Abstract
Congenital heart disease (CHD) is the most common anatomical malformation occurring live‐born infants and an increasing cause of morbidity and mortality across the lifespan and throughout the world. Population‐based observations have long described associations between maternal cardiometabolic disorders and the risk of CHD in the offspring. Here we review the epidemiological evidence and clinical observations relating maternal obesity and diabetes mellitus to the risk of CHD offspring with particular attention to mechanistic models of maternal‐fetal risk transmission and first trimester disturbances of fetal cardiac development. A deeper understanding of maternal risk factors holds the potential to improve both prenatal detection of CHD by identifying at‐risk pregnancies, along with primary prevention of disease by improving preconception and prenatal treatment of at‐risk mothers.
Keywords: cardiometabolic, congenital heart disease, maternal diabetes mellitus, maternal obesity
Subject Categories: Obesity; Pediatrics; Women; Diabetes, Type 2; Diabetes, Type 1
Among anatomical malformations present at birth, congenital heart disease (CHD) is the most common, occurring in 0.8% to 1% of live‐born infants, and is increasing in prevalence worldwide.1 In the current practice of neonatal and pediatric cardiothoracic surgery and perioperative care, the survival of children with CHD approaches 95% to 99% depending on the severity of disease.2 However, childhood survivors of CHD are impacted by neurodevelopmental differences,3 whereas adult survivors of CHD are burdened with adult‐onset cardiovascular disease,4 neuropsychiatric disease,5 and cancer.6 From infancy through adulthood, CHD continues to be an important and increasing proportion of the population at increased risk of morbidity and mortality.
Although the past decade has seen advances in our understanding of the genetic basis of CHD,7 maternal diabetes mellitus occurring during early pregnancy has been recognized as a risk factor for disease for many decades.8 More recently, population‐based observations have described associations between risk of CHD in the offspring with other maternal cardiometabolic disorders such as obesity.9 The significant phenotypic overlap between diabetes mellitus, obesity, and cardiometabolic risk is complex; it is not yet established which of these factors is causal for risk to the fetus when present in the mother during early pregnancy (Figure).
Figure 1.
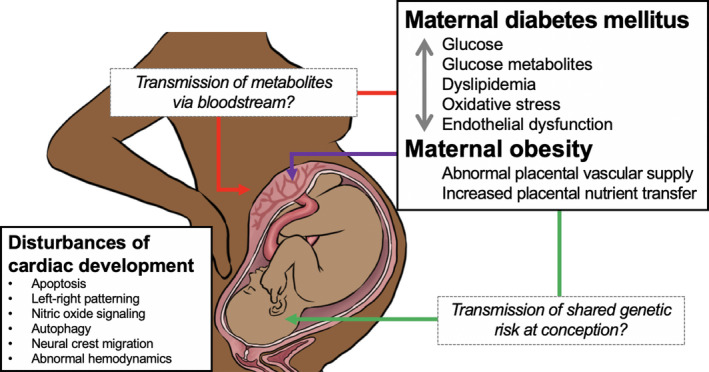
Potential mechanisms for transmission of maternal metabolic risk for congenital heart disease (CHD) in the fetus.
Illustration of potential mechanisms of transmission of maternal factors during pregnancy influencing risk for CHD in offspring. Maternal diabetes mellitus and obesity share a variety of intermediate phenotypes (bidirectional gray arrow), which could be transmissible from mother to fetus in the blood across the placenta (red arrow) or transmitted genetically at the time of conception by pleiotropic variants, conferring risk for both metabolic phenotypes and CHD (green arrow). Specific differences in placental function related to maternal obesity may also contribute to risk (purple arrow). Experimental models have suggested a variety of potential mechanisms by which maternal metabolic factors may disturb development of the heart, which occurs early in pregnancy during the first trimester.
General Relationship: Maternal Obesity
Mirroring trends in the general population, both the rate and severity of maternal obesity has increased at an alarming rate during recent years.10 In European countries, 7% to 25% of expectant mothers are overweight,11 and in the United States only 45% of mothers have a normal weight when becoming pregnant.10
Maternal obesity is associated with adverse pregnancy outcomes, neonatal complications, and morbidity. These include stillbirth,12 macrosomia,13, 14 shoulder dystocia,14 preterm delivery,15 and congenital malformations, such as neural tube defects,16, 17 omphalocele,17, 18and CHD.17, 18, 19, 20, 21, 22 Moreover, a dose‐dependent association has also been observed whereby severity of maternal obesity is directly associated with risk for adverse neonatal outcomes.9, 23
Several recent meta‐analyses consistently report a general association between maternal overweight and obesity and risk for congenital heart defects in the offspring.24, 25, 26 The increased risk associated with maternal obesity includes a wide range of different cardiac defects, including septal defects,9, 22, 27 aortic arch defects,9 persistent ductus arteriosus,9, 21 conotruncal defects,9, 27, 28, 29, 30 left ventricular outflow tract obstruction defects,29 and right ventricular outflow tract defects.21, 28 The association of risk regarding specific CHD subtypes, however, has not been universally consistent in different studies. One source of bias could be the fact that body mass index (BMI) estimations in many of these studies were based on retrospective of self‐reported data, which are associated with recall bias. In addition, many of the studies report only prenatally or neonatally diagnosed defects. Given that noncritical CHD may not cause symptoms at birth and diagnosed later in life, these studies might be under‐reporting CHD rates. Finally, many of the studies are case‐control studies, which provide estimates of risk that may be less reliable than prospective, population‐based cohort studies for estimating prevalence.
The largest single study thus far, including a national cohort of 2 050 491 live‐born singleton infants in Sweden, showed that maternal obesity measured at the first antenatal visit increased the risk to offspring for transposition of great arteries in those with a BMI of 35 to 40 and >40 kg/m2, aortic arch defects in those with a BMI of 30 to 35, 35 to 40, and >40 kg/m2, single‐ventricle heart in a group of mothers with a BMI of 30 to 35 kg/m2, and atrial septal defect and patent ductus arteriosus in mothers with a BMI of >25 kg/m2. In addition, the risk for pulmonary valve defects was increased in offspring of mothers with a BMI of 30 to 35 kg/m2.9 The strengths of this study included a population‐based design with prospectively collected data on both exposure and outcome in a country with publicly funded health care, but did not take into account pregnancies with CHD that resulted in termination or stillbirth.
The risk for CHD also appears to increase with the severity of obesity. The study by Persson et al from Sweden demonstrated that the risk for congenital malformations, including CHD, progressively increased with BMI from overweight to severe obesity.31 When focusing on specific CHD groups, aortic arch defects, atrial septal defect, and patent ductus arteriosus presented with a dose‐responsive association.9 In addition, a similar dose‐responsive association has been reported in hypoplastic left heart syndrome and right outflow tract defects.21
Potential Mechanisms of Risk in Maternal Obesity
The precise mechanism by which maternal obesity impacts critical stages of cardiac development is not known and is hypothesized to be multifactorial. Whereas the mechanisms of maternal obesity in later gestation on placental function and fetal growth have been under active research during recent years, early pregnancy has received less attention.
Maternal prepregnancy obesity is known to be associated with increased risk for gestational diabetes mellitus,32 and it is likely that some of the effect in obese individuals may be mediated by glycemic dysregulation. However, the CHD‐risk increase has remained significant even after adjusting for glucose levels, suggesting that abnormalities in glucose metabolism do not fully explain the risk in obese mothers.27 In addition to glycemic dysregulation, a wide range of metabolic abnormalities are present in obese individuals. Obesity is associated with hyperinsulinemia and insulin resistance,33, 34 dyslipidemia,33, 35 and oxidative stress.35 In pregnancy, gestational diabetes mellitus increases low‐density lipoprotein susceptibility to oxidation, and obesity has been further shown to exacerbate this effect.36 Compared with nonobese women, obese mothers may display differential fat distribution, where nonobese women accumulate fat in the lower body whereas obese women accumulate fat in the upper body.37 Upper‐body obesity is associated with reduced uptake and storage of fatty acids, along with increases in lipolysis.38 In contrast, lower‐body fat accumulation is associated with more‐favorable lipid and carbohydrate metabolic dysregulation39 and an overall lower‐risk metabolic profile.40 Thus, the potential negative effects of adverse metabolic changes related to fat accumulation during pregnancy are more profound in obese individuals, which may contribute to increased levels of adverse effects in the fetus.
Fetal macrosomia associated with maternal obesity has been proposed to arise from an increased placental nutrient transfer, related, at least partly, to adiponectin levels. Circulating adiponectin levels are lower in obese individuals41 and remain lower in obese individuals throughout pregnancy.42 Lower adiponectin levels during pregnancy have been associated with placental insulin resistance43, 44 and adverse placental function, in terms of increased placental nutrient transfer45, 46, 47 and increased fetal growth.48 Development of the pancreas and production of insulin do not occur until the beginning of the second trimester; therefore, during the period of heart development during the early first trimester, the fetus is unable to regulate glucose and may be susceptible to adiponectin‐related dysfunction in placental glucose transport.49
Endothelial cell dysfunction in mice lacking endothelial nitric oxide synthase during embryogenesis has been shown to cause CHD in mice.50, 51 Obesity causes chronic pre‐existing endothelial activation and impairment of endothelial function,33, 52, 53, 54 as well as inflammatory upregulation.33 Bioavailability of nitric oxide, a regulator of vascular tone, is decreased in endothelial cell dysfunction. Insulin55 and adiponectin56 activate endothelial nitric oxide synthase, whereas in obesity and diabetes mellitus these protective mechanisms are diminished. Maternal obesity is associated with increased abnormalities in placental vascular supply, and it has been shown to have an adverse effect on fetal vascular circulation.57 Thus, the endothelial dysregulation present in obese mothers may extend to the fetal circulation to impact developmental pathways in the fetus. Moreover, the effect may persist after birth, given that it has been observed that offspring of nonhuman primates exposed to a high‐fat diet during pregnancy have impaired endothelial function >1 year after birth.58
Finally, it has been proposed that some of the CHD risk increase is mediated by a lower diagnostic rate in pregnancy screenings in obese individuals, given that cardiac views during pregnancy are suboptimal in obese mothers. Decreased sensitivity of ultrasound for cardiac anatomy has been documented in obese mothers.59, 60, 61 Whereas rates of pregnancy termination are difficult to ascertain and compare between studies, it is possible that differences in diagnostic rates could affect termination rates, leading to a higher share of CHD pregnancies carried to birth in obese mothers with lower diagnostic rates. However, in a recent study of an advanced nation‐wide CHD screening program within a country with universal health coverage, obesity or other maternal risk factors for offspring severe heart disease did not appear to affect prenatal detection as such.30
Impact of Treatment and Prenatal Care
Lifestyle interventions aiming to restrict weight gain in obese women during pregnancy are seen as a means to reduce adverse outcomes related to obesity. A healthier diet during the year before pregnancy has been shown to decrease the risk for conotruncal and septal defects in the offspring,62 and one‐carbon‐rich dietary pattern during pregnancy, characterized by a high intake of fish and seafood, has been associated with a reduced risk of overall CHD.63 Maternal malnutrition and especially folate deficiency has been associated with CHD in the offspring,64 and there is evidence that obese mothers may have an insufficient response to folic acid supplementation for primary prevention of congenital anomalies.65, 66 Lifestyle interventions for expectant mothers with obesity and/or previous gestational diabetes mellitus during pregnancy have not, however, resulted in an effect on gestational weight gain, or obstetric or perinatal outcomes.67 It has been suggested that obesity could be associated with a lower compliance for following nutritional recommendations.68 Moreover, prepregnancy BMI is a stronger predictor for adverse outcomes as compared with gestational weight gain.69 These results indicate that lifestyle interventions should be increasingly aimed at mothers planning pregnancy. Interestingly, certain genetic risk variants have been shown to modify the effectiveness of lifestyle interventions,70 which might affect targeting of such interventions in the future.
Several animal studies have addressed interventions to improve the outcome of obese pregnancies. Exercise has been shown to prevent adverse effects of maternal obesity on placental vascularization and fetal growth.71 In a mouse model, exercise in obese pregnancy was beneficial to offspring cardiac function and structure.72 Adiponectin levels are lower in obese mothers, and adiponectin supplementation of mice during late pregnancy reversed the adverse effects of maternal obesity on placental function and fetal growth.73, 74 Moreover, although these interventions showed to be beneficial in terms of maternal and fetal health, none of these studies specifically addressed CHD as an outcome.
General Relationship: Glycemic Regulation
The association between maternal diabetes mellitus and CHD in offspring has been recognized for almost 80 years.8 The underlying pathology of diabetes mellitus is a mismatch between insulin production and response to insulin resulting in elevated glucose levels. Type 1 diabetes mellitus is attributable primarily to the absence of pancreatic insulin secretion originating from autoimmune destruction of beta cells. Type 2 and gestational diabetes mellitus arise from an increased requirement for insulin for intracellular transport of glucose in peripheral tissues, a now well‐described physiological phenomenon of insulin resistance implicated in the pathophysiology of a variety of adult‐onset diseases.75 Maternal diabetes mellitus is a risk factor for adverse maternal and fetal outcomes, including anatomical malformations such as CHD.76, 77 Risk for CHD in offspring is present in mothers with all types of disease, such as type 178, 79 or 279, 80 diabetes mellitus existing before pregnancy, along with gestational diabetes mellitus developing during pregnancy.81, 82
In large, population‐based studies, maternal diabetes mellitus appears to be a strong risk factor for any and all subtypes of CHD.81, 83 Individual studies hint at a higher risk for conotruncal and laterality subtypes of CHD83, 84; however, comparisons between subtypes are limited by low prevalence of individual malformations present even in large cohorts. For syndromic causes of CHD with a known genetic etiology, such as Down syndrome, maternal diabetes mellitus is not recognized as a cofactor for cardiac malformation in the fetus.85 On a population level, exposure to prepregnancy diabetes mellitus was estimated to be responsible for up to 4.2% of CHD within a regional Canadian health system.80
Cardiac development occurs during the first trimester and is largely complete by the sixth week of pregnancy; thus, maternal physiology and metabolism during the early first trimester is most relevant to the developing fetal heart. Hemoglobin A1C values measured during the first trimester are associated with risk for CHD in offspring,86, 87 and women with pre‐existing diabetes mellitus who experienced a greater number of diabetic complications or had a greater hemoglobin A1C appear to be at increased risk of having a child with CHD.78, 83 Our own recent data suggest that risk for CHD extends to pregnancies of women who may not carry a clinical diagnosis of diabetes mellitus; abnormalities of glucose metabolism below standard diagnostic thresholds for diabetes mellitus are associated with measurable risk for CHD in offspring.88, 89 Thus, risk of CHD in offspring is directly correlated with abnormalities in glucose metabolism in pregnancies with and without diabetes mellitus.
Mechanism of Risk
The mechanism by which presence of maternal diabetes mellitus during critical stages of cardiac development is not clear. The earliest experiments simply treated chicken and rodent embryos with exogenous glucose, which resulted in malformations in many organ systems including cardiac defects.90, 91 Experimentally supported mechanisms proposed to alter cardiac development include glucose‐mediated disturbances of left‐right patterning,92 increased apoptosis resulting from oxidative or other cellular stress,93, 94 deficiencies in nitric oxide signaling,95 impaired autophagy,96 and alterations of neural crest cell formation and migration.97, 98 Deriving from early descriptions of the teratogenic potential of glucose alone, ex vivo models of cardiac development have substituted treatment with supraphysiological levels of glucose as a proxy for maternal diabetes mellitus. However, alterations in maternal glucose are accompanied by changes in downstream metabolites of glycolysis, such as beta‐hydroyxbutyrate,99 and the impact of downstream metabolites of glucose upon cardiac development remains relatively unexplored.100, 101, 102 Accompanying these mechanistic hypotheses, experimental models of maternal diabetes mellitus have also described the disruption of canonical signaling pathways during mesodermal differentiation and cardiac development.103 The variety of proposed cellular models and molecular mechanisms, none of which are mutually exclusive, highlights the need for further research into how maternal diabetes mellitus disturbs fetal heart development (Figure).
Impact of Treatment and Prenatal Care
Like maternal obesity, maternal diabetes mellitus is associated with a variety of adverse pregnancy outcomes, including pre‐eclampsia, prematurity, fetal demise, and stillbirth.77 These outcomes have prompted public health efforts to improve preconception and prenatal diagnosis and treatment for diabetes mellitus. In a meta‐analysis studying prenatal care, standard treatment of maternal diabetes mellitus resulted in ~75% reduction in risk of anatomical malformations in offspring inclusive of CHD.104 Newer technological approaches to diabetes mellitus care, including continuous glucose monitoring and continuous subcutaneous insulin injection, are in common use by women of childbearing age,105 and randomized controlled trials using these technologies demonstrate incremental improvements in measures of glucose control and improvements in some measures of pregnancy and fetal outcome.106 Simulations suggest that in the US population, achieving glycemic control in all women before pregnancy has the potential to reduce rates of CHD by 3.8% or 2670 cases per year.107
In addition to standard care of diabetes mellitus before and during pregnancy, other routine interventions have been trialed in pregnant women with maternal diabetes mellitus with the goal of preventing adverse fetal and maternal outcomes. Exercise during pregnancy is safe and reduces maternal glucose levels,108 but there is inadequate evidence to assess any impact of maternal exercise on fetal outcomes.109 Trials to gauge the impact of dietary interventions during pregnancy upon maternal and fetal outcomes are ongoing.110 Conversely, observational studies suggest that exposure to either metformin or beta‐blockers during pregnancy, both of which reduce glucose levels, may actually increase the risk of certain types of CHD in the fetus.111, 112 In summary, routine adjunctive interventions targeted at glucose reduction in maternal diabetes mellitus have yet to demonstrate improvements in fetal outcomes, such as CHD, in appropriately controlled trials.
Novel interventions centered on proposed mechanisms of disease have arisen from experimental animal models of maternal diabetes mellitus. Pharmacological agents, which ameliorate oxidative stress, have been reported to prevent cardiac malformations; in a chick model of maternal diabetes mellitus, coinjection of N‐acetyl cysteine with glucose prevented heart malformations caused by injection of glucose alone.113 In a mouse model of type 1 diabetes mellitus, neural tube defects (also associated with maternal diabetes mellitus) were prevented by maternal ingestion of trehalose, a polysaccharide with antioxidant properties.114 Nitric oxide is a key vascular signaling molecule synthesized in the smooth muscle and endothelium, which is disturbed in diabetes mellitus115; oral supplementation of diabetic mice with a cofactor for nitric oxide synthase reduced the rates of CHD in offspring.95 However, given the observation that even clinically accepted interventions to reduce maternal glucose fail to impact the rate of fetal malformations during pregnancy and an absence of consensus on the mechanism of risk, the prospect of prenatal interventions derived from experimental animal models should be viewed with caution.
Knowledge Gaps and Future Directions
CHD causes high levels of physical, emotional, and economic burden for the patient, their family, and society at large. Although maternal obesity and glucose metabolism are clearly associated with the risk of CHD, the mechanisms by which risk is transmitted from mother to fetus and the causal factors which disturb fetal cardiac development remain poorly defined (Figure). Understanding the causal factors and mechanism of transmission will provide the necessary framework for addressing 2 important real‐world outcomes; primary prevention of CHD and improving prenatal screening for CHD.
Given that neonatal and childhood surgery are likely to be the mainstay of treatment for the foreseeable future, and that cardiopulmonary bypass and perioperative disturbances in physiology may contribute to the adverse health outcomes in long‐term survivors of CHD,116, 117 primary prevention of disease is an important goal with potentially significant benefit to public health. Obesity and diabetes mellitus are both potentially modifiable maternal risk factors for CHD, each with effective evidence‐based therapies generally and in the context of maternal health.118, 119 With a clear understanding of the mechanism of risk transmission from mother to fetus, large‐scale trials of public health interventions focused upon causal factors underlying maternal obesity and glucose metabolism with specific attention to fetal outcomes are needed. Where possible, fetal outcomes data inclusive of cardiac malformations should be scrutinized from ongoing trials of dietary interventions110 and innovations in glucose control106 in order to guide efforts prospective interventions for CHD. Lifestyle factors, such as weight, physical activity, and dietary habits, represent potential targets for preconception and prenatal interventions for CHD prevention.
A key component of prenatal care is the in utero identification of pregnancies with CHD as early as possible. An improved understanding of the maternal risk factors for carrying a pregnancy impacted by CHD holds the potential to improve both prenatal screening and postnatal care. Improved risk stratification of pregnant women may allow for better selection of pregnancies at the greatest risk of CHD for prenatal screening by fetal echocardiogram,120 particularly in health systems with less‐organized screening programs.30, 121 In pregnancies with CHD which are carried to term,122 prenatal detection also allows early referral to a tertiary center to optimize the delivery and early care, and thus improved prenatal screening is likely to improve early survival and long‐term outcomes of children affected with CHD.123, 124
Although the significance of maternal glucose metabolism and obesity as risk factors for CHD is clear, the mechanisms underlying these risks are not. A deep mechanistic understanding of causal maternal factors holds the potential to improve both prevention of CHD by preconception and prenatal treatment of causal maternal factors, and to improve prenatal screening and in utero identification of CHD by measuring causal maternal factors to identify pregnancies at highest risk. The molecular mechanisms of maternal risk and potential genetic modifiers of these factors represent an outstanding opportunity where advances from basic, translational, and clinical research are poised to yield real‐world applications to reduce the burden of disease.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e011541 DOI: 10.1161/JAHA.119.011541.)
References
- 1. Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta‐analysis of 260 studies. Int J Epidemiol. 2019;48:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin GR, Jonas RA. Surgery for congenital heart disease: improvements in outcomes. Am J Perinatol. 2018;35:557–560. [DOI] [PubMed] [Google Scholar]
- 3. Calderon J, Stopp C, Wypij D, DeMaso DR, Rivkin M, Newburger JW, Bellinger DC. Early‐term birth in single‐ventricle congenital heart disease after the fontan procedure: neurodevelopmental and psychiatric outcomes. J Pediatr. 2016;179:96–103. [DOI] [PubMed] [Google Scholar]
- 4. Saha P, Potiny P, Rigdon J, Morello M, Tcheandjieu C, Romfh A, Fernandes SM, McElhinney DB, Bernstein D, Lui GK, et al. Substantial cardiovascular morbidity in adults with lower‐complexity congenital heart disease. Circulation. 2019;139:1889–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kasmi L, Bonnet D, Montreuil M, Kalfa D, Geronikola N, Bellinger DC, Calderon J. Neuropsychological and psychiatric outcomes in dextro‐transposition of the great arteries across the lifespan: a state‐of‐the‐art review. Front Pediatr. 2017;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. [DOI] [PubMed] [Google Scholar]
- 7. Blue GM, Kirk EP, Giannoulatou E, Sholler GF, Dunwoodie SL, Harvey RP, Winlaw DS. Advances in the genetics of congenital heart disease: a clinician's guide. J Am Coll Cardiol. 2017;69:859–870. [DOI] [PubMed] [Google Scholar]
- 8. The infants of diabetic mothers. BMJ. 1940;1:1064. [Google Scholar]
- 9. Persson M, Razaz N, Edstedt Bonamy AK, Villamor E, Cnattingius S. Maternal overweight and obesity and risk of congenital heart defects. J Am Coll Cardiol. 2019;73:44–53. [DOI] [PubMed] [Google Scholar]
- 10. Deputy NP, Dub B, Sharma AJ. Prevalence and trends in prepregnancy normal weight—48 states, New York City, and District of Columbia, 2011–2015. MMWR Morb Mortal Wkly Rep. 2018;66:1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devlieger R, Benhalima K, Damm P, Van Assche A, Mathieu C, Mahmood T, Dunne F, Bogaerts A. Maternal obesity in Europe: where do we stand and how to move forward?: a scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur J Obstet Gynecol Reprod Biol. 2016;201:203–208. [DOI] [PubMed] [Google Scholar]
- 12. Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre‐pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112:403–408. [DOI] [PubMed] [Google Scholar]
- 13. Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Usha Kiran TS, Hemmadi S, Bethel J, Evans J. Outcome of pregnancy in a woman with an increased body mass index. BJOG. 2005;112:768–772. [DOI] [PubMed] [Google Scholar]
- 15. Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikström AK, Granath F. Maternal obesity and risk of preterm delivery. JAMA. 2013;309:2362–2370. [DOI] [PubMed] [Google Scholar]
- 16. Waller DK, Mills JL, Simpson JL, Cunningham GC, Conley MR, Lassman MR, Rhoads GG. Are obese women at higher risk for producing malformed offspring? Am J Obstet Gynecol. 1994;170:541–548. [DOI] [PubMed] [Google Scholar]
- 17. Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega‐Riz AM, Gallaway MS, Correa A; National Birth Defects Prevention Study . Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161:745–750. [DOI] [PubMed] [Google Scholar]
- 18. Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003;111:1152–1158. [PubMed] [Google Scholar]
- 19. Moore LL, Singer MR, Bradlee ML, Rothman KJ, Milunsky A. A prospective study of the risk of congenital defects associated with maternal obesity and diabetes mellitus. Epidemiology. 2000;11:689–694. [DOI] [PubMed] [Google Scholar]
- 20. Mills JL, Troendle J, Conley MR, Carter T, Druschel CM. Maternal obesity and congenital heart defects: a population‐based study. Am J Clin Nutr. 2010;91:1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madsen NL, Schwartz SM, Lewin MB, Mueller BA. Prepregnancy body mass index and congenital heart defects among offspring: a population‐based study. Congenit Heart Dis. 2013;8:131–141. [DOI] [PubMed] [Google Scholar]
- 22. Cedergren MI, Källén BAJ. Maternal obesity and infant heart defects. Obes Res. 2003;11:1065–1071. [DOI] [PubMed] [Google Scholar]
- 23. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta‐analysis. JAMA. 2014;311:1536–1546. [DOI] [PubMed] [Google Scholar]
- 24. Cai GJ, Sun XX, Zhang L, Hong Q. Association between maternal body mass index and congenital heart defects in offspring: a systematic review. Am J Obstet Gynecol. 2014;211:91–117. [DOI] [PubMed] [Google Scholar]
- 25. Zhu Y, Chen Y, Feng Y, Yu D, Mo X. Association between maternal body mass index and congenital heart defects in infants: a meta‐analysis. Congenit Heart Dis. 2018;13:271–281. [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Ding G, Yang W, Feng X, Li Y, Liu H, Zhang Q, Ji L, Li D. Maternal body mass index and risk of congenital heart defects in infants: a dose‐response meta‐analysis. Biomed Res Int. 2019;2019:1315796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brite J, Laughon SK, Troendle J, Mills J. Maternal overweight and obesity and risk of congenital heart defects in offspring. Int J Obes. 2014;38:878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Block SR, Watkins SM, Salemi JL, Rutkowski R, Tanner JP, Correia JA, Kirby RS. Maternal pre‐pregnancy body mass index and risk of selected birth defects: evidence of a dose‐response relationship. Paediatr Perinat Epidemiol. 2013;27:521–531. [DOI] [PubMed] [Google Scholar]
- 29. Simeone RM, Tinker SC, Gilboa SM, Agopian AJ, Oster ME, Devine OJ, Honein MA; National Birth Defects Prevention Study . Proportion of selected congenital heart defects attributable to recognized risk factors. Ann Epidemiol. 2016;26:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hautala J, Gissler M, Ritvanen A, Tekay A, Pitkänen‐Argillander O, Stefanovic V, Sarkola T, Helle E, Pihkala J, Pätilä T, et al. The implementation of a nationwide anomaly screening programme improves prenatal detection of major cardiac defects: an 11‐year national population‐based cohort study. BJOG. 2019;126:864–873. [DOI] [PubMed] [Google Scholar]
- 31. Persson M, Cnattingius S, Villamor E, Söderling J, Pasternak B, Stephansson O, Neovius M. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ. 2017;357:j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Torloni MR, Betrbn AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, Valente O. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta‐analysis. Obes Metabol. 2009;6:48–49. [DOI] [PubMed] [Google Scholar]
- 33. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–4237. [DOI] [PubMed] [Google Scholar]
- 34. Catalano PM, Ehrenberg HM. Review article: the short‐ and long‐term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. [DOI] [PubMed] [Google Scholar]
- 35. Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich‐Horvat P, Larson MG, Keaney JF Jr, Meigs JB, Lipinska I, Kathiresan S, et al. Visceral and subcutaneous adipose tissue volumes are cross‐sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. [DOI] [PubMed] [Google Scholar]
- 36. Sánchez‐Vera I, Bonet B, Viana M, Quintanar A, Martín MD, Blanco P, Donnay S, Albi M. Changes in plasma lipids and increased low‐density lipoprotein susceptibility to oxidation in pregnancies complicated by gestational diabetes: consequences of obesity. Metabolism. 2007;56:1527–1533. [DOI] [PubMed] [Google Scholar]
- 37. Ehrenberg HM, Huston‐Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189:944–948. [DOI] [PubMed] [Google Scholar]
- 38. Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. [DOI] [PubMed] [Google Scholar]
- 40. Jarvie E, Hauguel‐de‐Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci. 2010;119:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. [DOI] [PubMed] [Google Scholar]
- 42. Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander‐Hulthén L, Powell TL, Jansson T. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87:1743–1749. [DOI] [PubMed] [Google Scholar]
- 43. Jones HN, Jansson T, Powell TL. Full‐length adiponectin attenuates insulin signaling and inhibits insulin‐stimulated amino acid transport in human primary trophoblast cells. Diabetes. 2010;59:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aye IL, Gao X, Weintraub ST, Jansson T, Powell TL. Adiponectin inhibits insulin function in primary trophoblasts by PPARα‐mediated ceramide synthesis. Mol Endocrinol. 2014;28:512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T. Increased glucose and placental GLUT‐1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol. 2015;212:227–e1–7.. [DOI] [PubMed] [Google Scholar]
- 46. Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL. Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta. 2016;40:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity. 2015;23:1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rorsman P, Arkhammar P, Bokvist K, Hellerström C, Nilsson T, Welsh M, Welsh N, Berggren PO. Failure of glucose to elicit a normal secretory response in fetal pancreatic beta cells results from glucose insensitivity of the ATP‐regulated K+ channels. Proc Natl Acad Sci U S A. 1989;86:4505–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation. 2000;101:2345–2348. [DOI] [PubMed] [Google Scholar]
- 51. Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T, Yee SP. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation. 2002;106:873–879. [DOI] [PubMed] [Google Scholar]
- 52. Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92:969–975. [DOI] [PubMed] [Google Scholar]
- 53. Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction. 2010;140:373–385. [DOI] [PubMed] [Google Scholar]
- 54. Campia U, Tesauro M, Di Daniele N, Cardillo C. The vascular endothelin system in obesity and type 2 diabetes: pathophysiology and therapeutic implications. Life Sci. 2014;118:149–155. [DOI] [PubMed] [Google Scholar]
- 55. Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. [DOI] [PubMed] [Google Scholar]
- 57. Bar J, Kovo M, Schraiber L, Shargorodsky M. Placental maternal and fetal vascular circulation in healthy non‐obese and metabolically healthy obese pregnant women. Atherosclerosis. 2017;260:63–66. [DOI] [PubMed] [Google Scholar]
- 58. Fan L, Lindsley SR, Comstock SM, Takahashi DL, Evans AE, He GW, Thornburg KL, Grove KL. Maternal high‐fat diet impacts endothelial function in nonhuman primate offspring. Int J Obes. 2013;37:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ailes EC, Gilboa SM, Riehle‐Colarusso T, Johnson CY, Hobbs CA, Correa A, Honein MA; National Birth Defects Prevention Study . Prenatal diagnosis of nonsyndromic congenital heart defects. Prenat Diagn. 2014;34:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolfe HM, Sokol RJ, Martier SM, Zador IE. Maternal obesity: a potential source of error in sonographic prenatal diagnosis. Obstet Gynecol. 1990;76:339–342. [PubMed] [Google Scholar]
- 61. Hendler I, Blackwell SC, Bujold E, Treadwell MC, Wolfe HM, Sokol RJ, Sorokin Y. The impact of maternal obesity on midtrimester sonographic visualization of fetal cardiac and craniospinal structures. Int J Obes Relat Metab Disord. 2004;28:1607–1611. [DOI] [PubMed] [Google Scholar]
- 62. Botto LD, Krikov S, Carmichael SL, Munger RG, Shaw GM, Feldkamp ML. Lower rate of selected congenital heart defects with better maternal diet quality: a population‐based study. Arch Dis Child Fetal Neonatal Ed. 2016;101:43–49. [DOI] [PubMed] [Google Scholar]
- 63. Obermann‐Borst SA, Vujkovic M, de Vries JH, Wildhagen MF, Looman CW, de Jonge R, Steegers EAP, Steegers‐Theunissen RPM. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. BJOG. 2011;118:1205–1215. [DOI] [PubMed] [Google Scholar]
- 64. Carmichael SL, Yang W, Gilboa S, Ailes E, Correa A, Botto LD, Feldkamp ML, Shaw GM; the National Birth Defects Prevention Study . Elevated body mass index and decreased diet quality among women and risk of birth defects in their offspring. Birth Defects Res A. 2016;106:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mojtabai R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol. 2004;19:1029–1036. [DOI] [PubMed] [Google Scholar]
- 66. Werler MM, Louik C, Shapiro S, Mitchell AA. Prepregnant weight in relation to risk of neural tube defects. JAMA. 1996;275:1089–1092. [DOI] [PubMed] [Google Scholar]
- 67. Rönö K, Grotenfelt NE, Klemetti MM, Stach‐Lempinen B, Huvinen E, Meinilä J, Valkama A, Tiitinen A, Roine RP, Pöyhönen‐Alho M, et al. Effect of a lifestyle intervention during pregnancy—findings from the Finnish gestational diabetes prevention trial (RADIEL). J Perinatol. 2018;38:1157–1164. [DOI] [PubMed] [Google Scholar]
- 68. Case AP, Ramadhani TA, Canfield MA, Beverly L, Wood R. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36:335–341. [DOI] [PubMed] [Google Scholar]
- 69. Nohr EA, Vaeth M, Baker JL, Sørensen TI, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–1759. [DOI] [PubMed] [Google Scholar]
- 70. Grotenfelt NE, Wasenius NS, Rönö K, Laivuori H, Stach‐Lempinen B, Orho‐Melander M, Schulz CA, Kautiainen H, Koivusalo SB, Eriksson JG. Interaction between rs10830963 polymorphism in MTNR1B and lifestyle intervention on occurrence of gestational diabetes. Diabetologia. 2016;59:1655–1658. [DOI] [PubMed] [Google Scholar]
- 71. Son JS, Liu X, Tian Q, Zhao L, Chen Y, Hu Y, Chae SA, de Avila JM, Zhu MJ, Du M. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J Physiol. 2019;597:3333–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Beeson JH, Blackmore HL, Carr SK, Dearden L, Duque‐Guimarães DE, Kusinski LC, Pantaleão LC, Pinnock AG, Aiken CE, Giussani DA, et al. Maternal exercise intervention in obese pregnancy improves the cardiovascular health of the adult male offspring. Mol Metab. 2018;16:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aye ILMH, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A. 2015;112:12858–12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vaughan OR, Rosario FJ, Powell TL, Jansson T. Normalisation of circulating adiponectin levels in obese pregnant mice prevents cardiac dysfunction in adult offspring. Int J Obes. 2020;44:488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Reaven GM. Insulin resistance: from bit player to centre stage. CMAJ. 2011;183:536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schaefer‐Graf U, Napoli A, Nolan CJ; the Diabetic Pregnancy Study Group . Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia. 2018;61:1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sugrue R, Zera C. Pregestational diabetes in pregnancy. Obstet Gynecol Clin North Am. 2018;45:315–331. [DOI] [PubMed] [Google Scholar]
- 78. Lisowski LA, Verheijen PM, Copel JA, Kleinman CS, Wassink S, Visser GHA, Meijboom EJ. Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. Herz. 2010;35:19–26. [DOI] [PubMed] [Google Scholar]
- 79. Liu S, Joseph KS, Lisonkova S, Rouleau J, Van den Hof M, Sauve R, Kramer MS. Association between maternal chronic conditions and congenital heart defects. Circulation. 2013;128:583–589. [DOI] [PubMed] [Google Scholar]
- 80. Liu S, Rouleau J, León JA, Sauve R, Joseph KS, Ray JG; Canadian Perinatal Surveillance System . Impact of pre‐pregnancy diabetes mellitus on congenital anomalies, Canada, 2002–2012. Health Promot Chronic Dis Prev Can. 2015;35:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hoang TT, Marengo LK, Mitchell LE, Canfield MA, Agopian AJ. Original findings and updated meta‐analysis for the association between maternal diabetes and risk for congenital heart disease phenotypes. Am J Epidemiol. 2017;186:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leirgul E, Brodwall K, Greve G, Vollset SE, Holmstrøm H, Tell GS, Øyen N. Maternal diabetes, birth weight, and neonatal risk of congenital heart defects in Norway, 1994–2009. Obstet Gynecol. 2016;128:1116–1125. [DOI] [PubMed] [Google Scholar]
- 83. Øyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, Quertermous T, Wohlfahrt J, Melbye M. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133:2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hoang TT, Goldmuntz E, Roberts AE, Chung WK, Kline JK, Deanfield JE, Giardini A, Aleman A, Gelb BD, Neal MM, et al. The Congenital Heart Disease Genetic Network Study: cohort description. PLoS One. 2018;13:e0191319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bergström S, Carr H, Petersson G, Stephansson O, Bonamy AKE, Dahlström A, Halvorsen CP, Johansson S. Trends in congenital heart defects in infants with Down syndrome. Pediatrics. 2016;138:e20160123. [DOI] [PubMed] [Google Scholar]
- 86. Starikov R, Bohrer J, Goh W, Kuwahara M, Chien EK, Lopes V, Coustan D. Hemoglobin A1c in pregestational diabetic gravidas and the risk of congenital heart disease in the fetus. Pediatr Cardiol. 2013;34:1716–1722. [DOI] [PubMed] [Google Scholar]
- 87. Ludvigsson JF, Neovius M, Söderling J, Gudbjörnsdottir S, Svensson AM, Franzén S, Stephansson O, Pasternak B. Periconception glycaemic control in women with type 1 diabetes and risk of major birth defects: population based cohort study in Sweden. BMJ. 2018;362:k2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Helle EIT, Biegley P, Knowles JW, Leader JB, Pendergrass S, Yang W, Reaven GR, Shaw GM, Ritchie M, Priest JR. First trimester plasma glucose values in women without diabetes are associated with risk for congenital heart disease in offspring. J Pediatr. 2018;195:275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Priest JR, Yang W, Reaven G, Knowles JW, Shaw GM. Maternal midpregnancy glucose levels and risk of congenital heart disease in offspring. JAMA Pediatr. 2015;169:1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hughes AF, Freeman RB, Fadem T. The teratogenic effects of sugars on the chick embryo. J Embryol Exp Morphol. 1974;32:661–674. [PubMed] [Google Scholar]
- 91. Garnham EA, Beck F, Clarke CA, Stanisstreet M. Effects of glucose on rat embryos in culture. Diabetologia. 1983;25:291–295. [DOI] [PubMed] [Google Scholar]
- 92. Basu M, Zhu JY, LaHaye S, Majumdar U, Jiao K, Han Z, Garg V. Epigenetic mechanisms underlying maternal diabetes‐associated risk of congenital heart disease. JCI Insight. 2017;2:95085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kumar SD, Vijaya M, Samy RP, Thameem Dheen S, Ren M, Watt F, James Kang Y, Bay BH, Tay SSW. Zinc supplementation prevents cardiomyocyte apoptosis and congenital heart defects in embryos of diabetic mice. Free Radic Biol Med. 2012;53:1595–1606. [DOI] [PubMed] [Google Scholar]
- 94. Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes mellitus‐induced apoptosis and heart defects through restoration of impaired wnt signaling. Circ Cardiovasc Genet. 2015;8:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Engineer A, Saiyin T, Lu X, Kucey AS, Urquhart BL, Drysdale TA, Norozi K, Feng Q. Sapropterin treatment prevents congenital heart defects induced by pregestational diabetes mellitus in mice. J Am Heart Assoc. 2018;7:e009624 DOI: 10.1161/jaha.118.009624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang G, Huang WQ, Cui SD, Li S, Wang XY, Li Y, Chuai M, Cao L, Li JC, Lu DX, Yang X. Autophagy is involved in high glucose‐induced heart tube malformation. Cell Cycle. 2015;14:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Suzuki N, Svensson K, Eriksson UJ. High glucose concentration inhibits migration of rat cranial neural crest cells in vitro. Diabetologia. 1996;39:401–411. [DOI] [PubMed] [Google Scholar]
- 98. Wang XY, Li S, Wang G, Ma ZL, Chuai M, Cao L, Yang X. High glucose environment inhibits cranial neural crest survival by activating excessive autophagy in the chick embryo. Sci Rep. 2016;5:18321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Scholtens DM, Muehlbauer MJ, Daya NR, Stevens RD, Dyer AR, Lowe LP, Metzger BE, Newgard CB, Bain JR, Lowe WL Jr; Metabolomics reveals broad‐scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care. 2014;37:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hunter ES, Sadler TW, Wynn RE. A potential mechanism of DL‐beta‐hydroxybutyrate‐induced malformations in mouse embryos. Am J Physiol Endocrinol Metab. 1987;253:E72–E80. [DOI] [PubMed] [Google Scholar]
- 101. Miodovnik M, Skillman CA, Hertzberg V, Harrington DJ, Clark KE. Effect of maternal hyperketonemia in hyperglycemic pregnant ewes and their fetuses. Am J Obstet Gynecol. 1986;154:394–401. [DOI] [PubMed] [Google Scholar]
- 102. Moore DCP, Stanisstreet M, Clarke CA. Morphological and physiological effects of β‐hydroxybutyrate on rat embryos grown in vitro at different stages. Teratology. 1989;40:237–251. [DOI] [PubMed] [Google Scholar]
- 103. Basu M, Garg V. Maternal hyperglycemia and fetal cardiac development: clinical impact and underlying mechanisms. Birth Defects Res. 2018;110:1504–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wahabi HA, Alzeidan RA, Esmaeil SA. Pre‐pregnancy care for women with pre‐gestational diabetes mellitus: a systematic review and meta‐analysis. BMC Public Health. 2012;12:792 DOI: 10.1186/1471-2458-12-792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Polsky S, Wu M, Bode BW, DuBose SN, Goland RS, Maahs DM, Foster NC, Peters AL, Levy CJ, Shah VN, et al. Diabetes technology use among pregnant and nonpregnant women with T1D in the T1D exchange. Diabetes Technol Ther. 2018;20:517–523. [DOI] [PubMed] [Google Scholar]
- 106. Feig DS, Murphy HR. Continuous glucose monitoring in pregnant women with type 1 diabetes: benefits for mothers, using pumps or pens, and their babies. Diabet Med. 2018;35:430–435. [DOI] [PubMed] [Google Scholar]
- 107. Simeone RM, Devine OJ, Marcinkevage JA, Gilboa SM, Razzaghi H, Bardenheier BH, Sharma AJ, Honein MA. Diabetes and congenital heart defects: a systematic review, meta‐analysis, and modeling project. Am J Prev Med. 2015;48:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sklempe Kokic I, Ivanisevic M, Kokic T, Simunic B, Pisot R. Acute responses to structured aerobic and resistance exercise in women with gestational diabetes mellitus. Scand J Med Sci Sports. 2018;28:1793–1800. [DOI] [PubMed] [Google Scholar]
- 109. Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with pre‐existing diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev. 2017;12:CD012696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wattar BHA, Al Wattar BH, Dodds J, Placzek A, Spyreli E, Moore A, Hooper R, Beresford L, Roseboom TJ, Bes‐Rastrollo M, et al. Effect of simple, targeted diet in pregnant women with metabolic risk factors on maternal and fetal outcomes (ESTEEM): study protocol for a pragmatic multicentre randomised trial. BMJ Open. 2016;6:e013495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fisher SC, Van Zutphen AR, Werler MM, Lin AE, Romitti PA, Druschel CM, Browne ML; National Birth Defects Prevention Study . Maternal antihypertensive medication use and congenital heart defects: updated results from the National Birth Defects Prevention Study. Hypertension. 2017;69:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Given JE, Loane M, Garne E, Addor MC, Bakker M, Bertaut‐Nativel B, Gatt M, Klungsoyr K, Lelong N, Morgan M, et al. Metformin exposure in first trimester of pregnancy and risk of all or specific congenital anomalies: exploratory case‐control study. BMJ. 2018;361:k2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Roest PAM, van Iperen L, Vis S, Wisse LJ, Poelmann RE, Steegers‐Theunissen RPM, Molin DGM, Eriksson UJ, Gittenberger‐De Groot AC. Exposure of neural crest cells to elevated glucose leads to congenital heart defects, an effect that can be prevented by N‐acetylcysteine. Birth Defects Res A. 2007;79:231–235. [DOI] [PubMed] [Google Scholar]
- 114. Xu C, Li X, Wang F, Weng H, Yang P. Trehalose prevents neural tube defects by correcting maternal diabetes‐suppressed autophagy and neurogenesis. Am J Physiol Endocrinol Metab. 2013;305:E667–E678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, et al; International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators . Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gunn JK, Beca J, Hunt RW, Goldsworthy M, Brizard CP, Finucane K, Donath S, Shekerdemian LS. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch Dis Child. 2016;101:1010–1016. [DOI] [PubMed] [Google Scholar]
- 118. Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;(6):CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;11:CD010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Newham JJ, Glinianaia SV, Tennant PWG, Rankin J, Bell R. Improved antenatal detection of congenital anomalies in women with pre‐gestational diabetes: population‐based cohort study. Diabet Med. 2013;30:1442–1448. [DOI] [PubMed] [Google Scholar]
- 121. van Velzen CL, Clur SA, Rijlaarsdam MEB, Bax CJ, Pajkrt E, Heymans MW, Bekker MN, Hruda J, de Groot CJM, Blom NA, et al. Prenatal detection of congenital heart disease‐results of a national screening programme. BJOG. 2016;123:400–407. [DOI] [PubMed] [Google Scholar]
- 122. Lytzen R, Vejlstrup N, Bjerre J, Petersen OB, Leenskjold S, Dodd JK, Jørgensen FS, Søndergaard L. Live‐born major congenital heart disease in Denmark: incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiol. 2018;3:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Eckersley L, Sadler L, Parry E, Finucane K, Gentles TL. Timing of diagnosis affects mortality in critical congenital heart disease. Arch Dis Child. 2016;101:516–520. [DOI] [PubMed] [Google Scholar]
- 124. Khoshnood B, Lelong N, Houyel L, Bonnet D, Ballon M, Jouannic JM, Goffinet F; EPICARD Study group . Impact of prenatal diagnosis on survival of newborns with four congenital heart defects: a prospective, population‐based cohort study in France (the EPICARD Study). BMJ Open. 2017;7:e018285. [DOI] [PMC free article] [PubMed] [Google Scholar]


