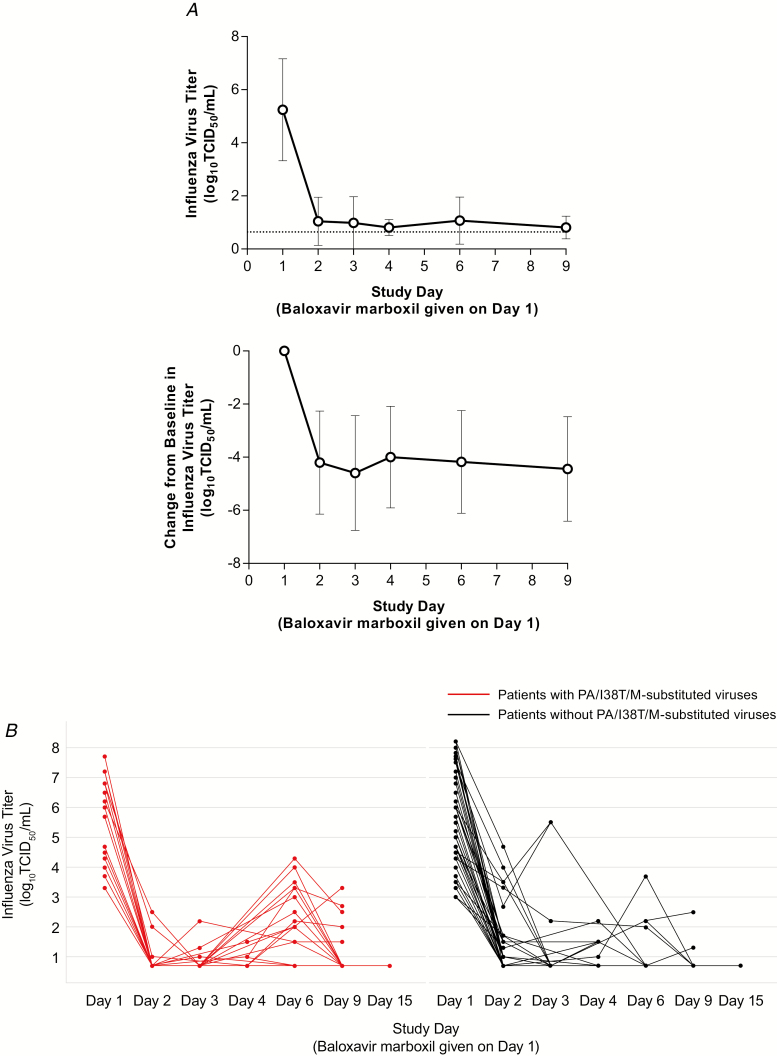
Figure 1. .
A, Influenza virus titer (upper panel) and change from baseline in influenza virus titer (lower panel). Data are presented as mean ± SD. The dotted line in upper panel represents lowest level of detection. B, Individual influenza virus titer in patients with PA/I38T/M-substituted viruses (red line) and without PA/I38T/M-substituted viruses (black line). Abbreviations: PA/I38T, isoleucine substituted by threonine at position 38 of virus polymerase acidic protein; PA/I38M, isoleucine substituted by methionine at position 38 of virus polymerase acidic protein; SD, standard deviation; TCID50, 50% tissue culture infective dose.
Table 4.
Median of Virus Titer (log10TCID50/mL) by Study Day
| Study Daya | Baloxavir Marboxil (N = 101)b |
|---|---|
| Day 1 | |
| n Median |
101 5.20 |
| Day 2 | |
| n Median |
101 0.70 |
| Day 3 | |
| n Median |
61 0.70 |
| Day 4 | |
| n Median |
52 0.70 |
| Day 6 | |
| n Median |
101 0.70 |
| Day 9 | |
| n Median |
101 0.70 |
Detection limit of virus titer: 0.7 log10TCID50/mL.Abbreviation: TCID50, 50% tissue culture infective dose.
aOptional visit: day 3 and day 4.
bThe patients with positive virus titer at day 1 were included in this analysis.
The frequency of HAI antibody seroconversions (≥4-fold increase) was 83.9% (73/87) in patients with A(H3N2) and 37.5% (3/8) in patients with B/Yamagata (Supplementary Table 5).
Amino Acid Substitutions at PA/I38
In the ITTI population, 77 had post-treatment and baseline samples available for sequencing. No PA/I38-substituted viruses were detected pretreatment. PA/I38T/M-substituted viruses were detected in 18 patients (23.4%), and their first detection was at days 3 (n = 1), 6 (n = 12) or 9 (n = 5). All were infected with A(H3N2) virus; variants included PA/I38T (n = 10), PA/I38T/I mixture (n = 5), PA/I38M (n = 2) and PA/I38I/M mixture (n = 1).
The median time to sustained cessation of viral shedding in patients with PA/I38T/M-substituted viruses was 180.0 hours (95% CI, 144.0–216.0 hours) compared with 24.0 hours (95% CI, 24.0–48.0 hours) in those without (Supplementary Table 6). By day 2 the mean change in virus titer from baseline was comparable in those with (4.75 log10 50% tissue culture infective dose [TCID50]/mL) or without (4.78 log10TCID50/mL) PA/I38T/M-substituted viruses. However, those with substitutions showed increased titers to a mean of 2.14 log10TCID50/mL (range, <0.7 (detection limit) to 4.30) at day 6 (Figure 1B). Increases in viral titer (>0.7) at day 3 or later were observed in 72.2% of patients with emergence of PA/I38T/M-substituted viruses compared with 13.6% of those without such viruses.
The median TTIA and fever resolution in patients with PA/I38T/M-substituted viruses were 79.6 and 29.5 hours, respectively, compared with 42.8 and 20.8 hours, respectively, in those without PA/I38T/M-substituted viruses (Figure 2, Supplementary Table 6). Transient fever and symptom recurrence after 72 hours postdose were observed in 23.5% and 37.5% of patients, respectively, with PA/I38T/M-substituted viruses compared with 8.5% and 20.5%, respectively, in those without (Supplementary Table 7); transient symptom score increases were also observed at 72 hours postdose (Supplementary Figure 2). Symptom recurrences resolved (score of 1 or 0) within 48 hours, with the exception of 2 patients without PA/I38T/M-substituted viruses.
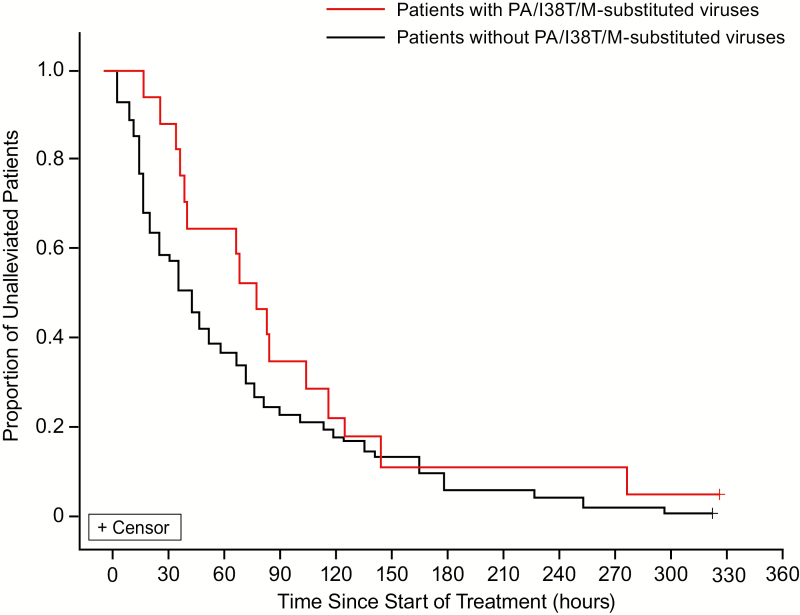
Figure 2. .
Kaplan–Meier analysis of the illness alleviation after treatment with baloxavir marboxil in patients with and without PA/I38T/M-substituted viruses. Influenza illness was composed of cough, nasal discharge/congestion, and body temperature. The median TTIA was 79.6 hours in patients with PA/I38T/M-substituted viruses (n = 18) and was 42.8 hours in patients without PA/I38T/M-substituted viruses (n = 59). Abbreviations: PA/I38T, isoleucine substituted by threonine at position 38 of virus polymerase acidic protein; PA/I38M, isoleucine substituted by methionine at position 38 of virus polymerase acidic protein; TTIA, time to illness alleviation.
Next-Generation-Sequencing Analysis
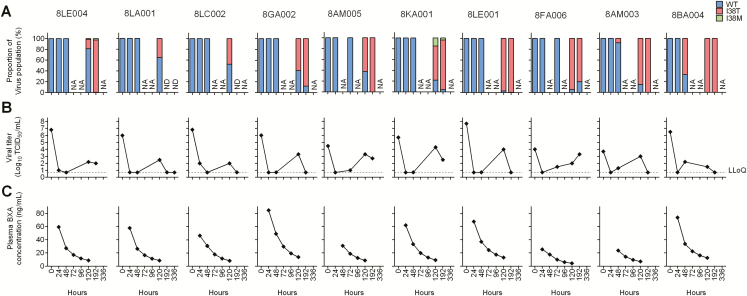
To assess time course of emergence of PA/I38T/M-substituted viruses, next-generation sequencing (NGS) was used for the analysis on swab samples from 10 selected patients with I38T-substituted viruses (Supplementary Figure 3). No detectable PA/I38 substitutions were confirmed in the viruses pretreatment (Figure 3A). In the post-treatment samples, PA/I38T substitutions were found to have emerged as early as 48 hours in 2 patients, and variant viruses emerged by 120 hours in 8 patients (Figure 3A). The PA/I38T viruses became dominant in most of the patients after 120 hours, except for 2 patients who had undetectable levels of viral RNAs. The emergence of PA/I38T-substituted viruses corresponded temporally with an increase in viral titers, which, in most cases, had been below the quantification limit for at least 1 day prior to PA/I38T-substituted virus emergence (Figure 3B). PA/I38T-substituted viruses were first detected when the plasma BXA concentration ranged from 8.34 to 23.5 ng/mL (Figure 3A, C).
Figure 3. .
Comparison between proportion (A) of PA/I38T/M-substituted viruses with viral titer (B) and plasma BXA concentration (C). A, Time courses of % proportion of PA/I38 T/M-substituted viruses in the swabs and B, viral titer (log10TCID50/mL) in the swabs were represented. C, BXA concentration in plasma (ng/mL) was plotted by a measured value at 24 hours and simulated value at 48, 72, and 96 hours, which were calculated by Bayesian inference with measured values. The individual patient numbers are shown on top. A threshold frequency of >1% was adopted for calling variant viruses in NGS analysis, and the LLoQ of viral titer was set at 0.7 log10TCID50/mL. Abbreviations: BXA, baloxavir acid; LLoQ, lower limit of quantification; NA, not applicable; ND, below detection limit of 2.05 log10 RNA copies/mL, or no PCR amplification observed, or no variant profiles obtained due to low coverage (<100); NGS, next-generation sequencing; PA/I38T, isoleucine substituted by threonine at position 38 of virus polymerase acidic protein; PA/I38M, isoleucine substituted by methionine at position 38 of virus polymerase acidic protein; WT, wild-type virus.
Factors Associated With Emergence of PA/I38T/M-Substituted Viruses
Post hoc analyses identified differences in age distribution, body weight, and baseline HAI antibody between patients with and without PA/I38T/M-substituted viruses (Table 5). A higher frequency of emergence of PA/I38T/M-substituted viruses occurred in patients with baseline A(H3N2) HAI antibody titer of less than 40 (42.3% [11/26]) than in those with titers of 40 or greater (17.1% [7/41]) (P = .0467; Table 6). Also, a higher frequency of PA/I38T/M-substituted virus detection was found in H3N2-infected children aged 5 years or younger (6/10) than in older children (12/57) (Table 5).
Table 5.
Baseline Characteristics in Patients Infected by A(H3N2) Virus With and Without PA/I38T/M-Substituted Viruses
| Characteristic | Patients With PA/I38T/M-Substituted Viruses (n = 18) |
Patients Without PA/I38T/M-Substituted Viruses (n = 49) |
Total (N = 67)a |
|---|---|---|---|
| Age, years | |||
| Median (range), years | 7.0 (2–11) | 9.0 (3–11) | 8.0 (2–11) |
| <5 years, n (%) | 6 (33.3) | 4 (8.2) | 10 (14.9) |
| ≥5 years, n (%) | 12 (66.7) | 45 (91.8) | 57 (85.1) |
| Body weight, kg | |||
| Mean (SD) | 22.81 (9.69) | 27.03 (8.59) | 25.89 (9.02) |
| <20 kg, n (%) | 8 (44.4) | 10 (20.4) | 18 (26.9) |
| ≥20 kg, n (%) | 10 (55.6) | 39 (79.6) | 49 (73.1) |
| BMI, kg/m2 | |||
| Mean (SD) | 15.96 (1.50) | 16.20 (2.44) | 16.13 (2.22) |
| Sex, n (%) | |||
| Male | 8 (44.4) | 30 (61.2) | 38 (56.7) |
| Sum of 2 symptom scores at baseline | |||
| Mean (SD) | 2.9 (1.0) | 3.3 (1.1) | 3.2 (1.0) |
| Body temperature at baseline, °C | |||
| Mean (SD) | 38.8 (0.5) | 38.8 (0.7) | 38.8 (0.6) |
| Infectious virus titer at baseline, log10TCID50/mL | |||
| Mean (SD) | 5.64 (1.37) | 5.80 (1.57) | 5.75 (1.51) |
| Time to treatment from symptom onset, n (%) | |||
| ≥0 to ≤12 hours | 13 (72.2) | 22 (44.9) | 35 (52.2) |
| >12 to ≤24 hours | 4 (22.2) | 18 (36.7) | 22 (32.8) |
| >24 to ≤36 hours | 1 (5.6) | 8 (16.3) | 9 (13.4) |
| >36 to ≤48 hours | 0 | 1 (2.0) | 1 (1.5) |
| Influenza vaccination, n (%) | |||
| Yes | 7 (38.9) | 11 (22.4) | 18 (26.9) |
| No | 11 (61.1) | 38 (77.6) | 49 (73.1) |
| HAI antibody titer, n (%) | |||
| <40 | 11 (61.1) | 15 (30.6) | 26 (38.8) |
| ≥40 | 7 (38.9) | 34 (69.4) | 41 (61.2) |
| Meal before administration | |||
| Yes | 12 (66.7) | 28 (57.1) | 40 (59.7) |
| No | 6 (33.3) | 21 (42.9) | 27 (40.3) |
| Duration between meal before administration, n (%) | |||
| <2 hours | 2 (16.7) | 2 (7.1) | 4 (10.0) |
| ≥2 to ≤4 hours | 4 (33.3) | 9 (32.1) | 13 (32.5) |
| >4 hours | 6 (50.0) | 17 (60.7) | 23 (57.5) |
| Meal after administration, n (%) | |||
| Yes | 14 (77.8) | 47 (95.9) | 61 (91.0) |
| No | 4 (22.2) | 2 (4.1) | 6 (9.0) |
| Duration between meal after administration, n (%) | |||
| <2 hours | 5 (35.7) | 22 (46.8) | 27 (44.3) |
| ≥2 to ≤4 hours | 3 (21.4) | 16 (34.0) | 19 (31.1) |
| >4 hours | 6 (42.9) | 9 (19.2) | 15 (24.6) |
Abbreviations: HAI, hemaggulutinin inhibition; PA/I38T, isoleucine substituted by threonine at position 38 of virus polymerase acidic protein; PA/I38M, isoleucine substituted by methionine at position 38 of virus polymerase acidic protein; SD, standard deviation; TCID50, 50% tissue culture infective dose.
aIntention-to-treat infected population with A(H3N2) virus infection and with available paired sequencing at baseline and post-treatment.
Table 6.
Identification of Prognostic Factors for Emergence of PA/I38T/M-Substituted Viruses by Logistic Regression Analysis
| Parameter | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Sex (male to female) | 0.474 | .122–1.838 | .2804 |
| Baseline body weight (by 1 kg) | 0.970 | .899–1.046 | .4299 |
| Baseline sum of symptom scores (by 1 score) | 0.765 | .405–1.445 | .4095 |
| Baseline body temperature (by 1°C) | 0.927 | .323–2.662 | .8886 |
| Baseline virus-specific HAI antibody titer (≥40 to <40) | 0.276 | .078–0.982 | .0467 |
| Baseline influenza virus titer (by 1.0 log10TCID50/mL) | 0.917 | .589–1.429 | .7017 |
| Time to treatment from influenza onset (by 12 hours)a | 0.428 | .145–1.265 | .1249 |
| Meal before administration on day of dosing (yes to no) | 1.109 | .276–4.450 | .8838 |
Patients with A(H3N2) virus who had paired-sequencing data available and had baseline characteristic data for potential risk factor were included in this analysis (N = 67). Abbreviations: CI, confidence interval; HAI, hemaggulutinin inhibition; PA/I38T, isoleucine substituted by threonine at position 38 of virus polymerase acidic protein; PA/I38M, isoleucine substituted by methionine at position 38 of virus polymerase acidic protein; TCID50, 50% tissue culture infective dose.
aTreated as ordered category of 4 levels.
The mean plasma C24 in patients with PA/I38T/M-substituted viruses (58.4 ng/mL) was similar to that in patients without such viruses (58.0 ng/mL). However, the mean C96 was 42–53% lower with 10 mg compared with higher doses and 46–57% lower than that in adults [20] (see Supplementary Materials).
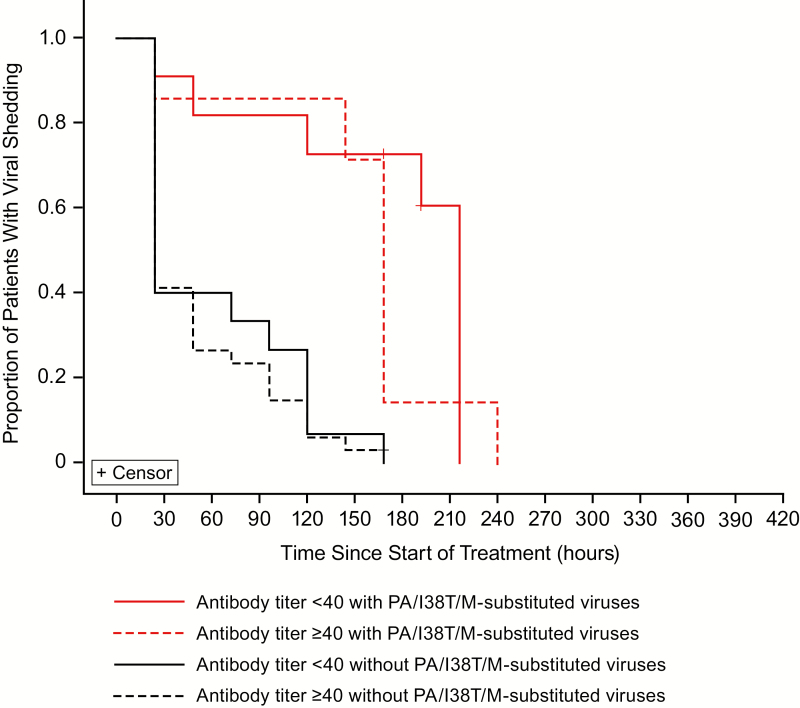
Among those with emergence of PA/I38T/M-substituted viruses, the median TTIA was 29.4 hours longer in those with baseline HAI antibody titer of less than 40 than in those with a higher HAI antibody titer (Figure 4). The time to sustained cessation of infectious viral shedding in patients with PA/I38T/M-substituted viruses was considerably longer than that in those without, regardless of baseline HAI antibody titer (Figure 5).
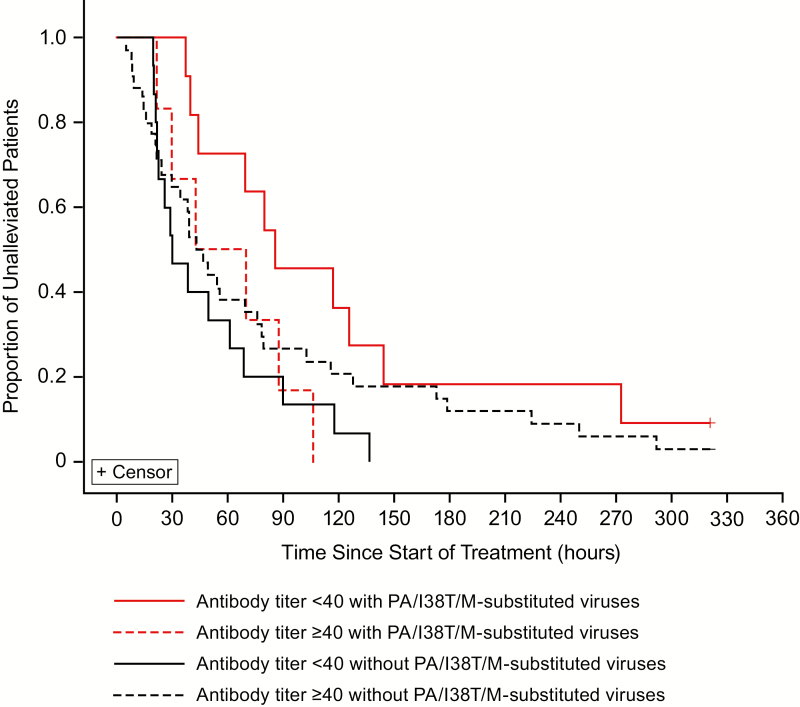
Figure 4. .
Kaplan–Meier analysis of the alleviation of the symptoms of influenza illness after treatment with baloxavir marboxil in patients with and without PA/I38T/M-substituted viruses by HAI virus antibody titer at baseline. Patients with A(H3N2) virus who had paired sequencing data available and with any observed diary data were included in this analysis. The median TTIA was 85.4 hours (antibody <40 with PA/I38T/M-substituted viruses [n = 11]), 29.9 hours (antibody <40 without PA/I38T/M-substituted viruses [n = 15]), 56.0 hours (antibody ≥40 with PA/I38T/M-substituted viruses [n = 6]), and 44.7 hours (antibody ≥40 without PA/I38T/M-substituted viruses [n = 34]). Abbreviations: HAI, hemagglutinin inhibition; PA/I38T, isoleucine substituted by threonine at position 38 of virus polymerase acidic protein; PA/I38M, isoleucine substituted by methionine at position 38 of virus polymerase acidic protein; TTIA, time to illness alleviation.
Figure 5. .
Kaplan–Meier analysis of the time to sustained cessation of viral shedding by virus titer after treatment with baloxavir marboxil in patients with and without PA/I38T/M-substituted viruses by HAI antibody titer at baseline. Patients with A(H3N2) virus who had paired-sequencing data available were included in this analysis. The median times to sustained cessation of viral shedding: 216.0 hours (antibody <40 with PA/I38T/M-substituted viruses [n = 11]), 24.0 hours (antibody <40 without PA/I38T/M-substituted viruses [n = 15]), 168.0 hours (antibody ≥40 with PA/I38T/M-substituted viruses [n = 7]), and 24.0 hours (antibody ≥40 without PA/I38T/M-substituted viruses [n = 34]). Abbreviations: HAI, hemagglutinin inhibition; PA/I38T, isoleucine substituted by threonine at position 38 of virus polymerase acidic protein; PA/I38M, isoleucine substituted by methionine at position 38 of virus polymerase acidic protein.
DISCUSSION
This is the first study to examine the safety, pharmacokinetics [19], and clinical and virological outcomes of oral baloxavir in pediatric patients with influenza virus infection. A single weight-based dose, targeted to provide C24 levels in children similar to those in adults receiving a 40-mg dose [20], was generally well tolerated in our patients, and all baloxavir recipients completed the study. In comparison, placebo-controlled of oseltamivir reported that 1.8–5.4% of patients discontinued studies due to adverse events, particularly gastrointestinal events such as vomiting [23, 24]. The frequency of emesis in our study (7.5%) was within the age-related range in placebo recipients (3.2%, 8.5%, and 18.6%) and generally lower than that in oseltamivir recipients (5.9%, 14.7%, and 29.2%) reported in 3 earlier double-blinded randomized controlled trials of oseltamivir treatment in children [23–25]. The C24 values of 3 patients who vomited at 0.25, 1.57, and 0.87 hours after administration were 46.6, 84.5, and 65.5 ng/mL, respectively, and were comparable to levels in those without emesis. The emesis occurring within 1 hour of administration did not appear to reduce baloxavir levels, suggesting rapid onset of absorption [21].
Because we lacked a control group, we are limited to comparing our findings with previous neuraminidase inhibitor (NAI) studies in children. In open-label studies in Japanese children, the median TTIA in this study was longer than the time to illness alleviation reported with single-dose intravenous peramivir (29.1 hours) in outpatients aged 28 days–15 years [26] but similar to that of inhaled laninamivir (56 hours) in children aged 3–9 years old [12]. The median time to resolution of fever (medians, 41–48 hours) and of illness (medians, 72–103) were longer in oseltamivir recipients from placebo-controlled studies in non-Japanese pediatric patients [23–25].
Recurrence of fever after initial resolution was noted in approximately 11% of baloxavir-treated children in our study. The proportions of NAI-treated children experiencing recurrent fever have ranged widely from 1.8% to 20% in previous studies in Japan [27–29]. However, those studies enrolled children with different types/subtypes of influenza virus infections, used different definitions of recurrence of fever, and had different age distributions of subjects. In addition, it is unclear how often recurrent fever was related to influenza illness or complications in these studies. Consequently, no definitive conclusions can be reached with regard to the relative frequencies of recurrent fever in baloxavir- or NAI-treated children at present. In this regard, a head-to-head comparison of single-dose baloxavir and a 5-day regimen of oseltamivir in outpatient children aged 1–12 years has been completed recently (NCT03629184).
An important finding in this study was the detection of treatment-emergent PA/I38T/M-substituted viruses in 23.4% of patients with paired-sequence data, a frequency over 2-fold higher than in baloxavir-treated adults with A(H3N2) infections [17]. Depending on the assay method, clinical isolates of A(H3N2) virus harboring I38T showed 65- to 155-fold reduced susceptibility in vitro [18, 30]. Emergence of these variants was associated with transient rises in viral titers and prolongation of virus detectability and sometimes illness (Supplementary Table 6 and Figure 1B). Such findings also raise concern about the potential risk of variant transmission to close contacts, an important public health issue [31]. One published case [32] and 2 additional cases of detecting PA/I38T-substituted viruses in non–baloxavir-treated patients have been reported recently in Japan [33]. Because these instances likely represent transmission from baloxavir-treated patients, monitoring and surveillance to determine the extent of transmission are ongoing.
Next-generation sequencing revealed that PA/I38T-substituted viruses emerged after 48 hours (day 3) post-treatment of baloxavir in association with decreasing plasma BXA concentrations, consistent with the previous report in adult and adolescent patients with influenza [18]. PA/I38T-substituted viruses emerged when plasma BXA concentrations declined to levels that inhibit viral replication of wild-type virus but not I38T-substituted viruses in vitro. Of note, children aged 1–5 years were more likely to have variant virus emergence and also to have received a drug dose that resulted in lower baloxavir concentrations by 96 hours. Consequently, a follow-up pediatric study was conducted during the 2018–2019 season to assess whether a higher dose (2 mg/kg) might reduce PA/I38X-substituted virus emergence (JapicCTI-194577). Also, our observations would support studies of alternative treatment regimens, such as repeat baloxavir dosing or combination with NAI, to reduce the risk of variant virus emergence.
Acquired immunity may also be involved in the selection of variants during the baloxavir treatment, in that we found that low baseline HAI antibody titers were significantly associated with higher risk of variant emergence. Of note, a clear prolongation of TTIA was only seen in patients with PA/I38T/M-substituted viruses with low baseline antibody titer. We speculate that despite subinhibitory baloxavir concentration for PA/I38X-substituted viruses, patients with higher baseline antibody titers were better able to control replication and reduce the risk of variant emergence. Low baseline HAI titers were associated with lack of vaccination and younger age (Supplementary Table 3). The possibility that the association between the emergence of PA/I38X variants and prolongation of symptom alleviation requires additional investigation, including data on innate and acquired cellular immune responses before and at the time of variant emergence.
In conclusion, we found that a single, weight-adjusted oral dose of baloxavir was well tolerated in pediatric patients with influenza. Further study is required to assess whether alternative dose regimens of baloxavir might reduce the frequency of PA/I38X variant virus emergence. While oseltamivir is currently recommended for influenza treatment in children [34], our study provides initial evidence for a promising alternative antiviral with a novel mechanism of action.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors were involved in the study design and interpretation of the data, and in the drafting, critical revision, and approval of the final version of the manuscript. N. H., C. S., and A. W. also contributed to data collection, and T. S. contributed to data analysis.
Acknowledgments. The authors thank all study participants and investigators who participated in the study. They also thank Masahiro Kinoshita, Keiko Kawaguchi, Hiroki Koshimichi, and Satoshi Kojima, all from Shionogi & Co, Ltd, and to Norio Sugaya, MD, from Keiyu Hospital Japan, who provided medical advice. Medical writing assistance funded by Shionogi was provided by Rebecca Lew, PhD, CMPP, and Hiroko Ebina, BPharm, Ph, MBA, of ProScribe–Envision Pharma Group with international guidelines for Good Publication Practice (GPP3). They also acknowledge Saskia Smits and her colleagues at Viroclinics Biosciences BV for next-generation sequencing analyses on swab samples.
Financial support. This work was supported by Shionogi & Co, Ltd (Osaka, Japan), the manufacturer/licensee of baloxavir marboxil.
Potential conflicts of interest. H. S., C. S., T. I., K. B., S. O., T. S., K. T., and T. U. are employees of Shionogi & Co, Ltd; T. I., K. B., T. S., and K. T. also own stock in the company. N. H. has received honoraria from Shionogi & Co, Ltd, for giving promotional lectures regarding influenza infection and is an advisor to Shionogi & Co, Ltd, regarding anti-influenza drug development. T. S. has a patent PCT/JP16/087170 pending. F. G. H. has received travel support from Shionogi, and the company has made charitable donations to the Robert Ford Haitian Orphanage and School for his consulting time. F. G. H. has received personal fees from the World Health Organization and the University of Alabama Antiviral Drug Discovery and Development Consortium, outside the submitted work; Seqirus has made charitable donations to the Robert Ford Haitian Orphanage and School for his consulting activities related to peramivir and influenza vaccine, outside the submitted work. F. G. H. reports fees paid to the University of Virginia from GSK, Celltrion, Cidara, and Vaccitech and has served as a nonpaid consultant to various other companies (CoCrystal, Farmak, FujiFilm/Toyama, Gilead, GSK, Janssen, MedImmune, Regeneron, resTORbio, Roche/Genentech, SAB Biotherapeutics, Vir, Visterra) that are developing investigational therapeutics for influenza, all outside the submitted work. A. W. has been involved with advisory boards and speaker's bureaus organized by Shionogi and has received research funding from Shionogi & Co, Ltd. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Uyeki TM, Bernstein HH, Bradley JS, et al. . Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis 2019; 68:e1–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Weekly Epidemiological Record (WER) 23. November 2012; 87: 461–476. Available at: https://www.who.int/wer/2012/wer8747/en/. Accessed 5 April 2019. [Google Scholar]
- 3. Wong KK, Jain S, Blanton L, et al. . Influenza-associated pediatric deaths in the United States, 2004-2012. Pediatrics 2013; 132:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nair H, Brooks WA, Katz M, et al. . Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917–30. [DOI] [PubMed] [Google Scholar]
- 5. Reed C, Katz JM, Hancock K, Balish A, Fry AM; H1N1 Serosurvey Working Group Prevalence of seropositivity to pandemic influenza A/H1N1 virus in the United States following the 2009 pandemic. PLoS One 2012; 7:e48187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayden FG, Belshe RB, Clover RD, Hay AJ, Oakes MG, Soo W. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N Engl J Med 1989; 321:1696–702. [DOI] [PubMed] [Google Scholar]
- 7. Jackson ML, Chung JR, Jackson LA, et al. . Influenza vaccine effectiveness in the United States during the 2015-2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sugaya N, Mitamura K, Yamazaki M, et al. . Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis 2007; 44:197–202. [DOI] [PubMed] [Google Scholar]
- 9. Sugaya N, Tamura D, Yamazaki M, et al. . Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis 2008; 47:339–45. [DOI] [PubMed] [Google Scholar]
- 10. Sato M, Saito R, Sato I, et al. . Effectiveness of oseltamivir treatment among children with influenza A or B virus infections during four successive winters in Niigata City, Japan. Tohoku J Exp Med 2008; 214:113–20. [DOI] [PubMed] [Google Scholar]
- 11. Samson M, Pizzorno A, Abed Y, Boivin G. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res 2013; 98:174–85. [DOI] [PubMed] [Google Scholar]
- 12. Sugaya N, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother 2010; 54:2575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurt AC, Hardie K, Wilson NJ, et al. . Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takashita E, Ejima M, Itoh R, et al. . A community cluster of influenza A(H1N1)pdm09 virus exhibiting cross-resistance to oseltamivir and peramivir in Japan, November to December 2013. Euro Surveill 2014; 19:20666. [DOI] [PubMed] [Google Scholar]
- 15. Noshi T, Kitano M, Taniguchi K, et al. . In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res 2018; 160:109–17. [DOI] [PubMed] [Google Scholar]
- 16. Omoto S, Speranzini V, Hashimoto T, et al. . Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep 2018; 8:9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 18. Uehara T, Hayden FG, Kawaguchi K, et al. . Treatment emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated influenza. J Infect Dis 2019. jiz244. [DOI] [PubMed] [Google Scholar]
- 19. Koshimichi H, Ishibashi T, Wajima T. Population pharmacokinetics of baloxavir marboxil in Japanese pediatric influenza patients. J Pharm Sci 2019; 108:3112–7. [DOI] [PubMed] [Google Scholar]
- 20. Koshimichi H, Tsuda Y, Ishibashi T, Wajima T. Population pharmacokinetic and exposure-response analyses of baloxavir marboxil in adults and adolescents including patients with influenza. J Pharm Sci 2019; 108:1896–904. [DOI] [PubMed] [Google Scholar]
- 21. Koshimichi H, Ishibashi T, Kawaguchi N, Sato C, Kawasaki A, Wajima T. Safety, tolerability, and pharmacokinetics of the novel anti-influenza agent baloxavir marboxil in healthy adults: phase I study findings. Clin Drug Investig 2018; 38:1189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noshi T, Satou K, Ishibashi T, et al. . Pharmacokinetic and pharmacodynamic analysis of S-033188/S-033447, a novel inhibitor of influenza virus cap-dependent endonuclease, in mice infected with influenza A virus [P1973]. In: Final Programme of the 27th European Congress of Clinical Microbiology and Infectious Diseases (Vienna). Basel: European Society of Clinical Microbiology and Infectious Diseases, 2017. [Google Scholar]
- 23. Whitley RJ, Hayden FG, Reisinger KS, et al. . Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001; 20:127–33. [DOI] [PubMed] [Google Scholar]
- 24. Heinonen S, Silvennoinen H, Lehtinen P, et al. . Early oseltamivir treatment of influenza in children 1-3 years of age: a randomized controlled trial. Clin Infect Dis 2010; 51:887–94. [DOI] [PubMed] [Google Scholar]
- 25. Fry AM, Goswami D, Nahar K, et al. . Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis 2014; 14:109–18. [DOI] [PubMed] [Google Scholar]
- 26. Sugaya N, Kohno S, Ishibashi T, Wajima T, Takahashi T. Efficacy, safety, and pharmacokinetics of intravenous peramivir in children with 2009 pandemic H1N1 influenza A virus infection. Antimicrob Agents Chemother 2012; 56:369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki E, Ichihara K. The course of fever following influenza virus infection in children treated with oseltamivir. J Med Virol 2008; 80:1065–71. [DOI] [PubMed] [Google Scholar]
- 28. Koseki N, Kaiho M, Kikuta H, et al. . Comparison of the clinical effectiveness of zanamivir and laninamivir octanoate for children with influenza A(H3N2) and B in the 2011-2012 season. Influenza Other Respir Viruses 2014; 8:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishiguro N, Koseki N, Kaiho M, et al. . Clinical effectiveness of four neuraminidase inhibitors (oseltamivir, zanamivir, laninamivir, and peramivir) for children with influenza A and B in the 2014-2015 to 2016-2017 influenza seasons in Japan. J Infect Chemother 2018; 24:449–57. [DOI] [PubMed] [Google Scholar]
- 30. Takashita E, Kawakami C, Morita H, et al. . Detection of influenza A(H3N2) viruses exhibiting reduced susceptibility to the novel cap-dependent endonuclease inhibitor baloxavir in Japan, December 2018. Euro Surveill 2019; 24:1800698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pharmaceuticals and Medical Devices Agency. 2018. Review report on Xofluza 17 January 2018. Available at: https://www.pmda.go.jp/files/000225380.pdf. Accessed 5 April 2019.
- 32. Takashita E, Kawakami C, Ogawa R, et al. . Influenza A(H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalised child without prior baloxavir treatment, Japan, January 2019. Euro Surveill 2019; 24:1900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Institute Infectious Disease. Infectious Agents Surveillance Report (IASR) 12 March 2019 Available at: https://www.niid.go.jp/niid/ja/flu-m/flu-iasrs/8664-470p01.html. Accessed 5 April 2019.
- 34. American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2017 – 2018. Pediatrics 2017; 140:e20173535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.