Abstract
Background
The Heart Team approach is ascribed a Class I recommendation in contemporary guidelines for revascularization of complex coronary artery disease. However, limited data are available regarding the decision‐making and outcomes of patients based on this strategy.
Methods and Results
One hundred sixty‐six high‐risk coronary artery disease patients underwent Heart Team evaluation at a single institution between January 2015 and November 2018. We prospectively collected data on demographics, symptoms, Society of Thoracic Surgeons Predicted Risk of Mortality/Synergy Between PCI with Taxus and Cardiac Surgery (STS‐PROM/SYNTAX) scores, mode of revascularization, and outcomes. Mean age was 70.0 years; 122 (73.5%) patients were male. Prevalent comorbidities included diabetes mellitus (51.8%), peripheral artery disease (38.6%), atrial fibrillation (27.1%), end‐stage renal disease on dialysis (13.3%), and chronic obstructive pulmonary disease (21.7%). Eighty‐seven (52.4%) patients had New York Heart Association III‐IV and 112 (67.5%) had Canadian Cardiovascular Society III‐IV symptomatology. Sixty‐seven (40.4%) patients had left main and 118 (71.1%) had 3‐vessel coronary artery disease. The median STS‐PROM was 3.6% (interquartile range 1.9, 8.0) and SYNTAX score was 26 (interquartile range 20, 34). The median number of physicians per Heart Team meeting was 6 (interquartile range 5, 8). Seventy‐nine (47.6%) and 49 (29.5%) patients underwent percutaneous coronary intervention and coronary artery bypass grafting, respectively. With increasing STS‐PROM (low, intermediate, high operative risk), coronary artery bypass graft was performed less often (47.9%, 18.5%, 15.2%) and optimal medical therapy was recommended more often (11.3%, 18.5%, 30.3%). There were no trends in recommendation for coronary artery bypass graft, percutaneous coronary intervention, or optimal medical therapy by SYNTAX score tertiles. In‐hospital and 30‐day mortality was 3.9% and 4.8%, respectively.
Conclusions
Integrating a multidisciplinary Heart Team into institutional practice is feasible and provides a formalized approach to evaluating complex coronary artery disease patients. The comprehensive assessment of surgical, anatomical, and other risk scores using a decision aid may guide appropriate, evidence‐based management within this team‐based construct.
Keywords: cardiac surgery, percutaneous coronary intervention, quality improvement, revascularization, team‐based care
Subject Categories: Quality and Outcomes, Percutaneous Coronary Intervention, Cardiovascular Surgery, Revascularization, Treatment
Clinical Perspective
What Is New?
We designed an institutional Heart Team approach for complex coronary artery disease—inclusive of a structured coronary artery disease heart team form and simultaneous Interventional Cardiology and Cardiac Surgery consultation—that culminated in a well‐attended interdisciplinary team meeting held for each high‐risk patient.
What Are the Clinical Implications?
Incorporating a structured Heart Team form that assesses formal risk stratification facilitates personalized decision‐making for complex coronary artery disease patients.
Nonstandard Abbreviations and Acronyms.
IQR interquartile range
HT Heart Team
OMT optimal medical therapy
STS‐PROM Society of Thoracic Surgeons Predicted Risk of Mortality
SYNTAX Synergy Between PCI with Taxus and Cardiac Surgery
Introduction
Determining the optimal treatment strategy for patients with complex coronary artery disease (CAD) requires assessment of each patient's presenting illness, clinical stability, coronary anatomy, comorbidities, and goals of care. An informed decision to pursue percutaneous or surgical revascularization in patients with multivessel or left main CAD may be optimized when appropriate stakeholders—primary care physicians, cardiologists, interventional cardiologists, cardiac surgeons, patients, and family members—communicate and proceed in a coordinated, interdisciplinary manner. Professional society guidelines, risk calculators, and appropriate use criteria provide a framework that may help guide management at the bedside.1, 2, 3, 4, 5 However, the complexities of integrating patient‐centered shared decision‐making with evidence‐based medicine in the context of multiple comorbid disease entities substantiate the potential benefit of a multidisciplinary team‐based approach to medical care.6, 7
The multidisciplinary “Heart Team” (HT) approach has been espoused and deployed for multiple conditions relevant to cardiovascular medicine: valvular heart disease, peripheral vascular disease, pulmonary embolism, and cardiogenic shock.8, 9, 10, 11, 12 European professional society guidelines first mandated a team‐based approach to decision‐making for multivessel CAD in 2010.13 This was followed by American‐centered guidelines in 2014 that provided a class I recommendation for the HT strategy in managing stable ischemic heart disease.1 These recommendations follow from the data and experience of multiple large randomized clinical trials that have compared percutaneous and surgical revascularization in patients with complex coronary disease. For instance, the Synergy Between PCI with Taxus and Cardiac Surgery (SYNTAX) trial included in its design a formalized protocol for assessment of patients by both interventional cardiology and cardiac surgery.14
Implementation of the rich evidence base guiding CAD management to the nuances of real‐world practice can be challenging, thus supporting the role of an interdisciplinary model of care for ischemic heart disease.14, 15, 16 In this study, we describe the structure and deployment of a multidisciplinary HT for patients with complex CAD, as well as the clinical outcomes at a single institution with the use of this approach over a 3‐year time period since its inception. Furthermore, in this team model, we created and employed a practical HT form that integrates the use of US and European practice guidelines, validated surgical and percutaneous coronary intervention (PCI) risk scores, and SYNTAX grading to aid in patient‐centered decision‐making. In addition, we implemented an interventional cardiology consultative service to provide formal recommendations for these patients with complex CAD presentations.
Methods
Multidisciplinary HT Structure and Workflow
Starting in January 2015, the Massachusetts General Hospital has offered a multidisciplinary CAD HT consultative service for patients diagnosed with complex CAD and deemed high risk for surgical or percutaneous revascularization. The HT may receive electronic notification requesting a formal consultation by either an inpatient or outpatient cardiology physician. This may be requested following a cardiac catheterization that has provided an up‐to‐date assessment of a patient's coronary anatomy and hemodynamics. Referring physicians may classify these patients as high risk based on several factors: multivessel distribution of coronary disease, left main involvement, low ejection fraction, chronic total occlusion, advanced patient age or frailty, socioeconomic factors, or other coexisting medical comorbidities. Once a consultation is requested, the patient is independently seen and assessed by an interventional cardiology service in tandem with the cardiac surgical service. Those participating physicians will then coordinate a multidisciplinary heart team meeting for that patient. The CAD HT was therefore developed based on a quality improvement initiative and has continued to operate at the Massachusetts General Hospital since its inception.
Furthermore, a structured HT form was designed to capture relevant clinical data before a formal interdisciplinary team meeting. Requisite information included laboratory data, coronary angiography +/− right heart hemodynamic data, echocardiography, and if warranted, noninvasive functional testing. A comprehensive risk profile was also conducted using validated risk stratification tools and documented in the “CAD Heart Team Decision Aid” shown in Figure 1. Within 24 to 48 hours of request, a scheduled multidisciplinary HT meeting is convened, consisting of a referring team physician, the patient's primary cardiologist, ≥2 interventional cardiologists, and ≥2 cardiothoracic surgeons. Depending on each patient's unique circumstance, additional participants may include other subspecialty consultants (eg, hematology, oncology, pulmonology, etc). The patient's presenting illness, history, noninvasive, and invasive data are reviewed in detail, and a formal recommendation regarding management strategy of CAD is coformulated by participating team members.
Figure 1.

Multidisciplinary heart team risk assessment and summary form.
Study Population and Data Collection
From January 2015 to November 2018, 166 patients deemed high risk for coronary revascularization underwent a HT consultation at our institution. We prospectively collected data on demographics, symptoms, comorbidities, noninvasive and cardiac catheterization results, and calculated Society of Thoracic Surgeons Predicted Risk of Mortality (STS‐PROM) and SYNTAX scores. We also recorded data on HT participation, including attendant members, subspecialties represented, recommendation provided with respect to treatment strategy, and final treatment/mode of revascularization delivered. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Partners Healthcare. REDCap is a secure, web‐based application designed to support data capture for research studies.17 This project was undertaken as a Quality Improvement Initiative at the Massachusetts General Hospital, and as such was not formally supervised by the Institutional Review Board per their policies. The authors declare that all supporting data are available within the article.
Practice Assessment/Outcomes
We retrospectively analyzed the revascularization strategy according to coronary anatomy complexity using the validated SYNTAX score calculated for each patient. We also stratified revascularization approach according to low (0–4%), intermediate (4–8%), and high (>8%) surgical risk groups using the estimated STS‐PROM score. Periprocedural data, in‐hospital mortality, and 30‐day mortality were tracked and recorded for all patients. We also assessed for 30‐day unplanned readmission rate for the overall cohort, and then stratified according to treatment strategy.
Statistical Analysis
Descriptive statistics were used to analyze demographics, comorbidities, results of noninvasive and invasive testing, HT meeting data, details of revascularization strategies, and outcomes of the study cohort. Categorical variables are expressed as frequencies and percentages and continuous variables as mean±SD and/or median (interquartile range [IQR]) as appropriate. Statistical analysis was performed using SPSS version 23.0 (IBM Corp, Armonk, NY).
Results
Baseline Characteristics
From January 2015 through November 2018, the total number of patients undergoing coronary revascularization at the Massachusetts General Hospital was 6120 (4331 PCI; 1789 CABG). During this time period, 166 of these CAD patients were deemed high‐risk and underwent formal HT consultation (166/6120, 2.7%). Table 1 depicts the patient demographics, comorbidities, presentation, noninvasive testing, and angiographic data for the cohort. The mean age was 70.0 (±11.8) years, and 122 (73.5%) patients were male. Prevalent comorbidities included diabetes mellitus (51.8%), peripheral artery disease (38.6%), atrial fibrillation (27.1%), cancer (24.1%), chronic obstructive lung disease (21.7%), end‐stage renal disease on dialysis (13.3%), and chronic liver disease (6.6%). More than one third of patients had at least mild frailty. The most common CAD presentation was non‐ST‐elevation acute coronary syndrome (111 [66.9%]), and 112 (67.5%) patients had Canadian Cardiovascular Society class III‐IV angina. The median left ventricular ejection fraction was 45% (IQR 32, 60), and 87 (52.4%) patients had New York Heart Association functional class III‐IV symptoms. The median STS‐PROM score was 3.6% (IQR 1.9, 8.0).
Table 1.
Baseline Characteristics
| Variable | n=166 |
|---|---|
| Age, y | |
| Mean±SD | 70.0±11.8 |
| Median (IQR) | 72 (63, 79) |
| Sex | |
| Male | 122 (73.5) |
| Female | 44 (26.5) |
| Body mass index, kg/m2 | 28.2 (24.3, 32.0) |
| Diabetes mellitus | 86 (51.8) |
| No therapy | 13 (7.8) |
| Oral therapy | 21 (12.7) |
| Insulin therapy | 55 (31.3) |
| Hemoglobin A1c | 6.8±1.2 |
| Chronic obstructive lung disease | 36 (21.7) |
| Home oxygen therapy | 9 (5.4) |
| Peripheral artery disease | 64 (38.6) |
| Serum creatinine, mg/dL | 1.1 (0.9, 1.5) |
| ESRD on dialysis | 22 (13.3) |
| Prior cerebrovascular disease | 45 (27.1) |
| Carotid artery disease | 29 (17.5) |
| Prior PCI | 63 (38.0) |
| Prior CABG | 24 (14.5) |
| Arrhythmia | |
| None | 106 (63.9) |
| Atrial fibrillation | 45 (27.1) |
| Atrial flutter | 2 (1.2) |
| VT/VF | 12 (7.2) |
| Hematocrit, % | 34.7 (30.0, 39.5) |
| Platelet count, ×103 per μL | 187 (143, 244) |
| Liver disease | 11 (6.6) |
| MELD score | 11 (8, 23) |
| Child‐Pugh Class | |
| A | 8 (4.8) |
| B | 2 (1.2) |
| C | 1 (0.6) |
| Cancer | 40 (24.1) |
| Immunocompromised | 20 (12.0) |
| CSHA Clinical Frailty Scale | 4 (3, 5) |
| ≥5 | 62 (37.4) |
| CAD presentation | |
| STEMI | 3 (1.8) |
| NSTEMI | 89 (53.6) |
| Unstable angina | 22 (13.3) |
| Stable angina | 24 (14.5) |
| No angina | 28 (16.9) |
| CCS angina class | |
| 0 | 20 (12.0) |
| I | 2 (1.2) |
| II | 4 (2.4) |
| III | 33 (19.9) |
| IV | 79 (47.6) |
| Heart failure | 116 (69.9) |
| NYHA functional class | |
| I | 7 (4.2) |
| II | 22 (13.3) |
| III | 34 (20.5) |
| IV | 53 (31.9) |
| Cardiogenic shock | 13 (7.8) |
| Cardiac arrest | 10 (6.0) |
| STS PROM score | 3.6 (1.9, 8.0) |
| Noninvasive assessment | |
| Echocardiogram | |
| LVEF, % | 45 (32, 60) |
| LVEDD, mm | 51 (45, 57) |
| Right ventricular dysfunction | 37 (22.3) |
| RVSP, mm Hg | 42 (35, 52) |
| ≥ moderate AS | 27 (16.3) |
| ≥ moderate MR | 50 (30.1) |
| Stress testing | |
| None | 115 (69.3) |
| Yes, without ischemia | 11 (6.6) |
| Yes, with ischemia | 40 (24.1) |
| Viability testing | |
| No | 130 (78.3) |
| Yes | 36 (21.7) |
| Coronary angiogram | |
| Coronary artery involvement | |
| LMCA | 67 (40.4) |
| LAD | 158 (95.2) |
| LCX | 142 (85.5) |
| RCA | 142 (85.5) |
| Bypass graft | 16 (9.6) |
| Number of diseased vessels | |
| 1 | 9 (5.4) |
| 2 | 37 (22.3) |
| 3 | 118 (71.1) |
| Chronic total occlusion | 65 (39.2) |
| SYNTAX Score (n=142) | 26 (20, 34) |
| Right heart catheterization (n=56) | |
| RAP, mm Hg | 8 (6, 11) |
| RVSP, mm Hg | 42 (32, 52) |
| PASP, mm Hg | 44 (32, 56) |
| PCWP, mm Hg | 18 (12, 27) |
| CO, L/min | 3.9 (3.2, 4.7) |
| CI, L/min per m2 | 2.1 (1.7, 2.5) |
Continuous variables are presented as means±SD or medians with interquartiles (Q1, Q3). AS indicates aortic stenosis; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; CI, cardiac index; CO, cardiac output; CSHA, Canadian Study on Health and Aging; IQR, interquartile range; ESRD, end‐stage renal disease; LAD, left anterior descending; LCX, left circumflex artery; LMCA, left main coronary artery; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MELD, Model for End‐Stage Liver Disease; MR, mitral regurgitation; NSTEMI, non–ST‐segment–elevation myocardial infarction; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; PCI, percutaneous coronary intervention; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; RCA, right coronary artery; RVSP, right ventricular systolic pressure; STEMI, ST‐segment–elevation myocardial infarction; STS PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; SYNTAX, Synergy between PCI with Taxus and Cardiac Surgery; VF, ventricular fibrillation; and VT, ventricular tachycardia.
With respect to coronary anatomic complexity, 67 (40.4%) patients had left main and 118 (71.1%) had 3‐vessel CAD. A chronic total occlusion was present in 65 (39.2%) patients. The median SYNTAX score in patients without prior history of CABG was 26 (IQR 20, 34). The distribution of HT patients based on SYNTAX tertiles (after excluding n=24 prior CABG patients) was: low tertile, 35.9%; intermediate tertile, 33.8%; and high tertile, 30.3%. Among the 56 (33.7%) patients who had right heart catheterization data available, the median cardiac index was 2.1 (IQR 1.7, 2.5). From a surgical perspective, the median STS‐PROM in the HT group was 3.6% (IQR 1.9, 8.0). The distribution of HT patients based on STS categories was: low risk, 53.6%; intermediate risk, 21.7%; and high risk, 24.7%.
CAD HT Profile and Clinical Outcomes
During the study period, the median number of physicians in attendance of the HT meeting was 6 (IQR 5, 8). The median number of interventional cardiologists and cardiac surgeons in attendance was 2 (IQR 2, 3) and 3 (IQR 1, 4), respectively (Table 2). Other specialties in attendance 12/166 (7.2%) included: pulmonary in 3 (1.8%); hematology in 2 (1.2%); oncology in 3 (1.8%); and infectious disease, vascular surgery, endocrine, and primary care in 1 each (0.6%). Palliative care participated in formal consultation in 15/166 (9.0%) of this high‐risk cohort.
Table 2.
Multidisciplinary Heart Team Meeting Data and Outcomes
| Patient status at the time of meeting | |
| Inpatient | 119 (71.7) |
| Outpatient | 47 (28.3) |
| Number of attendees | |
| Total | 6 (5, 8) |
| Cardiac surgeons | 3 (1, 4) |
| Interventional cardiologists | 2 (2, 3) |
| General cardiologists | 1 (1, 2) |
| Formal Heart Team recommendation | |
| OMT | 23 (13.9) |
| PCI | 64 (38.6) |
| CABG | 44 (26.5) |
| Hybrid | 2 (1.2) |
| Othera | 4 (2.4) |
| Deferb | 29 (17.5) |
| Outcomes | |
| Successful PCI | 79 (47.6) |
| Heart team meeting to PCI, d | 1 (0, 3) |
| Successful CABG | 49 (29.5) |
| Heart Team meeting to CABG, d | 3 (1, 6) |
| Hybrid | 1 (0.6) |
| OMT only | 34 (20.5) |
| Length of stay, d (n=113) | 16 (11, 24) |
| 30‐d readmission rate | 31 (18.7%) |
| Outcomes of patients who underwent revascularization (n=129) | |
| Myocardial infarction | 5 (3.9) |
| Stroke | 3 (2.3) |
| AKI requiring dialysis | 3 (2.3) |
| Transfusion | 42 (32.6) |
| In‐hospital mortality | 5 (3.9) |
| 30‐d postprocedure mortality (n=125) | 6 (4.8) |
AKI indicates acute kidney injury; CABG, coronary artery bypass grafting; ICD, implantable cardioverter‐defibrillator; LVAD, left ventricular assist device; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; and TAVR, transcatheter aortic valve replacement.
Other recommendations included TAVR (n=1), ICD (n=1), and LVAD (n=2).
Additional studies/evaluation needed.
Of the 166 patients discussed at HT meetings, eventually 79 (47.6%) underwent PCI, 49 (29.5%) underwent CABG, 1 (0.6%) underwent hybrid revascularization, and 34 (20.5%) were treated with optimal medical therapy (OMT) only. The median duration between HT meeting and PCI was 1 (IQR 0, 3) day and that between HT meeting and CABG was 3 (IQR 1, 6) days. Figure 2 depicts the HT recommendation stratified by STS and SYNTAX scores. When stratified by predicted STS surgical risk, CABG was most often recommended for patients in the lowest surgical risk stratum (47.9%) and PCI was most often recommended for patients in the intermediate risk stratum (63.0%). OMT as the primary strategy was infrequently recommended, but more often as the predicted surgical risk rose. By SYNTAX score, there were no apparent trends in the relative recommendation for CABG, PCI, or OMT. Of the 34 patients who received a recommendation for OMT, 21 were hospitalized as inpatients. Four of these patients died in‐hospital, 3 of whom were under comfort care only at the time of death.
Figure 2.
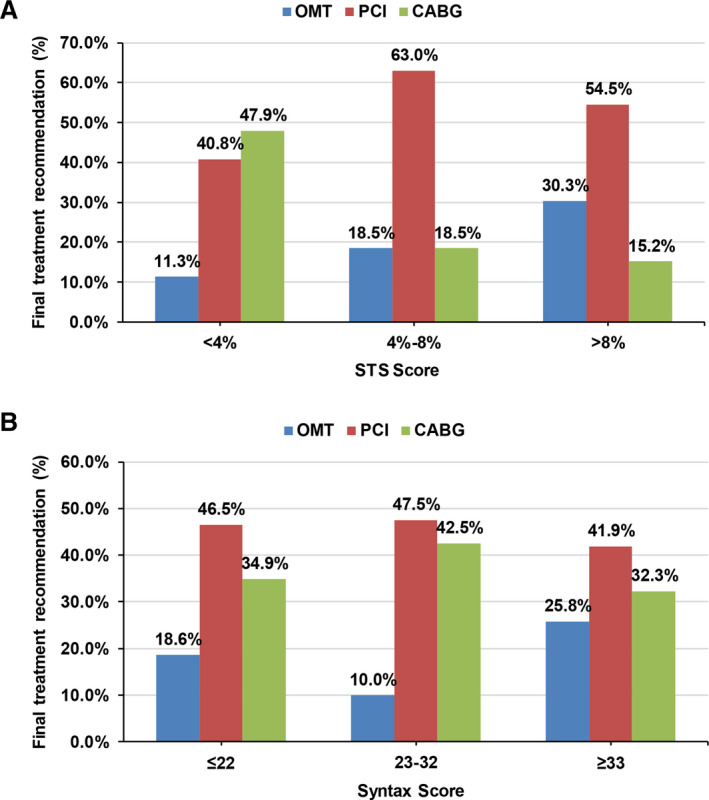
Heart team recommendation stratified by STS predicted risk of mortality (A) and SYNTAX scores (B).
CABG indicates coronary artery bypass grafting; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; and SYNTAX, Synergy Between PCI with Taxus and Cardiac Surgery.
Details of the revascularization strategies are shown in Table 3. In patients who underwent PCI, left main stenting was performed in 29 (36.7%). Drug‐eluting stents were used in a majority (96.2%) of the patients. Intracoronary imaging was used in 30 (37.9%), atherectomy in 38 (48.1%), and mechanical circulatory support in 23 (29.1%) patients. In patients who underwent CABG, the left anterior descending coronary artery was bypassed in 47 (95.9%). All procedures were performed on‐pump and mechanical circulatory support was used in 7 (14.3%) patients. Twelve (24.5%) patients underwent concurrent (eg, CABG+valve) cardiac surgical procedures.
Table 3.
Revascularization Strategies
| PCI | n=79 |
|---|---|
| Vessels treated | |
| LMCA | 29 (36.7) |
| LAD | 50 (63.3) |
| LCX | 40 (50.6) |
| Ramus | 3 (3.8) |
| RCA | 25 (31.6) |
| Bypass graft | 7 (8.8) |
| DES | 76 (96.2) |
| BMS | 4 (5.1) |
| No. of stents implanted | 2 (1, 4) |
| IVUS | 28 (35.4) |
| OCT | 2 (2.5) |
| Rotational atherectomy | 22 (27.8) |
| Orbital atherectomy | 13 (16.5) |
| Laser atherectomy | 3 (3.8) |
| Mechanical circulatory support | 23 (29.1) |
| IABP | 1 (1.3) |
| Impella | 20 (25.3) |
| ECMO | 1 (1.3) |
| LVAD | 2 (2.5) |
| Staged PCI | 7 (8.9) |
| CABG | n=49 |
|---|---|
| Vessels bypassed | |
| LAD | 47 (95.9) |
| LCX | 38 (77.6) |
| RCA | 33 (67.3) |
| LIMA graft | 49 (100.0) |
| On‐pump | 49 (100.0) |
| Mechanical circulatory support | 7 (14.3) |
| IABP | 6 (12.2) |
| Impella | 1 (2.0) |
| Concurrent surgical procedure | 12 (24.5) |
| AVR | 4 (8.2) |
| MVR | 2 (4.1) |
| Mitral annuloplasty ring | 2 (4.1) |
| LAA ligation | 5 (10.2) |
| Other | 3 (6.1) |
AVR indicates aortic valve replacement; BMS, bare metal stent; CABG, coronary artery bypass grafting; DES, drug‐eluting stent; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; IVUS, intravascular ultrasound; LAA, left atrial appendage; LAD, left anterior descending; LCX, left circumflex artery; LMCA, left main coronary artery; LVAD, left ventricular assist device; MVR, mitral valve replacement; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; and RCA, right coronary artery.
Among 129 patients who underwent revascularization (PCI or CABG), the in‐hospital mortality was 3.9%. Other in‐hospital major adverse events included myocardial infarction (5 [3.9%]), stroke (3 [2.3%]), acute kidney injury requiring dialysis (5 [3.9%]), and blood transfusion (42 [32.6%]). The overall 30‐day postprocedure mortality rate was 4.8% (Table 2). For patients who underwent CABG (n=49), the observed 30‐day mortality rate was 2.2%, while the expected 30‐day mortality based on estimated STS‐PROM was 4.1%. Therefore, the observed/expected mortality ratio for this group was 2.2/4.1=0.54.
For the entire high‐risk cohort, the 30‐day unplanned readmission rate was 31/166, or 18.7%. Specifically, 13/79 (16.4%) of PCI patients, 11/49 (22.4%) of CABG patients, and 6/34 (17.6%) of OMT patients were hospitalized within 30 days. The single patient who underwent hybrid surgical and percutaneous revascularization was also readmitted.
Discussion
The concept of the multidisciplinary “Heart Team” has been successfully employed and championed in several areas within the field of cardiovascular medicine. For instance, the structural HT evolved from the advent and dispersion of transcatheter aortic and mitral valve therapies. Institutional experiences of dedicated pulmonary embolism response teams have also been well‐described in the literature.9, 10 This interdisciplinary team‐based philosophy has even been extrapolated to conditions such as cardiogenic shock and critical limb ischemia.8, 18 These initiatives derive from the belief that patient care may best be optimized when leveraging the expertise and experience of subspecialists, each of whom can intimately understand and weigh in on the nuances of a patient's disease state relevant to his or her presenting illness.
Similar to other cardiovascular disease entities, complex CAD presents multiple challenges with respect to patient‐centered shared‐decision making, periprocedural risk stratification, antiplatelet and anticoagulant treatment strategy, and downstream clinical implications based on mode of revascularization.19 To date, the development and integration of the HT approach to the care of CAD patients has been previously published in a few single center observational studies, each with variable reports of use of predicted surgical risk and SYNTAX scoring in team‐based recommendations.20, 21, 22, 23, 24, 25 It is, however, unclear how widely adopted a formalized, team‐based strategy to coronary revascularization may be, despite its classification as a Class I recommendation in current professional society guidelines.1
We provide a report on the design, implementation, and 3‐year outcomes of a HT consultative service for the management of high risk, complex CAD patients at a single institution. We build upon prior HT studies by devising a succinct yet practical HT decision aid that integrates relevant patient data; SYNTAX score; STS‐PROM and PCI risk calculation; formalized interventional cardiology consultation service at the bedside, professional guidelines grading; and technical considerations for revascularization. We then deployed this clinical tool in the construct of our interdisciplinary model while longitudinally collecting outcomes following either medical therapy or revascularization in our complex CAD population.
Overall, patients who underwent HT consultation in our prospectively collected cohort were older; severely symptomatic by both New York Heart Association and Canadian Cardiovascular Society class; medically complex based on coexisting comorbidities (eg, chronic pulmonary disease, peripheral artery disease); anatomically complex by SYNTAX grading; and often intermediate or high surgical risk by STS risk score. Reassuringly, all patients referred for revascularization underwent successful CABG or PCI with an in‐hospital mortality rate that was relatively low (3.9%) for this high‐risk patient cohort. In the PCI subgroup, the use of intracoronary imaging, atherectomy, and mechanical circulatory support were frequent, thus attesting to the technical complexity required for PCI of HT patients. Meanwhile, for the CABG subgroup, approximately one quarter of these patients underwent a concurrent surgical procedure (eg, valve replacement). Furthermore, the actual 30‐day mortality rate was 2.2%, which was favorably lower than the mean estimated STS‐PROM of 4.1% (observed/expected 0.54). Notably, the 30‐day readmission rate was high across the entire cohort (18.7%) and among all management strategy subgroups (16.4% PCI, 22.4% CABG, 17.6% OMT). This attests to the high‐risk, medically complex nature of this population.
From this study, we first highlight the utilization of risk assessment scores and other validated instruments to provide quantitative data that could better inform the patient, referring cardiologist, and proceduralist regarding all potential therapeutic options including OMT. By incorporating STS PROM, SYNTAX scores, relevant calculated PCI risks, and American Heart Association and European Society of Cardiology guideline references, the HT has offered the opportunity to comprehensively discuss appropriate indications for revascularization as well as quantified risk assessment for each revascularization strategy. Furthermore, these HT meetings often incorporated technical discussions among proceduralists. For instance, a cardiac surgeon may articulate whether or not a patient would be a candidate for off‐pump bypass, hybrid CABG‐PCI options, or if coexisting valvular disease would also need to be treated in tandem. An interventional cardiologist on the team might discuss the role for atherectomy, extent of revascularization in a single or staged setting, radial versus femoral access, adjunctive hemodynamic support (ie, percutaneous ventricular assist device, heart failure/durable left ventricular assist device/transplantation assessment before PCI), or optimal dual antiplatelet therapy/triple therapy regimens post‐PCI.
We found that high‐risk CAD HT meetings were well attended by both interventional cardiologists and cardiac surgeons (median total attendees 6). This is important in that—beyond the consulting interventional cardiologist and cardiac surgeon—additional attendance was otherwise voluntary. When stratifying the HT recommendations by SYNTAX score, we found that a larger proportion of patients in the low (<23) and intermediate (23–32) SYNTAX tertiles received a recommendation for PCI relative to patients in the highest SYNTAX tertile (≥33). This practice pattern might suggest that the CAD HT operated in the context of contemporary trial data (ie, SYNTAX trial). Furthermore, when analyzing recommendations according to STS‐PROM, the recommendation for surgical revascularization decreased while optimal medical therapy proportionally increased with each STS‐PROM category (low <4%, intermediate 4–8%, and high >8%; Figure 2).
Limitations are present in this institutional case series. Data were generated from a single center, and randomization was not feasible as the new HT structure was adopted when first implemented. It would be challenging to capture how the efficacy of decision‐making evolves with institutional experience and maturation of the HT model. Furthermore, not all complex CAD patients during this study period may have been captured because of clinical urgency or other logistical issues. Longer‐term follow‐up and the impact of incomplete revascularization will be of importance in future investigations.
From the current experience, we conclude that the formulation of a multidisciplinary process by which all high‐risk CAD patients are comprehensively risk‐stratified and evaluated is safe and realistically achievable in a busy tertiary referral system. Interdisciplinary HT meetings were well‐attended by both interventional cardiologists and cardiac surgeons. Based on formal recommendations made in the context of the patient's stratified anatomic and surgical risks, the HT practice patterns regarding revascularization strategy appeared to account for anatomical and patient‐specific risks. Future study in this area of healthcare delivery may focus on whether or not such constructs have measurable, meaningful impacts on informed consent and patient‐reported outcomes.
Conclusions
The organization and deployment of a multidisciplinary HT for complex CAD is realistically achievable and helps provide a formalized construct to guide medical decision‐making for both healthcare providers and patients. The utilization of this interdisciplinary model of care enables individualized risk assessment as well as an evidence‐based approach to coronary revascularization. The integration of SYNTAX, STS‐PROM, and other validated risks scores into the comprehensive HT evaluation of complex CAD is feasible, informative, and effective for guiding care in high‐risk patients.
Sources of Funding
None
Disclosures
Jaffer reports research grant support from NIH/NHLBI, Canon, and Siemens; consultant for Boston Scientific, Abbott Vascular, Siemens, Philips, and Acrostak. Massachusetts General Hospital has a patent licensing arrangement with Canon, and Jaffer has the right to receive royalties. The remaining authors have no disclosures to report.
Acknowledgments
The authors appreciate the efforts of the members of the Cardiology Division and Cardiac Surgery Division of the Massachusetts General Hospital who have contributed to this quality initiative, and to Michael Bode, MD for assistance with the CAD Heart Team Form.
(J Am Heart Assoc. 2020;9:e014738 DOI: 10.1161/JAHA.119.014738.)
See Editorial by Holmes and Mack
For Sources of Funding and Disclosures, see page 9.
References
- 1. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 2. Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517–592. [DOI] [PubMed] [Google Scholar]
- 3. Coronary Revascularization Writing Group , Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA, Technical Panel, Masoudi FA, Dehmer GJ, Patel MR, et al. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Thorac Cardiovasc Surg. 2012;143:780–803. [DOI] [PubMed] [Google Scholar]
- 4. Teo KK, Cohen E, Buller C, Hassan A, Carere R, Cox JL, Ly H, Fedak PW, Chan K, Legare JF, et al. Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology/Canadian Society of Cardiac Surgery position statement on revascularization—multivessel coronary artery disease. Can J Cardiol. 2014;30:1482–1491. [DOI] [PubMed] [Google Scholar]
- 5. Farooq V, Brugaletta S, Serruys PW. Utilizing risk scores in determining the optimal revascularization strategy for complex coronary artery disease. Curr Cardiol Rep. 2011;13:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulman‐Marcus J, Peterson K, Banerjee R, Samy S, Yager N. Coronary revascularization in high‐risk stable patients with significant comorbidities: challenges in decision‐making. Curr Treat Options Cardiovasc Med. 2019;21:5. [DOI] [PubMed] [Google Scholar]
- 7. Holmes DR Jr, Rich JB, Zoghbi WA, Mack MJ. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61:903–907. [DOI] [PubMed] [Google Scholar]
- 8. Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB III, O'Neill W. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72:1972–1980. [DOI] [PubMed] [Google Scholar]
- 9. Kabrhel C, Jaff MR, Channick RN, Baker JN, Rosenfield K. A multidisciplinary pulmonary embolism response team. Chest. 2013;144:1738–1739. [DOI] [PubMed] [Google Scholar]
- 10. Zern EK, Young MN, Rosenfield K, Kabrhel C. A Pulmonary Embolism Response Team: initial experiences and future directions. Expert Rev Cardiovasc Ther. 2017;15:481–489. [DOI] [PubMed] [Google Scholar]
- 11. Thourani VH, Edelman JJ, Satler LF, Weintraub WS. Surgical aortic valve replacement in the transcatheter aortic valve replacement era: implications for the heart team. JACC Cardiovasc Interv. 2018;11:2157–2159. [DOI] [PubMed] [Google Scholar]
- 12. Kolte D, Parikh SA, Piazza G, Shishehbor MH, Beckman JA, White CJ, Jaff MR, Iribarne A, Nguyen TC, Froehlich JB, et al. Vascular teams in peripheral vascular disease. J Am Coll Cardiol. 2019;73:2477–2486. [DOI] [PubMed] [Google Scholar]
- 13. Task Force on Myocardial Revascularization of the European Society of Cardiology, the European Association for Cardio‐Thoracic Surgery, European Association for Percutaneous Cardiovascular Interventions , Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555.20802248 [Google Scholar]
- 14. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, et al. Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 15. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 16. Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM III, et al. Everolimus‐eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel RS. Team approach to critical limb ischemia care and research. Tech Vasc Interv Radiol. 2016;19:101–103. [DOI] [PubMed] [Google Scholar]
- 19. Doenst T, Haverich A, Serruys P, Bonow RO, Kappetein P, Falk V, Velazquez E, Diegeler A, Sigusch H. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol. 2019;73:964–976. [DOI] [PubMed] [Google Scholar]
- 20. Bonzel T, Schachinger V, Dorge H. Description of a Heart Team approach to coronary revascularization and its beneficial long‐term effect on clinical events after PCI. Clin Res Cardiol. 2016;105:388–400. [DOI] [PubMed] [Google Scholar]
- 21. Chu D, Anastacio MM, Mulukutla SR, Lee JS, Smith AJ, Marroquin OC, Sanchez CE, Morell VO, Cook CC, Lico SC, et al. Safety and efficacy of implementing a multidisciplinary heart team approach for revascularization in patients with complex coronary artery disease: an observational cohort pilot study. JAMA Surg. 2014;149:1109–1112. [DOI] [PubMed] [Google Scholar]
- 22. Domingues CT, Milojevic M, Thuijs D, van Mieghem NM, Daemen J, van Domburg RT, Kappetein AP, Head SJ. Heart Team decision making and long‐term outcomes for 1000 consecutive cases of coronary artery disease. Interact Cardiovasc Thorac Surg. 2019;28:206–213. [DOI] [PubMed] [Google Scholar]
- 23. Long J, Luckraz H, Thekkudan J, Maher A, Norell M. Heart team discussion in managing patients with coronary artery disease: outcome and reproducibility. Interact Cardiovasc Thorac Surg. 2012;14:594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pavlidis AN, Perera D, Karamasis GV, Bapat V, Young C, Clapp BR, Blauth C, Roxburgh J, Thomas MR, Redwood SR. Implementation and consistency of Heart Team decision‐making in complex coronary revascularisation. Int J Cardiol. 2016;206:37–41. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez CE, Dota A, Badhwar V, Kliner D, Smith AJ, Chu D, Toma C, Wei L, Marroquin OC, Schindler J, et al. Revascularization heart team recommendations as an adjunct to appropriate use criteria for coronary revascularization in patients with complex coronary artery disease. Catheter Cardiovasc Interv. 2016;88:E103–E112. [DOI] [PubMed] [Google Scholar]


