Abstract
Background
Resistive reserve ratio is a thermodilution‐based index which integrates both coronary flow and pressure. Resistive reserve ratio represents the vasodilatory capacity of interrogated vessels including both epicardial coronary artery and microvascular circulation. We evaluated the prognostic potential of resistive reserve ratio compared with pressure‐derived index (fractional flow reserve [FFR]) or flow‐derived index (coronary flow reserve [CFR]).
Methods and Results
A total of 1245 patients underwent coronary pressure and flow measurement using pressure‐temperature wire. Resistive reserve ratio was calculated by CFR adjusted using the ratio between resting and hyperemic distal coronary pressure ([resting mean transit time/hyperemic mean transit time]×[resting distal coronary pressure/hyperemic distal coronary pressure]). Clinical outcome was assessed by patient‐oriented composite outcome (POCO), a composite of any death, myocardial infarction, and revascularization at 5 years. At 5 years, the cumulative incidence of POCO was significantly different according to quartiles of resistive reserve ratio (9.9%, 11.3%, 17.2%, and 22.7% in quartiles 1 to 4, respectively, log rank P<0.001). Among patients with deferred revascularization, those with depressed resistive reserve ratio (<3.5) showed a significantly higher risk of POCO than those with preserved resistive reserve ratio (≥3.5) in patients with FFR>0.80 or patients with CFR>2.0. (FFR>0.80 group: 14.8% versus 6.0%; log rank P=0.001; CFR>2.0 group: 13.5% versus 7.1%; log rank P=0.045). Adding resistive reserve ratio into the model for 5‐year POCO showed significantly higher global Chi square value than FFR or CFR (P<0.001, respectively, for FFR and CFR). Resistive reserve ratio <3.5 was significantly associated with the risk of POCO at 5 years in multivariable model (adjusted hazard ratio 1.597, 95% CI, 1.098–2.271, P=0.014).
Conclusions
Resistive reserve ratio, which integrated both coronary flow and pressure, showed incremental prognostic implications in patients with coronary artery disease undergoing elective percutaneous coronary intervention guided by invasive physiologic evaluation.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03690713.
Keywords: coronary artery disease, coronary flow reserve, fractional flow reserve, prognosis, resistive reserve ratio
Subject Categories: Percutaneous Coronary Intervention, Physiology, Prognosis, Treatment, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- CFR
coronary flow reserve
- FFR
fractional flow reserve
- HR
hazard ratio
- IMR
index of microcirculatory resistance
- PCI
percutaneous coronary intervention
- RRR
resistive reserve ratio
- POCO
patient‐oriented composite outcome
Clinical Perspective
What Is New?
The current study demonstrated prognostic implication of resistive reserve ratio (RRR) as an integrated physiologic index of both pressure and flow. RRR showed an inverse relationship with the estimated risk of patient‐oriented composite outcome.
Among patients with deferred revascularization, those with depressed RRR (<3.5) showed a significantly higher risk of patient‐oriented composite outcome than those with preserved RRR (≥3.5) in patients with coronary flow reserve >2.0 or patients with fractional flow reserve>0.80.
Depressed RRR<3.5 was independently associated with higher risk of patient‐oriented composite outcome and RRR showed superior discrimination of 5‐year patient‐oriented composite outcome than those with fractional flow reserve or coronary flow reserve on top of clinical variables.
What Are the Clinical Implications?
Incremental prognostic impact of RRR imply that integration of both relative and absolute metrics of coronary circulatory indices would show more specific stratification of patients with myocardial ischemia and higher risk of future clinical events than flow‐derived or pressure‐derived indices alone.
Introduction
In the treatment of ischemic heart disease, it is important to interpret how epicardial coronary arterial stenosis is related with functional significance, which is related to improving ischemic symptoms and prognosis.1 The introduction of fractional flow reserve (FFR), an invasive physiologic index of functional stenosis severity that uses the trans‐stenotic pressure ratio as a surrogate of myocardial blood flow impairment, revolutionized decision‐making on coronary revascularization in the cardiac catheterization laboratory, leading to improved patient outcomes compared with angiography‐based decision making.2, 3, 4
Despite this, pressure‐derived indices are not free from limitations in assessing coronary circulatory function, since disagreements between pressure‐derived indices and flow measurements have been reported in several studies.5, 6 Coronary flow reserve (CFR) is an absolute ratio between resting and hyperemic coronary flow velocity or surrogate marker of flow velocity, and reflects the flow limitations of the entire coronary circulatory system, including both the epicardial and microvascular components.2, 7 Despite supportive study data on the prognostic impact of depressed CFR,7, 8, 9, 10, 11 heterogenous mechanisms of depressed CFR and higher variability of CFR values according to hemodynamic status have limited its clinical applicability.12 Considering both pressure‐derived and flow‐derived indices have their own limitations, integration of both coronary pressure and flow might provide superior discrimination of patient at higher risk of future clinical events. Since both coronary pressure and flow can be easily obtained using a pressure‐temperature sensor guide wire, calculation of integrated index of both CFR and distal coronary pressure can be achieved.
Recently, the concept of resistive reserve ratio (RRR), which is an integrated index of both thermodilution‐measured CFR and distal coronary pressure measured during resting and hyperemic status, has been suggested.13 RRR represents the vasodilatory capacity of the coronary circulation and reflects cumulative functional disease burden throughout the interrogated vessel (Figure 1). We hypothesize that RRR might provide incremental prognostic stratification of patients at higher risk of clinical events at long‐term follow‐up than either pressure‐derived (FFR) or flow‐derived (CFR) indices. In this regard, we sought to evaluate the prognostic implications of RRR compared with FFR or CFR in patients with coronary artery disease.
Figure 1. Concept of resistive reserve ratio.
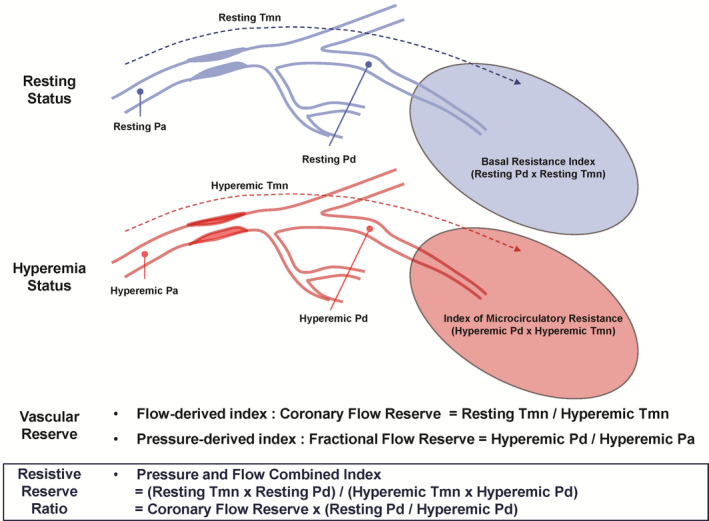
In assessment of coronary circulatory function, coronary flow reserve can be calculated by ratio between resting and hyperemic mean transit time which is a surrogate marker of coronary flow. Fractional flow reserve is calculated as the ratio between hyperemic distal coronary pressure and hyperemic aortic pressure which is also surrogate marker of coronary flow at hyperemia. Resistive reserve ratio is an integrated index of both thermodilution‐measured coronary flow reserve and distal coronary pressure measured during resting and hyperemic status and represents vasodilatory capacity of the coronary circulation and reflects cumulative functional disease burden throughout the interrogated vessel. Pa indicates aortic pressure; Pd, distal coronary pressure; and Tmn, mean transit time.
Methods
Anonymized patient level data will be made available after discussion in the executive committee for reasonable requests. Consent was not obtained for data sharing but the presented data are anonymized and the risk of identification is minimal.
Study Design and Population
The study population was derived from the International Collaboration of Comprehensive Physiologic Assessment Registry (NCT03690713). The registry was a patient‐level pooled cohort of 3 prospective registries whose results have been previously published.7, 11, 14, 15, 16 The registry was composed of 7 tertiary medical institutes in Korea (Seoul National University Hospital, Samsung Medical Centre, Inje University Ilsan Paik Hospital, Keimyung University Dongsan Medical Centre, and Ulsan University Hospital), Japan (Tsuchiura Kyodo General Hospital), and Spain (Hospital Clinico San Carlos). All patients were prospectively enrolled, and underwent clinically indicated invasive coronary angiography and comprehensive physiologic assessment for at least 1 vessel with intermediate stenosis.7, 11, 14, 15 In all studies, the same exclusion criteria were applied, and patients with hemodynamic instability, left ventricular dysfunction (ejection fraction <30%), or a culprit vessel of acute coronary syndrome were excluded. Individual patient data were collected using standardized spreadsheets. For all variables included, standardized definitions were used. Invasive physiologic indices were also cross‐checked and confirmed by each study's principal investigators.
Among the total 1694 vessels (1397 patients) enrolled overall, patients without available RRR were excluded from the current analysis, leaving 1484 vessels (1245 patients). Study protocols were designed in accordance with the Declaration of Helsinki (2013) and were authorized by the Institutional Review Boards or Ethics Committees at corresponding centers. The study protocol was registered at ClinicalTrials.gov (NCT03690713).
Angiographic Analysis and Coronary Physiologic Measurements
Coronary angiography was performed using standard techniques. Angiographic views were obtained following the administration of intracoronary nitrate (100 or 200 μg). All angiograms were analyzed at local core laboratories in masked fashion. Percent diameter stenosis (%DS), minimum luminal diameter, reference‐vessel size, and lesion length were measured.
All coronary physiologic measurements were performed after diagnostic angiography and before revascularization was performed.7, 11, 17 A guide catheter (5–7 Fr) without side holes was used to engage the coronary artery, and a pressure‐temperature sensor guide wire (Abbott Vascular, St. Paul, MN) was used to measure physiologic indices. After zeroing and equalizing the pressure sensor to the aortic pressure, the pressure sensor was positioned at the distal segment of the target vessel. Intracoronary nitrate (100 or 200 μg) was administered before each physiologic measurement. To derive resting mean transit time (Tmn), a thermodilution curve was obtained using 3 injections (4 mL each) of room‐temperature saline. Hyperemia was induced by intravenous infusion of adenosine (140 μg/kg per min) through a peripheral or central vein. Hyperemic proximal aortic pressure (Pa), distal coronary pressure (Pd), and hyperemic Tmn were measured during sustained hyperemia after the pressure curve reached a nadir point.18 The hyperemic period was recognized by a decreased Pd/aortic pressure pattern and a left shift in the Tmn. After measurements were complete, the guide wire was pulled back to the guide catheter, and the presence of a pressure drift was checked.
FFR was calculated as hyperemic Pd/aortic pressure, at the lowest average of 3 consecutive beats during maximal hyperemia. CFR was calculated as the resting Tmn/hyperemic Tmn.11 Index of microcirculatory resistance (IMR) was calculated as the hyperemic Pd×hyperemic Tmn and was corrected using the Yong formula.17 RRR was calculated as the ratio between basal resistance index (resting Tmn×resting Pd) and IMR, which can be transformed by CFR adjusted using ratio between resting and hyperemic distal coronary pressure ([resting Tmn/hyperemic Tmn]×[resting Pd/hyperemic Pd]).13 For lesions with low FFR (≤0.80), percutaneous coronary intervention was recommended according to the current guidelines. However, the final decision for percutaneous coronary intervention was at the discretion of the operators. Current analysis used only pre‐percutaneous coronary intervention physiologic indices.
Cut‐Off Values and Patient Classifications
Cut‐off values were FFR ≤0.80 (low FFR) and CFR ≤2.0 (low CFR), as previously described.7, 19, 20, 21 The cut‐off value of RRR was determined by optimal cut‐off value to predict the occurrence of POCO, which corresponded with the median value of RRR, and RRR <3.5 was defined as low RRR.
Patient Follow‐Up, Outcome Measurements, and Clinical Event Adjudications
Clinical data were obtained at outpatient clinic visits or by telephone contact if needed. An independent clinical events committee whose members were unaware of the clinical, angiographic, and physiologic data adjudicated all events. The primary outcome was the patient‐oriented composite outcome (POCO),22 which includes all‐cause mortality, any myocardial infarction, or any revascularization events during the 5‐year follow‐up. All clinical outcomes were defined according to the Academic Research Consortium, including the addendum to the definition of myocardial infarction.22 All deaths were considered cardiac unless an undisputable non‐cardiac cause was present. Repeat revascularization was also additionally adjudicated as to whether the event occurred in the initially interrogated vessel (target vessel‐related repeat revascularization). Clinical outcomes were analyzed based on the worst hierarchical order (death > myocardial infarction >revascularization).
Statistical Analysis
All discrete or categorical variables are presented as numbers and relative frequencies (percentages), and continuous variables as means and SDs or medians with interquartile ranges according to their distributions, which were checked by the Kolmogorov‐Smirnov test and visual inspection of Q‐Q plots. Correlation coefficient among physiologic indices was analyzed using Pearson or Spearman method, according to the normality of variables. Data were analyzed on a per‐patient basis for clinical characteristics and clinical outcomes at 5 years, and a per‐vessel basis for comparison of vessel‐related parameters. Among patients who underwent multivessel measurements, the vessel with the lowest FFR value was selected as a representative vessel of that patient for the per‐patient analysis. For per‐patient analyses, continuous variables were compared based on a 1‐way analysis of variance, and dichotomous variables were compared using Chi‐square tests or Fisher exact tests. For per‐vessel analyses, a generalized estimating equation with an independent correlation structure was used to adjust for intra‐subject variability among vessels from the same patient. Estimated means and SDs were presented as summary statistics. No post hoc adjustments were performed.
Event rates according to quartile and optimal cut‐off value of RRR were calculated based on Kaplan–Meier censoring estimates and presented with cumulative incidence at 5‐year follow‐up, and the log‐rank test or the Breslow test was used to compare survival curves between groups. The optimal cut‐off value of RRR to discriminate the occurrence of POCO was calculated based on maximizing the sum of sensitivity and specificity of RRR, and the derived cut‐off value was revalidated using a method using maximally selected log‐rank statistics as a sensitivity analysis. A Cox proportional hazard regression was used to calculate hazard ratio (HR) and 95% CI. The assumption of proportionality was assessed graphically by the log‐minus‐log plot, and the Cox proportional hazard models for all clinical outcomes satisfied the proportional hazards assumption. To explore the prognostic impact of RRR as continuous values, estimated event rates derived from the Cox proportional hazards regression model were plotted using the locally weighted scatterplot smoothing regression line according to RRR value, and the associations between RRR and estimated event rates were also adjusted by %DS, FFR, or multivariable analysis. Multivariable Cox proportional hazard models to identify independent predictors of POCO were constructed using all variables with a P<0.05 from the univariate analyses, and variables could be potentially relevant. Harrell c‐statistics with 95% CI were calculated to validate the discriminant function of the model.
All analyses were 2‐tailed, and clinical significance was defined as P<0.05. Statistical analyses were performed using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the Study Population
The overall characteristics of 1245 patients (1484 vessels) are outlined in Table S1. The mean age of the study population was 64.7±10.3 years and 76.9% were men. Revascularization was performed in 464 vessels from 458 patients (36.8% of patients [31.3% of vessels]). The distribution of physiologic indices is shown in Figure S1. According to the quartile of RRR value, the study population was classified into 4 groups: quartile 1 (Q1: RRR ≥5), quartile 2 (Q2: RRR 3.5–4.9), quartile 3 (Q3: RRR 2.5–3.4), and quartile 4 (Q4: RRR <2.5). Patient and lesion characteristics, according to quartile of RRR, are summarized in Table 1.
Table 1.
Patient and Lesion Characteristics According to Quartile of Resistive Reserve Ratio
| Quartile 1 (RRR≥5) | Quartile 2 (RRR 3.5–4.9) | Quartile 3 (RRR 2.5–3.4) | Quartile 4 (RRR<2.5) | P Value | |
|---|---|---|---|---|---|
| Per‐patient analysis (n=1245) | 258 (20.7%) | 356 (28.6%) | 276 (22.2%) | 355 (28.5%) | |
| General characteristics | |||||
| Age, y | 62.17±9.91 | 63.65±10.07 | 65.78±10.36 | 66.79±10.11 | <0.001 |
| Men | 217 (84.1%) | 285 (80.1%) | 195 (70.7%) | 261 (73.5%) | <0.001 |
| BMI, kg/m2 | 24.9±3.7 | 24.9±3.4 | 24.7±3.5 | 24.8±3.6 | 0.701 |
| Clinical presentation | 0.970 | ||||
| Stable ischemic heart disease | 230 (89.1%) | 321 (90.2%) | 246 (89.1%) | 318 (89.6%) | |
| Acute coronary syndrome | 28 (10.9%) | 35 (9.8%) | 30 (10.9%) | 37 (10.4%) | |
| Cardiovascular risk factors | |||||
| Hypertension | 156 (60.5%) | 231 (64.9%) | 180 (65.2%) | 250 (70.4%) | 0.079 |
| Diabetes mellitus | 73 (28.3%) | 107 (30.1%) | 110 (39.9%) | 155 (43.7%) | <0.001 |
| Hypercholesterolemia | 169 (65.5%) | 225 (63.2%) | 176 (63.8%) | 214 (60.3%) | 0.595 |
| Current smoker | 60 (23.3%) | 67 (18.8%) | 53 (19.2%) | 86 (24.2%) | 0.220 |
| Obesity (BMI>25 kg/m2) | 115 (45.5%) | 160 (45.1%) | 119 (44.2%) | 154 (44.6%) | 0.993 |
| Multivessel disease | 77 (29.8%) | 117 (32.9%) | 100 (36.2%) | 141 (39.7%) | 0.060 |
| Per‐vessel analysis (n=1484)a | 323 (21.8%) | 416 (28.0%) | 327 (22.0%) | 418 (28.2%) | |
| Target vessel location | 0.008 | ||||
| LAD | 191 (59.1%) | 270 (64.9%) | 216 (66.1%) | 273 (65.3%) | |
| LCX | 41 (12.7%) | 54 (13.0%) | 52 (15.9%) | 72 (17.2%) | |
| RCA | 91 (28.2%) | 92 (22.1%) | 59 (18.0%) | 73 (17.5%) | |
| Angiographic characteristics | |||||
| Reference diameter | 3.0±0.6 | 2.9±0.6 | 2.9±0.7 | 2.8±0.6 | <0.001 |
| Diameter stenosis, % | 42.9±15.6 | 45.3±16.4 | 45.9±16.4 | 49.5±17.8 | <0.001 |
| Lesion length, mm | 11.7±8.1 | 12.4±8.1 | 12.2±8.3 | 13.7±9.6 | 0.003 |
| Coronary physiologic parameters | |||||
| Resting Pd/Pa | 0.96±0.04 | 0.94±0.05 | 0.93±0.06 | 0.89±0.11 | <0.001 |
| Fractional flow reserve | 0.86±0.08 | 0.84±0.10 | 0.83±0.11 | 0.79±0.13 | <0.001 |
| Coronary flow reserve | 4.8±0.8 | 3.3±0.5 | 2.4±0.4 | 1.5±0.4 | <0.001 |
| Resting Tmn, sec | 1.10±0.45 | 0.90±0.41 | 0.76±0.44 | 0.62±0.44 | <0.001 |
| Hyperemic Tmn, sec | 0.23±0.09 | 0.28±0.13 | 0.33±0.22 | 0.43±0.33 | <0.001 |
| IMR, U | 16.8±7.6 | 19.0±9.2 | 21.8±12.6 | 25.6±19.9 | <0.001 |
| Resistive reserve ratio | 6.2±1.0 | 4.2±0.4 | 3.0±0.3 | 1.9±0.4 | <0.001 |
Values expressed as mean±SD or number (%). BMI indicates body mass index; IMR, index of microcirculatory resistance; LAD, left anterior descending artery; LCX, left circumflex artery; Pa, aortic pressure; Pd, distal coronary pressure; RCA, right coronary artery; and Tmn, mean transit time.
Per‐vessel analyses results (P values) were calculated from a generalized estimating equation.
Patients in quartiles 3 to 4 (lower value of RRR) were of older age, were more frequently women, and had a higher incidence of diabetes mellitus than those of patients in quartiles 1 to 2 (higher value of RRR). There were significant differences in the severity of the stenoses and the physiologic indices of the lesions across all groups. The diameter stenosis tended to be higher when the RRR value was lower. Overall, patients with lower RRR showed lower FFR, lower CFR, and higher IMR. Figure S2 shows the correlation of RRR with %DS, FFR, CFR, and IMR. RRR showed a modest correlation with %DS (R=−0.148, P<0.001), FFR (R=0.198, P<0.001), IMR (R=−0.268, P<0.001). Although RRR showed significant correlation with CFR (R=0.948, P<0.001), the classification agreement between CFR and RRR was only modest (Kappa value 0.605, P<0.001) and 28.5% of patients with CFR>2.0 showed low RRR (<3.5) (Figure S3).
Clinical Outcomes of Patients According to RRR
Table 2 and Figure 2 present the cumulative incidence of POCO during 5 years of follow‐up according to the quartile of RRR among total patients. Median follow‐up duration was 1422.0 days (Q1–Q3, 566.0–1855.0 days). At 5 years, the cumulative incidence of POCO was significantly different according to quartiles of RRR (9.9%, 11.3%, 17.2%, and 22.7% in Q1 to Q4, respectively, log‐rank P<0.001). Patients with low RRR had a higher risk of POCO than those with high RRR (Q3 versus Q1: HR 1.740, 95% CI, 1.004 to 3.014, P=0.048; Q4 versus Q1: HR 2.457, 95% CI, 1.482–4.072, P<0.001). Similarly, when patients were stratified into 2 groups by optimal cut‐off value (RRR<3.5), those with low RRR showed a higher risk of POCO than those with high RRR (HR 1.941, 95% CI, 1.387–2.714, P<0.001) (Figure S4).
Table 2.
Clinical Outcomes of Patients According to Quartile of Resistive Reserve Ratio
| Quartile 1 (RRR≥5) | Quartile 2 (RRR 3.5–4.9) | Quartile 3 (RRR 2.5–3.4) | Quartile 4 (RRR<2.5) | P Value | |
|---|---|---|---|---|---|
| Per‐patient analysis (n=1245) | 258 (20.7%) | 356 (28.6%) | 276 (22.2%) | 355 (28.5%) | |
| All‐cause death | 1.3% (3) | 2.7% (8) | 5.3% (10) | 6.6% (16) | 0.042 |
| Cardiac death | 0.9% (2) | 1.3% (4) | 2.2% (4) | 3.4% (8) | 0.338 |
| Myocardial infarction | 1.4% (3) | 2.3% (6) | 1.1% (2) | 4.1% (11) | 0.086 |
| Any revascularization | 8.8% (17) | 9.2% (26) | 12.6% (25) | 16.3% (42) | 0.029 |
| Target vessel‐related revascularizationa | 6.7% (11) | 5.8% (12) | 10.9% (15) | 13.8% (26) | 0.041 |
| Death or myocardial infarction | 2.2% (5) | 4.9% (14) | 6.0% (11) | 10.4% (27) | 0.003 |
| Patient‐oriented composite outcomeb | 9.9% (20) | 11.3% (33) | 17.2% (35) | 22.7% (61) | <0.001 |
Data expressed as cumulative incidence of clinical outcomes and numbers of events. Cumulative incidence of clinical outcomes represents Kaplan–Meier estimates during median follow‐up of 1422.0 days (Q1–Q3, 566.0–1855.0 days). P values for log‐rank or Breslow test in survival analysis. RRR indicates resistive reserve ratio.
Target vessel‐related revascularization denotes ischemia‐driven revascularization occurred in initially interrogated vessel.
Patient‐oriented composite outcomes include all‐cause mortality, any myocardial infarction, and any revascularization.
Figure 2. Comparison of patient‐oriented composite outcome according to quartiles of resistive reserve ratio.
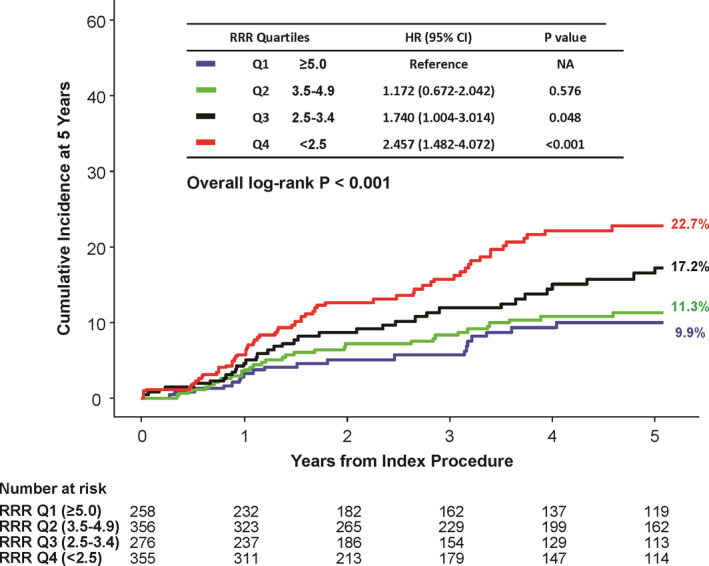
The cumulative incidence of patient‐oriented composite outcome at 5 years is compared according to quartile of resistive reserve ratio. There was a significant trend of higher RRR and lower risk of patient‐oriented composite outcome, and vice versa. HR indicates hazard ratio; Q1 to Q4, quartile 1 to 4; and RRR, resistive reserve ratio.
To evaluate the prognostic impact of RRR among deferred patients (n=787) with high FFR or high CFR, the risk of POCO was compared according to RRR in a subgroup of high FFR (n=643) or high CFR (n=597). Comparisons of baseline patient and vessel characteristics according to RRR in high FFR or high CFR groups are presented in Table S2. In the high FFR group, patients with RRR<3.5 showed significantly higher risk of POCO at 5 years (14.8% versus 6.0%, HR 2.556, 95% CI, 1.428–4.577, P=0.001) than those with RRR≥3.5. Similarly, patients with RRR<3.5 showed about a 2‐fold higher risk of POCO than those with RRR≥3.5 in the high CFR group (13.5% versus 7.1%, HR 1.849, 95% CI, 1.003–3.408, P=0.045) (Table 3 and Figure 3).
Table 3.
Clinical Outcomes of Patients With Deferred Revascularization According to Resistive Reserve Ratio in High FFR or High CFR Subgroups
| High FFR Population | High CFR Population | |||||||
|---|---|---|---|---|---|---|---|---|
| RRR≥3.5 | RRR<3.5 | HR (95% CI) | P Value | RRR≥3.5 | RRR<3.5 | HR (95% CI) | P Value | |
| Per‐patient analysis | 349/643 (54.3%) | 294/643 (45.7%) | 416/597 (69.7%) | 181/597 (30.3%) | ||||
| All‐cause death | 1.3% (4) | 5.0% (11) | 3.483 (1.109–10.94) | 0.023 | 1.7% (6) | 5.3% (7) | 2.857 (0.959–8.502) | 0.048 |
| Cardiac death | 0.6% (2) | 4.2% (9) | 5.709 (1.233–26.44) | 0.012 | 1.1% (4) | 3.3% (4) | 2.460 (0.615–9.839) | 0.188 |
| Myocardial infarction | 1.1% (3) | 1.6% (4) | 1.663 (0.372–7.435) | 0.501 | 1.4% (5) | 0.0% (0) | NA | NA |
| Any revascularization | 4.7% (13) | 10.3% (23) | 2.270 (1.149–4.482) | 0.015 | 5.5% (18) | 8.7% (11) | 1.511 (0.714–3.201) | 0.277 |
| Target vessel‐related revascularizationa | 2.5% (7) | 6.6% (14) | 2.568 (1.036–6.366) | 0.035 | 3.6% (12) | 5.7% (7) | 1.434 (0.565–3.644) | 0.448 |
| Death or myocardial infarction | 2.4% (7) | 6.2% (14) | 2.527 (1.020–6.265) | 0.038 | 2.8% (10) | 5.3% (7) | 1.713 (0.652–4.502) | 0.269 |
| Patient‐oriented composite outcomea | 6.0% (17) | 14.8% (34) | 2.556 (1.428–4.577) | 0.001 | 7.1% (24) | 13.5% (18) | 1.849 (1.003–3.408) | 0.045 |
Data expressed as cumulative incidence of clinical outcomes and numbers of events. Cumulative incidence of clinical outcomes represents Kaplan–Meier estimates during median follow‐up of 1422.0 days (Q1–Q3 566.0–1855.0 days). P values for log‐rank or Breslow test in survival analysis. CFR indicates coronary flow reserve; FFR, fractional flow reserve; HR, hazard ratio; and RRR, resistive reserve ratio.
Target vessel‐related revascularization denotes ischemia‐driven revascularization occurred in initially interrogated vessel.
Figure 3. Comparison of patient‐oriented composite outcome between preserved or depressed resistive reserve ratio, among high fractional flow reserve or high coronary flow reserve population with deferred revascularization.

The cumulative incidence of patient‐oriented composite outcome at 5 years is compared between preserved (≥3.5) and depressed resistive reserve ratio (<3.5) in subgroup of (A) high fractional flow reserve (>0.80) or (B) high coronary flow reserve (>2.0) among patients with deferred revascularization. CFR indicates coronary flow reserve; FFR, fractional flow reserve; HR, hazard ratio; and RRR, resistive reserve ratio.
Comparison of Additive Prognostic Impact of FFR, CFR, and RRR for Occurrence of POCO Among Patients With Deferred Revascularization
RRR showed significant association with the occurrence of POCO after adjustment with %DS or FFR. In addition, RRR was an independent predictor of POCO in the multivariable model and showed significant association with the estimated risk of POCO at 5 years (adjusted HR of RRR<3.5: 1.579, 95% CI, 1.098–2.271, P=0.014) (Table 4 and Figure 4).
Table 4.
Association of Resistive Reserve Ratio for Occurrence of Patient‐Oriented Composite Outcomes at 5 Years Among Patients With Deferred Revascularization
| Models | HR (95% CI) | P Value | Harrell C‐index |
|---|---|---|---|
| Unadjusted Model | 0.611 (0.554–0.668) | ||
| RRR<3.5 | 2.496 (1.531–4.068) | <0.001 | |
| Adjusted by Diameter stenosis | 0.672 (0.608–0.735) | ||
| RRR<3.5 | 2.543 (1.544–4.190) | <0.001 | |
| %DS | 1.028 (1.012–1.044) | <0.001 | |
| Adjusted by FFR | 0.652 (0.589–0.715) | ||
| RRR<3.5 | 2.415 (1.480–3.940) | <0.001 | |
| FFR (per 0.01 increase) | 0.946 (0.918–0.976) | <0.001 | |
| Full multivariable adjustmenta | 0.713 (0.670–0.756) | ||
| RRR<3.5 | 1.579 (1.098–2.271) | 0.014 | |
| FFR (per 0.01 increase) | 0.972 (0.955–0.989) | 0.001 | |
| Age (per 1.0 increase) | 1.031 (1.011–1.051) | 0.002 | |
| Diabetes mellitus | 1.479 (1.054–2.075) | 0.025 |
FFR indicates fractional flow reserve; and RRR, resistive reserve ratio.
Adjusted covariates included age, sex, hypertension, diabetes mellitus, hyperlipidemia, acute coronary syndrome, multivessel disease, fractional flow reserve, and % diameter stenosis.
Figure 4. Association of resistive reserve ratio with estimated risk of patient‐oriented composite outcome at 5 years among patients with deferred revascularization.
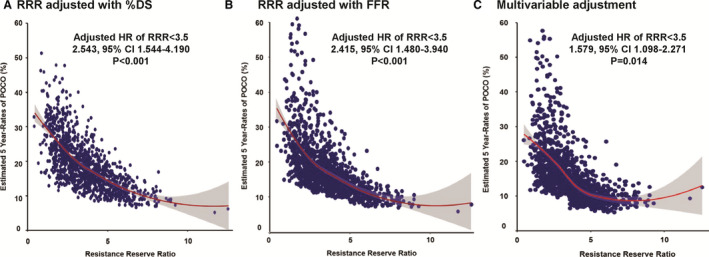
The non‐linear relationship between resistive reserve ratio and the estimated risk of patient‐oriented composite outcome at 5 years was plotted after adjustment with (A) percent diameter stenosis (%DS), (B) fractional flow reserve, or (C) multivariable adjustment. Adjusted covariates in the multivariable model are age, sex, hypertension, diabetes mellitus, hyperlipidemia, acute coronary syndrome, multivessel disease, fractional flow reserve, and % diameter stenosis. %DS indicates percent diameter stenosis; FFR, fractional flow reserve; and RRR, resistive reserve ratio.
In comparison of additive prognostic impact on top of clinical variables, models with FFR, CFR, or RRR showed significantly increased global Chi square value than model with clinical variables. However, model with RRR showed the highest global Chi square value than model with FFR or CFR (P<0.001 for both comparisons) (Figure 5).
Figure 5. Comparison of additive prognostic impact of FFR, CFR, and RRR for risk of patient‐oriented composite outcome at 5 years among patients with deferred revascularization.
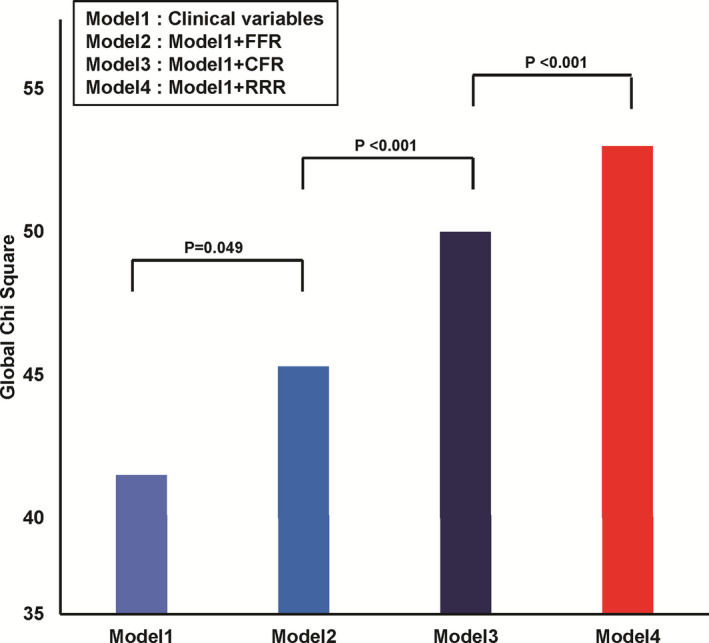
In addition to model with clinical variables, additive prognostic impact of fractional flow reserve, coronary flow reserve, or resistive reserve ratio was compared. Model 4 (clinical variables with resistive reserve ratio) showed significantly higher global Chi square value than Model 2 (clinical variables with fractional flow reserve) or Model 3 (clinical variables with coronary flow reserve). Included clinical variables were age, sex, hypertension, diabetes mellitus, hyperlipidemia, acute coronary syndrome, multivessel disease, and % diameter stenosis. CFR indicates coronary flow reserve, FFR, fractional flow reserve; and RRR, resistive reserve ratio.
Discussion
The current study evaluated the prognostic implications of RRR as an integrated physiologic index of both pressure and flow. RRR represents the vasodilatory capacity of the coronary circulation and reflects cumulative functional disease burden throughout the interrogated vessel. We found that RRR is a valuable index in predicting POCO at 5 years, with an inverse relationship between RRR and POCO rates, both in the overall study population and in those patients in whom revascularization was deferred. The significant difference in the risk of POCO according to RRR was also maintained in high FFR or high CFR groups. Multivariable analysis confirmed that the prognostic value of RRR in predicting long‐term POCO rates is independent of other indices. In addition, model with RRR showed significantly higher predictability for POCO at 5 years than the model with FFR or the model with CFR.
Coronary Circulation and Physiologic Indices
The coronary artery system has 3 components with different functions: conductive epicardial coronary arteries, arterioles, and capillaries, although the borders of each compartment cannot be clearly defined anatomically.23 In any condition wherein coronary blood flow fails to meet myocardial oxygen demand, myocardial ischemia can occur. Physiological interrogation of epicardial vessels with stenoses is typically performed with FFR, a well‐validated approach to set the indication of coronary revascularization to improve both angina symptoms and clinical outcomes.19, 24 However, lack of a functionally significant epicardial coronary stenosis does not exclude the possibility of microvascular disease as another potential domain of myocardial ischemia.7, 11, 25 Previous studies presented that the additional stratification using flow‐based index (CFR or coronary flow velocity reserve) with FFR could discriminate patients with myocardial ischemia but FFR>0.80.7, 10, 11, 15, 26, 27 However, heterogenous mechanism of depressed CFR and higher variability of flow‐based indices according to hemodynamic status have limited its clinical applicability.12
Rationale of Integrated Physiologic Index With Both Pressure and Flow
The concept of coronary flow capacity has been introduced to overcome the potential limitations of flow‐derived or pressure‐derived indices.14, 28, 29 Coronary flow capacity is a 2‐step stratified approach using CFR and absolute measure of hyperemic coronary blood flow. Previous studies indicated that coronary flow capacity is an effective way to define myocardial ischemia using non‐invasive measurement using positron emission tomography, and invasive measurement using Doppler or pressure‐temperature sensor guidewires.14, 28, 29 Because of additional stratification using absolute hyperemic coronary blood flow, coronary flow capacity essentially ruled out that depressed CFR originated from elevated resting coronary blood flow, and therefore, provides less sensitivity to changes in systemic hemodynamics during resting status than CFR alone. In addition, previous studies also presented that coronary flow capacity showed better risk stratification for patient's clinical outcomes than CFR or FFR alone.14, 28, 29 These results imply that integration of both relative and absolute metrics of coronary circulatory indices would show more specific stratification of patients with myocardial ischemia and higher risk of future clinical events than flow‐derived or pressure‐derived indices alone. However, coronary flow capacity requires 2‐dimensional mapping for interpreting its significance and the cut‐off value of absolute hyperemic coronary blood flow has not been clarified.
In this regard, the current study used the concept of RRR. RRR was proposed by Layland et al.13 They defined the RRR as the ratio between basal resistance index (resting Tmn×resting Pd) and hyperemic resistance index, IMR (hyperemic Tmn×hyperemic Pd). However, this equation can be transformed into CFR divided by ratio between resting and hyperemic Pd, and thus can be interpreted as CFR, adjusted by a surrogate marker of hyperemic coronary flow which was normalized by resting value. Therefore, this transformation enables the interpretation of RRR as a single surrogate value of coronary flow capacity. Using both pressure and flow measured in the target vessel, RRR represents cumulative inducible flow limitations and functional disease burden from epicardial coronary arteries and the microvasculature.
Clinical Relevance of Resistive Reserve Ratio
When physiologic characteristics according to quartiles of RRR were compared, patients with lower RRR showed lower FFR and CFR, higher diameter stenosis and IMR than those with higher RRR. In addition, RRR had a modest positive correlation with FFR, and a negative correlation with %DS and IMR. Although RRR showed significant correlation with CFR, the classification agreement between CFR and RRR was modest, and about one third of patients with preserved CFR showed depressed RRR (<3.5). In a comparison of the risk of POCO according to quartiles of RRR, there was a significant trend of higher RRR and lower POCO risk, and vice versa. These results were consistent when the patients were classified by an optimal cut‐off value of RRR (<3.5).
More importantly, patients with depressed RRR showed a significantly higher risk of POCO than those with preserved RRR among patients with high FFR and deferred revascularization. Similarly, patients with depressed RRR also showed significantly higher risk of POCO than those with preserved RRR among deferred patients with high CFR. Furthermore, RRR showed superior discrimination of 5‐year POCO than those with FFR or CFR on top of clinical variables. The current results support the improved risk stratification by RRR in patients with high FFR or high CFR after deferral of revascularization. Therefore, when revascularization was deferred based on FFR or CFR, RRR can be used as additional prognostic indicator. Further study is warranted to clarify the potential role of RRR in guiding treatment decision‐making in patients with stable ischemic heart disease.
Although previous studies indicated that only one physiologic index such as FFR, CFR, or IMR cannot fully discriminate patients at higher risk of clinical events, it is unclear how can we integrate multiple coronary physiologic indices. In this regard, the current study proposed the concept of RRR as an integrated physiologic index of both coronary flow and pressure. RRR can be discriminated from flow‐derived index (CFR), pressure‐derived index (FFR), or the lowest possible passive microvascular resistance (IMR). Compared with CFR alone, RRR can integrate the severity of epicardial coronary disease by incorporating distal coronary pressure, as with the concept of coronary flow capacity. Compared with FFR alone, RRR integrates the concept of coronary flow reserve, which cannot be reflected by pressure‐derived indices. In addition, as RRR reflects the dynamic nature of coronary arterial tone, it is less influenced by the amount of myocardium subtended to the location of the pressure‐temperature sensor, which was the shortcoming of IMR.30
Study Limitations
Some limitations of the current study should be noted. First, we could not compare non‐invasive stress tests according to the low or high RRR groups. Second, although the decision to perform percutaneous coronary intervention for target lesions was made based on the FFR value, the participating investigators were not masked to the physiologic indices and therefore may have been influenced in their decision‐making strategy. Nevertheless, all clinical events were independently adjudicated by the clinical events adjudication committee. Third, further investigations, including the external validation of cut‐off value and a longer follow‐up period, are needed to confirm our findings. Fourth, the majority of the current study population presented with stable ischemic heart disease, therefore, the results cannot be extrapolated to patients with ST‐segment–elevation myocardial infarction. However, there has been no previous study which evaluated prognostic implication of RRR in patients with stable ischemic heart disease. Fifth, 15.1% of deferred vessels showed an FFR ≤0.80 and were deferred revascularization based on operator's discretion. However, time to target vessel revascularization was not different according to FFR value at the index procedure (FFR ≤0.80 at index procedure: 876.2±518.3 days versus FFR >0.80 at index procedure: 780.6±469.9 days, P=0.583) and clinical events were defined according to objective criteria. Sixth, the discrimination ability of multivariable model was relatively low, probably because of a limited number of clinical events. Therefore, a further validation study would be warranted.
Conclusions
Resistive reserve ratio which integrates both coronary flow and pressure showed incremental prognostic implications in patients with coronary artery disease undergoing elective percutaneous coronary intervention guided by invasive physiologic evaluation.
Sources of Funding
None.
Disclosures
Dr Joo Myung Lee received a Research Grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. The remaining authors have no disclosures to report.
Supporting information
Appendix S1 Tables S1 and S2 Figures S1–S4
(J Am Heart Assoc. 2020;e015846 DOI: 10.1161/JAHA.119.015846.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Iskander S, Iskandrian AE. Risk assessment using single‐photon emission computed tomographic technetium‐99 m sestamibi imaging. J Am Coll Cardiol. 1998;32:57–62. [DOI] [PubMed] [Google Scholar]
- 2. van de Hoef TP, Siebes M, Spaan JA, Piek JJ. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur Heart J. 2015;36:3312–3319a. [DOI] [PubMed] [Google Scholar]
- 3. Lee JM, Koo BK, Shin ES, Nam CW, Doh JH, Hwang D, Park J, Kim KJ, Zhang J, Hu X, et al. Clinical implications of three‐vessel fractional flow reserve measurement in patients with coronary artery disease. Eur Heart J. 2018;39:945–951. [DOI] [PubMed] [Google Scholar]
- 4. Barbato E, Toth GG, Johnson NP, Pijls NH, Fearon WF, Tonino PA, Curzen N, Piroth Z, Rioufol G, Juni P, et al. A prospective natural history study of coronary atherosclerosis using fractional flow reserve. J Am Coll Cardiol. 2016;68:2247–2255. [DOI] [PubMed] [Google Scholar]
- 5. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, et al. Physiological basis and long‐term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. [DOI] [PubMed] [Google Scholar]
- 6. Lee JM, Hwang D, Park J, Zhang J, Tong Y, Kim CH, Bang JI, Suh M, Paeng JC, Cheon GJ, et al. Exploring coronary circulatory response to stenosis and its association with invasive physiologic indexes using absolute myocardial blood flow and coronary pressure. Circulation. 2017;136:1798–1808. [DOI] [PubMed] [Google Scholar]
- 7. Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, Jung HW, Cho YK, Yoon HJ, Song YB, et al. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv. 2018;11:1423–1433. [DOI] [PubMed] [Google Scholar]
- 8. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Hoef TP, Bax M, Damman P, Delewi R, Hassell ME, Piek MA, Chamuleau SA, Voskuil M, van Eck‐Smit BL, Verberne HJ, et al. Impaired coronary autoregulation is associated with long‐term fatal events in patients with stable coronary artery disease. Circ Cardiovasc Interv. 2013;6:329–335. [DOI] [PubMed] [Google Scholar]
- 11. Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67:1158–1169. [DOI] [PubMed] [Google Scholar]
- 12. de Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94:1842–1849. [DOI] [PubMed] [Google Scholar]
- 13. Layland J, Carrick D, McEntegart M, Ahmed N, Payne A, McClure J, Sood A, McGeoch R, MacIsaac A, Whitbourn R, et al. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non‐st‐segment‐elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:231–236. [DOI] [PubMed] [Google Scholar]
- 14. Hamaya R, Yonetsu T, Kanaji Y, Usui E, Hoshino M, Yamaguchi M, Hada M, Kanno Y, Murai T, Hirao K, et al. Diagnostic and prognostic efficacy of coronary flow capacity obtained using pressure‐temperature sensor‐tipped wire‐derived physiological indices. JACC Cardiovasc Interv. 2018;11:728–737. [DOI] [PubMed] [Google Scholar]
- 15. Echavarria‐Pinto M, Escaned J, Macias E, Medina M, Gonzalo N, Petraco R, Sen S, Jimenez‐Quevedo P, Hernandez R, Mila R, et al. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013;128:2557–2566. [DOI] [PubMed] [Google Scholar]
- 16. Mejia‐Renteria H, Lee JM, Lauri F, van der Hoeven NW, de Waard GA, Macaya F, Perez‐Vizcayno MJ, Gonzalo N, Jimenez‐Quevedo P, Nombela‐Franco L, et al. Influence of microcirculatory dysfunction on angiography‐based functional assessment of coronary stenoses. JACC Cardiovasc Interv. 2018;11:741–753. [DOI] [PubMed] [Google Scholar]
- 17. Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbourn R, Macisaac A, Kritharides L, Wilson A, et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53–58. [DOI] [PubMed] [Google Scholar]
- 18. Toth GG, Johnson NP, Jeremias A, Pellicano M, Vranckx P, Fearon WF, Barbato E, Kern MJ, Pijls NH, De Bruyne B. Standardization of fractional flow reserve measurements. J Am Coll Cardiol. 2016;68:742–753. [DOI] [PubMed] [Google Scholar]
- 19. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrom T, Kaab S, Dambrink JH, Rioufol G, et al. Five‐year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 20. Melikian N, Vercauteren S, Fearon WF, Cuisset T, MacCarthy PA, Davidavicius G, Aarnoudse W, Bartunek J, Vanderheyden M, Wyffels E, et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention. 2010;5:939–945. [PubMed] [Google Scholar]
- 21. Lee JM, Choi KH, Koo BK, Park J, Kim J, Hwang D, Rhee TM, Kim HY, Jung HW, Kim KJ, et al. Prognostic implications of plaque characteristics and stenosis severity in patients with coronary artery disease. J Am Coll Cardiol. 2019;73:2413–2424. [DOI] [PubMed] [Google Scholar]
- 22. Garcia‐Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel M‐A, van Es G‐A, Zuckerman B, et al. Standardized end point definitions for coronary intervention trials. Eur Heart J. 2018;39:2192–2207. [DOI] [PubMed] [Google Scholar]
- 23. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 24. Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, Juni P, Pijls NHJ, Hlatky MA; Investigators FT . Clinical outcomes and cost‐effectiveness of fractional flow reserve‐guided percutaneous coronary intervention in patients with stable coronary artery disease: three‐year follow‐up of the fame 2 trial (fractional flow reserve versus angiography for multivessel evaluation). Circulation. 2018;137:480–487. [DOI] [PubMed] [Google Scholar]
- 25. Lee JM, Doh JH, Nam CW, Shin ES, Koo BK. Functional approach for coronary artery disease: filling the gap between evidence and practice. Korean Circ J. 2018;48:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meuwissen M, Chamuleau SA, Siebes M, de Winter RJ, Koch KT, Dijksman LM, van den Berg AJ, Tijssen JG, Spaan JA, Piek JJ. The prognostic value of combined intracoronary pressure and blood flow velocity measurements after deferral of percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008;71:291–297. [DOI] [PubMed] [Google Scholar]
- 27. van de Hoef TP, Bax M, Meuwissen M, Damman P, Delewi R, de Winter RJ, Koch KT, Schotborgh C, Henriques JP, Tijssen JG, et al. Impact of coronary microvascular function on long‐term cardiac mortality in patients with acute ST‐segment‐elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:207–215. [DOI] [PubMed] [Google Scholar]
- 28. van de Hoef TP, Echavarria‐Pinto M, van Lavieren MA, Meuwissen M, Serruys PW, Tijssen JG, Pocock SJ, Escaned J, Piek JJ. Diagnostic and prognostic implications of coronary flow capacity: a comprehensive cross‐modality physiological concept in ischemic heart disease. JACC Cardiovasc Interv. 2015;8:1670–1680. [DOI] [PubMed] [Google Scholar]
- 29. Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5:430–440. [DOI] [PubMed] [Google Scholar]
- 30. Echavarria‐Pinto M, van de Hoef TP, Nijjer S, Gonzalo N, Nombela‐Franco L, Ibanez B, Sen S, Petraco R, Jimenez‐Quevedo P, Nunez‐Gil IJ, et al. Influence of the amount of myocardium subtended to a coronary stenosis on the index of microcirculatory resistance. Implications for the invasive assessment of microcirculatory function in ischaemic heart disease. EuroIntervention. 2017;13:944–952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Tables S1 and S2 Figures S1–S4


