Abstract
Aims
We investigated whether pre-existing diabetes, newly-diagnosed diabetes, and admission hyperglycemia were associated with COVID-19 severity independently from confounders.
Methods
We retrospectively analyzed data on patients with COVID-19 hospitalized between February and April 2020 in an outbreak hospital in North-East Italy. Pre-existing diabetes was defined by self-reported history, electronic medical records, or ongoing medications. Newly-diagnosed diabetes was defined by HbA1c and fasting glucose. The primary outcome was a composite of ICU admission or death.
Results
413 subjects were included, 107 of whom (25.6%) had diabetes, including 21 newly-diagnosed. Patients with diabetes were older and had greater comorbidity burden. The primary outcome occurred in 37.4% of patients with diabetes compared to 20.3% in those without (RR 1.85; 95%C.I. 1.33–2.57; p < 0.001). The association was stronger for newly-diagnosed compared to pre-existing diabetes (RR 3.06 vs 1.55; p = 0.004). Higher glucose level at admission was associated with COVID-19 severity, with a stronger association among patients without as compared to those with pre-existing diabetes (interaction p < 0.001). Admission glucose was correlated with most clinical severity indexes and its association with adverse outcome was mostly mediated by a worse respiratory function.
Conclusion
Newly-diagnosed diabetes and admission hyperglycemia are powerful predictors of COVID-19 severity due to rapid respiratory deterioration.
Keywords: Observational, SARS-CoV-2, Survival, Prediction, Mediation, Metabolism
1. Introduction
Diabetes worsens the outcome of virtually any acute or chronic medical condition, resulting in a shortened life expectancy [1]. Mortality from infectious disease is also increased in patients with diabetes, especially for sepsis and pneumonia [2]. Since the very beginning of the SARS-CoV-2 pandemic, diabetes emerged as one of the most common comorbidities and a potential driver of poor outcomes [3]. The prevalence of diabetes was higher among patients hospitalized for COVID-19 who were admitted to the intensive care unit (ICU) or died [4]. Meta-analyses of studies reporting the characteristics of patients according to COVID-19 severity in China found that diabetes conferred a 2–3 fold increased rate of poor disease outcome [5], [6]. These findings are in line with the available literature on the adverse prognostic impact of diabetes on other viral infections, including influenza [7], [8].
It remains unclear whether diabetes drove such excess risk independently from confounders, including its complications and comorbidities. It is conceivable that patients with diabetes experienced a worse COVID-19 outcome because of multiple organ damage due to micro- and macrovascular disease [9], [10]. On the other side, whether the impact of diabetes on COVID-19 outcome depends on glucose control is less appreciated. In a study conducted on 7337 Chinese patients, diabetes was associated with higher mortality, but patients with good glycemic control had lower mortality compared to those with poorer control [11]. Optimal glycemic management can therefore be crucial to improve COVID-19 outcome [12]. Finally, new-onset diabetes has been reported during COVID-19 [13], [14], [15], but its impact on disease outcome has not been assessed. In patients with acute medical conditions, such as acute myocardial infarction, newly-detected diabetes has an adverse prognostic effect [16], [17]. Of note, newly-diagnosed diabetes is often linked with occult organ damage that shortens survival [18]. In addition, stress hyperglycemia has been known for decades to drive an exaggerated inflammatory response in critically-ill individuals [19].
In the present study, we investigated the role of pre-existing diabetes, newly-diagnosed diabetes, and admission glucose levels on the outcome of patients hospitalized for COVID-19. We eventually explored which were the mediators of a poor outcome associated with diabetes.
2. Subjects, materials and methods
2.1. Study design
This retrospective study was conducted by collecting anonymized patient’s data from electronic medical records. The protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. In agreement with national regulation on retrospective studies, the protocol was notified to the local ethical committee (no. 0031264) and the need for patient’s informed consent was waived.
2.2. Data collection
We retrieved data on all consecutive patients hospitalized for COVID-19 between February 21st and April 20th 2020 at the University Hospital of Padova, which is located at the center of one of the first SARS-CoV-2 outbreaks in Northern Italy. We screened records of all patients admitted to the Hospital with a positive PCR test for SARS-CoV-2 on upper or lower airway sample. Pre-existing diabetes was defined based on self-reported history, prior electronic medical records reporting a diagnosis of diabetes, or ongoing therapy with glucose-lowering medications. Newly-diagnosed diabetes was defined by a HbA1c value of 48 mmol/mol (6.5%) or higher; in the absence of an HbA1c determination, a random glucose level of 11.1 mmol/l (200 mg/dl) or higher, accompanied by signs and symptoms of hyperglycemia was considered diagnostic.
For all patients, we recorded the following information: demographics (age, sex), concomitant cardiovascular risk factors (smoke, hypertension, dyslipidemia), comorbidities (chronic obstructive pulmonary disease and history of cancer), complications (cardiovascular disease and microangiopathy), presence or absence of COVID-19 related pneumonia or interstitial lung disease (ILD), ongoing therapies before hospitalization. We collected information on symptoms of COVID-19 upon admission (time from onset of symptoms to hospitalization; presence of fever, cough, dyspnea, and gastrointestinal symptoms). Vital signs were recorded at admission and at their worst timepoint: systolic and diastolic blood pressure, heart and respiratory rate, oxygen saturation, PaO2/FiO2 ratio. Key laboratory exams included: fasting plasma glucose, HbA1c, lipid profile (total cholesterol, HDL cholesterol, triglycerides, and calculated LDL cholesterol), and serum creatinine for calculation of estimated glomerular filtration rate (eGFR) using the CKD-EPI equation. We also collected admission levels and worse levels of the following biomarkers related to inflammation (white blood cell count [WBC], C-reactive protein [CRP], IL-6, pro-calcitonin), hematology and coagulation (hemoglobin, lymphocytes, platelets, D-dimer) and tissue injury (liver enzymes, troponin I, lactate). We reported the worst vital signs or biomarkers as the worst value measured during the entire hospital stay, regardless of whether it occurred at admission or during any other time of hospitalization. In some cases, worst values could coincide with admission values if admission values were the worst among all values recorded during the hospital stay. The following in-hospital treatments were recorded: low-flow and high-flow oxygen, non-invasive ventilation, intubation, use of specific drugs, such as lopinavir/ritonavir, azithromycin, other antibiotics, remdesivir, chloroquine/hydroxychloroquine, glucocorticoids, tocilizumab.
2.3. Outcome definition
The primary outcome was a composite of admission to the ICU (including all subjects needing mechanical ventilation) or death. Death from any cause, was considered as a separate outcome. Among those who were discharged alive, time to discharge was considered as additional secondary outcomes. Because duration of hospitalization was highly skewed and sometimes driven by non-clinical reasons (e.g. patients being unable to return to home because lacking social support), observation was censored at 30 days.
2.4. Statistical analysis
Continuous variables are reported as mean ± standard deviation (SD) or as median (inter-quartile range), while categorical variables have been reported has percentages. Between-group differences in clinical characteristics were evaluated with Student t-test or chi-Square for continuous and categorical variables, respectively. The association between diabetes, glucose levels and other variables of interest with categorical outcomes was evaluated with robust-error-variance Poisson regression models [20]. In order to identify possible confounding factors, these associations were tested in unadjusted models and in two different multivariable adjusted (MVA) models with increasing complexity. Model 1 (MVA1): adjusted for sex and age. Model 2 (MVA2): adjusted for sex, age and pre-existing conditions or pre-hospital medications associated with COVID-19 severity in MVA1. In order to test the MVA, full dataset of variables were needed, thus missing data were handled by means of multiple imputation (MI). MI was performed with a fully conditional specification (FCS) algorithm [21] obtaining 10 imputed datasets including only covariates with less than 60% of missing values. Outcome variables and main variables of interest (e.g. plasma glucose) were not imputed. Outcome analyses were performed on each imputed dataset and the pooled estimated effects are presented [22]. Cox proportional hazard model was used to compare the patient’s probability of being discharged alive.
The differences between the association of pre-existing diabetes and newly-diagnosed diabetes with the primary and secondary outcomes were evaluated by means of Z score (Z score = (estimates1 − esitmates2)/√(SE1 2 + SE2 2)). The differences in the association between glucose levels and primary outcome among subjects with or without diabetes was evaluated with the inclusion of an interaction term in the models. The association between fasting plasma glucose and the worst levels of clinical and laboratory parameters detected during hospital stay were represented graphically with scatter plots and described by Pearson’ r correlation coefficient.
We evaluated which of these parameters explained the association between glucose levels and COVID-19 severity outcome (i.e. being mediators). To be consider a candidate mediator, variables needed to be independently associated with both the primary outcome (tested using Poisson regression with robust error variance) and with glucose levels (tested with linear regression). For those variables meeting these two conditions, we then quantified the possible mediation effect (in percentage) as the differences in the association between glucose and the primary outcome in the model adjusted by the candidate mediator (RRADJUST) as compared to the association in the model not adjusted by the candidate mediator (RRNOT-ADJUST). Therefore the mediation analyses was evaluated as: mediation = 100 × [ln(RRNOT-ADJUST) − ln(RRADJUST)]/ln(RRNOT-ADJUST). Statistical analyses were conducted with a significance threshold of p < 0.05 and were done in SAS version 9.4 (TS1M4), GraphPad PRISM v8.3.0.
3. Results
3.1. Characteristics of the COVID-19 population by diabetes status
We included 413 patients who tested positive for SARS-CoV-2 upon PCR analysis. Of these, 107 (25.6%) had diabetes (86 pre-existing and 21 newly diagnosed). As compared to individuals without diabetes, those with diabetes were older, had a higher prevalence of hypertension, dyslipidemia, cardiovascular disease, and chronic kidney disease. In addition, pre-admission use of statins, antiplatelet drugs and beta-blockers were more common among subjects with diabetes (Table 1 ). Symptoms at admission were similar as were laboratory findings (except for FPG and HbA1c). Participants with diabetes had more compromised respiratory function with lower PaO2/FiO2 and higher respiratory rate (Table 2 ). In-hospital pharmacological treatments were similar in subjects with or without diabetes, except that diabetic patients more often received antibiotics other than macrolids (Table 2). Table S1 shows patients’ clinical characteristics further stratified by pre-existing versus newly-diagnosed diabetes status.
Table 1.
Characteristics of COVID-19 patients according to diabetes status. Data presented as mean (SD) or as percentage. In the column reporting data of all patients, data availability is also shown. Diabetes includes both pre-existing diabetes and newly-detected diabetes.
| All patients N = 413 |
No diabetes N = 306 |
Diabetes N = 107 |
p | ||
|---|---|---|---|---|---|
| Available | Value | Value | Value | ||
| Age, years | 100% | 64.9 ± 15.4 | 63.3 ± 15.5 | 69.7 ± 13.8 | <0.001 |
| Sex male, % | 100% | 245 (59.3%) | 175 (57.2%) | 70 (65.4%) | 0.136 |
| Concomitant risk factors, n (%) | |||||
| Hypertension | 100% | 212 (51.3%) | 139 (45.4%) | 73 (68.2%) | <0.001 |
| Current smoking | 46% | 53 (27.7%) | 38 (27.5%) | 15 (28.3%) | 0.916 |
| Dyslipidemia | 100% | 91 (22.0%) | 54 (17.6%) | 37 (34.6%) | <0.001 |
| Comorbidities, n (%) | |||||
| Cardiovascular disease | 97% | 72 (18.0%) | 45 (15.3%) | 27 (26.0%) | 0.015 |
| Atrial fibrillation | 97% | 44 (11.0%) | 32 (10.8%) | 12 (11.5%) | 0.830 |
| CKD | 100% | 30 (7.3%) | 16 (5.2%) | 14 (13.1%) | 0.007 |
| COPD | 97% | 29 (7.3%) | 20 (6.8%) | 9 (8.7%) | 0.527 |
| Cancer | 96% | 66 (16.6%) | 48 (16.3%) | 18 (17.5%) | 0.777 |
| Symptoms at admission | |||||
| Time from symptoms to hospitalization, days | 88% | 7.0 ± 4.7 | 7.1 ± 4.6 | 7.0 ± 4.9 | 0.907 |
| Fever, % | 100% | 272 (66.0%) | 212 (69.5%) | 60 (56.1%) | 0.012 |
| Body temperature (°C) | 44% | 38.5 ± 0.6 | 38.6 ± 0.6 | 38.4 ± 0.6 | 0.173 |
| Cough, % | 89% | 234 (63.9%) | 180 (65.2%) | 54 (60.0%) | 0.371 |
| Dyspnea, % | 91% | 232 (61.9%) | 169 (60.8%) | 63 (64.9%) | 0.468 |
| Pneumonia / ILD, % | 92% | 332 (87.1%) | 241 (85.2%) | 91 (92.9%) | 0.050 |
| GI symptoms, % | 87% | 102 (28.5%) | 78 (28.9%) | 24 (27.3%) | 0.771 |
| Medication before hospitalization | |||||
| ACE inhibitors, % | 100% | 71 (17.2%) | 48 (15.7%) | 23 (21.5%) | 0.170 |
| Angiotensin receptor blockers, % | 100% | 73 (17.7%) | 48 (15.7%) | 25 (23.4%) | 0.073 |
| Calcium channel blockers, % | 100% | 61 (14.8%) | 40 (13.1%) | 21 (19.6%) | 0.100 |
| Beta blockers, % | 100% | 87 (21.1%) | 49 (16.0%) | 38 (35.5%) | <0.001 |
| Anti-platelet agents, % | 100% | 73 (17.7%) | 46 (15.0%) | 27 (25.2%) | 0.017 |
| Statins, % | 100% | 81 (19.6%) | 47 (15.4%) | 34 (31.8%) | <0.001 |
| Warfarin, % | 100% | 23 (5.6%) | 19 (6.2%) | 4 (3.7%) | 0.337 |
| New anticoagulants, % | 100% | 22 (5.3%) | 15 (4.9%) | 7 (6.5%) | 0.516 |
| Antibiotics, % | 100% | 135 (32.7%) | 104 (34.0%) | 31 (29.0%) | 0.341 |
| NSAID, % | 100% | 24 (5.8%) | 19 (6.2%) | 5 (4.7%) | 0.559 |
| Gluocorticosteroids, % | 100% | 21 (5.1%) | 14 (4.6%) | 7 (6.5%) | 0.425 |
| Laboratory | |||||
| Fasting plasma glucose, mmol/l | 72% | 7.3 ± 3.5 | 6.0 ± 1.5 | 10.7 ± 4.9 | <0.001 |
| HbA1c, mmol/mol | 30% | 50.3 ± 17.8 | 39.5 ± 5.3 | 57.5 ± 19.6 | <0.001 |
| Total cholesterol, mmol/l | 29% | 3.5 ± 1.2 | 3.6 ± 1.1 | 3.4 ± 1.3 | 0.301 |
| HDL cholesterol, mmol/l | 25% | 1.0 ± 0.4 | 1.0 ± 0.5 | 0.9 ± 0.4 | 0.052 |
| Triglycerides, mmol/l | 30% | 1.4 ± 0.8 | 1.3 ± 0.5 | 1.8 ± 1.1 | 0.006 |
| LDL-cholesterol, mmol/l | 25% | 2.0 ± 0.9 | 2.0 ± 0.9 | 1.9 ± 1.0 | 0.422 |
| Serum creatinine, umol/l | 100% | 90.3 ± 67.5 | 91.1 ± 75.5 | 88.0 ± 36.1 | 0.573 |
| eGFR, ml/min/0.173 m2 | 100% | 78.2 ± 22.8 | 78.8 ± 22.7 | 76.4 ± 22.9 | 0.343 |
| Parameters at admission | |||||
| Systolic blood pressure, mm Hg | 98% | 130.1 ± 20.0 | 128.2 ± 18.8 | 135.7 ± 22.4 | 0.003 |
| Diastolic blood pressure, mm Hg | 98% | 76.8 ± 11.8 | 77.1 ± 11.4 | 76.0 ± 13.2 | 0.404 |
| Heart rate, bpm | 97% | 86.6 ± 15.8 | 86.7 ± 14.7 | 86.5 ± 18.7 | 0.906 |
| Respiratory rate /min | 74% | 20.8 ± 6.2 | 20.1 ± 5.6 | 23.2 ± 7.3 | 0.001 |
| Oxygen saturation, % | 98% | 94.6 ± 4.5 | 95.0 ± 4.3 | 93.4 ± 4.8 | 0.001 |
| PaO2 / FiO2 | 68% | 284.6 ± 97.5 | 295.8 ± 94.7 | 253.7 ± 99.1 | 0.001 |
| White blood cells, kel/µl | 70% | 6.7 ± 3.5 | 6.6 ± 3.7 | 7.0 ± 2.8 | 0.329 |
| Lymphocytes, kel/µl | 68% | 1.1 ± 0.9 | 1.1 ± 0.9 | 1.1 ± 0.8 | 0.901 |
| Hemoglobin, g/l | 72% | 13.5 ± 1.8 | 13.6 ± 1.8 | 13.3 ± 1.9 | 0.240 |
| Platelets, el/μl | 100% | 209.4 ± 84.6 | 208.6 ± 83.9 | 211.5 ± 87.1 | 0.766 |
| C-reactive protein, mg/dl | 84% | 6.3 (2.5–12.3) | 5.5 (2.2–11.4) | 7.8 (3.3–14.8) | 0.123 |
| D-dimer, μg/l | 90% | 223 (150–452) | 210 (150–380) | 284 (169–580) | 0.669 |
| ALT, U/l | 99% | 35.5 ± 28.8 | 35.7 ± 30.2 | 35.1 ± 24.3 | 0.847 |
| Troponin I, μg/l | 65% | 10 (4–30) | 9 (3–28) | 14 (5–38) | 0.653 |
| Lactate, mmol/l | 59% | 1.4 ± 0.5 | 1.4 ± 0.6 | 1.5 ± 0.5 | 0.218 |
CKD, chronic kidney disease. COPD, chronic obstructive pulmonary disease. ILD, interstitial lung disease. GI, gastrointestinal. ACE, angiotensin converting enzyme. NSAID, non-steroidal anti-inflammatory drugs. HDL, high density lipoprotein. LDL, low density lipoprotein. eGFR, estimated glomerular filtration rate. PaO2, partial oxygen pressure. FiO2, fractional inhaled O2. ALT, alanine aminotransferase.
Table 2.
Hospital treatments and outcomes according to diabetes status. In patients without diabetes and in those with diabetes (pre-existing and newly-diagnosed altogether), we show the worst value of clinical-laboratory parameters, as well as in-hospital treatments and outcomes.
| All patients N = 413 |
No diabetes N = 306 |
Diabetes N = 107 |
p | ||
|---|---|---|---|---|---|
| Worst in-hospital parameters | Available | Value | Value | Value | |
| Systolic blood pressure, mm Hg | 99% | 110.7 ± 17.7 | 108.3 ± 15.8 | 117.6 ± 21.0 | <0.0001 |
| Diastolic blood pressure, mm Hg | 99% | 66.1 ± 11.1 | 65.3 ± 10.5 | 68.5 ± 12.4 | 0.020 |
| Heart rate, bpm | 98% | 94.3 ± 17.8 | 94.1 ± 17.5 | 94.8 ± 18.7 | 0.711 |
| Respiratory rate/min | 83% | 24.5 ± 8.3 | 23.7 ± 8.2 | 26.9 ± 8.0 | 0.002 |
| Oxygen saturation, % | 100% | 91.6 ± 6.0 | 91.9 ± 5.5 | 90.7 ± 7.1 | 0.118 |
| PaO2/FiO2 | 75% | 221.2 ± 123.6 | 231.1 ± 124.2 | 194.1 ± 118.4 | 0.020 |
| WBC, kel/µl | 70% | 10.1 ± 7.8 | 9.2 ± 6.7 | 12.7 ± 9.8 | 0.005 |
| Lymphocytes, kel/µl | 69% | 0.9 ± 0.8 | 0.9 ± 0.8 | 0.7 ± 0.5 | 0.088 |
| Hemoglobin, g/l | 74% | 11.8 ± 2.1 | 11.9 ± 2.1 | 11.5 ± 2.2 | 0.093 |
| Platelets, kel/l | 100% | 180.6 ± 73.4 | 182.0 ± 74.4 | 176.5 ± 70.4 | 0.505 |
| C-reactive protein, mg/dl | 94% | 9.5 (4.3–16.0) | 8.8 (3.8–15.6) | 10.8 (6.2–19.0) | 0.130 |
| IL-6, pg/ml | 36% | 51 (12–171) | 46 (12–162) | 52 (15–194) | 0.489 |
| Pro-calcitonin, μg/l | 62% | 0.15 (0.1–0.6) | 0.13 (0.1–0.5) | 0.23 (0.1–0.9) | 0.076 |
| D-dimer, µg/l | 95% | 383 (188–1276) | 325 (180–1065) | 595 (243–2318) | 0.139 |
| ALT, U/l | 100% | 91.8 ± 209.8 | 98.0 ± 237.3 | 74.2 ± 94.1 | 0.147 |
| Troponin I, µg/l | 67% | 13 (4–40) | 10 (3–30) | 20 (8–77) | 0.458 |
| Lactate, mmol/l | 61% | 2.0 ± 2.2 | 1.9 ± 1.7 | 2.4 ± 3.1 | 0.205 |
| COVID-19 therapies, n (%) | |||||
| Low-flow oxygen | 100% | 305 (73.8%) | 221 (72.2%) | 84 (78.5%) | 0.203 |
| High-flow oxygen | 100% | 97 (23.5%) | 57 (18.6%) | 40 (37.4%) | <0.001 |
| Non-invasive ventilation | 100% | 70 (16.9%) | 38 (12.4%) | 32 (29.9%) | <0.001 |
| Invasive ventilation | 100% | 53 (12.8%) | 33 (10.8%) | 20 (18.7%) | 0.035 |
| Lopinavir/Ritonavir | 100% | 156 (38.0%) | 110 (36.2%) | 46 (43.0%) | 0.212 |
| Azithromycin | 100% | 264 (64.2%) | 202 (66.4%) | 62 (57.9%) | 0.115 |
| Antibiotics (other than AZT) | 100% | 287 (69.7%) | 204 (66.9%) | 83 (77.6%) | 0.039 |
| Remdesivir | 100% | 24 (5.8%) | 18 (5.9%) | 6 (5.6%) | 0.905 |
| Chloroquine | 100% | 350 (85.2%) | 260 (85.5%) | 90 (84.1%) | 0.723 |
| Glucocorticoids | 100% | 170 (41.4%) | 118 (38.8%) | 52 (48.6%) | 0.077 |
| Tocilizumab | 100% | 40 (9.7%) | 28 (9.2%) | 12 (11.2%) | 0.547 |
| Outcomes, n (%) | |||||
| Primary Outcome | 100% | 102 (24.7%) | 62 (20.3%) | 40 (37.4%) | <0.001 |
| Death | 100% | 48 (11.6%) | 33 (10.8%) | 15 (14.0%) | 0.367 |
| Discharged alive | 100% | 298 (72.2%) | 238 (77.8%) | 60 (56.1%) | <0.001 |
| Mean time to discharge * | 100% | 10.1 ± 5.7 | 10.1 ± 5.7 | 12.5 ± 7.2 | 0.021 |
| Mean days of hospitalization** | 100% | 12.3 ± 7.7 | 11.3 ± 7.1 | 15.0 ± 8.6 | <0.001 |
PaO2, partial oxygen pressure. FiO2, fractional inhaled O2. ALT, alanine aminotransferase. IL-6, interleukin-6. AZT, azithromycin. **Mean time to discharge evaluated in 298 (72.2%) patients discharged alive. **Mean days of Hospitalization evaluated in survivors only.
3.2. Diabetes and severity of COVID-19
Over a median observation time of 17 days (IQR 6–26), 102 patients (24.7%) showed a severe course, defined as the primary outcome, 48 of whom died (11.6%). In unadjusted analysis, presence of diabetes (including pre-existing and newly-diagnosed) compared to its absence was associated with a higher incidence of the primary outcome (37.4% vs 20.3%; RR 1.85; 95% C.I. 1.33–2.57; p < 0.001; Fig. 1 A). Several other pre-existing conditions were associated with COVID-19 severity, including hypertension, cardiovascular disease, atrial fibrillation, CKD and COPD (Table S2). Among pre-hospitalization treatments, use of ARBs, novel oral anticoagulants, and systemic glucocorticoids was more frequent among patients with the primary severity outcome (Table S2).
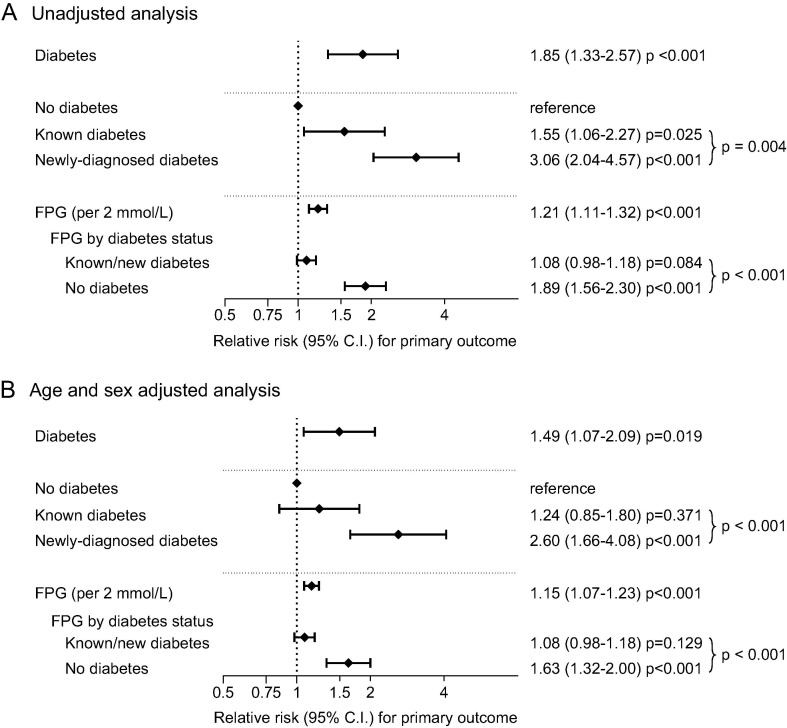
Fig. 1.
Forest plots of the association of diabetes/hyperglycemia on COVID-19 outcomes. Unadjusted (A) and age- and sex-adjusted (B) analysis of the association between diabetes, as compared to no diabetes, split known diabetes versus newly-diagnosed diabetes and hyperglycemia (for each 2 mmol/l increase) with severe COVID-19. The primary severity outcome was admittance to the intensive care unit, or need of mechanical ventilation, or death (results of model 2 are detailed in Table 3, and not are graphically represented here since results were very similar to model 1).
Compared to absence of diabetes, newly diagnosed diabetes (RR 3.06; 95% C.I. 2.04–4.57) showed a stronger association with the primary outcome than pre-existing diabetes (RR 1.55, 95% C.I. 1.06–2.27). The difference in the RR for the primary outcome between pre-existing and newly-diagnosed diabetes was statistically significant (p = 0.004). In addition, higher FPG at admission was associated with COVID-19 severity, with an increase in relative risk of 21% (RR 1.21; 95% C.I. 1.11–1.32; p < 0.001) for each 2 mmol/L (36 mg/dl) increase in FPG. This association was stronger among individuals without diabetes than in those with diabetes (p for interaction = 0.003; Fig. 1A)
After adjusting for age and sex, the magnitude of the association between diabetes (pre-existing and newly-diagnosed altogether) and COVID-19 severity remained significant (RR 1.49; 95% C.I. 1.07–2.09; p = 0.019; Fig. 1B). Other variables associated with severity in the age- and sex-adjusted analysis were chronic kidney disease and pre-hospitalization use of systemic glucocorticoids (Table S2).
The analysis was repeated splitting diabetes into pre-existing and newly-diagnosed. The association of pre-existing diabetes was attenuated and no longer significant, whereas the association between newly-diagnosed diabetes with the primary outcome remained statistically significant. The association between FPG and the primary outcome remained significant after age- and sex-adjustment and yet with a larger effect on outcome among non-diabetic than diabetic subjects (Fig. 1B).
3.3. Independent predictors of critical COVID-19 severity
We tested whether the associations of diabetes and FPG with COVID-19 severity was independent from additional confounders. As shown in Table S3, the association between diabetes and the primary outcome was confirmed in all models, including those with pre-existing comorbidities and medications used prior to hospitalization (RR 1.61; 95% C.I. 1.12–2.30; p = 0.010). Similarly, the association between FPG and the primary outcome was confirmed in all models (Table S3), including those with pre-existing comorbidities and medications used prior to hospitalization (RR per each 2 mmol/l = 1.14; 95% C.I. 1.06–1.23; p < 0.001).
3.4. Diabetes and COVID-19 mortality
We found a similar mortality rate among those with or without diabetes (10.8 vs 14.0%; unadjusted RR 1.30; 95% CI 0.74–2.30; p = 0.367). Conversely, a significant association was detected between FPG and mortality (RR for each 2 mmol/L increase 1.15; 95% CI 1.03–1.30; p = 0.017), but this association disappeared after adjustment for age and sex (RR 1.03; p = 0.668). We also found that a lower proportion of diabetic patients was discharged alive during the observation time (56.1% vs 77.8%; p < 0.001; Table 2) with a longer mean time to discharge (+2.3 days; p = 0.021; Table 2). In a time-to-event analysis, diabetes was associated with a 43% reduced probability of recovery after adjustment for age and sex (HR 0.58; 95% C.I. 0.43–0.77). Among survivors, the duration of hospitalization was significantly longer for patients with as compared to those without diabetes in unadjusted (+3.7 days; 95% C.I. 1.9–5.5, p < 0.001; Table 2) and age- and sex-adjusted models (+2.6 days; 95% C.I. 0.9–4.6, p = 0.003). For each 2 mmol/l higher FPG, there was a significant 19% lower probability of recovery (HR 0.82 95% C.I. 0.74–0.91; p < 0.001) and longer duration of hospitalization (+1.0 days; 95% C.I. 0.5–1.5; p < 0.001).
3.5. Mediators of association between glucose and COVID-19 severity
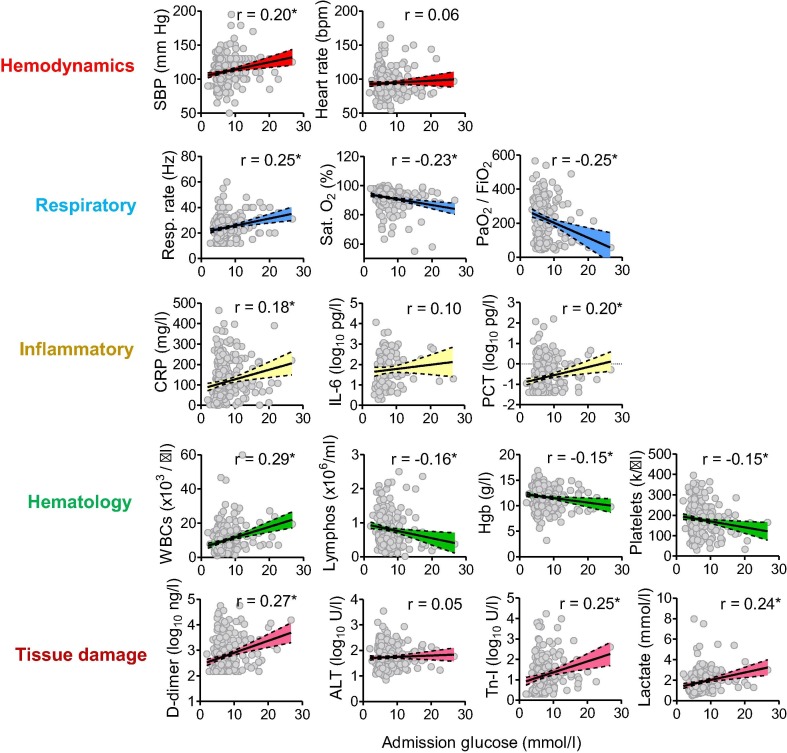
We found statistically significant correlations between admission glucose levels and most clinical-laboratory characteristics, recorded at their worst level, indicative of COVID-19 severity or progression, including hemodynamic, respiratory, hematologic, inflammatory, and tissue damage biomarkers (Fig. 2 ).
Fig. 2.
Correlation between admittance glucose levels and severity clinical variables. Clinical variables of COVID-19 severity are divided into those related to hemodynamics, respiratory, inflammatory, hematologic and tissue damage biomarkers. For each correlation plot, we show individual data points, the regression line with shadowed 95% C.I. between dashed lines, as well as the Pearson’s r correlation coefficient. *p < 0.05. When distribution of the dependent variable was highly skewed, the Y scale was log transformed.
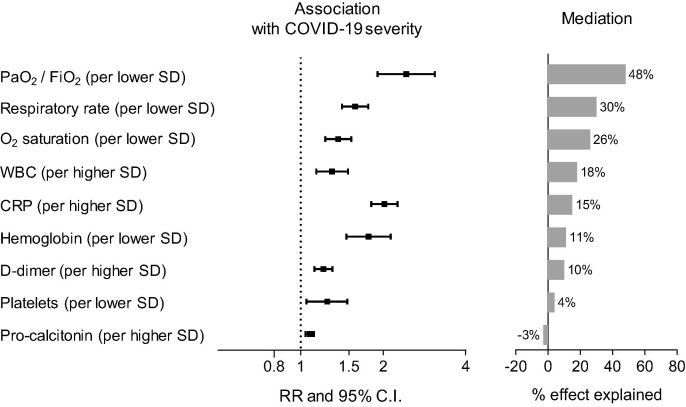
We then evaluated whether these correlations could partially explain (i.e. mediate) the association between FPG and COVID-19 severity outcome. We first identified clinical-laboratory characteristics (recorded at their worst level) significantly associated with COVID-19 severity (independently from age and sex). Then, we estimated what percentage of the effect of FPG on COVID-19 severity was explained by the association between FPG and these variables. As described in Table S4 and represented graphically in Fig. 3 , we found that a decline in respiratory function variables was the major determinant of the detrimental effect of hyperglycemia on COVID-19 severity.
Fig. 3.
Mediators of the effects of hyperglycemia on COVID-19 outcomes. For each candidate mediator, the forest plot shows the association with the primary COVID-19 severity outcome (per each higher or lower SD), along with a histogram of their mediation effect on the association between glucose levels and COVID-19 severity. Mediation is expressed as percentage of the glucose effect on the primary outcome. Further details on the methods are given in the text, whereas full data are presented in Table S4.
4. Discussion
In this study, we found that newly-detected diabetes and admission hyperglycemia were more strongly associated with COVID-19 severity outcome than pre-existing diabetes. A known diabetes status was more common among patients with severe COVID-19, defined by ICU admittance or death. Known diabetes remained associated with poor COVID-19 outcome independently from age and sex, but not when adjusted for other baseline clinical variables, suggesting that such effect was mostly driven by concomitant factors and complications. On the other side, newly-detected diabetes remained associated with COVID-19 severity in fully-adjusted analyses. Upon a formal comparison, the association with the primary outcome was stronger for newly-diagnosed than for pre-existing diabetes.
Admission glucose levels were closely related to most clinical and biochemical parameters of COVID-19 severity collected during hospitalization, including the PaO2/FiO2 ratio. Remarkably, for each 2 mmol/l (36 mg/dl) higher admission glucose, the probability of severe progression significantly increased by about 15% independently from any other clinical-biochemical variable. The association between hyperglycemia and COVID-19 severity was significantly stronger for patients with newly-diagnosed diabetes than for those with pre-existing diabetes.
Our finding is consistent with prior literature indicating that, in patients with acute medical conditions, newly-detected diabetes is a powerful predictor of poor outcomes [16], [23]. This may be at least in part driven by the fact that known diabetes is often associated with manifest organ damage that can be accounted for clinically and statistically [18]. Vice-versa, patients who are unaware of their diabetes status are most of the times unaware of ongoing organ damage too, as their treating physicians are. In addition, such occult organ damage cannot be accounted for in statistical adjustment. Yet, our data argue that newly-diagnosed diabetes was predictive of COVID-19 severity not only because of a possible masked multi-organ frailty, but also by the strong link between glucose levels and the outcome. We performed an analysis to evaluate which of the clinical-laboratory parameters of disease severity were mostly responsible for mediating the effect of hyperglycemia on the outcome. Top results included respiratory parameters and white blood cell count, whereas other markers of inflammation and tissue damage were not among top mediators, suggesting that hyperglycemia drove a worse progression of respiratory failure. Indeed, PaO2/FiO2 directly reflects respiratory failure and drives ICU admittance or mechanical ventilation [24].
Diabetes and hyperglycemia were previously found to cause a form of pulmonary disease known as “diabetic lung”, featuring changes in lung volumes and diffusion capacity [25]. Speculatively, patients with admission hyperglycemia upon hospitalization for COVID-19 might have a poorer outcome because of an underlying subclinical pulmonary remodeling [26]. The diabetic lung is supposed to be driven at least in part by obesity and metabolic syndrome [27], but systemic inflammation and platelet dysfunction have been implicated in hyperglycemic pulmonary microangiopathy [28]. Since inflammatory overactivation and coagulopathy are major features of severe COVID-19 [29], we speculate that hyperglycemia can directly accelerate disease course.
The study has limitations that do not allow us to exclude alternative explanations. First, some clinical variables were not collected for all patients, due to the setting where COVID-19 patients were followed, i.e. strictly isolated wards with limited contacts with health personnel. Absence of data on body mass index (BMI) prevented us from adequately considering the confounding role of obesity [30], [31]. In addition, HbA1c was not available for all patients, potentially biasing the detection of pre-existing hyperglycemia. Although we were able to control for the use of pre-admission use of glucocorticoids, in most cases it was impossible to judge the duration of hyperglycemia prior to hospitalization for COVID-19 in patients without known diabetes. Therefore, whether these patients had undiagnosed diabetes or stress hyperglycemia due to the cytokine storm remains unclear. Despite our attempts to control for major biases, we cannot rule out that residual confounding was driving a spurious non-causal association between hyperglycemia and severe COVID-19. For example, a more severe degree of inflammatory activation before hospitalization could drive both stress hyperglycemia and a subsequent severe disease course, even if hyperglycemia exerted no direct role on disease progression. In other terms, it is impossible to rule out that hyperglycemia is simply a biomarker of a more severe disease [23].
Nonetheless, our study has notable strengths, including the detailed clinical characterization of patients with inclusion of several biochemical inflammatory markers, and the rigorous statistical approach to bias. Furthermore, the mediation analysis allows speculating on the processes that drove poor outcome of COVID-19 patients presenting with admission hyperglycemia.
Finally, we wish to underline that, while this manuscript was under preparation, a study conducted on 453 patients hospitalized for COVID-19 at the Union Hospital in Wuhan (China) found that newly diagnosed diabetes was associated with a higher risk of mortality than known diabetes [32]. Interestingly, patients without known diabetes but presenting with hyperglycemia had the worst outcome. The similarities with our study are striking, providing evidence that COVID-19 presents with the same clinical features in distant countries and different healthcare models. This also markedly reinforces the clinical message that hyperglycemia is a strong prognostic factor for adverse outcomes during COVID-19.
Data availability
Original data are available from the corresponding author at a reasonable request.
Contribution statement
Study design: GPF, MLM, FB, PF, AA. Data collection and analysis: GPF, MLM, BMB, AM, LB, ES, GA, SP, FF, DF, LR, GV, SM, GC, FG, ST, AMC, AV, PF, RV, AA. Manuscript writing: GPF, MLM, FB. Manuscript revision: AM, PF, RV, AA. All authors approved the final version of the manuscript.
Funding
The study was supported by the University of Padova Department of Medicine with a grant from Fondazione CA.RI.PA.RO (COVIDIMED project), Italy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108374.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Rao Kondapally Seshasai S., Kaptoge S., Thompson A., Di Angelantonio E., Gao P., Sarwar N. Diabetes mellitus, fasting glucose, and risk of cause-specific death. New England J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoppini G., Fedeli U., Schievano E., Dauriz M., Targher G., Bonora E. Mortality from infectious diseases in diabetes. Nutrition, Metabolism, Cardiovascular Diseases: NMCD. 2018;28:444–450. doi: 10.1016/j.numecd.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. New England J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Baadani A.M., Elzein F.E., Alhemyadi S.A., Khan O.A., Albenmousa A.H., Idrees M.M. Characteristics and outcome of viral pneumonia caused by influenza and Middle East respiratory syndrome-coronavirus infections: A 4-year experience from a tertiary care center. Ann Thorac Med. 2019;14:179–185. doi: 10.4103/atm.ATM_179_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong K.W., Cheong H.J., Choi W.S., Lee J., Wie S.H., Baek J.H. Clinical courses and outcomes of hospitalized adult patients with seasonal influenza in Korea, 2011–2012: Hospital-based Influenza Morbidity & Mortality (HIMM) surveillance. J Infect Chemother. 2014;20:9–14. doi: 10.1016/j.jiac.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Whyte M.B., Vas P., Heiss C., Feher M.D. The contribution of diabetic micro-angiopathy to adverse outcomes in COVID-19. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q, Zhang X, Jiang F, Hu N, Bimu C, Feng J, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care 2020. [DOI] [PubMed]
- 11.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing Type 2 diabetes. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sardu C., D'Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V. Outcomes in patients with hyperglycemia affected by Covid-19: can we do more on glycemic control? Diabetes Care. 2020 doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potier L., Julla J.B., Roussel R., Boudou P., Gauthier D.C., Ketfi C. COVID-19 symptoms masking inaugural ketoacidosis of type 1 diabetes. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenzen M., Ryden L., Ohrvik J., Bartnik M., Malmberg K., Scholte Op Reimer W. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur Heart J. 2006;27:2969–2974. doi: 10.1093/eurheartj/ehl363. [DOI] [PubMed] [Google Scholar]
- 17.Barzilay J.I., Davis B.R., Bettencourt J., Margolis K.L., Goff D.C., Jr., Black H. Cardiovascular outcomes using doxazosin vs. chlorthalidone for the treatment of hypertension in older adults with and without glucose disorders: a report from the ALLHAT study. J Clin Hypertens (Greenwich) 2004;6:116–125. doi: 10.1111/j.1524-6175.2004.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis T.M., Coleman R.L., Holman R.R. Prognostic significance of silent myocardial infarction in newly diagnosed type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS) 79. Circulation. 2013;127:980–987. doi: 10.1161/CIRCULATIONAHA.112.000908. [DOI] [PubMed] [Google Scholar]
- 19.Marik P.E., Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–756. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4:287–295. doi: 10.6000/1929-6029.2015.04.03.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin D.B. Multiple imputation after 18+ Years. J Am Stat Assoc. 2012;91:473–489. [Google Scholar]
- 23.Umpierrez G.E., Isaacs S.D., Bazargan N., You X., Thaler L.M., Kitabchi A.E. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 24.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA; 2020. [DOI] [PMC free article] [PubMed]
- 25.Fuso L., Pitocco D., Antonelli-Incalzi R. Diabetic lung, an underrated complication from restrictive functional pattern to pulmonary hypertension. Diabetes Metab Res Rev. 2019;35 doi: 10.1002/dmrr.3159. [DOI] [PubMed] [Google Scholar]
- 26.Caruso I., Giorgino F. The diabetic lung: an easy target for SARS-CoV-2? Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3346. e3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiengo A., Fadini G.P., Avogaro A. The metabolic syndrome, diabetes and lung dysfunction. Diabetes Metab. 2008;34:447–454. doi: 10.1016/j.diabet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Jagadapillai R., Rane M.J., Lin X., Roberts A.M., Hoyle G.W., Cai L. Diabetic microvascular disease and pulmonary fibrosis: the contribution of platelets and systemic inflammation. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bello-Chavolla O.Y., Bahena-Lopez J.P., Antonio-Villa N.E., Vargas-Vazquez A., Gonzalez-Diaz A., Marquez-Salinas A. Predicting mortality due to SARS-CoV-2: A mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajifathalian K., Kumar S., Newberry C., Shah S., Fortune B., Krisko T. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York City. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H., Tian S., Chen T., Cui Z., Shi N., Zhong X. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data are available from the corresponding author at a reasonable request.