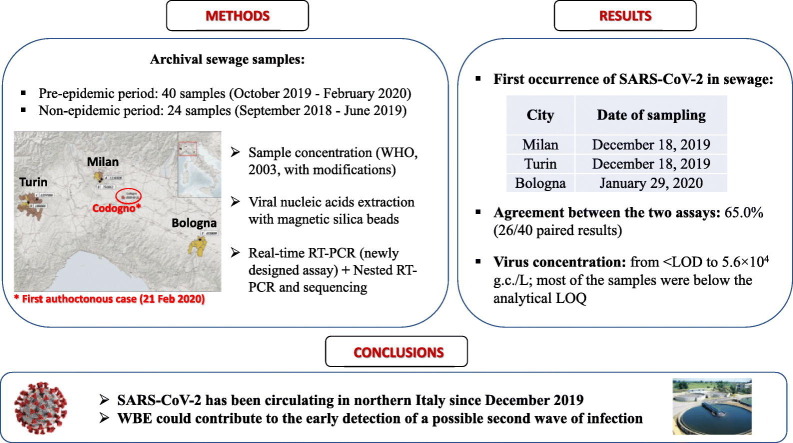
Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease COVID-19, a public health emergency worldwide, and Italy is among the most severely affected countries. The first autochthonous Italian case of COVID-19 was documented on February 21, 2020. We investigated the possibility that SARS-CoV-2 emerged in Italy earlier than that date, by analysing 40 composite influent wastewater samples collected - in the framework of other wastewater-based epidemiology projects - between October 2019 and February 2020 from five wastewater treatment plants (WWTPs) in three cities and regions in northern Italy (Milan/Lombardy, Turin/Piedmont and Bologna/Emilia Romagna). Twenty-four additional samples collected in the same WWTPs between September 2018 and June 2019 (i.e. long before the onset of the epidemic) were included as ‘blank’ samples. Viral concentration was performed according to the standard World Health Organization procedure for poliovirus sewage surveillance, with modifications. Molecular analysis was undertaken with both nested RT-PCR and real-rime RT-PCR assays. A total of 15 positive samples were confirmed by both methods. The earliest dates back to 18 December 2019 in Milan and Turin and 29 January 2020 in Bologna. Virus concentration in the samples ranged from below the limit of detection (LOD) to 5.6 × 104 genome copies (g.c.)/L, and most of the samples (23 out of 26) were below the limit of quantification of PCR.
Our results demonstrate that SARS-CoV-2 was already circulating in northern Italy at the end of 2019. Moreover, it was circulating in different geographic regions simultaneously, which changes our previous understanding of the geographical circulation of the virus in Italy. Our study highlights the importance of environmental surveillance as an early warning system, to monitor the levels of virus circulating in the population and identify outbreaks even before cases are notified to the healthcare system.
Keywords: SARS-CoV-2, Coronavirus, COVID-19, Sewage, Wastewater, Surveillance
Graphical abstract
1. Introduction
Coronaviruses (CoVs) belong to the Coronaviridae family and are enveloped, single-stranded RNA viruses, grouped into four main groups: alpha, beta, gamma and delta CoVs. Most human coronaviruses cause mild respiratory infections (CoV 229E, NL63, OC43, and HKU1). Some CoVs, however, are associated with severe symptoms and outbreaks. These are the beta coronavirus that causes Middle East Respiratory Syndrome (MERS-CoV), severe acute respiratory syndrome (SARS-CoV), and the recently discovered SARS-CoV-2 (the novel coronavirus that causes the coronavirus disease 2019, or COVID-19).
SARS-CoV-2 was discovered in December 2019 in China, and has then spread widely in many countries, to the point that, on 11 March 2020, the World Health Organization (WHO) declared COVID-19 a pandemic. Italy has been among the first, and most severely affected countries in the world with, as of August 11th, 2020, 250.973 COVID-19 cases diagnosed, and 35.644 deaths reported (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard). However, it is likely that, in Italy as well as in all other affected countries in the world, the true number of cases has been substantially greater than reported, as mild or asymptomatic infections have often been overlooked.
The first SARS-CoV-2 cases reported in Italy were two Chinese tourists who fell ill in January after flying in from Wuhan, where the epidemic began (Giovanetti et al., 2020a, Giovanetti et al., 2020b). These patients were immediately put into isolation, and are not believed to have infected anyone else. The first autochthonous patient was diagnosed one month later in Lombardy, on February 21. He was a 38-year-old man, from the town of Codogno, 60 km southeast of Milan. Initially, it was believed that “patient zero” might have been a colleague of his who had recently returned from a business trip to China. This colleague tested negative, however, so the first introduction of the virus into Italy remains unclear.
Identifying the first introduction of the virus is of epidemiological interest, for the tracking and mapping of COVID-19 spread in a country. In Italy, and elsewhere, there have been speculations to the effect that COVID-19 had been silently circulating before the first case was identified. Indeed, other countries have been trying to ascertain whether earlier infections had occurred. In France, where the COVID-19 epidemic was believed to have started in late January 2020, a retrospective analysis of a stored respiratory sample from a patient hospitalised in December 2019, demonstrated that the patient was positive for SARS-CoV-2, suggesting that, in France, the epidemic started much earlier than previously thought (Deslandes et al., 2020).
It is known that gastrointestinal symptoms are seen in patients with COVID-19 (between 16% to 33% in most studies), and that approximately 50% of patients with COVID-19 have detectable virus in their stool (Ouali et al., 2020). The viral load in the faeces of COVID-19 patients was estimated between 103 and 107 copies/mL, depending on the infection course (reviewed in Foladori et al., 2020). These patients have been shown to shed the virus in their stools even if asymptomatic or pre-symptomatic (Jiang et al., 2020; Park et al., 2020; Tang et al., 2020). Sewage samples can thus be used to monitor the levels of virus circulating in the population, an approach called wastewater-based epidemiology (WBE). Several studies performed in the Netherlands (Medema et al., 2020), the United States (Wu et al., 2020; Nemudryi et al., 2020; Sherchan et al., 2020), France (Wurtzer et al., 2020), Australia (Ahmed et al., 2020b), Spain (Randazzo et al., 2020; Chavarria-Mirò et al., 2020), Japan (Hata et al., 2020), Turkey (Kocamemi et al., 2020), and Israel (Bar-Or et al., 2020), and Brasil (Prado et al., 2020) have demonstrated that sewage surveillance can help understand the circulation of SARS-CoV-2 in human populations. In Italy, our group has previously found SARS-CoV-2 in sewage samples collected between the end of February (after the first autochthonous case) and April 2020 (La Rosa et al., 2020). Another Italian study confirmed the occurrence of the virus in sewage samples collected in April (Rimoldi et al., 2020). Thus far, all of the cited studies performed worldwide, have analysed wastewater samples collected during the pandemic, with the exception of the Spanish study of Chavarria-Mirò and co-worker, who also analysed frozen archival samples from 2018 (January–March) and 2019 (January, March, September–December) (Chavarria-Mirò et al., 2020). Similarly, in the current study we retrospectively searched for genomic traces of SARS-CoV-2 in a collection of sewage samples gathered from WWTPs in northern Italy between October 2019 and February 2020, in the framework of different projects on enteric viruses. The samples were analysed to ascertain whether SARS-CoV-2 was circulating in the weeks and months before the virus was believed to have arrived in Italy.
2. Materials and methods
2.1. Sampling and sample preparation.
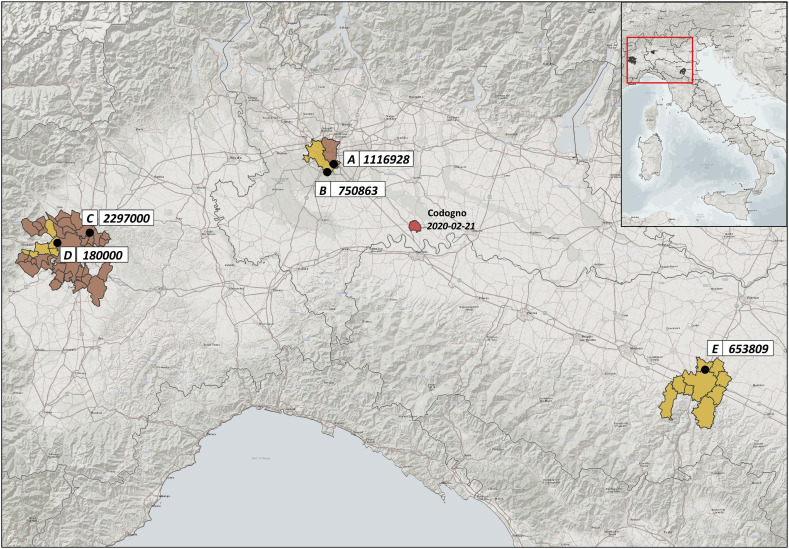
Forty sewage samples were analysed for the study. Samples were collected between 9th October 2019 and 28th February 2020 from five WWTPs located in Milan (20 samples from two distinct plants, referred to as A and B), Turin (16 samples from plants C and D), and Bologna (4 samples from plant E). The location and number of inhabitants (expressed as population equivalents) served by these WWTPs are summarised in Fig. 1 . Other 24 wastewater samples, collected from the same WWTPs in Milan, Turin and Bologna between 12th September 2018 and 19th June 2019 (i.e. before the emergence of SARS-CoV-2 as a human pathogen), were analysed as ‘blank’ samples.
Fig. 1.
Location and number of inhibitants served by the WTPs included in the study. Numbers in correspondence of the WTP code represent the inhibitants served by each plant.
Composite samples, representing 24-h periods, were collected raw, before treatments, stored at −20 °C, and dispatched frozen to Istituto Superiore di Sanità (the Italian National Institute of Health) for analysis. Precautions taken during sample treatment were reported elsewhere (La Rosa et al., 2020). Before sample concentration, a 30 min viral inactivation treatment at 56 °C was undertaken to increase the safety of the analytical protocol for both laboratory personnel and the environment. It has already been demonstrated that this treatment will not affect SARS-CoV-2 RNA detection (La Rosa et al., 2020). Sample concentration was performed using the two-phase (PEG-dextran) separation method recommended by the WHO Guidelines for environmental surveillance of poliovirus circulation (WHO, 2003), with modifications. Briefly, 250 mL of wastewater sample was centrifuged (30 min at 1200 ×g) to separate the pellet. The pellet was kept at 4 °C to be later combined with the concentrated supernatant. The clarified wastewater was neutralized (pH 7.0–7.5), mixed with dextran and polyethylene glycol (19.8 mL of 22% dextran, 143.5 mL 29% PEG 6000, and 17.5 mL 5 N NaCl), and after a constant agitation for 30 min using a horizontal shaker, the mixture was left to stand overnight at 4 °C in a separation funnel. Viruses, accumulated in the smaller bottom layer and/or at the boundary between the layers (interphase), were then collected drop-wise, and this concentrate was re-joined to the pellet retained after the initial centrifugation. In a previous study by our group on SARS-CoV-2 detection in sewage (La Rosa et al., 2020), the original WHO protocol was modified by omitting the chloroform treatment after collecting the concentrate, to avoid loss of SARS-CoV-2 particles, since lipid-containing viruses are chloroform sensitive. However, this resulted in PCR inhibition (median 29.1%; range 8.7% - 51.4%). Therefore, after performing comparative extraction experiments with and without chloroform, using samples spiked with the human Alphacoronavirus HCoV 229E and field samples (see Supplementary Material), the chloroform purification step was reintroduced to improve the purification of samples before RNA extraction, and obtain a higher detection sensitivity. The concentrated sample was then extracted with 20% (v/v) of chloroform by shaking vigorously for 10 min and centrifugation at 1400 ×g for 10 min. The total recovered volume (ranging from 7 to 10 mL) was then recorded, and half of the concentrate was subjected to genome extraction, the remaining being stored at −80 °C.
The recovery efficiency of the concentration and extraction procedure was assessed through separate spiking experiments performed in quadruplicate using the Alphacoronavirus HCoV 229E (ATCC VR-740) and the protocol detailed in Supplementary Materials. This was not done on field samples in order to avoid interferences with future virome analyses.
Genome extraction was performed using the NucliSENS miniMAG semi-automated extraction system with magnetic silica (bioMerieux, Marcy l'Etoile, France), with the following modifications to the manufacturer's protocol to adapt to large volumes: the quantity of lysis buffer added was the equivalent of twice the volume of the sample, the lysis phase was prolonged to 20 min, and 100 μL magnetic silica beads were used per sample. The subsequent washing phases were performed as per manufacturer's instructions. Before molecular tests, extracted RNAs were purified from residual PCR inhibitors using the OneStep PCR Inhibitor Removal Kit (Zymo Research, CA, USA).
2.2. Nested RT-PCR
RNAs were tested for the presence of SARS-CoV-2 by the nested RT-PCR assays in the ORF1ab region (Table 1 ) used to detect the first positive sewage samples in Italy (La Rosa et al., 2020).
Table 1.
Primers and amplification protocols used in the study.
| Target | Region | Primer name | Nucleotide sequence | Orientation | Usage | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | ORF1ab (nsp14) |
2274 - CO-FW1 | GTGCTAAACCACCGCCTG | + | First PCR | 368 | La Rosa, 2020 |
| 2275 - CO-REV1 | CAGATCATGGTTGCTTTGTAGGT | − | |||||
| 2276 - CO-FW2 | CGCCTGGAGATCAATTTAAACAC | + | Nested PCR | 332 | |||
| 2277 - CO-REV2 | ACCTGTAAAACCCCATTGTTGA | − | |||||
| SARS-CoV-2 | ORF1ab (nsp14) |
2297-CoV-2-F | ACATGGCTTTGAGTTGACATCT | + | Real-time RT-qPCR | − | This study |
| 2298-CoV-2-R | AGCAGTGGAAAAGCATGTGG | − | |||||
| 2299-CoV-2-P | FAM-CATAGACAACAGGTGCGCTC-MGBEQ | ||||||
| SARS Betacoronavirus | E gene | E_Sarbeco_F1 | ACAGGTACGTTAATAGTTAATAGCGT | + | Real-time RT-qPCR | − | Corman et al., 2020 |
| E_Sarbeco_R2 | ATATTGCAGCAGTACGCACACA | − | |||||
| E_Sarbeco_P1 | FAM-ACACTAGCCATCCTTACTGCGCTTCG-BHQ1 | ||||||
| SARS-CoV-2 | RdRp | RdRp_SARSr-F2 | GTGARATGGTCATGTGTGGCGG | + | Real-time RT-qPCR | − |
Corman et al., 2020 This study |
| RdRp_SARSr-R1mod | CARATGTTAAAAACACTATTAGCATA | − | |||||
| RdRp_SARSr-P2 | FAM-CAGGTGGAACCTCATCAGGAGATGC- BHQ1 |
FAM: 6-Carboxyfluorescein; MGBEQ: Minor Groove Binder Eclipse Quencher; BHQ1: Black Hole Quencher-1.
Primer RdRp_SARSr-R1mod was modified compared to Corman et al. (2020) by substituting the degenerate base in position 12, as suggested by Vogels et al. (2020) to increase sensitivity.
For the assay, first-strand cDNA was synthesized using Super Script IV Reverse Transcriptase (ThermoFisher Scientific) with the reverse primer, according to the manufacturer's instructions. PCR reaction was performed using 2.5 μL of cDNA in a final volume of 25 μL (Kit Platinum SuperFi Green PCR Master Mix, Thermo), using 1 μL of each primer (10 μM). The PCR conditions were as follows: 98 °C for 30 s; 35 cycles at 98 °C for 10 s, 54 °C for 10 s, and 72 °C for 30 s; final extension at 72 °C for 5 min. After the first round of PCR, nested PCR was performed using 2 μL of the first PCR product under the same conditions. A synthetic DNA fragment (Biofab Research, Italy) including the PCR target region was used as positive control. To avoid false-positive results, standard precautions were taken and results were confirmed in two independent experiments.
The PCR products were visualised by gel electrophoresis, were purified using a Montage PCRm96 Microwell Filter Plate (Millipore, Billerica, MA, USA), and were then sequenced on both strands (BioFab Research, Rome, Italy). Sequences were identified using BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). For comparison purposes, all Italian SARS-CoV-2 genome sequences available at the time of analysis (12th June 2020; n = 134) were retrieved from Gisaid (https://www.gisaid.org/) and aligned with the study sequences using the MEGA X software (Kumar et al., 2018). Sequences were submitted to NCBI GenBank with the following accession numbers: MT843229-MT843240.
2.3. Real-time RT-(q)PCR
Analysis by real-time RT-(q)PCR was undertaken with three different protocols (Table 1):
-
a)
Two published real-time RT-qPCR assays targeting the E gene of the SARS Betacoronavirus and the RdRp gene of SARS-CoV-2, respectively, as described previously (Corman et al., 2020) with slight modifications. The RT-qPCR mix (25 μL total volume) was prepared using the UltraSense one-step qRT-PCR System (Life Technologies, CA, USA), and 5 μL aliquots of sample RNA were analysed in reactions containing 1× buffer, 0.1× ROX reference dye, and 1.25 μL of RNA UltraSense enzyme mix. Primer/probe concentrations were as follows: 400 nM, 400 nM and 200 nM for E_Sarberco_F1, E_Sarberco_R2, and probe E_Sarberco_P1, respectively, and 600 nM, 800 nM, and 250 nM for RdRp-SARSr-F2, RdRp-SARSr-R1mod, and probe RdRp-SARSr-P2, respectively. Amplification conditions included reverse transcription for 30 min at 50 °C, inactivation for 5 min at 95 °C and 45 cycles of 15 s at 95 °C and 1 min at 58 °C. For standard curve construction, the two targeted regions were synthetized and quantified by Eurofins Genomics (Germany). Tenfold dilutions were used for standard curve construction (range 101–105 copies/μL).
-
b)
A newly developed real-time RT-(q)PCR designed using the Primer3 software (http://primer3.ut.ee/) targeting the ORF1ab region (nsp14; 3′-to-5′ exonuclease) of the SARS-CoV-2 genome (positions 18600-18699 of GenBank accession number NC_045512). Following optimization, the RT-qPCR mix (25 μL total volume) was prepared using the AgPath-ID One-Step RT-PCR (Life Technologies), and 5 μL of sample RNA were analysed in reactions containing 1× RT-PCR buffer, 1 μL of RT-PCR enzyme mix, 1.67 μL of detection enhancer, and 500 nM, 900 nM, and 250 nM of primer 2297-CoV-2-F, primer 2298-CoV-2-R, and probe 2299-CoV-2-P, respectively. Amplification conditions were: reverse transcription for 30 min at 50 °C, inactivation for 5 min at 95 °C and 45 cycles of 15 s at 95 °C and 30 s at 60 °C. For standard curve construction, the targeted region was synthetized and purified by BioFab Research (Italy), and was quantified by fluorometric measure (Qubit, Thermo Scientific). Tenfold dilutions were used for standard curve construction (range 5 × 100–5 × 104 copies/μL). In vitro synthetized RNA containing the target region was used as an external amplification control to check for PCR inhibition.
Reactions for quantitative analysis were performed in duplicate. Amplifications were considered acceptable if inhibition was ≤50% and if standard curves displayed a slope between −3.1 and − 3.6 and a R2 ≥ 0.98 (Hougs et al., 2017). All amplifications were conducted on a Quant Studio 12 K Flex instrument (Thermo Scientific). Molecular biology grade water served as the no-template control; two negative controls were included in each run to check for reagent contamination and for environmental contamination, respectively.
Since analysis on environmental matrices may occasionally display high fluorescence background or non-exponential amplification (fluorescence ‘drift’) during amplification, a conservative approach was applied for data analysis. All amplification plots were visually checked for exponential amplification, the threshold was manually set at the midpoint of the exponential phase, and a Cq cut-off value of 40 was applied to all results.
2.4. Specificity and sensitivity of nested RT-PCR and real-time RT-(q)PCR
Our in-house nested RT-PCR was evaluated for specificity using the European Virus Archive – EVA GLOBAL (EVAg) panel, kindly provided by the Erasmus University Medical Center (Rotterdam, The Netherlands), and consisting of RNAs from different Alfa- and Beta- coronaviruses (HCoV-NL63, HCoV-229E, HCoV-OC43, MERS-CoV, SARS-CoV and SARS-CoV-2). Moreover, all amplicons obtained by nested PCR were sequenced for confirmation and compared with those available in GeneBank and in Gisaid (https://www.gisaid.org/). The real-time RT-(q)PCR was evaluated for specificity using the GLOBA (EVAg) panel and, in addition, to exclude possible aspecific signals, specificity was also tested against a panel of nucleic acids from viruses (n = 32) and bacteria (n = 15), as detailed in Supplementary Material. Further to this, to assess specificity of the test on samples representative of the natural microbiota of sewage, 24 ‘blank’ sewage samples (i.e. samples collected between September 2018 and June 2019, long before the onset of the SARS-CoV-2 epidemic) were tested by both molecular methods.
As for sensitivity, in the absence of certified reference material for quantitative assays, SARS-CoV-2 RNA provided in the EVAg panel (quantified ~3 × 104 genome copies (g.c.)/μL using our in-house real-time RT-(q)PCR) was used to prepare serial dilutions to assess the sensitivity of the assays on pure target RNA. To evaluate their performance in wastewater samples, the same RNA dilutions were used to spike nucleic acids extracted from sewage concentrates that had tested negative for SARS-CoV-2. The dilutions were tested by nested RT-PCR (one replicate) to determine the lower detectable concentration of the method, and were analysed in quadruplicate to calculate the limit of detection (LOD50) and the limit of quantification (LOQ) of the real-time RT-(q)PCR assay. LOD50 was calculated according to Wilrich and Wilrich (2009), using the tools available in https://www.wiwiss.fu-berlin.de/fachbereich/vwl/iso/ehemalige/wilrich/index.html). LOQ was calculated as the last dilution level at which the relative repeatability standard deviation (RSDr) of the measurements was below 25% (Hougs et al., 2017).
3. Results
Our nested RT-PCR was able to detect SARS-CoV-2 RNA in spiked sewage samples in a concentration of 3.71 g.c./μL. On pure samples of target RNA, the real-time RT-(q)PCR yielded a LOD50 of 0.41 g.c./μL and a LOQ of 3.71 g.c./μL; in sewage samples, LOD50 and LOQ were 1.46 g.c./μL RNA and 7.35 g.c./μL respectively. Overall, in the real-time RT-(q)PCR runs, the standard curve slopes and the correlation coefficient R2 ranged from −3.32 to −3.47 and from 0.996 to 1.000, respectively.
As regards the specificity of the two assays, amplification was obtained only in reactions containing SARS-CoV-2 RNA (EVAg Coronavirus panel), and no aspecific amplification was detected for the other human coronaviruses, for the RNA/DNA panel of enteric viruses and bacteria, or for the 24 ‘blank’ sewage samples collected between September 2018 and June 2019.
The recovery efficiency of the concentration and extraction procedure, evaluated with seeded experiments performed in quadruplicate, using the Alphacoronavirus HCoV-229E (ATCC VR-740) showed an average recovery of 2.04 ± 0.70%. Sample inhibition, assessed by real-time RT-(q)PCR, ranged from null to 49.0%, with a median value of 3.2%.
With regard to the 40 sewage samples collected between October 2019 and February 2020 from the WWTPs in Milan, Turin and Bologna, SARS-CoV-2 RNA was detected by nested RT-PCR in 18/40 (45%) samples (amplicon sequences confirmed as SARS-CoV-2 by blast analysis) and in 26/40 (65%) samples by the newly developed real-time RT-(q)PCR (Table 2 ), with an overall agreement between the two assays of 65.0% (26/40 paired results). In 15 samples, SARS-CoV-2 RNA was detected by both methods. Only these samples, that tested positive by both nested and real-time PCR, were considered as confirmed positive samples. None of the samples tested positive using the previously published SARS-CoV-2 RdRp and Sarbeco E gene protocols.
Table 2.
SARS-CoV-2 detection in sewage samples, October 2019 – February 2020.
| Sample ID | Origin | Date of sampling | WWTP | Nested RT-PCR | Real-time RT-(q)PCR (c.g./L) |
|---|---|---|---|---|---|
| 3285 | Milan | 24/10/2019 | A | − | − |
| 3287 | Milan | 25/11/2019 | A | − | − |
| 3289 | Milan | 18/12/2019 | A | + | 4.1 × 103 |
| 3290 | Milan | 18/12/2019 | A2 | − | 8.7 × 102 |
| 3238 | Milan | 20/12/2019 | B | + | 1.2 × 103 |
| 3291 | Milan | 29/01/2020 | A | + | 2.3 × 103 |
| 3292 | Milan | 29/01/2020 | A2 | − | 2.2 × 103 |
| 3244 | Milan | 03/02/2020 | B | − | 6.1 × 102 |
| 3231 | Milan | 12/02/2020 | A | − | 1.6 × 103 |
| 3239 | Milan | 12/02/2020 | B | − | 2.8 × 103 |
| 3232 | Milan | 19/02/2020 | A | + | − |
| 3240 | Milan | 19/02/2020 | B | − | 2.6 × 103 |
| 3241 | Milan | 23/02/2020 | B | + | 1.5 × 103 |
| 3233 ⁎ | Milan | 24/02/2020 | A | + | 9.2 × 102 |
| 3230 | Milan | 25/02/2020 | A | + | 4.8 × 102 |
| 3237 | Milan | 25/02/2020 | A2 | − | 1.4 × 103 |
| 3234 | Milan | 26/02/2020 | A | + | 3.7 × 103 |
| 3242 | Milan | 26/02/2020 | B | − | 1.7 × 103 |
| 3235 | Milan | 28/02/2020 | A | − | − |
| 3243 ⁎ | Milan | 28/02/2020 | B | + | 1.3 × 103 |
| 3144 | Turin | 09/10/2019 | C | − | − |
| 3145 | Turin | 09/10/2019 | C | − | − |
| 3321 | Turin | 06/11/2019 | C | − | − |
| 3323 | Turin | 06/11/2019 | D | − | − |
| 3325 | Turin | 20/11/2019 | C | − | − |
| 3329 | Turin | 04/12/2019 | C | − | − |
| 3331 | Turin | 04/12/2019 | D | − | − |
| 3333 | Turin | 18/12/2019 | C | + | − |
| 3335 | Turin | 18/12/2019 | D | + | 1.2 × 103 |
| 3337 | Turin | 14/01/2020 | C | + | 7.4 × 102 |
| 3339 | Turin | 15/01/2020 | D | + | 1.2 × 103 |
| 3341 | Turin | 28/01/2020 | D | + | 5.6 × 102 |
| 3343 | Turin | 29/01/2020 | C | − | 6.0 × 102 |
| 3345 | Turin | 11/02/2020 | D | − | 4.7 × 102 |
| 3347 | Turin | 25/02/2020 | D | + | 2.9 × 102 |
| 3349 | Turin | 26/02/2020 | C | + | 5.6 × 104 |
| 3374 | Bologna | 21/11/2019 | E | − | − |
| 3375 | Bologna | 10/12/2019 | E | − | 2.9 × 104 |
| 3376 | Bologna | 29/01/2020 | E | + | 3.3 × 104 |
| 3377 | Bologna | 19/02/2020 | E | + | − |
Highlighted in bold are the first occurrences of SARS-CoV-2 in each of the urban areas included in the study. ‘A2’ represents a second branch of the ‘A' wastewater treatment plant. Samples below 5.9 × 103 g.c./L (LOQ) should be considered as estimated counts.
Samples detected as positive in a previous study (La Rosa et al., 2020) and confirmed as such by repeating both the extraction and the molecular analysis.
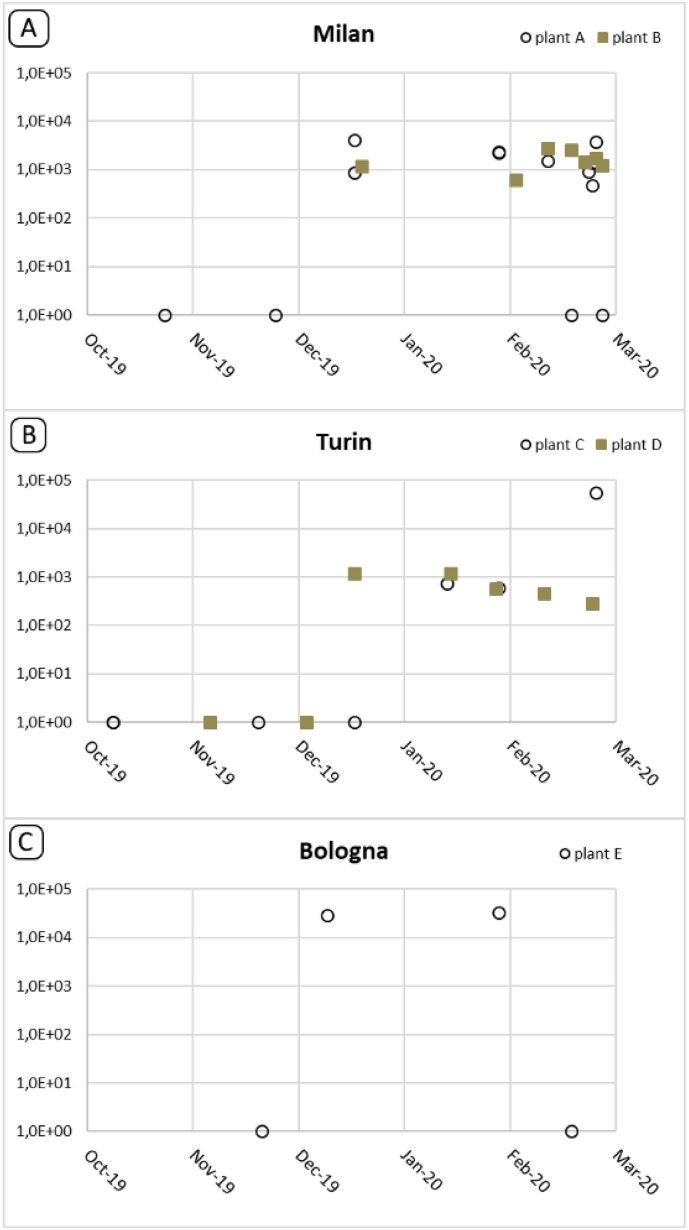
Of the 15 positive samples, 8 were taken earlier than February 21, i.e. before the first autochthonous Italian case was reported. Specifically, the first SARS-CoV-2 positive sewage samples were collected as early as 18 December 2019 in Milan and Turin and 29 January 2020 in Bologna. In all three cities, the virus was also detected in the samples collected in January and February, with only one exception - the February sample from Bologna. Here, however, the negative real-time RT-(q)PCR result may have been affected by the slightly higher-than-usual inhibition in this amplification (16.3%). Virus concentration in the samples (Table 2 and Fig. 2 ) ranged from <LOD to 5.6 × 104 g.c./L, and most of the positive samples (23/26) were below the analytical LOQ (5.9 × 103 g.c./L). The highest concentration was recorded in a sample collected in Turin, in February 2020 (plant C, 5.6 × 104 g.c./L).
Fig. 2.
Trend of the SARS-CoV-2 detection in Milan, Turin and Bologna during the observed period. All quatitative values obtained by real time RT-(q)PCR are reported, irrespectively of confirmation of positive results by nested RT-PCR.
4. Discussion
The COVID-19 pandemic first broke out in December 2019 in Wuhan, China, and then rapidly spread worldwide. As of 13th August 2020, more than 20 million cases of COVID-19 have been registered, and over 740 thousand deaths have been reported (https://www.worldometers.info/coronavirus/).
Italy is one of the first and most severely affected countries in Europe, with a high number of documented cases and deaths. The first documented cases were two Chinese tourists who fell ill in Italy in late January (30th January 2020) after flying in from Wuhan, where the epidemic began. The first autochthonous case of infection was recorded in Italy on 21st February 2020. A sustained local transmission has been documented, so that by 11th August 2020, 250.973 COVID-19 cases were diagnosed, with 35.6441 deaths (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard). As far as we know, COVID-19 first affected Lombardy and Veneto and, later on, all the other regions of Italy. The vast majority of cases were reported in Northern Italy. Phylogenetic analyses on SARS-CoV-2 sequences conducted at the beginning of the epidemic, clustered Italian sequences far from the first two Chinese tourists' strains, and suggested that there may have been multiple introductions of the virus into Italy (Bartolini et al., 2020; Giovanetti et al., 2020a, Giovanetti et al., 2020b; Stefanelli et al., 2020), followed by autochthonous transmission. A genomic characterisation and phylogenetic analysis performed on complete SARS-CoV-2 genomes isolated from patients involved in the first outbreak of COVID-19 in Lombardy, suggested that SARS-CoV-2 entered Northern Italy weeks before the first reported case of infection (Zehender et al., 2020).
To test this hypothesis, we analysed sewage samples collected between October 2019 and February 2020 in Northern Italy in the framework of projects on enteric viruses, and stored in the archive of the Department of Environment and Health at the Italian National Institute of Health. In a previous study, we demonstrated the presence of SARS-CoV-2 in untreated wastewaters in Italy by analysing samples collected during the early stages of the epidemic (February to April 2020) (La Rosa et al., 2020), and other studies around the world have demonstrated that SARS-CoV-2 surveillance in sewage may be considered a sensitive tool to monitor the spread of the virus in the population (Ahmed et al., 2020b; Hata et al., 2020; Medema et al., 2020; Randazzo et al., 2020; Wu et al., 2020; Wurtzer et al., 2020; Kocamemi et al., 2020; Bar-Or et al., 2020; Sherchan et al., 2020).
In this study, the analysis of archival samples showed that SARS-CoV-2 was already circulating in Italy, shed by symptomatic, asymptomatic or paucisymptomatic people, many weeks before the first documented autochthonous case, reported on February 21, 2020. Specifically, viral RNA first occurred in sewage samples collected on December 18th, in Milan (Lombardy) and Turin (Piedmont). Therefore, after mid-December 2019, SARS-CoV-2 had already been circulating in major urban centres surrounding the area (Codogno, in the province of Lodi) where the first case of COVID-19 was reported in February 2020. Significantly, all of these regions documented COVID-19 cases starting from 25th February (Protezione Civile, 2020).
Other findings supports the hypothesis that SARS-CoV-2 had been circulating in Italy, as well as in other countries, as early as the end of 2019. Indeed, the fact that the virus had been circulating in Europe in late December 2019 has already been demonstrated by a French study (Deslandes et al., 2020) that retrospectively analysed samples taken from intensive care patients with influenza-like symptoms in Paris, and found one SARS-CoV-2 positive respiratory sample in a French resident who had not visited China and who had been hospitalised on December 27, 2020. Considering the incubation period of COVID-19 - 6.4 days on average (Wang et al., 2020) - as well as evidence showing that viral shedding may occur in asymptomatic patients (Jiang et al., 2020; Park et al., 2020; Tang et al., 2020), it is conceivable that the virus was circulating and being released into the sewage in the Paris area roughly at the same time as in northern Italy, as indicated by our positive sewage samples.
A Spanish study in the region of Murcia detected SARS-CoV-2 RNA in wastewater before the first COVID-19 cases were declared by the local authorities in many of the cities where wastewaters have been sampled (Randazzo et al., 2020), revealing that members of the community were shedding SARS-CoV-2 RNA before the first cases were reported. A similar study conducted in France showed SARS-CoV-2 viral genome in raw sewage before the exponential phase of the epidemic, suggesting that presence of SARS-CoV-2 in wastewater anticipate the reporting of clinical cases (Wurtzer et al., 2020).
The hypothesis of SARS-CoV-2 circulation before the identification of the first clinical cases is supported by other epidemiological approaches as well: a seroprevalence study, conducted on healthy blood donors in the province of Milan during the COVID-19 epidemic showed that, between 12 and 17 February 2020, 2.0% of donors displayed IgG for SARS-CoV-2 (Percivalle et al., 2020). Given the temporal delay between infection and SARS-CoV-2 neutralising antibodies appearance, it might be hypothesised that the virus circulated well before the detection of the index case.
Evolutionary sequence analyses lend credibility to the scenario of an introduction of SARS-CoV-2 into the human population in the fourth quarter of 2019 (Duchene et al., 2020; Giovanetti et al., 2020a, Giovanetti et al., 2020b; Hill and Rambaut, 2020; Li et al., 2020; Lu et al., 2020; Volz et al., 2020). Recently, van Dorp and co-workers analysed the genomic diversity of SARS-CoV-2 in the global population since the beginning of the COVID-19 pandemic by comparing 7666 SARS-CoV-2 genomes covering a vast geographical area (van Dorp et al., 2020). Results showed that all sequences shared a common ancestor towards the end of 2019 (6 October 2019–11 December 2019), indicating this as the period when SARS-CoV-2 jumped into the human population, and that the virus may have been transmitted between human hosts for quite some time before it was identified.
Our study indicates that SARS-CoV-2 was present in Italy before the first imported cases were reported in late January 2020. Since faecal viral shedding occurs in both symptomatic and asymptomatic patients, the question remains whether the traces of SARS-CoV-2 RNA that we found in the sewage of Milan, Turin and Bologna reflected the presence of a significant number of asymptomatic carriers, or of symptomatic patients misdiagnosed as cases of influenza.
In the present study, several analytical issues had to be addressed. The method used for sample concentration is a modified protocol for the surveillance of poliovirus in sewage. Different volumes and concentration methods are being applied in the various studies assessing the occurrence of SARS-CoV-2: adsorption-extraction with different pre-treatment options, centrifugal concentration device methods, polyethylene glycol concentration, and ultrafiltration (Ahmed et al., 2020b). The concentration method used in this study, based on the two-phase (PEG-dextran) separation method, was selected despite the fact that recovery efficiencies seem to be lower than those obtained by other methods (Ahmed et al., 2020b). It is, however, recommended by the WHO Guidelines for environmental surveillance and is the standard for enteric virus sewage surveillance worldwide (WHO, 2003). This means that a number of laboratories already have both the know-how and the equipment necessary to perform it. Moreover, samples that are routinely collected and concentrated for poliovirus surveillance could be shared and used for SARS-CoV-2 surveillance as well, thus optimising economic and personnel resources.
As for the method used for SARS-CoV-2 detection and quantification, the nested RT-PCR targeting the ORF1ab region, previously published for the first detection of SARS-CoV-2 in wastewater in Italy (La Rosa et al., 2020), was tested in this study for specificity against a panel of human coronavirus RNAs and ‘blank’ samples. Moreover, as a routine procedure for all conventional PCRs, the identity of all amplified fragments was confirmed by sequencing.
In our previous study on SARS-CoV-2 in sewage in Italy (La Rosa et al., 2020), no positive results were obtained by a published real-time RT-qPCR, therefore no quantitative data could be provided for the positive samples. Therefore, in this study, a newly designed real-time RT-(q)PCR assay was evaluated. Indeed, sewage is a very complex matrix, and assays developed for clinical samples are not always suitable for use on environmental samples. The newly designed assay was shown to be specific for SARS-CoV-2 by testing against the human coronavirus panel, nucleic acids from relevant viruses and bacteria and ‘blank’ samples. While cross-reactivity with untested microorganisms or with uncharacterised viruses displaying sequences closely matching the target region may not be excluded in principle, the absence of any amplification in ‘blank’ samples supported the specificity of the reaction. Further tests on a larger variety of reference strains and complex matrices, however, should be performed for full validation of this method. The sensitivity of the assay was also assessed, and proved to be fit for use. On the other hand, none of the samples tested positive using the previously published SARS-CoV-2 RdRp and Sarbeco E gene protocols (Corman et al., 2020) recommended for the screening of clinical sample. There is still no consensus on the use of an assay to detect SARS-CoV-2 in environmental samples, and comparative studies with primers targeting different genome regions should be performed to assess the sensitivity and specificity of the different methods in environmental matrices.
Moreover, it should be noted that, in the absence of an internationally recognised standard for SARS-CoV-2 quantification (as available for other human viruses), a robust assessment of the sensitivity and accuracy of real-time RT-(q)PCR assays cannot be performed, as quantitative results are prone to error depending on both the amplification efficiency of the reactions and the trueness of the reference values attributed to standard curves. Indeed, several studies performing the simultaneous quantification of samples by multiple targets or protocols, as required for example in the CDC protocol testing for N1 and N2 (CDC, 2020), showed variability in the results from the different targets (Randazzo et al., 2020; Wu et al., 2020). Further method harmonization, the development of certified reference materials and a robust characterisation of the method's performance (including estimation of LOD, LOQ and measurement uncertainty) are required for a reliable use of real-time RT-(q)PCR in SARS-CoV-2 quantification in sewage, particularly in view of the use of these data for estimating the number of infected individuals shedding virus, as done in some recent studies (Ahmed et al., 2020a).
In this study, virus concentrations in the tested wastewater samples ranged from undetectable to 5.6 × 104 g.c./L, with most results in the order of 102–103 g.c./L. These results are consistent with the concentrations obtained by other authors who tested samples collected at a later stage of the pandemic (mid-January through May 2020) in different countries, finding values ranging from 102 to 106 g.c./L (Ahmed et al., 2020a; Randazzo et al., 2020; Wu et al., 2020; Wurtzer et al., 2020). In some of these studies, an upward trend in viral concentrations was observed over the course of the epidemic. Wurtzer et al. (2020) showed SARS-CoV-2 concentrations in Paris wastewaters to increase from 104 to 105 g.c./L at the beginning of the epidemic to 106–107 g.c./L after its peak. In other studies, perhaps due to shorter periods of observation, an almost constant concentration of SARS-CoV-2 in tested samples was reported following its first detection (Randazzo et al., 2020). While the high number of results below the LOQ obtained in our study did not allow for an accurate trend analysis, quantitative data in samples from Milan showed that, following the first occurrence of the virus, an almost constant concentration was reached in sewage samples, while in Turin, the different plants sampled – serving different districts of the metropolitan area – displayed different tendencies, with a more evident increase in concentrations in plant C. Further studies on samples collected from February 2020 are required to assess the trends in viral concentrations as the epidemic unfolded in the different cities. Moreover, possible differences between WWTPs and the areas they serve should be taken into account in future surveillance studies.
5. Conclusion
In conclusion, our study on archival samples collected before the first autochthonous case was detected in Italy confirms that SARS-COV-2 was already circulating after mid-December 2019. This study also demonstrates the potential of environmental surveillance as an early warning system capable of alerting public health authorities to the presence of an outbreak in a specific population. The activation of national WBE networks for the monitoring of SARS-CoV-2 could contribute to the early detection of a possible second wave of infection, so as to quickly coordinate and implement mitigation interventions, and could establish a surveillance system ready to operate in case of future epidemic events.
CRediT authorship contribution statement
Category 1 Conception and design of study: GLR, ES, LL, LB
Acquisition of data: MI, PM, GBF, CV
Analysis and/or interpretation of data: MI, PM, GBF, CV; GLR, ES, LB, LL
Category 2 Drafting the manuscript: GLR, ES
revising the manuscript critically for important intellectual content: GLR, ES
Category 3 Approval of the version of the manuscript to be published (the names of all authors must be listed): GLR, MI, PM, GBF, CV; LB, LL, ES
Declaration of competing interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. This statement is signed by all the authors to indicate agreement that the above information is true and correct
Acknowledgments
This publication was supported by the European Virus Archive Global (EVA-GLOBAL) project, which provided the Coronavirus RNA panel for the assessment of our in-house PCR. The EVA-GLOBAL project, in turn, received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 871029.
We also wish to acknowledge Prof. Annalaura Carducci of the University of Pisa for kindly providing the HuCoV 229E (ATCC VR-740) strain and Dr. Loredana Cozzi (Istituto Superiore di Sanità, Department of Food Safety, Nutrition and Veterinary public health) for virus replication.
We thank the personnel of the integrated water service for providing wastewater samples.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.141711.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E., Mannasse B., Shirazi R., Kramarsky-Winter E., Nir O., Abu-Ali H., Ronen Z., Rinott E., Lewis Y.E., Friedler E., Bitkover E., Paitan Y., Berchenko Y., Kushmaro A. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. medRxiv. 2020 doi: 10.1101/2020.04.26.20073569. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini B., Rueca M., Gruber C.E.M., Messina F., Carletti F., Giombini E., Lalle E., Bordi L., Matusali G., Colavita F., Castilletti C., Vairo F., Ippolito G., Capobianchi M.R., Di C.A. SARS-CoV-2 phylogenetic analysis, Lazio region, Italy, February-march 2020. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2608.201525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. 2020. https://www.fda.gov/media/134922/download [DOI] [PMC free article] [PubMed]
- Chavarria-Mirò, G., Anfruns-Estrada, E., Guix, S., Paraira, M., Galofrè, B., SÃanchez, G., Pintò, R., Bosch, A., 2020. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. medRxiv preprint. doi: 10.1101/2020.06.13.20129627. [DOI]
- Protezione Civile 2020. https://github.com/pcm-dpc/COVID-19/blob/master/dati-province/dpc-covid19-ita-province-20200225.csv
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van D. V., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes A., Berti V., Tandjaoui-Lambotte Y., Alloui C., Carbonnelle E., Zahar J.R., Brichler S., Cohen Y. SARS-CoV-2 was already spreading in France in late December 2019. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchene S., Featherstone L., Haritopoulos-Sinanidou L. 2020. Temporal Signal and the Evolutionary Rate of 2019 n-CoV Using 47 Genomes Collected by Feb 01 2020. [Google Scholar]
- Foladori P., Cutrupi F., Segata N., et al. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review [published online ahead of print, 2020 Jun 24] Sci. Total Environ. 2020;140444:743. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M., Angeletti S., Benvenuto D., Ciccozzi M. A doubt of multiple introduction of SARS-CoV-2 in Italy: a preliminary overview. J. Med. Virol. 2020:1–3. doi: 10.1002/jmv.25773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanetti M., Benvenuto D., Angeletti S., Ciccozzi M. The first two cases of 2019-nCoV in Italy: where they come from? J. Med. Virol. 2020;92(5):518–521. doi: 10.1002/jmv.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata, A., Honda, R., Hara-Yamamura, H., Meuchi, Y., 2020. Detection of SARS-CoV-2 in wastewater in Japan by multiple molecular assays-implication for wastewater-based epidemiology (WBE). medRxiv preprint. doi: 10.1101/2020.06.09.20126417. [DOI]
- Hill V., Rambaut A. 2020. Phylodynamic Analysis of SARS-CoV-2 | Update 2020-03-06. [Google Scholar]
- Hougs, L., Gatto, F., Goerlich, O., Grohmann, L., Lieske, K., Mazzara, M., Narendja, F., Ovesna, J., Papazova, N., Scholtens, I., Žel, J., 2017. Verification of analytical methods for GMO testing when implementing interlaboratory validated methods. EUR 29015 EN, publication Office of the European Union, Luxembourg, 2017, ISBN 978-92-79-77310-5, doi:10.2760/645114, JRC 109940.
- Jiang X., Luo M., Zou Z., Wang X., Chen C., Qiu J. Asymptomatic SARS-CoV-2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. J. Med. Virol. 2020 doi: 10.1002/jmv.25941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi, B. A., Kurtb, H., Hacıogluc S., Yaralıc C., Saatcid, A.M., Pakdemirli, B., 2020. First data-set on SARS-CoV-2 detection for Istanbul wastewaters in Turkey. medRxiv preprint. doi: 10.1101/2020.05.03.20089417doi. [DOI]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. The Science of the Total Environment. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang W., Zhao X., Zai J., Zhao Q., Li Y., Chaillon A. Transmission dynamics and evolutionary history of 2019-nCoV. J. Med. Virol. 2020;92:501–511. doi: 10.1002/jmv.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., du P.L., Liu Z., Hill V., Kang M., Lin H., Sun J., François S., Kraemer M.U.G., Faria N.R., McCrone J.T., Peng J., Xiong Q., Yuan R., Zeng L., Zhou P., Liang C., Yi L., Liu J., Xiao J., Hu J., Liu T., Ma W., Li W., Su J., Zheng H., Peng B., Fang S., Su W., Li K., Sun R., Bai R., Tang X., Liang M., Quick J., Song T., Rambaut A., Loman N., Raghwani J., Pybus O.G., Ke C. Genomic epidemiology of SARS-CoV-2 in Guangdong Province, China. Cell. 2020;181:997–1003. doi: 10.1016/j.cell.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 in sewage. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., et al. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Preprint. medRxiv. 2020 doi: 10.1101/2020.04.15.20066746. 2020.04.15.20066746. Published 2020 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouali S.E., Achkar J.P., Lashner B., Regueiro M. Gastrointestinal manifestations of COVID-19. Cleve. Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc049. [DOI] [PubMed] [Google Scholar]
- Park S.K., Lee C.W., Park D.I., Woo H.Y., Cheong H.S., Shin H.C., Ahn K., Kwon M.J., Joo E.J. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percivalle E., Cambiè G., Cassaniti I., et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi red zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25(24):2001031. doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., SimÃ3n P., Allende A., Sánchez, G SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:15. doi: 10.1016/j.scitotenv.2020.140621. (November 2020, Article number 140621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanelli P., Faggioni G., Lo P.A., Fiore S., Marchi A., Benedetti E., Fabiani C., Anselmo A., Ciammaruconi A., Fortunato A., De S.R., Fillo S., Capobianchi M.R., Gismondo M.R., Ciervo A., Rezza G., Castrucci M.R., Lista F., On Behalf Of Iss Covid-Study Group Whole genome and phylogenetic analysis of two SARS-CoV-2 strains isolated in Italy in January and February 2020: additional clues on multiple introductions and further circulation in Europe. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.13.2000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N., Wu W.J., Yuan C., Yu M.L., Li P., Yan J.B. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp L., Acman M., Richard D., Shaw L.P., Ford C.E., Ormond L., Owen C.J., Pang J., Tan C.C.S., Boshier F.A.T., Ortiz A.T., Balloux F. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels C.B.F., Brito A.F., Wyllie A.L., Fauver J, .R., Ott I.M., Kalinich C.C., Petrone M.E., Casanovas-Massana A., Muenker M.C., Moore A.J., Klein J., Lu P., Lu-Culligan A., Jiang X., Kim D.J., Kudo E., Mao T., Moriyama M., Eun Oh J., Park A., Silva J., Song E., Takehashi T., Taura M., Tokuyama M., Venkataraman A., Weizman O.-E., Wong P., Yang Y., Cheemarla N.R., White E., Lapidus S., Earnest R., Geng B., Vijayakumar P., Odio C., Fournier J., Bermejo S., Farhadian S., Dela Cruz C., Iwasaki A., Ko A.I., Landry M.-L., Foxman E.F., Grubaugh N.D. Analytical sensitivity and efficiency comparisons of SARS-COV-2 qRT-PCR primer-probe sets. medRxiv preprint. 2020 doi: 10.1101/2020.03.30.20048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E., Baguelin M., Bhatia S., et al. Report 5: phylogenetic analysis of SARS-CoV-2. Imperial College London. 2020 doi: 10.25561/77169. [DOI] [Google Scholar]
- Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020;92(6) doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, editor. Guidelines for Environmental Surveillance of Poliovirus Circulation. Edited by WHO/V&B/03.03. 2003. [Google Scholar]
- Wilrich C., Wilrich P.-Th. EXCEL program for the estimation of the POD function and the LOD of a qualitative microbiological measurement method. J. AOAC Int. 2009;92:1763–1772. http://www.wiwiss.fu-berlin.de/fachbereich/vwl/iso/ehemalige/wilrich/index.html [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5(4):e00614–e00620. doi: 10.1128/mSystems.00614-20. Published 2020 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv preprint. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehender G., Lai A., Bergna A., Meroni L., Riva A., Balotta C., Tarkowski M., Gabrieli A., Bernacchia D., Rusconi S., Rizzardini G., Antinori S., Galli M. Genomic characterization and phylogenetic analysis of SARS-COV-2 in Italy. J. Med. Virol. 2020 doi: 10.1002/jmv.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material