Hypertrophic cardiomyopathy (HCM) is the most common genetic heart disease occurring in up to 1 in 200 individuals, with a diverse phenotypic expression and clinical course.1, 2 Overall, HCM patients are at risk for a number of adverse disease‐related events; however, contemporary treatments allow for favorable outcomes and extended survival utilizing surgical myectomy and selectively alcohol septal ablation for reversal of heart failure (HF) symptoms secondary to left ventricular outflow obstruction,1, 2, 3 sudden death prevention utilizing a mature risk stratification algorithm and primary prevention implantable cardioverter defibrillators,1, 4, 5 and improved survival and quality of life in nonobstructive end‐stage disease with HF treatments including transplant.6 In this regard, while 40% to 50% of HCM patients at dedicated HCM centers have 1 or more disease‐related event, HCM‐related mortality is only 0.5%/year, and 90% to 95% of patients have no or mild (New York Heart Association class I or II) symptoms after treatment interventions.1, 2, 3
See Article by Xu et al.
Atrial fibrillation (AF) is the most common sustained arrhythmia in HCM patients, with symptomatic episodes occurring in 20% to 25% of patients and an annual incidence of 2% to 4%, with AF significantly more common in HCM than in the general population.7, 8 While in previous treatment eras AF was associated with substantial stroke risk and HCM‐related mortality, advances in treatment for HCM patients with AF allow for a low and a 4‐fold decrease in mortality rate (from 3%/year to <1%/year).7 However, there remain several unmet treatment needs related to AF in HCM.
Stroke is the most important potential sequel of AF, with initiation of anticoagulation in HCM allowing for substantial reduction of embolic stroke risk.1, 7 Notably, traditional embolic risk scoring tools in AF, such as CHA2DS2‐VASc score, are insensitive for identification of HCM patients with embolic events and therefore initiation of anticoagulation is recommended in all HCM patients after the initial AF episode.2, 7, 8 Nevertheless, an important subgroup of patients (2% of all HCM patients who develop AF and 25% of HCM patients with embolic events) have embolic events during the initial AF episode,7 making the identification of patients at high risk for AF and prevention of AF an important unmet need in HCM.
In addition, repetitive and unpredictable episodes of AF can substantially impair quality of life in HCM patients with worsening symptoms in AF related to loss of atrial contribution to ventricular filling, particularly symptomatic in the presence of left ventricular hypertrophy, diastolic dysfunction, and/or left ventricular outflow obstruction.1, 2, 7 In this regard, most HCM patients with AF require treatment to reduce AF frequency, including antiarrhythmic drugs (amiodarone, sotalol, disopyramide, or dofetilide), catheter ablation, or the Maze procedure performed at the time of surgical myectomy (Figure).1, 2, 7, 8, 9, 10 While these treatment interventions can substantially reduce symptomatic AF episodes in most patients, medications may be limited by side effects, efficacy of catheter ablation for AF is reduced in HCM,7, 9 and the more efficacious Maze procedure is utilized only for patients with left ventricular outflow tract obstruction who are candidates for surgical myectomy.10 Therefore in a small but important subset of HCM patients with AF (5–10%), treatment interventions are ineffective or not well tolerated.7, 8
Figure 1.
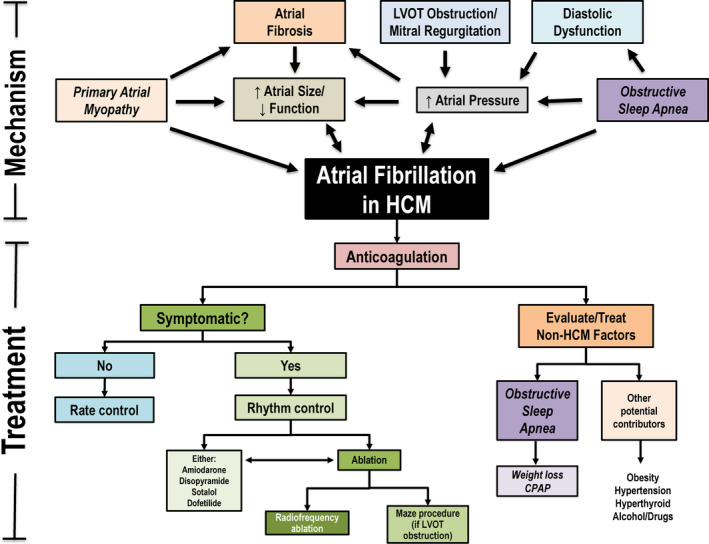
Mechanism and treatment of atrial fibrillation in hypertrophic cardiomyopathy.A complex interplay of HCM‐related features contributes to development of AF including a postulated primary atrial myopathy. Once AF develops, initiation of anticoagulation is recommended for all HCM patients, regardless of CHA2DS2‐VASc score, and patients should be evaluated for potential non‐HCM contributors to AF including obstructive sleep apnea. A decision for rate vs rhythm control should be made based on degree of symptoms in AF. AF indicates atrial fibrillation; CPAP, continuous positive airway pressure; HCM, hypertrophic cardiomyopathy; and LVOT, left ventricular outflow tract.
In the past, the mechanism of AF has predominantly been thought to be related to an interplay of HCM‐related abnormalities with little impact of noncardiac conditions on the development or recurrence of AF. Specifically, the hypertrophied left ventricle with poor compliance causes increase in afterload of the left atrium (LA), which results in progressive LA dilation.1, 2, 8, 11, 12, 13 In obstructive HCM, systolic anterior motion of the mitral valve with secondary mitral regurgitation further contribute to elevated LA pressure, stretch, and remodeling.1 Such increases in LA pressure and LA dilation in turn lead to shortening of the effective atrial refractory period and increase in the dispersion of depolarization, providing the ideal arrhythmogenic substrate primed for the development and maintenance of AF.6, 14 There is also evidence pointing towards a primary atrial myopathy in HCM, supported by a higher burden of atrial fibrosis as compared with other forms of heart disease,12, 13 which likely impairs propagation of sinus impulses through the atrium and serves as a substrate for slow conduction and intra‐atrial reentry. In addition, there is evidence for decreased LA function, and high frequency of recurrent AF after successful pulmonary vein isolation, implicating foci beyond just the pulmonary veins in HCM patients.9 Further supporting the theory of a primary atrial myopathy is that a subset of HCM patients develop AF as their only disease‐related complication despite normal LA size and without HF symptoms.1, 7, 8
It is well established that noncardiac comorbidities such as obesity, hypertension, and obstructive sleep apnea (OSA) are important contributors to AF in the general population, with treatment of these conditions an important aspect of AF management that is linked to decreased AF occurrence.14 However, given the multiple direct disease mechanisms that could be responsible for AF, the impact of non‐HCM factors may be overlooked in HCM. In particular, because OSA can worsen structural abnormalities already present in HCM patients including diastolic dysfunction as well as LA dilation and remodeling,14 it is perhaps not surprising that OSA may contribute to adverse events in this disease. In this regard, several studies have raised suggestion of the impact of OSA in modifying the clinical course in HCM including a case‐series reporting reduction in left ventricular outflow tract gradients and improvement in symptoms after treatment of OSA with continuous positive airway pressure therapy in HCM patients,15 while other studies have demonstrated that OSA was associated with a 4‐ to 5‐fold increase in prevalence of AF as compared with HCM patients without OSA.16
The study by Xu et al. in this issue of the Journal of the American Heart Association (JAHA) substantiates these previous findings, demonstrating a strong relationship between OSA and AF in a large cohort of 555 HCM patients.17 In this adult HCM cohort, the prevalence of AF is significantly higher in patients with OSA (24%) compared with patients without OSA (14%). Xu et al. also find the severity of OSA to be independently correlated with both the presence and severity of AF independent of other disease‐related variables linked to AF in HCM including age, LA size, left ventricular outflow tract obstruction, and severity of HF symptoms.17 Notably, the prevalence of OSA in this HCM cohort was high at 59%, but this may represent an overestimation of the prevalence of OSA in a general HCM population because of selection bias as to which HCM patients were referred for sleep studies. In addition, because 15% of HCM patients in this cohort without OSA also had AF, it would be unjustified to consider the primary mechanism of AF to be linked to OSA in HCM. Nevertheless, these findings in a very large and well‐described HCM cohort provide strong observational evidence that OSA is an important contributor to AF development in some HCM patients.
That OSA contributes to AF development in a subset of HCM patients raises several important management considerations. First, in the setting of new‐onset AF, screening for OSA should be considered in order to identify patients who would benefit from treatment. Indeed, in non‐HCM cohorts, treatment of OSA has a favorable effect on recurrence and progression of AF,14 and it is not unreasonable to consider that treatment of OSA may reduce overall AF burden in HCM patients and potentially prevent need or improve efficacy of antiarrhythmic medications and/or AF ablation. Given the potential for OSA to worsen the pathological features of HCM, it is also plausible that screening and treatment of OSA in at‐risk HCM patients may even prevent the development of AF in a subset of patients.
The relationship between OSA and AF in HCM appears to be similar to the impact of other non‐HCM comorbidities on adverse events in HCM patients.18, 19, 20 For example, obesity is highly prevalent and has also been demonstrated to be associated with higher rates of both HF symptoms and AF in HCM.18 Similarly, obstructive coronary atherosclerosis in HCM is linked to higher rates of adverse outcomes in comparison to non‐HCM cohorts with similar burden of coronary atherosclerosis.19 Additionally, the most common cause of mortality in the current HCM treatment era is no longer secondary to HCM but rather secondary to other non‐HCM‐related conditions.20 Therefore, a focus on preventative measures, lifestyle interventions, and treatment of other non‐HCM comorbidities, in conjunction with the primary care providers and other specialists, is an important component that should not be overlooked in the care of HCM patients.
In HCM, AF is an important adverse disease‐related event that is responsible for symptoms that interfere with lifestyle, leads to stroke risk requiring treatment with anticoagulation, and often antiarrhythmics and AF ablation procedures.1, 2, 7 While the predominant mechanism of AF in HCM is a result of disease‐related features, the clear association with OSA severity to AF development and burden raises awareness of the need for appropriate screening. While current treatments of AF in HCM are associated with substantial improvement in outcomes compared with previous eras, the identification and treatment of OSA may serve to further improve outcomes in HCM.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e016260 DOI: 10.1161/JAHA.120.016260.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 3.
References
- 1. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. [DOI] [PubMed] [Google Scholar]
- 2. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. [DOI] [PubMed] [Google Scholar]
- 3. Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph‐Edwards A, Rakowski H. Clinical and echocardiographic determinants of long‐term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–2041. [DOI] [PubMed] [Google Scholar]
- 4. Maron MS, Rowin EJ, Wessler BS, Mooney PJ, Fatima A, Patel P, Koethe BC, Romashko M, Link MS, Maron BJ. Enhanced American College of Cardiology/American Heart Association strategy for prevention of sudden cardiac death in high‐risk patients with hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4:644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schinkel AF, Vriesendorp PA, Sijbrands EJ, Jordaens LJ, ten Cate FJ, Michels M. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta‐analysis. Circ Heart Fail. 2012;5:552–559. [DOI] [PubMed] [Google Scholar]
- 6. Rowin EJ, Maron BJ, Abt P, Kiernan MS, Vest A, Costantino F, Maron MS, DeNofrio D. Impact of advanced therapies for improving survival to heart transplant in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2018;121:986–996. [DOI] [PubMed] [Google Scholar]
- 7. Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W, Rastegar H, Estes NAM, Maron MS, Maron BJ. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. 2017;136:2420–2436. [DOI] [PubMed] [Google Scholar]
- 8. Guttmann OP, Pavlou M, O'Mahony C, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, Garcia‐Pavia P, et al. Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart. 2017;103:672–678. [DOI] [PubMed] [Google Scholar]
- 9. Debonnaire P, Joyce E, Hiemstra Y, Mertens BJ, Atsma DE, Schalij MJ, Bax JJ, Delgado V, Marsan NA. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new‐onset atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e004052. [DOI] [PubMed] [Google Scholar]
- 10. Sivalokanathan S, Zghaib T, Greenland GV, Vasquez N, Kudchadkar SM, Kontari E, Lu DY, Dolores‐Cerna K, van der Geest RJ, Kamel IR, et al. Hypertrophic cardiomyopathy patients with paroxysmal atrial fibrillation have a high burden of left atrial fibrosis by cardiac magnetic resonance imaging. JACC Clin Electrophysiol. 2019;5:364–375. [DOI] [PubMed] [Google Scholar]
- 11. Ohtani K, Yutani C, Nagata S, Koretsune Y, Hori M, Kamada T. High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J Am Coll Cardiol. 1995;25:1162–1169. [DOI] [PubMed] [Google Scholar]
- 12. Santangeli P, Di Biase L, Themistoclakis S, Raviele A, Schweikert RA, Lakkireddy D, Mohanty P, Bai R, Mohanty S, Pump A, et al. Catheter ablation of atrial fibrillation in hypertrophic cardiomyopathy: long‐term outcomes and mechanisms of arrhythmia recurrence. Circ Arrhythm Electrophysiol. 2013;6:1089–1094. [DOI] [PubMed] [Google Scholar]
- 13. Boll G, Rowin EJ, Maron BJ, Wang W, Rastegar H, Maron MS. Efficacy of combined Cox‐Maze IV and ventricular septal myectomy for treatment of atrial fibrillation in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2020;125:120–126. [DOI] [PubMed] [Google Scholar]
- 14. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 15. Sengupta PP, Sorajja D, Eleid MF, Somers VK, Ommen SR, Parish JM, Khandheria B, Tajik AJ. Hypertrophic obstructive cardiomyopathy and sleep‐disordered breathing: an unfavorable combination. Nat Clin Pract Cardiovasc Med. 2009;6:14–15. [DOI] [PubMed] [Google Scholar]
- 16. Konecny T, Brady PA, Orban M, Lin G, Pressman GS, Lehar F, Tomas K, Gersh BJ, Tajik AJ, Ommen SR, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1597–1602. [DOI] [PubMed] [Google Scholar]
- 17. Xu H, Wang J, Yuan J, Hu F, Yang W, Guo C, Luo X, Liu R, Cui J, Gao X, et al. Implication of apnea‐hypopnea index, a measure of obstructive sleep apnea severity, for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Heart Assoc. 2020;9:e015013 DOI: 10.1161/JAHA.119.015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fumagalli C, Maurizi N, Day SM, Ashley EA, Michels M, Colan SD, Jacoby D, Marchionni N, Vincent‐Tompkins J, Ho CY, et al. Association of obesity with adverse long‐term outcomes in hypertrophic cardiomyopathy. JAMA Cardiol. 2019;1–8. DOI: 10.1001/jamacardio.2019.4268. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Berger PB, Tajik AJ. Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease. Circulation. 2003;108:2342–2348. [DOI] [PubMed] [Google Scholar]
- 20. Maron BJ, Rowin EJ, Casey SA, Garberich RF, Maron MS. What do patients with hypertrophic cardiomyopathy die from? Am J Cardiol. 2016;117:434–435. [DOI] [PubMed] [Google Scholar]


