Abstract
Background
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) are used to reduce low‐density lipoprotein (LDL) cholesterol. PCSK9i use after initiation, as well as persistence with or alterations to other LDL‐lowering therapy after PCSK9i initiation, is not well understood.
Methods and Results
We conducted a retrospective study of alirocumab or evolocumab (PCSK9i) new users from July 2015 to December 2017 in the MarketScan Early View database of US commercial insurance beneficiaries. We determined the prevalence of PCSK9i interruption (≥30‐day gap in supply) and LDL‐lowering therapy use in the year after PCSK9i initiation. The average age of 6151 patients initiating PCSK9i therapy was 63 years, 44.4% were women, and 76.8% had atherosclerotic cardiovascular disease. Overall, 52.2% (95% CI, 50.8%–53.7%) of patients had an interruption in PCSK9i therapy in the first year after treatment initiation and 62.5% remained on PCSK9i therapy at 1‐year postinitiation. Also, 27.7% of patients were taking a statin at the time of PCSK9i initiation, with only 22.4% on statin therapy at 1 year after PCSK9i initiation. Ezetimibe use decreased from 20.9% at the time of PCSK9i initiation to 12.0% a year later. By 1 year after PCSK9i initiation, 44.0% of patients had experienced an interruption in all LDL‐lowering therapies, and 26.6% were no longer on any LDL‐lowering therapies.
Conclusions
After PCSK9i initiation, statins were often discontinued, whereas more than half of patients experienced an interruption in PCSK9i therapy. These results suggest that many new PCSK9i users may remain at high risk for cardiovascular events because of interruptions in LDL‐lowering therapy.
Keywords: lipid lowering, proprotein convertase subtilisin/kexin type 9 inhibitors, statin, treatment interruption
Subject Categories: Quality and Outcomes, Health Services
Nonstandard Abbreviations and Acronyms
- ASCVD
atherosclerotic cardiovascular disease
- LDL
low‐density lipoprotein
- PCSK9i
proprotein convertase subtilisin/kexin type 9 inhibitors
Clinical Perspective
What Is New?
Many patients will discontinue statin or other lipid‐lowering therapy after initiating a proprotein convertase subtilisin/kexin type 9 inhibitor.
More than half of patients will experience an interruption in proprotein convertase subtilisin/kexin type 9 inhibitor therapy within 1 year of initiation.
What Are the Clinical Implications?
Many new users of proprotein convertase subtilisin/kexin type 9 inhibitor therapy may remain at high risk for atherosclerotic events because of statin discontinuation and/or interruptions in proprotein convertase subtilisin/kexin type 9 inhibitor therapy.
Introduction
The US Food and Drug Administration approved proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) therapies in 2015 for additional low‐density lipoprotein (LDL) lowering among adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease (ASCVD) who are on maximally tolerated statin therapy.1 Randomized controlled trials have demonstrated the efficacy of 2 PCSK9i therapies, evolocumab and alirocumab, in lowering LDL cholesterol and reducing rates of cardiovascular events in patients with atherosclerotic disease.2, 3, 4, 5, 6 Given their Food and Drug Administration–approved indications, patients initiating PCSK9i may have high LDL and a high risk for CVD events.
Treatment interruptions are common among users of statins and other long‐term therapies7, 8 and may be even more frequent among patients initiating a PCSK9i because of the potential burden of prior authorization paperwork to obtain refills9, 10 and the high cost of treatment.10, 11, 12, 13, 14 Indeed, poor persistence and adherence to statin therapy has been previously documented in various patient populations.15, 16, 17 As PCSK9i are potent LDL‐lowering agents, patients initiating PCSK9i may discontinue or down titrate other LDL‐lowering therapies. These patients may not be taking any LDL‐lowering therapy if they experience an interruption in PCSK9i. The goals of the current research were to (1) examine the prevalence of and factors associated with PCSK9i interruption after treatment initiation; (2) describe patterns of other LDL‐lowering therapy use before and after initiating a PCSK9i; and (3) assess the risk of interruption of all LDL‐lowering therapies among patients newly initiated on a PCSK9i.
Methods
We conducted a retrospective cohort study using prescription and medical claims from the IBM Truven Health MarketScan Early View database. The MarketScan database contains data from various employer‐sponsored healthcare insurance plans in the United States and Medicare supplemental plans. We restricted the analysis to patients initiating a PCSK9i, identified using National Drug Codes, from July 2015 through December 2017. The date of the first fill of a PCSK9i for each patient was defined as the index date. To examine LDL‐lowering therapy use before initiation of a PCSK9i, patients were required to have at least 365 days of continuous enrollment in their healthcare insurance plan before their index date. To ascertain PCSK9i interruption after treatment initiation, patients were required to have at least 90 days of continuous enrollment following their index date. Patients were followed up until they lost insurance coverage in the Early View database or December 31, 2017, whichever occurred first. Institutional review board approval was not necessary. The authors cannot make data and study materials available to other investigators for purposes of reproducing the results because of licensing restrictions. Interested parties, however, could obtain and license the data by contacting Truven Health Analytics Inc and Optum.
Claims data were available from January 1, 2013, through December 31, 2017. Comorbidities, including prior myocardial infarction, unstable angina, prior percutaneous coronary intervention or coronary artery bypass grafting, ischemic stroke, transient ischemic attack, cerebrovascular disease, peripheral artery disease, prior heart failure, diabetes mellitus, chronic kidney disease, and hypertension, were defined with International Classification of Diseases, Ninth Revision (ICD‐9), and International Classification of Diseases, Tenth Revision (ICD‐10), diagnosis codes and procedure codes. At least 1 inpatient, 2 outpatient diagnosis codes at least 30 days apart, or at least 1 procedure code (when applicable) was required to meet each comorbidity definition.
Medications were identified using National Drug Codes, and their use at the time of PCSK9i initiation was defined as having a days’ supply within the 30‐day period preceding the index date. Medication use before initiating a PCSK9i was defined as having at least 1 fill within the 31 to 365 days before the index PCSK9i fill date. We defined LDL‐lowering therapy interruption as a gap in supply (fill) of at least 30 days. The date of treatment interruption was set as the first day without medication available to take (Figure S1). We defined PCSK9i resumption as at least 1 fill of any PCSK9i medication following treatment interruption. Switching from one to the other PCSK9i (eg, alirocumab to evolocumab) was identified on the basis of the first claim for a different PCSK9i than the one filled on initiation, regardless of the duration between fills.
We defined the occurrence of statin intolerance via a claims‐based algorithm meeting any one of following criteria within 2 years following their first statin fill and before their PCSK9i initiation date: statin interruption or down titration followed by ezetimibe initiation; evidence of statin‐induced muscle events or adverse effect of any antihyperlipidemic agent followed by statin interruption or statin down titration; or a fill for at least ≥3 different statin types within the 2‐year evaluation period.18
Demographic and clinical characteristics of patients initiating PCSK9i were calculated as number and percentage for each categorical variable and mean and SD for continuous variables. In addition, the percentage of patients taking LDL‐lowering therapies, including statins and ezetimibe, in the year before PCSK9i initiation was calculated. We then examined the time to first interruption of a PCSK9i, the time to switching between the 2 PCSK9i (evolocumab and alirocumab), and the time to first reinitiation after interruption of a PCSK9i. The cumulative incidence was computed for each of these outcomes with loss to follow‐up as a competing risk. In addition, Cox proportional hazards regression models were used to determine demographic and clinical characteristics associated with these outcomes. For each outcome, the following characteristics were included in a single multivariable‐adjusted model: sex, age, census region, ASCVD, cerebrovascular disease, peripheral artery disease, multibed disease, diabetes mellitus, heart failure, chronic kidney disease, ezetimibe use as of PCSK9i initiation, no statin or ezetimibe use at PCSK9i initiation, ≥3 statin drugs in 12 months before PCSK9i initiation, discontinued statin before PCSK9i initiation, and number of hospitalizations in prior 12 months. Loss to follow‐up was censored in the Cox regression analyses.
A Sankey diagram was used to depict the dynamics of LDL‐lowering therapy use, including PCSK9i, at 3, 6, 9, and 12 months following PCSK9i initiation and the changes in LDL‐lowering therapies between these time points. The percentages of patients filling a certain LDL‐lowering therapy regimen is listed at each time point. The state transition probabilities in the Sankey diagram were estimated via inverse probability of censoring weighted estimation. The probability of censoring was estimated at each time point, and treatment state and patients transitioning out of each treatment state were up weighted by the inverse of the probability that they would be uncensored. Finally, we estimated the risk of a first interruption of all LDL‐lowering therapies using Cox proportional hazards regression. We reported demographic and clinical characteristics associated with this outcome, from the variables listed above.
All analyses were completed, and the Sankey diagram was programmed using R version 3.5.2.
Results
Patient Characteristics at PCSK9i Initiation
Between July 2015 and December 2017, a total of 6151 patients initiated a PCSK9i. After PCSK9i initiation, these patients had a mean (SD) follow‐up of 390 (195) days. The mean age of patients initiating a PCSK9i was 63 years, 44.4% were women, and most (76.8%) of patients had ASCVD, including 22.5% with evidence of a prior myocardial infarction, 13.9% with unstable angina, 12.8% with prior percutaneous coronary intervention or coronary artery bypass grafting, 12.3% with ischemic stroke, 4.0% with transient ischemic attack, and 14.8% with peripheral artery disease (Table). More than one third of patients initiating a PCSK9i had diabetes mellitus (36.9%) or chronic kidney disease (38.0%). Also, 30.7% of patients initiating a PCSK9i were taking an antiplatelet agent, and 49.9% were taking antihypertensive medications. On average, patients were taking a mean of 3.3 other medications concurrently at the time of PCSK9i initiation.
Table 1.
Demographic and Clinical Characteristics of Patients Initiating a PCSK9i
| Variable | Patients Initiating PCSK9i (N=6151), Mean (SD) or No. (%) |
|---|---|
| Demographics | |
| Age, ya | 63.0 (10.2) |
| Women | 2731 (44.4) |
| Geographic region | |
| Midwest | 1221 (19.9) |
| Northeast | 1353 (22.0) |
| South | 3068 (49.9) |
| West | 501 (8.1) |
| Missing | 8 (0.1) |
| Insurance type | |
| Medicare (supplemental) | 2423 (39.4) |
| Commercial | 3728 (60.6) |
| Comorbidities | |
| Atherosclerotic CVDb | 4724 (76.8) |
| Prior MI | 1382 (22.5) |
| Unstable angina | 857 (13.9) |
| Prior PCI or CABG | 787 (12.8) |
| Ischemic stroke | 759 (12.3) |
| TIA | 247 (4.0) |
| Cerebrovascular disease | 839 (13.6) |
| PAD | 908 (14.8) |
| Prior HF | 824 (13.4) |
| Diabetes mellitus | 2267 (36.9) |
| Chronic kidney disease | 2335 (38.0) |
| Hypertension | 4957 (80.6) |
| Current medications | |
| β Blockers | 3170 (51.5) |
| Anticoagulants | 331 (5.4) |
| Antiplatelet agent (not including aspirin alone) | 1886 (30.7) |
| Antihypertensives | 3072 (49.9) |
| Insulin | 520 (8.5) |
| Antidepressants | 1415 (20.3) |
| No. of concurrent medicationsa | 3.3 (2.5) |
CABG indicates coronary artery bypass grafting; CVD, cardiovascular disease; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; and TIA, transient ischemic attack.
All numbers in table are number (percentage), except age, which is mean (SD).
Composite of MI, unstable angina, ischemic stroke, PAD, TIA, or CABG/PCI.
During the 31 to 365 days before PCSK9i initiation, 46.6% of patients had filled at least 1 lipid‐lowering prescription, including 29.3% who filled a statin (12.8% high‐intensity statin), 8.5% who filled ezetimibe, and 10.1% who filled other medications, including bile acid sequestrant, niacin, fibrates, and prescription‐strength omega‐3 fatty acids (Figure 1). At the time of PCSK9i initiation, 27.7% of patients were taking a statin and 20.9% of patients were taking ezetimibe; 61.3% were not taking any additional LDL‐lowering therapy (Figure 2). Overall, 13.6% of patients met our definition of experiencing a statin‐associated adverse event before initiating a PCSK9i, including 9.2% who initiated ezetimibe after discontinuing or down titrating statin therapy, 4.3% who used ≥3 statin drug types over the past 24 months, 0.1% who had a muscle event, and 0.1% with an adverse effect related to any LDL‐lowering therapy followed by statin down titration or interruption.
Figure 1. Use of lipid‐lowering medication within 31 to 365 days before initiation of a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i).
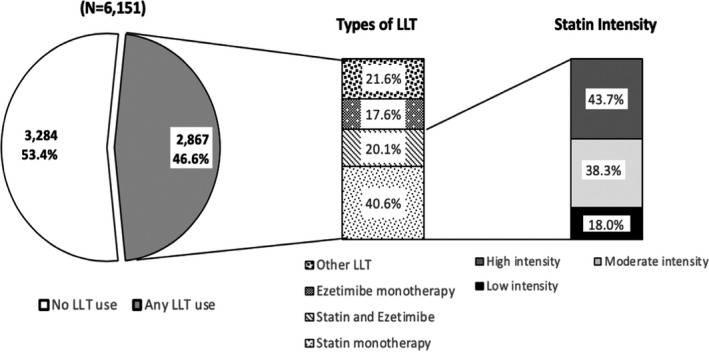
This figure depicts which lipid‐lowering medications were used in the 31 to 365 days before initiation of a PCSK9i, including the statin intensity. High intensity statins included atorvastatin, ≥40 mg/d, rosuvastatin, ≥20 mg/d, and simvastatin, 80 mg/d. Moderate‐intensity statins included pitavastatin, ≥2 mg, rosuvastatin, 10 mg, atorvastatin, 10 or 20 mg, pravastatin, ≥40 mg, fluvastatin, >80 mg, simvastatin, 20 to 40 mg, and lovastatin, ≥40 mg. Low‐intensity statins included pitavastatin, 1 mg, rosuvastatin, 5 mg, pravastatin, ≤40 mg, fluvastatin, ≤80 mg, simvastatin, ≤20 mg, and lovastatin, ≤40 mg. LLTT indicates lipid‐lowering therapy.
Figure 2. Sankey diagram of lipid‐lowering treatment transitions in the year after proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) initiation.
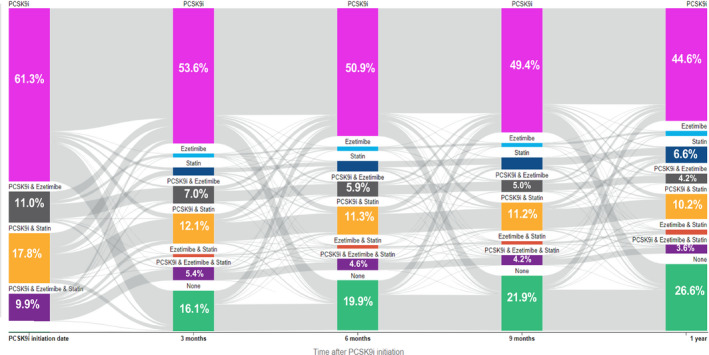
This figure illustrates the Sankey diagram of lipid‐lowering therapy in the year after PCSK9i initiation. The flow of patients occurs from left to right with calendar time. The width of the gray areas connecting the bars is directly proportional to the number of patients transitioning from one low‐density lipoprotein (LDL)–lowering therapy regimen to another LDL‐lowering therapy regimen 3 months later. The percentages of patients filling a certain LDL‐lowering therapy regimen are listed at each time point for each LDL‐lowering therapy category.
PCSK9i Interruption, Resumption After Interruption, and Switch
Within 1 year after PCSK9i initiation, 52.2% of patients (95% CI, 50.8%–53.7%) had a treatment interruption of at least 30 days. Interruption of PCSK9i therapy occurred at a mean (SD) of 155 (135.5) days after initiation. In a multivariable model, older age and residence in the South and West census regions of the United States versus the Northeast census region were associated with higher likelihood of PCSK9i interruption, whereas patients on ezetimibe at the time of PCSK9i initiation were associated with lower likelihood of PCSK9i interruption (Figure 3).
Figure 3. Factors associated with the risk of proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) therapy interruption.
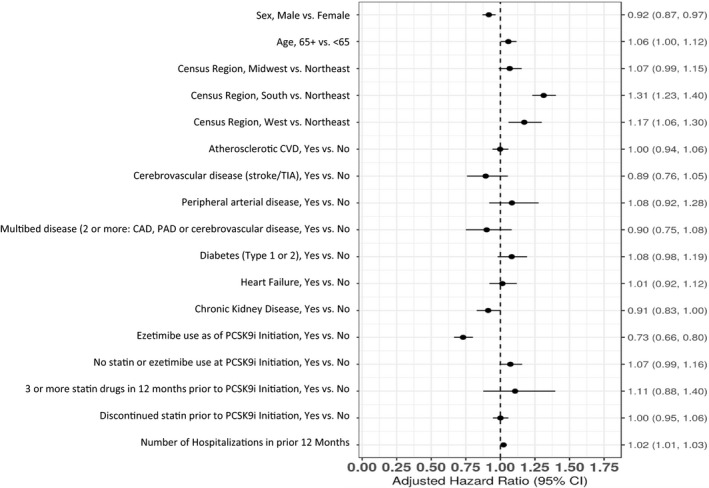
In this figure, older age and residence in the South and West census regions of the United States vs the Northeast census region were associated with higher likelihood of PCSK9i interruption, whereas patients on ezetimibe at the time of PCSK9i initiation were associated with lower likelihood of PCSK9i interruption. CAD indicates coronary artery disease; CVD, cardiovascular disease; PAD, peripheral artery disease; and TIA, transient ischemic attack.
Overall, 27.2% (95% CI, 25.4%–28.9%) of patients resumed treatment within 6 months after PCSK9i interruption, and 50.4% (95% CI, 48.1%–52.7%) resumed treatment within 1 year after interruption. In the multivariable model, residence in the South versus Northeast census region was the only factor associated with a lower likelihood of treatment resumption, and no factors were associated with a higher likelihood of treatment resumption (Figure S2). Switches between PCSK9i medications (evolocumab to alirocumab or alirocumab to evolocumab) occurred in 3.9% (95% CI, 3.4%–4.5%) of patients.
Use of LDL‐Lowering Therapies After PCSK9i Initiation
At 1 year following initiation, 62.5% of patients were on a PCSK9i. As shown in the Sankey diagram (Figure 2), 26.6% of patients were not on any LDL‐lowering therapy at 1 year after PCSK9i initiation. The proportion of statin‐treated patients decreased over time, from 27.7% at the time of PCSK9i initiation to 22.4% at 1 year after PCSK9i initiation. Also, 3.5% of patients taking a statin had down titrated their statin dose within a year after initiating a PCSK9i. Ezetimibe use decreased from 20.9% at the time of PCSK9i initiation to 12.0% 1 year later.
During the year following PCSK9i initiation, 43.8% (95% CI, 42.4%–45.2%) of patients experienced an interruption in all LDL‐lowering therapies. Patients aged ≥65 years living in the South versus Northeast census region and patients not taking a statin or ezetimibe at the time of PCSK9i initiation were more likely to have an interruption in all LDL‐lowering therapies in the year following initiation (Figure 4). Patients taking ezetimibe at the time of PCSK9i initiation were less likely to have an interruption in all LDL‐lowering therapies.
Figure 4. Factors associated with the risk of low‐density lipoprotein (LDL)–lowering therapy interruption.
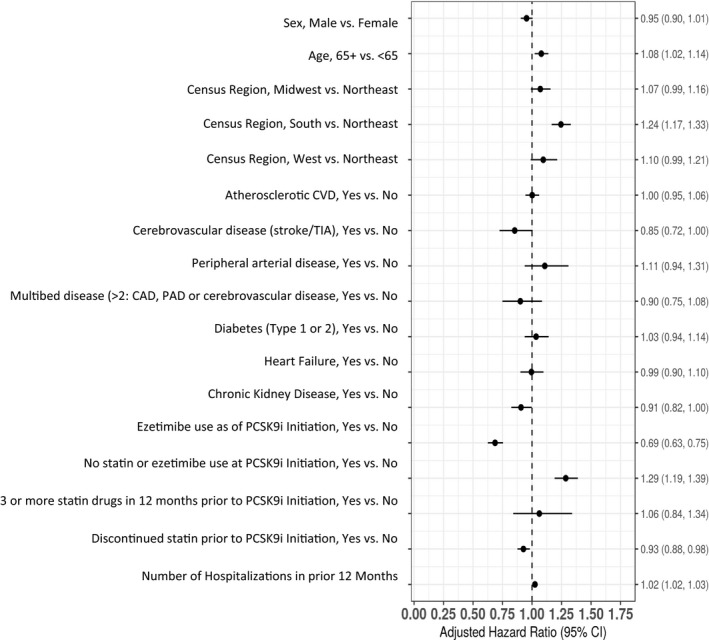
In this figure, patients aged ≥65 years living in the South vs Northeast census region and patients not taking a statin or ezetimibe at the time of proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) initiation were more likely to have an interruption in all LDL‐lowering therapies in the year following initiation. CAD indicates coronary artery disease; CVD, cardiovascular disease; PAD, peripheral artery disease; and TIA, transient ischemic attack.
Discussion
In the current analysis, more than half of patients initiated on PCSK9i experienced an interruption in PCSK9i treatment and only 63% of patients remained on a PCSK9i 1 year following initiation. The proportion of patients taking a statin or ezetimibe decreased after PCSK9i initiation. By 1 year after PCSK9i initiation, 44% of patients had experienced an interruption in all LDL‐lowering therapies, and 27% of patients were no longer on any LDL‐lowering therapies 1 year following treatment initiation. Patients aged >65 years, living in the South census region of the United States, and not taking LDL‐lowering therapy at PCSK9i initiation were more likely to experience interruption of all LDL‐lowering therapies at some point in the year after PCSK9i initiation. The current findings raise concern that many new PCSK9i users are undertreated and are therefore at higher risk of adverse cardiovascular events.
Most patients initiated on a PCSK9i had ASCVD. In the year before initiating a PCSK9i, a large proportion of patients had filled at least 1 prescription for a lipid‐lowering treatment, yet only 28% were on a statin at the time of PCSK9i initiation and 13% were treated with a high‐intensity statin in the past year. These results suggest an at‐risk patient population with ASCVD who may be initiating PCSK9i because of inability to tolerate statins or reach a guideline‐recommended statin dose. A recent analysis of physician‐reported treatment patterns in early users of PCSK9i therapies reported that 31.6% of patients on a PCSK9i were taking a statin and that patients prescribed a PCSK9i more frequently had ASCVD than patients treated with other lipid‐lowering therapies.6 These data suggest early adopters of PCSK9i therapy are likely patients at high cardiovascular risk for whom consistent and intense LDL lowering is critical.
At 1 year after PCSK9i initiation, only 22% of patients were taking statin therapy. Ezetimibe use rates were also lower 1 year after PCSK9i initiation. Patients may choose to discontinue these LDL‐lowering therapies because of the perception of adequate lipid lowering and ASCVD risk reduction with a PCSK9i, difficulties adhering to a complex medication regimen, or belief that they are taking too many medications. In the current study, of which >75% had a history of ASCVD, patients were on an average of 3 other medications in addition to the PCSK9i, likely making medication persistence more challenging.
A key finding of our study was the high rate of PCSK9i therapy interruption within the first year after initiation. Approximately half of all PCSK9i new users experienced an interruption in treatment, with a low proportion resuming treatment. There are likely many explanations for PCSK9i treatment interruption. High out‐of‐pocket costs have limited initial PCSK9i prescription for many patients in the early days after Food and Drug Administration approval.14, 19, 20 A prior study showed that 3 of 4 patients with a copay exceeding $375 abandoned a prescription for a PCSK9i therapy at the pharmacy, and the authors noted that the pharmacy abandonment rate was almost completely accounted for by the copay.10 Financial burden likely contributes as well to PCSK9i interruptions beyond the initial fill. Our study showed that patients on ezetimibe (nongeneric during the study period) at PCSK9i initiation were less likely to interrupt treatment, whereas those residing in the South and West were more likely to interrupt therapy. Use of ezetimibe may be associated with greater ability to afford medications with higher copays. Geographic variation in the prescribing of and adherence to high‐intensity statin therapy has been previously demonstrated.21, 22 Geographic variation in practice patterns and patient beliefs and regional variation in insurance plans and their corresponding reauthorization criteria have been cited as explanations for this variation in prescribing practices and adherence. Prior studies estimate that 50% of patients are nonadherent to statin therapy within 1 year after initiation,15, 23, 24 without differences in adherence between patients prescribed generic versus brand statins.16 This suggests that although cost is certainly an issue, clinicians need to be vigilant for other triggers of lipid‐lowering therapy discontinuation. In the current study, patients aged ≥65 years were more likely to experience a PCSK9i interruption and interruption of all LDL‐lowering therapies. Poor long‐term persistence to statin therapy has also been previously demonstrated in older adults.17 Older patients may experience interruptions in PCSK9i therapy because of a variety of factors, including a high medication burden, fixed incomes, and cognitive and physical declines that limit patients’ ability to self‐administer biweekly injections.
With high rates of PCSK9i interruption as well as discontinuation of statins or ezetimibe after PCSK9i initiation, 27% of patients were on no LDL‐lowering therapy by 1 year after PCSK9i initiation, and 44% of patients experienced interruptions of all LDL‐lowering therapies for at least 30 days. These results caution clinicians against complacency after we have successfully initiated the patient on a PCSK9i. As mentioned previously, most new PCSK9i users had prior ASCVD and it is critical that these patients do not go completely untreated when the PCSK9i is interrupted or discontinued. The 2018 American Heart Association/American College of Cardiology Guidelines on the Management of Blood Cholesterol recommend that patients taking PCSK9i therapy should be on concomitant statin and ezetimibe therapy, where possible.25 The association of statin nonadherence with worse clinical outcomes, including death and recurrent cardiovascular events, has been demonstrated.26 Clinicians need to closely monitor and educate patients about the importance of continuing LDL‐lowering medications. This discussion should include the rationale for reducing blood cholesterol, the independent benefits of statin therapy beyond LDL lowering, and the need to maintain long‐term secondary prevention, which may require combination therapy of multiple LDL‐lowering medications.
There are several important limitations of the current study. The data are limited to pharmacy fills; whether patients took all the medications they filled could not be evaluated. Interruptions in fills may represent intentional discontinuation decisions made by the clinician or the patient or lack of fill for other reasons. We did not have 1‐year follow‐up on all patients after PCSK9i initiation. However, we used the inverse probability of censoring weights to account for this in the Sankey diagrams. The Sankey diagram only allows us to determine the number of patients on an LDL‐lowering therapy at each time point. In addition, the prescription claims data do not capture medication fills paid for in cash or samples of drugs given to patients. Therefore, the current analysis may underestimate LDL‐lowering therapy use. However, a previous analysis has demonstrated that few statin users are missed because of prescriptions being paid for in cash.27 In addition, the statin intolerance definition defined in the Methods section may not fully capture all patients who had adverse events to statins; indeed, we are not able to determine if statin down titration or interruption with PCSK9i therapy could represent a statin intolerant patient. Although we used an accepted algorithm for statin intolerance, multiple statin fills could be attributable to changing to a more potent or less potent statin therapy. Furthermore, we could not ascertain the reasons why a PSCK9i or other LDL‐lowering therapy was interrupted and the impact of PCSK9i or LDL‐lowering therapy on clinical outcomes. Finally, the costs of PCSK9i have declined more recently; our study extended through 2017, when PCSK9i therapy was more cost prohibitive.
Conclusions
In the current study, more than half of patients initiated on a PCSK9i experienced an interruption in therapy within the first year after initiation. Also, after PCSK9i initiation, many patients discontinue or down titrate other LDL‐lowering therapies, such as statins or ezetimibe. These findings raise concern that many new PCSK9i users may remain at high risk of cardiovascular events because of interruptions or discontinuations in LDL‐lowering therapy.
Sources of Funding
The design and conduct of the study, analysis, interpretation of the data, and preparation of the article were supported through research support from Amgen Inc.
Disclosures
Dr Rymer was supported during the conduct of this study by an American College of Cardiology grant (William F. Keating award) and has received research grants from Boston Scientific and Abbott Vascular. Dr Mues is an employee and stockholder of Amgen, Inc. Dr Monda is an employee and stockholder of Amgen, Inc. Dr Bratton is an employee of IQVIA. Dr Wirtz is an employee and stockholder of Amgen, Inc, and a stockholder of Teva Pharmaceuticals. Dr Okerson was an employee and stockholder of Amgen, Inc. Dr Brookhart has served as a scientific advisor to AbbVie, Amgen, Inc, The Brigham and Women's Hospital, Fibrogen, Genentech, Gilead, Merck, RxAnte, TargetPharma, and World Health Information Consultants; and he holds equity in NoviSci, Inc. Dr Muntner receives research grant support from Amgen, Inc. Dr Wang reports research grant support from AstraZeneca, Boston Scientific, CryoLife, Daiichi Sankyo, Eli Lilly, Gilead, Novartis, and Regeneron; educational support from AstraZeneca, Bristol Myers Squibb, Gilead, and Merck; and consulting from Pfizer and Sanofi‐Aventis. Dr Overman has no disclosures to report.
Supporting information
Figures S1–S2
(J Am Heart Assoc. 2020;9:e014347 DOI: 10.1161/JAHA.119.014347.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. FDA Approval of Evolocumab . Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf. Accessed April 10, 2020.
- 2. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 4. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 5. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 7. Brookhart MA, Patrick AR, Schneeweiss S, Avorn J, Dormuth C, Shrank W, van Wijk BL, Cadarette SM, Canning CF, Solomon DH. Physician follow‐up and provider continuity are associated with long‐term medication adherence: a study of the dynamics of statin use. Arch Intern Med. 2007;167:847–852. [DOI] [PubMed] [Google Scholar]
- 8. Brookhart MA, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Patrick AR, Mogun H, Solomon DH. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120:251–256. [DOI] [PubMed] [Google Scholar]
- 9. Doshi JA, Puckett JT, Parmacek MS, Rader DJ. Prior authorization requirements for proprotein convertase subtilisin/kexin type 9 inhibitors across US private and public payers. Circ Cardiovasc Qual Outcomes. 2018;11:e003939. [DOI] [PubMed] [Google Scholar]
- 10. Navar AM, Taylor B, Mulder H, Fievitz E, Monda KL, Fievitz A, Maya JF, Lopez JAG, Peterson ED. Association of prior authorization and out‐of‐pocket costs with patient access to PCSK9 inhibitor therapy. JAMA Cardiol. 2017;2:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arrieta A, Page TF, Veledar E, Nasir K. Economic evaluation of PCSK9 inhibitors in reducing cardiovascular risk from health system and private payer perspectives. PLoS One. 2017;12:e0169761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arrieta A, Hong JC, Khera R, Virani SS, Krumholz HM, Nasir K. Updated cost‐effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER Trial. JAMA Cardiol. 2017;2:1369–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hlatky MA, Kazi DS. PCSK9 inhibitors: economics and policy. J Am Coll Cardiol. 2017;70:2677–2687. [DOI] [PubMed] [Google Scholar]
- 14. Mastey V, Johnstone BM. Cost‐effectiveness of PCSK9 inhibitor therapy. JAMA. 2016;316:2151–2152. [DOI] [PubMed] [Google Scholar]
- 15. Colantonio LD, Huang L, Monda KL, Bittner V, Serban MC, Taylor B, Brown TM, Glasser SP, Muntner P, Rosenson RS. Adherence to high‐intensity statins following a myocardial infarction hospitalization among Medicare beneficiaries. JAMA Cardiol. 2017;2:890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien EC, McCoy LA, Thomas L, Peterson ED, Wang TY. Patient adherence to generic versus brand statin therapy after acute myocardial infarction: insights from the Can Rapid Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines Registry. Am Heart J. 2015;170:55–61. [DOI] [PubMed] [Google Scholar]
- 17. Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long‐term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. [DOI] [PubMed] [Google Scholar]
- 18. Colantonio LD, Kent ST, Huang L, Chen L, Monda KL, Serban MC, Manthripragada A, Kilgore ML, Rosenson RS, Muntner P. Algorithms to identify statin intolerance in Medicare administrative claim data. Cardiovasc Drugs Ther. 2016;30:525–533. [DOI] [PubMed] [Google Scholar]
- 19. Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, van Hout B. Cost‐effectiveness of LDL‐C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol. 2016;39:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tice JA, Ollendorf DA, Cunningham C, Pearson SD, Kazi DS, Coxson PG, Moran AE, Penko J, Guzman D, Bibbins‐Domingo K. PCSK9 inhibitors for treatment of high cholesterol: effectiveness, value, and value‐based price benchmarks. Institute for Clinical and Economic Review. https://icer-review.org/wp-content/uploads/2016/01/Final-Report-for-Posting-11-24-15-1.pdf. Accessed September 29, 2018.
- 21. Brooks JM, Cook EA, Chapman CG, Kulchaitanaroaj P, Chrischilles EA, Welch S, Robinson J. Geographic variation in statin use for complex acute myocardial infarction patients: evidence of effective care? Med Care. 2014;52:S37–S44. [DOI] [PubMed] [Google Scholar]
- 22. Erickson SR, Lin YN. Geospatial analysis of statin adherence using pharmacy claims data in the state of Michigan. J Manag Care Spec Pharm. 2014;20:1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long‐term statin therapy? Curr Atheroscler Rep. 2013;15:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathews R, Wang TY, Honeycutt E, Henry TD, Zettler M, Chang M, Fonarow GC, Peterson ED. Persistence with secondary prevention medications after acute myocardial infarction: insights from the TRANSLATE‐ACS study. Am Heart J. 2015;170:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. DOI: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colantonio LD, Kent ST, Kilgore ML, Delzell E, Curtis JR, Howard G, Safford MM, Muntner P. Agreement between Medicare pharmacy claims, self‐report, and medication inventory for assessing lipid‐lowering medication use. Pharmacoepidemiol Drug Saf. 2016;25:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


