Abstract
Over the past decade, the field of valvular heart disease (VHD) has rapidly transformed, largely as a result of the development and improvement of less invasive transcatheter approaches to valve repair or replacement. This transformation has been supported by numerous well‐designed randomized trials, but they have centered almost entirely on devices and procedures. Outside this scope of focus, however, myriad aspects of therapy and management for patients with VHD have either no guidelines or recommendations based only on expert opinion and observational studies. Further, research in VHD has often failed to engage patients to inform study design and identify research questions of greatest importance and relevance from a patient perspective. Accordingly, the National Heart, Lung, and Blood Institute convened a Working Group on Patient‐Centered Research in Valvular Heart Disease, composed of clinician and research experts and patient advocacy experts to identify gaps and barriers to research in VHD and identify research priorities. While recognizing that important research remains to be done to test the safety and efficacy of devices and procedures to treat VHD, we intentionally focused less attention on these areas of research as they are more commonly pursued and supported by industry. Herein, we present the patient‐centered research gaps, barriers, and priorities in VHD and organized our report according to the “patient journey,” including access to care, screening and diagnosis, preprocedure therapy and management, decision making when a procedure is contemplated (clinician and patient perspectives), and postprocedure therapy and management. It is hoped that this report will foster collaboration among diverse stakeholders and highlight for funding bodies the pressing patient‐centered research gaps, opportunities, and priorities in VHD in order to produce impactful patient‐centered research that will inform and improve patient‐centered policy and care.
Keywords: aortic valve, heart valve, heart valve surgery, mitral valve, patient‐centered care, shared decision making, transcatheter valve implantation
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Pharmacology, Heart Failure, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms.
AS aortic stenosis
BP blood pressure
HF heart failure
RCT randomized clinical trial
SDM shared decision making
TAVR transcatheter aortic valve replacement
VHD valvular heart disease
Introduction
There has been an explosion in valvular heart disease (VHD) research over the past few decades with a shift in the evidence base from expert opinion alone, with virtually no randomized clinical trials (RCTs), to numerous RCTs addressing the safety and efficacy of devices to relieve stenosis or reduce regurgitation.1, 2, 3 However, many guideline recommendations for VHD are still only supported by expert opinion and observational studies. Further, as is true of many areas of cardiovascular research, studies of patients with VHD are driven primarily by clinicians and often fail to answer the questions of most importance to patients.
Patient‐centered research is characterized both by its orientation and the process by which that research is formulated and executed. While acknowledging that basic science research involving cells and animals is relevant to patients, as the long‐term goal of those avenues of investigation is often to prevent or slow VHD progression, patient‐centered research involves and studies patients either prospectively or retrospectively. Every bit as important, though, patient‐centered research ought to involve patients at each stage of the research process, from identifying research questions to prioritizing outcome measures to implementation into clinical practice. Although researchers and patients will agree on many questions and outcomes, patients often identify other issues that may not have been considered.
Rationale and Working Group Goals
The National Heart, Lung, and Blood Institute convened a Working Group on Patient‐Centered Research in Valvular Heart Disease in July 2019 to identify gaps in patient‐centered VHD research, develop a list of important patient‐centered research questions, and consider any barriers that discourage investigators from pursuing these questions. Predictably, there are areas of overlap and distinctiveness with respect to these issues for patients with VHD versus other forms of cardiovascular disease. Because considerable attention has recently focused on devices and procedures, we concentrated less on important questions surrounding device performance and procedural optimization (acknowledging that these are patient‐centered lines of investigation) and more on knowledge gaps regarding preprocedural and postprocedural management, decision making, and the opportunity to consider other end points for device trials. We also recognize that there is overlap between patient‐centered care, patient‐centered research, and patient‐centered policy—the focus of this Working Group is the “research” piece, recognizing that an ultimate goal of this research is to inform healthcare delivery and policy.
To meet the objectives for this Working Group, we included representatives from VHD‐related patient organizations, clinicians with expertise in VHD, and researchers with active studies on VHD, while recognizing that many other areas of expertise are included in a Heart Valve Team and in caring for patients with VHD. We chose to frame our discussion in terms of the “patient journey” from diagnosis to long‐term management (Figure 1). The specific aims of this Working Group were to: (1) identify knowledge gaps and generate a list of patient‐centered VHD research questions spanning the patient journey from the initial diagnosis to long‐term outcomes; (2) identify gaps in patient‐oriented information about VHD and effective decision aids and implementation strategies for shared decision making; (3) identify barriers to patient‐centered VHD research; and (4) disseminate an open access summary to researchers, clinicians, policymakers, the general public, and patient interest groups.
Figure 1. Context for patient‐centered research in valvular heart disease (VHD)—the patient journey.
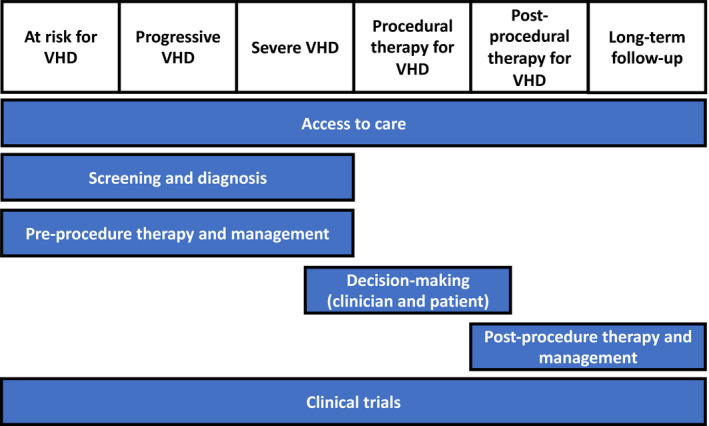
This figure outlines the patient journey and puts the sections of our report in context of this journey.
Access to Care
Access to care for patients with VHD is not equitable with, for example, documented racial disparities in diagnosis and treatment of black patients with severe aortic stenosis (AS) (Table 1).4, 5, 6, 7 Black patients with severe AS are more likely to decline AVR when recommended, raising questions about trust, historical discrimination, and delivery of care.6, 8 Understanding the role that access to care has in the mechanisms of these outcome differences is difficult, since black patients also have a higher prevalence of risk factors for VHD than white patients, including hypertension, diabetes mellitus, and chronic renal insufficiency. Importantly, black patients are not the only racial minorities affected, with emerging data of undertreatment of valve disease among Latino populations and Native Americans.
Table 1.
Access to Care—Patient‐Centered Research Questions in VHD
Disparities in care delivery
|
Telemedicine
|
Heart valve centers
|
VHD indicates valvular heart disease.
Sex disparities in care are also seen in patients with VHD. Women have higher mortality than men after mitral surgery and present with higher case complexity, possibly because of less guideline‐directed surveillance.9, 10 For patients with AS, the relative mortality benefit of transcatheter aortic valve replacement (TAVR) versus surgical aortic valve replacement appears to be greater for women compared with men.11
Innovation in care delivery is being studied, although there are few links to reduction in disparities. Electronic consults have been shown to be amenable to clinical questions about valve disease with cardiologists reviewing electronic data and images (eg, echocardiogram) in a shared electronic medical record and then providing detailed clinical recommendations in the electronic medical record to the referring clinician without an office visit.12, 13 Cardiology electronic consults are cost‐saving relative to traditional care14 and associated with fewer emergency department visits in a cluster‐randomized trial.14 Overall, evidence suggests that cardiology electronic consults improve access to outpatient cardiology care.15 As such, electronic consults and other alternatives to office‐based visits may improve access to care for patients with VHD. However, differences in valve‐specific end points in electronic consults are unknown.
Specialized comprehensive valve centers are recommended in guidelines for patients with asymptomatic severe VHD, patients who may benefit from repair versus replacement, and patients with multimorbid disease.1, 16 This recommendation is based on a known surgical volumes‐outcomes relationship as well as high rates of mitral repair for mitral valve prolapse at some centers.17, 18 In addition, more recent data suggest that mortality following transfemoral TAVR is higher and more variable at lower‐volume centers.19 Lower‐volume centers treat greater proportions of rural patients, black patients, and Hispanic patients.19
The implications of concentrating VHD care at high‐volume comprehensive valve centers are unclear. For coronary artery disease, centers of excellence do not appear to have better outcomes.20 Adding nuance, the focus of high‐volume comprehensive valve centers is on the procedural aspect of care for patients with VHD. However, there may be value to patients with VHD being followed in more specialized heart valve clinics during the progressive stage of disease and after a valve procedure.21, 22, 23 How this specialized longitudinal care would be integrated into a system of care that might concentrate expertise and procedures in certain centers (that may be less practical to access for patients longitudinally) is unclear. Optimizing and integrating care for patients with VHD along the continuum of disease before and potentially after an intervention is fraught with challenges and uncertainties, particularly in a healthcare environment of increasingly restricted lines of referral mandated by insurance providers or other forces.16 The cost implications of various models are also uncertain but inevitably intersect with considerations of quality and access to care. These issues are clearly not unique to patients with VHD, but there are some particular ways in which these system‐of‐care issues may specifically affect them. Diverse stakeholders need to engage Centers for Medicare & Medicaid Services (CMS) and other policy makers to ensure that policies are developed that are evidence based and in the best interests of our patients.
Screening and Diagnosis
A comprehensive understanding of risk factors for VHD will allow for a more targeted approach to screening and diagnosis as well as prevention (Table 2). Current screening of VHD primarily relies on patient symptoms and physical examination, despite wide variation in clinical practice and lack of accuracy for diagnosis of VHD, leading to variations in treatment.24 In a study comparing auscultation by primary care clinicians and cardiologists against echocardiography, both groups had poor sensitivity for detecting mild or significant VHD (22–32%) with suboptimal specificity ranging from 67% to 83%.25 Accordingly, tools other than auscultation are needed to effectively screen for VHD. Notably, undiagnosed VHD appears to be more common in lower socioeconomic groups, but the reasons for this are not fully understood.
Table 2.
Screening and Diagnosis—Patient‐Centered Research Questions in VHD
Risk factors for VHD
|
Tools to screen for VHD
|
Scope of screening for VHD
|
Integrated screening for VHD
|
Consequences of screening for VHD
|
Accurate diagnosis of significant VHD
|
VHD indicates valvular heart disease.
Screening for VHD using echocardiography and advanced imaging approaches has not been well studied. Among individuals 65 years and older without a prior diagnosis of VHD, systematic echocardiography identified 51% with mild or more left‐sided VHD or moderate or severe right‐sided VHD, including 6.4% with significant (moderate or more) VHD.26 The increasing availability of handheld ultrasound machines and application of artificial intelligence algorithms is likely to lower costs. Research is needed to determine optimal screening algorithms, including the scope of these efforts, cost‐effectiveness, tools utilized, how to leverage new technologies, and how these efforts may need to be adapted based on geography, clinical setting, and available resources. Important areas for study are determining which patient populations will benefit from screening (eg, relatives of those with VHD and age‐based or risk‐based [based on genetics, biomarkers, or comorbidities] subgroups) and how detection of VHD early in the pathophysiological process (eg, mild in severity) impacts costs and patient outcomes and how this may differ depending on the type of VHD. Whether screening should be focused on identifying only more significant (eg, moderate to severe) VHD versus mild disease needs to be considered and will likely depend on the specific VHD and whether interventions are available to prevent or slow progression of earlier‐stage disease. Finally, there is wide variation among practitioners with respect to monitoring for progression of diagnosed VHD.27 Patients who are women, black, or on Medicaid are less likely to be screened for progression of VHD at appropriate intervals.10 Further studies are needed to clarify optimal monitoring timeframes and the factors underlying variations in surveillance for progression of VHD. The role of multimodality imaging in the diagnosis and assessment of severity of VHD requires additional research.28, 29, 30, 31
Preprocedure Therapy and Management
Valve lesions, such as AS and mitral regurgitation, are commonly viewed as mechanical problems requiring a mechanical solution with a transcatheter or surgical procedure (Table 3). This is attributable to the fact that there have been no medical therapies proven to prevent, slow, or reverse primary VHD to date.1, 32 There was enthusiasm that statins might play such a role for patients with aortic sclerosis or AS based on preclinical studies, but several clinical trials demonstrated a lack of clinical benefit.33, 34 Progress is being made in elucidating underlying mechanisms of valve disease, but these discoveries have yet to be translated into effective therapies.32, 35, 36 In some cases, promising targets and therapies exist, but they have not been tested in patients with VHD. For example, elevated Lipoprotein(a) is associated with incident and progressive AS and emerging data indicate a potential role for PCSK9 in valve calcification. Therapies targeting these molecules are available, but they have not been tested as potential medical therapies to prevent or slow progression of AS.37, 38
Table 3.
Preprocedure Therapy and Management—Patient‐Centered Research Questions in VHD
Prevent/slow/reverse VHD with medical therapy
|
Prevent/slow/reverse maladaptive ventricular remodeling and dysfunction with medical therapy
|
“Prehabilitation” in frail patients
|
Blood pressure targets in patients with VHD
|
Activity recommendations and restrictions in patients with VHD before a procedure
|
VHD indicates valvular heart disease.
The morbidity and mortality of valve disease often stems from how pressure or volume overload affects the ventricle. The sequelae of VHD overlap significantly with heart failure (HF) with preserved ejection fraction and HF with reduced ejection fraction both in terms of ventricular remodeling and dysfunction as well as clinical manifestations and symptoms. Most patients with VHD develop manifestations and symptoms of HF before an intervention on their valve and many have residual HF after a valve procedure. Even if the primary valve abnormality progresses, perhaps medical therapy targeting the maladaptive ways in which the ventricle responds to pressure or volume overload could delay the onset of HF symptoms or leave the heart in a healthier structural and functional place after a valve procedure is performed to mitigate HF after a procedure. For example, in patients with AS, excessive hypertrophy and the presence and extent of myocardial fibrosis are associated with increased HF, worse left ventricular function, and increased mortality.39, 40 Accordingly, medical therapy targeting maladaptive hypertrophy or fibrosis may promote ventricular health and improve survival even if the AS progresses and valve replacement is still needed. Although the mechanism for the potential benefit is unclear, there are retrospective studies suggesting that renin‐angiotensin system blockade may be associated with improved survival and a lower risk of cardiovascular events.41
Several tools will be needed to elucidate pathobiology in the valve and the ventricle and to test medical therapies directed at promising targets. Phenotyping should include circulating biomarkers (including ‐omic approaches), multimodality and molecular imaging, tissue analyses (eg, myocardium, valve), studies done under resting and stress (eg, exercise) conditions, and invasive hemodynamics.
Beyond medical therapy targeting the valve and ventricle, there are other knowledge gaps pertinent to the stage of progressive valve disease related to the blood pressure (BP) and physical activity goals and guidelines. With respect to BP goals, the VHD guidelines defer to BP guidelines for the general population and offer no specific targets for patients with VHD.1 However, for AS, while hypertension is a risk factor for incident AS and faster progression, a post hoc analysis of SEAS (Simvastatin Ezetimibe in Aortic Stenosis Study) showed that event rates were higher for those with a systolic BP <120 mm Hg or diastolic BP <70 mm Hg.42, 43, 44
Guidelines on physical activity and restrictions for patients with progressive VHD are generally based on expert consensus, but further research could refine and improve those recommendations.45 At the other end of the spectrum, given the increased procedural risk and postoperative events associated with impaired physical function and frailty, it is unclear whether “prehabilitation” (rehabilitation before an intervention) before a valve procedure may reduce risk and improve outcomes.
DECISION MAKING When a Procedure is Contemplated (Clinician Perspective)
There are a number of factors a clinician must consider in order to determine whether to recommend a valve procedure or surgery to a patient: appropriateness, timing, feasibility, and approach, and whether the recommendation is reflective of the patient's goals and preferences. Each of these areas has potential for important research questions (Table 4). Particularly among younger patients, considering the longitudinal patient journey and the potential need for multiple interventions over the patient's life, consideration needs to be given to and research directed at clarifying the optimal treatment path when multiple procedures over a lifetime can be predicted.
Table 4.
Decision Making (Clinician and Patient Perspectives) When a Procedure is Contemplated—Patient‐Centered Research Questions in VHD
| Clinician Perspective |
|---|
Optimal timing of a valve procedure
|
Nonresponders to a valve procedure
|
Futility of a valve procedure caused by comorbidities and frailty
|
Clarifying the relationship between valve disease and symptoms and anticipated benefit of a procedure
|
Health status assessment
|
Approach to valve procedures
|
| Patient Perspective |
|---|
Patient goals and preferences and integration into VHD trials
|
Selection of outcomes for SDM trials in VHD
|
Strategies to support an SDM process
|
Impact of policy on delivery of care to patients with VHD
|
AS indicates aortic stenosis; HF, heart failure; SDM, shared decision making; and VHD, valvular heart disease.
A critical step in the decision‐making process for clinicians is to determine whether the procedure is appropriate. Determining the appropriateness of a procedure centers on assessing whether the anticipated benefits of the procedure are likely to outweigh the risks, which is inextricably linked to understanding the patient's goals and preferences and determining whether the procedure has a reasonable likelihood of achieving these goals. Notably, the research suggests that clinicians often make a “preference misdiagnosis,” and thus tools and skill sets to clarify patient values are needed.46 There are generally 2 broad categories of inappropriate (or ineffective) procedures: (1) futility of a valve procedure because of comorbidities and frailty—even if the procedure is technically successful, the patient will die soon or experience an ongoing decline in health status; or (2) nonresponder to a valve procedure—even if the procedure is technically successful, it does not improve health status, survival, or other goals of the patient. The first scenario is easier to conceptualize; an example of the second from another cardiovascular specialty would be the lack of clinical response to cardiac resynchronization therapy among patients with a nonleft bundle block QRS morphology.47 While we are gaining more insight into patients for whom TAVR may be futile, much work remains to be done to clarify which patients will not benefit from mitral or tricuspid procedures.
Timing of the procedure is also an important step in decision making: Does the patient meet criteria for treatment of the valve? Our current indications for treatment with transcatheter therapies reflect practice patterns when surgery is the only option. With less invasive treatments and increasing options for repeat procedures, the optimal timing of intervention should be questioned. With the introduction of TAVR and the opportunity for valve‐in‐valve TAVR in the treatment of AS, strategy trials are important to better understand whether TAVR may be beneficial earlier in the disease, ie, before symptoms (eg, EARLY TAVR [Evaluation of Transcatheter Aortic Valve Replacement Compared to Surveillance for Patients With Asymptomatic Severe Aortic Stenosis] NCT 03042104], or in symptomatic moderate disease (eg, TAVR UNLOAD [Transcatheter Aortic Valve Replacement to Unload the Left Ventricle in Patients With Advanced Heart Failure: A Randomized Trial] NCT 02661451). Similar questions about timing exist for the treatment of mitral and tricuspid valve disease. The technology, however, is at an earlier stage in defining the efficacy of approaches and devices.
An additional step in the decision process is to determine the best approach to treating the valve. The best approach might depend on technical feasibility (eg, is the left ventricular outflow tract too large or small, are the mitral [or tricuspid] leaflets amenable to clipping, how much mitral annular calcification is too much) but also consider other issues. The choice of a transcatheter versus surgical versus hybrid approach, optimal choice of valve, simultaneous versus sequential procedures for multiple valve disorders, and whether concurrent cardiac conditions (eg, coronary disease) need to be addressed depend on the patients’ medical condition, procedural risk, age, and cardiac function, as well as patient preferences and values.
To improve decision making from the clinician perspective, the emphasis should be on identifying factors and developing and validating risk models that will inform, influence, and guide clinical decisions and actions regarding: (1) timing of a procedure (perform it now versus later); (2) whether to recommend a procedure when futility is anticipated (either because of frailty and impaired physical function or a predicted lack of clinical response to the intervention); or (3) whether a specific adjunctive intervention should be employed in a subgroup of patients alongside a procedure to optimize outcomes. For example, a risk prediction tool for poor outcome after TAVR identified 8.4% of patients with a ≥70% predicted risk of a poor 1‐year outcome; of those very high‐risk patients, 60.3% were dead and an additional 16.9% had poor quality of life or quality of life decline by 1 year after TAVR.48 Given that average 1‐year mortality in patients with symptomatic severe AS not getting TAVR is ≈50%, knowing that a patient is in this very high‐risk subgroup may inform shared decision‐making conversations regarding whether to perform TAVR.49 Similarly, a risk score for outcomes after TAVR that includes a frailty component is useful not so much because it improves discrimination of mortality (eg, improved c‐statistic), but because it identifies patients at very high risk for death or disability at 1 year for whom TAVR may be futile and also identifies patients for whom an aggressive rehabilitation plan is particularly important as an adjunct to TAVR for outcomes to be optimized.50
DECISION MAKING When a Procedure is Contemplated (Patient Perspective)
The expansion of treatment options for VHD and the increase in the number of older adults with multiple competing comorbid conditions make shared decision making (SDM) increasingly relevant (Table 4).51 SDM is a process in which clinicians and patients deliberate reasonable treatment alternatives and collaborate on a final treatment plan, with the final choice informed by patients’ goals and preferences.52 An SDM process is most applicable for preference‐sensitive decisions, defined as those in which more than one reasonable option exists; there remains uncertainty in the evidence; or patient preferences vary between patients or compared with clinicians. In these types of medical decisions, patients’ values and preferences play a significant role in identifying which treatment may be best for them.53
SDM is distinct from patient education, which is a 1‐way stream of information from clinician to patient. SDM involves listening to the values and preferences of informed patients incorporating this into decision making.54 There is consistent evidence that clinicians do not elicit patient values and preferences, nor adjust care to preferences.55, 56
SDM research, pioneered and rigorously evaluated in fields including oncology and orthopedics over the past 3 decades, includes the study of strategies to improve patient‐clinician communication when making medical decisions.57, 58, 59, 60 Numerous randomized trials on the effectiveness of decision aids to promote an SDM process have demonstrated improvement in patient‐centered outcomes including knowledge, satisfaction, and decisions consistent with patients’ values.57 Decision aids, which may include paper handouts, videos, websites, or tools embedded in the electronic health record, raise awareness there is a choice to be made, provide information on risks and benefits, and may also assist in values clarification.61, 62 However, large‐scale implementation projects identify that while decision aids are helpful, clinician skill sets in SDM—combined with positive clinician and leadership attitudes towards meaningful change in healthcare delivery—are critical for effective SDM.54 An SDM approach is consistently advocated across multiple disease conditions in cardiology by professional society guidelines, yet there remains a lack of recommendations regarding best practices or most effective tools for implementation.16
It is essential that validated frameworks and measures are used in study conceptualization, design, deployment, evaluation, and implementation of SDM interventions, such as patient decision aids.61, 62, 63, 64 Study designs often include cluster randomized trials, quasi‐experimental designs with pre‐post testing, or repeated observations over time.65 While a review of all measures of the quality of decision making is outside the scope of this review, examples include independent, third‐party review of audiotaped clinical encounters, patient surveys, or simply noting that a decision aid was used in the visit.66
Because some of the research in SDM is striving to describe natural phenomena, including clinician and patient attitudes, beliefs, and behaviors, qualitative research is also utilized. These studies may employ nominal group technique, semistructured interviews leading to framework‐guided qualitative analysis, or more traditional focus groups.56, 67, 68 The National Quality Forum provides additional best practices to help guide implementation efforts of evidence‐based tools, such as decision aids.53 SDM is the “science of allocating time for care,” and time will remain a significant barrier until SDM is no longer seen as “a ‘nice‐to‐have’ extra for which new time needs to be found.”69 This requires an investment in research into healthcare delivery innovations that embed the process of SDM into our existing structures, valuing the outcomes that reflect high‐quality decisions so that patient engagement returns to its rightful place as intrinsic to our actions as clinicians.
Postprocedure Therapy and Management
Continuity of care and seamless management of the complexity of VHD after an intervention are central to ensuring patients derive their expected benefits of treatment (Table 5). For example, the 3M TAVR (Multimodality, Multidisciplinary, But Minimalist TAVR) study recently demonstrated the safety and reproducibility of a clinical pathway inclusive of minimalist periprocedure approach, a standardized postprocedure protocol of rapid mobilization and reconditioning, and criteria‐driven discharge to achieve safe next‐day discharge.70 These findings reflect the experience of single‐center observational studies that prioritize a bundle of care that promotes the mitigation of postprocedure risks in the mostly elderly patient population with VHD.71 The development and evaluation of health service delivery interventions is complex because of the multiple interacting components, the number and difficulty of behaviors required by those delivering or receiving the interventions, the number of organizational levels targeted by the intervention, and the measurement of outcomes that must be reflective of and responsive to the intervention.72
Table 5.
Postprocedure Therapy and Management—Patient‐Centered Research Questions in VHD
| Supporting a Safe Recovery |
|---|
Getting home safely—improving transitions of care
|
Getting better after a heart valve procedure—rehabilitation and improving physical functioning
|
Managing complications and the long‐term sequelae of valve procedures
|
| Managing Heart Disease Related to VHD |
|---|
Treating HF and abnormal ventricular structure and function after a valve procedure
|
Blood pressure targets in patients with VHD
|
Anticoagulation and antiplatelet therapy after valve procedures
|
| Device Surveillance and Durability |
|---|
Valve durability and surveillance
|
HF indicates heart failure; and VHD, valvular heart disease.
The CMS, Joint Commission, and the Institute of Medicine have consistently highlighted that the failure of ensuring appropriate transition of care—the movement of patients between healthcare practitioners, settings, and home as their condition and care needs change—can have devastating effects on patients.73, 74 We currently lack evidence to guide and risk‐stratify the use of postprocedure pathways, determine the optimal length of stay, and support patient‐centered discharge planning in an increasingly heterogenous VHD population. There is a pressing need to focus research on strategies to address patients’ vulnerabilities in the early recovery period and optimize care transitions in healthcare systems to improve outcomes. Given the high prevalence of frailty in patients with VHD and its association with poor outcomes after valve procedures, effective strategies to improve postprocedural physical function are sorely needed. However, enrollment in center‐based cardiac rehabilitation programs is low and there are numerous barriers to participation.75 Novel approaches that leverage technology and can be implemented entirely or partially at home may be more effective.76 More research is also needed regarding the consequences of and how to monitor for complications of valve procedures.
Many patients continue to have a poor quality of life and adverse outcomes after intervention for VHD. Maladaptive left ventricular remodeling and dysfunction in response to pressure or volume overload does not always reverse toward normal after the valve is fixed, which is associated with worse outcomes.39, 40, 77, 78 Research to elucidate mechanisms of persistent maladaptive ventricular remodeling and dysfunction after a valve procedure may identify novel targets for medical therapy to improve outcomes as an adjunct to a procedure.49, 79, 80, 81 Optimal BP targets after a valve procedure may differ than those for the general population, but further work is needed to clarify these relationships and appropriate goals.82, 83
It is also critical to identify best practices for antiplatelet and anticoagulant medications after different valve procedures. While valve thrombosis does occur after TAVR and may influence valve durability, indiscriminate treatment with anticoagulation is associated with harm.84 The rapid increase in the number and types of devices to treat VHD also emphasizes the need for research to rigorously assess device durability, identify best practices for surveillance of device performance, and determine the clinical significance and appropriate treatment of abnormalities identified.
Clinical Trials in VHD—Challenges and Opportunities
With the introduction of transcatheter options to treat VHD as an alternative to surgery, a rapid succession of numerous well‐designed RCTs have been completed, providing a robust evidence base particularly for the role of TAVR in the treatment of AS (Table S1). Most trials in VHD over the past decade are device‐focused. While there is an ongoing need for more device and procedure‐related trials, there is also an urgent need for RCTs to address many nondevice research questions in VHD along the full spectrum of the patient's journey from screening to long‐term postprocedure management. Indeed, many of the questions included herein could be optimally addressed by RCTs. Yet, there are several challenges to performing clinical trials, particularly those addressing questions not related to a device. Leveraging existing registries (eg, TVT [Transcatheter Valve Therapy] and STS [Society of Thoracic Surgeons]) to perform pragmatic trials could be a good starting point. These registries capture an extensive number of data but they are designed for tracking quality and outcomes and less as a vehicle for prospective research. Incorporating use of their data into prospective studies is currently onerous and expensive.
Barriers to Patient‐Centered Research in VHD
There are many barriers to patient‐centered research in VHD as summarized in Table 6. Until recently, VHD was not recognized as a common and important clinical condition and there are no defined training pathways for clinical expertise in VHD. Research on VHD tends to be spread across different specialty scientific meetings and medical journals, which are organized by the type of research rather than the patient with VHD (eg, the disease not the method). Similarly, the concept that patients should be involved in clinical research is relatively new and has yet to gain wide acceptance, although some medical journals now require a statement about patient involvement.64 Some patient‐centered research questions and outcomes seem “soft” compared with the traditional “hard” end points of clinical trials; researchers and reviewers are often unfamiliar and uncomfortable with standards for performing and reporting qualitative data.63 Investigator‐initiated funding for VHD research is difficult to obtain given this lack of expertise and priority by funding agencies. Many of these barriers can be reduced or eliminated by promotion of training and research in VHD; education of researchers, reviewers, and journal editors about patient‐centered research; increased funding opportunities for VHD research; and closer collaboration between researchers, clinicians, and patients with VHD.
Table 6.
Barriers to Effective Patient‐Centered Research on VHD
| Barrier | Impact |
|---|---|
| Lack of recognition of VHD as a specific area of expertise |
|
| Limited funding and lack of recognition of need for VHD research |
|
| Lack of patient involvement in VHD research priorities, study design, and implementation |
|
| Lack of diversity in VHD researchers |
|
| Lack of inclusion of patients with VHD in clinical trials of HF, hypertension, arrhythmias, and other cardiac conditions |
|
| Lack of validated VHD‐specific patient‐reported outcome measures |
|
| Few measures of effectiveness of approaches to improving outcomes in patients with VHD |
|
| Traditional views on diagnosis and treatment of VHD |
|
| Healthcare system inertia in the approach to provision of care to patients with VHD |
|
| Silos based on type of physician and type of medical center |
|
| Lack of diversity in the clinical VHD workforce |
|
| Difficulty in publishing patient‐centered research in cardiology journals |
|
HF indicates heart failure; and VHD, valvular heart disease.
Conclusions
Over the past decade, an explosion of research in VHD has centered on new opportunities to perform valve procedures less invasively utilizing transcatheter approaches. There is little doubt that, on the whole, this is good for patients. Numerous opportunities exist to build on these advances and improve outcomes for patients with VHD. Herein, we have outlined knowledge gaps and research priorities for patient‐centered research in VHD, recognizing that the patient ought to be the center of our attention and not simply a valve or device (Figure 2). There are a number of barriers that impede progress, but also numerous opportunities for collaboration and progress among diverse stakeholders who can be united with a common purpose (Table S2). Ultimately, patient‐centered research needs to intersect with, promote, and provide evidence for patient‐centered care and policy to yield the greatest benefits for those who have the most at stake: our patients.
Figure 2. Patient‐centered research in valvular heart disease (VHD).
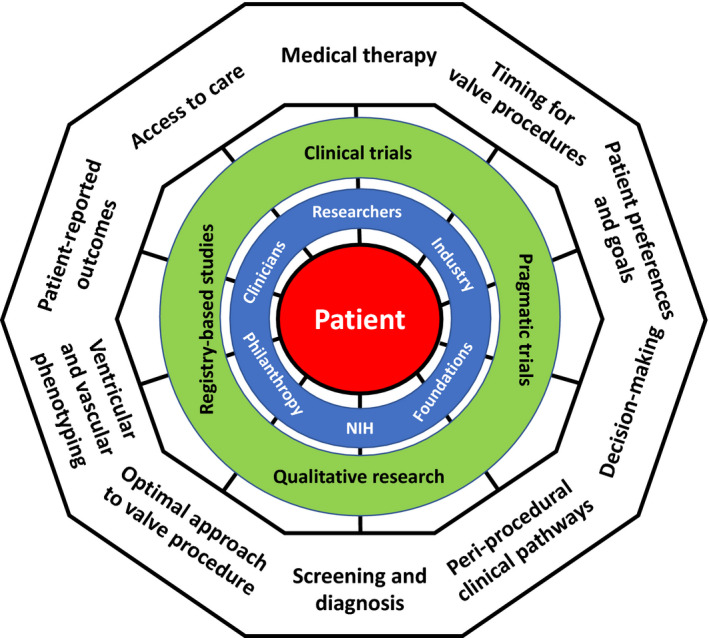
This figure shows the multifaceted aspects of what we define and characterize as patient‐centered research in VHD. The patient (red) is a participant in and focus of the research. The outer ring represents some of the many research questions and knowledge gaps in the field. The most common research tools and methodologies to address those knowledge gaps are shown in the next inner circle (green). Those doing and funding the research are shown in the final inner circle (blue).
Disclosures
Dr Lindman has served on the scientific advisory board for Roche Diagnostics, has received research grants from Edwards Lifesciences and Roche Diagnostics, and has consulted for Medtronic. Ms Clark and Ms Peschin are both employees of the Alliance for Aging Research, which has received educational support from Edwards Lifesciences and Edwards Lifesciences Foundation. Dr Coylewright has received research grants from Edwards Lifesciences and Boston Scientific, and honoraria from W.L. Gore. Drs Evans and Sachdev are both employees of the National Institutes of Health. Dr Lauck has been a consultant for Edwards Lifesciences and Abbott. Dr Wasfy is funded by an American Heart Association grant (18 CDA 34110215). Heart Valve Voice US has received educational support from Abbott, Boston Scientific, Edwards Lifesciences, Edwards Lifesciences Foundation, and Medtronic. The remaining authors have no disclosures to report. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Supporting information
Tables S1–S2
(J Am Heart Assoc. 2020;9:e015975 DOI: 10.1161/JAHA.119.015975.)
For Disclosures, see page 12.
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, et al; Group ESCSD . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 4. Cruz Rodriguez B, Acharya P, Salazar‐Fields C, Horne A Jr. Comparison of frequency of referral to cardiothoracic surgery for aortic valve disease in blacks, Hispanics, and whites. Am J Cardiol. 2017;120:450–455. [DOI] [PubMed] [Google Scholar]
- 5. Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE, Jacobs JP, et al; Registry SAT . 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69:1215–1230. [DOI] [PubMed] [Google Scholar]
- 6. McNeely C, Zajarias A, Fohtung R, Kakouros N, Walker J, Robbs R, Markwell S, Vassileva CM. Racial comparisons of the outcomes of transcatheter and surgical aortic valve implantation using the Medicare database. Am J Cardiol. 2018;122:440–445. [DOI] [PubMed] [Google Scholar]
- 7. Patel DK, Green KD, Fudim M, Harrell FE, Wang TJ, Robbins MA. Racial differences in the prevalence of severe aortic stenosis. J Am Heart Assoc. 2014;3:e000879 DOI: 10.1161/JAHA.114.000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeung M, Kerrigan J, Sodhi S, Huang PH, Novak E, Maniar H, Zajarias A. Racial differences in rates of aortic valve replacement in patients with severe aortic stenosis. Am J Cardiol. 2013;112:991–995. [DOI] [PubMed] [Google Scholar]
- 9. Vassileva CM, McNeely C, Mishkel G, Boley T, Markwell S, Hazelrigg S. Gender differences in long‐term survival of Medicare beneficiaries undergoing mitral valve operations. Ann Thorac Surg. 2013;96:1367–1373. [DOI] [PubMed] [Google Scholar]
- 10. Tanguturi VK, Bhambhani V, Picard MH, Armstrong K, Wasfy JH. Echocardiographic surveillance of valvular heart disease in different sociodemographic groups. JACC Cardiovasc Imaging. 2019;12:751–752. [DOI] [PubMed] [Google Scholar]
- 11. Williams M, Kodali SK, Hahn RT, Humphries KH, Nkomo VT, Cohen DJ, Douglas PS, Mack M, McAndrew TC, Svensson L, et al. Sex‐related differences in outcomes after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis: insights from the PARTNER Trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol. 2014;63:1522–1528. [DOI] [PubMed] [Google Scholar]
- 12. Wasfy JH, Rao SK, Chittle MD, Gallen KM, Isselbacher EM, Ferris TG. Initial results of a cardiac e‐consult pilot program. J Am Coll Cardiol. 2014;64:2706–2707. [DOI] [PubMed] [Google Scholar]
- 13. Wasfy JH, Rao SK, Kalwani N, Chittle MD, Richardson CA, Gallen KM, Isselbacher EM, Kimball AB, Ferris TG. Longer‐term impact of cardiology e‐consults. Am Heart J. 2016;173:86–93. [DOI] [PubMed] [Google Scholar]
- 14. Olayiwola JN, Anderson D, Jepeal N, Aseltine R, Pickett C, Yan J, Zlateva I. Electronic consultations to improve the primary care‐specialty care interface for cardiology in the medically underserved: a cluster‐randomized controlled trial. Ann Fam Med. 2016;14:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oseran AS, Wasfy JH. Early experiences with cardiology electronic consults: a systematic review. Am Heart J. 2019;215:139–146. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura RA, O'Gara PT, Bavaria JE, Brindis RG, Carroll JD, Kavinsky CJ, Lindman BR, Linderbaum JA, Little SH, Mack MJ, et al. 2019 AATS/ACC/ASE/SCAI/STS expert consensus systems of care document: a proposal to optimize care for patients with valvular heart disease: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:2609–2635. [DOI] [PubMed] [Google Scholar]
- 17. LaPar DJ, Ailawadi G, Isbell JM, Crosby IK, Kern JA, Rich JB, Speir AM, Kron IL; Virginia Cardiac Surgery Quality I . Mitral valve repair rates correlate with surgeon and institutional experience. J Thorac Cardiovasc Surg. 2014;148:995–1003; discussion 1003‐4. [DOI] [PubMed] [Google Scholar]
- 18. Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg. 2012;144:308–312. [DOI] [PubMed] [Google Scholar]
- 19. Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, Kumbhani DJ, Ruiz CE, Thourani VH, Hanzel G, et al. Procedural volume and outcomes for transcatheter aortic‐valve replacement. N Engl J Med. 2019;380:2541–2550. [DOI] [PubMed] [Google Scholar]
- 20. Khatana SAM, Nathan AS, Dayoub EJ, Giri J, Groeneveld PW. Centers of excellence designations, clinical outcomes, and characteristics of hospitals performing percutaneous coronary interventions. JAMA Intern Med. 2019. DOI: 10.1001/jamainternmed.2019.0567. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lancellotti P, Rosenhek R, Pibarot P, Iung B, Otto CM, Tornos P, Donal E, Prendergast B, Magne J, La Canna G, et al. ESC Working Group on Valvular Heart Disease position paper—heart valve clinics: organization, structure, and experiences. Eur Heart J. 2013;34:1597–1606. [DOI] [PubMed] [Google Scholar]
- 22. Zilberszac R, Lancellotti P, Gilon D, Gabriel H, Schemper M, Maurer G, Massetti M, Rosenhek R. Role of a heart valve clinic programme in the management of patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2017;18:138–144. [DOI] [PubMed] [Google Scholar]
- 23. Lancellotti P, Magne J, Dulgheru R, Clavel MA, Donal E, Vannan MA, Chambers J, Rosenhek R, Habib G, Lloyd G, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. 2018;3:1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Webb J, Thoenes M, Chambers JB. Identifying heart valve disease in primary care: differences between practice in Germany, France and the United Kingdom. Eur J Cardiovasc Med. 2014;3:388–392. [Google Scholar]
- 25. Gardezi SKM, Myerson SG, Chambers J, Coffey S, d'Arcy J, Hobbs FDR, Holt J, Kennedy A, Loudon M, Prendergast A, et al. Cardiac auscultation poorly predicts the presence of valvular heart disease in asymptomatic primary care patients. Heart. 2018;104:1832–1835. [DOI] [PubMed] [Google Scholar]
- 26. d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson‐Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, et al. Large‐scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanguturi VK, Hidrue MK, Picard MH, Atlas SJ, Weilburg JB, Ferris TG, Armstrong K, Wasfy JH. Variation in the echocardiographic surveillance of primary mitral regurgitation. Circ Cardiovasc Imaging. 2017;10:e006495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, Gurram S, Jain K, Subero M, Jang JJ, et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65:1078–1088. [DOI] [PubMed] [Google Scholar]
- 29. Kammerlander AA, Wiesinger M, Duca F, Aschauer S, Binder C, Zotter Tufaro C, Nitsche C, Badre‐Eslam R, Schonbauer R, Bartko P, et al. Diagnostic and prognostic utility of cardiac magnetic resonance imaging in aortic regurgitation. JACC Cardiovasc Imaging. 2019;12:1474–1483. [DOI] [PubMed] [Google Scholar]
- 30. Clavel MA, Burwash IG, Pibarot P. Cardiac imaging for assessing low‐gradient severe aortic stenosis. JACC Cardiovasc Imaging. 2017;10:185–202. [DOI] [PubMed] [Google Scholar]
- 31. Pawade T, Clavel MA, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L, Renard C, Gun M, Jenkins WS, Macron L, et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging. 2018;11:e007146. [DOI] [PubMed] [Google Scholar]
- 32. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid‐lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. [DOI] [PubMed] [Google Scholar]
- 34. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. [DOI] [PubMed] [Google Scholar]
- 35. Aikawa E, Libby P. A rock and a hard place: chiseling away at the multiple mechanisms of aortic stenosis. Circulation. 2017;135:1951–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine RA, Hagege AA, Judge DP, Padala M, Dal‐Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia‐Naji N, Bruneval P, et al; Leducq Mitral Transatlantic N . Mitral valve disease–morphology and mechanisms. Nat Rev Cardiol. 2015;12:689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, White AC, Timmers N, Hjortnaes J, Rogers MA, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73:2150–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poggio P, Songia P, Cavallotti L, Barbieri SS, Zanotti I, Arsenault BJ, Valerio V, Ferri N, Capoulade R, Camera M. PCSK9 involvement in aortic valve calcification. J Am Coll Cardiol. 2018;72:3225–3227. [DOI] [PubMed] [Google Scholar]
- 39. Chin CW, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10:1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. [DOI] [PubMed] [Google Scholar]
- 41. Nadir MA, Wei L, Elder DH, Libianto R, Lim TK, Pauriah M, Pringle SD, Doney AD, Choy AM, Struthers AD, et al. Impact of renin‐angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol. 2011;58:570–576. [DOI] [PubMed] [Google Scholar]
- 42. Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, Tu JV, Wijeysundera HC, Ko DT. A population‐based study on the associations between traditional cardiovascular risk factors and incident aortic stenosis: the CANHEART Aortic Stenosis Study. J Am Coll Cardiol. 2017;69:1523–1532. [DOI] [PubMed] [Google Scholar]
- 43. Tastet L, Capoulade R, Clavel MA, Larose E, Shen M, Dahou A, Arsenault M, Mathieu P, Bedard E, Dumesnil JG, et al. Systolic hypertension and progression of aortic valve calcification in patients with aortic stenosis: results from the PROGRESSA study. Eur Heart J Cardiovasc Imaging. 2017;18:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nielsen OW, Sajadieh A, Sabbah M, Greve AM, Olsen MH, Boman K, Nienaber CA, Kesaniemi YA, Pedersen TR, Willenheimer R, et al. Assessing optimal blood pressure in patients with asymptomatic aortic valve stenosis: the SEAS Study. Circulation. 2016;134:455–468. [DOI] [PubMed] [Google Scholar]
- 45. Bonow RO, Nishimura RA, Thompson PD, Udelson JE; American Heart Association E, Arrhythmias Committee of Council on Clinical Cardiology CoCDiYCoC, Stroke Nursing CoFG, Translational B and American College of C . Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 5: valvular heart disease: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e292–e297. [DOI] [PubMed] [Google Scholar]
- 46. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572. [DOI] [PubMed] [Google Scholar]
- 47. Cunnington C, Kwok CS, Satchithananda DK, Patwala A, Khan MA, Zaidi A, Ahmed FZ, Mamas MA. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non‐left bundle branch block QRS morphology: meta‐analysis of randomised controlled trials. Heart. 2015;101:1456–1462. [DOI] [PubMed] [Google Scholar]
- 48. Arnold SV, Afilalo J, Spertus JA, Tang Y, Baron SJ, Jones PG, Reardon MJ, Yakubov SJ, Adams DH, Cohen DJ; Investigators USC . Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arnold SV, Cohen DJ, Dai D, Jones PG, Li F, Thomas L, Baron SJ, Frankel NZ, Strong S, Matsouaka RA, et al. Predicting quality of life at 1 year after transcatheter aortic valve replacement in a real‐world population. Circ Cardiovasc Qual Outcomes. 2018;11:e004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Afilalo J, Lauck S, Kim DH, Lefevre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY‐AVR Study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 51. Bavaria JE, Tommaso CL, Brindis RG, Carroll JD, Deeb GM, Feldman TE, Gleason TG, Horlick EM, Kavinsky CJ, Kumbhani DJ, et al. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:340–374. [DOI] [PubMed] [Google Scholar]
- 52. Elwyn G, Lloyd A, May C, van der Weijden T, Stiggelbout A, Edwards A, Frosch DL, Rapley T, Barr P, Walsh T, et al. Collaborative deliberation: a model for patient care. Patient Educ Couns. 2014;97:158–164. [DOI] [PubMed] [Google Scholar]
- 53. Forum NQ . National Quality Partners Playbook: Shared Decision Making in Healthcare. National Quality Forum; 2018. [Google Scholar]
- 54. Joseph‐Williams N, Lloyd A, Edwards A, Stobbart L, Tomson D, Macphail S, Dodd C, Brain K, Elwyn G, Thomson R. Implementing shared decision making in the NHS: lessons from the MAGIC programme. BMJ. 2017;357:j1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Couët N, Desroches S, Robitaille H, Vaillancourt H, Leblanc A, Turcotte S, Elwyn G, Légaré F. Assessments of the extent to which health‐care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18:542–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coylewright M, O'Neill ES, Dick S, Grande SW. PCI Choice: cardiovascular clinicians’ perceptions of shared decision making in stable coronary artery disease. Patient Educ Couns. 2017;100:1136–1143. [DOI] [PubMed] [Google Scholar]
- 57. Stacey D, Legare F, Lewis K, Barry MJ, Bennett CL, Eden KB, Holmes‐Rovner M, Llewellyn‐Thomas H, Lyddiatt A, Thomson R, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Probst MA, Noseworthy PA, Brito JP, Hess EP. Shared decision‐making as the future of emergency cardiology. Can J Cardiol. 2018;34:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coylewright M, Shepel K, Leblanc A, Pencille L, Hess E, Shah N, Montori VM, Ting HH. Shared decision making in patients with stable coronary artery disease: PCI choice. PLoS One. 2012;7:e49827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Allen LA, McIlvennan CK, Thompson JS, Dunlay SM, LaRue SJ, Lewis EF, Patel CB, Blue L, Fairclough DL, Leister EC, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE‐LVAD randomized clinical trial. JAMA Intern Med. 2018;178:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ipdas C . International Patient Decision Aid Standards (IPDAS). Published 2015.
- 62. Sepucha KR, Abhyankar P, Hoffman AS, Bekker HL, LeBlanc A, Levin CA, Ropka M, Shaffer VA, Sheridan SL, Stacey D, et al. Standards for UNiversal reporting of patient Decision Aid Evaluation studies: the development of SUNDAE Checklist. BMJ Qual Saf. 2018;27:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O'Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245–1251. [DOI] [PubMed] [Google Scholar]
- 64. Boivin A, Richards T, Forsythe L, Gregoire A, L'Esperance A, Abelson J, Carman KL. Evaluating patient and public involvement in research. BMJ. 2018;363:k5147. [DOI] [PubMed] [Google Scholar]
- 65. Coylewright M, O'Neill E, Sherman A, Gerling M, Adam K, Xu K, Grande SW, Dauerman HL, Dodge SE, Sobti NK, et al. The learning curve for shared decision‐making in symptomatic aortic stenosis. JAMA Cardiol. 2020. DOI: 10.1001/jamacardio.2019.5719. Published online January 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sepucha KR, Scholl I. Measuring shared decision making: a review of constructs, measures, and opportunities for cardiovascular care. Circ Cardiovasc Qual Outcomes. 2014;7:620–626. [DOI] [PubMed] [Google Scholar]
- 67. Col NF, Solomon AJ, Springmann V, Garbin CP, Ionete C, Pbert L, Alvarez E, Tierman B, Hopson A, Kutz C, et al. Whose preferences matter? A patient‐centered approach for eliciting treatment goals. Med Decis Making. 2018;38:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grande SW, O'Neill ES, Sherman AE, Coylewright M. Are older adults willing to consider new strategies to reduce stroke risk? Qual Health Res. 2017;29:568‐576. [DOI] [PubMed] [Google Scholar]
- 69. Pieterse AH, Stiggelbout AM, Montori VM. Shared decision making and the importance of time. JAMA. 2019;322:25–26. [DOI] [PubMed] [Google Scholar]
- 70. Wood DA, Lauck SB, Cairns JA, Humphries KH, Cook R, Welsh R, Leipsic J, Genereux P, Moss R, Jue J, et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) clinical pathway facilitates safe next‐day discharge home at low‐, medium‐, and high‐volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. JACC Cardiovasc Interv. 2019;12:459–469. [DOI] [PubMed] [Google Scholar]
- 71. Kotronias RA, Teitelbaum M, Webb JG, Mylotte D, Barbanti M, Wood DA, Ballantyne B, Osborne A, Solo K, Kwok CS, et al. Early versus standard discharge after transcatheter aortic valve replacement: a systematic review and meta‐analysis. JACC Cardiovasc Interv. 2018;11:1759–1771. [DOI] [PubMed] [Google Scholar]
- 72. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M; Medical Research Council G . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Naylor MD, Bowles KH, McCauley KM, Maccoy MC, Maislin G, Pauly MV, Krakauer R. High‐value transitional care: translation of research into practice. J Eval Clin Pract. 2013;19:727–733. [DOI] [PubMed] [Google Scholar]
- 74. Daughtridge GW, Archibald T, Conway PH. Quality improvement of care transitions and the trend of composite hospital care. JAMA. 2014;311:1013–1014. [DOI] [PubMed] [Google Scholar]
- 75. Patel DK, Duncan MS, Shah AS, Lindman BR, Greevy RA Jr, Savage PD, Whooley MA, Matheny ME, Freiberg MS, Bachmann JM. Association of cardiac rehabilitation with decreased hospitalization and mortality risk after cardiac valve surgery. JAMA Cardiol. 2019;4:1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home‐based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. [DOI] [PubMed] [Google Scholar]
- 77. Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, Sheikh A, Lopez B, Gonzalez A, Manisty C, et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lindman BR, Stewart WJ, Pibarot P, Hahn RT, Otto CM, Xu K, Devereux RB, Weissman NJ, Enriquez‐Sarano M, Szeto WY, et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv. 2014;7:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lindman BR, Alexander KP, O'Gara PT, Afilalo J. Futility, benefit, and transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014;7:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al; Investigators C . Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 81. Inohara T, Manandhar P, Kosinski AS, Matsouaka RA, Kohsaka S, Mentz RJ, Thourani VH, Carroll JD, Kirtane AJ, Bavaria JE, et al. Association of renin‐angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter aortic valve replacement. JAMA. 2018;320:2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lindman BR, Otto CM, Douglas PS, Hahn RT, Elmariah S, Weissman NJ, Stewart WJ, Ayele GM, Zhang F, Zajarias A, et al. Blood pressure and arterial load after transcatheter aortic valve replacement for aortic stenosis. Circ Cardiovasc Imaging. 2017;10:e006308. [DOI] [PubMed] [Google Scholar]
- 83. Lindman BR, Goel K, Bermejo J, Beckman J, O'Leary J, Barker CM, Kaiser C, Cavalcante JL, Elmariah S, Huang J, et al. Lower blood pressure after transcatheter or surgical aortic valve replacement is associated with increased mortality. J Am Heart Assoc. 2019;8:e014020 DOI: 10.1161/JAHA.119.014020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dangas GD, Tijssen JGP, Wohrle J, Sondergaard L, Gilard M, Mollmann H, Makkar RR, Herrmann HC, Giustino G, Baldus S, et al; Investigators G . A controlled trial of rivaroxaban after transcatheter aortic‐valve replacement. N Engl J Med. 2020;382:120–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


