Abstract
Background
Epidemiological data suggest a link between psychological stress and increased cardiovascular disease risk; however, the underlying mechanisms remain incompletely understood. The purpose of this investigation was to directly examine the influence of daily psychosocial stress on microvascular adrenergic vasoconstrictor responsiveness in healthy adults. We hypothesized increased daily psychosocial stress would be positively related to increased norepinephrine‐induced vasoconstriction.
Methods and Results
Eighteen healthy adults (19–36 years; 10 women) completed a daily psychosocial experiences telephone interview for 8 consecutive evenings in order to document their exposure and emotional responsiveness to common stressors (eg, arguments, work stress) over the preceding 24 hrs. On the last interview day, red cell flux (laser Doppler flowmetry) was measured during graded intradermal microdialysis perfusion of norepinephrine (10−12 to 10−2 mol/L) and expressed as a percentage of baseline vascular conductance. Exogenous norepinephrine elicited progressive and robust vasoconstriction in all individuals (maximal vasoconstriction: 71±4%base; cumulative vasoconstriction [area under the curve]: 118±102 arbitrary units). Participants experienced a stressor on 51±5% of days and a total of 5.2±0.9 stressors over the 8‐day time frame. Increased daily frequency of stressor exposure was positively related to both maximal (R 2=0.26; P=0.03) and cumulative (R 2=0.31; P=0.02) vasoconstrictor responsiveness. Likewise, the total number of stressors was associated with increased maximal (R 2=0.40; P<0.01) and cumulative (R 2=0.27; P=0.03) norepinephrine‐induced vasoconstriction. Neither stressor severity nor stress‐related emotions were related to vasoconstrictor responsiveness.
Conclusions
Collectively, these data suggest that daily psychosocial stressor exposure by itself is sufficient to adversely influence microvascular vasoconstrictor function, regardless of the perceived severity or emotional consequences of the stressor exposure.
Keywords: microdialysis, sympathetic reactivity, microvascular function, skin blood flow, daily stress
Subject Categories: Vascular Biology, Physiology
Clinical Perspective
What Is New?
The novel finding of this study is that increased cumulative exposure to common naturally occurring everyday psychosocial stressors was positively related to increased norepinephrine‐induced vasoconstriction in healthy young adults.
However, neither stressor severity nor stressor‐related emotional or cognitive processes were related to end‐organ vasoconstrictor responsiveness to exogenous norepinephrine.
What Are the Clinical Implications?
These data suggest that daily psychosocial stressor exposure by itself is sufficient to adversely influence microvascular vasoconstrictor function, regardless of the perceived severity, control over the stressor, or the emotional consequences of the exposure.
These findings add to the growing body of literature linking naturalistic daily psychosocial stress to impaired microvascular function and provide additional mechanistic insight into stress‐related cardiovascular risk.
Introduction
In response to stress, multiple cognitive, behavioral, and physiological feedback mechanisms are activated in order to protect the body from perceived threats and maintain homeostasis. However, it is now evident that a dysregulated acute stress response or chronic exposure to psychological stress can precipitate the development of both physical and mental health problems.1, 2, 3, 4, 5 In line with this, the INTERHEART study, spanning 52 countries and including ~25 000 individuals, prospectively linked chronic stress to increased cardiovascular risk,6 an association that was independent of, and similar in magnitude to, that imposed by traditional cardiovascular risk factors (eg, aging, hypertension, hypercholesterolemia, etc.). Despite this compelling epidemiological evidence, the mechanisms converting psychological stress into pathophysiological cardiovascular outcomes remain incompletely understood.
Activation of the sympathetic‐adrenal‐medullary axis is a critical component of the acute stress response. In this regard, acute psychological stress elicits large increases in plasma norepinephrine, at least in some individuals,7, 8, 9 the downstream effects of which include activation of adrenergic receptor‐mediated signaling pathways and subsequent increases in vasoconstrictor tone. However, excessive stress‐induced increases in sympathetic activity have been proposed to mediate the link between stress and cardiovascular disease,3, 7, 10, 11, 12 likely via the deleterious effect of excessive sympathetic outflow on the vasculature and its contribution to sustained increases in tonic blood pressure. Psychological stress causes transient impairments in vascular endothelial function,13, 14, 15 in part, via overactivation of vasoconstrictor mechanisms.15 Interestingly, longitudinal data suggest that heightened cardiovascular responsiveness to stress‐induced sympathetic activation is predictive of the future development of hypertension only in individuals with high amounts of daily life stress.16 This suggests that antecedent psychological stress exposure is potentially causally related to dysregulated sympathetic control of the vasculature. Although there is some evidence supporting a link between high amounts of daily life stress and increased stress‐induced norepinephrine release,17 to our knowledge, no studies have directly examined the influence of daily psychosocial stress on microvascular adrenergic vasoconstrictor responsiveness. Thus, the purpose of the present investigation was to assess whether common and mundane everyday psychosocial stressors modulate microvascular reactivity to direct exogenous administration of the sympathetic transmitter norepinephrine in healthy young adults. We hypothesized that increased daily psychosocial stress exposure would be positively related to increased norepinephrine‐induced vasoconstriction.
Methods
Participants
The institutional review board at The Pennsylvania State University (IRB 9617) and the Food and Drug Administration (IND 125 994) approved all experimental procedures and protocols. Verbal and written informed consent was obtained voluntarily from all participants in accordance with the guidelines set forth by the Declaration of Helsinki. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Eighteen young adults participated (10 women; 19–36 years; body mass index 25.0±3.4 kg/m2; resting blood pressure 117±2 / 72±1 mm Hg; resting heart rate 70±2 bpm). All participants were free of cardiovascular, renal, metabolic, and neurological disease; did not use tobacco products; were recreationally active; and were not taking prescription medications, with the exception of hormonal birth control (n=8). Norepinephrine‐induced vasoconstriction in these 8 women was not different from that in the 10 other participants (P>0.05); thus, these data were analyzed collectively. A urine pregnancy test confirmed the absence of pregnancy.
Assessment of Daily Psychosocial Stress
As previously described in detail,18, 19 daily psychosocial stressors were assessed every day for 8 consecutive days (the experimental visit described subsequently occurred on day 8) using the Daily Inventory of Stressful Events, which consists of stem questions asking whether any of 7 types of daily stressors occurred in the past 24 hours: argument, argument avoidance, stressful event at work or school, stressful event at home, discrimination, network stress (ie, stressful event that happened to a close friend or relative), or any other stressful event. Thus, this assessment of daily psychosocial stress captures specific events that occurred within the 24 hours prior to each telephone interview and is not intended to assess the effects of chronic stress occurring over much longer durations (eg, living in poverty, being in an abusive relationship). Obtaining daily psychosocial stressor information over this short‐term time frame helps in alleviating concerns regarding ecological validity and retrospective memory distortions that can occur over longer periods of time.19 All participants completed every daily interview.
A dichotomous variable was created to indicate the occurrence of any stressor that day (1=yes, 0=no). From these data, two stressor exposure indicators were calculated: stressor frequency (ie, percentage of interview days during which at least one stressor occurred) and total stressors (ie, total number of stressors reported across all interview days). Using a 4‐point Likert scale (0=none at all, 1=a little, 2=some, 3=a lot), participants also rated the severity of the stressor, the amount of time they spent thinking about the stressor (ie, rumination), and the amount of control they felt over the stressor, as well as the contribution of the stressor to any of four negative emotions (angry, sad, nervous, shameful; 0=not at all, 1=not very, 2=somewhat, 3=very). The mean of these indicators was calculated by averaging the ratings for all stressors within that day and then aggregating scores across the 8 interview days. In those individuals who did not experience any stressors, these stressor indices were not calculated.
Daily affect was assessed using a validated scale.20, 21 Participants rated the frequency of 14 positive (in good spirits, cheerful, extremely happy, calm and peaceful, satisfied, full of life, close to others, like you belong, enthusiastic, attentive, proud, active, and confident) and 14 negative (restless or fidgety, nervous, worthless, so sad nothing could cheer you up, everything was an effort, hopeless, lonely, afraid, jittery, irritable, ashamed, upset, angry, and frustrated) emotions using a 5‐point scale (0=none of the time, 1=a little of the time, 2=some of the time, 3=most of the time, 4=all of the time). The emotion item ratings were averaged to obtain daily positive and negative affect scores and scores were aggregated for the 8 interview days. Participants also indicated whether they had experienced any of 28 physical symptoms each day (eg, headache, fatigue, cough, etc.); the responses were summed to provide the number of daily stress‐related physical symptoms.22
Assessment of Sympathetic Microvascular Reactivity
Participants refrained from alcoholic and caffeinated beverages for 12 hours and vigorous physical activity for 24 hours before the experimental visit. One intradermal microdialysis probe (CMA Linear 30 probe, 6 kDa; Harvard Apparatus, Cambridge, MA) was inserted into the dermal layer of the ventral forearm using sterile technique for the local delivery of pharmacological agents, as previously described in detail and standardized in our laboratory.23, 24, 25 Norepinephrine was prepared immediately before use, dissolved in lactated Ringer solution with 0.5 mg/mL (2.8 mmol/L) ascorbic acid (Sigma, St. Louis, MO) as a preservative,23, 26, 27, 28 filtered using sterile syringe microfilters (Acrodisc, Pall, Port Washington, NY), wrapped in foil to prevent photodegradation, and subsequently perfused through the microdialysis probes at a rate of 2 μL/min (Bee Hive controller and Baby Bee microinfusion pumps, BASi, West Lafayette, IN). Although local ascorbic acid perfusion has been shown to blunt the adrenergic vasoconstrictor response to local cooling in human skin,29 in our laboratory, the administration of this low concentration of ascorbic acid alone does not influence cutaneous vasomotor tone; therefore, it is unlikely that the addition of ascorbic acid as a preservative contributed to the observed responses in this study.
During an initial hyperemia resolution period (~60 minutes) and during baseline measures (~10 minutes), lactated Ringer solution perfused the fiber. Thereafter, increasing concentrations of norepinephrine (10−12–10−2 mol/L; United States Pharmacopeia, Rockville, MD) were sequentially perfused for 5 minutes each. Red blood cell flux, an index of cutaneous blood flow, was continuously measured directly over the microdialysis fiber using an integrated laser Doppler flowmeter probe placed in a local heating unit (VP12 and VHP2, Moor Instruments, Wilmington, DE) set to thermoneutrality (33°C). Automated brachial blood pressure (Spot Connex, Welch Allyn, Skaneateles Falls, NY) was measured every 5 minutes throughout the protocol.
Data and Statistical Analysis
Red cell flux was recorded at 40 Hz (PowerLab and LabChart, AD Instruments, Sydney, Australia). Vascular conductance was calculated as laser Doppler flux (perfusion units) divided by mean arterial pressure, normalized as a percentage of baseline (%base), and averaged during 5 minutes of baseline and the last 1 minute of each norepinephrine dose.23, 26, 27, 28 Vasoconstriction at the highest dose of norepinephrine was used as an index of maximal adrenergic vasoconstriction. Given that exogenous intradermal microdialysis perfusion of the selected range of concentrations of norepinephrine yields a biphasic response (ie, vasodilation and/or loss of vasoconstrictor tone at lower doses and progressive vasoconstriction at higher doses),23, 26, 27, 28, 30 owing to the differential effects of α‐ and β‐adrenergic receptors,23 the net area under the curve was calculated as the negative peak area subtracted from the positive peak area and used as to reflect cumulative adrenergic vasoconstriction (Prism 8, GraphPad, La Jolla, CA). Linear regression was used to determine the relation between daily psychosocial stress and norepinephrine‐induced vasoconstriction (Prism 8, GraphPad). Given the robust nature of these physiological measures,23, 26, 27 using an estimated standard deviation of 0.15 perfusion units·mmHg−1 and a physiologically meaningful slope of 0.1, we calculated a priori that 15 subjects would be necessary to observe a medium effect size given varying daily stressor exposure (G*Power 3.1.9.4). The sample size was increased to account for potential subject dropout and/or missing daily interview data. Significance was set at α<0.05 and data are presented as mean±standard error of the mean.
Results
Consistent with previous reports,23, 26, 27, 28 exogenous norepinephrine elicited a biphasic response, whereby low concentrations caused modest vasodilation and/or loss of vasoconstrictor tone and higher concentrations elicited progressive vasoconstriction (Figure 1). Despite increased variability at the lowest doses of norepinephrine, this response was reliably observed in all subjects. Maximal norepinephrine‐induced vasoconstriction was 71±4%base (range: 42–96%base). The net area under the curve was 118±102 arbitrary units, though there was significant interindividual heterogeneity in this parameter owing to a prominent initial dilatory response in some individuals (range: −1096–615 arbitrary units).
Figure 1. Group summary data for exogenous norepinephrine (NE)‐induced cutaneous vasoconstriction in healthy young adults (n=18; 10 women).
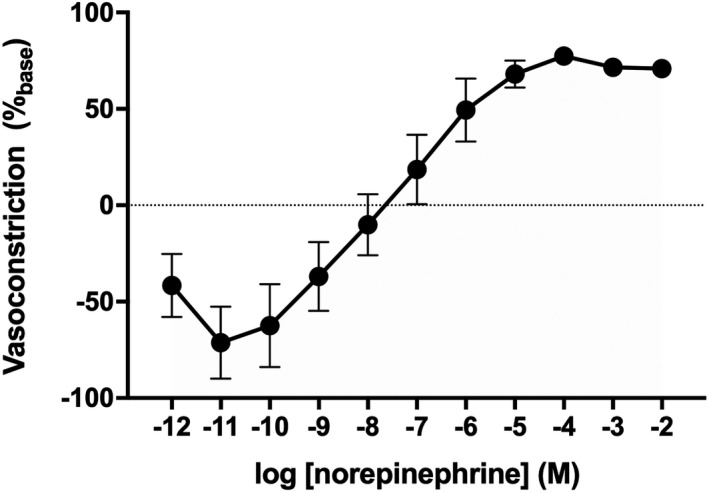
Data are mean±SE and are expressed as a percentage of baseline vascular conductance.
On average, over the 8‐day time frame of daily stress interviews, participants experienced a psychosocial stressor on 51±6% (range: 0–88%) of days and a total of 5.2±0.9 (range: 0–14) stressors. Additional daily stress processes are presented in the Table. Increased daily frequency of exposure to at least one psychosocial stressor was positively related to both increased maximal vasoconstriction (Figure 2A; P=0.03) and increased cumulative responsiveness (Figure 2B; P=0.02). Further, the total number of psychosocial stressors was likewise positively related to increased maximal norepinephrine‐induced vasoconstriction (Figure 3A; P<0.01), as well as cumulative vasoconstrictor responsiveness over the full range of doses of norepinephrine (Figure 3B; P=0.03). Neither maximal nor cumulative adrenergic vasoconstrictor responsiveness were related to stressor severity, stressor‐related rumination, the amount of control over the stressor, stressor‐induced emotional responsiveness, or physical symptoms (Table; all P>0.05), with the exception of a significant relation between net area under the curve and negative affect (Table; P=0.04).
Table 1.
The Relation Between Daily Psychosocial Stress and Vasoconstrictor Responsiveness to Norepinephrine
| Stress Process | Mean | Maximal Vasoconstriction (%base) | Cumulative Vasoconstriction (AU) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | y‐Intercept | R 2 | P Value | Slope | y‐Intercept | R 2 | P Value | ||
| Stressor severity (range 0–3) | 2.1±0.2 | −5.7 | 85.2 | 0.07 | 0.32 | 28.6 | 125.6 | 0.01 | 0.82 |
| Stressor‐related rumination (range 0–3) | 1.9±0.2 | 2.5 | 67.3 | 0.02 | 0.61 | 144.5 | −97.0 | 0.14 | 0.13 |
| Amount of control over stressor (range 0–3) | 1.7±0.2 | −1.6 | 73.6 | 0.01 | 0.71 | 46.0 | 39.5 | 0.01 | 0.70 |
| Stressor‐related negative emotions (range 0–3) | 1.2±0.2 | 4.3 | 65.8 | 0.04 | 0.43 | 221.4 | −142.9 | 0.14 | 0.13 |
| Negative affect (range 0–4) | 1.0±0.2 | 4.6 | 66.1 | 0.06 | 0.31 | 243.4 | −135.3 | 0.24 | 0.04 |
| Positive affect (range 0–4) | 3.0±0.1 | −13.4 | 110.6 | 0.14 | 0.12 | −342.7 | 1132.0 | 0.12 | 0.15 |
| No. of daily physical symptoms | 0.7±0.1 | 5.9 | 66.5 | 0.05 | 0.38 | 250.4 | −67.8 | 0.12 | 0.16 |
Data are mean±SE. AU indicates arbitrary units.
Figure 2. The relation between the frequency of psychosocial stress exposure and maximal (A) and cumulative (B) norepinephrine (NE)‐induced vasoconstriction in healthy young adults (n=18; 10 women).
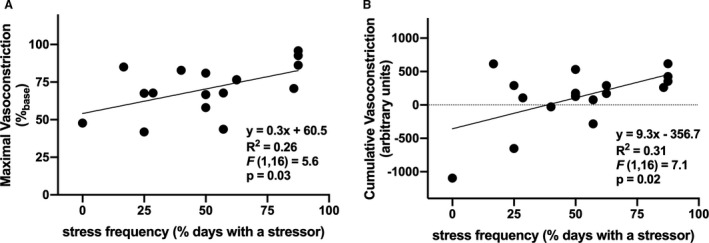
Increased psychosocial stress exposure was positively related to increased norepinephrine‐induced vasoconstriction.
Figure 3. The relation between the total number of psychosocial stressors and maximal (A) and cumulative (B) norepinephrine (NE)‐induced vasoconstriction in healthy young adults (n=18; 10 women).
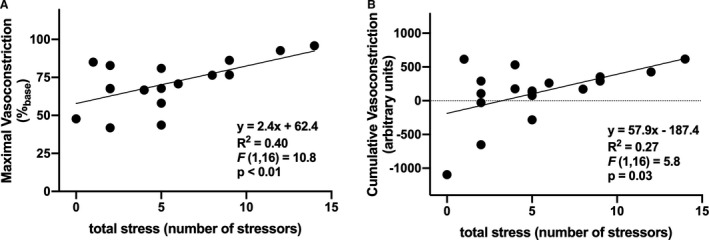
Increased total psychosocial stress was positively related to increased vasoconstrictor responsiveness to exogenous norepinephrine.
Discussion
The primary novel finding of the present study is that increased cumulative exposure to common naturally occurring everyday psychosocial stressors was positively related to increased norepinephrine‐induced vasoconstriction in healthy young adults. However, neither stressor severity nor stressor‐related emotional or cognitive processes were related to end‐organ vasoconstrictor responsiveness to exogenous norepinephrine. Taken together, these data suggest that daily psychosocial stressor exposure by itself is sufficient to adversely influence microvascular vasoconstrictor function, regardless of the perceived severity, control over the stressor, or the emotional consequences of the exposure. These findings add to the growing body of literature linking naturalistic daily psychosocial stress to impaired microvascular function24, 31, 32 and provide additional mechanistic insight to stress‐related cardiovascular risk.
Exposure to stress is a powerful activator of the sympathetic nervous system,1 and the resultant release of the sympathetic neurotransmitter norepinephrine is essential for the cardiovascular alterations that prepare an organism for “fight or flight.”33 In the laboratory setting, application of acute psychological stressors (eg, mental arithmetic, anxiety‐provoking speech tasks, cognitive challenges, etc.) elicits significant increases in blood pressure.34, 35, 36 The stress‐induced pressor response is thought to be mediated, in large part, by increased sympathetic nervous system activity, though there is substantial heterogeneity in sympathetic reactivity to acute psychological stress.7, 37 Nevertheless, sympathetic hyperreactivity to stress has been prospectively linked to increased cardiovascular disease risk,3 clearly highlighting the clinical importance of stress‐induced overactivation of the sympathetic nervous system. Moreover, it appears that heightened cardiovascular responsiveness to stress is most predictive of the future development of high blood pressure in individuals with high amounts of daily life stress,16 suggesting an important link between persistent chronic life stress and dysregulation of the acute stress response.
In this regard, increased daily psychosocial stress has been associated with autonomic dysfunction.18, 38 Broadly consistent with these previous reports, the novel findings of the present study demonstrate that cumulative exposure to common everyday psychosocial stressors was positively related to increased microvascular vasoconstrictor responsiveness to exogenous norepinephrine. This linear association was evident for maximal vasoconstriction, as well as for cumulative adrenergic vasoconstrictor responsiveness assessed across the broad range of concentrations of norepinephrine utilized in this study. The increased transduction of a given sympathetic stimulus into the resultant vasoconstrictor response may therefore potentially contribute increased stress‐induced cardiovascular risk via its adverse effect on vascular health.1, 12 This concept is largely consistent with the evidence indicating that psychological stress impairs vascular endothelial function.13, 14, 15 Our laboratory recently confirmed and extended these findings, demonstrating that acute exposure (within 1 day) to any naturally occurring everyday psychosocial stressor was related to more severe deficits in microvascular endothelial function in young adults with depression.24 Thus, the collective vascular consequences of daily psychosocial stressor exposure—heightened sympathetic vasoconstriction coupled with impaired endothelium‐dependent dilation—likely work in concert to the detriment of cardiovascular health.
Previous studies that have attempted to examine the extent to which antecedent chronic life stress influences end‐organ responsiveness to sympathetic stimulation are equivocal.17, 39, 40 An important caveat to these studies is their reliance on nonspecific (ie, blood pressure) and indirect (ie, plasma norepinephrine) indices of sympathetic activation in response to acute stress. Instead, we directly measured target effector organ responsiveness to discrete sympathetic stimulation and quantified the resultant vasoconstrictor response in relation to daily psychosocial stress exposure, which we assessed using an ecologically valid comprehensive daily interview paradigm.18, 19, 24 This stress assessment methodology captures aspects of daily stress as they unfold, namely stressor frequency, severity, and related emotions, allowing the quantification of exposure to, as well as the cognitive appraisal of, minor stressful events in everyday life. Further, given their relatively short timeframes, daily interview studies address concerns regarding potential memory bias.41 Our data clearly demonstrate that increased exposure to daily psychosocial stress translates to increased vascular reactivity to direct sympathetic stimulation in healthy young adults. Speculatively, the accumulation of daily stressor exposure and subsequent increase in allostatic load and vasoconstrictor tone may therefore catalyze accelerated vascular aging and cardiovascular disease trajectories. Large‐scale prospective longitudinal studies are clearly necessary to better understand the mechanistic link between psychosocial stress exposure and microvascular sympathetic reactivity in contributing to cardiovascular risk.
We did not observe a significant relation between any cognitive or emotional processes associated with daily psychosocial stress exposure and vasoconstrictor responsiveness. Taken together, these findings suggest that daily stress exposure by and of itself is sufficient to precipitate enhanced microvascular adrenergic vasoconstrictor responsiveness, whereas the perception of severity and cognitive and emotional processes related to stressor exposure do not appear to influence vascular reactivity to direct sympathetic stimulation, at least in healthy young adults. These findings were somewhat surprising given that emotional responsiveness (ie, affective reactivity) to psychosocial stress may be even more predictive of long‐term disease risk than stressor exposure per se.18, 22, 41, 42 For example, midlife and older adults who perceived their stressors to be more severe or who demonstrated greater increases in negative affect when faced with stressors exhibited blunted heart rate variability, whereas the frequency of daily stressors did not affect cardiac autonomic control.18 The authors interpret their findings to suggest that perception and emotional responsiveness to daily stressors are relatively more important for cardiovascular health than exposure to stressful events. Interestingly, we did detect a significant relation between negative affect and cumulative microvascular responsiveness to norepinephrine, suggesting that the detrimental emotional toll of daily stress may indeed amplify the vascular response to stress‐induced sympathetic activation and contribute to a proatherogenic milieu. Larger studies that include individuals with a more diverse health history are required to better understand the link between daily psychosocial stress “affective reactivity” and sympathetic‐mediated vasoconstriction.
Although adrenergic receptors play a critical role in mediating the response to stress, the coordinated action of multiple blood pressure regulatory systems is required for adequate cardiovascular regulation during exposure to stressful events. Data from an animal model demonstrate that endothelin‐1 contributes to the pressor response to chronic stress, though whether this effect is mediated independently of increased adrenergic sensitivity remains unclear.43, 44 In humans, acute psychological stress‐induced vascular dysfunction occurs, in part, via activation of endothelin‐A receptors.15 The current study was not designed to probe the specific downstream signaling pathways affected by direct administration of norepinephrine to the microvasculature. However, given the aforementioned potential of an interaction between norepinephrine‐ and endothelin‐1‐mediated vasoconstriction, coupled with notable stress‐induced increases in plasma concentrations of these signaling molecules, this warrants future investigation.
Perspectives and Conclusions
Investigations of the mechanisms by which chronic stress increases cardiovascular risk are technically challenging and often not feasible given that experimentally imposing long‐term psychological stress in humans is unethical. Moreover, naturalistic chronic stress study designs are complex and difficult to conduct and involve inherent potential for self‐selection bias. To circumvent this, the majority of studies that have examined stress‐induced alterations in vascular function have assessed responsiveness to the application of acute laboratory‐based psychological stressors, on the assumption of a link between reactivity to acute laboratory stressors and naturalistic stress.36 However, evidence for such generalizability remains controversial,36 though recent investigations that have used technological advances in methodology to simultaneously collect ambulatory cardiovascular data in conjunction with episodic stress exposure indicate better generalizability between lab and life stress.45, 46 We posit that our approach in the present investigation—the coupling of an ecologically valid assessment of short‐lived but pervasive naturally occurring everyday psychosocial stressors with the direct measurement of microvascular responsiveness to sympathetic stimulation using a targeted in vivo pharmacological approach—reflects an important conceptual advancement in the assessment of the link between psychosocial stress and cardiovascular regulation. Interestingly, intervention strategies to counter the detrimental cognitive and emotional consequences of stressor exposure appear to secondarily reduce basal sympathetic outflow.47 Whether increasing psychological resiliency to stress also translates to reduced microvascular reactivity to sympathetic stimulation has not yet been examined but represents an exciting avenue for future research.
Sources of Funding
This work was supported by National Institutes of Health (NIH) awards HL133414 (JLG), T32 AG049676 (AS), and the National Center for Advancing Translational Sciences UL1 TR002014, and the Penn State Social Science Research Institute (JLG, LMA, DMA).
Disclosures
None.
Acknowledgments
We greatly appreciate the effort expended by the volunteer participants. We thank Susan Slimak, RN, Jane Pierzga, MS, Gabrielle Dillon, BS, and Tina Hoy, BS for their assistance.
(J Am Heart Assoc. 2020;9:e015697 DOI: 10.1161/JAHA.119.015697.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. [DOI] [PubMed] [Google Scholar]
- 2. Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta‐analysis of prospective evidence. Hypertension. 2010;55:1026–1032. [DOI] [PubMed] [Google Scholar]
- 3. Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18‐year follow‐up study. Hypertension. 2008;52:336–341. [DOI] [PubMed] [Google Scholar]
- 4. Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress—a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547–560. [DOI] [PubMed] [Google Scholar]
- 5. Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. [DOI] [PubMed] [Google Scholar]
- 6. Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐Amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
- 7. Dimsdale JE, Moss J. Short‐term catecholamine response to psychological stress. Psychosom Med. 1980;42:493–497. [DOI] [PubMed] [Google Scholar]
- 8. Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Long‐term stability of cardiovascular and catecholamine responses to stress tests: an 18‐year follow‐up study. Hypertension. 2010;55:131–136. [DOI] [PubMed] [Google Scholar]
- 9. Trico D, Fanfani A, Varocchi F, Bernini G. Endocrine and haemodynamic stress responses to an arithmetic cognitive challenge. Neuro Endocrinol Lett. 2017;38:182–186. [PubMed] [Google Scholar]
- 10. Boudou N, Despas F, Rothem JV, Lairez O, Elbaz M, Vaccaro A, Lebrin M, Pathak A, Carrie D. Direct evidence of sympathetic hyperactivity in patients with vasospastic angina. Am J Cardiovasc Dis. 2017;7:83–88. [PMC free article] [PubMed] [Google Scholar]
- 11. Lambert E, Dawood T, Straznicky N, Sari C, Schlaich M, Esler M, Lambert G. Association between the sympathetic firing pattern and anxiety level in patients with the metabolic syndrome and elevated blood pressure. J Hypertens. 2010;28:543–550. [DOI] [PubMed] [Google Scholar]
- 12. Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiedorowicz JG, Ellingrod VL, Kaplan MJ, Sen S. The development of depressive symptoms during medical internship stress predicts worsening vascular function. J Psychosom Res. 2015;79:243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. [DOI] [PubMed] [Google Scholar]
- 15. Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin‐A receptors. Circulation. 2002;105:2817–2820. [DOI] [PubMed] [Google Scholar]
- 16. Light KC, Girdler SS, Sherwood A, Bragdon EE, Brownley KA, West SG, Hinderliter AL. High stress responsivity predicts later blood pressure only in combination with positive family history and high life stress. Hypertension. 1999;33:1458–1464. [DOI] [PubMed] [Google Scholar]
- 17. Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintick J, Costlow C, Irwin MR. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med. 1997;59:447–457. [DOI] [PubMed] [Google Scholar]
- 18. Sin NL, Sloan RP, McKinley PS, Almeida DM. Linking daily stress processes and laboratory‐based heart rate variability in a national sample of midlife and older adults. Psychosom Med. 2016;78:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: an interview‐based approach for measuring daily stressors. Assessment. 2002;9:41–55. [DOI] [PubMed] [Google Scholar]
- 20. Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalences and trends in non‐specific psychological distress. Psychol Med. 2002;32:959–976. [DOI] [PubMed] [Google Scholar]
- 21. Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. J Pers Soc Psychol. 1998;75:1333–1349. [DOI] [PubMed] [Google Scholar]
- 22. Leger KA, Charles ST, Ayanian JZ, Almeida DM. The association of daily physical symptoms with future health. Soc Sci Med. 2015;143:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Lack of limb or sex differences in the cutaneous vascular responses to exogenous norepinephrine. J Appl Physiol. 2014;117:1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greaney JL, Koffer RE, Saunders EFH, Almeida DM, Alexander LM. Self‐reported everyday psychosocial stressors are associated with greater impairments in endothelial function in young adults with major depressive disorder. J Am Heart Assoc. 2019;8:e010825 DOI: 10.1161/JAHA.118.010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greaney JL, Saunders EFH, Santhanam L, Alexander LM. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res. 2019;124:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greaney JL, Kenney WL, Alexander LM. Neurovascular mechanisms underlying augmented cold‐induced reflex cutaneous vasoconstriction in human hypertension. J Physiol. 2017;595:1687–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Impaired increases in skin sympathetic nerve activity contribute to age‐related decrements in reflex cutaneous vasoconstriction. J Physiol. 2015;593:2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension. 2017;70:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamazaki F. Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J Appl Physiol. 2010;108:328–333. [DOI] [PubMed] [Google Scholar]
- 30. Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clin Sci (Lond). 2017;131:2777–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Aart CJC, Nawrot TS, Sioen I, De Boever P, Zaqout M, De Henauw S, Michels N. Longitudinal association between psychosocial stress and retinal microvasculature in children and adolescents. Psychoneuroendocrinology. 2018;92:50–56. [DOI] [PubMed] [Google Scholar]
- 32. Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO III. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76:125–130. [DOI] [PubMed] [Google Scholar]
- 33. Cannon WB. The wisdom of the body, 2nd ed Oxford, England: Norton & Co.; 1939. [Google Scholar]
- 34. Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carter JR, Durocher JJ, Kern RP. Neural and cardiovascular responses to emotional stress in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1898–R1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carter JR, Goldstein DS. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol. 2015;5:119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol. 2009;296:H847–H853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. 2005;46:1201–1206. [DOI] [PubMed] [Google Scholar]
- 39. Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory‐induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull. 2008;134:829–885. [DOI] [PubMed] [Google Scholar]
- 40. Matthews KA, Gump BB, Owens JF. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychol. 2001;20:403–410. [PubMed] [Google Scholar]
- 41. Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci. 2010;65:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiang JJ, Turiano NA, Mroczek DK, Miller GE. Affective reactivity to daily stress and 20‐year mortality risk in adults with chronic illness: findings from the National Study of Daily Experiences. Health Psychol. 2018;37:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fox BM, Becker BK, Loria AS, Hyndman KA, Jin C, Clark H, Johns R, Yanagisawa M, Pollock DM, Pollock JS. Acute pressor response to psychosocial stress is dependent on endothelium‐derived endothelin‐1. J Am Heart Assoc. 2018;7:e007863 DOI: 10.1161/JAHA.117.007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Becker BK, Speed JS, Powell M, Pollock DM. Activation of neuronal endothelin B receptors mediates pressor response through alpha‐1 adrenergic receptors. Physiol Rep. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnston DW, Tuomisto MT, Patching GR. The relationship between cardiac reactivity in the laboratory and in real life. Health Psychol. 2008;27:34–42. [DOI] [PubMed] [Google Scholar]
- 46. Kamarck TW, Schwartz JE, Janicki DL, Shiffman S, Raynor DA. Correspondence between laboratory and ambulatory measures of cardiovascular reactivity: a multilevel modeling approach. Psychophysiology. 2003;40:675–683. [DOI] [PubMed] [Google Scholar]
- 47. Park J, Lyles RH, Bauer‐Wu S. Mindfulness meditation lowers muscle sympathetic nerve activity and blood pressure in African‐American males with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2014;307:R93–R101. [DOI] [PMC free article] [PubMed] [Google Scholar]


