Abstract
Background
Obstructive sleep apnea (OSA) is common and independently associated with atrial fibrillation (AF) in patients with hypertrophic cardiomyopathy (HCM). This study aimed to investigate the relationship between apnea‐hypopnea index (AHI), a measure of OSA severity, and prevalence of AF in a large series of patients with HCM.
Methods and Results
A total of 555 patients with HCM who underwent sleep evaluations were retrospectively included. Data from polysomnography studies, echocardiography, and baseline clinical characteristics were collected. OSA was present in 327 patients (58.9%). Patients with OSA or higher AHI quartiles were older, more often male, had a higher body mass index, and more clinical comorbidities. The prevalence of AF increased in patients with OSA (23.9% versus 13.6%, P=0.003) or across AHI quartiles (9.4%, 17.3%, 26.6%, and 25.2%, respectively; P for trend <0.001). After adjustment for age, sex, body mass index, New York Heart Association class, left atrial diameter, hypertension, oxygen desaturation index, and obstructive HCM, highest AHI quartile (odds ratio, 4.42; 95% CI, 1.35–14.52 [P=0.014]) or moderate to severe OSA (odds ratio, 3.03; 95% CI, 1.28–7.20 [P=0.012]) but not presence of OSA (odds ratio, 1.58; 95% CI, 0.84–2.97 [P=0.153]) were significantly associated with AF. Higher AHI levels were also factors associated with persistent or permanent AF (highest AHI quartile with odds ratio, 10.96; 95% CI, 1.07–111.85).
Conclusions
Severity of AHI level is independently associated with AF in patients with HCM. Clinical trials are required to determine the benefits of OSA treatment on AF in patients with HCM.
Keywords: apnea‐hypopnea index, atrial fibrillation, hypertrophic cardiomyopathy, obstructive sleep apnea
Subject Categories: Atrial Fibrillation, Cardiomyopathy, Hypertrophy, Risk Factors
Clinical Perspective
What Is New?
Obstructive sleep apnea (OSA) was highly prevalent in this large series of patients with hypertrophic cardiomyopathy (HCM).
The prevalence of atrial fibrillation increased in patients with HCM who have OSA or across apnea‐hypopnea index quartiles.
Higher apnea‐hypopnea index levels as well as moderate to severe OSA were independent factors for atrial fibrillation prevalence in multivariate analysis.
What Are the Clinical Implications?
Apnea‐hypopnea index might be a potential sleep parameter for risk stratification of atrial fibrillation development in patients with HCM.
More attention should be paid and treatment decisions should be made in patients with HCM who have more severe OSA.
The findings of the study motivate clinical trials aimed at investigating whether treatment of OSA has the potential to prevent future atrial fibrillation development and reduce the incidence of cardiovascular death especially in patients with HCM who have higher apnea‐hypopnea index levels.
Nonstandard Abbreviations and Acronyms .
AF atrial fibrillation
AHI apnea‐hypopnea index
BMI body mass index
HCM hypertrophic cardiomyopathy
LAD left atrial diameter
LV left ventricular
LVOTG left ventricular outflow tract gradient OSA obstructive sleep apnea
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in individuals with hypertrophic cardiomyopathy (HCM). Patients with HCM who have AF have increased rates of morbidity and mortality attributable to heart failure and stroke.1 Identifying comorbidities that exacerbate the occurrence of AF in patients with HCM is of great importance.2 Obstructive sleep apnea (OSA) is characterized by recurrent episodes of either partial or complete upper airway obstruction during sleep, leading to episodes of interruption of respiration and intermittent hypoxia.3 A number of studies have implicated OSA as an independent risk factor for AF in the general population.4, 5, 6 Moreover, OSA is extremely common among patients with HCM, with a prevalence ranging from 32% to 71%, and is independently associated with AF in patients with HCM.7, 8, 9, 10 However, these studies were conducted in a relatively small sample size of <100 patients and there are a lack of data from a large HCM population. In addition, the association between severity of OSA and AF in patients with HCM is still unclear.
In the present study, we aimed to determine the impacts of the presence of OSA as well as apnea‐hypopnea index (AHI), a measure of OSA severity, on the prevalence of AF in a large series of patients with HCM. A total of 555 patients with HCM who underwent an overnight diagnostic sleep study from 2010 to 2018 at Fuwai Hospital were retrospectively studied. Patients were grouped according to the diagnosis of OSA or AHI quartiles. This is to date the largest study evaluating the effects of sleep disorders on AF in patients with HCM.
Methods
Study Population
This retrospective study included patients who were diagnosed with HCM and underwent a first overnight diagnostic sleep examination in the inpatient department at Fuwai Hospital between February 2010 and December 2018. The data that support the findings of this study are available from the corresponding author upon reasonable request. All patients were clinically stable who did not undergo changes in New York Heart Association functional class over the past 30 days. All patients did not previously undergo continuous positive airway pressure treatment. The diagnosis of HCM was made based on typical clinical, ECG, and echocardiographic features. Diagnostic criteria of HCM were consistent with the 2011 American Heart Association/American College of Cardiology and 2014 European Society of Cardiology guidelines, which mainly include unexplained septal hypertrophy with a thickness of at least 15 mm. Cardiac magnetic resonance imaging was conducted in more than 80% of patients in our study to help diagnosis. Patients were excluded if they had >50% of central respiratory events, incomplete sleep recording data, were younger than 18 years, had previous septal reduction therapy (septal myectomy or alcohol septal ablation), or history of heart transplantation surgery.
Patient demographics and clinical data such as age, sex, height, weight, medication use, history of coronary artery disease, diabetes mellitus, hyperlipidemia, hypertension, smoking, and previous surgical septal myectomy or alcohol septal ablation were retrospectively reviewed. All patients provided informed consent before enrollment. The study was approved by the ethics committee of Fuwai Hospital. All studies were conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Definition of AF
The diagnosis of AF was made in accordance with the definitions of the 2016 European Society of Cardiology guidelines.11 Data including medical history, ECG, Holter ECG, or history of AF were collected to help diagnosis. An episode lasting at least 30 seconds was necessary for the diagnosis of AF. Diagnosis of AF was made before the time of sleep monitoring. Paroxysmal AF was defined as AF that terminated spontaneously or with intervention within 7 days of onset. Persistent AF was defined when AF episodes lasted longer than 7 days, including episodes that were terminated by cardioversion, either with drugs or by direct current cardioversion, after ≥7 days. Long‐standing persistent AF that lasted at least 1 year when decided to adopt a rhythm control strategy was classified as persistent AF in our study. Permanent AF was defined when AF was accepted by the patients and physicians and further attempts were stopped to restore or maintain sinus rhythm.
Sleep Study
An overnight polysomnography was performed in all included patients in Fuwai Hospital by using the portable monitoring system Embletta (Medcare Flaga). This device records nasal airflow by an airflow pressure transducer, finger pulse oximetry, thoracic and abdominal movement, body position, snoring, heart rate, and ECG, and has been validated against full polysomnography.12 A continuous recording of respiration and oxyhemoglobin enabled the evaluation of sleep fragmentation and desaturation. The sleep was monitored automatically, starting from 30 minutes after the patients went to bed. Polysomnography data were scored manually by trained personnel. Hypopnea was defined as a 50% or discernible decrement in airflow lasting 10 seconds with oxygen desaturation of 4%. Apnea was defined when cessation of airflow or airflow reduction to ≤10% of the baseline value lasted for ≥10 seconds (whether central, obstructive, or mixed). Obstructive apneas were classified on the basis of the presence of thoracic efforts. The total recording time was used in the denominator to calculate the AHI. AHI was defined as the total number of apneas and hypopneas occurring per hour of sleep. The oxygen desaturation index was defined as the number of oxygen level drops 3% from baseline per hour. In addition, mean and minimal oxygen saturation, average pulse frequency, and snoring proportion were also recorded. Diagnosis of OSA was made solely when the AHI in the recorded study was ≥5 events per hour, irrespective of daytime OSA symptoms, which allowed objective evaluation of the disease severity.13 The severity of OSA was stratified according to AHI quartiles. We also grouped patients into mild OSA (AHI: 5.0–14.9 events per hour) and moderate to severe OSA (AHI ≥15.0 events per hour) based on widely accepted OSA severity thresholds in regression analysis.
Echocardiographic Study
Transthoracic echocardiography was performed using a GE Vivid 7 or E‐9 ultrasound machine (GE Healthcare) with a multifrequency phased‐array transducer. Echocardiographic examinations were performed by experienced physicians. Diameters of the cardiac chambers were expressed as the maximum value of the anteroposterior diameter in cardiac cycles. The ascending aorta diameter was 4 cm above the aortic valve during diastole. The thickness of the interventricular septum and ventricular wall was determined during diastole. Aside from the maximum thickness, the representative thickness of the interventricular septum, which was usually the thickness of the point 25 mm under the right coronary sinus nadir, was recorded to indicate overall thickness. Left ventricular (LV) outflow tract gradient (LVOTG) was measured in the apical views by continuous‐wave Doppler echocardiography under resting conditions and during provocative maneuvers, including Valsalva, treadmill exercise, and/or amyl nitrite inhalation, to elicit latent obstruction, as previously reported.14 The measurements of LV volume, LV ejection fraction and left atrial diameter (LAD) were determined following the American Society of Echocardiography recommendations.15
Statistical Analysis
The results were expressed as mean±SD, median (interquartile range), or number (percentage). Continuous variables were tested for normal distribution with the Kolmogorov–Smirnov test. Comparison of categorical variables was performed using χ2 or Fisher exact test, as appropriate. Differences between the 2 groups in continuous variables were compared using Student unpaired t test or Mann–Whitney U test. Differences among the 4 groups were compared using 1‐way analysis of variance or the Kruskal–Wallis H test, as appropriate. Comparisons among different groups were performed using χ2 Mantel–Haenszel test for trend in the distribution of AF. Univariate and multivariate logistic regression analyses were used to determine the association between the presence of OSA or severity of AHI and AF. Variables were included into multivariate analysis because of their known clinical importance or because they were previously accepted as clinical risk factors for AF.1, 16, 17 Models were presented as minimally adjusted (model 1 adjusted for age, sex, and body mass index [BMI]) and multivariate adjusted (model 2 adjusted for age, sex, BMI, New York Heart Association class, LAD, hypertension, oxygen desaturation index, and obstructive HCM). All reported probability values were 2‐tailed, and a P value <0.05 was considered statistically significant. SPSS version 24.0 (IBM Corp.) and GraphPad Prism version 7.0 (GraphPad Software Inc.) were used for calculations and illustrations, respectively.
Results
Population Characteristics
A total of 555 patients with HCM were included in the analysis (Figure 1), with 327 (58.9%) diagnosed with OSA. The median AHI value of the whole study population was markedly elevated (7.5 events per hour; interquartile range, 2.0–18.8 events per hour). Table 1 shows the baseline characteristics of the study population in the presence of OSA or across quartiles of AHI value. Patients with OSA or higher AHI quartiles were older, more likely to be male, had a higher BMI, were smokers, and had more comorbidities. The levels of blood pressure, fasting blood sugar, high‐sensitivity C‐reactive protein, and creatinine were increased with the presence of OSA or across AHI quartiles. The frequency of patients with obstructive HCM, the value of LVOTG at rest, and intraventricular septal thickness decreased in patients with OSA or higher AHI quartiles. Patients with OSA or higher AHI quartiles were associated with enlarged LAD, LV end‐diastolic diameter, and ascending aorta diameter. There were also significant differences in medications at discharge among the study patients. Compared with patients without OSA, the prevalence of AF was higher in patients with OSA (23.9% versus 13.6%, P=0.003) (Table 1 and Figure 2A). Most importantly, there was a dose‐response relationship between AHI quartiles and prevalence of AF (9.4% in quartile 1, 17.3% in quartile 2, 26.6% in quartile 3, and 25.2% in quartile 4; P for trend <0.001) (Figure 2B). Prevalence of persistent or permanent AF was also higher in patients with OSA or across AHI quartiles (Table 1). With multivariate analysis (Table S1), age (odds ratio [OR], 1.05; 95% CI, 1.03–1.07), BMI (OR, 1.25; 95% CI, 1.17–1.33), hypertension (OR, 2.01; 95% CI, 1.27–3.19), and hyperlipidemia (OR, 2.36; 95% CI, 1.43–3.90) were independently associated with OSA. Patients with HCM who had AF are characterized in Table S2. Patients with AF were older, had a higher prevalence of stroke, had a higher level of N‐terminal pro–brain natriuretic peptide, and had a lower prevalence of obstructive HCM compared with patients without AF. LAD was markedly elevated and use of anticoagulation therapy as well as class III antiarrhythmic drugs was more common in patients with AF.
Figure 1.
Study flow diagram.
HCM indicates hypertrophic cardiomyopathy; PSG, polysomnography.
Table 1.
Baseline Characteristics of Patients With HCM Grouped by the Presence of OSA or AHI Quartiles
| Variables | No OSA (n=228) | OSA (n=327) | P Value | Quartile 1 (n=138) AHI <2.0 events per h | Quartile 2 (n=139) AHI ≥2.0 to <7.5 events per h | Quartile 3 (n=139) AHI ≥7.5 to <18.8 events per h | Quartile 4 (n=139) AHI ≥18.8 events per h | P Value |
|---|---|---|---|---|---|---|---|---|
| Men | 146 (64.0) | 249 (76.1) | 0.002 | 81 (58.7) | 90 (64.7) | 104 (74.8) | 120 (86.3) | <0.001 |
| Age, y | 45.4±13.7 | 53.7±11.2 | <0.001 | 42.2±13.7 | 51.5±12.1 | 53.2±11.5 | 54.0±10.7 | <0.001 |
| BMI, kg/m2 | 24.6±3.2 | 27.2±3.7 | <0.001 | 23.9±3.4 | 25.7±2.9 | 26.6±3.3 | 28.0±4.0 | <0.001 |
| Cigarette use | 84 (36.8) | 170 (52.0) | <0.001 | 48 (34.8) | 55 (39.6) | 61 (43.9) | 90 (64.7) | <0.001 |
| Hypertension | 64 (28.1) | 197 (60.2) | <0.001 | 33 (23.9) | 54 (38.8) | 78 (56.1) | 96 (69.1) | <0.001 |
| Hyperlipidemia | 42 (18.4) | 153 (46.8) | <0.001 | 18 (13.0) | 42 (30.2) | 64 (46.0) | 71 (51.1) | <0.001 |
| Diabetes mellitus | 17 (7.5) | 48 (14.7) | 0.009 | 9 (6.5) | 12 (8.6) | 20 (14.4) | 24 (17.3) | 0.019 |
| Coronary heart disease | 19 (8.3) | 59 (18.0) | 0.001 | 8 (5.8) | 17 (12.2) | 16 (11.5) | 37 (26.6) | <0.001 |
| Peripheral vascular disease | 8 (3.5) | 20 (6.1) | 0.167 | 6 (4.3) | 5 (3.6) | 6 (4.3) | 11 (7.9) | 0.349 |
| Stroke | 7 (3.1) | 30 (9.2) | 0.005 | 4 (2.9) | 8 (5.8) | 10 (7.2) | 15 (10.8) | 0.066 |
| NYHA class II or III | 145 (63.6) | 228 (69.7) | 0.130 | 87 (63.0) | 94 (67.9) | 88 (63.3) | 104 (74.8) | 0.127 |
| Familial history of HCM | 32 (14.0) | 29 (8.9) | 0.056 | 23 (16.7) | 14 (10.1) | 12 (8.6) | 12 (8.6) | 0.100 |
| Familial history of SCD | 8 (3.5) | 13 (4.0) | 0.777 | 4 (2.9) | 4 (2.9) | 9 (6.5) | 4 (2.9) | 0.297 |
| Syncope | 25 (11.0) | 39 (11.9) | 0.727 | 17 (12.3) | 14 (10.1) | 18 (12.9) | 15 (10.8) | 0.868 |
| AF | 31 (13.6) | 78 (23.9) | 0.003 | 13 (9.4) | 24 (17.3) | 37 (26.6) | 35 (25.2) | 0.001 |
| Paroxysmal AF | 23 (10.1) | 47 (14.4) | 0.135 | 11 (8.0) | 16 (11.5) | 23 (16.5) | 20 (14.4) | 0.158 |
| Persistent or permanent AF | 8 (3.5) | 31 (9.5) | 0.007 | 2 (1.4) | 8 (5.8) | 14 (10.1) | 15 (10.8) | 0.008 |
| Ventricular tachycardia | 27 (11.8) | 52 (15.9) | 0.178 | 17 (12.3) | 14 (10.1) | 28 (20.1) | 20 (14.4) | 0.095 |
| Systolic pressure, mm Hg | 124.5±16.6 | 133.8±20.6 | <0.001 | 125.6±16.8 | 125.6±17.5 | 134.3±21.3 | 134.4±20.2 | <0.001 |
| Diastolic pressure, mm Hg | 74.0±11.4 | 80.3±13.1 | <0.001 | 75.0±11.7 | 74.5±12.1 | 79.9±12.4 | 81.4±13.5 | <0.001 |
| Fasting blood sugar, mmol/L | 4.8±1.2 | 5.3±1.5 | <0.001 | 4.7±1.1 | 5.1±1.3 | 5.2±1.3 | 5.6±1.8 | <0.001 |
| hs‐CRP, mg/L | 0.8 (0.4–1.7) | 1.5 (0.7–2.9) | <0.001 | 0.7 (0.3–1.5) | 1.0 (0.4–2.5) | 1.1 (0.6–2.1) | 1.6 (0.9–3.5) | <0.001 |
| LDL‐C, mmol/L | 2.7±0.8 | 2.7±0.8 | 0.788 | 2.7±0.8 | 2.8±0.8 | 2.8±0.8 | 2.6±0.8 | 0.078 |
| Creatinine, mmol/L | 82.4±15.8 | 87.3±19.2 | 0.002 | 82.4±15.1 | 83.1±17.0 | 86.7±18.2 | 89.9±20.7 | 0.014 |
| NT‐proBNP, pg/mL | 975.5 (477.4–2103.0) | 627.1 (223.4–1565.5) | <0.001 | 1096 (572.5–2195.0) | 821.9 (285.0–1750.0) | 616.0 (248.8–1471.0) | 587.0 (159.0–1478.3) | <0.001 |
| Echocardiographic data | ||||||||
| Obstructive HCM | 143 (62.7) | 156 (47.7) | <0.001 | 90 (65.2) | 85 (61.2) | 69 (49.6) | 55 (39.6) | <0.001 |
| LVOTG at rest, mm Hg | 41.0 (0.0–73.5) | 18.0 (0.0–61.0) | <0.001 | 48.0 (0.0–72.5) | 41.0 (0.0–76.0) | 16.0 (0.0–82.0) | 0.0 (0.0–50.0) | <0.001 |
| LAD, mm | 41.9±6.5 | 43.1±7.2 | 0.103 | 41.1±6.1 | 42.9±6.6 | 43.6±7.8 | 42.9±7.0 | 0.049 |
| LVEDD, mm | 43.8±6.3 | 46.6±6.0 | <0.001 | 42.8±6.4 | 44.7±5.8 | 46.3±5.8 | 47.8±6.1 | <0.001 |
| IVST, mm | 18.7±5.5 | 17.3±4.9 | 0.002 | 18.9±4.5 | 18.4±6.1 | 17.4±5.3 | 16.8±4.7 | 0.001 |
| LVEF, % | 66.5±8.8 | 65.4±9.0 | 0.051 | 67.0±8.8 | 66.1±7.9 | 66.1±8.4 | 64.3±10.3 | 0.086 |
| AAD, mm | 30.4±5.0 | 33.0±4.3 | <0.001 | 29.4±5.0 | 32.0±4.2 | 32.7±4.8 | 33.4±4.2 | <0.001 |
| Moderate or severe MR | 88 (38.6) | 89 (27.2) | 0.005 | 54 (39.1) | 56 (40.3) | 34 (24.5) | 33 (23.7) | 0.001 |
| Medical therapy | ||||||||
| β‐Blockers | 204 (89.5) | 275 (84.1) | 0.070 | 128 (92.8) | 123 (88.5) | 118 (84.9) | 110 (79.1) | 0.009 |
| CCBs | 38 (16.7) | 112 (34.3) | <0.001 | 16 (11.6) | 31 (22.3) | 40 (28.8) | 63 (45.3) | <0.001 |
| ACEIs/ARBs | 47 (20.6) | 120 (36.7) | <0.001 | 21 (15.2) | 36 (25.9) | 54 (38.8) | 56 (40.3) | <0.001 |
| Spirolactone | 27 (11.8) | 36 (11.0) | 0.761 | 13 (9.4) | 15 (10.8) | 17 (12.2) | 18 (12.9) | 0.798 |
| Class III antiarrhythmic drugs | 30 (13.2) | 42 (12.8) | 0.914 | 15 (10.9) | 21 (15.1) | 22 (15.8) | 14 (10.1) | 0.370 |
| Anticoagulation therapy | 30 (13.2) | 53 (16.2) | 0.322 | 12 (8.7) | 23 (16.5) | 22 (15.8) | 26 (18.7) | 0.105 |
Values are presented as mean±SD, median (interquartile range), or number (percentage). AAD indicates ascending aorta diameter; ACEIs, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; AHI, apnea‐hypopnea index; ARBs, angiotensin II receptor blockers; BMI, body mass index; CCBs, calcium channel blockers; HCM, hypertrophic cardiomyopathy; hs‐CRP, high‐sensitivity C‐reactive protein; IVST, interventricular septum thickness; LAD, left atrial diameter; LDL‐C, low‐density lipoprotein cholesterol; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVOTG, left ventricular outflow tract gradient; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; NYHA, New York Heart Association; OSA, obstructive sleep apnea; and SCD, sudden cardiac death.
Figure 2.
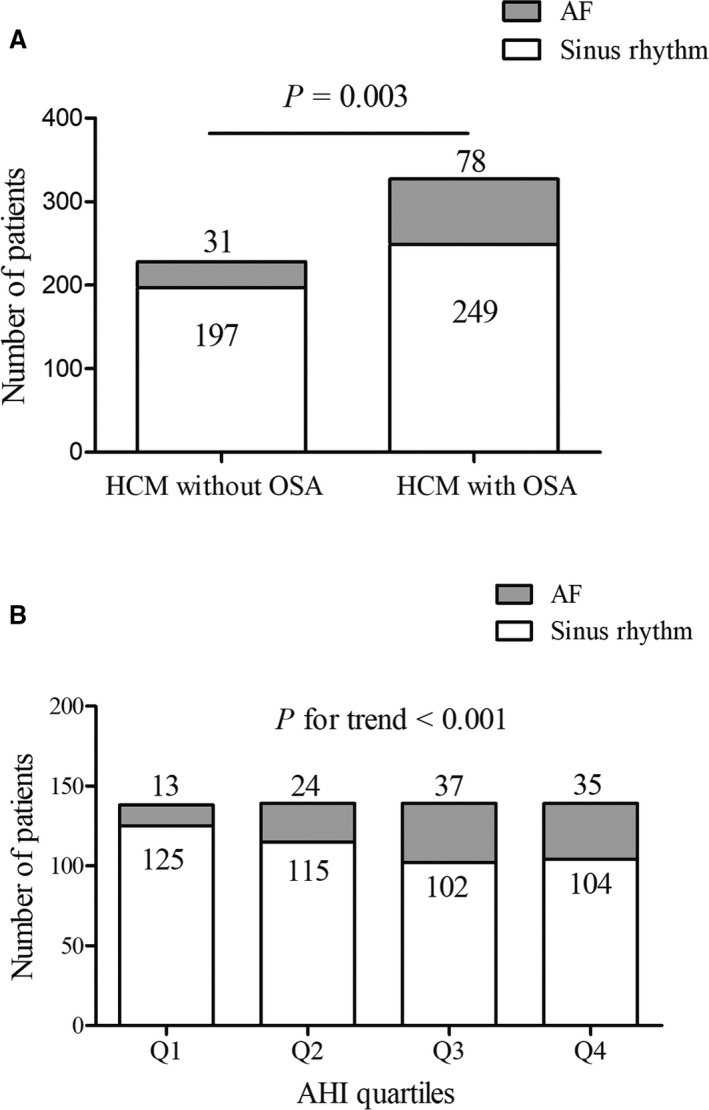
Prevalence of atrial fibrillation (AF) in patients with hypertrophic cardiomyopathy (HCM) grouped by presence of obstructive sleep apnea (OSA) (A) or apnea‐hypopnea index (AHI) severity stratified by AHI quartiles (B).
Sleep Parameters
Sleep study parameters are summarized in Table 2. The value of AHI, oxygen desaturation index, snoring time ratio, longest apnea/hypopnea time, time spent with oxygen saturation <90%, mean oxygen decline degree, and heart rate during sleep were all significantly higher in patients with OSA than those without OSA. These parameters were also significantly increased across AHI quartiles. Lowest oxygen saturation and mean oxygen saturation reflecting nocturnal oxygen level were decreased in patients with OSA or higher AHI quartiles.
Table 2.
Polysomnographic Parameters of Patients With HCM Grouped by the Presence of OSA or AHI Quartiles
| Variables | No OSA (n=228) | OSA (n=327) | P Value | Quartile 1 (n=138) AHI <2.0 events per h | Quartile 2 (n=139) AHI ≥2.0 to <7.5 events per h | Quartile 3 (n=139) AHI ≥7.5 to <18.8 events per h | Quartile 4 (n=139) AHI ≥18.8 events per h | P Value |
|---|---|---|---|---|---|---|---|---|
| AHI, events per h | 1.5 (0.6–2.9) | 16.6 (9.0–31.1) | <0.001 | 0.8 (0.3–1.4) | 4.3 (2.9–5.4) | 12.4 (9.2–15.5) | 33.7 (25.2–44.3) | <0.001 |
| OAI, events per h | 0.4 (0.1–1.0) | 5.0 (1.9–13.2) | <0.001 | 0.2 (0.0–0.5) | 1.0 (0.4–1.9) | 3.2 (1.1–6.0) | 16.2 (7.5–25.4) | <0.001 |
| CAI, events per h | 0.0 (0.0–0.0) | 0.0 (0.0–1.2) | <0.001 | 0.0 (0.0–0.0) | 0.0 (0.0–0.1) | 0.0 (0.0–0.8) | 0.4 (0.0–3.4) | <0.001 |
| Hypopnea index, events per h | 0.7 (0.2–1.8) | 8.0 (4.7–13.4) | <0.001 | 0.3 (0.1–0.7) | 2.5 (1.5–3.9) | 7.7 (5.1–10.2) | 12.3 (7.2–19.5) | <0.001 |
| ODI, events per h | 2.3 (1.0–4.3) | 16.4 (9.1–30.4) | <0.001 | 0.5 (1.5–2.3) | 4.9 (3.2–6.7) | 11.8 (9.1–15.9) | 33.0 (23.1–42.5) | <0.001 |
| Snoring time ratio, % | 3.0 (0.4–5.9) | 8.2 (2.3–15.4) | <0.001 | 2.0 (0.3–5.6) | 4.1 (0.4–9.7) | 7.3 (2.3–14.1) | 12.4 (3.8–22.0) | <0.001 |
| Longest apnea/hypopnea time, s | 36.7 (24.5–53.7) | 70.0 (53.5–91.7) | <0.001 | 27.2 (19.3–40.1) | 50 (40.0–73.5) | 65.3 (51.0–82.3) | 78.2 (58.0–95.0) | <0.001 |
| Lowest SaO2, % | 89.0 (86.0–90.0) | 82.0 (76.0–86.0) | <0.001 | 89.0 (87.0–91.0) | 87.0 (84.0–89.0) | 84 (80.0–86.0) | 78.0 (72.0–82.0) | <0.001 |
| Mean SaO2, % | 94.5 (94.0–95.5) | 93.2 (92.2–94.4) | <0.001 | 95.0 (94.1–95.7) | 94.0 (93.1–95.0) | 93.6 (92.8–94.6) | 93.0 (91.6–94.0) | <0.001 |
| Time spent with SaO2 <90%, % | 0.1 (0.0–1.5) | 5.3 (1.1–13.5) | <0.001 | 0.0 (0.0–1.0) | 0.5 (0.0–2.0) | 3.0 (0.9–8.3) | 12.1 (4.4–21.7) | <0.001 |
| Mean oxygen decline degree, % | 4.2 (3.8–4.7) | 5.5 (4.8–6.6) | <0.001 | 4.2 (3.8–4.7) | 4.4 (4.0–4.9) | 5.0 (4.8–5.7) | 6.5 (5.6–7.5) | <0.001 |
| HR during sleep | 60.1±7.2 | 61.3±7.5 | 0.001 | 58.9±6.5 | 61.2±7.3 | 60.7±7.0 | 62.3±8.5 | <0.001 |
| Supine time, min | 208.0 (168.8–269.3) | 208.0 (136.0–239.0) | 0.005 | 234.5 (181.0–312.3) | 208.0 (153.0–239.0) | 208.0 (137.0–254.0) | 206.0 (120.0–213.0) | <0.001 |
| Total recording time, min | 494.0 (420.0–540.0) | 451.0 (409.0–501.0) | <0.001 | 500.0 (439.3–551.5) | 473.0 (420.0–525.0) | 455.0 (414.0–512.0) | 434.0 (404.9–479.0) | <0.001 |
Values are presented as mean±SD, median (interquartile range), or number (percentage). AHI indicates apnea‐hypopnea index; CAI, central apnea index; HCM, hypertrophic cardiomyopathy; HR: heart rate; OAI, obstructive apnea index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; and SaO2, oxygen saturation.
Association Between AHI and AF
The AHI value was significantly higher in patients with AF compared with those with sinus rhythm (median, 13.0 [interquartile range, 3.8–23.2 events per hour] versus median, 6.4 [interquartile range, 1.7–18.0 events per hour]; P=0.002) (Figure 3). In addition, the AHI value was highest in patients with persistent or permanent AF compared with patients with paroxysmal AF or patients without AF (P=0.005) (Figure S1). In univariate analyses, significant associations were observed between prevalence of AF and the diagnosis of OSA or OSA severity measures (Table 3). Patients with OSA were associated with an OR of 1.99 (95% CI, 1.26–3.14) compared with patients with no OSA. Highest AHI quartile was associated with an OR of 3.24 (95% CI, 1.63–6.44) compared with the lowest AHI quartiles, whereas moderate to severe OSA was associated with an OR of 2.42 (95% CI, 1.46–4.00) compared with no OSA. After controlling for age, sex, and BMI (model 1), the association between diagnosis of OSA and AF was no longer statistically significant. In contrast, the adjusted relationship between AF and higher AHI quartiles (OR, 2.54; 95% CI, 1.16–5.57) or moderate to severe OSA (OR, 1.99; 95% CI, 1.13–3.51) remained significant. After additionally controlling for New York Heart Association class, LAD, hypertension, oxygen desaturation index, and obstructive HCM (model 2), the association was still significant (highest AHI quartile: OR, 4.43 [95% CI, 1.35–14.52] or moderate to severe OSA: OR, 3.03 [95% CI, 1.28–7.20]). The fully adjusted analysis also showed evidence of increasing risk of persistent or permanent AF with increasing AHI quartiles (highest AHI quartile: OR, 10.96; 95% CI, 1.07–111.85).
Figure 3.
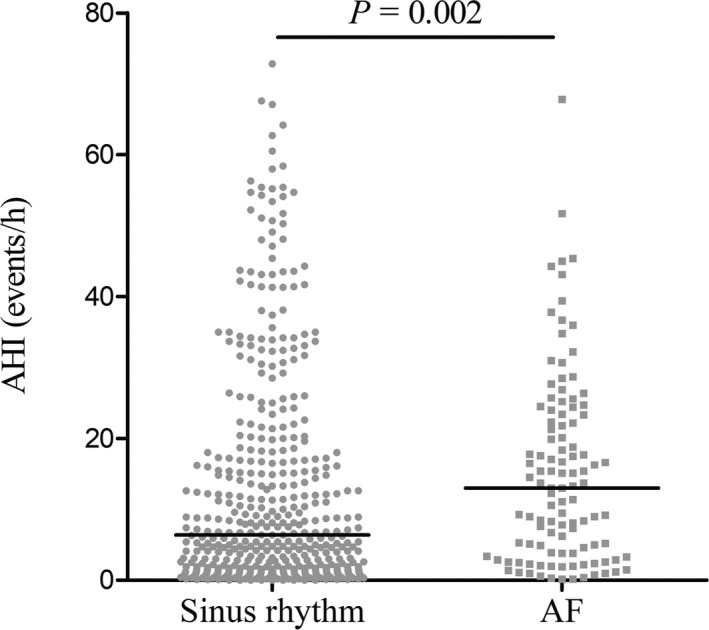
The value of apnea‐hypopnea index (AHI) number in patients with hypertrophic cardiomyopathy (HCM) according to the presence of atrial fibrillation (AF).
Table 3.
Association Between AF and Presence of OSA or AHI Quartiles or Standard OSA Severity Classification in Patients With HCM
| Item | Univariate | P Value | Model 1 | P Value | Model 2 | P Value |
|---|---|---|---|---|---|---|
| AF | ||||||
| Presence of OSA | ||||||
| No OSA | Reference | Reference | Reference | |||
| OSA | 1.99 (1.26–3.14) | 0.003 | 1.52 (0.92–2.52) | 0.106 | 1.58 (0.84–2.97) | 0.153 |
| AHI quartiles | 0.001 | 0.038 | 0.045 | |||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | 2.01 (0.98–4.13) | 0.058 | 1.50 (0.71–3.21) | 0.292 | 1.58 (0.70–3.55) | 0.271 |
| Quartile 3 | 3.49 (1.76–6.91) | <0.001 | 2.62 (1.24–5.52) | 0.011 | 3.01 (1.28–7.06) | 0.011 |
| Quartile 4 | 3.24 (1.63–6.44) | 0.001 | 2.54 (1.16–5.57) | 0.020 | 4.43 (1.35–14.52) | 0.014 |
| Standard OSA severity classification | 0.003 | 0.044 | 0.041 | |||
| No OSA | Reference | Reference | Reference | |||
| Mild OSA | 1.65 (0.94–2.89) | 0.081 | 1.25 (0.69–2.26) | 0.470 | 1.44 (0.74–2.82) | 0.282 |
| Moderate to severe OSA | 2.42 (1.46–4.00) | 0.001 | 1.99 (1.13–3.51) | 0.017 | 3.03 (1.28–7.20) | 0.012 |
| Persistent/permanent AF | ||||||
| Presence of OSA | ||||||
| No OSA | Reference | Reference | Reference | |||
| OSA | 2.88 (1.30–6.39) | 0.009 | 2.30 (0.98–5.44) | 0.057 | 1.92 (0.57–6.46) | 0.289 |
| AHI quartiles | 0.025 | 0.056 | 0.190 | |||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | 4.15 (0.87–19.92) | 0.075 | 3.40 (0.68–16.97) | 0.136 | 4.23 (0.70–25.62) | 0.117 |
| Quartile 3 | 7.62 (1.70–34.18) | 0.008 | 6.40 (1.32–30.90) | 0.021 | 7.59 (1.16–49.83) | 0.035 |
| Quartile 4 | 8.23 (1.84–36.69) | 0.006 | 7.51 (1.50–37.56) | 0.014 | 10.96 (1.07–111.85) | 0.043 |
| Standard OSA severity classification | 0.013 | 0.047 | 0.304 | |||
| No OSA | Reference | Reference | Reference | |||
| Mild OSA | 2.03 (0.78–5.28) | 0.145 | 1.60 (0.59–4.33) | 0.356 | 1.75 (0.48–6.36) | 0.394 |
| Moderate to severe OSA | 3.49 (1.51–8.08) | 0.004 | 3.02 (1.20–7.60) | 0.019 | 3.34 (0.72–15.46) | 0.123 |
Data are presented as odds ratios (95% CIs). Model 1: adjusted for age, sex, and body mass index (BMI). Model 2: adjusted for age, sex, and BMI and further adjusted for New York Heart Association class, left atrial diameter, hypertension, oxygen desaturation index, and obstructive hypertrophic cardiomyopathy (HCM). AF indicates atrial fibrillation; AHI, apnea‐hypopnea index; and OSA, obstructive sleep apnea.
Discussion
The present study evaluated the influence of OSA and severity of AHI value on prevalence of AF in a large series of patients with HCM. Our study showed that 58.9% of patients with HCM had OSA and the prevalence of AF in this population was 19.6%. AF was more common in patients with OSA and there was a dose‐response relationship between AHI quartiles and rate of AF. In multivariate analysis, higher AHI quartiles or OSA severity stratified by widely used clinical cut points rather than the presence of OSA were independent factors for AF. In addition, higher AHI quartiles were also independently associated with persistent or permanent AF.
The relationship between sleep‐disordered breathing and HCM was reported more than 10 years ago.10, 18 In 2010, Pedrosa and his colleagues9 first illustrated a high prevalence of OSA in patients with HCM. To produce good generalizability, we performed the study in a large HCM population and the number of patients in our study was almost 7‐fold larger than that in Pedrosa et al's. The prevalence of OSA in our study was 58.9%, which was in accordance with previously reported results indicating that OSA was a much more common clinical condition in patients with HCM.7, 10, 19
Previous studies have implicated the association between OSA and AF in community‐based populations.20, 21 Unlike in the general population, patients with HCM were more susceptible to AF, and the presence of AF is associated with increased morbidity and mortality.22 According to our study, 19.6% of patients with HCM had AF, which was consistent with the high rate of AF in patients with HCM as previously reported.1, 23 Atrial arrhythmogenesis in HCM is commonly thought to be a process secondary to elevated filling pressures from LV diastolic failure as well as the presence of an LVOTG and associated mitral regurgitation. Moreover, accumulating evidence suggests the presence of an underlying intrinsic atrial myopathy in patients with HCM.2, 24, 25, 26 Left atrial dysfunction, enlargement, and fibrosis could occur in these patients independent of LV remodeling. In our study, prevalence of AF increased with apnea severity, suggesting that non–HCM‐related comorbidities may play a role in AF development. Additionally, our results also showed that patients with AF had higher AHI values compared with patients with sinus rhythm, indicating a close relationship between sleep disorder and AF. OSA could predispose to AF through several mechanisms: hypoxemia, nocturnal hypertension, oxidative stress, intrathoracic pressure fluctuations, sympathetic activation, and chronic inflammation.27, 28 OSA is also associated with electrophysiologic changes in atrium such as reduction in voltage, conduction slowing, and longer sinus node recovery.27 We propose that all of these factors together with the underlying atrial myopathy predispose patients to electrical and structural atrial remodeling over time and result in elevated risk of AF on HCM. Interestingly, prevalence of ventricular tachycardia was not significantly different between patients with or without OSA or across AHI quartiles in our study. We speculate that atrium might be more susceptible to OSA‐related arrhythmias than ventricle in HCM.
Clinical parameters such as female sex, age, LAD, New York Heart Association class, and hypertension had been demonstrated to be predictors of incident AF in a cohort of 4248 patients with HCM.16 In the present study, all of these parameters together with other factors were included in the multivariate adjusted regression model and the association between AHI quartiles or moderate to severe OSA and AF remained significant. Furthermore, we also found an association of increasing AHI quartiles with persistent or permanent AF. As persistent or permanent AF had a worse prognosis compared with paroxysmal AF,29, 30 our study first showed the relationship between sleep disorder and more severe AF types in patients with HCM. Previous studies have shown that patients with persistent or permanent AF were associated with severity of sleep‐disordered breathing and that treatment of OSA using continuous positive airway pressure therapy could prevent AF progression from a paroxysmal pattern to more permanent forms.31, 32 The AHI value in our study was also higher in patients with persistent or permanent AF compared with those with paroxysmal AF, which was consistent with previous studies. These results indicate that serious sleep disorders are associated with more severe AF patterns in patients with HCM.
However, no significant association was obtained when comparing patients with versus those without OSA, which was different from the study by Pedrosa and colleagues. We speculated that the discrepancy between our study and Pedrosa et al's arose from the differences in cutoff value of AHI for the diagnosis of OSA. In the present study, we used AHI ≥5 events per hour as the value for diagnosis of OSA, while in the study by Pedrosa and colleagues, the value was 15 events per hour, which equaled the category of moderate to severe OSA in our study. In addition, patients with diagnosed OSA using the threshold of AHI ≥5 events per hour usually had a wide range of AHI distribution. Even patients who were not at high risk for AF were included. For example, as shown in our study, there was no significant association between mild OSA and AF. Taken together, the present study was in broad agreement with Pedrosa and colleagues, providing evidence that severity rather than diagnosis of OSA was independently associated with AF in patients with HCM. In line with these previous studies, we found an enlarged LAD, LV end‐diastolic diameter, and ascending aorta diameter in patients with HCM who had OSA or higher AHI quartiles compared with patients without OSA or lower AHI quartiles. In contrast to what would be expected from previous studies,33, 34 our findings showed no increase in LVOTG at rest and interventricular septal thickness in patients with OSA. We presume that a higher level of blood pressure together with increased systemic resistance suppress LVOTG in patients with OSA or higher AHI quartiles. In addition, genetic determinants of LV hypertrophic site that characterize HCM might overcome the influence of OSA on LV remodeling.
Apart from AHI value, several other sleep parameters closely related to oxygen saturation were also shown to be associated with AF. Kendzerska et al4 and Cadby et al6 used time with oxygen saturation <90% to measure OSA severity and found it to be an independent predictor of incident AF.4, 6 In a study by Gami et al,5 the decrease in nocturnal oxygen saturation was used. However, patients with OSA not only experience oxygen desaturation but also harmful hemodynamic changes during sleep. Based on the reason that AHI is used to diagnose and assess the severity of OSA, we categorized patients into AHI quartiles. We found that all parameters related to nocturnal hypoxia were more severe across AHI quartiles, indicating that our measurement was effective to stratify severity of oxygen desaturation. In addition, using AHI quartiles to describe the degree of sleep disorder overcame the limitation of using an arbitrary cutoff value. Therefore, AHI might be a potential sleep parameter for risk stratification of AF development in patients with HCM.
At present, most cases of OSA remain undiagnosed and the rate of OSA further increases with age and obesity.3, 35 As prevalence of OSA is high and associated with increased risk of AF in patients with HCM, we expect that a high degree of suspicion for OSA is warranted and clinicians should have a low threshold to refer for diagnostic sleep evaluation. Importantly, a recent study found that treatment of OSA using continuous positive airway pressure was safe in patients with HCM.36 Therefore, clinical trials are required to determine whether treatment of OSA has the potential to prevent future AF development and reduce the incidence of cardiovascular death especially in patients with HCM who have higher AHI levels.
Limitations
The present study has several limitations. First, all of the patients in our study received only 1 night of sleep study. In fact, parameters of OSA showed a considerable night‐to‐night variability, and single‐night diagnostic sleep study is prone to miscategorize the severity of OSA.37 Night‐to‐night variability may also lead to misdiagnosis, especially in patients with mild OSA, as there are often false‐negative sleep studies in such patients.38 In that case, repeated measurements will help to improve patient categorization and make treatment decisions. Second, the generalizability of our findings was limited by its single‐center design, and the study population may not reflect that of the general HCM population because of selection bias, as they presented to a tertiary medical center for their care and many were symptomatic. Third, the diagnosis of AF was made before the time of sleep evaluation, limiting the ability to draw a conclusion of causation between the degree of sleep disorder and AF. Future work is needed to investigate the causative effects of OSA on new‐onset AF. Fourth, our study grouped patients into AHI quartiles and did not produce a certain cutoff value, which might hamper its comparability and clinical applicability. Finally, because of the cross‐sectional and retrospective nature of the present study, there were limitations related to availability of data on important confounders such as daily alcohol consumption and diagnosis of primary pulmonary disease. It was not possible to ensure that all confounding variables were fully adjusted in multivariate analysis.
Conclusions
OSA is highly prevalent in patients with HCM, and higher AHI levels rather than the diagnosis of OSA are independently associated with AF, a risk factor for cardiovascular death in this population. These results suggest that more attention should be paid and treatment decisions should be made in patients with HCM who have more severe OSA. Further studies are needed to investigate the impact of OSA on future cardiovascular complications as well as the safety and efficacy of OSA treatment in these patients.
Sources of Funding
This study was supported by a grant from the National Natural Science Foundation of China (81370327).
Disclosures
None.
Supporting information
Tables S1 and S2 Figure S1
(J Am Heart Assoc. 2020;9:e015013 DOI: 10.1161/JAHA.119.015013.)
See Editorial by Rowin and Sridharan
For Sources of Funding and Disclosures, see page 11.
References
- 1. Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W, Rastegar H, Estes NA, Maron MS, Maron BJ. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. 2017;136:2420–2436. [DOI] [PubMed] [Google Scholar]
- 2. Philipson DJ, Rader F, Siegel RJ. Risk factors for atrial fibrillation in hypertrophic cardiomyopathy. Eur J Prev Cardiol. 2019;2047487319828474. [E‐pub ahead of publication]. [DOI] [PubMed] [Google Scholar]
- 3. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin JL, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature‐based analysis. Lancet Respir Med. 2019;7:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kendzerska T, Gershon AS, Atzema C, Dorian P, Mangat I, Hawker G, Leung RS. Sleep apnea increases the risk of new hospitalized atrial fibrillation: a historical cohort study. Chest. 2018;154:1330–1339. [DOI] [PubMed] [Google Scholar]
- 5. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 6. Cadby G, McArdle N, Briffa T, Hillman DR, Simpson L, Knuiman M, Hung J. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep‐clinic cohort. Chest. 2015;148:945–952. [DOI] [PubMed] [Google Scholar]
- 7. Konecny T, Somers VK. Sleep‐disordered breathing in hypertrophic cardiomyopathy: challenges and opportunities. Chest. 2014;146:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nerbass FB, Pedrosa RP, Danzi‐Soares NJ, Drager LF, Arteaga‐Fernandez E, Lorenzi‐Filho G. Obstructive sleep apnea and hypertrophic cardiomyopathy: a common and potential harmful combination. Sleep Med Rev. 2013;17:201–206. [DOI] [PubMed] [Google Scholar]
- 9. Pedrosa RP, Drager LF, Genta PR, Amaro AC, Antunes MO, Matsumoto AY, Arteaga E, Mady C, Lorenzi‐Filho G. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010;137:1078–1084. [DOI] [PubMed] [Google Scholar]
- 10. Konecny T, Brady PA, Orban M, Lin G, Pressman GS, Lehar F, Tomas K, Gersh BJ, Tajik AJ, Ommen SR, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1597–1602. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 12. Santos‐Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, Bittencourt LR. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sleep‐related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 14. Marwick TH, Nakatani S, Haluska B, Thomas JD, Lever HM. Provocation of latent left ventricular outflow tract gradients with amyl nitrite and exercise in hypertrophic cardiomyopathy. Am J Cardiol. 1995;75:805–809. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 16. Guttmann OP, Pavlou M, O'Mahony C, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, Garcia‐Pavia P, et al. Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart. 2017;103:672–678. [DOI] [PubMed] [Google Scholar]
- 17. Guttmann OP, Rahman MS, O'Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. 2014;100:465–472. [DOI] [PubMed] [Google Scholar]
- 18. Banno K, Shiomi T, Sasanabe R, Otake K, Hasegawa R, Maekawa M, Ito T. Sleep‐disordered breathing in patients with idiopathic cardiomyopathy. Circ J. 2004;68:338–342. [DOI] [PubMed] [Google Scholar]
- 19. Eleid MF, Konecny T, Orban M, Sengupta PP, Somers VK, Parish JM, Mookadam F, Brady PA, Sullivan BL, Khandheria BK, et al. High prevalence of abnormal nocturnal oximetry in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:1805–1809. [DOI] [PubMed] [Google Scholar]
- 20. Tung P, Levitzky YS, Wang R, Weng J, Quan SF, Gottlieb DJ, Rueschman M, Punjabi NM, Mehra R, Bertisch S, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc. 2017;6:e004500 DOI: 10.1161/JAHA.116.004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. [DOI] [PubMed] [Google Scholar]
- 22. Garg L, Gupta M, Sabzwari SRA, Agrawal S, Agarwal M, Nazir T, Gordon J, Bozorgnia B, Martinez MW. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical impact, and management. Heart Fail Rev. 2019;24:189–197. [DOI] [PubMed] [Google Scholar]
- 23. Vaidya K, Semsarian C, Chan KH. Atrial fibrillation in hypertrophic cardiomyopathy. Heart Lung Circ. 2017;26:975–982. [DOI] [PubMed] [Google Scholar]
- 24. Kallergis EM, Goudis CA, Vardas PE. Atrial fibrillation: a progressive atrial myopathy or a distinct disease? Int J Cardiol. 2014;171:126–133. [DOI] [PubMed] [Google Scholar]
- 25. Sivalokanathan S, Zghaib T, Greenland GV, Vasquez N, Kudchadkar SM, Kontari E, Lu DY, Dolores‐Cerna K, van der Geest RJ, Kamel IR, et al. Hypertrophic cardiomyopathy patients with paroxysmal atrial fibrillation have a high burden of left atrial fibrosis by cardiac magnetic resonance imaging. JACC Clin Electrophysiol. 2019;5:364–375. [DOI] [PubMed] [Google Scholar]
- 26. Farhad H, Seidelmann SB, Vigneault D, Abbasi SA, Yang E, Day SM, Colan SD, Russell MW, Towbin J, Sherrid MV, et al. Left atrial structure and function in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. J Cardiovasc Magn Reson. 2017;19:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, Antic N, Thornton A, Saint DA, McEvoy D, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–327. [DOI] [PubMed] [Google Scholar]
- 28. Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Levy P, Kalman JM, Sanders P. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3:532–540. [DOI] [PubMed] [Google Scholar]
- 29. Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian P, Green MS, Humphries KH, Klein GJ, Sheldon R, et al. Progression of paroxysmal to persistent atrial fibrillation: 10‐year follow‐up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm. 2017;14:801–807. [DOI] [PubMed] [Google Scholar]
- 30. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Dan GA, Kalarus Z, Tavazzi L, Maggioni AP, et al. ‘Real‐world’ management and outcomes of patients with paroxysmal vs. non‐paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme‐Atrial Fibrillation (EORP‐AF) General Pilot Registry. Europace. 2016;18:648–657. [DOI] [PubMed] [Google Scholar]
- 31. Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation‐results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Am Heart J. 2015;169:647–654.e642. [DOI] [PubMed] [Google Scholar]
- 32. Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–1669. [DOI] [PubMed] [Google Scholar]
- 33. Sengupta PP, Sorajja D, Eleid MF, Somers VK, Ommen SR, Parish JM, Khandheria B, Tajik AJ. Hypertrophic obstructive cardiomyopathy and sleep‐disordered breathing: an unfavorable combination. Nat Clin Pract Cardiovasc Med. 2009;6:14–15. [DOI] [PubMed] [Google Scholar]
- 34. Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi‐Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–1386. [DOI] [PubMed] [Google Scholar]
- 35. Bostanci A, Bozkurt S, Turhan M. Impact of age on intermittent hypoxia in obstructive sleep apnea: a propensity‐matched analysis. Sleep Breath. 2018;22:317–322. [DOI] [PubMed] [Google Scholar]
- 36. Nerbass FB, Salemi VMC, Pedrosa RP, Portilho NP, Ferreira‐Filho JCA, Moriya HT, Antunes MO, Arteaga‐Fernandez E, Drager LF, Lorenzi‐Filho G. Acute effects of nasal CPAP in patients with hypertrophic cardiomyopathy. Chest. 2016;150:1050–1058. [DOI] [PubMed] [Google Scholar]
- 37. Stoberl AS, Schwarz EI, Haile SR, Turnbull CD, Rossi VA, Stradling JR, Kohler M. Night‐to‐night variability of obstructive sleep apnea. J Sleep Res. 2017;26:782–788. [DOI] [PubMed] [Google Scholar]
- 38. Dean RJ, Chaudhary BA. Negative polysomnogram in patients with obstructive sleep apnea syndrome. Chest. 1992;101:105–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2 Figure S1


