Abstract
Background
Vorapaxar as an adjunct to dual antiplatelet therapy (DAPT) reduces thrombotic events in patients with prior myocardial infarction at the expense of increased bleeding. Withdrawal of aspirin has emerged as a bleeding reduction strategy. The pharmacodynamic effects of vorapaxar with potent P2Y12 inhibitors as well as the impact of dropping aspirin is unexplored and represented the aim of the VORA‐PRATIC (Vorapaxar Therapy in Patients With Prior Myocardial Infarction Treated With Newer Generation P2Y12 Receptor Inhibitors Prasugrel and Ticagrelor) study.
Methods and Results
Post–myocardial infarction patients (n=130) on standard DAPT (aspirin+prasugrel or ticagrelor) were randomized to 1 of 3 arms: (1) triple therapy: aspirin+prasugrel/ticagrelor+vorapaxar; (2) dual therapy (drop aspirin): prasugrel/ticagrelor+vorapaxar; (3) DAPT: aspirin+prasugrel/ticagrelor. Pharmacodynamic assessments were performed at 3 time points (baseline and 7 and 30 days). Vorapaxar reduced CAT (collagen‐ADP‐TRAP)–induced platelet aggregation, a marker of platelet‐mediated global thrombogenicity (triple therapy versus DAPT at 30 days: mean difference=–27; 95% CI,–35 to –19; P<0.001; primary end point). This effect was attenuated but still significant in the absence of aspirin (dual therapy versus DAPT at 30 days: mean difference=–15; 95% CI,–23 to –7; P<0.001; between‐group comparisons, P<0.05). Vorapaxar abolished TRAP–induced aggregation (P<0.001), without affecting thrombin generation and clot strength. There were no differences in markers of P2Y12 reactivity. Markers sensitive to aspirin‐induced effects increased (P<0.001) in the dual‐therapy arm.
Conclusions
In post–myocardial infarction patients treated with potent P2Y12 inhibitors, vorapaxar reduces platelet‐driven global thrombogenicity, an effect that persisted, albeit attenuated, in the absence of aspirin and without affecting markers of P2Y12 reactivity or clot kinetics. The clinical implications of these PD observations warrant future investigation.
Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02545933.
Keywords: aspirin, myocardial infarction, pharmacodynamic, prasugrel, ticagrelor, vorapaxar
Subject Categories: Platelets, Pharmacology, Percutaneous Coronary Intervention
Nonstandard Abbreviations and Acronyms
- BARC
Bleeding Academic Research Consortium
- CAT
collagen‐ADP‐thrombin receptor–activating peptide
- DAPT
dual antiplatelet therapy
- LTA
light transmittance aggregometry
- MA
clot strength as maximum amplitude
- MI
myocardial infarction
- MPA
maximum platelet aggregation
- PRI
platelet reactivity index
- R
thrombin generation as reaction time
- TRA 2P–TIMI 50
Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events—Thrombolysis in Myocardial Infarction 50
- VASP
vasodilator‐stimulated phosphoprotein
- VORA‐PRATIC
Vorapaxar Therapy in Patients With Prior Myocardial Infarction Treated With Newer Generation P2Y12 Receptor Inhibitors Prasugrel and Ticagrelor
Clinical Perspective
What Is New?
Adding vorapaxar to stabilized post–myocardial infarction patients who are on maintenance therapy with one of the potent P2Y12 inhibitors, prasugrel or ticagrelor, is feasible and has no major safety concerns.
While not as suppressed as with triple therapy (aspirin+prasugrel or ticagrelor+vorapaxar), platelet‐mediated thrombogenicity during treatment with dual therapy with vorapaxar and either prasugrel or ticagrelor (without aspirin), was significantly reduced compared with standard dual antiplatelet therapy.
What Are the Clinical Implications?
Although our findings confirm that alternative antithrombotic treatment regimens cannot replace the selective effects of aspirin on platelet cyclooxygenase‐1 blockade, the observations from VORA‐PRATIC (Vorapaxar Therapy in Patients With Prior Myocardial Infarction Treated With Newer Generation P2Y12 Receptor Inhibitors Prasugrel and Ticagrelor) do provide pharmacodynamic support of a strategy of dropping aspirin in patients following myocardial infarction treated with vorapaxar and potent P2Y12 inhibition (ie, prasugrel or ticagrelor), as this leads to reduced platelet‐mediated global thrombogenicity compared with DAPT.
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor represents the standard of care for the prevention of atherothrombotic events in patients with myocardial infarction (MI).1 However, thrombotic complications continue to occur, suggesting a contributing role of platelet signaling pathways not inhibited by DAPT.2, 3 Thrombin is a potent inducer of platelet activation, and its levels are increased following an acute coronary event.4, 5 Accordingly, strategies aimed at modulating thrombin‐mediated effects have been extensively investigated.6 Vorapaxar selectively targets the protease‐activated receptor‐1 on the platelet membrane inhibiting thrombin‐induced platelet activation.4, 7 Notably, in patients with previous atherothrombosis, in particular those with a prior MI, vorapaxar in addition to standard‐of‐care antiplatelet therapy, including DAPT, reduces recurrent thrombotic events.8, 9
Guidelines recommend the preferential use of the newer generation P2Y12 inhibitors (ie, prasugrel or ticagrelor) over clopidogrel in patients following MI.1, 10 However, regulatory approval of vorapaxar was based on a clinical trial in which clopidogrel was the predominant P2Y12 inhibitor (99.3% of patients), and to date there is limited experience on the use of vorapaxar in combination with the newer generation P2Y12 inhibitors.8 Moreover, despite the efficacy of vorapaxar in reducing thrombotic complications, this benefit occurs at the expense of an increased risk of bleeding complications.8 In the presence of potent antithrombotic therapies, withdrawal of aspirin has emerged as a strategy to reduce the risk of bleeding.11 However, the effects of stopping aspirin in patients treated with vorapaxar and a newer‐generation P2Y12 inhibitor is unknown. The aim of this study was to assess the pharmacodynamic effects of vorapaxar in addition to a newer‐generation P2Y12 inhibitor (prasugrel or ticagrelor) with and without aspirin in a real‐world setting of patients following MI.
Methods
Study Design and Participants
VORA‐PRATIC (Vorapaxar Therapy in Patients With Prior Myocardial Infarction Treated With Newer Generation P2Y12 Receptor Inhibitors Prasugrel and Ticagrelor) was a prospective, randomized, parallel‐design, open‐label study aimed to assess the pharmacodynamic effects of the adjunctive use of vorapaxar in a real‐world clinical setting of patients following MI on maintenance DAPT with aspirin and either prasugrel or ticagrelor (Clinicaltrials.gov identifier: NCT02545933). The study was conducted in patients following MI in line with the approved indication and dosing regimen for the use of vorapaxar (2.5 mg/day).12 In brief, patients between 18 and 75 years of age who had experienced an MI within the previous 12 months and were on maintenance DAPT (aspirin [81 mg/day] plus either prasugrel [10 mg/day] or ticagrelor [90 mg twice daily]) as per standard‐of‐care were considered for the study. In line with the TRA 2P–TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events—Thrombolysis in Myocardial Infarction 50) trial design, post‐MI patients needed to be on DAPT for at least 2 weeks without experiencing any complication (ischemic or bleeding) following their index event.12 Patients with a prior cerebrovascular event, at high risk for bleeding, or with a contraindication to vorapaxar were not considered eligible for the study.12 The rationale for the exclusion of elderly patients (aged ≥75 years) and patients with a low body weight (≤60 kg) is that prasugrel is generally not recommended in these patient populations.1, 10 Specific study inclusion and exclusion criteria are provided in Data S1.
The study was performed at the University of Florida Health Science Center (Jacksonville, FL). Patients were screened and recruited at the outpatient clinics of our institution. Although the study had an open‐label design, laboratory personnel performing pharmacodynamic testing were blinded to treatment assignment. The study complied with the Declaration of Helsinki and was approved by the Western Institutional Review Board, and all patients gave their written informed consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
After providing written informed consent, patients meeting study entry criteria were randomly assigned in a 1:1:1 fashion to one of the following 3 treatment regimens: (1) triple therapy: aspirin+prasugrel or ticagrelor+vorapaxar; (2) dual therapy (ie, drop aspirin): prasugrel or ticagrelor+vorapaxar; (3) DAPT: aspirin+prasugrel or ticagrelor. Randomized treatment was maintained for 30±3 days. Randomization was stratified according to type of P2Y12 inhibitor to include at least 35% of patients on prasugrel. The rationale for predicting a higher number of randomized patients on ticagrelor derives from the broader clinical indications of ticagrelor compared with prasugrel.1, 10 Blood sampling for pharmacodynamic testing was conducted at 3 time points: (1) baseline (while patients were on standard DAPT and before randomization); (2) after 7 to 10 days of randomized study treatment; (3) after 30±3 days of randomized study treatment. At each time point, blood was collected before the morning dose of prasugrel/ticagrelor and vorapaxar to measure trough levels of platelet inhibition. During study treatment, major adverse cardiac events (death, MI, stroke, and urgent revascularization procedures) and serious adverse events (bleeding and other adverse events) were collected. Bleeding was defined by the BARC (Bleeding Academic Research Consortium) definition.13 After study completion, patients resumed an antiplatelet treatment regimen at the discretion of the treating physician. A flow diagram of the study design is illustrated in Figure 1.
Figure 1. Study design.
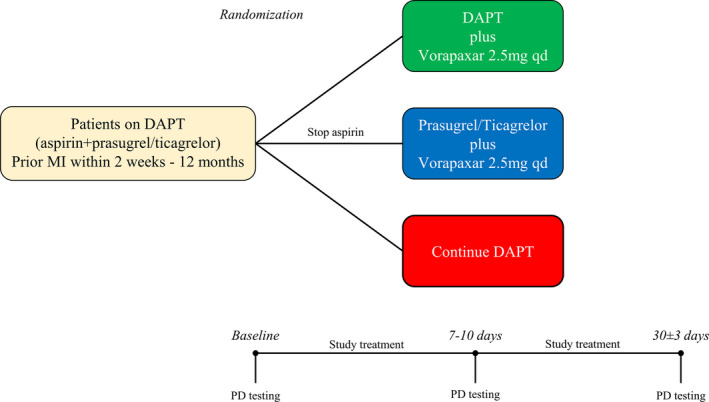
DAPT indicates dual antiplatelet therapy; MI, myocardial infarction; and PD, pharmacodynamic.
Blood Sampling and Laboratory Assessments
Peripheral venous blood samples were drawn through a short venous catheter inserted into a forearm vein and collected in citrate, EDTA, and serum tubes as appropriate for assessments. The first 2 to 4 mL of blood were discarded to avoid spontaneous platelet activation. Blood sampling for pharmacodynamic assessments was performed at 3 time points as indicated above in the study design section. A number of assays were used, including light transmittance aggregometry (LTA); whole blood vasodilator‐stimulated phosphoprotein; Thrombelastograph Coagulation Analyzer TEG 6s Series system, which also included the Platelet Mapping assay using ADP; and ELISA‐based assessment of serum thromboxane B2.14, 15, 16, 17 A detailed description of the assays is provided in Data S1. Assessments were performed and described with the following objectives: (1) to define the pharmacodynamic effect of vorapaxar on thrombin‐mediated effects on platelets and systemically; to this extent, LTA following thrombin receptor–activating peptide (TRAP, 15 μmol/L) stimuli and markers of clot kinetics using the TEG 6s system were used, respectively; (2) to define the pharmacodynamic effect of vorapaxar on platelet‐mediated global thrombogenicity; to this extent, LTA following stimuli with combination of 2 μg/mL collagen‐related peptide +5 μmol/L ADP +15 μmol/L TRAP (CAT) was used; (3) to define the pharmacodynamic effect of vorapaxar on P2Y12 inhibition induced by prasugrel/ticagrelor and the impact of aspirin withdrawal; to this extent, LTA following stimuli with ADP (20 μmol/L) and vasodilator‐stimulated phosphoprotein (VASP), and markers sensitive to cyclooxygenase‐1 blockade, including LTA following arachidonic acid (1 mmol/L) and collagen (3 μg/mL) stimuli, as well as measurement of serum thromboxane B2 levels, respectively, were assessed. LTA results were reported as maximum platelet aggregation (MPA, %), VASP results as platelet reactivity index, thrombin generation as reaction time, clot strength as maximum amplitude (mm), and serum thromboxane B2 levels in picograms per milliliter.14, 15, 16, 17
Study End Points and Sample Size Calculation
The primary end point of our study is the comparison of CAT‐induced MPA between DAPT plus vorapaxar and DAPT after 30±3 days of treatment. We hypothesized that adjunctive vorapaxar would result in a significant reduction of CAT‐induced platelet aggregation, a marker of platelet‐mediated thrombogenicity. Assuming a 10% absolute reduction in CAT‐induced MPA with a common standard deviation of 13%, 37 patients per group with valid primary end point data were required to detect a significant difference between DAPT plus vorapaxar and DAPT with a 90% power and 2‐sided α=0.05. Considering up to 30% to 35% rate of invalid results attributable to hemolysis or dropout and the 3 arms of treatment, up to 146 patients were estimated to be randomized. Since at the time of study design there were no preliminary data in this particular setting, the sample size of our study was calculated based on previous data of vorapaxar in addition to DAPT with aspirin and clopidogrel.17 This approach is in agreement with recommendations for pilot investigations.18 Our exploratory outcomes included the comparison of CAT‐induced MPA between vorapaxar in addition to a P2Y12 receptor inhibitor and vorapaxar in addition to standard DAPT (aspirin and prasugrel/ticagrelor). We hypothesized that stopping aspirin would not lead to significant differences in CAT‐induced MPA after 30±3 days of treatment.
Other objectives included the comparisons among the 3 groups of all pharmacodynamic parameters measured by multiple assays at every time point, as well as intragroup comparisons of pharmacodynamic parameters to evaluate the variability over time of vorapaxar pharmacodynamic effects, as well as how pharmacodynamic measures were affected by aspirin withdrawal.
Statistical Analysis
Categorical variables are expressed as frequencies and percentages. Continuous variables are presented as mean±SD. Continuous variables were analyzed for normal distribution with the Kolmogorov–Smirnov test. Comparisons between categorical variables was performed using 2‐tailed Fisher's exact test or the Pearson's chi‐square test. Student t test and Mann–Whitney U‐test were used to compare continuous variables when appropriate. An analysis of variance method with a general linear model was used to evaluate the overall difference among the 3 groups and all between‐group comparisons, including the primary end point. In line with similar pharmacodynamic studies, considering the exploratory nature of comparisons other than the primary end point, adjustment for multiple comparisons was not performed.14, 16 A repeated measure ANOVA was used to evaluate intragroup comparisons. A 2‐tailed P<0.05 was considered to indicate a statistically significant difference for all the analyses performed. Statistical analysis was performed by our group using SPSS version 25.0 software (SPSS Inc., Chicago, IL). The first and corresponding authors had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Results
Patient Population
Between February 2016 and April 2019, 238 post‐MI patients were screened, and a total of 131 patients on maintenance DAPT with aspirin and either prasugrel or ticagrelor therapy agreed to participate in the study; 1 patient was not eligible for randomization due to the presence of an exclusion criteria. Therefore, a total of 130 patients were randomized and exposed to at least 1 dose of study medication (triple therapy, n=44; dual therapy, n=43; DAPT, n=43). At randomization, 57 patients were on prasugrel‐based DAPT, and 73 were on ticagrelor‐based DAPT. Of these, 115 patients (triple therapy, n=37; dual therapy, n=39; DAPT, n=39) had valid primary end point data (Figure 2). There were no significant differences in baseline characteristics between the study groups (Table). Mean time from index event to randomization was 42±57 days, without any significant difference among groups (triple therapy, 51±69; dual therapy, 36±41; DAPT, 39±57; P=0.471). No ischemic or BARC type 2 to 5 bleeding events were observed; 7 patients (5.4%) had a BARC type 1 bleeding (triple therapy, n=6 [13.6%]; dual therapy, n=1 [2.3%]; DAPT, n=0; P=0.010), which led to study drug discontinuation in 1 patient receiving triple therapy. Twelve patients (9%) had nonbleeding adverse events (Table S1).
Figure 2. Trial profile.
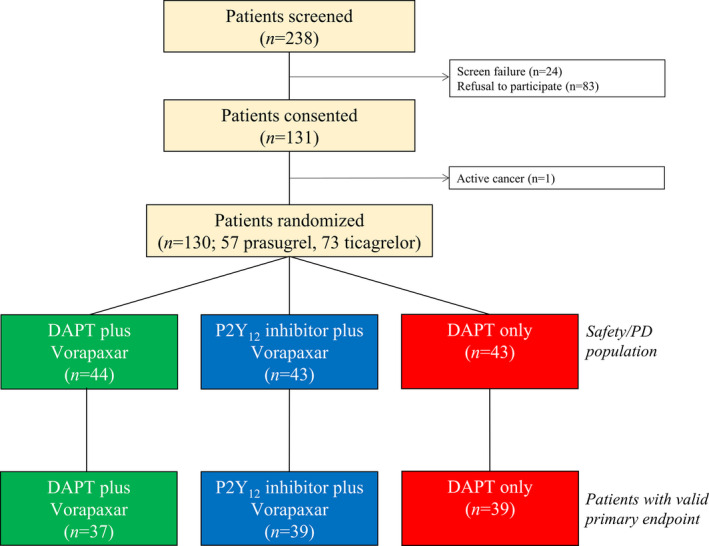
DAPT indicates dual antiplatelet therapy; and PD, pharmacodynamic.
Table 1.
Baseline Characteristics
| Vorapaxar+DAPT (n=44) | Vorapaxar+P2Y12 Inhibitor (n=43) | DAPT (n=43) | P Value | |
|---|---|---|---|---|
| Age, y | 57±9 | 56±9 | 56±10 | 0.953 |
| Sex, male, n (%) | 30 (68) | 32 (74) | 29 (67) | 0.740 |
| BMI, kg/m2 | 31±9 | 33±6 | 30±6 | 0.090 |
| Race, n (%) | 0.873 | |||
| White | 24 (54) | 26 (60) | 29 (67) | |
| Black | 17 (39) | 14 (33) | 13 (30) | |
| Other | 3 (7) | 3 (7) | 1 (2) | |
| P2Y12 inhibitor, n (%) | 0.425 | |||
| Prasugrel | 19 (43) | 22 (51) | 16 (37) | |
| Ticagrelor | 25 (57) | 21 (49) | 27 (63) | |
| Hypertension, n (%) | 28 (64) | 35 (81) | 30 (70) | 0.177 |
| Diabetes mellitus, n (%) | 11 (25) | 12 (28) | 10 (23) | 0.882 |
| Dyslipidemia, n (%) | 27 (61) | 34 (80) | 31 (72) | 0.187 |
| Active smoking, n (%) | 14 (32) | 10 (23) | 17 (39) | 0.267 |
| Prior PCI, n (%) | 42 (96) | 42 (98) | 42 (98) | 0.786 |
| Prior CABG, n (%) | 3 (7) | 3 (7) | 3 (7) | 0.778 |
| PAD, n (%) | 1 (2) | 1 (2) | 0 (0) | 0.605 |
| LVEF (%) | 47±14 | 48±11 | 49±12 | 0.578 |
| Creatinine, mg/dL | 0.9±0.2 | 1.0±0.3 | 0.9±0.3 | 0.788 |
| CrCL, mL/min | 108±39 | 118±38 | 114±49 | 0.569 |
| Platelet count, ×103/μL | 247±61 | 225±54 | 226±54 | 0.111 |
| Hematocrit, % | 40±4 | 40±5 | 40±5 | 0.821 |
| Hemoglobin, g/dL | 13.4±1.5 | 13.1±1.7 | 13.4±1.7 | 0.656 |
| Medications, n (%) | ||||
| Insulin therapy | 3 (7) | 6 (14) | 5 (12) | 0.548 |
| OAD | 9 (21 | 9 (21) | 7 (16) | 0.834 |
| β‐Blockers | 41 (93) | 42 (98) | 41 (95) | 0.607 |
| ACEI/ARB | 30 (68) | 32 (74) | 34 (79) | 0.510 |
| Statins | 43 (98) | 43 (100) | 42 (98) | 0.605 |
| PPI | 10 (23) | 15 (35) | 6 (14) | 0.073 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CrCl, creatinine clearance; DAPT, dual antiplatelet therapy; LVEF, left ventricular ejection fraction; OAD, oral antidiabetic drug; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; and PPI, proton pump inhibitor.
Pharmacodynamic Findings
Effects of Vorapaxar on Thrombin‐Mediated Effects
Adjunctive treatment with vorapaxar was associated with complete blockade of TRAP–induced platelet aggregation in both the triple‐ and dual‐therapy groups compared with DAPT (P<0.001; Figure 3A). Vorapaxar had only transient and modest effects on thrombin generation (P=0.028 at 7 days), which did not persist at 30 days (Figure 3B). Adjunctive treatment with vorapaxar did not result in any differences in clot strength (Figure 3C). These overall findings did not differ significantly irrespective of P2Y12 inhibitor used (prasugrel or ticagrelor) (P for interaction >0.05 for all assays).
Figure 3. Thrombin‐mediated effects.
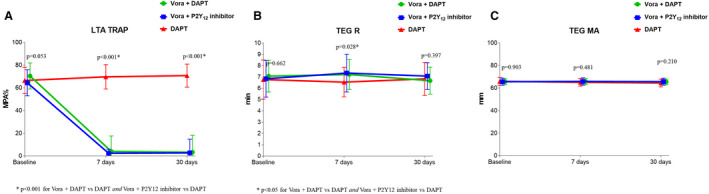
A, TRAP‐induced maximal platelet aggregation (MPA%) measured by LTA. B, Reaction time (R) measured by TEG using kaolin as agonist. C, Clot strength (MA) measured by TEG using kaolin as agonist. P‐values represent the comparisons among the 3 groups at each time point. Data are presented as mean; error bars indicate standard deviation. Offset between symbols and error bars is to improve readability. DAPT indicates dual antiplatelet therapy; LTA, light transmittance aggregometry; MA, maximal amplitude; TEG, thromboelastography; and Vora, vorapaxar.
Effects of Vorapaxar on Global Platelet‐Mediated Thrombogenicity
Adjunctive treatment with vorapaxar significantly reduced CAT‐induced platelet aggregation. Intragroup comparisons showed that CAT‐induced platelet aggregation was significantly reduced by 7 days (compared with baseline; P<0.001), and platelet aggregation continued to decline at 30 days (compared with 7 days; P=0.011) in the triple‐therapy group. In the dual‐therapy group, CAT‐induced aggregation was numerically lower than baseline at 7 days (P=0.055) and significantly lower at 30 days (P=0.011) (Figure 4). Intergroup comparisons showed that adding vorapaxar was associated with a significant reduction in CAT‐induced aggregation both at 7 and 30 days, which was enhanced in patients receiving aspirin (all between‐group comparisons had a significant P<0.05). Compared with DAPT, the triple‐therapy group had significantly lower CAT‐induced aggregation at 7 days (triple therapy versus DAPT: mean difference=–18; 95% CI, ‐26 to –11; P<0.001) and 30 days (triple therapy versus DAPT: mean difference=–27; 95% CI, ‐35 to ––19; P<0.001; primary end point) (Figure 4). The dual‐therapy group also had significantly lower CAT‐induced aggregation at 7 days (dual therapy versus DAPT: mean difference=–9; 95% CI, –16 to –1; P=0.015) and 30 days (dual therapy versus DAPT: mean difference=–15; 95% CI, –23 to –7; P<0.001) (Figure 4). Aspirin withdrawal was associated with significantly higher CAT‐induced aggregation at both 7 (P=0.015) and 30 days (P=0.003) compared with triple therapy. These overall findings did not differ significantly irrespective of P2Y12 inhibitor used (prasugrel or ticagrelor) (P for interaction >0.05).
Figure 4. Platelet‐mediated global thrombogenicity.
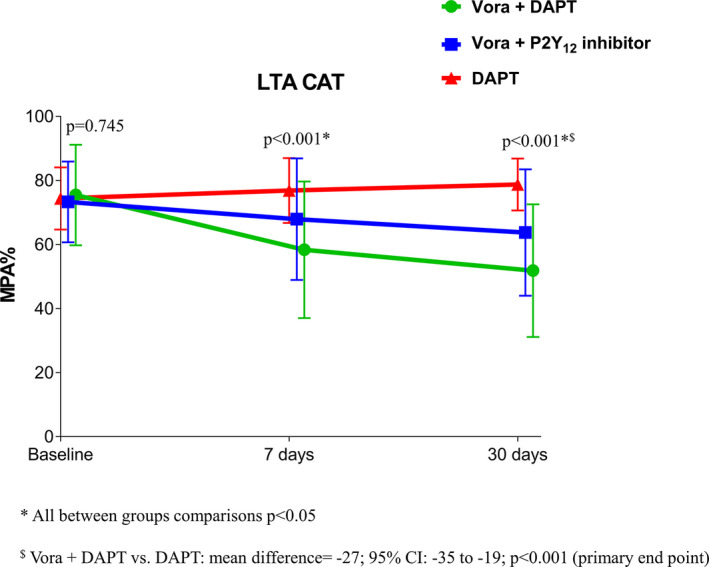
CAT‐induced maximal platelet aggregation (MPA%) measured by LTA. P values represent the comparisons among the 3 groups at each time point. Data are presented as mean; error bars indicate standard deviation. The agonist CAT is a combination of collagen‐related peptide, ADP and TRAP. Offset between symbols and error bars is to improve readability. DAPT indicates dual antiplatelet therapy; LTA, light transmittance aggregometry; and Vora, vorapaxar.
Effects of Vorapaxar on Modulating P2Y12 Inhibition Induced by Prasugrel/Ticagrelor in the Presence and Absence of Aspirin
Adding vorapaxar to DAPT did not affect markers assessing P2Y12 inhibition using all assays, including LTA‐ADP, VASP, and thromboelastography platelet mapping. These findings were consistent in the triple‐ and dual‐therapy groups (Figure 5 and Figure S1). Aspirin withdrawal was associated with a marked increase in makers sensitive to cyclooxygenase‐1 blockade, including arachidonic acid– and collagen‐induced aggregation as well as serum thromboxane B2 levels (P<0.001) (Figure 6 and Figure S2). These overall findings did not differ significantly irrespective of P2Y12 inhibitor used (prasugrel or ticagrelor) (P for interaction >0.05 for all assays).
Figure 5. Markers of P2Y12 signaling.
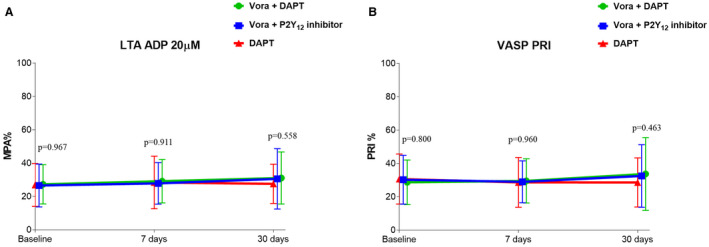
A, ADP‐induced maximal platelet aggregation (MPA%) measured by LTA. B, Platelet reactivity index (PRI) measured by VASP. P values represent the comparisons among the 3 groups at each time point. Data are presented as mean; error bars indicate standard deviation. Offset between symbols and error bars is to improve readability. DAPT indicates dual antiplatelet therapy; LTA, light transmittance aggregometry; VASP, whole blood vasodilator‐stimulated phosphoprotein; and Vora, vorapaxar.
Figure 6. Markers sensitive to cyclooxygenase‐1 blockade.
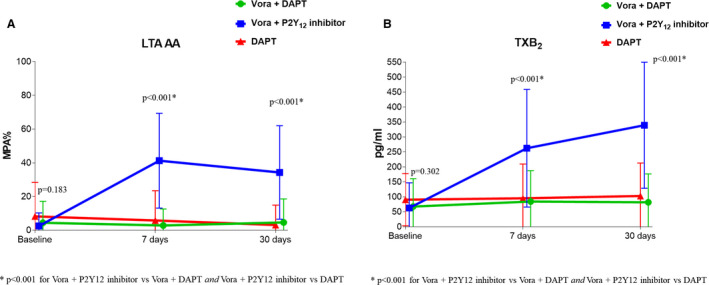
A, Arachidonic acid (AA)‐induced maximal platelet aggregation (MPA%) measured by LTA. B, Serum thromboxane B2. P values represent the comparisons among the 3 groups at each time point. Data are presented as mean; error bars indicate standard deviation. Offset between symbols and error bars is to improve readability. DAPT indicates dual antiplatelet therapy; LTA, light transmittance aggregometry; and Vora, vorapaxar.
Discussion
Vorapaxar is a selective platelet protease‐activated receptor‐1 inhibitor approved for the reduction of thrombotic cardiovascular events in patients with a history of MI.4, 8, 9, 12 In line with the TRA‐2P trial design that led to its approval, vorapaxar must be used in addition to standard‐of‐care antiplatelet therapy, which may include aspirin and a P2Y12 inhibitor.8, 9, 12 However, to date, clinical trial experience with vorapaxar has been almost exclusively with the P2Y12 receptor inhibitor clopidogrel, and the effects of vorapaxar in combination with guideline‐recommended agents in the post‐MI setting (ie, prasugrel or ticagrelor) is largely unexplored. This may indeed represent a limitation for the uptake of vorapaxar in current‐day clinical practice, where prasugrel and ticagrelor are preferentially used over clopidogrel.1, 10 In the present investigation, we demonstrate the feasibility of adding vorapaxar to stabilized patients following MI who were on maintenance therapy with aspirin and either prasugrel or ticagrelor. Although the study was not designed for clinical outcomes, this treatment regimen was overall well tolerated and did not raise any major safety concerns, with the exception of an increase in minor bleeding events (BARC type I), which was observed when vorapaxar was added to DAPT but not when aspirin was discontinued. From a pharmacodynamic perspective, our study confirmed the platelet‐specific, and not systemic, nature of vorapaxar on modulating thrombin‐mediated effects. In fact, while vorapaxar completely abolished TRAP–induced platelet aggregation, it only had modest and transient effects on thrombin generation. In line with prior investigations, this explains why platelet‐mediated thrombogenicity was markedly reduced with vorapaxar, while clot strength, which is the result of platelet‐fibrin binding, was not affected.14 In fact, levels of circulating thrombin, hence downstream fibrin, grossly unaffected by vorapaxar, have a pivotal role on thrombus formation and stabilization, which is why ultimately clot strength is not affected even if platelet‐mediated thrombogenicity is reduced.4, 14 Moreover, despite the known interplay between protease‐activated receptor‐1 and P2Y12‐mediated signaling, adjunctive treatment with vorapaxar did not interfere with markers of P2Y12 receptor blockade.19 This finding is consistent with other studies modulating systemic levels of thrombin by means of oral anticoagulant therapies and may be attributed to the fact that these patients are already on P2Y12‐inhibiting therapy, thus precluding our ability to detect such interplay previously observed in nonmedicated platelets.20, 21, 22, 23, 24, 25
The potent antiplatelet effects achieved with the novel P2Y12 receptor inhibitors has questioned the role of their combined use with aspirin.11, 26, 27 Prasugrel and ticagrelor are effective in inhibiting both P2Y12‐dependent and thromboxane A2‐dependent pathways of platelet activation.26, 27 In particular, platelet inhibition achieved by combining aspirin and a potent P2Y12‐receptor blocker was shown to be no greater than that produced by the P2Y12‐receptor blocker alone in healthy volunteers.26, 27 Other studies conducted in animals using a variety of thrombogenic stimuli suggest a limited effect of aspirin in reducing thrombus formation on a background of ongoing P2Y12 blockade.28 Most recently, these experimental observations were extended to a high‐risk human population undergoing coronary stenting showing that, after completing a 3‐month post–percutaneous coronary intervention period of DAPT, ticagrelor monotherapy (without aspirin) significantly reduced clinically relevant bleeding compared with ticagrelor plus aspirin, without any increase in ischemic events.29 Overall, these findings raise interest on the potential role of vorapaxar as part of a dual‐therapy treatment regimen in combination with either prasugrel or ticagrelor, but without aspirin, as a strategy to further reduce thrombotic events while minimizing the risk of bleeding. This is noteworthy given that trials of adjunctive treatment with vorapaxar have consistently shown an increase in bleeding complications.8, 30 Notably, numerous studies have demonstrated the adverse prognostic implications associated with bleeding, including increased mortality, and thus over the past years safety aspects with antiplatelet therapy and need to define treatment regimens with reduced bleeding while preserving efficacy have gained increased importance.31 However, it is important to underscore that clinical trial development of novel antiplatelet agents, including the more potent P2Y12 inhibitors and vorapaxar, was on top of standard‐of‐care therapies, in particular aspirin. The enhanced antithrombotic effects of these more potent antiplatelet agents have not only questioned the relative added contribution of aspirin on efficacy outcomes, but overall its clinical value in light of aspirin's well‐established association with gastrointestinal toxicity, a key driver of gastrointestinal bleeding.11
In our study, we showed that platelet‐mediated thrombogenicity associated with dual therapy with vorapaxar and either prasugrel or ticagrelor (without aspirin), while not as suppressed as with triple therapy, was significantly reduced compared with standard DAPT. This observation is in line with a prior study of adjunctive treatment with vorapaxar conducted in patients with and without diabetes mellitus treated with clopidogrel in which platelet‐mediated global thrombogenicity increased following aspirin withdrawal.14 However, the magnitude of such increase in thrombogenicity was higher in patients with diabetes mellitus compared with patients without diabetes mellitus. Moreover, in this setting of combined use of vorapaxar and clopidogrel among patients with diabetes mellitus, withdrawal of aspirin also led to an increase in markers of P2Y12 signaling, a finding not observed in our current study using prasugrel or ticagrelor in which markers of P2Y12 signaling remained unchanged in patients in whom aspirin was discontinued.14 Nevertheless, withdrawal of aspirin did not affect clot strength. Overall, our study findings are rather consistent with another pharmacodynamic investigation conducted in high‐risk patients undergoing percutaneous coronary intervention, which showed that withdrawal of aspirin on a background of ticagrelor therapy, while associated with an increase in markers sensitive to cyclooxygenase‐1 blockade, did not affect markers of P2Y12 signaling or ex vivo platelet‐dependent thrombus formation under dynamic flow conditions of shear stress that mimic moderate arterial stenosis (ie, Badimon perfusion chamber).32 Collectively, these observations from both in vitro and ex vivo investigations suggest that the synergism that is known to occur between the cyclooxygenase‐1 and P2Y12 pathways may be less relevant in the presence of more potent P2Y12 blockade.26, 27, 32, 33 Nonetheless, our findings showing that markers specifically assessing cyclooxygenase‐1 enzyme activity increase with aspirin withdrawal further support that alternative antithrombotic treatment regimens cannot replace the selective effects of aspirin on platelet cyclooxygenase‐1 blockade. This should indeed caution on strategies of aspirin withdrawal in the absence of effective alternative antithrombotic treatment. It is important to note that our investigation was not designed to test vorapaxar as an alternative to aspirin, but rather to investigate the pharmacodynamic effects associated with aspirin withdrawal on background of vorapaxar and a potent P2Y12 inhibitor. Indeed, the observations from VORA‐PRATIC do provide pharmacodynamic support of a strategy of dropping aspirin in patients following MI treated with vorapaxar and potent P2Y12 inhibition (ie, prasugrel or ticagrelor), as this leads to reduced platelet‐mediated global thrombogenicity compared with DAPT. However, if these pharmacodynamic observations (Figure 4) with a dual‐therapy strategy (ie, vorapaxar+prasugrel or ticagrelor) can translate into a reduction in thrombotic complications in patients following MI without increasing bleeding compared with a standard DAPT approach (aspirin+prasugrel or ticagrelor) warrants dedicated investigations.
Study Limitations
Our study was not designed to assess clinical outcomes. Therefore, the favorable safety profile observed with the use of dual (ie, prasugrel or ticagrelor+vorapaxar) compared with triple (ie, aspirin+prasugrel or ticagrelor+vorapaxar) therapy needs to be interpreted with caution. In addition, we conducted our pharmacodynamic investigation after carefully excluding patients at high risk for bleeding complications, in line with the product label indications for vorapaxar.12 Moreover, we extended the duration of treatment up to 30 days, which allowed us to assess the tolerability of this treatment regimen, with which nonbleeding side effects were rare. Ultimately, our study was specifically conducted in patients following MI treated with potent P2Y12 inhibitors and how our pharmacodynamic findings would directly compare with clopidogrel‐treated subjects cannot be deduced from our investigation.
Conclusions
Adjunctive treatment with vorapaxar reduces platelet‐mediated thrombogenicity without affecting clot kinetics in prior patients with MI on maintenance DAPT with aspirin and a potent P2Y12 inhibitor (ie, prasugrel or ticagrelor). In this setting of combined treatment with vorapaxar and potent P2Y12 inhibitor blockade, although withdrawal of aspirin led to an increase in markers specific to cyclooxygenase‐1 enzyme activity, platelet‐mediated global thrombogenicity was significantly reduced compared with DAPT. The clinical implications of these pharmacodynamic observations warrant future investigation.
Sources of Funding
The present study was investigator‐initiated. The study was originally funded by a grant from Merck until transfer of marketing rights of vorapaxar, including grant responsibilities, to Aralez. At the time of transfer, Merck had funded 58% of overall grant costs. Following such transfer, Aralez filed for bankruptcy and did not cover any of the residual grant costs, which was completed using research funds from the Division of Cardiology, University of Florida College of Medicine–Jacksonville. Upon completion of the study, Deerfield acquired marketing rights of vorapaxar and provided a nominal fee for the final phases of investigator‐initiated research studies conducted with vorapaxar at our institution (NCT02548650 and NCT02545933).
Disclosures
Dr Franchi has received payment as an individual for consulting fee or honorarium from AstraZeneca and Sanofi. Dr Rollini has received payment as an individual for consulting fee or honorarium from Chiesi. Dr Angiolillo has received payment as an individual for reports receiving payments as an individual for (1) consulting fee or honorarium from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; (2) participation in review activities from CeloNova and St. Jude Medical; and institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli‐Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. Dr Jennings reports receiving payments as an individual for: consulting fee or honorarium from Bayer, Janssen, PhaseBio, and Portola and institutional payments for grants from Janssen and from In Motion Musculoskeletal Institute. Dr Baber has received personal fees/honoraria/advisory board from Boston Scientific, Astra Zeneca, and Amgen; research funding to institution from Astra Zeneca. Dr Mehran has received institutional research grant support from AstraZeneca, Bayer, Beth Israel Deaconess Medical Center, Bristol‐Myers Squibb, CSL Behring, Eli Lilly/Daiichi‐Sankyo, Inc., Medtronic, Novartis Pharmaceuticals, and OrbusNeich; has served on the executive committee of Janssen Pharmaceuticals and Osprey Medical Inc.; has served on the data safety monitoring board of Watermark Research Partners; has served as a consultant for Abbott Laboratories, Abiomed (spouse), Boston Scientific, Cardiovascular Systems, Inc., Medscape, Siemens Medical Solutions, The Medicines Company (spouse), Roivant Sciences, Inc, Volcano Corporation, and Sanofi; and has equity in Claret Medical Inc. and Elixir Medical Corporation. The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1
Figures S1–S2
References 14–17
(J Am Heart Assoc. 2020;00:e015865. DOI: 10.1161/JAHA.120.015865.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.015865
For Sources of Funding and Disclosures, see page 10.
References
- 1. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72:2915–2931. [DOI] [PubMed] [Google Scholar]
- 2. Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. [DOI] [PubMed] [Google Scholar]
- 3. Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597–607. [DOI] [PubMed] [Google Scholar]
- 4. Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. 2010;31:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brummel‐Ziedins K, Undas A, Orfeo T, Gissel M, Butenas S, Zmudka K, Mann KG. Thrombin generation in acute coronary syndrome and stable coronary artery disease: dependence on plasma factor composition. J Thromb Haemost. 2008;6:104–110. [DOI] [PubMed] [Google Scholar]
- 6. Moon JY, Nagaraju D, Franchi F, Rollini F, Angiolillo DJ. The role of oral anticoagulant therapy in patients with acute coronary syndrome. Ther Adv Hematol. 2017;8:353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franchi F, Rollini F, Park Y, Angiolillo DJ. Platelet thrombin receptor antagonism with vorapaxar: pharmacology and clinical trial development. Future Cardiol. 2015;11:547–564. [DOI] [PubMed] [Google Scholar]
- 8. Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 9. Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, Mehrhof F, Merlini PA, Murphy SA, Sabatine MS, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 P‐TIMI 50 trial. Lancet. 2012;380:1317–1324. [DOI] [PubMed] [Google Scholar]
- 10. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. [DOI] [PubMed] [Google Scholar]
- 11. Capodanno D, Mehran R, Valgimigli M, Baber U, Windecker S, Vranckx P, Dangas G, Rollini F, Kimura T, Collet JP, et al. Aspirin‐free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15:480–496. [DOI] [PubMed] [Google Scholar]
- 12. Magnani G, Bonaca MP, Braunwald E, Dalby AJ, Fox KA, Murphy SA, Nicolau JC, Oude Ophuis T, Scirica BM, Spinar J, et al. Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc. 2015;4:e001505 DOI: 10.1161/JAHA.114.001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 14. Franchi R, Rollini F, Kairouz V, Rivas J, Rivas A, Agarwal M, Briceno M, Wali M, Nawaz A, Silva G, et al. Pharmacodynamic effects of vorapaxar in patients with and without diabetes mellitus treated with dual antiplatelet therapy: results of the optimizing anti‐Platelet therapy in diabetes MellitUS (OPTIMUS)‐5 study. J Am Coll Cardiol Basic Trans Science. 2019;4:763–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Capodanno D, Patel A, Dharmashankar K, Ferreiro JL, Ueno M, Kodali M, Tomasello SD, Capranzano P, Seecheran N, Darlington A, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv. 2011;4:180–187. [DOI] [PubMed] [Google Scholar]
- 16. Franchi F, Rollini F, Aggarwal N, Hu J, Kureti M, Durairaj A, Duarte VE, Cho JR, Been L, Zenni MM, et al. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)‐4 study. Circulation. 2016;134:780–792. [DOI] [PubMed] [Google Scholar]
- 17. Storey RF, Kotha J, Smyth SS, Moliterno DJ, Rorick TL, Moccetti T, Valgimigli M, Dery JP, Cornel JH, Thomas GS, et al. Effects of vorapaxar on platelet reactivity and biomarker expression in non–ST‐elevation acute coronary syndromes. The TRACER pharmacodynamic substudy. Thromb Haemost. 2014;111:883–891. [DOI] [PubMed] [Google Scholar]
- 18. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–312. [DOI] [PubMed] [Google Scholar]
- 19. Nylander S, Mattsson C, Ramström S, Lindahl TL. Synergistic action between inhibition of P2Y12/P2Y1 and P2Y12/thrombin in ADP‐ and thrombin‐induced human platelet activation. Br J Pharmacol. 2004;142:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franchi F, Rollini F, Cho JR, King R, Phoenix F, Bhatti M, DeGroat C, Tello‐Montoliu A, Zenni MM, Guzman LA, et al. Effects of dabigatran on the cellular and protein phase of coagulation in patients with coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel. Results from a prospective, randomised, double‐blind, placebo‐controlled study. Thromb Haemost. 2016;115:622–631. [DOI] [PubMed] [Google Scholar]
- 21. Franchi F, Rollini F, Garcia E, Rivas Rios J, Rivas A, Agarwal M, Kureti M, Nagaraju D, Wali M, Briceno M, et al. Effects of edoxaban on the cellular and protein phase of coagulation in patients with coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel: results of the EDOX‐APT study. Thromb Haemost. 2020;120:83–93. [DOI] [PubMed] [Google Scholar]
- 22. Borst O, Münzer P, Alnaggar N, Geue S, Tegtmeyer R, Rath D, Droppa M, Seizer P, Heitmeier S, Heemskerk JWM, et al. Inhibitory mechanisms of very low‐dose rivaroxaban in non–ST‐elevation myocardial infarction. Blood Adv. 2018;2:715–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furugohri T, Isobe K, Honda Y, Kamisato‐Matsumoto C, Sugiyama N, Nagahara T, Morishima Y, Shibano T. DU‐176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost. 2008;6:1542–1549. [DOI] [PubMed] [Google Scholar]
- 24. Honda Y, Kamisato C, Morishima Y. Edoxaban, a direct factor Xa inhibitor, suppresses tissue‐factor induced human platelet aggregation and clot‐bound factor Xa in vitro: comparison with an antithrombin‐dependent factor Xa inhibitor, fondaparinux. Thromb Res. 2016;141:17–21. [DOI] [PubMed] [Google Scholar]
- 25. Wong PC, Jiang X. Apixaban, a direct factor Xa inhibitor, inhibits tissue‐factor induced human platelet aggregation in vitro: comparison with direct inhibitors of factor VIIa, XIa and thrombin. Thromb Haemost. 2010;104:302–310. [DOI] [PubMed] [Google Scholar]
- 26. Armstrong PC, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, Warner TD. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011;9:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirkby NS, Leadbeater PD, Chan MV, Nylander S, Mitchell JA, Warner TD. Antiplatelet effects of aspirin vary with level of P2Y12 receptor blockade supplied by either ticagrelor or prasugrel. J Thromb Haemost. 2011;9:2103–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vilahur G, Gutiérrez M, Casani L, Lambert C, Mendieta G, Ben‐Aicha S, Capdevila A, Pons‐Lladó G, Carreras F, Carlsson L, et al. P2Y12 antagonists and cardiac repair post‐myocardial infarction: global and regional heart function analysis and molecular assessments in pigs. Cardiovasc Res. 2018;114:1860–1870. [DOI] [PubMed] [Google Scholar]
- 29. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, et al. Ticagrelor with or without aspirin in high‐risk patients after PCI. N Engl J Med. 2019;381:2032–2042. [DOI] [PubMed] [Google Scholar]
- 30. Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, et al. Thrombin‐receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. [DOI] [PubMed] [Google Scholar]
- 31. Buccheri S, Capodanno D, James S, Angiolillo DJ. Bleeding after antiplatelet therapy for the treatment of acute coronary syndromes: a review of the evidence and evolving paradigms. Expert Opin Drug Saf. 2019;18:1171–1189. [DOI] [PubMed] [Google Scholar]
- 32. Baber U, Zafar U, Dangas G, Escolar G, Angiolillo DJ, Sharma SK, Kini AS, Sartori S, Joyce L, Vogel B, et al. Ticagrelor with or without aspirin in high‐risk patients after PCI: a nested substudy of the TWILIGHT trial. J Am Coll Cardiol. 2020;75:578–586. [DOI] [PubMed] [Google Scholar]
- 33. Cadroy Y, Bossavy JP, Thalamas C, Sagnard L, Sakariassen K, Boneu B. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation. 2000;101:2823–2828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figures S1–S2
References 14–17


