Abstract
Background
The protective effects of polyamines on cardiovascular disease have been demonstrated in many studies. However, the roles of spermidine, a natural polyamine, in abdominal aortic aneurysm (AAA) disease have not been studied. In this study, we investigated the influence and potential mechanisms of spermidine treatment on experimental AAA disease.
Methods and Results
Experimental AAAs were induced in 8‐ to 10‐week‐old male C57BL/6J mice by transient intra‐aortic infusion of porcine pancreatic elastase. Spermidine was administered via drinking water at a concentration of 3 mmol/L. Spermidine treatment prevented experimental AAA formation with preservation of medial elastin and smooth muscle cells. In immunostaining, macrophages, T cells, neutrophils, and neovessels were significantly reduced in aorta of spermidine‐treated, as compared with vehicle‐treated elastase‐infused mice. Additionally, flow cytometric analysis showed that spermidine treatment reduced aortic leukocyte infiltration and circulating inflammatory cells. Furthermore, we demonstrated that spermidine treatment promoted autophagy‐related proteins in experimental AAAs using Western blot analysis, immunostaining, and transmission electron microscopic examination. Autophagic function was evaluated for human abdominal aneurysmal and nonaneurysmal adjacent aortae from AAA patients using Western blot analysis and immunohistochemistry. Dysregulated autophagic function, as evidenced by increased SQSTM1/p62 protein and phosphorylated mTOR, was found in aneurysmal, as compared with nonaneurysmal, aortic segments.
Conclusions
Our results suggest that spermidine supplementation limits experimental AAA formation associated with preserved aortic structural integrity, attenuated aortic inflammatory infiltration, reduced circulating inflammatory monocytes, and increased autophagy‐related proteins. These findings suggest that spermidine may be a promising treatment for AAA disease.
Keywords: abdominal aortic aneurysm, autophagy, experimental animal models, inflammation, spermidine
Subject Categories: Vascular Disease, Aneurysm
Nonstandard Abbreviations and Acronyms
- AAA
abdominal aortic aneurysm
- ACS
aortic cross section
- CR
calorie restriction
- CRM
calorie restriction mimetic
- EVG
Verhoeff's Van Gieson
- Keap1
Kelch‐like ECH‐associated protein 1
- LC3
microtubule‐associated protein 1 light chain 3
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- mTOR
mammalian target of rapamycin
- PPE
porcine pancreatic elastase
- SMC
smooth muscle cell
Clinical Perspective
What Is New?
Our present study found that autophagic function was dysregulated in clinical abdominal aortic aneurysm tissues and that spermidine, as an autophagy inducer, limited experimental abdominal aortic aneurysm by attenuating aortic inflammatory infiltration and upregulating autophagy‐related proteins.
What Are the Clinical Implications?
Given that spermidine is a natural polyamine present in daily diet with proven safety and tolerability, our study may have a potential translational significance for treating abdominal aortic aneurysm disease.
Abdominal aortic aneurysm (AAA) is defined as a maximal infrarenal abdominal aortic diameter ≥30 mm or at least 1.5 times the normal diameter and is characterized by progressive dilatation and rupture of the aortic wall.1 In people aged 75 to 79 years, global AAA age‐specific prevalence and incidence rate were 2.75% and 0.2%, respectively.2, 3 Currently, patients with a large AAA with aortic diameter >55 mm in men and 50 mm in women are usually treated clinically by surgical repair, whereas patients with a small AAA (<55 mm in diameter) have to wait for the aortic diameter to reach the size suitable for surgery.4 However, no pharmacological treatments have been convincingly shown to limit AAA growth or reduce the risk of rupture. Thus, patients with small AAAs or those deemed unfit for AAA repair lack active treatment options.5, 6 Hence, safe and effective medications that restrict small AAA expansion are anticipated.7
Spermidine, a naturally occurring polyamine, is widely found in vegetables, cereals, legumes, cheese, mushrooms, and other foods and particularly enriched in Mediterranean and Asian diets.8, 9 In epidemiological studies, higher intake of polyamines in Mediterranean and Asian diets increases longevity and lowers mortality.8, 10 Spermidine is rapidly resorbed from the intestine and distributed in the body without degradation,11 resulting in its subsequent increased concentration in the blood.12 Spermidine, as a histone acetyl transferase inhibitor, induces protein deacetylation and autophagy, and is involved in an array of biological events including autophagy induction, DNA stability, transcription, translation, and apoptosis.13 Previous studies have shown that spermidine prolongs mouse lifespan and exerts multiple cardioprotective effects in an autophagy‐dependent manner.14 In mice, spermidine reversed arterial aging by increasing nitrogen oxide bioavailability, reducing oxidative stress, modifying structural factors, and enhancing autophagy.15 In humans, high dietary intake of spermidine is associated with reduced incidence of atherosclerosis and other cardiovascular disease.16 Additionally, spermidine also activates adenosine monophosphate–activated protein kinase and inhibits mTORC1,17 all involved in autophagy and AAAs.18 However, whether exogenous spermidine supplementation alleviates AAAs has not been explored. In this study, we explored the autophagy function in human AAAs and further evaluated the influence and potential mechanisms of spermidine supplementation on the development of experimental AAAs.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Clinical AAA Tissue and Aorta Tissue Specimens
The protocol for collection of clinical AAAs and nearby artery of AAAs in the same patient was approved by the Ethics Committee of the Xiangya Hospital, Central South University (IRB Approval Number: 201803480). Informed consent was obtained from all participants or their legal guardians. Fresh aortic wall tissue samples were collected from 11 patients with AAA who underwent open AAA repair surgery at Xiangya Hospital, Central South University. The demographic and clinical characteristics of AAA patients are shown in Table. After tissue harvest, attached intramural thrombi were removed and the specimens were immediately frozen in liquid nitrogen and stored at −80°C for further analysis, or fixed in 10% formalin for embedding and sectioning. The study conforms to the declaration of Helsinki.
Table 1.
Main Characteristics of 11 Aneurysm Patients
| Variable | Result |
|---|---|
| Male/female | 10/1 |
| Age, y | 63.9±14.3 |
| Smoking | 7 (63.6%) |
| Aortic diameter, mm | 6.69±1.43 |
| Luminal thrombus | 8 (72.7%) |
| Hypertension | 7 (63.6%) |
| Systolic pressure, mm Hg | 141.1±23.6 |
| Diastolic pressure, mm Hg | 87.8±16.9 |
| Hyperlipidemia | 3 (27.3%) |
| COPD | 1 (9.1%) |
| Diabetes mellitus | 0 |
| Antihypertension medication | 7 (63.6%) |
| Lipid‐lowering medication | 2 (18.2%) |
| Diabetic medications | 0 |
Categorical variables are presented as number (%). Continuous variables are presented as mean±SD. COPD indicates chronic obstructive pulmonary disease.
Experimental Animals
Eight‐ to 10‐week‐old male C57BL/6 mice were purchased from the Xiangya School of Medicine, Central South University. All animals were housed under specific pathogen‐free conditions in a 12‐hour light/dark cycle with free access to food and water ad libitum. All experiments were carried out in accordance with the animal care guidelines and regulation of the Department of Laboratory Animals in Central South University and approved by the Animal Care and Use Committee of Ethics of the Xiangya Hospital, Central South University (IRB Approval Number: 201803481). All mouse experiments conform to the guidelines formulated by the European Community for experimental animal use (Directive 2010/63/EU).
Experimental Aneurysm Creation
AAAs were created via intraluminal infusion of porcine pancreatic elastase (PPE) into the infrarenal aorta as previously described.19 Briefly, mice were anesthetized with inhaled 2% isoflurane and considered as adequately anesthetized with no attempt to withdraw the limb after pressure. Under operative magnification, the infrarenal abdominal aorta was isolated and subjected to transient infusion of type I PPE (4.0 U/mL; E1250, Sigma‐Aldrich, St. Louis, MO) dissolved in phosphate‐balanced saline at the pressure of 150 mm Hg via a PE‐10 polyethylene microcatheter. The 11‐0 nylon sutures (Jinhuan, Shanghai, China) were used to close the aortotomy after infusion. Outer aortic diameter was measured under laparotomy at day 0 and day 14 after PPE infusion using S‐Eye software (Hayear, Shenzhen, China.). AAAs were defined as a ≥50% increase in maximal outer aortic diameter over the baseline level. At the end of the study, mice were anesthetized with ketamine/xylazine (100 and 20 mg/kg ip, respectively) and euthanized by exsanguination before aorta tissue collection at day 14.
Spermidine Treatment
C57BL/6 mice were randomly divided into 3 groups: the sham group mice infused with normal saline receiving regular drinking water, experimental AAA vehicle group mice receiving regular drinking water, and spermidine group mice receiving spermidine (S0266, Sigma‐Aldrich, St. Louis, MO) via drinking water at a concentration of 3 mmol/L. Spermidine at this dose has been proven to effective in cardioprotection and reversing arterial aging.14, 15 SPD treatment started on the day for, or 3 days after, PPE infusion, and continued until euthanasia. Drinking water was replaced every other day by diluting 1 mol/L stock (spermidine/HCl pH 7.4) solution with drinking water as previously described.14, 15
Determination of Plasma Spermidine Concentration in Mice
SPD concentration was determined using liquid chromatography‐mass spectrometry (LC/MS).14, 20 To measure spermidine, blood samples were centrifuged at 4°C, 3000g for 10 minutes. The supernatant (30 μL) was mixed with acetonitrile (120 μL) and 10 μmol/L 1,6‐diaminohexane (H11696, Sigma) solution in acetonitrile (30 μL), vortexed for 30 s, and centrifuged again at 3000g for 5 minutes. Then the solvent of supernatant was evaporated with nitrogen. The residue was redissolved in 150 μL of 0.1 mol/L sodium tetraborate (229946, Sigma) and then reacted with an equal volume of 40 mmol/L 4‐(N,N‐dimethylaminosulfonyl)‐7‐fluoro‐2,1,3‐benzoxadiazole (DBD‐F) (A5595, J&K Scientific Ltd., Beijing, China) in acetonitrile at 60°C for 30 minutes. Thereafter, the reaction mixtures were analyzed by Triple Quad 6500+ LC‐MS/MS System (AB Sciex, Framingham, MA) coupled to Analyst software 1.6.3.
Histochemical and Immunohistochemical Staining
Mouse aortae were harvested at 14 days after PPE infusion, fixed in 10% formalin, embedded in paraffin, and horizontally cut into sections (4‐μm thickness) from the middle of the AAA. Medial elastin retention, collagen, and smooth muscle cell (SMC) destruction were evaluated using Verhoeff's Van Gieson (EVG) solution (Servicebio, G1005, China) and Masson (Servicebio, G1006, China), respectively. Standard 3‐step biotin–streptavidin–peroxidase tissue immunostaining was performed to evaluate medial SMC destruction, angiogenesis, mural leukocyte infiltration, and autophagy. Primary antibodies for immunostaining are summarized in Table S1. Staining specificity for each antibody has been documented in previous studies. Other reagents, including biotinylated anti‐rabbit secondary antibodies, streptavidin–peroxidase conjugates, aminoethylcarbazole or diaminobenzidine peroxidase substrate kit were purchased from the ZSGB‐biotechnology Co., Ltd., Beijing, China. Based on EVG, Masson, and SMC α actin staining, destruction of medial elastin and SMCs was graded as I (mild) to IV (severe) as previously described.21, 22 Data about mural T cells, macrophages, neutrophils and angiogenesis were quantified as CD3+ cells, CD68+ cells, myeloperoxidase (MPO)‐positive cells, and CD31+ blood vessels per aortic cross section (ACS), respectively. Images of sections from the middle of the AAA were captured under uniform settings using an upright brightfield microscope.
Western Blot Analysis
Tissues were lysed for 30 minutes at 4°C in RIPA lysis buffer (P0013B, Beyotime, Shanghai, China). Total protein concentration was determined using bicinchoninic acid reagent. Then 50 μg of total protein was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane, and subjected to immunoblot analysis. The membranes were incubated with primary antibodies followed by incubation with horseradish peroxidase–conjugated secondary antibody (1:5000 dilution, Santa Cruz). Primary antibodies for Western blot are summarized in Table S1. The bands were visualized using enhanced chemiluminescence reagents and analyzed with a gel documentation system (Bio‐Rad Gel Doc1000 and Multi‐Analyst version 1.1). After visualizing the band of mammalian target of rapamycin (mTOR), the membrane was incubated by stripping buffer (P0025B; Beyotime, Shanghai, China) to wash out antibodies, blocked and incubated with a rabbit anti‐mTOR (7C10) monoclonal antibody.
Transmission Electron Microscopic Examination
Mouse aortae were harvested at 14 days after PPE infusion and sliced into 1×1×3 mm3 in size. Then the specimens were fixed in 2.5% glutaraldehyde with Millonig's phosphate buffer (pH=7.3) for transmission electron microscopic laboratory examination. Briefly, specimens were washed with Millonig's phosphate buffer and incubated for 1 hour in 1% osmium tetroxide. Then dehydration of the samples was carried out in a graded series of acetone and followed by a sample resin soaking, embedding, and solidifying process. Ultrathin sections (50 to 100 nm) of specimens were made with an ultramicrotome and a diamond knife. After double staining of 3% uranyl acetate and lead nitrate, the specimens were examined and photographed on a Hitachi HT‐7700 electron microscope.
Flow Cytometric Analysis
Flow cytometry was used to quantify macrophages and other leukocytes in aneurysmal aorta and peripheral blood. Single cell suspensions from peripheral blood and aneurysmal aortae were prepared at euthanasia as previously described.23, 24 Cells were incubated with appropriate antibody cocktail for 20 minutes at room temperature and followed by lysis of erythrocytes with red blood cell lysis buffer. All antibodies were purchased from Biolegend (San Diego, CA) and summarized in Table S1. Staining data were acquired on a Cytek Athena Dxp flow cytometer with Flowjo CE software and analyzed using FlowJo software (Ver X.0.7; FlowJoLLC, Eugene, OR).
Statistical Analysis
GraphPad Prism 8.0.1 (GraphPad Prism Software, Inc., San Diego, CA) software was used for all statistical analysis. All continuous data were reported as the mean± SD. The paired t test, unpaired t test, and nonparametric Mann–Whitney test were used to determine differences between groups according to data characteristics with an P<0.05 considered statistically significant. Differences in feed intake, body weight, and percentage of p62‐positive area in mice aorta were determined by 2‐way ANOVA, and the differences in aneurysm incidence were determined by Fisher exact test.
Results
Spermidine Oral Administration Does Not Alter Mouse Feed Intake and Body Weight Gain
To verify whether spermidine oral administration increases plasma spermidine levels in mice, we performed liquid chromatography/mass spectrometry (LC/MS) analysis. Anticipatively, plasma spermidine concentration was significantly increased in spermidine supplemented, as compared with vehicle, mice (Figure 1A). In addition, food consumption was calculated by weighing each cage of food every 3 days. As demonstrated in Figure 1B, food consumption decreased significantly after PPE infusion, recovered at day 4, and then gradually increased. Neither food consumption (Figure 1B) nor body weight (Figure 1C) were affected by spermidine treatment.
Figure 1. Spermidine oral administration does not alter mouse feed intake and body weight gain.
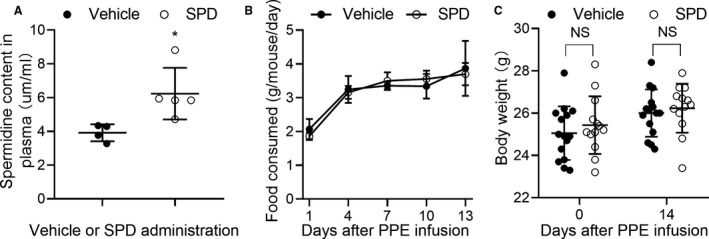
C57BL/6 male mice (8–10 weeks old) were administered 3 mmol/L spermidine in drinking water or normal drinking water as vehicle before PPE infusion to day 14 after PPE infusion. A, Plasma spermidine concentrations were measured by LC/MS. Unpaired t test, *P<0.05 vs vehicle group. n=4 to 5 mice/group. B, No difference in food intake in 2 treatment groups during whole experimental process. Two‐way ANOVA with the Bonferroni correction, P=0.879 vs vehicle group. C, No difference in body weight at the baseline level (P=0.665) and 14 days (P=0.857) following treatment with vehicle (n=15) or spermidine (n=13). Two‐way ANOVA. NS for nonsignificance, vs vehicle group. All data are means±SD. LC/MS indicates liquid chromatography‐mass spectrometry; PPE, porcine pancreatic elastase; and SPD, spermidine.
Spermidine Treatment Prevents Development of Experimental AAAs
Maximal outer aortic diameters were significantly smaller in spermidine‐treated than those in vehicle‐treated mice (1.26±0.12 mm versus 1.67±0.29 mm) (Figure 2A and 2B). Within 14 days following PPE infusion, AAAs developed in all vehicle‐treated mice (100%, 16/16) and 13 mice (72.22%, 13/18) in the spermidine‐treated group (Figure 2C). As shown in Figure 2D and 2E, both elastin destruction and SMC depletion, as evaluated by histological scores, were significantly attenuated in mice treated with spermidine as compared with mice treated with vehicle. Similar results were noted when the positive areas for both medial elastin and SMC were quantified with ImageJ software, and were consistent with the above results (Figure S1). In Masson staining, spermidine treatment promoted the presence of collagen (blue stain) in aneurysmal aortic media (Figure 2D and 2E). These results histologically confirmed AAA suppression by spermidine treatment.
Figure 2. Spermidine treatment prevents development of experimental AAA.
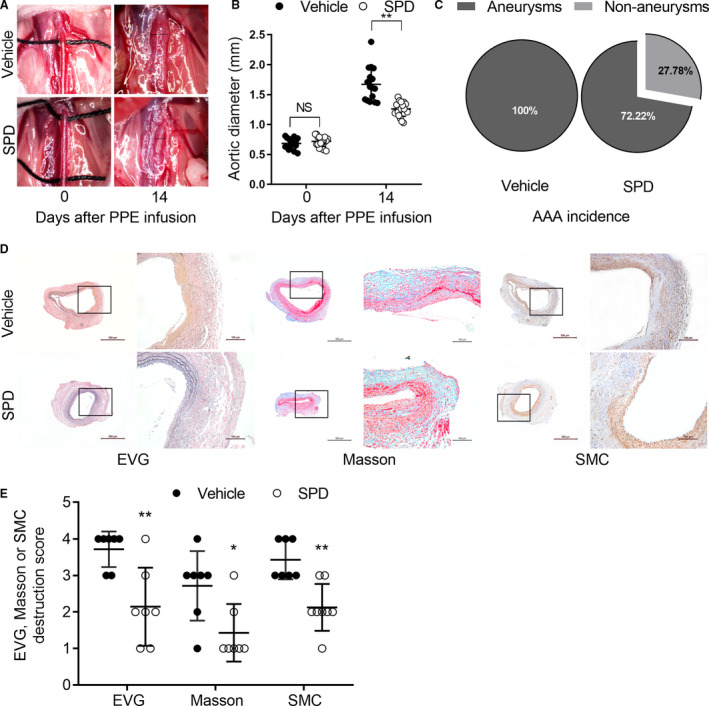
External aortic diameter was measured by computer software under laparotomy. A, Representative aortic images for the baseline (day 0) and 14 days after PPE infusion. B, Mean and SD of aortic diameter (mm) in mice treated with vehicle (n=16) or SPD (n=18) at day 0 and day 14. Unpaired t test, **P<0.01 vs vehicle group. C, AAA incidence. Fisher exact test, P=0.046 vs vehicle group. D and E, Representative images as well as mean±SD of destruction scores for EVG (P=0.004), Masson's (P=0.017), and SMC α actin staining (P=0.001). Scale bar=100 and 500 μm. Medial elastin and SMC destruction were graded as I (mild) to IV (severe). Nonparametric Mann‐Whitney test, **P<0.01 and *P<0.05 vs vehicle group, n=7 to 8 mice/group. AAA indicates abdominal aortic aneurysm; EVG, Verhoeff's Van Gieson; PPE, porcine pancreatic elastase; SMC, smooth muscle cell; and SPD, spermidine.
Spermidine Treatment Reduces Aortic Inflammatory Cell Infiltration and Angiogenesis
Mural inflammatory cell infiltration and angiogenesis are the histopathologic features of abdominal aortic degeneration. As shown in Figure 3, immunohistological analysis revealed that spermidine attenuated mural accumulation of CD3+ T cells and CD68+ macrophages and MPO‐positive neutrophils. A reduction in mural neoangiogenesis was also present, as indicated by reduced CD31+ mural capillary vessels in spermidine‐treated mice. Meanwhile, flow cytometric analysis further revealed that spermidine treatment markedly reduced aortic accumulation of CD45+ leukocytes, myeloid cells (CD45+CD11b+), and macrophages (CD45+CD11b+F4/80+) (Figure 4). These results suggest that reduced mural inflammatory cell infiltration and angiogenesis are partly involved in AAA inhibition by spermidine.
Figure 3. Spermidine treatment reduces aortic inflammatory cell infiltration and angiogenesis.
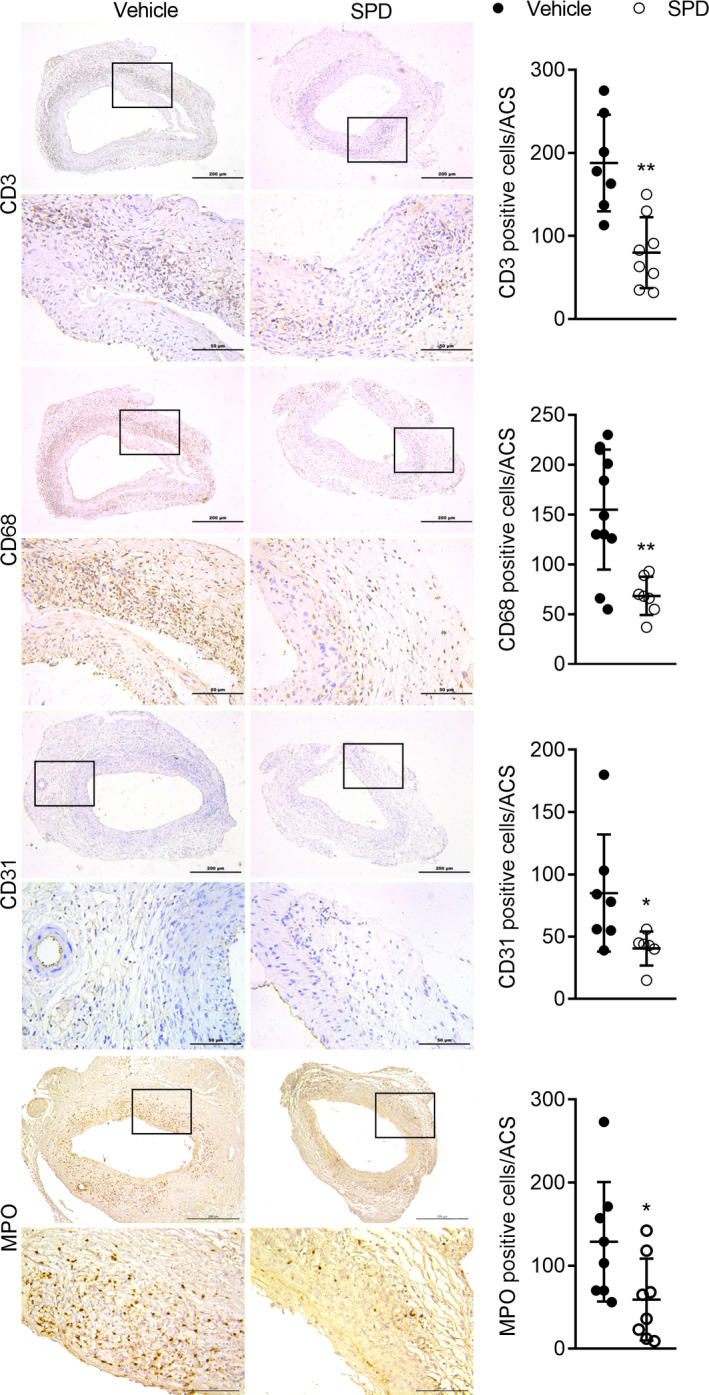
Aortic sections from mice 14 days after PPE infusion were stained with antibodies against CD3, CD68, MPO, and CD31. Representative immunostaining images for CD3+ T cells, CD68+ macrophages, MPO + neutrophils, and CD31+ blood vessels. Scale bar=50 μm and 200 μm. Mean and SD of aortic T cells (P=0.001), macrophages (P=0.001), neutrophils (P=0.041), and angiogenesis (P=0.048) quantified as the positive stain cells or vessels per ACS. Unpaired t test, **P<0.01 and *P<0.05 vs vehicle group, n=7 to 12 mice/group. ACS indicates aortic cross section; MPO, myeloperoxidase; and PPE, porcine pancreatic elastase.
Figure 4. Spermidine attenuates infiltration of leukocytes and macrophages into aneurysmal aorta.
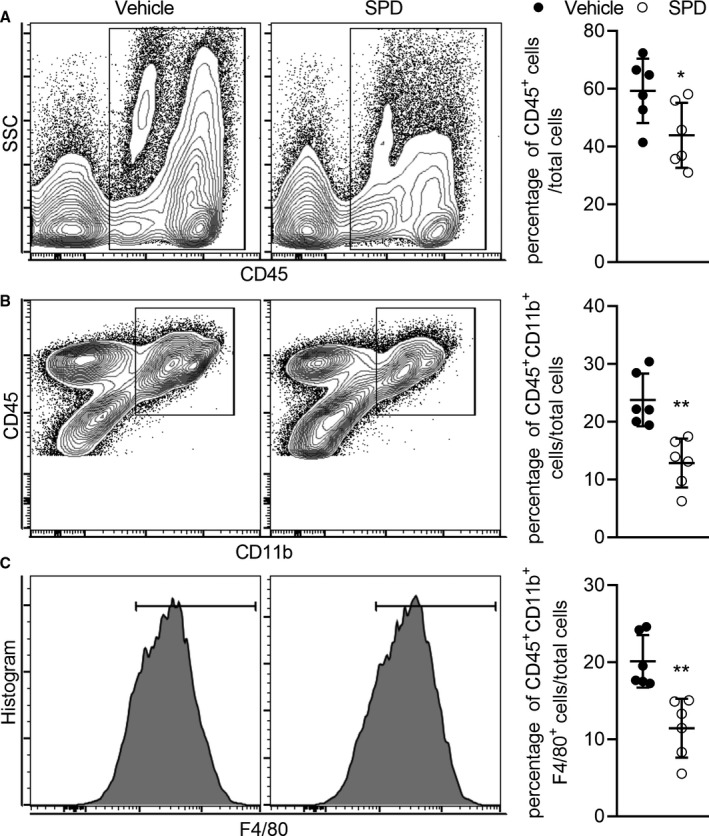
Aortae were collected 14 days after PPE infusion. After digestion into single cells, the aortae were stained with monoclonal antibodies against CD45, CD11b, and F4/80. Leukocytes (CD45+) (A), myeloid cells (CD45+ CD11b+) (B), and macrophages (CD45+ CD11b+F4/80+) (C) were analyzed by flow cytometry. Data presented as mean and SD of the percentages of leukocytes (P=0.039), myeloid cells (P=0.002), and macrophages (P=0.002) in total aortic cells. Unpaired t test with Welch's correction, **P<0.01 and *P<0.05 vs vehicle‐treated group, n=6 mice/group. PPE indicates porcine pancreatic elastase; and SPD, spermidine.
Spermidine Treatment Reduces Circulating Inflammatory Monocytes and Neutrophils
Since circulating inflammatory monocytes are precursors of tissue macrophages, flow cytometric analysis was used to quantify the relative number of circulating inflammatory monocytes (CD11b+Ly‐6Chigh cells) and neutrophils (CD11b+Ly‐6G+ cells) in PPE‐infused mice treated with either spermidine or vehicle. As shown in Figure 5, spermidine treatment reduced circulating inflammatory monocytes and neutrophils as compared with vehicle treatment. Since neutrophils and monocyte/macrophages participate in AAA formation and inflammatory cell infiltration, these results suggest that spermidine‐mediated aneurysm inhibition may be partially associated with reduced circulating inflammatory monocytes and neutrophils.
Figure 5. Spermidine reduces circulating inflammatory cells in experimental abdominal aortic aneurysms.
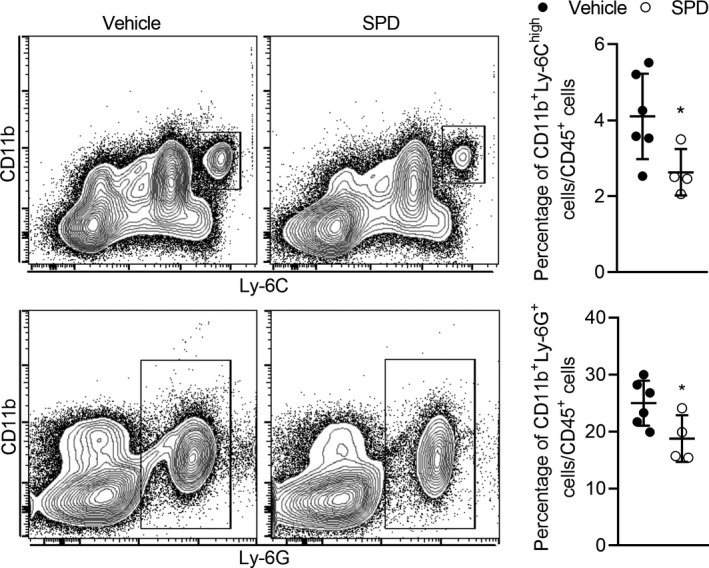
Whole blood samples collected from heart 14 days after PPE infusion were stained with monoclonal antibodies against CD45, CD11b, Ly‐6C, and Ly‐6G. Inflammatory monocytes (CD11b+Ly‐6Chigh) and neutrophils (CD11b+Ly‐6G+) in blood samples in SPD or vehicle group were analyzed by flow cytometry. Data presented as mean and SD of the percentages of inflammatory monocytes (P=0.046) and neutrophils (P=0.043) in total leukocytes gated by forward scatter and CD45. Unpaired t test with Welch's correction, *P<0.05 vs vehicle‐treated group, n=4 to 6 mice/group. PPE indicates porcine pancreatic elastase; and SPD, spermidine.
Spermidine Treatment Increases Autophagy‐Related Protein in Experimental AAA
The SQSTM1/p62 protein, a link between LC3 (microtubule‐associated protein 1 light chain 3) and ubiquitinated substrates, serves as an index of autophagic degradation.15 To address whether spermidine promotes autophagy, we evaluated autophagy‐related protein levels of LC3‐I, LC3‐II, and p62 in sham and 2 experimental AAA groups. As shown in Figure 6A and 6B, the ratio of LC3 II/I was upregulated in spermidine‐treated as compared with vehicle‐treated mice (P<0.05). In addition, compared with the sham group, p62 protein was increased in PPE‐perfused mice with vehicle treatment (P<0.05). Furthermore, Keap1 (Kelch‐like ECH‐associated protein 1), a substrate adaptor protein binding to the Keap1 interacting region of p62 and degrading together with p62 through the autophagy pathway, showed an increased tendency in the expression in PPE‐infused mice. While those tendencies can be stopped by spermidine compared with the vehicle group, spermidine treatment affected neither p62 nor Keap1 compared with the sham group. In immunohistochemical staining, less p62 protein accumulated in aortic media and adventitial in spermidine‐treated as compared with vehicle‐treated mice (Figure 6C and 6D). Furthermore, 3‐color immunofluorescent staining revealed that p62 protein accumulation was reduced in vascular SMCs. In electron microscopic examination, more autophagosomes were noted in medial SMCs and adventitial macrophages in spermidine‐treated as compared with vehicle‐treated mice (Figure 6E). Collectively, these data demonstrated that spermidine treatment increases autophagy‐related protein in experimental AAAs.
Figure 6. Spermidine treatment increases autophagy‐related proteins in experimental AAAs.
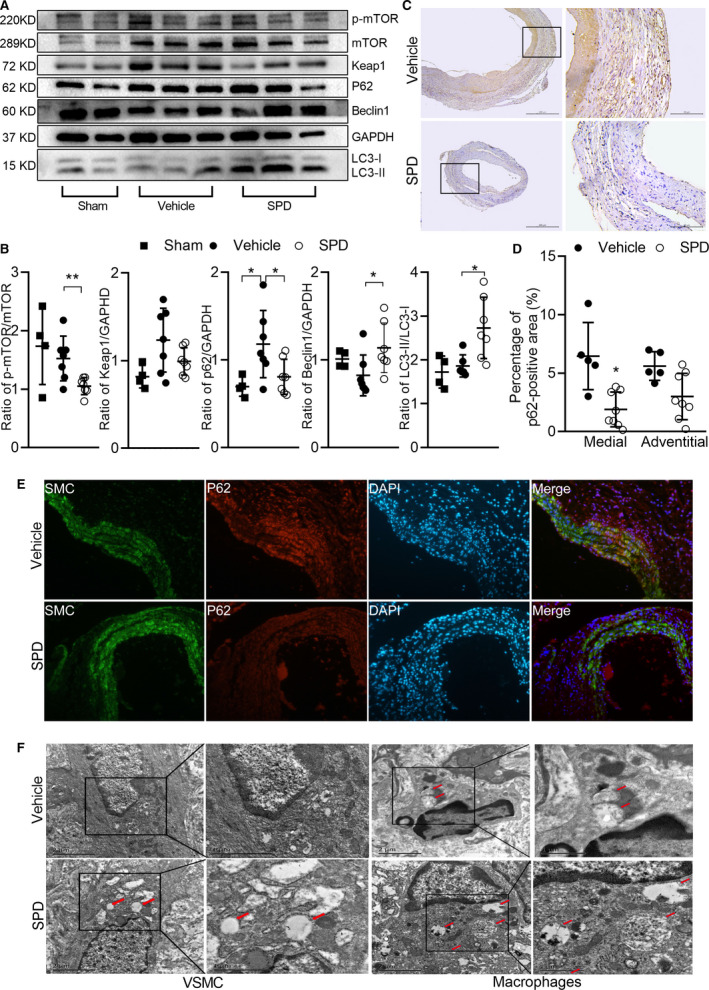
Aortae were collected 14 days after PPE infusion. Western blot, immunohistochemical staining and transmission electron microscopic examination were performed to evaluate the autophagy in experimental AAA. Protein levels of p‐mTOR, mTOR, Keap1, p62, Beclin1, GAPDH, LC3‐I, and LC3‐II were detected by Western blot (A) and quantified by densitometry (B). Data are presented as mean±SD. Unpaired t test, **P<0.01 and *P<0.05 vs vehicle group, n=4 to 7 in each group. C, Representative immunostaining images for p62 protein in vehicle (n=5) and SPD (n=8) groups. Scale bar=50 μm, 100 μm. D, p62 protein in medial (P=0.001) and adventitial (P=0.054) segments of aneurysm was quantified as p62‐positive area. Two‐way ANOVA, *P<0.05 vs vehicle group. E, Representative immunofluorescence staining with antibodies against p62 protein conjugated with Alexa Fluor 594 (red color), and antibodies against SMA conjugated with Alexa Fluor 488 (green color), with the nucleus counterstained with DAPI (blue color). F, Representative transmission electron microscopy of aorta in two groups. AAA indicates abdominal aortic aneurysm; DAPI, 4′,6‐diamidino‐2‐phenylindole; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; Keap1, kelch‐like ECH associated protein 1; LC3, microtubule‐associated protein 1 light chain 3; mTOR, mammalian target of rapamycin; PPE, porcine pancreatic elastase; and SPD, spermidine.
To further explore how spermidine treatment modulates autophagy in experimental AAAs, we analyzed total mTOR, p‐mTOR, and Beclin1 by Western blot analysis. Spermidine treatment reduced mTOR phosphorylation, which is crucial in autophagic flux inhibition, and increased expression of Beclin1, a partner involved in the initial step of autophagosome formation (Figure 6A and 6B). These data suggest that the SPD alters autophagy in experimental AAAs, probably via mTOR and Beclin1 pathways.
Evidence for Dysregulated Autophagy in Clinical AAAs
Finally, to see whether autophagy is altered in clinical AAAs, we analyzed autophagy‐related protein levels of LC3‐I, LC3‐II, p62, p‐mTOR, and mTOR in aneurysmal and nonaneurysmal aortic segments obtained from the same patients. As shown in Figure 7A and 7B, we found p62 protein (P=0.0102) and phosphorylated mTOR (P=0.0155) levels were increased in aneurysmal, as compared with nonaneurysmal, aortic segments. Immunohistochemical staining also revealed that p62 protein accumulation was increased in the medial and adventitial segments of aneurysmal, as compared with nonaneurysmal, aortic segments (Figure 7C and 7D). Although the reduced ratio of LC3‐II to LC3‐I was noted in some aneurysmal aortic segments, no statistical difference was seen between aneurysmal and nonaneurysmal aortic segments (P=0.14) (Figure 7A and 7B). To clarify whether this difference in autophagy was caused by the different cellular composition of the specimens, Masson staining and immunohistochemical staining for CD3, CD68, and CD31 were performed. No significant difference in muscle fiber and infiltrated inflammatory cells was noted between clinical aneurysmal and nonaneurysmal aortic segments (Figure S2), suggesting that the difference in autophagy might not be caused by the different cellular composition of the specimens. Collectively, these results indicate that autophagy is dysregulated in clinical AAAs.
Figure 7. Evidence for dysregulated autophagy in clinical AAAs.
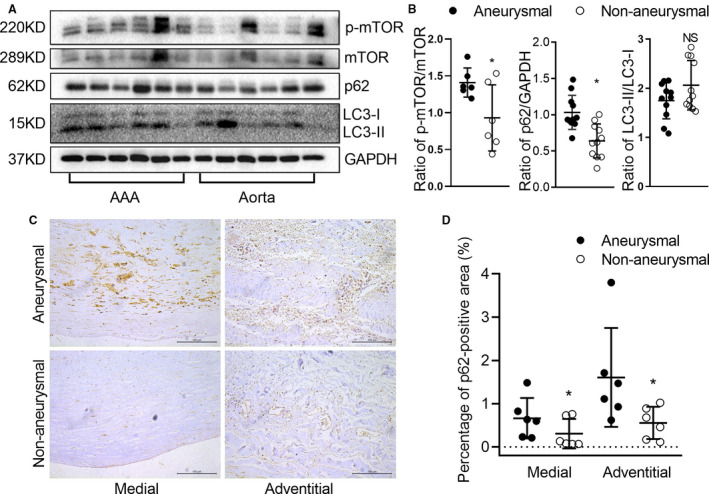
Western blot and immunostaining were performed to evaluate the autophagy of AAA and adjacent normal aortas from patients who underwent open AAA repair surgery. Protein levels of p‐mTOR, mTOR, p62, LC3‐I, and LC3‐II were detected by Western blot (A) and quantified by densitometry (B). Data are presented as mean±SD. Paired t test, NS for nonsignificance, *P<0.05 vs AAA, n=6 to 11. C, Representative aortic histology images of immunohistochemical staining of p62 protein. D, Analysis of p62 protein in medial (P=0.025) and adventitial (P=0.036) in adjacent normal and aneurysmal human aortae. Scale bar=100 μm. Data are presented as mean±SD. Paired t test, *P<0.05 vs AAA, n=6 in each group. AAA indicates abdominal aortic aneurysm; LC3, microtubule‐associated protein 1 light chain 3; and mTOR, mammalian target of rapamycin.
Spermidine Treatment Reduces Aortic Remodeling in Established Experimental AAA
To investigate the translational potential of SPD treatment in existing AAAs, treatment of SPD or vehicle alone was initiated 3 days after PPE infusion and continued until euthanasia. As shown in Figure S3A and S3B, delayed treatment with SPD was effective in reducing aneurysm enlargement in established AAAs, although lack of significance was likely because of large variations within the responses of individual mice. On histologic analysis, spermidine treatment preserved medial elastin and SMCs and diminished mural leukocyte infiltration (Figure S3C and S3D). Thus, these data suggest that spermidine might attenuate aortic remodeling and thus has therapeutic potential for established AAAs.
Discussion
In this study, we discovered that oral spermidine supplementation increased plasma spermidine levels and suppressed the development of experimental AAAs with preservation of aortic structural integrity. Spermidine‐mediated aneurysm inhibition was accompanied by attenuation of mural angiogenesis, leukocytes infiltration, and circulating inflammatory cells. Furthermore, spermidine upregulated autophagy in experimental AAA with decreased p62 protein accumulation and phosphorylated mTOR and increased Beclin1. We also demonstrated that the dysregulated autophagy in clinical AAAs was characterized by increased p62 protein accumulation and phosphorylated mTOR. These results underscore the specific role of autophagy in AAA pathogenesis and place spermidine as a promising translational pharmacological strategy for AAA disease suppression.
Main pathological features of AAA include extracellular matrix degradation and loss of vascular SMCs as a result of the inflammatory cells infiltration, and eventually lead to vascular remodeling and weakening of the aortic wall.25 Chronic aortic inflammation plays a critical role in AAAs through the release of matrix‐degrading enzymes, free radicals, and proinflammatory cytokines.26, 27, 28 Previous studies demonstrated that SPD levels and autophagy function declined with aging and disease,29, 30 and our present study also indicated a dysregulated autophagy function in clinical AAA specimens. Polyamines, primarily spermidine and spermine, regulate inflammatory responses.31 Although the mechanism of anti‐inflammatory and anti‐oxidative effects has not been fully elucidated, spermidine has been shown to exert anti‐inflammatory effects in several disease models.14, 32, 33, 34 In previous in vivo studies, spermidine suppressed subclinical inflammation and reduced circulating tumor necrosis factor in aged mice,14 protected against oxidative stress and recruitment of neutrophils and macrophages in both zebrafish32 and 12‐O‐tetradecanoylphorbol‐13‐acetate‐induced skin inflammation in mice,33 and inhibited the expression levels of pro‐inflammatory cytokines and macrophage activation by targeting the NF‐κB pathway with experimental autoimmune encephalomyelitis.34 In previous in vitro studies, spermidine treatment reduced reactive oxygen species accumulation and the levels of inflammatory cytokines such as nitric oxide, tumor necrosis factor‐α, and interleukin‐1β in lipopolysaccharide (LPS)‐stimulated RAW264.7 murine macrophages.32, 33 Moreover, spermidine inhibited the secretion of inflammatory cytokines such as tumor necrosis factor‐α and MCP‐1 in lipopolysaccharide‐induced NR8383 macrophage35 as well as inhibited lipopolysaccharide‐stimulated inflammation in microglia by blocking the NF‐κB, PI3K/Akt, and MAPKs signaling pathways.36 In agreement with those studies, we found that oral spermidine supplementation attenuated mural inflammatory cell infiltration in experimental AAAs as presented by immunohistochemical staining and flow cytometric analyses. Spermidine treatment also reduced circulating inflammatory monocytes and neutrophils, thereby diminishing the population of inflammatory cells available for migration and aortic localization. Those results suggest that the inhibitory effect of spermidine on AAAs was attributed to reduced systemic inflammation and migration of immune cells. However, whether the effects of spermidine on retaining aortic structure and alleviating mural inflammation are associated with autophagic flux is not fully understood.
Autophagy is a conserved intracellular process of recycling damaged cytoplasmic constituents, the primary mechanism by which hosts maintain protein homeostasis and protect against stressful conditions. It has been shown that autophagy is associated with many basic biological processes and dysregulated autophagy is involved in a variety of chronic AAA diseases.37 Moreover, basal autophagy, as an essential in vivo process, mediates proper vascular function and serves as an important survival pathway in endothelial cells and SMCs.38 Despite the protective effects of autophagy in arterial aging, atherosclerosis, and other cardiovascular diseases, the role of autophagy in AAA disease remains incompletely resolved.38 In previous research, the expressions of LC3 protein and autophagy‐related genes such as Beclin1/Atg6 were markedly upregulated in both clinical and experimental aneurysmal lesions,39, 40 suggesting autophagy was induced in these tissues. However, autophagic flux is a dynamic and multistep process that involves autophagosome formation, fusion with lysosomes and degradation, and its changes in AAA need to be further confirmed. In recent studies, defective autophagy in vascular SMCs has been shown to increase the susceptibility of SMCs to cell death and the severity of aortic disease with more obvious pathological changes such as aortic degradation and atherosclerosis lesions in mice with SMC‐specific deletion of gene atg741 and atg5.42 Mice with SMC‐specific ATG7 deficiency also exhibited exacerbated adverse aortic remodeling and larger suprarenal aortic diameters following angiotensin II infusion.43 Although no overt differences in the development of dissecting AAA are found in this study, it suggests an essential role of normal autophagy in maintaining normal structure of the aorta. However, chloroquine, a well‐known autophagy inhibitor, was found to have no effect on angiotensin II‐induced AAA formation and pathological features.44 This may be because of anti‐inflammatory effects of chloroquine, considering its anti‐inflammatory effects as an immunomodulator for the treatment of autoimmune diseases. In addition, previous studies have reported that several anti‐inflammatory agents, also confirmed as potent autophagy inducers, such as rapamycin,21 resveratrol,45 and everolimus,46 attenuated experimental AAAs. These studies indicate that autophagy might be an adaptive response to pathogenic factors and a potential therapeutic target in AAA disease.
In our study, we discovered impaired autophagy in clinical aneurysmal segments characterized by increased p62 protein accumulation and phosphorylated mTOR as compared with nonaneurysmal segments. Inconsistent with the previous study,39 there is no change in expression of LC3 protein, probably because we choose the adjacent normal aorta from the same patients instead of the normal aorta from organ donors as control. In addition, we found that autophagy‐related protein in experimental AAAs was increased by spermidine as evidenced by the downregulated p62 protein and upregulated LC3 II to LC3 I conversion following spermidine treatment. To further explore the potential mechanism of spermidine‐induced autophagy in experimental AAAs, Western blot analysis revealed decreased phosphorylation of mTOR and increased Beclin1 in the spermidine group. Although spermidine‐induced autophagy has been reported in an mTOR‐independent manner,47 our results are consistent with previous studies in which spermidine treatment alleviated myocardial infarction via enhanced cardiac autophagic flux by targeting the adenosine monophosphate–activated protein kinase/mTOR signaling pathway48 and promoted arterial autophagy in aged mice.15
SPD is also considered a calorie restriction mimetic (CRM) because of induction of deacetylation and autophagy. Calorie restriction (CR), which consists of decreasing caloric intake without causing malnutrition, protected experimental AAAs.49 Along with CR and most CRMs, spermidine treatment mimics the effects of starvation with regard to acetyl coenzyme A depletion and consequently protein deacetylation. CR49 and several CRMs such as rapamycin,21 resveratrol,45 metformin,50 and curcumin,51 also protected against experimental AAAs. In our study, spermidine supplementation has no effect on food consumption and body weight, suggesting spermidine‐induced AAA inhibition was likely not mediated by altering calorie intake directly.
In current drug research on AAA disease, spermidine might be very promising in clinical applications for multiple reasons. First, spermidine is a natural compound present in the daily diet; thus its oral supplementation is an effective means for clinical use. Second, spermidine can be synthesized in mammalian cells52 and mainly excreted through the kidneys after metabolism, suggesting that adverse reactions are unlikely to occur after exogenous supplementation. In fact, in a 3‐month randomized Phase II clinical trial, supplementation with spermidine‐rich plant extracts (equivalent to a daily spermidine dose of 1.2 mg) was safe and well tolerated in older adults.53 Third, 2 prospective population‐based studies (summarized in the same paper) reported for the first time that nutritional spermidine uptake is also linked to reduced overall, cardiovascular, and cancer‐related mortality in humans.29
CONCLUSIONS
In conclusion, our present study demonstrates that spermidine inhibited the development of experimental AAA, accompanied by preservation of aortic structural integrity, reduced inflammatory cell infiltration, and circulating inflammatory monocytes, as well as increased autophagy‐related protein. These findings suggest that spermidine might have therapeutic potential for AAA diseases.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (No. 81873525 to W. Wang), Clinical Research Project of Xiangya Hospital, Central South University (No. 201603 to W. Wang); and the project sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 201507 to W. Wang).
Disclosures
None.
Supporting information
Table S1
Figures S1–S3
Acknowledgments
We thank Dr Yongping Bai at Department of Cardiovascular Medicine, Xiangya Hospital, Central South University, for guidance of the experiments.
(J Am Heart Assoc. 2020;e014757 DOI: 10.1161/JAHA.119.014757.)
For Sources of Funding and Disclosures, see page 13.
References
- 1. Aggarwal S, Qamar A, Sharma V, Sharma A. Abdominal aortic aneurysm: a comprehensive review. Exp Clin Cardiol. 2011;16:11–15. [PMC free article] [PubMed] [Google Scholar]
- 2. Sampson UK, Norman PE, Fowkes FG, Aboyans V, Yanna S, Harrell FE Jr, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, et al. Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010. Glob Heart. 2014;9(171–180):e110. [DOI] [PubMed] [Google Scholar]
- 3. Huang T, Liu S, Huang J, Xu B, Bai Y, Wang W. Meta‐analysis of the growth rates of abdominal aortic aneurysm in the Chinese population. BMC Cardiovasc Disord. 2019;19:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e72. [DOI] [PubMed] [Google Scholar]
- 5. Golledge J, Norman PE, Murphy MP, Dalman RL. Challenges and opportunities in limiting abdominal aortic aneurysm growth. J Vasc Surg. 2017;65:225–233. [DOI] [PubMed] [Google Scholar]
- 6. Kokje VB, Hamming JF, Lindeman JH. Editor's Choice—pharmaceutical management of small abdominal aortic aneurysms: a systematic review of the clinical evidence. Eur J Vasc Endovasc Surg. 2015;50:702–713. [DOI] [PubMed] [Google Scholar]
- 7. Yu J, Liu S, Huang J, Wang W. Current theories and clinical trial evidence for limiting human abdominal aortic aneurysm growth. Curr Drug Targets. 2018;19:1302–1308. [DOI] [PubMed] [Google Scholar]
- 8. Binh PNT, Soda K, Maruyama C, Kawakami M. Relationship between food polyamines and gross domestic product in association with longevity in Asian countries. Health. 2010;2:1390–1396. [Google Scholar]
- 9. Soda K. Polyamine intake, dietary pattern, and cardiovascular disease. Med Hypotheses. 2010;75:299–301. [DOI] [PubMed] [Google Scholar]
- 10. Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, Werner P, Ruckenstuhl C, Iglseder B, Weger S, et al. Higher spermidine intake is linked to lower mortality: a prospective population‐based study. Am J Clin Nutr. 2018;108:371–380. [DOI] [PubMed] [Google Scholar]
- 11. Milovic V. Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol. 2001;13:1021–1025. [DOI] [PubMed] [Google Scholar]
- 12. Soda K, Kano Y, Sakuragi M, Takao K, Lefor A, Konishi F. Long‐term oral polyamine intake increases blood polyamine concentrations. J Nutr Sci Vitaminol (Tokyo). 2009;55:361–366. [DOI] [PubMed] [Google Scholar]
- 13. Ren J, Zhang Y. Targeting autophagy in aging and aging‐related cardiovascular diseases. Trends Pharmacol Sci. 2018;39:1064–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LaRocca TJ, Gioscia‐Ryan RA, Hearon CM Jr, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forte A, Balistreri CR, De Feo M, Della Corte A, Hellstrand P, Persson L, Nilsson BO. Polyamines and microbiota in bicuspid and tricuspid aortic valve aortopathy. J Mol Cell Cardiol. 2019;129:179–187. [DOI] [PubMed] [Google Scholar]
- 17. Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso‐Santano M, et al. Regulation of autophagy by cytosolic acetyl‐coenzyme A. Mol Cell. 2014;53:710–725. [DOI] [PubMed] [Google Scholar]
- 18. Yang L, Shen L, Gao P, Li G, He Y, Wang M, Zhou H, Yuan H, Jin X, Wu X. Effect of AMPK signal pathway on pathogenesis of abdominal aortic aneurysms. Oncotarget. 2017;8:92827–92840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W, Xu B, Xuan H, Ge Y, Wang Y, Wang L, Huang J, Fu W, Michie SA, Dalman RL. Hypoxia‐inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg. 2018;68:1538–1550.e1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsutsui H, Mochizuki T, Inoue K, Toyama T, Yoshimoto N, Endo Y, Todoroki K, Min JZ, Toyo'oka T. High‐throughput LC‐MS/MS based simultaneous determination of polyamines including N‐acetylated forms in human saliva and the diagnostic approach to breast cancer patients. Anal Chem. 2013;85:11835–11842. [DOI] [PubMed] [Google Scholar]
- 21. Rouer M, Xu BH, Xuan HJ, Tanaka H, Fujimura N, Glover KJ, Furusho Y, Gerritsen M, Dalman RL. Rapamycin limits the growth of established experimental abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47:493–500. [DOI] [PubMed] [Google Scholar]
- 22. Yu J, Liu R, Huang J, Wang L, Wang W. Inhibition of phosphatidylinositol 3‐kinase suppresses formation and progression of experimental abdominal aortic aneurysms. Sci Rep. 2017;7:15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, et al. Flt3 signaling‐dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. [DOI] [PubMed] [Google Scholar]
- 24. Xu B, Iida Y, Glover KJ, Ge Y, Wang Y, Xuan H, Hu X, Tanaka H, Wang W, Fujimura N, et al. Inhibition of VEGF (vascular endothelial growth factor)‐A or its receptor activity suppresses experimental aneurysm progression in the aortic elastase infusion model. Arterioscler Thromb Vasc Biol. 2019;39:1652–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raffort J, Lareyre F, Clement M, Hassen‐Khodja R, Chinetti G, Mallat Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol. 2017;14:457–471. [DOI] [PubMed] [Google Scholar]
- 26. Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16:225–242. [DOI] [PubMed] [Google Scholar]
- 27. Peng H, Zhang K, Liu Z, Xu Q, You B, Li C, Cao J, Zhou H, Li X, Chen J, et al. VPO1 modulates vascular smooth muscle cell phenotypic switch by activating extracellular signal‐regulated kinase 1/2 (ERK 1/2) in abdominal aortic aneurysms. J Am Heart Assoc. 2018;7:e010069 DOI: 10.1161/JAHA.118.010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Q, Huang JH, Xia RP, Duan XH, Jiang YB, Jiang Q, Sun WJ. Suppression of experimental abdominal aortic aneurysm in a rat model by the phosphodiesterase 3 inhibitor cilostazol. J Surg Res. 2011;167:e385–e393. [DOI] [PubMed] [Google Scholar]
- 29. Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359:eaan2788. [DOI] [PubMed] [Google Scholar]
- 30. Minois N, Carmona‐Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY). 2011;3:716–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hussain T, Tan B, Ren W, Rahu N, Dad R, Kalhoro DH, Yin Y. Polyamines: therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids. 2017;49:1457–1468. [DOI] [PubMed] [Google Scholar]
- 32. Jeong JW, Cha HJ, Han MH, Hwang SJ, Lee DS, Yoo JS, Choi IW, Kim S, Kim HS, Kim GY, et al. Spermidine protects against oxidative stress in inflammation models using macrophages and zebrafish. Biomol Ther (Seoul). 2018;26:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013;62:681–688. [DOI] [PubMed] [Google Scholar]
- 34. Yang Q, Zheng C, Cao J, Cao G, Shou P, Lin L, Velletri T, Jiang M, Chen Q, Han Y, et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016;23:1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perez‐Cano FJ, Franch A, Castellote C, Castell M. Immunomodulatory action of spermine and spermidine on NR8383 macrophage line in various culture conditions. Cell Immunol. 2003;226:86–94. [DOI] [PubMed] [Google Scholar]
- 36. Choi YH, Park HY. Anti‐inflammatory effects of spermidine in lipopolysaccharide‐stimulated BV2 microglial cells. J Biomed Sci. 2012;19:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhong Z, Sanchez‐Lopez E, Karin M. Autophagy, NLRP3 inflammasome and auto‐inflammatory/immune diseases. Clin Exp Rheumatol. 2016;34:12–16. [PubMed] [Google Scholar]
- 38. De Meyer GR, Grootaert MO, Michiels CF, Kurdi A, Schrijvers DM, Martinet W. Autophagy in vascular disease. Circ Res. 2015;116:468–479. [DOI] [PubMed] [Google Scholar]
- 39. Zheng YH, Tian C, Meng Y, Qin YW, Du YH, Du J, Li HH. Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. J Cell Physiol. 2012;227:127–135. [DOI] [PubMed] [Google Scholar]
- 40. Salmon M, Spinosa M, Zehner ZE, Upchurch GR, Ailawadi G. Klf4, Klf2, and Zfp148 activate autophagy‐related genes in smooth muscle cells during aortic aneurysm formation. Physiol Rep. 2019;7:e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osonoi Y, Mita T, Azuma K, Nakajima K, Masuyama A, Goto H, Nishida Y, Miyatsuka T, Fujitani Y, Koike M, et al. Defective autophagy in vascular smooth muscle cells enhances cell death and atherosclerosis. Autophagy. 2018;14:1991–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clement M, Chappell J, Raffort J, Lareyre F, Vandestienne M, Taylor AL, Finigan A, Harrison J, Bennett MR, Bruneval P, et al. Vascular smooth muscle cell plasticity and autophagy in dissecting aortic aneurysms. Arterioscler Thromb Vasc Biol. 2019;39:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramadan A, Singh KK, Quan A, Plant PJ, Al‐Omran M, Teoh H, Verma S. Loss of vascular smooth muscle cell autophagy exacerbates angiotensin ii‐associated aortic remodeling. J Vasc Surg. 2018;68:859–871. [DOI] [PubMed] [Google Scholar]
- 44. Ramadan A, Wheatcroft MD, Quan A, Singh KK, Lovren F, Dhingra N, Teoh H, Al‐Omran M, Leong‐Poi H, Verma S. Effects of long‐term chloroquine administration on the natural history of aortic aneurysms in mice. Can J Physiol Pharmacol. 2015;93:641–648. [DOI] [PubMed] [Google Scholar]
- 45. Moran CS, Biros E, Krishna SM, Wang Y, Tikellis C, Morton SK, Moxon JV, Cooper ME, Norman PE, Burrell LM, et al. Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin‐converting enzyme 2. Arterioscler Thromb Vasc Biol. 2017;37:2195–2203. [DOI] [PubMed] [Google Scholar]
- 46. Moran CS, Jose RJ, Moxon JV, Roomberg A, Norman PE, Rush C, Korner H, Golledge J. Everolimus limits aortic aneurysm in the apolipoprotein E‐deficient mouse by downregulating C‐C chemokine receptor 2 positive monocytes. Arterioscler Thromb Vasc Biol. 2013;33:814–821. [DOI] [PubMed] [Google Scholar]
- 47. Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Benit P, Rustin P, Criollo A, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yan J, Yan JY, Wang YX, Ling YN, Song XD, Wang SY, Liu HQ, Liu QC, Zhang Y, Yang PZ, et al. Spermidine‐enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting AMPK/mTOR signaling pathway. Br J Pharmacol. 2019;176:3126–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu Y, Wang TT, Zhang R, Fu WY, Wang X, Wang F, Gao P, Ding YN, Xie Y, Hao DL, et al. Calorie restriction protects against experimental abdominal aortic aneurysms in mice. J Exp Med. 2016;213:2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Z, Guo J, Han X, Xue M, Wang W, Mi L, Sheng Y, Ma C, Wu J, Wu X. Metformin represses the pathophysiology of AAA by suppressing the activation of PI3K/AKT/mTOR/autophagy pathway in ApoE(‐/‐) mice. Cell Biosci. 2019;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hao Q, Chen X, Wang X, Dong B, Yang C. Curcumin attenuates angiotensin II‐induced abdominal aortic aneurysm by inhibition of inflammatory response and ERK signaling pathways. Evid Based Complement Alternat Med. 2014;2014:270930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwarz C, Stekovic S, Wirth M, Benson G, Royer P, Sigrist SJ, Pieber T, Dammbrueck C, Magnes C, Eisenberg T, et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY). 2018;10:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S3


