Abstract
Background
The whole Plasmodium falciparum sporozoite (PfSPZ) vaccine is being evaluated for malaria prevention. The vaccine is administered intravenously for maximal efficacy. Direct venous inoculation (DVI) with PfSPZ vaccine has been safe, tolerable, and feasible in adults, but safety data for children and infants are limited.
Methods
We conducted an age de-escalation, dose-escalation randomized controlled trial in Siaya County, western Kenya. Children and infants (aged 5–9 years, 13–59 months, and 5–12 months) were enrolled into 13 age-dose cohorts of 12 participants and randomized 2:1 to vaccine or normal saline placebo in escalating doses: 1.35 × 105, 2.7 × 105, 4.5 × 105, 9.0 × 105, and 1.8 × 106 PfSPZ, with the 2 highest doses given twice, 8 weeks apart. Solicited adverse events (AEs) were monitored for 8 days after vaccination, unsolicited AEs for 29 days, and serious AEs throughout the study. Blood taken prevaccination and 1 week postvaccination was tested for immunoglobulin G antibodies to P. falciparum circumsporozoite protein (PfCSP) using enzyme-linked immunosorbent assay.
Results
Rates of AEs were similar in vaccinees and controls for solicited (35.7% vs 41.5%) and unsolicited (83.9% vs 92.5%) AEs, respectively. No related grade 3 AEs, serious AEs, or grade 3 laboratory abnormalities occurred. Most (79.0%) vaccinations were administered by a single DVI. Among those in the 9.0 × 105 and 1.8 × 106 PfSPZ groups, 36 of 45 (80.0%) vaccinees and 4 of 21 (19.0%) placebo controls developed antibodies to PfCSP (P < .001).
Conclusions
PfSPZ vaccine in doses as high as 1.8 × 106 can be administered to infants and children by DVI, and was safe, well tolerated, and immunogenic.
Clinical Trials Registration
Keywords: malaria, vaccine, sporozoite, safety, infants
In a double-blind, placebo-controlled dose-escalation, age de-escalation trial of Plasmodium falciparum sporozoite (PfSPZ) vaccine, doses as high as 1.8 × 106 PfSPZ were found to be safe and immunogenic in infants and children aged 5 months to 9 years.
Keywords. xxx; xxx; xxx.
With recent stalled progress in reducing the global malaria burden [1], additional malaria prevention approaches are needed. Malaria burden is highest among children aged <5 years; vaccines targeting infants could prevent substantial morbidity and mortality. Whole Plasmodium falciparum sporozoite (PfSPZ) vaccines are promising, with advances in vaccine manufacturing and optimization of administration route and dose increasing vaccine efficacy (VE) [2–7].
PfSPZ vaccine (Sanaria, Rockville, Maryland, USA) consists of live, radiation-attenuated, aseptic, purified, cryopreserved PfSPZ. PfSPZ vaccine has been shown to be safe and well tolerated in adults [2, 4, 7]. Administration through intradermal or subcutaneous routes elicited low-level immunity and limited protection against controlled human malaria infection (CHMI) [8]; intravenous (IV) administration in nonhuman primates induced significantly higher hepatic CD8+ T-cell responses [8]. IV administration resulted in sterile protection against CHMI in 6 of 6 malaria-naive volunteers [6], 29% VE against natural exposure by proportional analysis, and 52% by time-to-event analysis at 24 weeks in Malian adults [7], providing proof of concept for this immunization route with PfSPZ vaccine. Increasing the dose of PfSPZ vaccine is important for increasing VE.
Notably, vaccine immunogenicity is lower in malaria-exposed African adults than in malaria-naive volunteers [7, 9], possibly because prior exposure downregulates immune responses to the vaccine [10, 11]. This suggests that vaccinating infants with less malaria exposure may yield better immune responses. Infants aged 5–12 months might be an appropriate target group, as maternal antibodies wane by about 6 months [12, 13], and immune responses may be enhanced compared to responses soon after birth; immune responses and efficacy were higher among children 5–17 months old compared to those 6–12 weeks old following vaccination with the subunit P. falciparum circumsporozoite protein (PfCSP) RTS,S/AS01 vaccine [14]. Thus, immune responses in infants need to be explored, and there is limited experience with PfSPZ vaccine in children and infants [15]. Therefore, we conducted a pilot study in children and infants to evaluate the safety, tolerability, and immunogenicity of various dose regimens of PfSPZ vaccine prior to conducting a larger phase 2 safety, feasibility, and efficacy study in infants against naturally transmitted malaria in western Kenya.
METHODS
Study Setting
This study took place in Siaya County, western Kenya, which has year-round malaria transmission with 2 peaks following the rainy seasons (June–July and November–December). Malaria infection prevalence among children <15 years was 26.7% by microscopy in mid-2015 [16]. The area has been the site of several malaria vaccine trials, including the phase 3 RTS,S/AS01 malaria vaccine trial [14]. Participants in the current trial were recruited from a 10-km radius around Siaya County Referral Hospital.
Study Design and Participants
We conducted an age de-escalation, dose-escalation, randomized, placebo-controlled, double-blind trial that included children 5 months through 9 years of age, from July 2016 to February 2017. Five doses of PfSPZ vaccine (1.35 × 105, 2.7 × 105, 4.5 × 105, 9.0 × 105, and 1.8 × 106 PfSPZ) were tested in regimens involving 1 vaccination (1.35 × 105, 2.7 × 105, 4.5 × 105 PfSPZ) or 2 vaccinations (9.0 × 105 and 1.8 × 106 PfSPZ) separated by 8 weeks. Participants and study staff were blinded to treatment assignment (vaccine or normal saline placebo) but not to dose group.
Exclusion Criteria
Participants were screened for chronic illnesses and excluded if known to be human immunodeficiency virus (HIV) positive or HIV exposed, given the need for cotrimoxazole prophylaxis, which has antimalarial activity [17]. Medical history was taken, a physical examination was done, and baseline complete blood count, alanine aminotransferase (ALT), and creatinine were assessed. A baseline electrocardiogram (ECG) was evaluated by a pediatric cardiologist, except for the first group of twelve 5- to 9-year-olds, as ethical clearance for ECGs was obtained after this group was vaccinated. Children with predefined laboratory or ECG abnormalities (ALT >84 U/L, hemoglobin <8 g/dL, leukocytes <1500 cells/μL, neutrophils <750 cells/μL, platelet count <75 000 cells/μL, creatinine >0.9 mg/dL, pathological arrhythmias, long QTc, other significant ECG abnormalities) were excluded. Other exclusion criteria included use of systemic immunosuppressants, history of splenectomy, known inherited red blood cell disorders (eg, sickle cell disease, thalassemia, glucose-6-phosphate dehydrogenase deficiency), or evidence of serious underlying medical conditions.
Randomization and Vaccination
Twelve eligible participants in each age-dose group were randomized immediately prior to vaccination using permuted block randomization in R [18] to receive PfSPZ vaccine (vaccinees) or normal saline placebo (controls) in a 2:1 ratio. Initially, a group of 12 children 5–9 years old (8 vaccinees, 4 controls), received 1 dose of 4.5 × 105 PfSPZ or placebo. Approximately 2 weeks after this dose was assessed to be safe and well tolerated, 12 more 5- to 9-year-olds were randomized to receive 9 × 105 PfSPZ/placebo, and after another 2 weeks a third group to 1.8 × 106 PfSPZ/placebo. At this time, the first cohort of 13- to 59-month-olds received a dose of 1.35 × 105 PfSPZ/placebo and thereafter, 4 more cohorts of 13- to 59-month-olds and 5 cohorts of 5- to 12-month-old infants received escalating doses using a staggered design (Supplementary Figure 1).
Immediately before vaccination, capillary blood was drawn for a blood smear (read after 2 weeks). No prevaccination antimalarials were given, unless febrile children had a positive rapid diagnostic test (First Response Malaria Antigen P. falciparum [HRP2] Card Test); these children were treated and vaccination deferred for 2 weeks. The vaccine was prepared by unblinded study pharmacy staff. PfSPZ vaccine vials were thawed and diluted with phosphate-buffered saline containing human serum albumin to a final volume of 0.5 mL in a 1-mL syringe; placebo consisted of 0.5 mL of normal saline in an indistinguishable 1-mL syringe. Vaccinations were administered by DVI using a 25-gauge needle or an IV cannula by trained study staff. The cannula was flushed with 1 mL and 3 mL of normal saline preinjection and postinjection, respectively. When veins could not be seen easily, a portable vein viewer (Vein Viewer Flex, Christie Medical) was used. Participants were replaced when venous access failed following 3 vaccination attempts postrandomization or when study product/placebo was partially injected into interstitial tissue (vs all 0.5 mL injected intravenously). Children with partial injections were monitored for adverse events (AEs) for 29 days. Data were collected on ease of venous access and difficulties during vaccination.
Monitoring for Safety and Tolerability
After each vaccination (day 1), children were monitored for AEs for 2 hours at the study clinic. Local and systemic solicited and unsolicited AEs were recorded during this time, during home visits on days 2–6, and at a day 8 clinic visit. On day 8, blood samples were taken for laboratory tests, and a repeat ECG was performed after the last vaccination for all participants except the first group of 5- to 9-year-olds. Physical examination was performed on days 3, 8, and 29. Passive surveillance for unsolicited AEs continued for 29 days after the first vaccination, at which point children scheduled to receive only 1 vaccination were closed out. At the time of vaccination 2 (2 highest dose groups only), active and passive surveillance for AEs resumed for 8 and 29 days, respectively. Caregivers were encouraged to bring children to the clinic at any time for any illness until closeout. Serious adverse events (SAEs) were reported during the entire study period. Unsolicited and solicited AEs were graded according to US National Institutes of Health grading tables [19, 20] (Supplementary Table 1). Simple febrile seizures were reported as SAEs of special interest.
Immunogenicity
Blood for immunogenicity testing for the 2 highest dose groups was drawn during screening (0–4 weeks before vaccination) and 1 week postvaccination; after clotting for at least 30 minutes, serum was separated and frozen at −20°C within 1 hour of collection. Immunoglobulin G antibodies to PfCSP were assessed by enzyme-linked immunosorbent assay [5, 6]. The serum dilution at which optical density (OD) was 1.0, the difference between the post-OD and pre-OD (net OD), and the ratio of post-OD to pre-OD were reported. An individual was considered to have seroconverted if net OD was ≥50 and the OD ratio was ≥3.0 [5]. We analyzed differences between vaccinees and controls using 2-tailed Barnard tests or Fisher exact test for seroconversion rates and Wilcoxon rank-sum tests for net OD and OD ratios.
Data Management and Statistical Analysis
Data were collected on paper forms and entered into CommCare (Dimagi, Cambridge, Massachusetts) or, for AE forms, transcribed to teleforms and scanned into an Access database. The sample size was based on convention in dose escalation trials to find serious common safety concerns with increased dosing, and not to detect statistically significant differences among study groups [21]. Participants receiving any vaccine/placebo, including partial injections, were included in safety, tolerability, and feasibility analyses; those with complete second doses in immunogenicity analyses; and those with failed venous access only in selected feasibility analyses. Proportions of participants with AEs and frequencies of individual AEs were calculated.
Ethical Considerations
Written informed consent was obtained from each child's parent/guardian. The protocol was approved by the institutional review boards of the Kenya Medical Research Institute and the US Centers for Disease Control and Prevention, with regulatory oversight from the Kenya Pharmacy and Poisons Board. The study was conducted under a US Food and Drug Administration Investigational New Drug application and registered at ClinicalTrials.gov (NCT02687373). An independent data safety and monitoring board and local safety monitor were appointed by the trial sponsor (Sanaria).
RESULTS
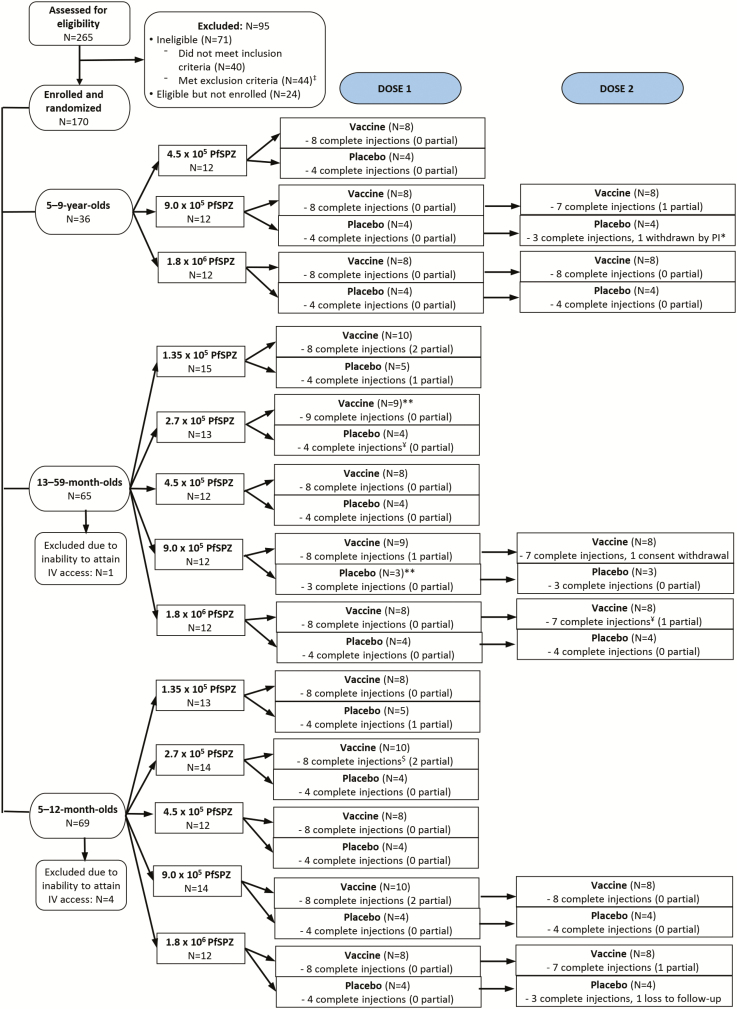
A total of 265 children and infants were screened for eligibility; 170 were enrolled. Of these, 5 failed venous access (Figure 1). Of 165 participants receiving study product, 5 received an initial partial injection. Among 71 participants assigned to receive 2 vaccinations, 68 received the second dose (Figure 1). Overall, 159 of 165 (96.3%) participants receiving any vaccine/placebo completed the study.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of participant enrollment. Abbreviations: IV, intravenous; PfSPZ, Plasmodium falciparum sporozoite; PI, principal investigator.
Safety
Solicited AEs
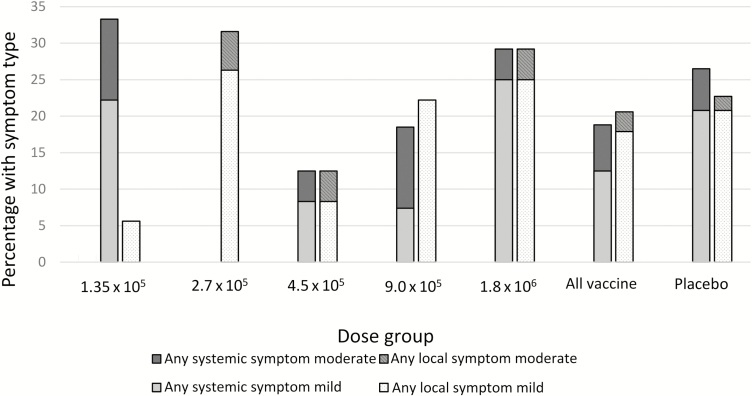
Forty of 112 (35.7%) children receiving any vaccine and 22 of 53 (41.5%) receiving any placebo had at least 1 solicited AE within 7 days of immunization; 26.8% of vaccinees and 35.8% of controls experienced a related solicited AE (Table 1). Systemic solicited AEs, primarily measured fever (Table 2), occurred in 18.8% of vaccinees and 26.4% of controls; fewer were considered related in vaccinees (10/112 [8.9%]) compared to controls (8/53 [15.1%]) (Table 1). Solicited injection site symptoms, primarily mild pain upon touch and swelling (Table 2), occurred in 20.5% of vaccinees and 22.6% of controls. All solicited local events were considered related except where there was an obvious alternative cause (ie, insect bites). No grade 3 solicited AEs occurred. The prevalence of solicited AEs did not differ substantially by dose (Table 2 and Figure 2) or age group (Supplementary Table 2).
Table 2.
Local and Systemic Solicited Adverse Events by Dose Group and Maximum Severity, All Ages Combined
| Dose Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | Severity | 1.35 × 105 (n = 18) | 2.7 × 105 (n = 19) | 4.5 × 105 (n = 24) | 9 × 105 (n = 27) | 1.8 × 106 (n = 24) | All Vaccine (n = 112) | After Dose 1 (9 × 105 and 1.8 × 106) (n = 51) | After Dose 2 (9 × 105 and 1.8 × 106) (n = 47) | Placebo (n = 53) |
| Any symptom | Mild | 5 (27.8) | 5 (26.3) | 4 (16.7) | 6 (22.2) | 10 (41.7) | 30 (26.8) | 12 (23.5) | 8 (17.0) | 18 (34.0) |
| Moderate | 2 (11.1) | 1 (5.3) | 2 (8.3) | 3 (11.1) | 2 (8.3) | 10 (8.9) | 3 (5.9) | 1 (2.1) | 4 (7.5) | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Any systemic symptom | Mild | 4 (22.2) | 0 | 2 (8.3) | 2 (7.4) | 6 (25.0) | 14 (12.5) | 6 (11.8) | 3 (6.4) | 11 (20.8) |
| Moderate | 2 (11.1) | 0 | 1 (4.2) | 3 (11.1) | 1 (4.2) | 7 (6.3) | 3 (5.9) | 0 | 3 (5.7) | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Allergic rash/urticaria/ generalized pruritusa | Mild | 2 (11.1) | 0 | 0 | 0 | 1 (4.2) | 3 (2.9) | 1 (2.0) | 0 | 3 (6.1) |
| Moderate | 0 | 0 | 0 | 1 (3.7) | 0 | 1 (1.0) | 1 (2.0) | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Drowsiness | Mild | 0 | 0 | 0 | 1 (3.7) | 2 (8.3) | 3 (2.7) | 3 (5.9) | 0 | 3 (5.7) |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Irritability/fussiness | Mild | 1 (5.6) | 0 | 1 (4.2) | 1 (3.7) | 0 | 3 (2.7) | 1 (2.0) | 0 | 1 (1.9) |
| Moderate | 1 (5.6) | 0 | 0 | 1 (3.7) | 0 | 2 (1.8) | 1 (2.0) | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Inability to eat | Moderate | 2 (11.1) | 0 | 1 (4.2) | 1 (3.7) | 1 (4.2) | 5 (4.5) | 2 (3.9) | 0 | 3 (5.7) |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fever (axillary) | Mild | 2 (11.1) | 0 | 3 (12.5) | 3 (11.1) | 5 (20.8) | 13 (11.6) | 5 (9.8) | 3 (6.4) | 10 (18.9) |
| Moderate | 0 | 0 | 0 | 1 (3.7) | 0 | 1 (0.9) | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Any local symptom | Mild | 1 (5.6) | 5 (26.3) | 2 (8.3) | 6 (22.2) | 6 (25.0) | 20 (17.9) | 7 (13.7) | 6 (12.8) | 11 (20.8) |
| Moderate | 0 | 1 (5.3) | 1 (4.2) | 0 | 1 (4.2) | 3 (2.7) | 0 | 1 (2.1) | 1 (1.9) | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pain at injection site | Mild | 1 (5.6) | 0 | 2 (8.3) | 3 (11.1) | 1 (4.2) | 7 (6.3) | 4 (7.8) | 0 | 5 (9.4) |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.9) | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pruritus/itchiness at injection sitea | Mild | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Swelling at injection site | Mild | 0 | 5 (26.3) | 1 (4.2) | 4 (14.8) | 6 (25.0) | 16 (14.3) | 5 (9.8) | 6 (12.8) | 7 (13.2) |
| Moderate | 0 | 1 (5.3) | 0 | 0 | 1 (4.2) | 2 (1.8) | 0 | 1 (2.1) | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Redness (erythema) at injection sitea | Mild | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.0) |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Bruising at injection site | Mild | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Induration at injection site | Mild | 0 | 0 | 0 | 0 | 1 (4.2) | 1 (0.9) | 0 | 1 (2.1) | 1 (1.9) |
| Moderate | 0 | 0 | 1 (4.2) | 0 | 0 | 1 (0.9) | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Only relevant age groups are included within each dose group; 1.35 × 105 and 2.7 × 105 groups include only 5- to 12-month-olds and 13- to 59-month-olds; other dose groups include all 3 age groups.
aAllergic rash/urticaria/generalized pruritus, pruritus/itchiness at injection site, and redness (erythema) at injection site were not collected for 5- to 9-year-olds receiving 4.5 × 105 or placebo.
Table 1.
Summary of Solicited and Unsolicited Adverse Events, All Ages Combined (N = 165 Immunized Participants)
| Adverse Event | All Vaccine (n = 112) | All Placebo (n = 53) | ||
|---|---|---|---|---|
| All AEs | Possibly, Probably, or Definitely Related AEs | All AEs | Possibly, Probably, or Definitely Related AEs | |
| Participants with at least 1 solicited AE within 7 d of immunization | 40 (35.7) | 30 (26.8) | 22 (41.5) | 19 (35.8) |
| Participants with a solicited grade 3 AE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Participants with at least 1 solicited local AE | 23 (20.5) | 23 (20.5) | 12 (22.6) | 12 (22.6) |
| Participants with at least 1 solicited systemic AE | 21 (18.8) | 10 (8.9) | 14 (26.4) | 8 (15.1) |
| Participants with at least 1 unsolicited AE within 28 d of immunization | 94 (83.9) | 9 (8.0) | 49 (92.5) | 1 (1.9) |
| Participants with an unsolicited grade 3 AE | 3 (2.7) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Participants experiencing an SAE | 3 (2.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total No. of SAEs (maximum severity grade) | 3 (grade 3) | 0 (…) | 0 (…) | 0 (…) |
Data are presented as no. (%). Unsolicited, nonserious AEs are included in this summary if they occurred within 28 days postvaccination, but serious AEs are summarized for the duration of the study period in accordance with the protocol.
Abbreviations: AE, adverse event; SAE, serious adverse event.
Figure 2.
Solicited symptoms, including systemic and local, by dose group and maximum severity, all ages combined.
Unsolicited and Serious AEs
Unsolicited AEs during 29 days following each vaccination were common, occurring among 94 of 112 (83.9%) vaccinees and 49 of 53 (92.5%) controls (Table 1). The most common unsolicited AEs were upper respiratory tract infections/flu/tonsillitis, occurring in 52.7% of vaccinees and 66.1% of controls; confirmed malaria (31.2% of vaccinees and 32.0% of controls); and gastroenteritis (22.3% of vaccinees and 15.1% of controls) (Supplementary Table 3). Nine of 112 (8.0%) vaccinees and 1 control (1.9%) experienced a possibly or probably related unsolicited AE (mostly reported fever) (Table 1 and Supplementary Table 4); all were mild, occurred within 7 days of vaccination, and resolved.
One grade 3 unsolicited AE and 3 SAEs occurred; all were considered unrelated and resolved (Supplementary Table 5). One 8-year-old boy who received 1 injection of placebo developed a finger abscess following a capillary blood draw that healed after incision and drainage. This was considered related to a study procedure but not study product. One 9-month-old girl developed severe malarial anemia 44 days after her first dose of 9.0 × 105 PfSPZ. After hospitalization, transfusion, and treatment, she recovered fully. A 6- and 16-month-old each experienced simple febrile seizures associated with malaria, 4 and 26 days, respectively, after vaccination with 1 dose of 1.8 × 106 PfSPZ vaccine. Neither child was admitted nor fulfilled criteria of severe malaria; both recovered fully. All 3 children with SAEs had asymptomatic parasitemia on vaccination day, determined by retrospectively read blood smears.
Laboratory Abnormalities
No grade 3 laboratory abnormalities were detected on day 8. The most common laboratory abnormality was grade 1 or 2 reduced hemoglobin, present in 23 of 111 (20.7%) vaccinees and 15 of 53 (28.3%) controls (Table 3); 33 of 38 (86.5%) had either preexisting anemia or recent malaria confirmed by blood smear. All grade 2 hemoglobin values improved with treatment to >9 g/dL (per Kenya guidelines, grade 1 hemoglobin, 9.0–9.9, was not treated). Grade 1 or 2 neutropenia occurred in 7.2% of vaccinees and 7.5% of controls, all among the older 2 age groups. Leukopenia, thrombocytopenia, and elevated creatinine or ALT were all uncommon but slightly more common in controls. One possibly related grade 2 thrombocytopenia (platelets of 67 000, without accompanying malaria) occurred 8 days after a 13-month-old received dose 2 of placebo (Supplementary Table 6). A probably related grade 2 neutropenia (neutrophils of 640) occurred in a 6-month-old 8 days after the first dose of 9.0 × 105 PfSPZ (Supplementary Table 6). Both abnormalities resolved on follow-up testing. Apart from the predefined laboratory abnormalities above, two 13- to 59-month-olds in the 2 lowest dose groups had significant eosinophilia after vaccination. One was associated with allergic dermatitis and considered possibly related to vaccination; the other occurred in a child with ascariasis. The eosinophilia resolved after treating each underlying condition.
Table 3.
Day 8 Laboratory Safety Parameters, by Dose Group
| Laboratory Parameter | 1.35 × 105 (n = 18) | 2.7 × 105 (n = 18) | 4.5 × 105 (n = 24) | 9 × 105 (n = 27) | 1.8 × 106 (n = 24) | All Vaccine (n = 111) | Placebo (n = 53) |
|---|---|---|---|---|---|---|---|
| Hemoglobin | 1 (5.6) | 5 (27.8) | 6 (25.0) | 6 (22.2) | 5 (20.8) | 23 (20.7) | 15 (28.3) |
| White blood cells | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (11.1) | 0 (0.0) | 3 (2.7) | 3 (5.7) |
| Platelets | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (3.7) | 0 (0.0) | 2 (1.8) | 2 (3.8) |
| Neutrophil count | 1 (5.6) | 3 (16.7) | 1 (4.2) | 3 (11.1) | 0 (0.0) | 8 (7.2) | 4 (7.5) |
| ALT | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 2 (3.8) |
| Creatinine | 0 (0.0) | 0 (0.0) | 1 (4.2) | 0 (0.00) | 1 (4.2) | 2 (1.8) | 1 (1.9) |
Data are no. (%) of subjects with abnormal laboratory values. One subject in the 2.7 × 105 group terminated early before follow-up labs were collected. Abnormal laboratory values are all grade 1 or grade 2, defined per the protocol-defined toxicity ranges postvaccination (Supplementary Tables 1 and 2). No grade 3 laboratory abnormalities occurred.
Abbreviation: ALT, alanine aminotransferase.
ECG Results
Overall, 215 ECGs were obtained prior to vaccination. Three children were excluded due to abnormal screening ECGs: 1 with left ventricular hypertrophy, 1 with biventricular hypertrophy, and 1 with first-degree atrioventricular block. Eight screening ECGs were abnormal with left/right axis deviation, first-degree heart block, and possible right ventricular hypertrophy, which the study cardiologist considered clinically insignificant; these children were included [22]. Of 149 postvaccination ECGs, 5 were abnormal: 3 patients had abnormalities at baseline, and 2 had new borderline abnormalities (1 possible left and 1 possible right ventricular hypertrophy) classified as unlikely to be study related. Ventricular hypertrophy by voltage criteria on ECG has poor sensitivity and low positive predictive value for actual ventricular hypertrophy; it is often affected by lead placement and body habitus [23–25].
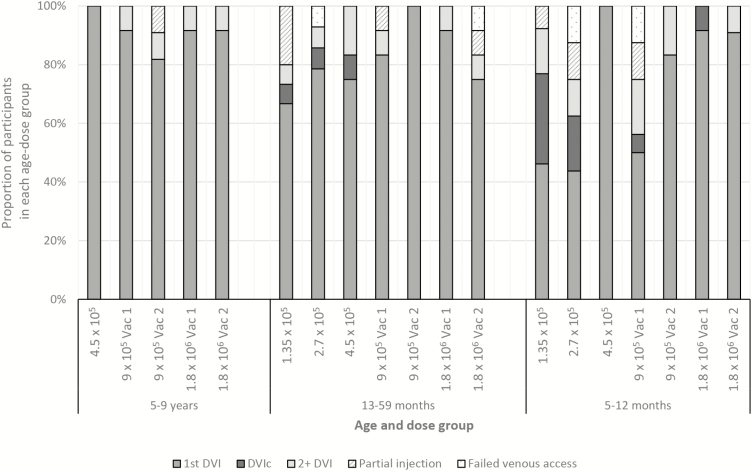
Administration by Direct Venous Inoculation
Of 238 vaccination attempts, 188 (79.0%) were administered with a single DVI, although cannulation or multiple intravenous injections were required more often in younger age groups (Figure 3). As study clinicians gained more experience, injection success rate improved, indicated by better DVI success in higher dose groups (Figure 3). Most injections in 5- to 9-year-olds were given in the antecubital fossa, but the back of the hand or wrist was more commonly used in the 2 younger age groups (Supplementary Table 7), likely due to use of the vein viewer (in 39/174 [22.4%] vaccinations in the youngest 2 groups vs 1/59 [1.7%] in 5- to 9-year-olds), which more easily displayed blood vessels in the hand vs antecubital fossa. In the majority (78.0%) of vaccinations of 5- to 9-year-olds where any vaccine/placebo was injected, mothers rated the DVI procedure as painless for their child, as did mothers for 54.0% of vaccinations in 13- to 59-month-olds and 27.2% of vaccinations in infants (Supplementary Table 7).
Figure 3.
Number of injection attempts and use of indwelling catheter, by age group, among all enrolled participants. Partial injection means that less than the full 0.5 mL of study product or normal saline placebo was injected intravenously; failed venous access means that no study product or normal saline placebo was injected intravenously. The order of the bar charts from left to right generally indicates the chronological order of study group enrollment (although the lowest dose group of the 13- to 59-month-olds were vaccinated at the same time as the highest dose group of the 5- to 9-year-olds, and the lowest dose group of the 5- to 12-month-olds was vaccinated at the same time as the 4.5 × 105 dose group of the 13- to 59-month-olds). Each dose group contains placebo and vaccine recipients. Abbreviations: DVI, direct venous inoculation; DVIc, direct venous inoculation using an intravenous cannula; 2+ DVI, 2 or more direct venous inoculations required; Vac 1, vaccine dose 1; Vac 2, vaccine dose 2.
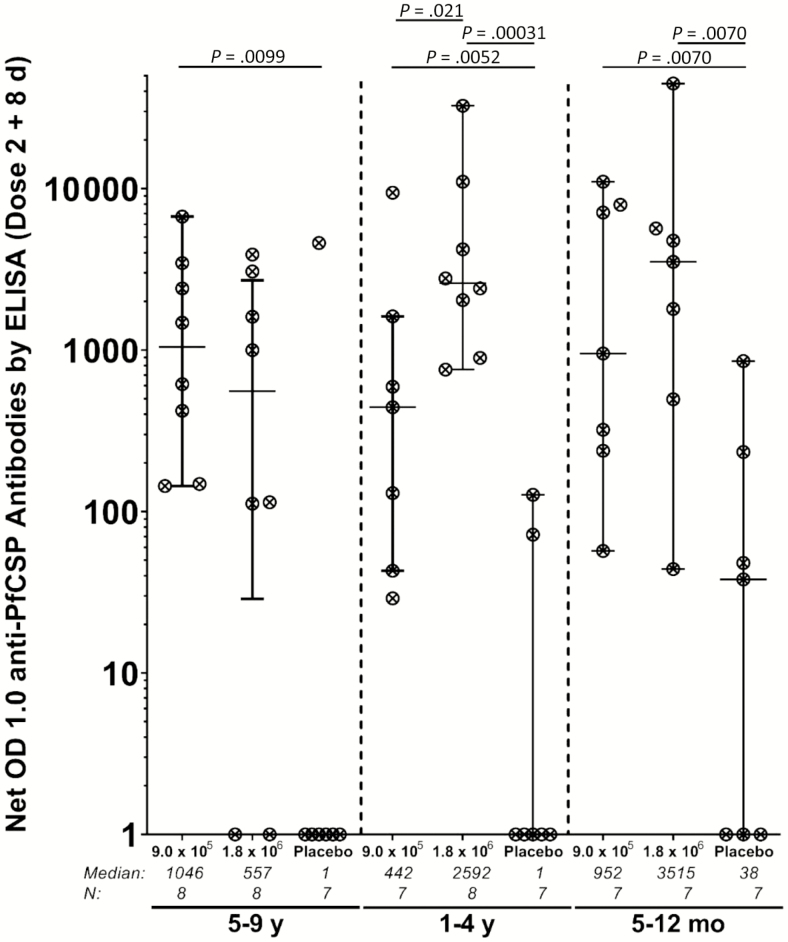
Immunogenicity
In the 2 highest dose groups, 36 of 45 (80.0%) vaccinees and 4 of 21 (19.0%) controls developed antibodies to PfCSP after the second dose (P = .000002). The rate of seroconversion ranged from 4 of 7 (57.1%) in 13- to 59-month-olds who received 9.0 × 105 PfSPZ to 8 of 8 (100%) in 13- to 59-month-olds who received 1.8 × 106 PfSPZ and 7 of 7 (100%) in 5- to 12-month-olds receiving 9.0 × 105 PfSPZ (Supplementary Table 8 and Figure 4). Median net OD and median OD ratio ranged from 442 and 4.7 in 13- to 59-month-olds who received 9.0 × 105 PfSPZ to 3515 and 92.7 in 5- to 12-month-olds receiving 1.8 × 106 PfSPZ. Among controls, 1 of 7 (14.3%) 5- to 9-year-olds, 1 of 7 (14.3%) 13- to 59-month-olds, and 2 of 7 (28.6%) 5- to 12-month-olds met seroconversion criteria after 2 doses. Three of these 4 participants had malaria during the study.
Figure 4.
Antibodies to Plasmodium falciparum circumsporozoite protein (PfCSP) measured 8 days after the second dose of P. falciparum sporozoite (PfSPZ) vaccine in vaccinees and normal saline controls in the 9.0 × 105 and 1.8 × 106 PfSPZ groups. Statistical analysis was done with Wilcoxon rank-sum test. Only P values <.05 are shown. Optical density (OD) 1.0 is the serum dilution at which the optical density was 1.0. Net OD 1.0 is the difference between the OD 1.0 eight days after the second immunization and the OD 1.0 prior to the first immunization. Abbreviation: ELISA, enzyme-linked immunosorbent assay.
DISCUSSION
Administration of PfSPZ vaccine in doses up to 1.8 × 106 PfSPZ was safe and well tolerated across multiple age cohorts including 5- to 12-month-old infants. Solicited AEs, including possibly or probably related AEs, were no more common in participants receiving PfSPZ vaccine than in those receiving placebo. No related SAEs, grade 3 AEs, or grade 3 laboratory abnormalities were detected postvaccination. Related AEs were infrequent and not dose-dependent.
Although the rates of AEs were similar between vaccinees and controls, the rate of solicited AEs overall was higher than in an earlier study of the vaccine in Tanzania, in which no solicited AEs were reported in children 6 months–10 years old during 8 days postvaccination [15]. This could be partly explained by the greater number of home visits with measured AEs (vs self-reported AEs by phone call in Tanzania) and higher malaria transmission in Siaya compared with Bagamoyo, Tanzania.
The administration of PfSPZ vaccine by DVI showed limited reactogenicity, consistent with reports in adults showing very low reactogenicity [7, 9, 15]. Data from larger trials of PfSPZ vaccine will need to confirm its limited reactogenicity and favorable tolerability profile in younger age groups. PfSPZ vaccine was successfully administered by DVI to most children and infants; feasibility is explored further in Oneko et al (manuscript in preparation). While DVI was initially more challenging in younger children and infants, injectors progressively improved with subsequent cohorts within each age group, as was noted in Tanzania [15].
Among participants receiving 2 doses of 9.0 × 105 or 1.8 × 106, 80.0% seroconverted. The highest levels of antibodies were seen in infants, as in Tanzania [15], consistent with findings that natural malaria exposure limits the vaccine response. This has been most clearly documented in adults. When the same PfSPZ vaccine dosage regimen was given to adults in the United States [2], Mali [7], Tanzania [9], and Equatorial Guinea [26], antibody responses to PfCSP were up to 30.6-fold higher in US vs African vaccinees. When the same dosage regimen was given to US and Tanzanian adults, CD4 T-cell responses to PfSPZ were approximately 6 times higher in US compared with Tanzanian adults [27]. We believe that these differences in immune responses are due to immune dysregulation from long-standing malaria exposure.
CONCLUSIONS
This Kenyan trial represents the largest numbers of children and infants vaccinated with PfSPZ vaccine to date and provides the first data on PfSPZ vaccine doses as high as 1.8 × 106 in infants and young children. Results indicate that administration of PfSPZ vaccine by DVI was possible, had an acceptable safety and tolerability profile, and was immunogenic for antibody responses in these age groups.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors sincerely thank all of the study staff, participants, and their caregivers who made this study possible. The authors are very grateful to the data safety and monitoring board members for their careful review of safety data and recommendations: Kent Kester (chair), Anna Durbin, Malcolm Molyneux, Michele Spring, Jody Ciolino, and Juliana Otieno (local safety monitor). The authors thank the Sanaria Manufacturing and Quality Teams for providing the Plasmodium falciparum Sporozoite (PfSPZ) vaccine for this study and Wathsala Wijayalath for Pharmaceutical Operations support. The authors also thank the Sanaria Clinical and Regulatory team for supporting this trial.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institutes of Health (NIH) Vaccine Research Center. Manufacturing and quality control release and stability assays for PfSPZ vaccine were supported in part by the National Institute of Allergy and Infectious Diseases, NIH (Small Business Innovation Research grant numbers 5R44AI055229-09A1 and 2R44AI058375-06A1 to S. L. H.).
Potential conflicts of interest. T. L. R., N. K. C., T. M., L. W. P. C., B. K. L. S., P. F. B., E. R. J., Y. A., S. L. H. are employees of Sanaria (Rockville, Maryland), which manufactures the vaccine tested in this study. S. L. H. and B. K. L. S. are named inventor on patents related to PfSPZ vaccine. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2017. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 2. Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight 2017; 2:e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ishizuka AS, Lyke KE, DeZure A, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lyke KE, Ishizuka AS, Berry AA, et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A 2017; 114:2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mordmüller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017; 542:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seder RA, Chang LJ, Enama ME, et al. VRC 312 Study Team Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359–65. [DOI] [PubMed] [Google Scholar]
- 7. Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 2017; 17:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8⁺ T cell immunity. Science 2011; 334:475–80. [DOI] [PubMed] [Google Scholar]
- 9. Jongo SA, Shekalaghe SA, Church LWP, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg 2018; 99:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greenwood B. Progress with the PfSPZ vaccine for malaria. Lancet Infect Dis 2017; 17:463–4. [DOI] [PubMed] [Google Scholar]
- 11. Illingworth J, Butler NS, Roetynck S, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol 2013; 190:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Apinjoh TO, Anchang-Kimbi JK, Mugri RN, et al. Determinants of infant susceptibility to malaria during the first year of life in south western Cameroon. Open Forum Infect Dis 2015; 2:ofv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dobbs KR, Dent AE. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 2016; 143:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jongo SA, Church LWP, Mtoro A, et al. Safety and differential antibody and T cell responses to PfSPZ vaccine by age in Tanzanian adults, adolescents, children and infants. Am J Trop Med Hyg 2019; 100:1433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Malaria Control Programme, Kenya National Bureau of Statistics, ICF International. Kenya malaria indicator survey 2015. Nairobi, Kenya and Rockville, MD: NMCP, KNBS, and ICF International, 2016.
- 17. Manyando C, Njunju EM, D'Alessandro U, Van Geertruyden JP. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One 2013; 8:e56916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snow G. Blockrand: randomization for block random clinical trials . R package version 1.3 Vienna, Austria: The Comprehensive R Achieve Network, 2013. [Google Scholar]
- 19. US Department of Health and Human Services. Division of Microbiology and Infectious Diseases pediatric toxicity tables. Bethesda, MD: NIH, 2007. [Google Scholar]
- 20. US Department of Health and Human Services. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, version 2.0. Bethesda, MD: DAIDS, 2014. [Google Scholar]
- 21. US Food and Drug Administration, Center for Drug Evaluation and Research, Office of New Drugs. Good review practice: clinical review of investigational new drug applications. Washington, DC: FDA, 2013. [Google Scholar]
- 22. Ravi P, Ashwath R, Strainic J, Li H, Steinberg J, Snyder C. Clinical and financial impact of ordering an echocardiogram in children with left axis deviation on their electrocardiogram. Congenit Heart Dis 2016; 11:110–4. [DOI] [PubMed] [Google Scholar]
- 23. Bratincsák A, Williams M, Kimata C, Perry JC. The electrocardiogram is a poor diagnostic tool to detect left ventricular hypertrophy in children: a comparison with echocardiographic assessment of left ventricular mass. Congenit Heart Dis 2015; 10:E164–71. [DOI] [PubMed] [Google Scholar]
- 24. Killian L, Simpson JM, Savis A, Rawlins D, Sinha MD. Electrocardiography is a poor screening test to detect left ventricular hypertrophy in children. Arch Dis Child 2010; 95:832–6. [DOI] [PubMed] [Google Scholar]
- 25. Tague L, Wiggs J, Li Q, et al. Comparison of left ventricular hypertrophy by electrocardiography and echocardiography in children using analytics tool. Pediatr Cardiol 2018; 39:1378–88. [DOI] [PubMed] [Google Scholar]
- 26. Olotu A, Urbano V, Hamad A, et al. Advancing global health through development and clinical trials partnerships: a randomized, placebo-controlled, double-blind assessment of safety, tolerability, and immunogenicity of PfSPZ vaccine for malaria in healthy Equatoguinean men. Am J Trop Med Hyg 2018; 98:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jongo SA, Church LWP, Mtoro AT, et al. Safety and differential antibody and T-cell responses to the Plasmodium falciparum sporozoite malaria vaccine, PfSPZ vaccine, by age in Tanzanian adults, adolescents, children, and infants. Am J Trop Med Hyg 2019; 100:1433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.