Abstract
Background
Multiple biomarkers have been independently and additively associated with major adverse cardiovascular events in patients with coronary artery disease. We investigated the prognostic value of suPAR (soluble urokinase‐type plasminogen activator receptor) and hsTnI (high‐sensitivity troponin I) levels in symptomatic patients with no obstructive coronary artery disease. We hypothesized that high levels of these biomarkers will be associated with the risk of future adverse outcomes.
Methods and Results
Plasma levels of suPAR and hsTnI were measured in 556 symptomatic patients with no obstructive coronary artery disease. A biomarker risk score was calculated by counting the number of biomarkers above the median in this cohort (suPAR>2523 pg/mL and hsTnI>2.7 pg/mL). Survival analyses were performed with models adjusted for traditional risk factors. All‐cause death and major adverse cardiovascular events (cardiovascular death, myocardial infarction, stroke, and heart failure) served as clinical outcomes over a median follow‐up of 6.2 years. Mean age was 57±10 years, 49% of the cohort patients were female, and 68% had a positive stress test. High suPAR and hsTnI levels were independent predictors of all‐cause death (hazard ratio=3.2 [95% CI, 1.8–5.7] and 1.3 [95% CI, 1.0–1.7], respectively; both P<0.04) and major adverse cardiovascular events (hazard ratio=2.7 [95% CI, 1.4–5.4] and 1.5 [95% CI, 1.2–2.0], respectively; both P<0.002). Compared with a biomarker risk score of 0, biomarker risk scores of 1 and 2 were associated with 19‐ and 14‐fold increased risk of death and development of major adverse cardiovascular events, respectively.
Conclusions
Among symptomatic patients with no obstructive coronary artery disease, higher levels of suPAR and hsTnI were independently and additively associated with an increased risk of adverse events. Whether modification of these biomarkers will improve risk in these patients needs further investigation.
Keywords: adverse outcomes, angina, hsTnI, INOCA, ischemia, no obstructive coronary artery disease, suPAR
Subject Categories: Biomarkers, Ischemia
Clinical Perspective
What Is New?
Patients with angina and no obstructive coronary artery disease or ischemia with no obstructive coronary artery disease are challenging to assess, diagnose, and manage because of the lack of evidence‐based guidelines.
About 60% of patients with angina or ischemia and no obstructive coronary artery disease have coronary microvascular dysfunction.
What Are the Clinical Implications?
High levels of soluble urokinase‐type plasminogen activator receptor and high‐sensitivity troponin I can independently and additively identify patients at risk of adverse long term outcomes.
Therefore, these biomarkers may assist in identification for both high‐risk and low‐risk individuals from within a common but heterogeneous population of patients.
Nonstandard Abbreviations and Acronyms.
ANOCAangina and no obstructive coronary arteries
BMIbody mass index
BRSbiomarker risk score
CADcoronary artery disease
CMDcoronary microvascular dysfunction
HRhazard ratio
hsCRPhigh‐sensitivity C‐reactive protein
hsTnIhigh‐sensitivity troponin I
INOCAischemia and no obstructive coronary arteries
MACEmajor adverse cardiovascular events
suPARsoluble urokinase‐type plasminogen activator receptor
Introduction
Patients with angina and no obstructive coronary arteries (ANOCA) and those with signs of ischemia and no obstructive coronary arteries (INOCA), who account for over a third of the patients undergoing coronary angiography, are a diagnostic and therapeutic enigma.1, 2 About 60% of patients with ANOCA and INOCA have coronary microvascular dysfunction (CMD).3 Although the long‐term outcomes of these patients are better than those for people with obstructive coronary artery disease (CAD), their outlook is not completely benign, as a number of these patients are at an increased risk of adverse cardiovascular events, have impaired quality of life, and are often subject to higher healthcare resource utilization.1, 4, 5, 6, 7, 8, 9 Identifying the highest‐risk subgroup from within this population remains a challenge. In previous studies we have identified multiple biomarkers that were independently and additively predictive of adverse cardiovascular events in patients with CAD. These pathophysiologically specific biomarkers included hsCRP (high‐sensitivity C‐reactive protein), suPAR (soluble urokinase‐type plasminogen activator receptor), and hsTnI (high‐sensitivity troponin I).10, 11, 12, 13 Furthermore, we have shown that a biomarker risk score developed by aggregating the number of elevated biomarkers that are indicative of activation of various pathophysiologic pathways is a powerful predictor of future adverse outcome.11, 12
Elevated levels of suPAR, a proinflammatory biomarker, are associated with diabetes mellitus, hypertension, and peripheral arterial disease and are independently predictive of CAD severity, CMD, and development of future adverse events.14, 15, 16, 17, 18 Similarly, elevated levels of hsCRP, an inflammatory biomarker, are linked with a greater risk of adverse cardiovascular events in patients with and without known CAD.19, 20 Assays designed to measure hsTnI levels provide a precise measurement of very low concentrations of troponin, below those provided by current standard assays, and represent myocardial stress or ischemia but not necrosis.21, 22, 23 Higher levels of hsTnI have been associated with CAD severity, plaque burden, and increased risk of adverse cardiovascular outcomes as well as with abnormal coronary endothelial dysfunction in patients with no obstructive CAD.21, 22, 23, 24, 25, 26, 27, 28
Because smaller studies have demonstrated a link between nonobstructive CAD and elevation of these biomarkers,18, 24, 29, 30 we hypothesized that these biomarkers will be predictive of adverse outcomes in symptomatic patients with insignificant CAD and that elevation of multiple biomarkers will be linked to even greater risk. For this purpose we studied the relationship between circulating suPAR and hsTnI levels, both individually and in combination, and incident of cardiovascular events in patients with no obstructive CAD.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. Patients referred for clinically indicated cardiac catheterization who had no obstructive CAD (<50% epicardial stenosis) were enrolled into the Emory Cardiovascular BioBank (NCT00378924) between May 2004 and August 2014.31 ANOCA was defined as stable angina or angina equivalent and INOCA as evidence of ischemia on stress test (ECG changes during exercise, echocardiography, or nuclear imaging) and no obstructive CAD.32 Exclusion criteria included (1) history of cardiac transplantation, (2) severe valvular heart disease, (3) congenital heart disease, (4) obstructive CAD (defined as epicardial artery stenosis >50%) or history of coronary revascularization, (5) history of heart failure or left ventricular ejection fraction <50%, (6) chronic kidney disease with estimated glomerular filtration rate <60 mL/min per 1.73 m², and (7) malignancy. Demographic and clinical data obtained from questionnaires and medical records included age, sex, race, body mass index (BMI), history of smoking, diabetes mellitus (HbA1c>6.5 or treatment with insulin or oral antidiabetic medications), hypertension (systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or treatment with antihypertensive medications), dyslipidemia (total cholesterol ≥200 mg/dL, low‐density lipoprotein >130 mg/dL, high‐density lipoprotein <40 mg/dL, or treatment with lipid‐lowering medications) as previously described.31 The study complies with the Declaration of Helsinki, and each subject provided written informed consent as approved by the institutional review board at Emory University, Atlanta, GA.
Follow‐Up
A standardized protocol‐directed follow‐up was conducted by direct telephone contact and/or chart review and query of the Social Security Death Index and state records. Two independent cardiologists who were blinded to patient and biomarker data adjudicated adverse outcome events. Events were classified as all‐cause death and major adverse cardiovascular events (MACE), which included the first occurrence of cardiovascular‐related death (death secondary to sudden cardiac death of presumed cardiovascular cause in high‐risk patients, fatal myocardial infarction, stroke, or peripheral vascular disease), nonfatal myocardial infarction, hospitalization for heart failure, or nonfatal stroke.
Sample Collection
During cardiac catheterization, fasting blood samples were collected from the femoral arterial sheath, centrifuged, and stored at −80°C for future analysis. hsCRP levels were determined using a sandwich immunoassay by FirstMark, Inc (San Diego, CA). Minimum detectable hsCRP concentrations were 0.1 mg/L. Plasma suPAR levels were measured using an ELISA assay (suPARnostic kit; ViroGates, Copenhagen, Denmark) that has a detection limit of 100 pg/mL with intra‐ and interassay coefficients of variation of 2.75% and 9.17%, respectively.17 Plasma hsTnI levels were measured using the Abbott ARCHITECT analyzer (Abbott Laboratories, North Chicago, IL); the limit of detection was 1.2 pg/mL with an interassay coefficient of variation of <10% at 4.7 pg/mL. The 99th percentile values of the upper reference limit ranges between 24 and 30 pg/mL in healthy populations.33, 34, 35
Statistical Analysis
Subject characteristics were reported as descriptive values with means, medians, SDs, and ranges. Differences between groups were assessed using the t test for continuous variables and chi‐squared for categorical variables where appropriate. For nonnormally distributed variables such as hsCRP, suPAR, and hsTnI levels, the Mann‐Whitney U test was used to compare groups in unadjusted analyses. For multivariate analyses, hsCRP, suPAR, and hsTnI levels were examined as continuous variables after log transformation to achieve normality. The median values for suPAR and hsTnI were 2523 and 2.7 pg/mL, respectively. We also analyzed hsTnI data using sex‐specific cutoff values of 4.2 pg/mL for men and 2.8 pg/mL for women.36 Kaplan‐Meier curves, Cox proportional hazards models, and Fine and Gray subdistribution hazard models were used to examine the association among hsCRP, suPAR, and hsTnI levels and all‐cause death and MACE after adjustment for clinical variables such as age, BMI, history of hypertension, dyslipidemia, diabetes mellitus, and medications with and without the biomarkers. Fine and Gray subdistribution hazard models were performed for MACE, with noncardiovascular deaths treated as competing events. Sensitivity analyses were performed to explore whether the association between biomarker risk score and outcomes differed according to the presence of ANOCA or INOCA, presence of angiographic CAD, and cardiovascular risk factors. The C‐statistic, continuous net reclassification improvement, and integrated discrimination improvement were calculated to evaluate the improvement in predictive ability of the models with and without the biomarkers. All analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC) and R (version 3.5.1, http://www.R-project.org). Two‐sided P<0.05 were considered statistically significant.
Results
Baseline characteristics of the 556 patients are summarized in Table 1. The mean age was 57±10 years, 373 (67%) had 0% to 30%, and 183 (33%) had 30% to 50% epicardial artery stenosis. Of the entire cohort, reversible ischemia during stress testing (INOCA) was present in 380 (68%) patients, whereas the remaining 176 (32%) were referred for persistent anginal symptoms (ANOCA). There was no difference in the demographic, clinical, laboratory, angiographic or biomarkers levels between the 2 groups (Table 1) (Figure 1). Correlation analyses showed that suPAR weakly correlated with hsTnI and hsCRP ([r=0.1; P=0.02] and [r=0.23; P<0.00001], respectively). hsTnI and hsCRP levels were not correlated (r=−0.01, P=0.87).
Table 1.
Baseline Characteristics
| All Patients (n=556) | ANOCA (n=176) | INOCA (n=380) | P Value | |
|---|---|---|---|---|
| Age, y, mean (SD) | 57 (10) | 57 (10) | 57 (11) | 0.93 |
| Male, n (%) | 281 (51) | 93 (53) | 188 (49) | 0.63 |
| Race | ||||
| White, n (%) | 430 (77) | 136 (77) | 294 (77) | 0.76 |
| Black, n (%) | 134 (25) | 44 (25) | 90 (24) | 0.83 |
| Other, n (%) | 14 (3) | 5 (3) | 9 (2) | 0.78 |
| Body mass index, kg/m2, mean (SD) | 31 (7) | 31 (8) | 32 (7) | 0.37 |
| Systolic blood pressure, mm Hg, mean (SD) | 135 (19) | 134 (17) | 136 (20) | 0.54 |
| Estimated GFR, mL/min per 1.73 m2, mean (SD) | 87 (15) | 88 (15) | 87 (15) | 0.49 |
| Smoking, n (%) | 336 (60) | 102 (58) | 234 (62) | 0.28 |
| Diabetes mellitus, n (%) | 111 (20) | 32 (18) | 79 (21) | 0.44 |
| Hypertension, n (%) | 357 (64) | 114 (65) | 243 (64) | 0.98 |
| Dyslipidemia, n (%) | 325 (58) | 101 (57) | 224 (59) | 0.56 |
| Ejection fraction, %, mean (SD) | 59 (6) | 59 (6) | 59 (6) | 0.77 |
| Total cholesterol, mg/dL | 182 (39) | 183 (35) | 182 (40) | 0.36 |
| Triglycerides, mg/dL | 139 (85) | 140 (83) | 138 (86) | 0.78 |
| High‐density lipoprotein, mg/dL | 46 (14) | 47 (15) | 46 (14) | 0.62 |
| Low‐density lipoprotein, mg/dL | 109 (33) | 109 (31) | 109 (35) | 0.59 |
| ACE/ARB use, n (%) | 206 (37) | 60 (34) | 146 (38) | 0.25 |
| Aspirin use, n (%) | 277 (50) | 87 (49) | 190 (50) | 0.72 |
| Clopidogrel use, n (%) | 22 (4) | 8 (5) | 14 (4) | 0.66 |
| Statin use, n (%) | 249 (45) | 73 (41) | 176 (46) | 0.21 |
| β‐blocker use, n (%) | 201 (36) | 64 (36) | 137 (36) | 0.91 |
| suPAR, pg/mL, median (IQR) | 2523 (2047–3220) | 2464 (2044–3218) | 2542 (2046–3227) | 0.75 |
| hsTnI, pg/mL, median (IQR) | 2.7 (1.9–4.5) | 2.8 (1.9–4.8) | 2.6 (1.9–4.3) | 0.13 |
| hsCRP, mg/dL, median (IQR) | 2.7 (1.2–6.1) | 3.1 (1.4–6.8) | 2.6 (1.2–6.0) | 0.06 |
P level of significance was calculated by t test for continuous variables and by chi‐squared test for categorical variables. For nonnormally distributed variables, a Mann‐Whitney U test was used to compare groups.
ACE indicates angiotensin‐converting enzyme inhibitor; ANOCA, angina and no obstructive coronary artery disease; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate; hsCRP, high‐sensitivity C‐reactive protein; hsTnI, high‐sensitivity troponin I, INOCA, ischemia and no obstructive coronary artery diseases; IQR, interquartile range; and suPAR, soluble urokinase‐type plasminogen activator receptor.
Figure 1.
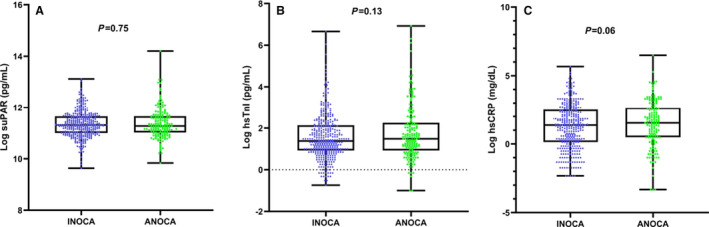
Box plots demonstrating the difference in biomarkers levels (log transformed) between patients with angina and no obstructive coronary artery disease (ANOCA) and those with ischemia and no obstructive coronary artery disease (INOCA). A, Soluble urokinase‐type plasminogen activator receptor (suPAR), (B) high‐sensitivity troponin I (hsTnI), and (C) hsCRP (high‐sensitivity C‐reactive protein).
Clinical and Demographic Predictors of Adverse Outcomes
During a median period of 6.2 years (interquartile range 3.0‐8.1) follow‐up, there were 38 all‐cause deaths and 28 MACE (cardiovascular death [N=18], myocardial infarction [N=4], new‐onset heart failure hospitalization [N=3], and stroke [N=3]). In Cox and in Fine and Gray subdistribution hazard models for clinical predictors of all‐cause death and MACE events, the following variables were included: age, BMI, history of hypertension, dyslipidemia, diabetes mellitus, and statin use. Age and use of statin therapy were predictors of all‐cause death, and histories of dyslipidemia and diabetes mellitus were predictors of MACE (Table 2).
Table 2.
Association Between Biomarkers Levels and All‐Cause Death and MACE
| All‐Cause Death (38 Events)a | MACE (28 Events)b | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Clinical risk factors | ||||
| Age (per 10y higher) | 1.6 (1.1–2.1) | 0.005 | 1.3 (0.8–2.1) | 0.24 |
| BMI (per 5 units higher) | 0.8 (0.6–1.0) | 0.06 | 1.2 (0.8–1.6) | 0.4 |
| History of diabetes mellitus | 1.9 (1.0–3.7) | 0.06 | 2.7 (1.3–5.7) | 0.009 |
| History of hypertension | 0.87 (0.6–1.7) | 0.66 | 1.3 (0.5–3.1) | 0.58 |
| Dyslipidemia | 0.8 (0.4–1.5) | 0.44 | 2.4 (1.0–5.7) | 0.041 |
| Statin therapy | 0.45 (0.2–0.9) | 0.03 | 0.5 (0.2–1.2) | 0.11 |
| Clinical risk factors+individual biomarkers | ||||
| Model 1: hsCRP (100% higher) | 1.2 (0.9–1.5) | 0.14 | 1.0 (0.8–1.3) | 0.99 |
| Model 2: suPAR (100% higher) | 3.9 (2.3–6.8) | <0.0001 | 2.4 (1.2–4.7) | 0.01 |
| Model 3: hsTnI (100% higher) | 1.5 (1.2–1.8) | 0.0006 | 1.6 (1.3–1.9) | <0.0001 |
| Model 4: clinical risk factors+suPAR and hsTnI | ||||
| suPAR (100% higher) | 3.2 (1.8–5.7) | 0.0002 | 2.7 (1.4–5.4) | 0.0047 |
| hsTnI (100% higher) | 1.3 (1.0–1.7) | 0.038 | 1.5 (1.2–2.0) | 0.002 |
| Clinical risk factors+biomarker score | ||||
| BRS=0 [reference] | … | … | ||
| BRS=1 | 8.2 (1.1–61.8) | 0.041 | 5.6 (0.7–43.3) | 0.09 |
| BRS=2 | 19.4 (2.6–144.2) | 0.004 | 14.4 (1.9–108.8) | 0.01 |
BRS=0 where both biomarkers were below the median, BRS=1 with elevation of either suPAR or hsTnI levels above median, and BRS=2 when both biomarker levels were above median values. BMI indicates body mass index; BRS, biomarker risk score, which was calculated using the median of each biomarker (suPAR=2523 and hsTnI=2.7); HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; hsTnI, high‐sensitivity troponin I; MACE, major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, heart failure, and stroke); and suPAR, soluble urokinase‐type plasminogen activator receptor.
Cox proportional hazards models were performed to examine the association between biomarkers and all‐cause death.
Fine and Gray subdistribution hazard models were performed to examine the association between biomarkers and MACE.
Relationship Between Individual Biomarkers and Adverse Outcomes
An elevated hsCRP level was not a predictor of incident death or MACE (Model 1, Table 2). In unadjusted analysis a doubling in suPAR level was associated with a higher risk of all‐cause death and MACE (hazard ratio [HR]=3.6 [95% CI, 2.2–5.8] and 2.9 [95% CI, 1.6–5.1], respectively; both P<0.0001) (Figure 2). After adjustment for the aforementioned clinical variables, the suPAR level remained an independent predictor of death and MACE (Model 2, Table 2). In unadjusted analysis each doubling in hsTnI level was associated with a higher risk of all‐cause death (HR=1.5 [95% CI, 1.2–1.9], P<0.0001) and MACE (HR=1.6 [95% CI, 1.3–2.0], P<0.0001) (Figure 3). These relationships remained significant after adjustment for clinical variables (Model 3, Table 2) and were also similarly significant using sex‐specific cutoff values of hsTnI36 (HR all‐cause death=3.1 [95% CI, 1.5–6.4]; P=0.002, and HR MACE=3.2 [95% CI, 1.3–8.2]; P=0.015). Even after addition of both suPAR and hsTnI levels to the clinical risk factors in a combined model, both biomarkers remained independent predictors of all‐cause death and MACE (Model 4, Table 2).
Figure 2.
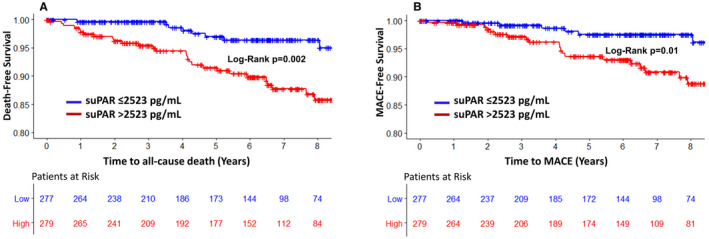
Kaplan‐Meier curves for association between levels of soluble urokinase‐type plasminogen activator receptor (suPAR) identified by the median (2523 pg/mL) for all‐cause death (A) and major adverse cardiovascular events (MACE) (B).
Figure 3.
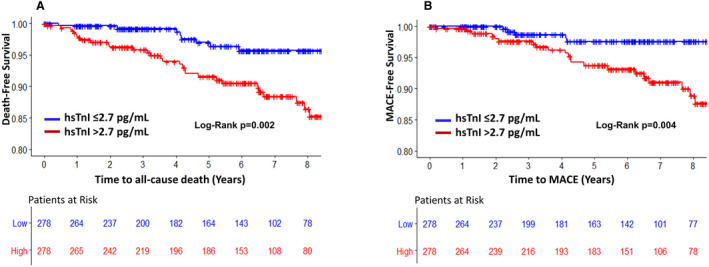
Kaplan‐Meier curves for association between levels of high‐sensitivity troponin I (hsTnI) identified by the median (2.7 pg/mL) for all‐cause death (A) and major cardiovascular adverse events (MACE) (B).
Relationship Between Biomarker Risk Score and Adverse Outcomes
We investigated the value of combining both suPAR and hsTnI levels as predictors of adverse outcomes by creating a biomarker risk score (BRS) with patients divided into groups with none, either, or both biomarkers elevated above their individual median values. Thus, 146 patients had a BRS of 0, where both biomarkers were below the median, 264 patients had a BRS=1, with elevation of either suPAR or hsTnI level above median, and 146 with a BRS=2, where both biomarker levels were above median values. The relationships between the BRS and clinical variables are shown in Table 3. Patients with a BRS of 2 were more likely to be older, have a higher BMI, and have a history of diabetes mellitus or hypertension compared with those with a BRS of 0. After adjustment for the aforementioned covariates, compared with BRS=0, patients with BRS=2 were at 19‐fold higher risk of death and 14‐fold higher risk of developing MACE (Table 2, Figure 4). Patients with BRS=0 had no death or MACE in the first 3 years of follow‐up.
Table 3.
Relationship Between Biomarkers Risk Score and Clinical Variables
| Number of Elevated Biomarkers | ||||
|---|---|---|---|---|
| 0 (N=146) | 1 (N=264) | 2 (N=146) | P Value | |
| Age, y, mean (SD) | 54±9 | 56±10 | 61±11 | <0.0001 |
| Male, n (%) | 80 (53%) | 127 (48%) | 67 (44%) | 0.47 |
| White, n (%) | 115 (76%) | 205 (77%) | 104 (68%) | 0.11 |
| BMI, kg/m2, mean (SD) | 30±6 | 31±7 | 33±8 | 0.014 |
| Diabetes mellitus, n (%) | 21 (14%) | 40 (15%) | 47 (31%) | 0.0002 |
| Hypertension, n (%) | 77 (51%) | 157 (59%) | 111 (73%) | 0.001 |
| Dyslipidemia, n (%) | 88 (58%) | 145 (55%) | 83 (55%) | 0.70 |
| Smoking, n (%) | 89 (59%) | 144 (55%) | 96 (63%) | 0.052 |
| Ejection fraction, %, mean (SD) | 60±5 | 59±6 | 59±6 | 0.07 |
P level of significance in t test for continuous variables and chi‐squared for categorical variables. BMI indicates body mass index.
Figure 4.
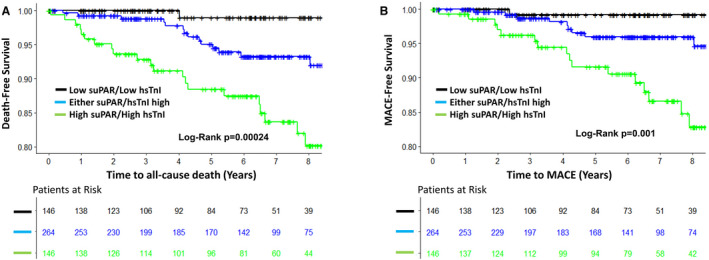
Kaplan‐Meier curves for association between biomarkers risk score and all‐cause death (A) and major cardiovascular adverse events (MACE) (B).
hsTnI indicates high‐sensitivity troponin I; suPAR, soluble urokinase‐type plasminogen activator receptor.
Risk Prediction Performance
We tested the incremental value of adding both biomarkers to a model with the aforementioned clinical variables in predicting adverse outcomes. Addition of both biomarkers improved the C‐statistic from 0.74 to 0.82; Δ=0.082 (95% CI, 0.001–0.163) for all‐cause death. There was trend toward improvement of the continuous net reclassification (0.562 [95% CI, −0.021 to 0.862; P=0.056]) and the integrated discrimination improvement (0.14 [95% CI, −0.025 to 0.318; P=0.066]) in predicting all‐cause death.
Sensitivity Analysis
We examined the heterogeneity in HR for all‐cause death and MACE on the basis of age, BMI, history of hypertension, diabetes mellitus, dyslipidemia, statin use, presentation (ANOCA or INOCA), and CAD severity. There was no significant interaction (P>0.05) among these factors and the predictive value of the BRS (Figure 5).
Figure 5.
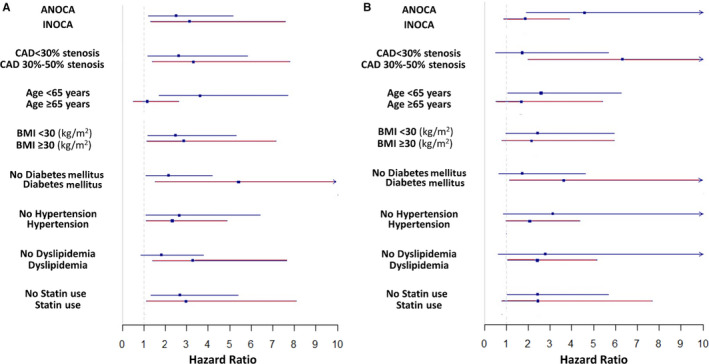
Forest plot of interaction with cardiovascular risk factors for 1 unit of biomarker risk score for outcomes of death (A) and major cardiovascular adverse events (B).
ANOCA indicates angina and no obstructive coronary artery disease; BMI, body mass index; CAD, coronary artery disease; and INOCA, ischemia and no obstructive coronary artery disease.
Discussion
Novel findings of our study are that, among patients with ANOCA and/or INOCA, elevation of circulating suPAR or hsTnI levels, but not of hsCRP, was an independent predictor of incident death and MACE. Elevation of either or both suPAR and hsTnI values was associated with worse long‐term outcomes in this population. The mortality risk in the subgroup with elevation of both these biomarkers was 19‐fold higher compared with those with low levels of both markers. Thus, activation of pathophysiologic pathways that are represented by suPAR and hsTnI, involving the immune/inflammatory and myocardial stress/ischemia pathways, identifies a subgroup at highest risk of developing adverse outcomes. Importantly, lack of activation of these pathways, which was present in up to 25% of these patients, identified a very low‐risk group that had no events in the following 3 years.
suPAR plays an important role in immune regulation and inflammation and is associated with increased inflammatory activity in atherosclerotic plaque.15 It is produced by the cleavage of membrane‐bound urokinase‐type plasminogen activator receptor, which is expressed on monocytes, T‐lymphocytes, macrophages, fibroblasts, and endothelial cells. Its soluble form has chemotactic properties that recruit inflammatory cells including neutrophils and monocytes and can mobilize hematopoietic stem cells.37, 38, 39 In the general population without known CAD, suPAR predicts adverse cardiovascular outcomes independent of hsCRP levels and the Framingham risk score.40, 41 We and others have previously demonstrated that elevated suPAR levels are associated with microvascular dysfunction, development and progression of CAD, peripheral vascular disease, and renal dysfunction. 11 , 14 , 17 , 18 , 42 We extend these findings in this study by investigating the prognostic value of suPAR in a large cohort of patients with confirmed nonobstructive CAD.
Furthermore, we recently investigated the prognostic role of hsTnI in patients with predominantly obstructive CAD.21 We found that high levels of hsTnI are associated with coronary atherosclerosis and progression of CAD and are linked to a higher risk of death and other adverse cardiovascular events. This study investigates the relationship between hsTnI levels and adverse clinical outcomes, specifically in patients with no obstructive CAD. The availability of high‐sensitivity assays is a powerful tool that enables detection of troponin in the absence of myocardial necrosis. The exact mechanism of elevated troponin levels in the absence of myocardial necrosis is still not well understood. Although some studies employing intravascular ultrasound suggested that patients with INOCA usually have diffuse nonobstructive atherosclerosis, 43 , 44 other studies failed to show an association between hsTnI levels and a left ventricular scar on cardiac magnetic resonance imaging 45 or elevated coronary calcium score. 46 A small increase in hsTnI can identify those with subclinical myocardial injury due to structural heart disease or subclinical ischemia. In fact, elevated hsTnI levels were found in patients with ventricular hypertrophy and impaired left ventricular systolic function. 46 However, these patients were excluded from our study. In the absence of significant atherosclerosis, elevated levels of hsTnI were also found in patients with coronary endothelial dysfunction.24 The latter can result in recurrent myocardial ischemia through alterations in epicardial and microvascular vasomotor regulation, leading to flow‐demand mismatch. Although microvascular function was not assessed in this study, it is known that more than 60% of patients with no obstructive CAD have CMD.3
In patients with stable CAD we have recently shown that a circulating hsTnI below the cutoff level of 2.5 pg/mL identifies those who are unlikely to develop myocardial ischemia during stress testing and at no risk of developing MACE during the follow‐up period of 3 years.22 In the present cohort we found that of the 11 deaths that occurred during the first 3 years of follow‐up, 10 were in patients with hsTnI ≥2.7 pg/mL (median). Several studies have shown that in patients presenting with chest pain, elevated hsTnI levels are associated with adverse events, but CAD severity and ischemia evaluation have not been available in these studies. 23 , 47 Further investigation is needed to identify the precise mechanisms underlying chronic hsTnI elevation and whether this is modifiable in patients with no obstructive CAD.
In additional analyses we also used previously published sex‐specific cutoffs for hsTnI levels. Compared with men with hsTnI levels <4.2 pg/mL and women with levels <2.8 pg/mL,36 men and women with higher levels were at 3‐fold higher risk of all‐cause death and at 5‐fold increased risk of developing MACE. Future studies to identify sex‐specific cutoffs for suPAR are needed.
Finally, we investigated the robust predictive value of adding both biomarkers in a composite BRS. Compared with patients with low levels of both biomarkers, those with elevated levels of both biomarkers had a >19‐fold higher risk of death and 14‐fold higher risk of developing MACE during follow‐up, demonstrating the additive value of combining multiple biomarkers, presumably representing activation of distinct pathophysiologic pathways. This knowledge may help physicians in the future to risk stratify patients with ANOCA or INOCA for more aggressive interventions. Interestingly, we found that patients with both biomarkers below median represent a low‐risk group who had no adverse events in the first 3 years during follow‐up.
Our study should be interpreted in the context of its strengths and limitations. This was a large, carefully phenotyped cohort of patients with no obstructive CAD that was confirmed by coronary angiography presenting with INOCA or only ANOCA in whom the strong predictive value of both suPAR and hsTnI levels was defined. We excluded all conditions that might explain the elevation of suPAR and/or hsTnI, such as autoimmune disease, chronic kidney disease, valvular disease, heart failure, cardiac transplant, and acute coronary syndromes. Although CMD and coronary spasm were not systematically evaluated in all our patients, previous studies have demonstrated the relationship between these 2 biomarkers and CMD.18, 24, 30 Biomarker measurements were made at only 1 time point, which may not reflect their potential fluctuation over time. Furthermore, the number of events in our study was relatively small with respect to the number of variables in the adjusted models. The study by Vittinghoff and McCulloch 48 found that the confidence interval coverage and estimation bias under the scenarios with 5 to 9 events per predictor variable were usually comparable to those with 10 to 16 events per predictor variable and further suggested that the rules relating to events per predictor variable can be relaxed. Regardless, we recognize that there is still a potential for overfitting. Finally, we derived biomarker cutoffs from a prespecified cohort, who were referred from clinically indicated cardiac catheterization. Therefore, these cutoffs may need to be confirmed in an independent cohort. Whether early aggressive management of patients with no obstructive coronary artery disease and elevated biomarkers is beneficial needs to be further investigated.
Conclusions
Among patients with INOCA and/or ANOCA, high suPAR and hsTnI levels were independently associated with a higher risk of all‐cause death and MACE. The BRS score, representing inflammatory and myocardial ischemia pathways, provides a robust tool for risk stratification of patients with no obstructive CAD. These observations may assist in identification of both high‐risk and low‐risk individuals from within a common but heterogeneous population of patients.
Sources of Funding
This work was supported by American Heart Association Postdoctoral Fellowship Award Grant (18POST34080330), the Abraham J. & Phyllis Katz Foundation grant (Atlanta, GA), National Institute on Aging grant (AG051633), National Institutes of Health grants (5P01HL101398‐02, 1P20HL113451‐01, 1R56HL126558‐01, 1RF1AG051633‐01, R01 NS064162‐01, R01 HL89650‐01, HL095479‐01, 1U10HL110302‐01, 1DP3DK094346‐01, and 2P01HL086773‐06A1). This work is also supported partially by Abbott Laboratories and Cardiorisk. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or of the National Institutes of Health.
Disclosures
Beshiri and Murtagh are full‐time employees and shareholders of Abbott Diagnostics. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:e015515 DOI: 10.1161/JAHA.119.015515.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence‐based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 4. Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in‐hospital mortality in the American College of Cardiology–National Cardiovascular Data Registry. Circulation. 2008;117:1787–1801. [DOI] [PubMed] [Google Scholar]
- 5. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, et al. Insights from the NHLBI‐sponsored women's ischemia syndrome evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender‐based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–S29. [DOI] [PubMed] [Google Scholar]
- 6. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 7. Tavella R, Cutri N, Tucker G, Adams R, Spertus J, Beltrame JF. Natural history of patients with insignificant coronary artery disease. Eur Heart J. 2016;2:117–124. [DOI] [PubMed] [Google Scholar]
- 8. Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored women's ischemia syndrome evaluation. Circulation. 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 9. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook‐Wiens G, Reis SE, Kelsey SF, Bittner V, et al. Impact of abnormal coronary reactivity on long‐term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghasemzadeh N, Brooks MM, Vlachos H, Hardison R, Sikora S, Sperling L, Quyyumi AA, Epstein SE. An aggregate biomarker risk score predicts high risk of near‐term myocardial infarction and death: findings from BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes). J Am Heart Assoc. 2017;6:e003587 DOI: 10.1161/JAHA.116.003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghasemzedah N, Hayek SS, Ko YA, Eapen DJ, Patel RS, Manocha P, Al Kassem H, Khayata M, Veledar E, et al. Pathway‐specific aggregate biomarker risk score is associated with burden of coronary artery disease and predicts near‐term risk of myocardial infarction and death. Circ Cardiovasc Qual Outcomes. 2017;10:e001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, Nanjundappa RA, Sikora S, Malayter D, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandesara PB, O'Neal WT, Tahhan AS, Hayek SS, Lee SK, Khambhati J, Topel ML, Hammadah M, Alkhoder A, et al. Comparison of the association between high‐sensitivity troponin I and adverse cardiovascular outcomes in patients with versus without chronic kidney disease. Am J Cardiol. 2018;121:1461–1466. [DOI] [PubMed] [Google Scholar]
- 14. Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, Veledar E, Le NA, Pielak T, et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3:e001118 DOI: 10.1161/JAHA.114.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edsfeldt A, Nitulescu M, Grufman H, Gronberg C, Persson A, Nilsson M, Persson M, Bjorkbacka H, Goncalves I. Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnerable human atherosclerotic plaque. Stroke. 2012;43:3305–3312. [DOI] [PubMed] [Google Scholar]
- 16. Persson M, Ostling G, Smith G, Hamrefors V, Melander O, Hedblad B, Engstrom G. Soluble urokinase plasminogen activator receptor: a risk factor for carotid plaque, stroke, and coronary artery disease. Stroke. 2014;45:18–23. [DOI] [PubMed] [Google Scholar]
- 17. Samman Tahhan A, Hayek SS, Sandesara P, Hajjari J, Hammadah M, O'Neal WT, Kelli HM, Alkhoder A, Ghasemzadeh N, et al. Circulating soluble urokinase plasminogen activator receptor levels and peripheral arterial disease outcomes. Atherosclerosis. 2017;264:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mekonnen G, Corban MT, Hung OY, Eshtehardi P, Eapen DJ, Al‐Kassem H, Rasoul‐Arzrumly E, Gogas BD, McDaniel MC, et al. Plasma soluble urokinase‐type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non‐obstructive coronary artery disease. Atherosclerosis. 2015;239:55–60. [DOI] [PubMed] [Google Scholar]
- 19. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C‐reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. [DOI] [PubMed] [Google Scholar]
- 20. Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, Smeeth L, Deanfield JE, Lowe GD, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samman Tahhan A, Sandesara P, Hayek SS, Hammadah M, Alkhoder A, Kelli HM, Topel M, O'Neal WT, Ghasemzadeh N, et al. High‐sensitivity troponin I levels and coronary artery disease severity, progression, and long‐term outcomes. J Am Heart Assoc. 2018;7:e007914 DOI: 10.1161/JAHA.117.007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammadah M, Kim JH, Tahhan AS, Kindya B, Liu C, Ko YA, Al Mheid I, Wilmot K, Ramadan R, et al. Use of high‐sensitivity cardiac troponin for the exclusion of inducible myocardial ischemia: a cohort study. Ann Intern Med. 2018;169:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Januzzi JL Jr, Suchindran S, Hoffmann U, Patel MR, Ferencik M, Coles A, Tardif JC, Ginsburg GS, Douglas PS, et al; PROMISE Investigators. Single‐molecule hsTnI and short‐term risk in stable patients with chest pain. J Am Coll Cardiol. 2019;73:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El Sabbagh A, Prasad M, Zack CJ, Widmer RJ, Karon BS, Lerman A, Jaffe AS. High‐sensitivity troponin in patients with coronary artery endothelial dysfunction. J Invasive Cardiol. 2018;30:406–410. [PubMed] [Google Scholar]
- 25. Sinning C, Keller T, Zeller T, Ojeda F, Schluter M, Schnabel R, Lubos E, Bickel C, Lackner KJ, et al; Gutenberg Health Study. Association of high‐sensitivity assayed troponin I with cardiovascular phenotypes in the general population: the population‐based Gutenberg Health Study. Clin Res Cardiol. 2014;103:211–222. [DOI] [PubMed] [Google Scholar]
- 26. Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin I levels measured with a high‐sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Coll Cardiol. 2013;61:1906–1913. [DOI] [PubMed] [Google Scholar]
- 27. Schulz O, Reinicke M, Berghoefer GH, Bensch R, Kraemer J, Schimke I, Jaffe AS. High‐sensitive cardiac troponin I (hs‐cTnI) values in patients with stable cardiovascular disease: an initial foray. Clin Chim Acta. 2010;411:812–817. [DOI] [PubMed] [Google Scholar]
- 28. Korosoglou G, Lehrke S, Mueller D, Hosch W, Kauczor HU, Humpert PM, Giannitsis E, Katus HA. Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart. 2011;97:823–831. [DOI] [PubMed] [Google Scholar]
- 29. Hung OY, Lee SK, Eshtehardi P, Samady H. Novel biomarkers of coronary microvascular disease. Future Cardiol. 2016;12:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corban MT, Prasad A, Nesbitt L, Loeffler D, Herrmann J, Lerman LO, Lerman A. Local production of soluble urokinase plasminogen activator receptor and plasminogen activator inhibitor‐1 in the coronary circulation is associated with coronary endothelial dysfunction in humans. J Am Heart Assoc. 2018;7:e009881 DOI: 10.1161/JAHA.118.009881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ko YA, Hayek S, Sandesara P, Samman Tahhan A, Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB). BMJ Open. 2017;7:e018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST‐elevation myocardial infarction: executive summary. Circulation. 2007;116:148–304. [Google Scholar]
- 33. Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–1581. [DOI] [PubMed] [Google Scholar]
- 34. Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth‐Zotz S, Warnholtz A, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. [DOI] [PubMed] [Google Scholar]
- 35. Zeller T, Tunstall‐Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, Woodward M, Struthers A, Hughes M, et al. High population prevalence of cardiac troponin I measured by a high‐sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–281. [DOI] [PubMed] [Google Scholar]
- 36. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, Maggioni A, Domanski MJ, Rouleau JL, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. [DOI] [PubMed] [Google Scholar]
- 37. Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25:450–455. [DOI] [PubMed] [Google Scholar]
- 38. Rijneveld AW, Levi M, Florquin S, Speelman P, Carmeliet P, van Der Poll T. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J Immunol. 2002;168:3507–3511. [DOI] [PubMed] [Google Scholar]
- 39. Selleri C, Montuori N, Salvati A, Serio B, Pesapane A, Ricci P, Gorrasi A, Li Santi A, Hoyer‐Hansen G, et al. Involvement of urokinase receptor in the cross‐talk between human hematopoietic stem cells and bone marrow microenvironment. Oncotarget. 2016;7:60206–60217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eugen‐Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, Petersen J, Pielak T, Møller LN, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. [DOI] [PubMed] [Google Scholar]
- 41. Sehestedt T, Lyngbaek S, Eugen‐Olsen J, Jeppesen J, Andersen O, Hansen TW, Linneberg A, Jorgensen T, Haugaard SB, et al. Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis. 2011;216:237–243. [DOI] [PubMed] [Google Scholar]
- 42. Hodges GW, Bang CN, Eugen‐Olsen J, Olsen MH, Boman K, Ray S, Kesaniemi AY, Jeppesen JL, Wachtell K. suPAR predicts postoperative complications and mortality in patients with asymptomatic aortic stenosis. Open Heart. 2018;5:e000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute‐Sponsored Women's Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. 2010;23:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eggers KM, Lind L, Ahlstrom H, Bjerner T, Ebeling Barbier C, Larsson A, Venge P, Lindahl B. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population‐based sample of elderly subjects. Eur Heart J. 2008;29:2252–2258. [DOI] [PubMed] [Google Scholar]
- 46. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roos A, Bandstein N, Lundback M, Hammarsten O, Ljung R, Holzmann MJ. Stable high‐sensitivity cardiac troponin T levels and outcomes in patients with chest pain. J Am Coll Cardiol. 2017;70:2226–2236. [DOI] [PubMed] [Google Scholar]
- 48. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. [DOI] [PubMed] [Google Scholar]


