Abstract
BACKGROUND
Medical therapy for heart failure with reduced ejection fraction evolved since trials validated the use of implantable cardioverter–defibrillators (ICDs). We sought to evaluate the performance of ICDs in reducing mortality in the era of modern medical therapy by means of a systematic review and meta‐analysis of contemporary randomized clinical trials of drug therapy for heart failure with reduced ejection fraction.
METHODS AND RESULTS
We systematically identified randomized clinical trials that evaluated drug therapy in patients with heart failure with reduced ejection fraction that reported mortality. Studies that enrolled <1000 patients, patients with left ventricular ejection fraction >40%, or patients in the acute phase of heart failure and study treatment with devices were excluded. We identified 8 randomized clinical trials, including 31 701 patients of whom 3631 (11.5%) had an ICD. ICDs were associated with a lower risk of all‐cause mortality (relative risk [RR], 0.85; 95% CI, 0.78–0.94) and sudden cardiac death (RR, 0.49; 95% CI, 0.40–0.61). Results were consistent among studies published before and after 2010. In meta‐regression analysis, the proportion of nonischemic etiology did not affect the associated benefit of ICD.
CONCLUSIONS
In our meta‐analysis of contemporary randomized trials of drug therapy for heart failure with reduced ejection fraction, the rate of ICD use was low and associated with a decreased risk in both all‐cause mortality and sudden cardiac death. This benefit was still present in trials with new medical therapy.
Keywords: all‐cause mortality, heart failure with reduced ejection fraction, implantable cardioverter–defibrillators, sudden cardiac death
Subject Categories: Sudden Cardiac Death, Ventricular Fibrillation, Arrhythmias
Nonstandard Abbreviations and Acronyms
- ACEI
angiotensin‐converting‐enzyme inhibitors
- ARB
antiotensin‐2 receptor blockers
- CRT
cardiac resynchronization therapy
- HFrEF
heart failure with reduced ejection fraction
- ICD
implantable cardioverter–defibrillator
- LVEF
left ventricular ejection fraction
- SCD
sudden cardiac death
Clinical Perspective
What Is New?
Implantable cardioverter–defibrillator use was associated with reduced all‐cause mortality and sudden cardiac death in patients enrolled in contemporary heart failure with reduced ejection fraction trials.
Implantable cardioverter–defibrillator benefit was not influenced by nonischemic etiology.
Implantable cardioverter–defibrillators were only used in 12% of patients.
What Are the Clinical Implications?
Implantable cardioverter–defibrillator use still appears to reduce mortality among patients with heart failure with reduced ejection fraction on top of contemporary optimal medical therapy.
The findings support current implantable cardioverter–defibrillator guidelines.
In patients with heart failure with reduced ejection fraction (HFrEF), sudden cardiac death (SCD) is a major contributor for the burden of all‐cause mortality.1 Medical therapy with modulators of neurohormonal systems involved in the triggering and progression of HFrEF is the mainstay of the treatment to reduce the risks of SCD and all‐cause mortality.2
Implantable cardioverter–defibrillators (ICDs) further reduce these risks and are currently recommended in patients with New York Heart Association functional class II or III and a left ventricular ejection fraction (LVEF) of 35% or less for the primary prevention of SCD and to prolong life.3, 4, 5 However, the rate of ICD use in clinical practice is suboptimal, even in populations enrolled in contemporary clinical trials of drug therapy for HFrEF.2, 6
We sought to evaluate the performance of ICDs in reducing SCD and all‐cause mortality in populations enrolled in contemporary randomized clinical trials of drug therapy for HFrEF by means of a systematic review and meta‐analysis.
METHODS
Search of the Studies
All data and materials have been made publicly available at the Dataverse Project and can be accessed at https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi%3A10.7910%2FDVN%2FVU8QNE.
A systematic review and trial‐level meta‐analysis of randomized clinical trials that evaluated drug therapy in patients with HFrEF were performed according to the Preferred Reporting Items for Systematic reviews and Meta‐Analyses guidelines.7 Potential trials were identified from PubMed, EMBASE, and CENTRAL (Cochrane Central Register of Controlled Trials) from December 1996, the date of the publication of the first clinical trial demonstrating the efficacy of ICD in reducing mortality,8 through February 8, 2019 using the following search terms ([heart failure OR cardiac failure OR cardiomyopathy] AND [reduced ejection fraction OR ejection fraction less OR systolic] AND [randomized OR randomised OR randomly] AND [mortality OR death]). We excluded studies enrolling <1000 patients, patients with left ventricular ejection fraction >40%, patients enrolled in the acute phase of heart failure, and study treatments with devices.
Data Extraction
Two authors (F.G., J.F.) independently assessed titles, abstracts, and full texts, when appropriate, for eligibility and data extraction. Disagreements were resolved by consensus or, if necessary, by a third author (F.C./J.C.). Details on the study design, patient characteristics, treatments, and outcome measures of all‐cause death and SCD were collected from the main publication9, 10, 11, 12, 13, 14, 15, 16 and subsequent subanalysis publications.2, 17, 18
Assessment of Risk of Bias
Two authors (J.C., F.C.) independently assessed the risk of bias within the trials using the Cochrane Collaboration risk‐of‐bias tool. The reviewers evaluated the risk of bias as “low,” “high,” or “unclear” for the following 6 domains: random sequence generation, allocation concealment, participants and personnel blinding, outcome assessment blinding, selective reporting, and other sources of bias.
Statistical Analysis
Random effects were used to pool the relevant studies and summarize the evidence because of the variability of demographic, clinical, and pharmacologic treatment baseline characteristics among the included study populations (Table 1). The results were presented as relative risk (RR) with 95% CIs. A 2‐sided P<0.05 was considered significant.
Table 1.
Study Design, Baseline Characteristics, Duration of Follow‐Up, and Major Outcomes of Included Studies
| Randomized Clinical Trial | BEST9 | CHARM‐Added10 | CHARM‐Alternative11 | CORONA12 | EMPHASIS‐HF13 | WARCEF14 | PARADIGM‐HF15 | ATMOSPHERE16 |
|---|---|---|---|---|---|---|---|---|
| Year of publication | 2001 | 2003 | 2003 | 2007 | 2011 | 2012 | 2014 | 2016 |
| Design | ||||||||
| Target population | ACEI treated HF patients | ACEI treated HF patients | ACEI intolerant HF patients | Older, ischemic and stable HF patients | Optimized medical therapy HF patients | Optimized medical therapy HF patients | Enalapril treated HF patients | Optimized medical therapy HF patients |
| Target LVEF (%) | ≤35 | ≤40 | ≤40 | ≤40 | ≤30 (≤35 if QRS >130 ms) | ≤35 | ≤40 | ≤35 |
| Study treatments | Bucindolol vs placebo | Candesartan vs placebo | Candesartan vs placebo | Rosuvastatin vs placebo | Eplerenone vs placebo | Warfarin vs aspirin | Sacubitril‐valsartan vs enalapril | Aliskiren+enalapril vs aliskiren vs enalapril |
| Population | ||||||||
| Total (N) | 2708 | 2548 | 2028 | 5011 | 2737 | 2293 | 8442 | 7016 |
| Age, y | 60 | 64.1 | 66.6 | 73 | 68.7 | 61 | 63.8 | 63.3 |
| Female, % | 22 | 21.3 | 31.9 | 24 | 22.3 | 20 | 21.8 | 21.7 |
| Hypertension, % | 41 | 51.8 | 55.1 | 63 | 66.5 | 59.6 | 70.7 | 61.7 |
| Diabetes mellitus, % | 35.5 | 29.8 | 27 | 29.5 | 31.4 | 31.5 | 34.7 | 27.7 |
| Nonischemic, % | 41.5 | 37.6 | 30.2 | 0 | 31 | 57 | 40 | 44 |
| Atrial fibrillation, % | 11.5 | 46.1 | 74.6 | 23.5 | 30.9 | 3.7 | 36.8 | 40.6 |
| Mean eGFR, mL/min per 1.73 m2 | 65.5±23.2 | 71.5±28 | 71.5±28 | 58±15 | 70.8±21.8 | 68.4±20.5 | 67.6±18.5 | 74±24 |
| NYHA III/IV, % | 100 | 75.9 | 52.4 | 62.9 | 0 | 30.9 | 24.6 | 36.7 |
| LVEF, % | 23 | 28 | 29.9 | 31 | 26.2 | 25.0 | 29.5 | 28.4 |
| Beta‐blockers, % | 50 | 55.5 | 54.5 | 75.2 | 86.7 | 89.9 | 92.5 | 91.7 |
| ACEI/ARB, % | 97.6 | 99.9 | 50 | 91.8 | 93.4 | 98.4 | 100 | 66.6 |
| Mineralocorticoid antagonist, % | 3.5 | 17.2 | 23.8 | 39.2 | 49.8 | 60.4 | 55.3 | 37.1 |
| ICD, % | 3.4 | 3.9 | 3.4 | 2.7 | 15.4 | 18.2 | 14.7 | 14.9 |
| CRT, % | NR | NR | NR | NR | 8.5 | NR | 6.8 | 5.6 |
| Follow‐up, mo | 24 | 41 | 33.7 | 32.8 | 21 | 22.8 | 27 | 36.6 |
| All‐cause death (100 patient/years) | 15.88 | 9.06 | 9.85 | 10.86 | 8.02 | 11.71 | 8.18 | 8.86 |
| Sudden death (100 patient/years) | ··· | 3.65 | 3.35 | 4.69 | 2.84 | 4.07 | 2.97 | ··· |
ACEI indicates angiotensin‐converting‐enzyme inhibitors; ARB, antiotensin‐2 receptor blockers; ATMOSPHERE, Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure; BEST, Beta‐Blocker Evaluation of Survival Trial; CHARM ‐ Added, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity ‐ Added Trial; CHARM ‐ Alternative, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity ‐ Alternative Trial; CORONA, Controlled Rosuvastatin Multinational Trial in Heart Failure; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; EMPHASIS‐HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; HF, heart failure; ICD, implantable cardioverter–defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; NR, not reported; and WARCEF, Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction.
We assessed for heterogeneity using the Cochran Q test, which was quantified by the I2 statistic. A fixed‐effects model was additionally used in case of heterogeneity across studies.
We conducted sensitivity analyses in studies published before and after 2008, studies including patients with LVEF 35% to 40% and only patients with LVEF <35%, and studies reporting the use of cardiac resynchronization therapy (CRT) and not reporting the use of this device. We set the median of the search period of studies (January 2008) as the landmark to differentiate older from newer studies.
We performed random‐effects meta‐regression with the baseline characteristics of study populations (prevalence for categorical variables and mean/median value for continuous variables) as a covariate to assess their potential effects on the impact of the ICD on sudden death.
We looked for potential publication bias using funnel plots and Egger's test.
The primary analyses were performed using RevMan version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark).
RESULTS
Studies Included
After manual screenings of the titles, abstracts, and full texts, we identified 8 studies that fulfilled the inclusion and exclusion criteria with outcome measures of interest for patients with and without ICD (Figure 1). In the ATMOSPHERE (Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure)16 study, the results were reported together for 1048 patients with ICDs and 107 patients with isolated CRT pacemakers. The WARCEF (Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction) study data refer to a propensity‐matched analysis with the entire cohort of ICD patients and 44.6% of the cohort without an ICD.18
Figure 1. Flowchart diagram illustrating studies selection methodology.
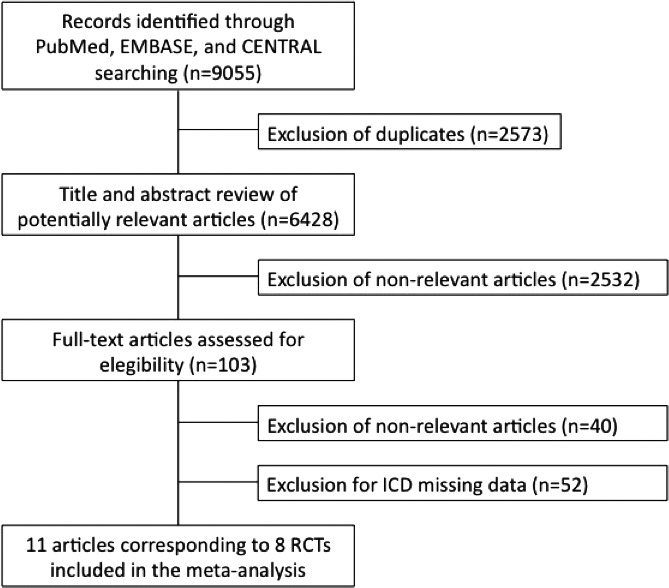
ICD indicates implantable cardioverter–defibrillator; and RCT, randomized clinical trial.
The SHIFT (Ivabradine and outcomes in chronic heart failure) study, although reporting univariable association of ICDs with all‐cause mortality using a hazard ratio (HR) (0.85; 95% CI, 0.58–1.58) could not be included because of missing absolute data.19
We included a total of 31 701 patients in this analysis, of whom 3631 (11.5%) had an ICD. Table 1 provides a comprehensive description of each included study.
All‐Cause Death
In 8 studies there were 715 deaths in 3631 patients (19.7%) with ICDs and 7086 deaths in 28 070 patients (25.2%) without ICDs (random‐effects risk ratio, 0.85; 95% CI, 0.78–0.94; I2=24%) (Figure 2). Mortality per 100 patient/year was 8.00 and 9.84, respectively. Fixed‐effects risk ratio was 0.86 (95% CI, 0.80–0.92) (Figure S1).
Figure 2. Forest plots comparing patients with ICD vs without ICD for the outcome all‐cause death.
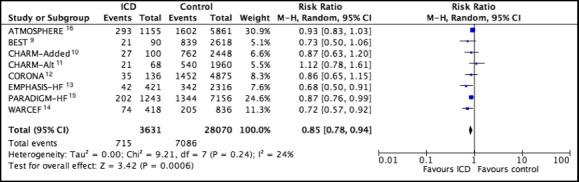
Pooled estimates were calculated by random effects. ATMOSPHERE indicates Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure; BEST, Beta‐Blocker Evaluation of Survival Trial; CHARM‐Added, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity ‐ Added Trial; CHARM‐Alt, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity ‐ Alternative Trial; CORONA, Controlled Rosuvastatin Multinational Trial in Heart Failure; EMPHASIS‐HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; ICD, implantable cardioverter–defibrillator; M–H, Mantel‐Haenszel methods; PARADIGM‐HF, Prospective Comparison of ARNI (Angiotensin Receptor‐Neprilysin Inhibitor) with ACEI (Angiotensin‐Converting Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial; and WARCEF, Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction.
Sudden Cardiac Death
In 6 studies there were 91 SCDs in 2386 patients (3.8%) with ICDs and 1855 SCDs in 19 591 patients (9.5%) without ICDs (random‐effects risk ratio, 0.49; 95% CI, 0.40–0.61; I2=0%) (Figure 3). SCDs per 100 patient/year was 1.74 and 3.79, respectively. Fixed‐effects risk ratio was 0.48 (95% CI, 0.39–0.60) (Figure S2).
Figure 3. Forest plots comparing patients with ICD vs without ICD for the outcome sudden cardiac death.
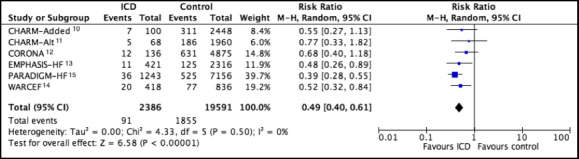
Pooled estimates were calculated by random effects. CHARM‐Added indicates Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity ‐ Added Trial; CHARM‐Alt, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity ‐ Alternative Trial; CORONA, Controlled Rosuvastatin Multinational Trial in Heart Failure; EMPHASIS‐HF, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; ICD, implantable cardioverter–defibrillator; M–H, Mantel‐Haenszel methods; PARADIGM‐HF, Prospective Comparison of ARNI (Angiotensin Receptor‐Neprilysin Inhibitor) with ACEI (Angiotensin‐Converting Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial; and WARCEF, Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction.
Sensitivity Analysis
The results were consistent among studies published before and after 2008, those that did or did not include patients with LVEF between 35% and 40%, and studies that did or did not report the use of CRT devices (Table 2).
Table 2.
Sensitivity Analysis for Studies Published Before and After 2010 and Studies That Did or Did Not Include Patients With LVEF Between 35% and 40%
| Risk Ratio and 95% CI | P Value for Interaction | |
|---|---|---|
| All‐cause death | ||
| Studies published before 2008 | 0.88 (0.75–1.04) | 0.57 |
| Studies published after 2008 | 0.83 (0.73–0.94) | ··· |
| Studies with EF 35% to 40% patients | 0.89 (0.79–0.99) | 0.27 |
| Studies with only EF <35% patients | 0.79 (0.65–0.94) | ··· |
| Studies reporting the use of CRT | 0.86 (0.76–0.98) | 0.70 |
| Studies not reporting the use of CRT | 0.83 (0.72–0.96) | ··· |
| Sudden cardiac death | ||
| Studies published before 2008 | 0.66 (0.45–0.97) | 0.09 |
| Studies published after 2008 | 0.44 (0.34–0.56) | ··· |
| Studies with LVEF 35% to 40% patients | 0.52 (0.38–0.73) | 0.89 |
| Studies without LVEF 35% to 40% patients | 0.51 (0.35–0.74) | ··· |
| Studies reporting the use of CRT | 0.41 (0.31–0.55) | 0.08 |
| Studies not reporting the use of CRT | 0.60 (0.44–0.81) | ··· |
CRT indicates cardiac resynchronization therapy; EF, Ejection Fraction; and LVEF, left ventricular ejection fraction.
The sensitivity analysis for all‐cause death after exclusion of the ATMOSPHERE study showed similar results (RR, 0.83; 95% CI, 0.75–0.91; I2=11%).
Meta‐Regression Analysis
Meta‐regression analyses investigating the potential effects of demographic and clinical characteristics on the all‐cause death associated with ICD compared to no ICD revealed significant effects of atrial fibrillation (P=0.013), left ventricular ejection fraction (P=0.040), and use of angiotensin‐converting‐enzyme inhibitors (ACEI) or antiotensin‐2 receptor blockers (ARB) (P=0.035) (Figure S3). There was no significant impact of age, sex, hypertension, diabetes mellitus, nonischemic etiology, New York Heart Association III to IV functional classes, use of beta‐blockers, or mineralocorticoid antagonists on the effect of ICDs on all cause‐death.
Risk of Bias
All of the included studies did not randomize ICD implantation. No publication bias was found using visual inspection of a funnel plot and an Egger test (P= 0.302) (Figure S4).
DISCUSSION
In this systematic review and meta‐analysis of contemporary clinical trials for drug therapy of HFrEF, the use of ICDs was scarce but associated with a significant reduction in all‐cause death and SCD when compared to no use.
Current guidelines supporting ICD implantation for primary prevention are backed up on long‐standing trials, namely, the MADIT II (Multicenter Automatic Defibrillator Implantation Trial II)20 and the SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial)3 that showed up to a 31% reduction in all‐cause mortality. However, HFrEF patients faced dramatic changes over time regarding epidemiology, etiology, and optimal medical therapy concept, including beta‐blockers, ACEI/ARB, mineralocorticoid antagonists, sacubitril‐valsartan, and even mechanical support concerning end‐stage heart failure. These medical advances culminated in the progressive reduction of all‐cause death and SCD,2 challenging the present additional benefit of ICDs.
Our study analyzed contemporary randomized clinical trials that were performed within a highly selected population where those with significant comorbidities, such as significant renal or hepatic diseases and any other condition that substantially reduces life expectancy, are often excluded and where compliance toward optimal medical therapy is assured in greater proportion than in a real‐word setting. This investigation showed a relative reduction both in all‐cause death and SCD by 15% and 50%, respectively, on top of the optimal conditions associated with the use of ICDs. These results are consistent with previous studies,3, 4, 20 even though a theoretic different HFrEF population sample.
The absence of significant heterogeneity among the studies analyzed emphasizes the association between ICD use and lower risk of all‐cause death. The RR of all‐cause death was not favorable to the use of ICD only in the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) Alternative study, which may be related to the small number of ICD patients, only 68, that may have generated a random distribution effect on events. In the SHIFT study, ICD use was also associated with 15% RR reduction in all‐cause death,19 which is superimposable to that observed in our meta‐analysis. The association with reduced risk of SCD revealed no heterogeneity among the 6 studies included in the analysis. The lower risk of SCD observed in ICD patients should be framed in a possible context of perceived higher risk of death in these patients. Despite the fact that PARADIGM HF (Prospective Comparison of ARNI [Angiotensin Receptor‐Neprilysin Inhibitor] with ACEI [Angiotensin‐Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial) and ATMOSPHERE have excluded patients with ventricular arrhythmias occurring 3 months prior to screening that were untreated, it is possible that ICD implantation has been performed for the secondary prevention of sudden death, and no study has excluded these patients.9, 10, 11, 12, 13, 14, 15, 16 The rates of recurrent ventricular arrhythmias and mortality remain high in patients who received an ICD for secondary prevention.21
The growing adherence of evidence‐based heart failure medication throughout the years should be highlighted. When comparing the older (BEST [Beta‐Blocker Evaluation of Survival Trial], CHARM‐Added, and CHARM‐Alternative)9, 10, 11 against the newer trials (PARADIGM‐HF and ATMOSPHERE),15, 16 both beta‐blockers and mineralocorticoid antagonists almost doubled their compliance rates. It is a different case scenario regarding ACEI/ARB adherence because it remained persistently high from early trials, with the exception of CHARM‐Alternative,11 where half of the population with intolerance to ACEI received an ARB, and ATMOSPHERE,16 where 2 of 3 patients were on ACEI, although all received a renin‐angiotensin‐aldosterone system inhibitor blockage (enalapril and/or aliskiren). Since 2008, the utilization rate of ACEI/ARB was >93%, beta‐blockers >86%, and mineralocorticoid antagonists >50%. However, sensitivity analysis did not reveal significant differences in all‐cause death or SCD in studies published either before9, 10, 11, 12 or after 2008.13, 14, 15, 16 These results reinforce the impact of ICD on preventing sudden death, even in patients with contemporary guideline optimal medical therapy. The results were also consistent in studies that included patients with LVEF between 35% and 40%. As the indication for ICD is based on the presence of LVEF <35%, the inclusion of patients with LVEF between 35% and 40% in the group without ICD and therefore with a lower risk of SCD could reduce its benefit in these studies.
The reproducibility of our results is shown in the recently published real‐word SwedeHF (Swedish Heart Failure) Registry that enrolled 1305 patients fulfilling European Society of Cardiology criteria for ICD implantation for primary prevention.22 After 1:1 propensity score matching to non‐ICD recipients, all‐cause mortality risk within 1 and 5 years reduced 27% and 12%, respectively. The results were consistent with all subgroup analysis, but data on SCD were not reported.
The ICD rate of use was 11.5%, which is low for a class I recommended treatment in patients with HFrEF.5 However, the use of this device therapy consistently increased >4‐fold between studies published before and after 2008. One study reported improvement in LVEF, limited life expectancy, New York Heart Association functional class IV, and patient refusal as the main causes for nonimplantation of ICDs.23 Patients with HFrEF followed in primary health care are less often referred for ICD implantation because of the poor perception of benefits against contraindications.22 The implementation of quality control and performance measure programs has been shown to increase adherence to the primary prevention of SCD with ICD.6 Only 3 studies, all published after 2008, reported the use of CRT, whose prevalence ranged from 5.6% to 8.5%.13, 15, 16 Although CRT amplifies the benefit of ICD in reducing mortality in patients with HFrEF,24 our sensitivity analysis with studies reporting the use of CRT showed no significant differences in all‐cause death or SCD compared to studies not reporting the use of CRT.
Meta‐regression analyses demonstrated that increasing the proportion of patients with atrial fibrillation attenuates the benefit of ICD in reducing death from any cause. In patients with HFrEF, atrial fibrillation has been described as a marker of disease severity,25 so its presence may lead to a higher risk of death from pump failure, with the consequent lower benefit of ICD in reducing mortality.26
Clinical trials with lower LVEF mean values showed a greater reduction in all‐cause death associated with ICD use, which is in line with the inverse relationship between LVEF and the risk of SCD described in the literature.27, 28
Widespread use of ACEI/ARB was associated with a lower risk of all‐cause death in patients with ICD compared to patients without ICD, highlighting the complementarity of pharmacological therapy with devices in patients with HFrEF. In a post hoc analysis of the COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure) trial, the authors reported that both ACEI and ARB lowered the risk of appropriate ICD therapy, thus demonstrating a synergistic effect between these therapies.29
An important issue regarding the effectiveness of ICDs is the relative uncertainty of their benefit in reducing all‐cause death in patients with nonischemic HFrEF.30 The WARCEF study was the only study that published results on the relative benefit of ICD use compared to nonuse on all‐cause death in patients with nonischemic etiology.18 The use of ICD was associated with no significant benefit on all‐cause death in those patients (HR, 0.984; 95% CI, 0.641–1.509; P=0.941), unlike that observed in patients with ischemic etiology (HR, 0.640; 95% CI, 0.448–0.915; P=0.015). Notwithstanding these facts, meta‐regression analysis showed no significant correlation between changes in the proportion of nonischemic etiology and the relative benefit on all‐cause death of ICD use compared to nonuse.
Limitations
This is a meta‐analysis of aggregate data from estimates of individual studies, and therefore individual participant data were not available. Although the studies have different designs, populations, pharmacological treatments, and follow‐up, any significant heterogeneity on the reported outcomes was not found, and sensitivity analyses were consistent with the main results. In the absence of randomization, it is possible that patients without ICD could have more comorbidity than patients with ICDs, leading to higher all‐cause death. However, it is well known that patients enrolled in clinical trials generally do not have many comorbidities, which is confirmed by the baseline characteristics of the population with and without ICD before propensity matching included in the WARCEF study.18 The clinical trials included in this systematic review and meta‐analysis did not report the type of ICD implanted or the incidence of appropriate and inappropriate shocks during the study follow‐up. Recognizing that ICD shocks have a negative impact on prognosis31 and the subcutaneous ICD is for selected patients,32 the inclusion of these variables in sensitivity analyses could bring new insights to the results. Finally, the use of CRT in ICD patients may have amplified the benefit of this device, although concomitant use was relatively low.
CONCLUSIONS
In our meta‐analysis of contemporary randomized trials of drug therapy for HFrEF, the rate of ICD use was low. The use of ICDs was associated with significant reductions in all‐cause death and SCD, and the results were consistent in all sensitivity analyses. Our results call for the need to adhere to the current guidelines for the management of HFrEF.
Sources of Funding
None.
Disclosures
Dr Cavaco has consulting fees under 2000 dollars/year from being an advisory board member of Boston Scientific. Dr Morgado has consulting fees under 1000 dollars/year from being proctor and speaker of Johnson & Johnson and Microport, respectively. The remaining authors have no disclosures to report.
Supporting information
Figures S1–S4
References 9–16
(J Am Heart Assoc. 2020;e015177 DOI: 10.1161/JAHA.119.015177.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. McMurray JJV. Systolic heart failure. N Engl J Med. 2010;362:228–238. [DOI] [PubMed] [Google Scholar]
- 2. Shen Li, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Køber L, et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 3. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 4. Shun‐Shin MJ, Zheng SL, Cole GD, Howard JP, Whinnett ZI, Francis DP. Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta‐analysis of 8567 patients in the 11 trials. Eur Heart J. 2017;38:1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 6. Shah B, Hernandez AF, Liang L, Al‐Khatib SM, Yancy CW, Fonarow GC, Peterson ED, Get With The Guidelines Steering Committee . Hospital variation and characteristics of implantable cardioverter defibrillator use in patients with heart failure: data from the GWTG‐HF (Get With The Guidelines‐Heart Failure) registry. J Am Coll Cardiol. 2009;53:416–422. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG and the PRISMA Group* . Preferred reporting items for systematic reviews and meta‐analyses. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 8. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 9. Beta‐Blocker Evaluation of Survival Trial Investigators , Eichhorn EJ, Domanski MJ, Krause‐Steinrauf H, Bristow MR, Lavori PW. A trial of the beta‐blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S. Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function taking angiotensin‐converting‐enzyme inhibitors: the CHARM‐Added trial. Lancet. 2003;362:767–771. [DOI] [PubMed] [Google Scholar]
- 11. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K. Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the CHARM‐Alternative trial. Lancet. 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 12. Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 13. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.21073363 [Google Scholar]
- 14. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 16. McMurray JJ, Krum H, Abraham WT, Dickstein K, Køber LV, Desai AS, Solomon SD, Greenlaw N, Ali MA, Chiang Y, et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016;374:1521–1532. [DOI] [PubMed] [Google Scholar]
- 17. Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, et al. Effect of the angiotensin‐receptor‐neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–1997. [DOI] [PubMed] [Google Scholar]
- 18. Lee TC, Qian M, Mu L, Di Tullio MR, Graham S, Mann DL, Nakanishi K, Teerlink JR, Lip GYH, Freudenberger RS, et al. Association between mortality and implantable cardioverter‐defibrillators by aetiology of heart failure: a propensity‐matched analysis of the WARCEF trial. ESC Heart Fail. 2019;6:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ford I, Robertson M, Komajda M, Böhm M, Borer JS, Tavazzi L, Swedberg K. Top ten risk factors for morbidity and mortality in patients with chronic systolic heart failure and elevated heart rate: the SHIFT Risk Model. Int J Cardiol. 2015;184:163–169. [DOI] [PubMed] [Google Scholar]
- 20. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 21. Sabbag A, Suleiman M, Laish‐Farkash A, Samania N, Kazatsker M, Goldenberg I, Glikson M, Beinart R. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real‐world setting: from the Israeli ICD Registry. Heart Rhythm. 2015;12:2426–2433. [DOI] [PubMed] [Google Scholar]
- 22. Schrage B, Uijl A, Benson L, Westermann D, Ståhlberg M, Stolfo D, Dahlström U, Linde C, Braunschweig F, Savarese G. Association between use of primary prevention implantable cardioverter‐defibrillators and mortality in patients with heart failure: a prospective propensity‐score matched analysis from the Swedish Heart Failure Registry. Circulation. 2019;140:1530–1539. [DOI] [PubMed] [Google Scholar]
- 23. LaPointe NM, Al‐Khatib SM, Piccini JP, Atwater BD, Honeycutt E, Thomas K, Shah BR, Zimmer LO, Sanders G, Peterson ED. Extent of and reasons for nonuse of implantable cardioverter defibrillator devices in clinical practice among eligible patients with left ventricular systolic dysfunction. Circ Cardiovasc Qual Outcomes. 2011;4:146–151. [DOI] [PubMed] [Google Scholar]
- 24. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, et al. Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med. 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 25. Verma A, Kalman JM, Callans DJ. Treatment of patients with atrial fibrillation and heart failure with reduced ejection fraction. Circulation. 2017;135:1547–1563. [DOI] [PubMed] [Google Scholar]
- 26. Mustafa U, Dherange P, Reddy R, DeVillier J, Chong J, Ihsan A, Jones R, Duddyala N, Reddy P, Dominic P. Atrial fibrillation is associated with higher overall mortality in patients with implantable. Cardioverter‐defibrillator: a systematic review and meta‐analysis. J Am Heart Assoc. 2018;7:e010156 DOI: 10.1161/JAHA.118.010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buxton AE. Should everyone with an ejection fraction less than or equal to 30% receive an implantable cardioverter‐defibrillator? Not everyone with an ejection fraction <30% should receive an implantable cardioverter‐defibrillator. Circulation. 2005;111:2537–2549. [DOI] [PubMed] [Google Scholar]
- 28. Di Tullio MR, Qian M, Thompson JL, Labovitz AJ, Mann DL, Sacco RL, Pullicino PM, Freudenberger RS, Teerlink JR, Graham S, et al. Left ventricular ejection fraction and risk of stroke and cardiac events in heart failure data from the Warfarin Versus Aspirin in Reduced Ejection Fraction Trial. Stroke. 2016;47:2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, Feldman AM, Galle E, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation. 2006;114:2766–2772. [DOI] [PubMed] [Google Scholar]
- 30. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 31. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA III, Greenberg H, Hall WJ, Huang DT, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 32. Chang PM, Doshi R, Saxon LA. Subcutaneous implantable cardioverter defibrillator. Circulation. 2014;129:e644–e646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S4
References 9–16


