Abstract
Objective
To explore the neural mechanisms of brain impairment in type 2 diabetes mellitus (T2DM), abnormal changes to the functional connections between brain regions in the resting state were investigated based on a meta‐analysis.
Methods
Resting‐state functional magnetic resonance imaging (fMRI) and neuropsychological assessment were performed on 38 patients with T2DM and 33 healthy controls (HCs). Functional connectivity between regions based on a meta‐analysis and other voxels in the brain was calculated and compared between the two groups using a two‐sample t test. A correlation analysis was conducted between clinical/cognitive variables and functional connection values from the regions with significant differences in the above comparison.
Results
Patients in the T2DM group showed a significantly decreased functional connection between the right posterior cerebellum and the right middle/inferior occipital gyrus, left middle temporal gyrus, left superior frontal gyrus, left middle frontal gyrus, left insula, left precuneus, and right paracentral lobule/left precuneus when compared with HC group. The functional connection values between the right insula and left medial frontal gyrus, left supplementary motor area, and between the left lingual gyrus and right middle/inferior occipital gyrus in patients with T2DM were significantly decreased. Moreover, the functional connection values between the right posterior cerebellum and left middle frontal gyrus, and between the right posterior cerebellum and left precuneus were negatively correlated with HbA1c in the T2DM group (r = −.356, p = .03; r = −.334, p = .043).
Conclusions
Our study showed a wide range of cerebellar–cerebral circuit abnormalities in patients with T2DM, which provides a new direction to investigate the neuropathological mechanisms of T2DM from the perspective of the cerebellum.
Keywords: functional connectivity, magnetic resonance imaging, resting state, type 2 diabetes mellitus
The posterior cerebellum is involved in a variety of brain function impairments in T2DM patients.It is suggested that the cerebellar–cerebral circuit may be involved in the neuropathological basis of brain dysfunction in patients with T2DM, which provides new insight into the neural mechanisms of brain dysfunction in patients with T2DM from the perspective of the cerebellum.
![]()
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is one of the most common chronic diseases that can lead to cognitive dysfunction in attention, executive function, visual information processing, etc. (Macpherson et al., 2017), potentially increasing the risk of dementia (Biessels, Deary, & Ryan, 2008). So far, the neural mechanisms that underlie brain impairments in patients with T2DM remain unclear.
Abnormal neuronal activity is widely recognized as the neural basis of cognitive impairment (De Felice & Lourenco, 2015; Iyalomhe et al., 2017;). Resting‐state functional magnetic resonance imaging (fMRI) is a tool that can be used to explore the spontaneous activity of neurons and has been widely used to examine the pathogenesis of various neuropsychiatric disorders (Avissar et al., 2017; Cheke et al., 2017; Du, Fryer, et al., 2018). During past years, several studies have used regional homogeneity and amplitude of low‐frequency fluctuation to explore spontaneous brain changes in patients with T2DM (Chen, Liu, & Ma, 2014; Cui et al., 2015; Liu et al., 2016; Peng et al., 2016; Wang et al., 2014; Xia et al., 2013; Zhou et al., 2014), but the results were inconsistent. Zhang et al. (2018) performed an activation‐likelihood estimation (ALE) meta‐analysis and found that the left lingual gyrus, right posterior cerebellum, left postcentral gyrus, and right insula had abnormal activity in patients with T2DM in a robust way during the resting state. These brain regions are not only involved in cognitive function (Guell, Gabrieli, & Schmahmann, 2018; Stoodley & Schmahmann, 2009), but are also closely related to the clinical manifestation of T2DM, such as abnormal feeding behavior (Woolley et al., 2007). Therefore, a comprehensive investigation into the effect of regional brain dysfunction on whole‐brain function could provide more useful information to understand the neural mechanisms of brain impairment in patients with T2DM.
Resting‐state functional connectivity, which is based on synchronous and low‐frequency (0.01–0.08 Hz) fluctuations of blood oxygen level‐dependent fMRI (Biswal et al., 1995), is commonly used to evaluate interregional cooperation between different brain regions. A number of previous studies have used the posterior cingulate cortex (Musen et al., 2012), hippocampus (Sun et al., 2018), thalamus (Du, Fryer, et al., 2018), and amygdala (Xia et al., 2018) as regions of interest (ROI) to reveal abnormal functional connectivity in the brains of patients with T2DM from different perspectives. However, the choice of ROI in these studies was hypothesis‐driven. Although this approach has the advantage of providing direct information regarding the network of regions that are most strongly correlated with the seed region (Cole, Smith, & Beckmann, 2010), hypothesis‐driven ROI depends on subjective experience and prior knowledge less objectively than data‐driven ROI (Du, Fryer, et al., 2018). To the best of our knowledge, no studies in patients with T2DM have used data‐driven ROI to explore their functional connectivity with the whole brain.
Hence, in the present study, we used data‐driven ROI based on the results of a meta‐analysis, as these could more comprehensively reflect abnormal functional connectivity in brain regions in patients with T2DM. We speculated that, compared with healthy controls (HCs), brain regions in patients with T2DM have disrupted functional connectivity with the whole brain, which may reveal the neural mechanism of diabetic brain damage.
2. MATERIALS AND METHODS
2.1. Study population
From February 2018 to January 2019, we recruited 40 patients with T2DM from the Department of Endocrinology at Shaanxi Provincial People's Hospital and 35 HCs from the community. Patients were diagnosed according to the criteria of the American Diabetes Association in 2014. All subjects were aged between 40 and 70 years and were right‐handed. Exclusion criteria in both groups included a self‐reported history of known brain injury, epilepsy, stroke, alcohol and other substance dependence, Parkinson's disease, major depression, or other disorders that could affect cognitive function, major medical illnesses (e.g., cancer), and MRI contraindications. Patients with hypoglycemia (blood glucose < 3.9 mmol/L) or hyperglycemia (blood glucose > 33.3 mmol/L) during the hospital stay were excluded from the study and the examination was not rescheduled. Two patients and two HCs were subsequently excluded as the limits for head motion were exceeded during data preprocessing.
All patients took medicines or insulin injections on time on the day of the scan and arrived at the department for MRI between 06:30 and 7:00 p.m. after dinner. First, structured clinical interviews and a series of psychological tests were carried out for approximately 30 min. Patients underwent MRI within 2 hr of dinner and required a postprandial blood glucose concentration of <33.3 mmol/L. The postprandial blood glucose concentration of patients in this study ranged from 7.9 mmol/L to 21.8 mmol/L. Only one patient was scheduled for MRI each day to ensure that each patient's MRI scan was completed between 7:30 and 8:30 p.m. Eight of the 38 patients had no complications, and the remaining 30 patients had one or more complications. The complications of patients with T2DM and the therapeutic agents used to treat them are shown in Table 1. The study was approved by the Ethics Committee of Shaanxi Provincial People's Hospital. All subjects were informed of the study protocol and informed consent was obtained from each patient before participation in the study.
TABLE 1.
Demographic, clinical, and cognitive data in the T2DM and HC groups
| Items | T2DM group (n = 38) | HC group (n = 33) | p |
|---|---|---|---|
| Age (years) | 55.71 ± 6.32 | 54.01 ± 4.99 | .231 |
| Gender (M/F) | 30/8 | 26/7 | .987 |
| Education (years) | 13.29 ± 2.50 | 15.18 ± 1.88 | .001* |
| Disease duration (years) | 8.13 ± 5.88 | – | – |
| BMI (kg/m2) | 24.59 ± 2.81 | 24.61 ± 3.10 | .970 |
| Systolic BP (mmHg) | 126.21 ± 12.82 | 123.24 ± 8.66 | .264 |
| Diastolic BP (mmHg) | 79.63 ± 9.84 | 82.18 ± 6.10 | .202 |
| HbA1c (%) | 8.10 ± 1.86 | 5.67 ± 0.54 | <.001* |
| FBG (mmol/L) | 9.16 ± 2.90 | 5.37 ± 0.87 | <.001* |
| TG (mmol/L) | 2.18 ± 1.28 | 1.74 ± 1.17 | .495 |
| TC (mmol/L) | 4.67 ± 1.53 | 4.93 ± 0.92 | .396 |
| MMSE | 28.00 ± 2.01 | 28.46 ± 1.60 | .301 |
| MoCA | 26.50 ± 2.67 | 27.02 ± 1.48 | .311 |
| TMT‐A | 74.50 ± 27.04 | 71.58 ± 26.51 | .866 |
| CDT | 17.88 ± 6.94 | 19.36 ± 5.70 | .333 |
| T2DM complications | |||
| Retinopathy | 8 | – | – |
| Peripheral neuropathy | 21 | – | – |
| Nephropathy | 22 | – | – |
| T2DM therapeutic agents | |||
| Dietary restriction | 12 | – | – |
| Oral medication | 18 | – | – |
| Insulin | 2 | – | – |
| Insulin + oral medication | 6 | – | – |
Abbreviations: BMI, body mass index; CDT, Clock Drawing Test; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; TC, total cholesterol; TG, triglycerides; TMT‐A, trail making test A.
p < .05.
2.2. Data collection
2.2.1. Biochemical characteristics
Medical history and clinical data from patients with T2DM were obtained from medical records and questionnaires. Clinical data from HCs were also collected from the outpatient medical examination center, which included weight, height, blood pressure, and body mass index (BMI). Blood pressure was measured while sitting at three different time points during the day and then averaged. After an overnight fast of at least 8 hr, blood samples were obtained to measure the levels of fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC), and glycated hemoglobin (HbA1c).
2.2.2. Neuropsychological tests
Neuropsychological tests were used to evaluate participants’ general mental statuses and cognitive domains. The Mini‐Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were used to assess general cognitive function. The information processing speed was tested using trail making test A (TMT‐A). Visual space, visual memory, and executive function were evaluated using the Clock Drawing Test (CDT). Neuropsychological tests were carried out by a psychiatrist.
2.2.3. MRI measurements
MRI scans were performed using a 3.0 Tesla MRI scanner (Philips Ingenia) using a 16‐channel phased‐array head coil. All subjects were instructed to keep their eyes closed and to stay awake during scanning. Foam pads and headphones were used to control head motion and decrease scanner noise as much as possible. Conventional T2‐weighted images and fluid‐attenuated inversion recovery scans were acquired to exclude visible brain lesions. Sagittal three‐dimensional T1‐weighted images were acquired with the following parameters: repetition time (TR) = 7.5 ms, echo time (TE) = 3.5 ms, flip angle (FA) = 8°, field of view (FOV) = 250 × 250 mm2, matrix = 256 × 256, slice thickness = 1 mm, no gap, and 328 sagittal slices. Resting‐state functional blood oxygen level‐dependent images were obtained using a gradient‐echo planar sequence with the following parameters: TR = 2,000 ms, TE = 30 ms, slices = 34, thickness = 4 mm, gap = 0 mm, FOV = 230 × 230 mm2, matrix = 128 × 128, FA = 90°, and 200 volumes.
Functional data analyses were conducted using DPABI 2.3 programs based on statistical parametric mapping 12 (SPM12, http://www.fil.ion.ucl.ac.uk/spm). After discarding the first 10 time points, the slice timing and realignment for head motion correction were performed. Any subjects with a head motion of >1.5 mm or translation of >1.5° rotation in any direction were excluded. Normalization was then performed based on the resulting images using unified segmentation of anatomical images (resampling voxel size = 3 × 3 × 3 mm3). Multiple regression models were employed to remove the effect of covariance of no interests, which included 24 motion parameters, cerebrospinal fluid signals, and white matter signals. The obtained images were smoothened with an isotropic Gaussian smooth kernel with a full width at half maximum of 6 mm, followed by detrending and filtering (0.01–0.08 Hz) in order.
The ROI was obtained from the previous ALE meta‐analysis (Zhang et al., 2018). ALE meta‐analysis data processing was completed in the MNI standard space coordinate system, and the results were corrected using the false discovery rate (p < .05, cluster size of >200 mm3). We presented the corrected ALE image results in the MNI standard space using DPABI software and saved the four clusters in the results map as MASK. The four ROI were the left lingual gyrus (−4, −74, −2, cluster size = 800 mm3), right cerebellar posterior lobe (28, −184, −14, cluster size = 488 mm3), left postcentral gyrus (−16, −30, 76, cluster size = 368 mm3), and right insula (46, −18, 10, cluster size = 256 mm3). For each ROI, a correlation analysis was carried out between the mean signal change and the time series of every voxel of the whole brain. The resulting r values were converted using Fisher's r‐to‐z transformation to improve their Gaussian distribution.
2.3. Statistical analysis
The statistical analysis was carried out using SPSS 17.0. A two‐tailed independent samples t test was used for normally distributed variables, while the Mann–Whitney U test was used for non‐normally distributed data. Simultaneously, the chi‐squared test was used for categorical variables. A p value of <.05 was considered statistically significant. Voxel‐wise two‐sample t test embedded in DPABI was performed to evaluate the intergroup differences for each ROI after controlling for years of education. Significance was determined using the Gaussian random field (GRF) correction method with a p value of <.05 (voxel p < .001, cluster size > 29). The mean value of functional connections of functionally altered brain regions between groups was extracted from patients in the T2DM group. Partial correlation analyses were conducted between the mean values and clinical/cognitive variables after controlling for years of education.
3. RESULTS
3.1. Clinical and neuropsychological data
A total of 38 patients with T2DM and 33 HCs were enrolled in the final analysis. Demographic, clinical, and cognitive information from patients in the T2DM group and the HC group are listed in Table 1. There were no significant intergroup differences in age, sex, BMI, TC, TG, blood pressure, or cognitive score (p > .05). Education level was higher in patients in the HC group compared with patients in the T2DM group (p = .001). Compared with HC group, patients in the T2DM group had elevated levels of FBG and HbA1c (p < .001 for both).
3.2. Functional connectivity analysis
3.2.1. ROI: right posterior cerebellum
Compared with the HC group, the functional connections between the right posterior cerebellum and right middle/inferior occipital gyrus, left middle temporal gyrus, superior frontal gyrus, left middle frontal gyrus, left insula, left precuneus, and right paracentral lobule/left precuneus showed a significant decrease in the T2DM group (Figure 1 and Table 2).
FIGURE 1.
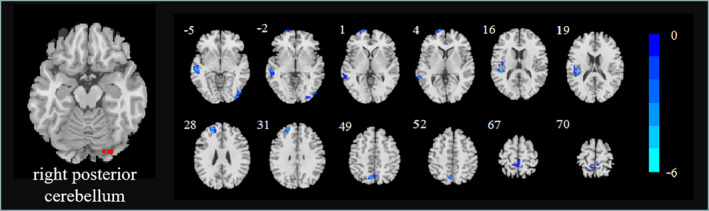
Significant differences were observed in right posterior cerebellum functional connectivity between the T2DM and HC groups. Thresholds were set using GRF correction at a p value of <.05 (voxel p < .001, cluster size > 29)
TABLE 2.
Aberrant functional connectivity in the T2DM group compared with the HC group
| Seed ROI | Brain regions | Peak MNI coordinates | Voxels | BA | Peak t score | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Right posterior cerebellum | R middle/inferior occipital gyrus | 36 | −93 | −6 | 57 | 18/19 | −4.55 |
| L middle temporal gyrus | −57 | −24 | −6 | 82 | 21/22 | −4.99 | |
| L superior frontal gyrus | −15 | 69 | 3 | 46 | 10 | −4.84 | |
| L insula | −39 | −21 | 18 | 44 | 13 | −5.04 | |
| L middle frontal gyrus | −24 | 51 | 27 | 43 | 9/10 | −5.34 | |
| L precuneus | −6 | −66 | 51 | 33 | 7 | −4.94 | |
| R paracentral lobule/L precuneus | −9 | −42 | 69 | 33 | 4/5 | −4.33 | |
| Right insula | L medial frontal gyrus | 0 | 51 | 24 | 49 | 9 | −4.20 |
| L supplementary motor area | −12 | 15 | 66 | 38 | 6 | −5.98 | |
| Left lingual gyrus | R middle/inferior occipital gyrus | 42 | −87 | 3 | 71 | 18/19 | −4.83 |
3.2.2. ROI: right insula
The functional connections between the right insula and left medial frontal gyrus, supplementary motor area showed a significant decrease in the T2DM group (Figure 2 and Table 2).
FIGURE 2.
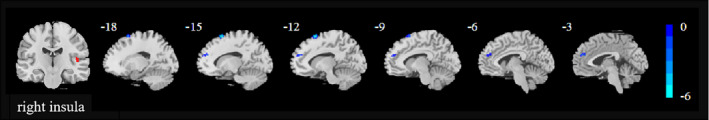
Significant differences were observed in right insula functional connectivity between the T2DM and HC groups. Thresholds were set using GRF correction at a corrected p value of <.05 (voxel p < .001, cluster size > 29)
3.2.3. ROI: left lingual gyrus
A significant decrease in the functional connection between the left lingual gyrus and right middle/inferior occipital gyrus was observed in the T2DM group (Figure 3 and Table 2).
FIGURE 3.
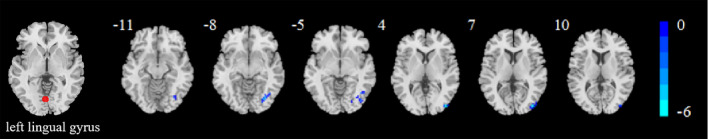
Significant differences were observed in left lingual gyrus functional connectivity between the T2DM and HC groups. Thresholds were set using GRF correction at a corrected p value of <.05 (voxel p < .001, cluster size > 29)
3.2.4. ROI: left postcentral gyrus
The functional connection between the left postcentral gyrus and other brain regions were not statistically significantly different between groups.
3.3. Correlation analysis
In the T2DM group, the functional connectivity between the right posterior cerebellum and left middle frontal gyrus, and between the right posterior cerebellum and left precuneus was negatively correlated with HbA1c (r = −.356, p = .03; r = −0.334, p = .043). However, the former significant correlation did not remain significant after Bonferroni correction. There was no correlation between abnormal functional connectivity and cognitive scores in patients with T2DM.
4. DISCUSSION
The present study used brain regions from the previous meta‐analysis as the ROIs to explore the functional connectivity patterns of these ROIs with the whole brain in patients with T2DM. We found multiple abnormal connections between different brain regions, especially the right cerebellar posterior lobe and extensive cerebral regions. In addition, the functional connectivity between the right posterior cerebellum and left middle frontal gyrus and left precuneus was negatively correlated with HbA1c in patients with T2DM. This suggests that the cerebellar–cerebral loop may be involved in neuropathological mechanisms of brain function in T2DM.
4.1. Decreased connectivity of the right posterior cerebellum in T2DM
Previous theories have suggested that the cerebellum is mainly associated with the somatosensory motor system. However, several recent studies have reported the location of motor‐related functional regions in the anterior cerebellum (Koziol et al., 2014; Stoodley & Schmahmann, 2018). In contrast, the posterior cerebellum is involved in several cognitive processes, including attention (Diano et al., 2016; Stoodley & Schmahmann, 2009), language processing (Guell et al., 2018; Stoodley & Schmahmann, 2009), working memory (Guell et al., 2018), and social cognition (Adamaszek et al., 2014; Guell et al., 2018). In contrast with the other three ROIs located in the cerebrum, the posterior cerebellum had the most widespread influence on the functioning of the whole brain in patients with T2DM in this study. Therefore, we speculated that the posterior cerebellum might participate in multiple pathways of brain dysfunction in T2DM, which deserves further verification in future research.
Studies of neuroanatomy have revealed a bidirectional projection between the cerebellum and the prefrontal cortex (Strick, Dum, & Fiez, 2009), suggesting a closed information loop of prefrontal–cerebellar in the human brain. This in turn provides the neural basis for the cerebellum to participate in multiple cognitive functions. Studies have confirmed that the prefrontal–cerebellar loop is related to working memory (Chanraud, Pitel, Muller‐Oehring, Pfefferbaum, & Sullivan, 2013) and the language network (Berl et al., 2014). Lower task‐based fMRI activation in the middle frontal gyrus is associated with poorer working memory, language, and executive function (Li et al., 2017). Our results show that the strength of functional connectivity between the right posterior cerebellum and the left middle frontal gyrus is negatively correlated with HbA1c, suggesting that the prefrontal–cerebellar loop may be involved in the neuropathology of T2DM.
The precuneus and middle temporal gyrus belong to default mode networks (DMN) and the posterior cerebellum is functionally coupled to DMN (Buckner et al., 2011). Multiple studies (Musen et al., 2012; Yang et al., 2016; Zhang et al., 2015) confirm abnormal functional connections within DMN and between DMN and other regions in patients with T2DM. In addition, one study (Yang et al., 2016) found that the connection between the bilateral posterior lobe of the cerebellum and DMN decreased and was negatively correlated with HbA1c in patients with T2DM. Our study found similar results and suggested that the decreased functional connection between the right posterior cerebellum and left precuneus was negatively correlated with HbA1c, which provides further evidence for the destruction of the connection between the cerebellum and DMN in patients with T2DM, especially the disconnection between the right posterior cerebellum and left precuneus. Patients with depression have abnormal functional connections between the cerebellum and precuneus and may predict suicidal tendencies (Zhang et al., 2016). Several studies (Stuart & Baune, 2012; O'Connor et al., 2009) suggest that the relationship between depression and T2DM is bidirectional or comorbidity. Therefore, we speculate that disconnection between the right posterior cerebellum and left precuneus may be related to emotional abnormalities in patients with T2DM.
4.2. Decreased connectivity of the right insula in T2DM
The eating patterns in T2DM are commonly altered and sometimes unhealthy, and the insula is closely related to food intake. It has been found that the insula, which contains the primary taste cortex, recognizes aromas, and has the ability to integrate smell (Cornier, 2011; Hinton et al., 2004). In addition, increased neural activity in the insula after stimulation by food cues involves the process of food compensation and manages feeding behavior (Cornier et al., 2012). The medial frontal gyrus (BA9) belongs to the dorsolateral prefrontal lobe. Current studies have demonstrated that (Gluck, Viswanath, & Stinson, 2017) the dorsolateral prefrontal lobes played an important role in diet control and food craving. Thus, the reduced functional connection between the insula and the medial frontal gyrus may be associated with a decreased ability to control food intake and abnormal eating behaviors in patients with T2DM.
A meta‐analysis of 1,768 functional neuroimaging experiments confirmed the involvement of the insula in motor function (Kurth, Zilles, Fox, Laird, & Eickhoff, 2010) and a direct structural connection between the insula and the motor cortices (Showers & Lauer, 1961). Our results show that decreased functional connectivity between the insula and the supplementary motor area (BA6) might impair motor function in patients with T2DM. It has been reported that patients with T2DM have poorer muscle performance (Bassil et al., 2011) and leg muscle strength (Park et al., 2006). In addition, one study found that female patients with T2DM have decreased fine motor function (Espeland et al., 2011). This research is consistent with our speculation.
4.3. Decreased connectivity of the left lingual gyrus in T2DM
Multiple studies (Cui et al., 2014; Wang et al., 2017) have confirmed the visual processing area of the occipital lobe is the most vulnerable region of the brain to T2DM. Cui et al. (2016) found diffusely decreased connectivity in the lingual gyrus‐related visual network in patients with T2DM. Our results were consistent with their findings. The lingual gyrus and middle occipital gyrus are important nodes of the visual network, which are crucial for the visual information processing relating to visual cognition (Zhen et al., 2018). Reduced functional connection between the lingual gyrus and middle/inferior occipital gyrus indicates visual impairment in patients with T2DM. However, due to the presence of diabetic retinopathy patients in this study, we cannot determine whether this abnormal functional connection occurs before or reflects the neural basis of retinopathy. In future studies, we will try to clarify the neural mechanism of this abnormality.
There are some limitations of the present study. First, the patient cohort in this study was relatively small. Increasing the sample size may increase the credibility of our results. Second, the blood glucose was not measured directly before neuropsychological assessment and MRI examination, further studies should take it into consideration to better elucidate the relationship between glycemic control and neural dysfunction. Third, most patients with T2DM in this study were revisiting and had a long disease duration, so our results may not be extended to patients with a short disease duration. Fourth, because all the subjects included in the previous meta‐analysis were Chinese, the results of this study may not be reproducible in other countries or ethnic populations. Finally, we did not control the T2DM complications and treatment methods during the enrollment process, which may have had a certain bias on the results. For example, metformin may affect the cognitive function of patients with diabetes (Moore et al., 2013).
5. CONCLUSIONS
In this study, data‐driven ROI was used to identify extensive changes in functional connectivity in brain regions of patients with T2DM, especially in the cerebellar–cerebral circuit. It is suggested that the cerebellar–cerebral circuit may be involved in the neuropathological basis of brain dysfunction in patients with T2DM, which provides new insight into the neural mechanisms of brain dysfunction in patients with T2DM from the perspective of the cerebellum.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
AUTHOR CONTRIBUTIONS
Dongsheng Zhang drafted the manuscript, designed the experiment, and performed the statistical analysis. Jie Gao contributed to performing the experiments and revised the manuscript. Min Tang, Xia Zhe, and Xuejiao Yan collected the data. Miao Cheng and Weibo Chen provided technical support. Xiaoling Zhang made contributions to the design of the experiment and revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank all volunteers and patients who participated in the study and the staff of the Department of MRI at the Shaanxi Provincial People's Hospital in Xi'an.
Zhang D, Gao J, Yan X, et al. Altered functional connectivity of brain regions based on a meta‐analysis in patients with T2DM: A resting‐state fMRI study. Brain Behav. 2020;10:e01725 10.1002/brb3.1725
Dongsheng Zhang and Jie Gao are contributed equally to this work.
Jie Gao is the co‐first author of this study.
The peer review history for this article is available at https://publons.com/publon/10.10.1002/brb3.1725.
Funding information
This research was supported by the National Natural Science Foundation of China (81270416), the Key Research and Development Program of Shaanxi Province of China (2018ZDXM‐SF‐038), and the Social Development Science and Technology Research Project of Shaanxi Province of China (2019SF‐131).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on requests from the corresponding author.
REFERENCES
- Adamaszek, M. , D'Agata, F. , Kirkby, K. C. , Trenner, M. U. , Sehm, B. , Steele, C. J. , … Strecker, K. (2014). Impairment of emotional facial expression and prosody discrimination due to ischemic cerebellar lesions. Cerebellum, 13, 338–345. 10.1007/s12311-013-0537-0 [DOI] [PubMed] [Google Scholar]
- Avissar, M. , Powell, F. , Ilieva, I. , Respino, M. , Gunning, F. M. , Liston, C. , & Dubin, M. J. (2017). Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimulation, 10, 919–925. 10.1016/j.brs.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil, M. , Marliss, E. B. , Morais, J. A. , Pereira, S. , Chevalier, S. , & Gougeon, R. (2011). Postprandial hyperaminoacidaemia overcomes insulin resistance of protein anabolism in men with type 2 diabetes. Diabetologia, 54, 648–656. 10.1007/s00125-010-1980-9 [DOI] [PubMed] [Google Scholar]
- Berl, M. M. , Mayo, J. , Parks, E. N. , Rosenberger, L. R. , VanMeter, J. , Ratner, N. B. , … Gaillard, W. D. (2014). Regional differences in the developmental trajectory of lateralization of the language network. Human Brain Mapping, 35, 270–284. 10.1002/hbm.22179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels, G. J. , Deary, I. J. , & Ryan, C. M. (2008). Cognition and diabetes: A lifespan perspective. The Lancet Neurology, 7, 184–190. 10.1016/S1474-4422(08)70021-8 [DOI] [PubMed] [Google Scholar]
- Biswal, B. , Yetkin, F. Z. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34, 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Buckner, R. L. , Krienen, F. M. , Castellanos, A. , Diaz, J. C. , & Yeo, B. T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 2322–2345. 10.1152/jn.00339.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud, S. , Pitel, A. L. , Muller‐Oehring, E. M. , Pfefferbaum, A. , & Sullivan, E. V. (2013). Remapping the brain to compensate for impairment in recovering alcoholics. Cerebral Cortex, 23, 97–104. 10.1093/cercor/bhr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheke, L. G. , Bonnici, H. M. , Clayton, N. S. , & Simons, J. S. (2017). Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia, 96, 137–149. 10.1016/j.neuropsychologia.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. C. , Xia, W. , Qian, C. , Ding, J. , Ju, S. , & Teng, G. J. (2015). Thalamic resting‐state functional connectivity: Disruption in patients with type 2 diabetes. Metabolic Brain Disease, 30, 1227–1236. 10.3389/fnagi.2015.00233 [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Liu, M. , & Ma, L. (2014). Resting‐state brain functional magnetic resonance imaging in patients with type 2 diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao, 34, 1083–1091. 10.3969/j.issn.1673-4254.2014.08.02 [DOI] [PubMed] [Google Scholar]
- Cole, D. M. , Smith, S. M. , & Beckmann, C. F. (2010). Advances and pitfalls in the analysis and interpretation of resting‐state FMRI data. Frontiers in Systems Neuroscience, 4, 8 10.3389/fnsys.2010.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier, M. A. (2011). Is your brain to blame for weight regain? Physiology & Behavior, 104, 608–612. 10.1016/j.physbeh.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier, M. A. , Melanson, E. L. , Salzberg, A. K. , Bechtell, J. L. , & Tregellas, J. R. (2012). The effects of exercise on the neuronal response to food cues. Physiology & Behavior, 105, 1028–1034. 10.1016/j.physbeh.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Jiao, Y. , Chen, H. J. , Ding, J. , Luo, B. , Peng, C. Y. , … Teng, G. J. (2015). Aberrant functional connectivity of default‐mode network in type 2 diabetes patients. European Radiology, 25(11), 3238–3246. 10.1007/s00330-015-3746-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Jiao, Y. , Chen, Y. C. , Wang, K. , Gao, B. , Wen, S. , … Teng, G. J. (2014). Altered spontaneous brain activity in type 2 diabetes: A resting‐state functional MRI study. Diabetes, 63, 749–760. 10.2337/db13-0519 [DOI] [PubMed] [Google Scholar]
- Cui, Y. , Li, S. F. , Gu, H. , Hu, Y. Z. , Liang, X. , Lu, C. Q. , … Teng, G. J. (2016). Disrupted brain connectivity patterns in patients with type 2 diabetes. American Journal of Neuroradiology, 37, 2115–2122. 10.3174/ajnr.A4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice, F. G. , & Lourenco, M. V. (2015). Brain metabolic stress and neuroinflammation at the basis of cognitive impairment in Alzheimer's disease. Frontiers in Aging Neuroscience, 7, 94 10.3389/fnagi.2015.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano, M. , D'Agata, F. , Cauda, F. , Costa, T. , Geda, E. , Sacco, K. , … Geminiani, G. C. (2016). Cerebellar clustering and functional connectivity during pain processing. Cerebellum, 15, 343–356. 10.1007/s12311-015-0706-4 [DOI] [PubMed] [Google Scholar]
- Du, Y. , Fryer, S. L. , Fu, Z. , Lin, D. , Sui, J. , Chen, J. , … Calhoun, V. D. (2018). Dynamic functional connectivity impairments in early schizophrenia and clinical high‐risk for psychosis. NeuroImage, 180, 632–645. 10.1016/j.neuroimage.2017.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , Fu, Z. , & Calhoun, V. D. (2018). Classification and prediction of brain disorders using functional connectivity: Promising but challenging. Frontiers in Neuroscience, 12, 525 10.3389/fnins.2018.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland, M. A. , Miller, M. E. , Goveas, J. S. , Hogan, P. E. , Coker, L. H. , Williamson, J. , … Resnick, S. M. (2011). Cognitive function and fine motor speed in older women with diabetes mellitus: Results from the women's health initiative study of cognitive aging. Journal of Women's Health, 20, 1435–1443. 10.1089/jwh.2011.2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck, M. E. , Viswanath, P. , & Stinson, E. J. (2017). Obesity, appetite, and the prefrontal cortex. Current Obesity Reports, 6, 380–388. 10.1007/s13679-017-0289-0 [DOI] [PubMed] [Google Scholar]
- Guell, X. , Gabrieli, J. D. E. , & Schmahmann, J. D. (2018). Triple representation of language, working memory, social and emotion processing in the cerebellum: Convergent evidence from task and seed‐based resting‐state fMRI analyses in a single large cohort. NeuroImage, 172, 437–449. 10.1016/j.neuroimage.2018.01.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton, E. C. , Parkinson, J. A. , Holland, A. J. , Arana, F. S. , Roberts, A. C. , & Owen, A. M. (2004). Neural contributions to the motivational control of appetite in humans. The European Journal of Neuroscience, 20, 1411–1418. 10.1111/j.1460-9568.2004.03589.x [DOI] [PubMed] [Google Scholar]
- Iyalomhe, O. , Swierczek, S. , Enwerem, N. , Chen, Y. , Adedeji, M. O. , Allard, J. , … Obisesan, T. O. (2017). The role of hypoxia‐inducible factor 1 in mild cognitive impairment. Cellular and Molecular Neurobiology, 37, 969–977. 10.1007/s10571-016-0440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol, L. F. , Budding, D. , Andreasen, N. , D'Arrigo, S. , Bulgheroni, S. , Imamizu, H. , … Yamazaki, T. (2014). Consensus paper: The cerebellum's role in movement and cognition. Cerebellum, 13, 151–177. 10.1007/s12311-013-0511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth, F. , Zilles, K. , Fox, P. T. , Laird, A. R. , & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Structure & Function, 214, 519–534. 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Wang, W. , Wang, A. , Li, P. , Zhang, J. , Tao, W. , & Zhang, Z. (2017). Vulnerability of the frontal and parietal regions in hypertensive patients during working memory task. Journal of Hypertension, 35, 1044–1051. 10.1097/HJH.0000000000001250 [DOI] [PubMed] [Google Scholar]
- Liu, D. , Duan, S. , Zhang, J. , Zhou, C. , Liang, M. , Yin, X. , … Wang, J. (2016). Aberrant brain regional homogeneity and functional connectivity in middle‐aged T2DM patients: A resting‐state functional MRI study. Frontiers in Human Neuroscience, 10, 490 10.3389/fnhum.2016.00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson, H. , Formica, M. , Harris, E. , & Daly, R. M. (2017). Brain functional alterations in type 2 diabetes ‐ a systematic review of fMRI studies. Frontiers in Neuroendocrinology, 47, 34–46. 10.1016/j.yfrne.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Moore, E. M. , Mander, A. G. , Ames, D. , Kotowicz, M. A. , Carne, R. P. , Brodaty, H. , … Investigators, A. (2013). Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care, 36, 2981–2987. 10.2337/dc13-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen, G. , Jacobson, A. M. , Bolo, N. R. , Simonson, D. C. , Shenton, M. E. , McCartney, R. L. , … Hoogenboom, W. S. (2012). Resting‐state brain functional connectivity is altered in type 2 diabetes. Diabetes, 61, 2375–2379. 10.2337/db11-1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, P. J. , Crain, A. L. , Rush, W. A. , Hanson, A. M. , Fischer, L. R. , & Kluznik, J. C. (2009). Does diabetes double the risk of depression? The Annals of Family Medicine, 7, 328–335. 10.1370/afm.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. W. , Goodpaster, B. H. , Strotmeyer, E. S. , de Rekeneire, N. , Harris, T. B. , Schwartz, A. V. , … Newman, A. B. (2006). Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes, 55, 1813–1818. 10.2337/db05-1183 [DOI] [PubMed] [Google Scholar]
- Peng, J. , Qu, H. , Peng, J. , Luo, T.‐Y. , Lv, F.‐J. , chen, L. I. , … Cheng, Q.‐F. (2016). Abnormal spontaneous brain activity in type 2 diabetes with and without microangiopathy revealed by regional homogeneity. European Journal of Radiology, 85, 607–615. 10.1016/j.ejrad.2015.12.024 [DOI] [PubMed] [Google Scholar]
- Showers, M. J. , & Lauer, E. W. (1961). Somatovisceral motor patterns in the insula. The Journal of Comparative Neurology, 117, 107–115. 10.1002/cne.901170109 [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2009). Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. NeuroImage, 44, 489–501. 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2018). Functional topography of the human cerebellum. Handbook of Clinical Neurology, 154, 59–70. 10.1016/B978-0-444-63956-1.00004-7 [DOI] [PubMed] [Google Scholar]
- Strick, P. L. , Dum, R. P. , & Fiez, J. A. (2009). Cerebellum and nonmotor function. Annual Review of Neuroscience, 32, 413–434. 10.1146/annurev.neuro.31.060407.125606 [DOI] [PubMed] [Google Scholar]
- Stuart, M. J. , & Baune, B. T. (2012). Depression and type 2 diabetes: Inflammatory mechanisms of a psychoneuroendocrine co‐morbidity. Neuroscience & Biobehavioral Reviews, 36, 658–676. 10.1016/j.neubiorev.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Sun, Q. , Chen, G. Q. , Wang, X. B. , Yu, Y. , Hu, Y. C. , Yan, L. F. , … Cui, G. B. (2018). Alterations of white matter integrity and hippocampal functional connectivity in type 2 diabetes without mild cognitive impairment. Frontiers in Neuroanatomy, 12, 21 10.3389/fnana.2018.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. X. , Fu, K. L. , Liu, H. J. , Xing, F. , & Zhang, S. Y. (2014). Spontaneous brain activity in type 2 diabetics revealed by amplitude of low‐frequency fluctuations and its association with diabetic vascular disease: A resting‐state fMRI study. PLoS One, 9, e108883 10.1371/journal.pone.0108883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. L. , Zou, L. , Lu, Z. W. , Xie, X. Q. , Jia, Z. Z. , Pan, C. J. , … Ge, X. M. (2017). Abnormal spontaneous brain activity in type 2 diabetic retinopathy revealed by amplitude of low‐frequency fluctuations: A resting‐state fMRI study. Clinical Radiology, 72(4), 340.e1–340.e7. 10.1016/j.crad.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Woolley, J. D. , Gorno‐Tempini, M. L. , Seeley, W. W. , Rankin, K. , Lee, S. S. , Matthews, B. R. , & Miller, B. L. (2007). Binge eating is associated with right orbitofrontal‐insular‐striatal atrophy in frontotemporal dementia. Neurology, 69, 1424–1433. 10.1212/01.wnl.0000277461.06713.23 [DOI] [PubMed] [Google Scholar]
- Xia, W. , Luo, Y. , Chen, Y. C. , Zhang, D. , Bo, F. , Zhou, P. , … Ma, J. (2018). Disrupted functional connectivity of the amygdala is associated with depressive mood in type 2 diabetes patients. Journal of Affective Disorders, 228, 207–215. 10.1016/j.jad.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Xia, W. , Wang, S. , Sun, Z. , Bai, F. , Zhou, Y. I. , Yang, Y. , … Yuan, Y. (2013). Altered baseline brain activity in type 2 diabetes: A resting‐state fMRI study. Diabetologia, 56, S41–S42. 10.1016/j.psyneuen.2013.05.012 [DOI] [PubMed] [Google Scholar]
- Yang, S. Q. , Xu, Z. P. , Xiong, Y. , Zhan, Y. F. , Guo, L. Y. , Zhang, S. , … Zhu, W. Z. (2016). Altered intranetwork and internetwork functional connectivity in type 2 diabetes mellitus with and without cognitive impairment. Scientific Reports, 6, 32980 10.1038/srep32980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. S. , Gao, J. , Zhe, X. , Yan, X. J. , Tang, M. , Yang, J. , & Zhang, X. L. (2018). Altered spontaneous brain activity in type 2 diabetes mellitus: An activation likelihood estimation Meta‐analysis (article in Chinese). Chinese Journal of Radiology, 52, 241–246. 10.3760/cma.j.issn.1005-1201.2018.04.001 [DOI] [Google Scholar]
- Zhang, H. , Hao, Y. , Manor, B. , Novak, P. , Milberg, W. , Zhang, J. , … Novak, V. (2015). Intranasal insulin enhanced resting‐state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes, 64, 1025–1034. 10.2337/db14-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Chen, J. M. , Kuang, L. , Cao, J. , Zhang, H. , Ai, M. , … Fang, W. D. (2016). Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry, 16, 337 10.1186/s12888-016-1047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen, D. , Xia, W. , Yi, Z. Q. , Zhao, P. W. , Zhong, J. G. , Shi, H. C. , … Pan, P. L. (2018). Alterations of brain local functional connectivity in amnestic mild cognitive impairment. Translational Neurodegeneration, 7, 26 10.1186/s40035-018-0134-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Zhang, J. , Chen, Y. , Ma, T. , Wang, Y. , Wang, J. , & Zhang, Z. (2014). Aggravated cognitive and brain functional impairment in mild cognitive impairment patients with type 2 diabetes: A resting‐state functional MRI study. Journal of Alzheimer's Disease, 41, 925–935. 10.3233/JAD-132354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on requests from the corresponding author.


