Abstract
The coronavirus disease 2019 (COVID-19) pandemic has infected millions of people around the globe. The outbreak caused by the novel coronavirus (SARS-CoV-2) poses a great health risk to the public. Therefore, rapid and accurate diagnosis of the virus plays a crucial role in treatment of the disease and saving lives. The current standard method for coronavirus detection is the reverse transcription polymerase chain reaction (RT-PCR) method. However, laboratory-based RT-PCR test for SARS-COV-2 requires complex facilities and elaborate training of operators, thus suffering from limit testing capacity and delayed results. Consequently, isothermal PCR such as loop-mediated isothermal amplification (LAMP) has been emerging as a great alternative to the RT-PCR method. LAMP possesses some fundamental advantages such as amplification at a constant temperature, exclusion of a thermal cycler, a faster test result, and potentially a larger diagnostic capacity, while maintaining similar sensitivity and specificity, thus making it more suitable than the RT-PCR for monitoring a pandemic. Starting with a brief introduction of the working principle of LAMP method, this review summarizes recent progress in LAMP-enabled SARS-CoV-2 viral RNA detection. Lastly, future research directions are discussed. This critical review will motivate biosensor community in furthering the present research, which may pave the road for rapid and large-scale screening of SARS-CoV-2.
Key words: RT-LAMP, COVID-19, Molecular diagnosis, Isothermal amplification, Rapid
1. Introduction
In early December 2019, there was an outbreak of a new virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China. This new virus is what causes coronavirus disease 2019 (COVID-19), the disease that is currently classified as a pandemic. The symptoms of COVID-19 are strikingly similar to other respiratory diseases, and some patients can be asymptomatic [1], so tracking the novel coronavirus is a difficult task. Another challenging aspect of tracing SARS-CoV-2 is the infectivity of the virus, as it is fairly easy for the virus to be transmitted to other humans. As of June 2020, according to the John Hopkins Coronavirus Resource Center, there are over 8 million confirmed COVID-19 cases and about 437,000 deaths, indicating how quickly the virus spreads and how urgently we need to contain the virus. Since the novel coronavirus has no vaccine or cure up to date, there is a drastic need for rapid and sensitive SARS-CoV-2 detection.
A current method for SARS-CoV-2 diagnosis is quantitative reverse transcription polymerase chain reaction (qRT-PCR) test. This analysis detects the presence of virial nucleic acids in nasopharyngeal swab samples with high sensitivity and specificity. The World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) approved the qRT-PCR test as the standard for SARS-CoV-2 detection [2], [3], [4]. However, this assay does have some shortcomings, as the RT-PCR requires complex equipment, extensive training for potential users, and multiple hours to complete the procedure. These limitations are further accentuated by the rapid growth of the pandemic, as the qRT-PCR does not have the screening capacity to keep pace [5]. The other assay widely utilized by the public is COVID-19 serology tests, which detect antibodies or antigen that are associated with the virus infection. These tests are easy to use with rapid results, as well as have minimal expenses, but serology immunoassays lack the necessary accuracy to be a reliable SARS-CoV-2 diagnostic test due to its low sensitivity and high false negative/positive rates. New solutions for COVID-19 detection are in high demand, and one method seems to be the answer - loop-mediated isothermal amplification (LAMP). This novel process is similar to conventional PCR tests, with the exception that the nucleic acid amplification occurs at one temperature, so certain mandatory equipment for PCR, such as a thermal cycler, is not required any more. This unique nucleic acid amplification method endows the LAMP-enabled viral RNA/DNA assay to being quicker, easier to use, and more cost effective than qRT-PCR assays in the scenario of diagnosis. Other advantages of LAMP method include wide pH and temperature ranges that are acceptable, the ability of the assay to accept non-processed samples, and the flexibility of the readout methods, all while maintain specificity and sensitivity that is about equal to that of the PCR tests [6,7].
In this review, we aim at introducing the detection principle of LAMP-based nucleic acid assays and then summarizing different LAMP procedures that have been developed and examined for use in diagnosing SARS-CoV-2. Future trends of LAMP-enabled COVID-19 detection are also discussed. We hope this review could provide general information to researchers who have interests to develop LAMP-based methods for COVID-19 detection, as well as give a better picture on the potential of using the LAMP assays for battling the current pandemic.
2. Working principle of LAMP assay for nucleic acid detection
Loop-mediated isothermal amplification is a nucleic acid amplification technique that is primarily used for diagnosis and detection of diseases. It is a powerful amplification method that is carried out in an isothermal setting, therefore changes in the temperature typically required by conventional PCR is not necessary. Amplification and detection of the nucleic acid could be completed at the same time, in a single step, by incubating the nucleic acid sample, 4 (or 6) specifically designed primers, and Bst DNA polymerase in the same test tube at about 60 to 65 °C, depending on the optimal LAMP temperature [8].
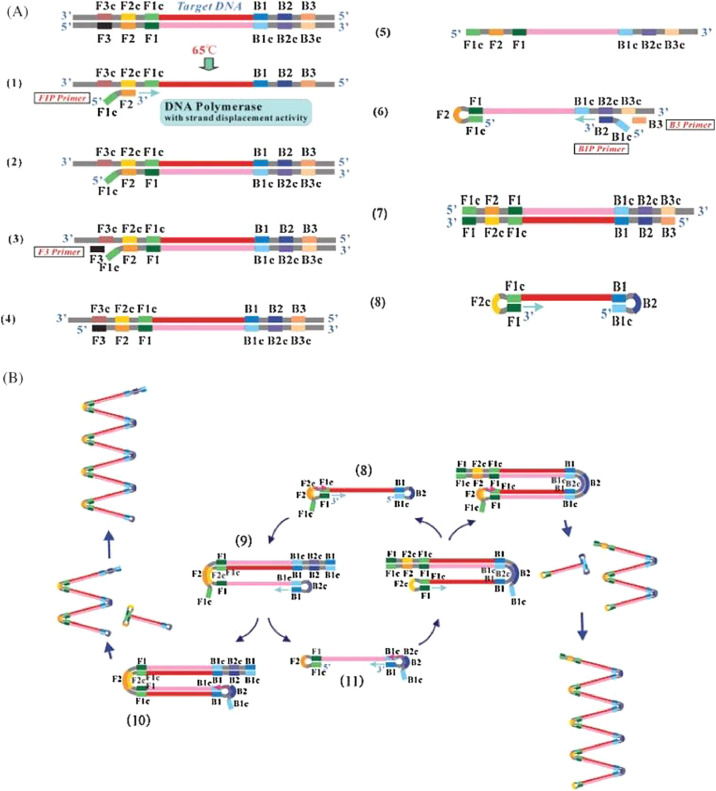
As shown in Fig. 1 , the primer set used for a typical LAMP assay consists of two inner and two outer primers that recognize six distinct regions of the target DNA sequence. The forward inner primer (FIP) consists of the F2 region and a complementary sequence of the F1 (F1c) region, while the backward inner primer (BIP) consists of the B2 region and a complementary sequence of the B1 (B1c) region. The forward outer primer (F3) and backward outer primer (B3) have the sequences that are complementary to the sequences of the F3c and B3c regions, respectively. These regions surround the desired amplified sequence. The primers used for any LAMP assay must be optimized by a series of factors, such as the nucleotide base pair concentration and locations, the distance between the DNA regions, the thermodynamics of the primers, etc. [9].
Fig. 1.
Schematic drawing of the LAMP amplification process [9]. Arrowing from the primer signifies direction of DNA synthesis. The desired sequence for amplification is shown in dark red. A. Initial steps to form dumbbell-like structure. B. Cyclic amplification of LAMP. (Reprinted from ref. 9).
The LAMP amplification process starts with the FIP complexing with the target DNA at its F2 region to form double-stranded DNA, in equilibrium at around 65 ∘C [9]. The DNA polymerase with strand displacement activity then initiates DNA synthesis from the FIP, simultaneously displacing a single strand of DNA, if any. After this initiation step, the F3 primer then binds to its complementary F3c region and displaces the FIP-complementary strand. Because of the F1c sequence in the FIP, the FIP strand is able to self-anneal and form a loop structure at one end of the DNA. This strand then serves as the target for BIP-initiated DNA synthesis and subsequent strand displacement from B3-primed DNA synthesis. This allows the other end of the single DNA strand to form a loop structure, thus resulting in a dumbbell-like DNA structure. It serves as the template for subsequent amplification.
After a dumbbell-like structure formed, exponential amplification of the dumbbell structure is then initiated, with the DNA polymerase starting DNA synthesis at the F1 region. FIP also hybridizes to the one-loop structure from the F2 region, and DNA synthesis of that primer cause the F1-primed strand to be displaced and self-bind into a loop structure. Finally, from the B1 region in the new loop, self-primed DNA synthesis is again started, amplifying the current template while also creating a new one from the displacement of FIP-complementary strand. From this repeating process, it is possible for massive amplification to be achieved, as DNA can be amplified as much as 109 times within an hour [9,10]. To further improve the amplification efficiency, six-primer (loop primer) LAMP is also developed. In LAMP with loop primers, six primers are used instead of four primers, with the forward and backward loop primers (LF and LB) annealing to regions between the F1/F2 and B1/B2 regions, respectively [9]. The loop primers are proven to enhance the LAMP process, as amplification is fastened from the increased starting points for DNA synthesis [10].
To extend LAMP from DNA diagnosis to RNA diagnosis as well as to realize multiplexed detection, several improvements were added to the original LAMP protocol to develop different methods, including reverse transcription LAMP (RT-LAMP) and multiplex LAMP. In RT-LAMP, RNA sequences are detected instead of DNA. Reverse transcriptase is added to the LAMP mixture, to help in the conversion of virial RNA into complementary DNA (cDNA) that will be used for amplification. This procedure has provided great help in diagnosing a multitude of RNA viruses [10]. Multiplexed LAMP assays were developed in order to detect multiple pathogens in the same test tube, with the use of more primers or with unique fluorescent signals [11,12]. All these iterations of the LAMP procedure show how promising it is in the virial diagnostic field. Consequently, it has great potential in SARS-CoV-2 detection, which will be summarized in the subsequent section.
3. Recent progress in RT-LAMP enabled COVID-19 detection
3.1. Rapid detection of COVID-19 with RT-LAMP under 30 min
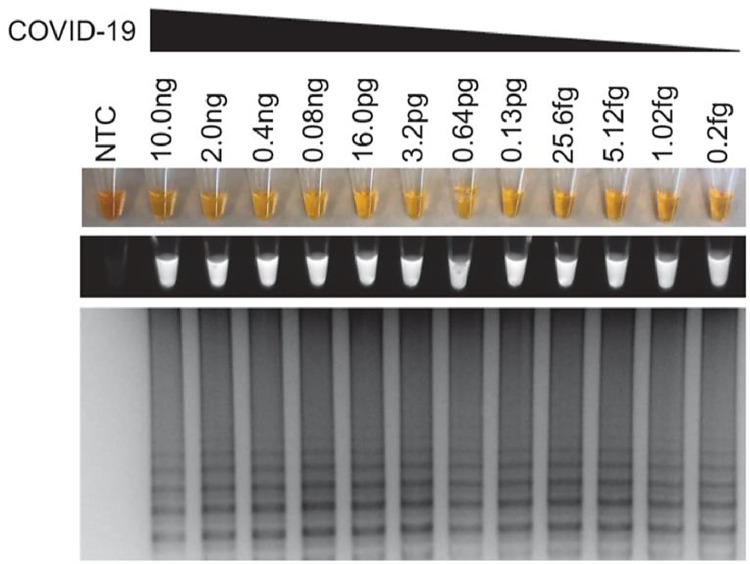
Recently, Lamb et al. [7] reported to develop a rapid screening LAMP test for COVID-19 with an assay time under 30 min. To achieve this goal, the consensus sequences of 23 different identified COVID-19 strains, according to online databases such as GenBank, were identified. The first step to develop the RT-LAMP assay is to choose the target sequences of the virus and design suitable LAMP primers. Using the genetic sequencing of the virus and LAMP Designer 1.15 (Premier Biosoft), RT-LAMP primers were designed to target consensus sequences that were shared among all COVID-19 strains but differed in similarly sequenced viruses, such as the Bat SARS-like coronavirus. Primer-Blast was also conducted against genomes of interests. To validate the developed assay, healthy human samples spiked with an oligonucleotide of GenBank MN908947.3 was employed as “simulated COVID-19 samples”. Similar simulated samples were also prepared for MERS viruses or other coronaviruses tests, without RNA isolation from samples. Readout of the RT-LAMP assay was monitored through three methods: color change, fluorescence, and gel electrophoresis (Fig. 2 ).
Fig. 2.
Sensitivity test for COVID-19 simulated samples. All readout methods are presented (top to bottom: color change, fluorescence under UV light, gel electrophoresis)[7]. (Reprinted from ref. 7).
The procedure was first optimized to run at 63 ∘C for 30 min. The RT-LAMP test was shown to have high specificity towards COVID-19, as no signal was observed in multiple tests using other viruses such as MERS, MHV, and BtCoV. The specificity of LAMP primers is further proven by sequence comparison with the sequences of SARS and other coronaviruses. The primer used had 0% mismatch with all COVID-19 strains, while 27–54% mismatch was observed with other common coronaviruses. Assay sensitivity was also investigated with the limit of detection as low as 1.02 fg/reaction using the simulated samples. Lastly, the process was confirmed to have high clinic utility, as the procedure correctly screened for COVID-19, no matter the spiked sample type. Serum, urine, saliva, oropharyngeal swab, and nasopharyngeal swab samples with spiked COVID-19 sequence were all correctly identified. It shows the potential for rapid diagnosis of COVID-19.
3.2. Rapid RT-LAMP for ORF1ab, S gene and N gene of COVID-19
To develop a fast and simple-to-use COVID-19 test kit, Huang et al. designed 4 sets of LAMP primers that targeted different gene regions of SARS-CoV-2 [8]. Two primer sets (N1 and N15) target the N gene of the virus, while the other two (S17 and O117) target the S gene and ORF1ab gene of SARS-CoV-2, respectively. The ORF1ab gene targeted is close to the 5′-end of the virial RNA, while the N gene is close to the 3′-end. It was hypothesized that detection performance among the different regions may be different, as degradation of the RNA occurs from the 5′-end to the 3′-end during the RNA sampling and extraction process. A fluorescent or colorimetric readout can be used to differentiate the positive and negative samples, but colorimetric is preferred to negate the necessity of specific instruments and to avoid ambiguous readings from the fluorescent signal background. To realize naked-eye based colorimetric detection, a pH-sensitive dye such as phenol red is used for reaction readout, which is cheap and is not difficult to read as our naked-eye can do the job. However, some clinical factors may interfere with the colorimetric readout, such as different samples having different pH levels. Therefore, FIP primers were conjugated with 6-carboxyfluorescein (FAM). This method was proven to be effective and reliable for both fluorescent and colorimetric detection of the developed RT-LAMP.
The specificity of all primers for COVID-19 detection was confirmed to be high, as they do not cross react with human DNA, indicating that these primers can be used in the detection of clinical samples. The accuracy of the tests is acceptable, as the test results are in good agreement with the conventional RT-qPCR. In detecting virial RNA, the N1 primer set performed the best with a sensitivity of 2 RNA copies per reaction of 25 µL in 20 min of reaction time. The N15 and S17 primers have the same detection limit of 2 RNA copies per reaction, but in 30 min of reaction time. The O117 primer set could only detect 20 RNA copies per reaction in 30 min. An additional one-step test using stem cells was administered, in order to investigate if RNA extraction can be omitted from the RT-LAMP based nucleic acid amplification. As observed, 40 min of the RT-LAMP process caused a color change visible to naked eye, indicating that it is possible and feasible to run RT-LAMP without the RNA extraction step. Impressively, both the N15 and O117 primer sets accurately detected all 8 positive and all 8 negative clinical samples, indicating that this developed test can be used to replace the classic RT-PCR tests for COVID-19 detection.
3.3. iLACO assay for COVID-19
Recently, Yu et al. [13,14] have developed an iLACO assay (isothermal LAMP based method for COVID-19) to target on ORF1ab gene for molecular diagnosis of the novel coronavirus. The iLACO is vastly faster than the conventional PCR method due to its high amplification efficiency. The iLACO detection process takes 15–40 min, depending on the virial load in the sample. In this experiment, the optimal primer set was determined from testing as well as software-enabled comparison between the sequences of designed primers and other viruses’ genome. In readout of the amplification step, SYBR green dye was first used for the enhanced fluorescence resulted from the intercalation of dyes in dsDNA. However, in order to increase the sensitivity of fluorescence detection at lower dilutions of viral RNA, GeneFinder dye with blue light was also used (Fig. 3 ).
Fig. 3.

Readout signal of COVID-19 samples with GeneFinder dye under blue light. Left are negative controls and right are positive samples[14]. (Reprinted from ref. 14).
Using synthesized RNA equivalent to COVID-19, the limit of detection of iLACO assay is about 10 copies of the ORF1ab gene, which is on par with Taqman based qPCR assay. In addition, the as-developed iLACO was subject to the challenge of testing 43 positive clinical samples confirmed by RT-qPCR in Shenyang, China. The sensitivity of this diagnostic test is reasonable with 97.6% (42/43) of 43 positive samples being correctly detected except a singular negative. A low viral RNA load may result in the false negative result, which may be attributed to the viral RNA loading below the limit of detection. How the sample amount affected the assay was also investigated. When the sample volume increased from 1 μL to 5 μL, variable results were observed, which may be attributed to the presence of either Tris or EDTA used in the RNA dilution buffer. Therefore, concentration of the RNA buffer would have to be optimized to improve the accuracy. Overall, this study demonstrated that well-designed RT-LAMP primer set possess high specificity and can generate high sensitivity and high accuracy in COVID-19 clinical sample test.
3.4. One-pot RT-LAMP assay for COVID-19
One-pot RT-LAMP assays were also developed in order to rapidly detect the N gene of COVID-19 with simplicity [15]. One-pot assay has all the procedures occur in one tube and there is no RNA extraction step. Due to lack of RNA extraction, the risk of infection from the RNA of COVID-19 is mitigated and the procedure is simplified. Two different one-pot assays are tested at the optimized temperature of 59 ∘C: a one-pot real-time RT-LAMP assay and a one-pot visual RT-LAMP assay. The real-time fluorescence signal detection was accomplished using a StepOne™ System, while the end-point visual color changes was realized by using color indicator.
As the RNA extraction was omitted and only the plasmid containing N gene was used in the study, it was difficult to evaluate how inhibitors in the samples would affect the amplification of the viral gene. To study the effect of inhibitors in blood samples on RT-LAMP amplification effectivity, different volume of blood samples was dissolved into 10 µL 2019-nCoV-Fast-Sample Nucleic Acid Releasing Agent and then added to RT-LAMP reaction system for amplification. It was concluded that it was best to use 5 μL of blood sample volume for a 50 μL reaction, as it is the highest sample volume added that still showed acceptable amplification of nucleic acid. The detection limit of both assays was found to be 6 copies of nucleic acid (~0.2 fg), and the LAMP assay seems to be very stable, as strikingly similar plots were collected for experiments conducted a month apart, indicating good robustness and reproducibility.
3.5. Mismatch-Tolerant RT-LAMP technique
In order to increase the quality and reliability of the detection results, one way was in implementation of mismatch-tolerant LAMP assay, which can be realized through the addition of 0.15 U of high-fidelity DNA polymerase [16]. This DNA polymerase prevents making and cultivating sequencing mistakes by removing mismatching base pairs, increasing the efficiency and sensitivity of the RT-LAMP process. In this study, the RdRp primers showed higher amplification efficiency, thus being selected for subsequent mismatch-tolerant RT-LAMP assay for COVID-19 viral RNA. For readout of results, a pH-sensitive indicator dye called cresol red was used to indicate that the ORF1ab virial gene was successful amplified due to a significant pH change associated with LAMP amplification.
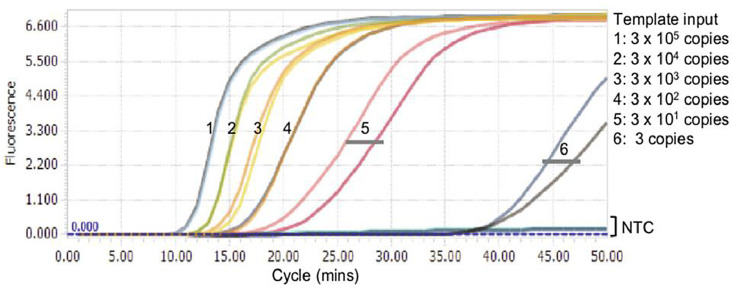
The sensitivity and specificity of this mismatch-tolerant RT-LAMP assay for SARS-CoV-2 seems promising. In the specificity test with COVID-19 and 17 common human respiratory viruses, only the sample collected from a COVID-19 patient had a distinct amplification signal. The sensitivity also seems great for the as-developed colorimetric assay, as 30 RNA copies of the viral RdRp fragment per reaction can be detected by naked eye in 40 min, though as low as 3 copies can be detected at around 50 min (Fig. 4 ). When compared to a commercial RT-qPCR assay with 24 clinical samples, the novel RT-LAMP assay showed 100% consistency, validating that this assay can detect SARS-CoV-2 RNA as reliably as RT-PCR method.
Fig. 4.
Sensitivity results for mismatch-tolerant RT-LAMP process[16]. (Reprinted from ref. 16).
3.6. Barcoded RT-LAMP (LAMP-Seq) for COVID-19
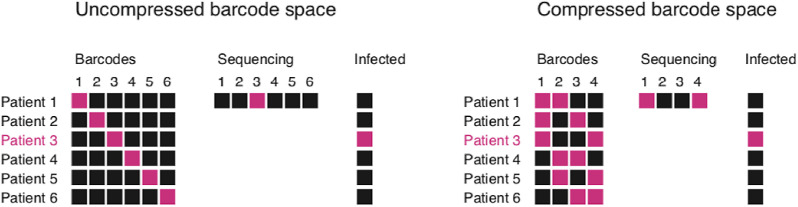
It has been suggested that population scale testing can help contain COVID-19 pandemic through identification and quarantine of infected persons. However, current COVID-19 detection methods such as RT-PCR do not have such capability. In order to detect the SARS-CoV-2 on a large scale, a barcoded RT-LAMP procedure called LAMP-Seq was developed [5]. In a typical detection protocol, a swab sample is first added to a RT-LAMP reaction, which contains the 6 different LAMP primers targeting the N gene. The forward inner primer (FIP) is barcoded with a compressed barcode space of 10 nucleotides, in order to label the specific sample for identification (Fig. 5 ). The results indicate that the presence of a 10-nt barcode inserted into the FIP primers does not have effects on the detection performance such as sensitivity and the amount of amplification product. Typically, a singular barcode is used, but in the barcode space, there can be one to five barcodes, so identification can be done with the number of unique barcodes that exist being kept minimum. After the RT-LAMP amplification is conducted for 30 min at 65 ∘C, the products are heated for 10 min at 95 ∘C to terminate the reaction. Then, the sample is pooled into batches of 1000–10,000 samples. For each pool, two 12-cycle PCR tests with specific barcoding primers are conducted, to amplify the amount of SARS-CoV-2 DNA as well as label each individual batch with a unique barcode sequence. Finally, the products are sequenced, and the barcodes associated with the correct viral sequence is determined computationally, to identify the positive patient.
Fig. 5.
Barcoding concept for LAMP-Seq procedure[5]. (Reprinted from ref. 5).
There is no clinical data available, as the final performance analysis of this test was done computationally. The authors assessed this protocol by interpreting the barcoding system as a modified Bloom filter, a probabilistic data structure that identifies elements in a group. Parameters that were optimized include the number of unique barcodes, batch size, number of barcodes per patient, and the probability of false positives/negatives, while also factoring in possible error in either adding or reading the barcodes. In doing this, it was concluded that if at most 1.3% of the population is infected, then using 5 barcodes per sample and detecting 3 barcodes, while splitting samples into 10 pools of 10,000 samples each, has a false-negative and false-positive rate below 0.2% with an estimated cost of less than $7 per sample, which may boost the screening capacity significantly.
3.7. Sample inactivation & purification for RT-LAMP based COVID-19 detection
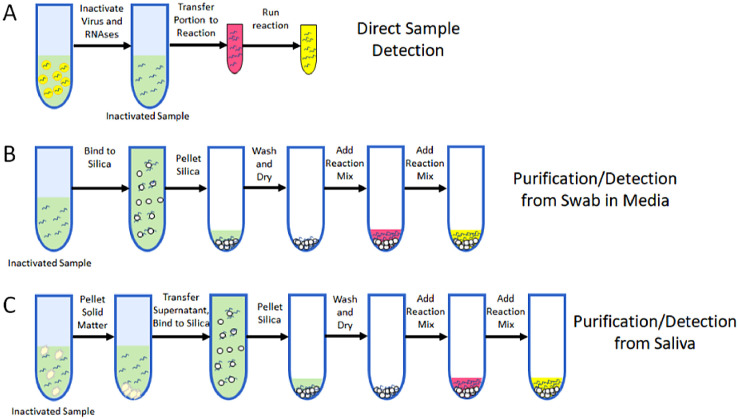
To achieve highly reliable detection of COVID-19, the quality of viral RNA is of paramount importance. Therefore, unique sample preparation steps can be added to LAMP assays [17], in order to increase sensitivity of the assay, while also increasing the stability of the virial RNA samples and lowering the risks of handling the infectious virus (Fig. 6 ). This is accomplished by disintegrating virions, releasing viral RNA, and deactivating ribonucleases (RNase) which catalyze RNA degradation. In a study by Rabe and Cepko, a lysis buffer to lyse virions, typically containing detergent (Tween20 or TritionX100) or salts such as sodium iodide (NaI) or guanidinium thiocyanate (GuSCN), can be integrated into the RT-LAMP reaction protocol. An inactivation step was added to deactivate RNase activity by adding a mixture of TCEP and EDTA to a 5 μL sample, followed by thermal treatment at 95 ∘C for 5 min before the RT-LAMP reaction. A purification procedure was simultaneously investigated to aid in assay sensitivity. This procedure included a silica particle suspension called “glass milk” that binds to RNA and DNA in the presence of the aforementioned salts, thus purifying the nucleic acids, which can be re-suspended in RT-LAMP reaction mixture for subsequent amplification. Color change and fluorescent readout were used to collect results.
Fig. 6.
Diagram of purification protocol, with colorimetric readout[17]. A) Testing without purification B) Purification from swab sample C) Purification from saliva sample. (Reprinted from ref. 17).
In assessing the sensitivity of using different primer sets, it was found that novel primer sets developed in this study (HMS Assay 1 and HMS Assay 1e) worked the best. While the acronym of HMS is not defined, the difference between the assays stem from modification to the forward and backward inner primers. At the level of 100 and 200 viral RNA copies in each reaction, HMS Assay 1 detected 26/40 and 36/40, respectively while HMS Assay 1e detected 31/40 and 39/40, respectively. All reactions with positive samples show obvious color change from orange to yellow. Also the presence or absence of 50 mM CuSCN makes no difference in sensitivity.
For inactivation of the virions, it was first found that the RT-LAMP reaction is tolerant of the detergents such as Tween 20 and TritinX100, as amplification can be detected for both detergents up to 3%. It was further found that chaotropic salt tolerance up to 50 mM for CuSCN or at least 50 mM for NaI was the threshold to achieve significant amplification. When this buffer was used to compare primer sets, the HMS Assay 1e showed the greatest sensitivity. Use of the inactivation protocol helped reliably improve the Assay 1e sensitivity from 100 copies/μL to 50 copies/μL, and the use of the purification protocol lowered the sensitivity to an astonishing 1 copy/μL. Finally, RNase activity was confirmed to be inhibited after thermal treatment, as inactivated samples that were spiked with RNA showed positive results.
3.8. Penn-RAMP for COVID-19
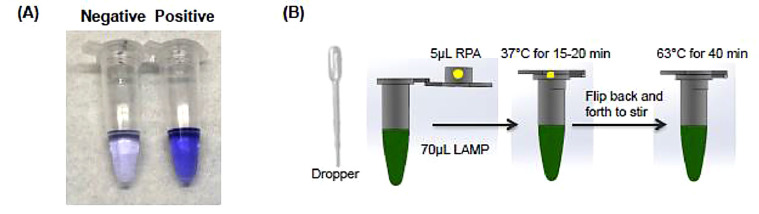
Other isothermal amplification methods can also be combined with the LAMP process for COVID-19 detection with enhanced sensitivity. Recently, a novel single and two-stage isothermal amplification assay, namely Penn-RAMP, was developed to target the ORF1ab gene of viral RNA with enhanced performance [6]. In the Penn-RAMP method as shown in Fig. 7 , first a recombinase polymerase amplification (RPA) process is conducted in the cap of a test tube at 38 ∘C. During this RPA process, an enzyme called a recombinase assists the F3 and B3 LAMP primers in locating the targeted sequence of the sample. After 15–20 min of amplification, the RPA mixture is blended into LAMP reaction reagents that were pre-loaded in the test tube, either through centrifuge or flipping the tube over. Inhibition of the LAMP reaction from the RPA contents are prevented by maintaining a volume ratio of 1:9 for RPA to LAMP mixtures. After mixing, a LAMP procedure was conducted at 63 ∘C for 40 min.
Fig. 7.
Visual detection after Penn-LAMP (A) and schematic drawing of Penn-RAMP process (B)[6]. (Reprinted from ref. 6).
In comparison of the three methods (COVID-19 Penn-RAMP, COVID-19 LAMP, and COVID-19 RT-PCR) using synthetic COVID-19 sequences, it was observed that the novel Penn-RAMP method is the best diagnostic procedure. First, unlike RT-PCR tests and similar to RT-LAMP assays, it is conducted at isothermal conditions, so the overall costs of operation are reduced. Furthermore, the Penn-RAMP assay has the greatest sensitivity. The detection limit of the novel Penn-RAMP assay is 7 copies/reaction, while both COVID-19 RT-PCR and COVID-19 LAMP methods have a detection limit of 70 copies/reaction. This higher sensitivity is attributed to the ability of Penn-RAMP to possess the advantages of both RPA and LAMP process while overcoming their disadvantages, such as RPA's high tolerance to inhibitors while overcoming RPA's tendency to produce spurious amplicons, as well as LAMP's high specificity.
As the authors did not have access to COVID-19 clinical samples, the Penn-RAMP assay was re-designed and tested using inactivated HIV particles for the purpose of proof-of-concept. The results show that Penn-RAMP also has the best clinical utility, as the sensitivity did not change when using healthy swab samples spiked with inactivated HIV particles. As a comparison, the RT-LAMP and RT-PCR tests had a detection limit of 700 copies/reaction for the unprocessed samples, indicating the robustness of the Penn-RAMP assay. The specificity of the assay was also confirmed to be high, as no signal was detected in any LAMP or Penn-RAMP assay with different coronaviruses (IBV, PDCoV, PEDV, and TGEV) available in their lab. This study offers a potential method for COVID-19 detection with reduced false negative results.
3.9. Integrated RT-LAMP and CRISPR-Cas12 method for COVID-19 detection
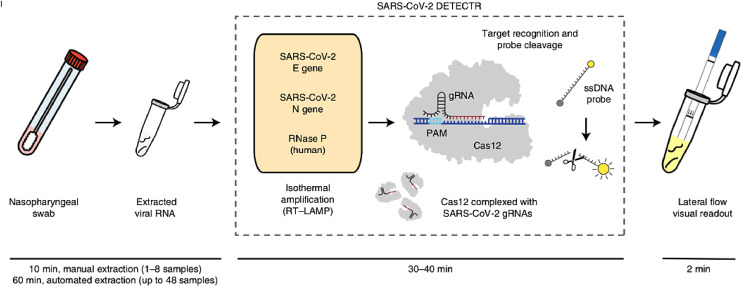
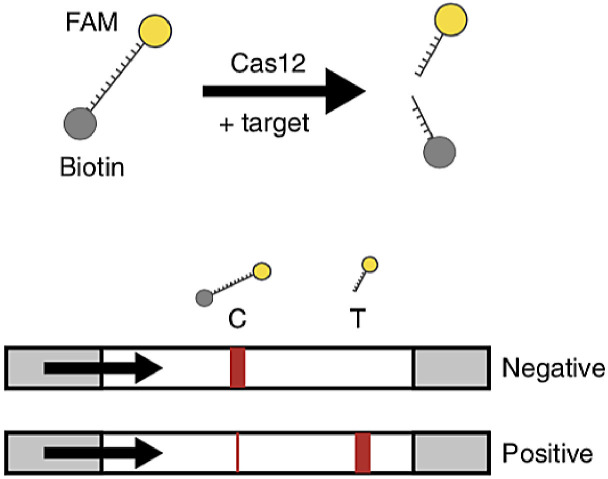
Another COVID-19 detection scheme is to combine the RT-LAMP amplification with a CRISPR-Cas12 diagnostic method [2], as shown in Fig. 8 . This novel CRISPR-enabled detection method, called SARS-CoV-2 DNA Endonuclease-Targeted CRISPR Trans Reporter (DETECTR), utilizes a Cas12 enzyme after an RT-LAMP procedure to detect a specific E gene and N gene sequence in the amplified virus RNA and indiscriminately cleave nearby structures once complexed. The N gene-targeted DETECTR assay only detects the SARS-CoV-2 virus, while the E gene-targeted assay detected SARS-CoV-2, SARS-CoV, and bat-SL-CoVZC45 viruses. The RNase P gene was used as a control. Visualization of the assay is achieved using a FAM-biotin reporter molecule and lateral flow strips designed to capture labeled nucleic acids (Fig. 9 ). Uncleaved reporter was captured at the control line while the Cas12 cleavage activity results in a visible band at the test line. The combination of both RT-LAMP and CRISPR-Cas12 technology improves on nucleic acid diagnostics, from the lack of complex equipment required, the extremely high specificity, and the quick versatility. The use of different guide RNAs for the Cas12 enzyme can also lead to accurate multiplexing experiments.
Fig. 8.
Diagram of RT-LAMP and CRISPR-Cas12 based COVID-19 detection[2]. (Reprinted from ref. 2).
Fig. 9.
Illustration of the FAM-biotin reporter used in SARS-CoV-2 DNA Endonuclease-Targeted CRISPR Trans Reporter (DETECTR) in conjunction with lateral flow detection platform [2]. When not cleaved, the reporter will bind to the control line in the LFA, confirming the absence of the target gene. (Reprinted from ref. 2).
Comparison of DETECTR methods using a fluorescence plate reader and a lateral flow test strip indicates that the fluorescent detection method was faster with time to result (TTR) after RT-LAMP amplification, as fluorescence signal was distinguishable in less than a minute, instead of in five minutes using the lateral flow strip. Both methods are in good agreement with each other, as 23 of 24 samples have similar results. There is also high agreement with the approved qRT-PCR method, as 95% of the positive predictive agreement and 100% of the negative predictive agreement results from 83 respiratory swab samples were obtained between the CDC qRT-PCR assay and the developed DETECTR assay. Matrix effects were defined, as increasing the concentration of the raw sample buffer (≥10% UTM or ≥ PBS by volume) results in the limit of detection to increase from the 10 copies/μL to 15,000 and 500 copies per μL, respectively. This study provides a new avenue to rapidly detect COVID-19 clinical samples in 30 to 40 min through the combination of RT-LAMP technology with CRISPR–Cas12 DETECTR technology.
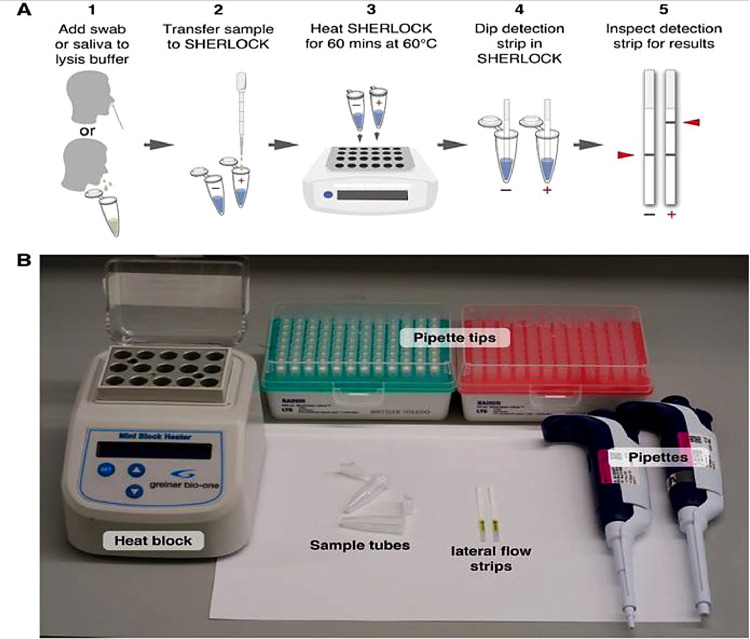
3.10. SHERLOCK with integrated RT-LAMP for COVID-19 detection
In furthering the integration of CRISPR detection with LAMP as an one-pot process, a novel, unpublished testing method for COVID-19 was developed [18]. This process, named STOPCovid (SHERLOCK testing in one pot for COVID-19), includes less complexity and sample handling steps than the typical SHERLOCK method, as well as eliminating the RNA extraction step (Fig. 10 ). The integrated RT-LAMP and SHERLOCK (specific high sensitivity enzymatic reporter unlocking) process consists of isothermal amplification of virial RNA and sequential detection of amplicon from CRISPR-mediated reporter cleaving. After the saliva or nasopharyngeal sample is collected for the STOPCovid test, lysis of the virial sample is carried out in a commercial lysis buffer at either 60 ∘C or room temperature for 10 min. Before RT-LAMP amplification, proteinase K, a compound that inhibits SHERLOCK reactions in lysis buffer, was either inactivated using proteinase K inhibitor or thermal deactivation at 95 °C for 5 min. After that, the STOPCovid reaction was conducted at 60 ∘C for an hour. A lateral flow strip is finally added to the tube for two minutes, in order to detect whether the reporter in the system was cleaved or not. For fluorescent readings, a fluorescent reporter is used instead of using a lateral flow test strip.
Fig. 10.
The overview of STOPCovid method. [18] (Reprinted from ref. 18).
The optimal LAMP N-gene primer set, Cas12 enzyme (AapCas12b), and guide RNA (AacCas12b-based) for SARS-CoV-2 detection were identified by comparing fluorescence results of healthy samples spiked with virial RNA. The STOPCovid assay possesses high stability and specificity, as reagent functionality remained after multiple freezing cycles and no cross reactivity is observed with the genomes of other coronaviruses (SARS-CoV and MERS-CoV). The limit of detection for SARS-CoV-2 in the lateral flow STOPCovid assay is 100 copies per reaction, which was confirmed with 30 trials of the test. With clinical samples, the STOPCovid assay reliably detected the virus, as 3 out of 3 replicates for 11/12 patient samples, and at least 2 out of 3 replicates for 12/12 patient samples were correctly diagnosed. In addition, all 5 negative samples in the test returned as negatives, demonstrating the accuracy and precision of this test for clinical samples. This study further shows how the combination of different existing diagnostic methods can help in monitoring the novel coronavirus.
As a comparison, Table 1 summarize some critical information of the reviewed RT-LAMP enabled COVID-19 nucleic acid detection papers, which can provide a clearer picture on the recent progress of RT-LAMP assays for COVID-19.
Table 1.
Comparison of different RT-LAMP enabled COVID-19 detection.
| Method | Enzymes/Master Mixes | LAMP primers | LAMP primer targeting gene (N, S, ORF, RdRp, etc.) | Sensitivity | Reference |
|---|---|---|---|---|---|
| RT-LAMP | Bst 2.0 DNA Polymerase WarmStart Reverse Transcriptase |
F3: TCCAGATGAGGATGAAGAAGA B3: AGTCTGAACAACTGGTGTAAG FIP: AGAGCAGCAGAAGTGGCACAGGTGATTGTGAAGAAGAAGAG BIP: TCAACCTGAAGAAGAGCAAGAACTGATTGTCCTCACTGCC LF: CTCATATTGAGTTGATGGCTCA LB: ACAAACTGTTGGTCAACAAGAC |
N gene (speculated from the nucleotide position in the paper) | 1.02 fg/reaction | 7 |
| iLACO | Super- Script® III Reverse Transcriptase First-Strand Synthesis System WarmStart Colormetric LAMP 2X Master Mix |
F3: CCACTAGAGGAGCTACTGTA B3: TGACAAGCTACAACACGT FIP: AGGTGAGGGTTTTCTACATCACTATATTGGAACAAGCAAATTCTATGG BIP: ATGGGTTGGGATTATCCTAAATGTGTGCGAGCAAGAACAAGTG LF: CAGTTTTTAACATGTTGTGCCAACC LB: TAGAGCCATGCCTAACATGCT |
ORF1ab gene | 10 copies/µL | 13, 14 |
| One-pot RT-LAMP | Bst 3.0 DNA/RNA Polymerase |
F3: GCCAAAAGGCTTCTACGCA B3: TTTGGCCTTGTTGTTGTTGG FIP: TCCCCTACTGCTGCCTGGAGTTTTCGGCAGTCAAGCCTCTTC BIP: TCCTGCTAGAATGGCTGGCAATTTTTTTTGCTCTCAAGCTGGTTCA LF: CGACTACGTGATGAGGAACGA LB: GCGGTGATGCTGCTCT |
N gene | 6 copies/μL | 15 |
| Mismatch-tolerant RT-LAMP | WarmStart Reverse Transcriptase Q5® High-Fidelity DNA Polymerase |
F3: TTTGCATTACAGTAGGGATGA B3: TGACAAATATCACTACATCTTTTGG FIP: GCATCGACCACAGAGATAGACACTCAAATGAATCTTAAGTATGCCA BIP: ATAGCCGCCACTAGAGGAGCGCAAATTCTATGGTGGTTGG LF: TAGTGCAAAGAATAGAGCTCGCAC LB: TACTGTAGTAATTGGAACAA |
RdRP in ORF1ab gene | 30 copies/reaction (40 min) 3 copies/reaction (50 min) |
16 |
| LAMP-Seq | Bst 3.0 DNA Polymerase NEBNext 2x Master Mix |
F3: TGGCTACTACCGAAGAGCT B3: TGCAGCATTGTTAGCAGGAT FIP: TCTGGCCCAGTTCCTAGGTAGTNNNNNNNNNNCCAGACGAATTCGTGGTGG BIP: AGACGGCATCATATGGGTTGCACGGGTGCCAATGTGATCT LF: GGACTGAGATCTTTCATTTTACCGT LB: ACTGAGGGAGCCTTGAATACA |
N gene | N/A | 5 |
| RT-LAMP plus sample inactivation (A1) | WarmStart LAMP Kit |
F3: CGGTGGACAAATTGTCAC B3: CTTCTCTGGATTTAACACACTT FIP: TCAGCACACAAAGCCAAAAATTTATCTGTGCAAAGGAAATTAAGGAG BIP: TATTGGTGGAGCTAAACTTAAAGCCCTGTACAATCCCTTTGAGTG LF: TTACAAGCTTAAAGAATGTCTGAACACT LB: TTGAATTTAGGTGAAACATTTGTCACG |
ORF1ab gene | 50 copies/µL | 17 |
| RT-LAMP plus sample inactivation and purification (A1e) | WarmStart LAMP Kit |
F3: Same as A1 B3: Same as A1 FIP: TCAGCACACAAAGCCAAAAATTTATTTTTCTGTGCAAAGGAAATTAAGGAG BIP: TATTGGTGGAGCTAAACTTAAAGCCTTTTCTGTACAATCCCTTTGAGTG LF: Same as A1 LB: Same as A1 |
ORF1ab gene | 1 copy/µL | 17 |
| Penn-RAMP | Isothermal Master Mix (ISO-001, OptiGene) |
F3: TGCTTCAGTCAGCTGATG B3: TTAAATTGTCATCTTCGTCCTT FIP: TCAGTACTAGTGCCTGTGCCCACAATCGTTTTTAAACGGGT BIP: TCGTATACAGGGCTTTTGACATCTATCTTGGAAGCGACAACAA LF: CTGCACTTACACCGCAA LB: GTAGCTGGTTTTGCTAAATTCC |
ORF1ab gene | 7 copies/reaction | 6 |
| RT-LAMP + CRISPR-Cas12 | Bst 2.0 DNA Polymerase WarmStart Reverse Transcriptase |
F3: AACACAAGCTTTCGGCAG B3: GAAATTTGGATCTTTGTCATCC FIP: CGCATTGGCATGGAAGTCACTTTGATGGCACCTGTGTAG BIP: TGCGGCCAATGTTTGTAATCAGCCAAGGAAATTTTGGGGAC LF: TTCCTTGTCTGATTAGTTC LB: ACCTTCGGGAACGTGGTT |
N gene | 10 copies/ μL | 2 |
| STOPCovid | Bst 2.0 WarmStart DNA Polymerase WarmStart Reverse Transcriptase |
F3: GCTGCTGAGGCTTCTAAG B3: GCGTCAATATGCTTATTCAGC FIP: GCGGCCAATGTTTGTAATCAGTAGACGTGGTCCAGAACAA BIP: TCAGCGTTCTTCGGAATGTCGCTGTGTAGGTCAACCACG LF: CCTTGTCTGATTAGTTCCTGGT LB: TGGCATGGAAGTCACACC |
N gene | 100 copies/reaction | 18 |
| Rapid RT-LAMP (N1) | WarmStart Colormetric LAMP 2x Master Mix (DNA & RNA) |
F3: TGGACCCCAAAATCAGCG B3: GCCTTGTCCTCGAGGGAAT FIP: CCACTGCGTTCTCCATTCTGGTAAATGCACCCCGCATTACG BIP: CGCGATCAAAACAACGTCGGCCCTTGCCATGTTGAGTGAGA LF: TGAATCTGAGGGTCCACCAAA LB: GGTTTACCCAATAATACTGCGTCTT |
N gene | 2 copies/reaction (20 min) | 8 |
| Rapid RT-LAMP (N15) | WarmStart Colormetric LAMP 2x Master Mix (DNA & RNA) |
F3: AGATCACATTGGCACCCG B3: CCATTGCCAGCCATTCTAGC FIP: TGCTCCCTTCTGCGTAGAAGCCAATGCTGCAATCGTGCTAC BIP: GGCGGCAGTCAAGCCTCTTCCCTACTGCTGCCTGGAGTT LF: GCAATGTTGTTCCTTGAGGAAGTT LB: GTTCCTCATCACGTAGTCGCAACA |
N gene | 2 copies/reaction (30 min) | 8 |
| Rapid RT-LAMP (S17) | WarmStart Colormetric LAMP 2x Master Mix (DNA & RNA) |
F3: TCTTTCACACGTGGTGTT B3: GTACCAAAAATCCAGCCTC FIP: CATGGAACCAAGTAACATTGGAAAACCTGACAAAGTTTTCAGATCC BIP: CTCTGGGACCAATGGTACTAAGAGGACTTCTCAGTGGAAGCA LF: GAAAGGTAAGAACAAGTCCTGAGT LB: CTGTCCTACCATTTAATGATGGTGT |
S gene | 2 copies/reaction (30 min) | 8 |
| Rapid RT-LAMP (O117) | WarmStart Colormetric LAMP 2x Master Mix (DNA & RNA) |
F3: CCCCAAAATGCTGTTGTT B3: TAGCACGTGGAACCCAAT FIP: GGTTTTCAAGCCAGATTCATTATGGATGTCACAATTCAGAAGTAGGA BIP: TCTTCGTAAGGGTGGTCGCAGCACACTTGTTATGGCAAC LF: TCGGCAAGACTATGCTCAGG LB: TTGCCTTTGGAGGCTGTGT |
ORF1ab gene | 20 copies/reaction (30 min) | 8 |
Conclusion and future trends
Rapid, low-cost, and user-friendly molecular diagnostic methods are prerequisite to address the outbreaks of infectious diseases. Especially during the outbreak of COVID-19 pandemic, there is an urgent need to build the global testing capacity up to 100-fold above what is achievable with current standard approaches [19]. LAMP is an innovative gene amplification technique that shows great promise as a detection tool, especially during this coronavirus pandemic. Here, we summarize recent progress in the RT-LAMP assays for rapid and accurate detection of SARS-CoV-2 nucleic acids. Even though false results are still present, the advantages of using the LAMP process are still immense. One major benefit is the speed of the analysis, as the confirmation of results for the typical RT-LAMP procedure is faster than that of the RT-PCR, by a wide margin [9]. Omission of a denaturing step and the integration of the amplification and detection step help in making the assay be conducted with faster time to results (TTR). Another benefit is the overall ease of use, as the isothermal characteristic of the amplification test ensures that simple and low-cost equipment may be used. The isothermal condition also helps the LAMP to have high amplification efficiency, from the lack of time loss due to thermal cycling in conventional RT-PCR [10]. Lastly, the readout for the results are also relatively simple, as either turbidity or a pH dye can be used to easily observe amplification by the naked eye. The procedure's speed, simplicity, and cost-effectiveness lend it to be a logical candidate to monitor spread of the SARS-CoV-2 virus, as mass amounts of people can swiftly use it, with relative ease. Due to aforementioned advantages, this assay can be deployed as a great point-of-care test, filling a role that is vital to track the virus spread.
Besides improvement of sensitivity and specificity in LAMP primers design, to further endow this technique for large-scale screening of COVID-19 and battling the pandemic, one potential trend is to develop “true” one-step, closed-tube isothermal PCR assay, which only requires the end-user to swirl sampling swab into the reaction mixture, followed by a close-tube amplification at a constant temperature. Thus, it may allow the detection of COVID-19 at residential homes with unlimited detection capacity. Recently, we investigated the feasibility to run one-step, closed-tube RT-LAMP for RNA detection at home [19]. By employing kitchen Range Oven's “Keep Warm” function as the heating source, RT-LAMP can be successfully conducted in kitchen Range Oven without sacrificing the detection capability and performance. Furthermore, by pre-loading the concentrated RT-LAMP reaction reagents and sample collection solution to the cap cavity and the bottom of a PCR tube, respectively, a potential one-step, closed-tube RT-LAMP was realized in an oven, which may allow us to develop a home-use assay only requiring the end-user to open the tube once for loading swab sample at the beginning. It is worth noting that recently, the U.S. Food and Drug Administration (FDA) authorized the first coronavirus sample collection tube from LabCorp that lets people collect a sample at home, thus strongly supporting the feasibility of all-in-one RT-LAMP test tube concept with the loading of swab sample by the end-users. To achieve such “true” one-step, closed-tube detection for virus particles with high sensitivity, a lysis solution is also typically required to lyse viruses, destroy RNase, and release viral RNA. Currently proteinase K containing lysis buffer is commercially available for lysis of virus with high efficiency. However, after virus lysis, the lysis buffer requires a high-temperature treatment step (e.g., 95 °C for 5 to 10 min) to deactivate proteinase K (typically thermal stable). Otherwise, proteinase K can digest enzymes such as reverse transcriptase/DNA polymerase in RT-LAMP reagents, thus significantly interfering with subsequent isothermal amplification. However, the enzymes used in RT-LAMP reaction will be deactivated at such high temperature as well. To combine these two steps (enzymatic lysis and RT-LAMP reaction) in a “true” one-tube platform which can be operated at residential homes, a potential solution is to use thermal-labile proteinase K (e.g., from New England Biolabs) in lysis solution, which is inactivated at 55 °C (a temperature lower than RT-LAMP temperature), thus allowing “all-in-one” RT-LAMP feasible. Considering the desperate need of increasing the screening capacity for containing COVID-19 pandemic, such home-use RT-LAMP methodology offers a new avenue in molecular diagnosis. As all operations are simple with minimum involvement of end-users at residential homes without the need of skilled personnel, the testing capacity is unlimited and has the potential to screen a large population in short time. Such home-based molecular diagnosis concept is also appropriate to the detection of any other infectious disease by using the corresponding LMAP primer set. Therefore, it could play an important role in combatting the outbreaks of pandemics nowadays as well as in future. We strongly believe that home-based molecular diagnosis will become truly affordable tests in the near future, and it will not only boost the detection capacity, but also significantly reduce the cost associated with the diagnosis, thus helping in the fight against the coronavirus.
Declaration of Competing Interest
There is no Conflict of Interest to declare.
Acknowledgment
We greatly appreciate the support from the University of Connecticut.
Footnotes
This article is handled by one Associate Editor.
References
- 1.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J.B., Chan W.C.W., COVID-19 Diagnosing. The disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 2.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020 doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. Rna. 2020 doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen M., Zhou Y., Ye J., Abdullah AL-maskri A.A., Kang Y., Zeng S., Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020;10:97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid-Burgk J.L., Li D., Feldman D., Slabicki M., Borrajo J., Strecker J., Cleary B., Regev A., Zhang F. LAMP-Seq: population-Scale COVID-19 diagnostics using a compressed barcode space. BioRxiv. 2020 doi: 10.1101/2020.04.06.025635. [DOI] [Google Scholar]
- 6.El-Tholoth M., Bau H.H., Song J. A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry. ChemRxiv. 2020 doi: 10.26434/CHEMRXIV.11860137.V1. [DOI] [Google Scholar]
- 7.Lamb L.E., Bartolone S.N., Ward E., Chancellor M.B. Rapid detection of novel coronavirus (COVID19) by reverse transcription-loop-mediated isothermal amplification. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3539654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W.E., Lim B., Hsu C.C., Xiong D., Wu W., Yu Y., Jia H., Wang Y., Zeng Y., Ji M., Chang H., Zhang X., Wang H., Cui Z. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020 doi: 10.1111/1751-7915.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parida M.M., Sannarangaiah S., Dash P.K., Rao P.V.L., Morita K. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008 doi: 10.1002/rmv.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahoo P.R., Sethy K., Mohapatra S., Panda D. Loop mediated isothermal amplification: an innovative gene amplification technique for animal diseases. Vet. World. 2016;9:465–469. doi: 10.14202/vetworld.2016.465-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foo P.C., Chan Y.Y., Mohamed M., Wong W.K., Nurul Najian A.B., Lim B.H. Development of a thermostabilised triplex LAMP assay with dry-reagent four target lateral flow dipstick for detection of Entamoeba histolytica and non-pathogenic Entamoeba spp. Anal. Chim. Acta. 2017 doi: 10.1016/j.aca.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Mahony J., Chong S., Bulir D., Ruyter A., Mwawasi K., Waltho D. Multiplex loop-mediated isothermal amplification (M-LAMP) assay for the detection of influenza A/H1, A/H3 and influenza B can provide a specimen-to-result diagnosis in 40min with single genome copy sensitivity. J. Clin. Virol. 2013 doi: 10.1016/j.jcv.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Yu L., Wu S., Hao X., Li X., Liu X., Ye S., Han H., Dong X., Li X., Li J., Liu N., Liu J., Zhang W., Pelechano V., Chen W.-.H., Yin X. Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran- scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform: iLACO. MedRxiv. 2020 doi: 10.1101/2020.02.20.20025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L., Wu S., Hao X., Dong X., Mao L., Pelechano V., Chen W.-.H., Yin X. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin. Chem. 2020:3–10. doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D. One-pot detection of COVID-19 with real-time reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay and visual RT-LAMP assay. BioRxiv. 2020 doi: 10.1101/2020.04.21.052530. [DOI] [Google Scholar]
- 16.Lu R., Wu X., Wan Z., Li Y., Zuo L., Qin J., Jin X., Zhang C. Development of a novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Virol. Sin. 2020;12250 doi: 10.1007/s12250-020-00218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabe B.A., Cepko C. SARS-CoV-2 detection using an isothermal amplification reaction and a rapid, inexpensive protocol for sample inactivation and purification. MedRxiv. 2020 doi: 10.1101/2020.04.23.20076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joung J., Ladha A., Saito M., Segel M., Bruneau R., Huang M.W., Kim N.-.G., Yu X., Li J., Walker B.D., Greninger A.L., Jerome K.R., Gootenberg J.S., Abudayyeh O.O., Zhang F. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. MedRxiv. 2020 doi: 10.1101/2020.05.04.20091231. [DOI] [Google Scholar]
- 19.Y. Lei, Kitchen range oven enabled One-tube RT-LAMP for RNA detection at home - a potential solution for large-scale screening of COVID-19, (2020). doi:10.31224/osf.io/ed85s.