Abstract
Background
Timely recognition of patients with acute coronary syndromes (ACS) is important for successful treatment. Previous research has suggested that women with ACS present with different symptoms compared with men. This review assessed the extent of sex differences in symptom presentation in patients with confirmed ACS.
Methods and Results
A systematic literature search was conducted in PubMed, Embase, and Cochrane up to June 2019. Two reviewers independently screened title‐abstracts and full‐texts according to predefined inclusion and exclusion criteria. Methodological quality was assessed using the Newcastle‐Ottawa Scale. Pooled odds ratios (OR) with 95% CI of a symptom being present were calculated using aggregated and cumulative meta‐analyses as well as sex‐specific pooled prevalences for each symptom. Twenty‐seven studies were included. Compared with men, women with ACS had higher odds of presenting with pain between the shoulder blades (OR 2.15; 95% CI, 1.95–2.37), nausea or vomiting (OR 1.64; 95% CI, 1.48–1.82) and shortness of breath (OR 1.34; 95% CI, 1.21–1.48). Women had lower odds of presenting with chest pain (OR 0.70; 95% CI, 0.63–0.78) and diaphoresis (OR 0.84; 95% CI, 0.76–0.94). Both sexes presented most often with chest pain (pooled prevalences, men 79%; 95% CI, 72–85, pooled prevalences, women 74%; 95% CI, 72–85). Other symptoms also showed substantial overlap in prevalence. The presence of sex differences has been established since the early 2000s. Newer studies did not materially change cumulative findings.
Conclusions
Women with ACS do have different symptoms at presentation than men with ACS, but there is also considerable overlap. Since these differences have been shown for years, symptoms should no longer be labeled as “atypical” or “typical.”
Keywords: acute coronary syndrome, diagnosis, meta‐analysis, sex differences, symptoms, systematic review
Subject Categories: Epidemiology, Women
Clinical Perspective
What Is New?
Symptoms experienced by men and women with confirmed acute coronary syndromes show substantial overlap.
Yet some sex differences in symptoms exist as women have higher odds of experiencing pain between the shoulder blades, nausea or vomiting and shortness of breath, and lower odds of experiencing chest pain or diaphoresis.
These differences and similarities between women and men with confirmed acute coronary syndromes in symptoms experienced have been established in literature for more than a decade.
What Are the Clinical Implications?
Symptoms of acute coronary syndromes should no longer be labeled as “typical” or “atypical” for women and/or men.
Attention for sex differences in symptoms of acute coronary syndromes should be proportional to the large overlap in symptoms of acute coronary syndromes between women and men.
Introduction
Ischemic heart disease (IHD) is the world's leading cause of eath accounting for an estimated 9 million deaths in 2015.1, 2 Acute coronary syndrome (ACS) is an umbrella term for unstable angina (UA), non–ST‐segment–elevation myocardial infarction (NSTEMI) or ST‐segment–elevation myocardial infarction (STEMI) and is a substantial component of IHD.3 In recent decades ACS mortality has decreased, because of advancements in treatment, lifestyle changes, and a focus on primary prevention, but rates remain high.1, 4
Effective treatment of ACS is available with reperfusion therapies, preferably with percutaneous coronary intervention and if not available thrombolysis or coronary artery bypass grafting.3 The efficacy of ACS treatment depends on timely initiation of the required treatment to minimize myocardial damage.3 Delayed symptom recognition, both by patients and medical professionals, is an important contributor to delay in treatment and subsequent ACS mortality.5 Despite several awareness campaigns, referral delay in women with ACS has persisted over time.6, 7
Previous studies, but not all, have reported that women with ACS experience different symptoms compared with men with ACS.8, 9, 10 Additionally, other studies emphasize the overlap in symptoms between men and women with ACS.11 Symptoms experienced by women with ACS are often labeled as “atypical” if these are different to those experienced by men. Previous systematic reviews of sex differences in symptoms of patients with ACS have been inconsistent, with varying in‐ and exclusion criteria and studies lacking standardized data collection.12, 13 Recent studies have attempted to solve these issues, with the development of standardized data collection surveys.11, 14
The purpose of this systematic review and meta‐analysis is to assess the presence and extent of sex differences in symptom presentation in patients with confirmed ACS. It updates and extends earlier meta‐analyses on this topic.12, 15 Furthermore, through cumulative meta‐analyses in which studies are added to the pooled findings in order of publication date, we aim to determine for how long evidence, where it exists, has been established in the literature. As much has been published on this topic, we hypothesize that recent studies have mainly been confirmatory. Throughout this review, “sex” will be used when referring to differences between men and women. “Gender” is not used, as social and cultural differences are not examined.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Search Strategy
A systematic literature search was performed in PubMed, EMBASE, and the Cochrane Library up to June 2019. A combination of the search terms symptom, presentation, gender, sex, acute coronary syndrome, myocardial infarction and unstable angina and synonyms of these terms were used (Data S1). Duplicates were removed using Mendeley Reference Manager. Titles and abstracts and, subsequently, the full‐texts of the articles were screened. Screening and study selection were done by 2 independent reviewers (RO and AB) according to predefined inclusion and exclusion criteria and discrepancies were resolved through discussion between these 2 reviewers. In addition, references of included articles and previous reviews on symptom presentation in patients with ACS were checked, and citation tracking was performed on the included studies.
Inclusion and Exclusion Criteria
We included all studies that reported on symptom presentation in both women and men with confirmed ACS. ACS was defined as either myocardial infarction (STEMI or NSTEMI) or UA, in accordance with American Heart Association (AHA) clinical guidelines.16 ACS is diagnosed by the presence of symptoms of myocardial infarction, new ECG changes and elevated levels of cardiac enzymes. Studies were excluded if they reported on patients with other cardiac conditions or if specific symptoms were required for inclusion in the study. Further exclusion criteria were full texts being unavailable, other publication type such as conference abstracts or reviews, and articles published in languages other than English, Dutch, French, or German. If 2 studies examined the same study population, the study with the largest sample size was included.
Quality Assessment
An adapted version of the Newcastle‐Ottawa Scale was used to assess the quality of the included studies. The scoring system is based on 3 components, namely Selection, Comparability, and Outcome.17 Studies using random or consecutive patient selection were allocated 4 stars for Selection. Studies adjusting for multiple covariables, including age, were allocated 2 stars for Comparability. Studies that independently assessed the symptoms experienced were allocated 1 star for Outcome (Data S2). Studies with a high risk of selection bias, defined as all other ways of population selection except a random or consecutive approach, were excluded.
Data Collection and Extraction
The study design, method of data collection, patient population, sample size, demographic characteristics and covariable adjustments were extracted for all included studies and reviewed by 2 authors (RO and AB). All reported symptoms were derived from the studies. An overview of the symptoms reported in the individual studies can be found in Table S1. The following symptoms were combined: dizziness or lightheadedness, left arm and left shoulder pain, nausea or vomiting, right arm and right shoulder pain, and stomach or epigastric pain. In 1 study,18 the presence of symptoms was categorized as present, unknown, or absent. In this case, the unknown symptoms were treated as if the symptom was absent. The outcomes of interest were the symptoms experienced when presenting with ACS. This was summarized as the odds ratio (OR) of the symptom being present in women relative to men and, if available, the OR adjusted for at least age. If the OR was not provided by a study, it was calculated using the provided data.
Statistical Analysis
Aggregated and cumulative meta‐analyses were performed for both the crude and adjusted OR using a random effects model with inverse variance weighting. To assess when a possible significant observed sex difference was established in literature, cumulative meta‐analysis was performed for symptoms that showed a significant sex difference in the aggregated meta‐analysis. For the cumulative meta‐analysis, we identified the year after which the pooled OR was statistically significant and did not notably change by adding later studies. Sex‐specific prevalence for all symptoms and its variance was calculated for each study. Meta‐analysis for the prevalence was performed with a random effects model with inverse variance weighting, 2 separate models were fitted for men and women. Heterogeneity was assessed by visual inspection and using the I2 statistics. I2 values <40% were considered to represent low heterogeneity, 30% to 60% moderate heterogeneity, 50% to 90% substantial heterogeneity and >75% as considerable heterogeneity.19 To explore possible heterogeneity by age, random effects meta‐regression was performed for symptoms reported by ≥10 studies. Analyses were performed by mean age of the participants (≤65 years and >65 years). Details of subgroup division are provided in Table S2. A P value of P<0.05 is considered statistically significant. Publication bias was assessed using funnel plots if ≥10 studies were available for an individual symptom. All statistical analyses were performed using the “Metafor” package in R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Selection and Quality Assessment
The systematic search yielded 3750 unique articles. After title‐abstract, full‐text screening, and citation tracking 31 studies remained. Results of the quality assessment can be found in Table S3. The majority of studies scored between 5 and 6 out of 7 stars. Four studies20, 21, 22, 23 were excluded after quality assessment because of a high risk of selection bias attributable to the use of convenience sampling. Thus, 27 studies were included in the review (Figure 1). The funnel plots for most symptoms were fairly symmetric, indicating a low risk of publication bias (Figures S1–S10). Diaphoresis was an exception, where there seems to be an overrepresentation of smaller studies with a higher odds for women to present with diaphoresis (Figure S11).
Figure 1.
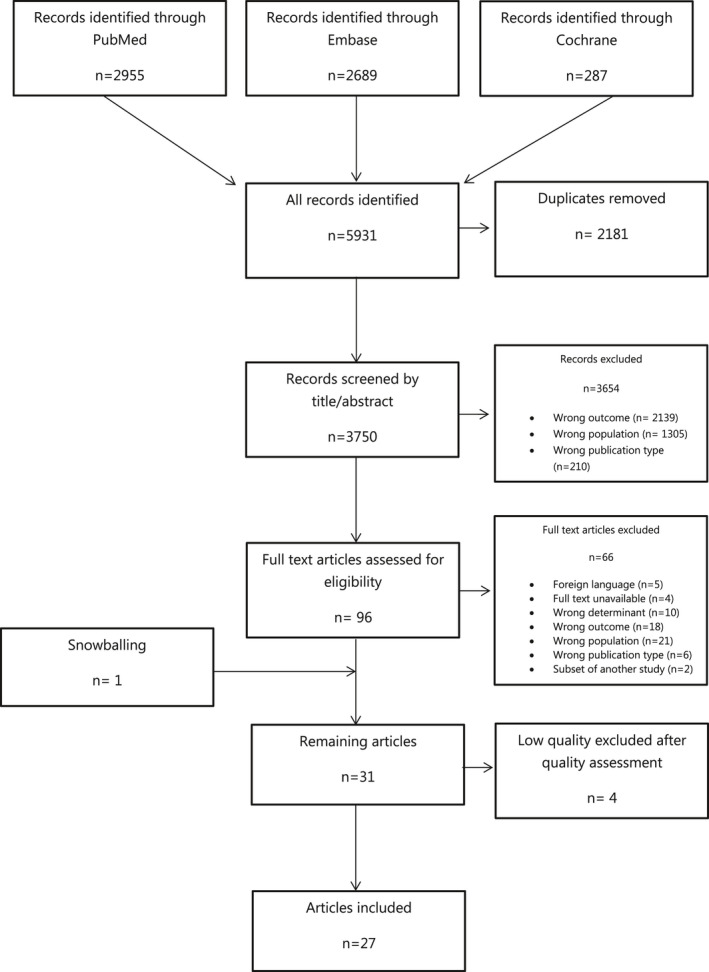
Flow diagram of search results and study selection.
Characteristics of Included Studies
Baseline characteristics of the 27 included studies are summarized in Table 1.3, 8, 9, 10, 18, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 The majority of studies were conducted in Europe or the United States with study years ranging between 1985 and 2017. Data on symptom presentation were collected via the review of medical records (n=10), questionnaires/surveys/checklists (n=9), patient interviews (n=4), voice recordings (n=2), or a combination of a questionnaire and interview (n=2). The sample sizes of studies ranged from 82 to 1 143 513 patients and total sample size was 1 226 163. The mean age of patients ranged from 47 to 78 years in women and from 47 to 69 years in men. Twelve studies adjusted for covariables, with 4 studies adjusting for age only and 8 for other factors in addition to age.
Table 1.
Baseline Characteristics of the Included Studies
| Study | Country | Study Design | Data Collection | Population | Establishment of ACS | Study, y | Sample Size (Men/Women) | Mean Age Men/Women, y | Inclusion Criteria | Exclusion Criteria | Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tunstall‐Pedoe et al. 199618 | Scotland | Cross‐sectional | Medical records | MI | ECG changes and cardiac enzyme levels exceeding twice the upper limit of normal | 1985–1991 | 5541 (3991/1551) | 55.5/57.0 | −25 to 64 years old | None | |
| Meischke et al, 199824 | United States | Cross‐sectional | Medical records | MI | ECG changes or enzyme elevation | 1991–1993 | 4497 (2970/1527) | Median: 64/73 | – Clinically stable | – Missing symptom information | None |
| Goldberg et al, 199825 | United States | Cross‐sectional | Medical records | MI | ≥2 of the following: clinical history of chest pain, elevated serum levels of CK or LDH and ECG changes | 1986–1988 | 1360 (810/550) | 64.7/72.1 | – MI developed during surgery | Age, medical history | |
| Milner et al, 199926 | United States | Cross‐sectional | Patient interview | ACI or MI |
ACI: ECG changes and lack of cardiac enzyme elevation MI: ECG changes and cardiac enzyme elevation |
1995–1997 | 217 (127/90) | 63.0/68.8 | − Diagnosis of ACI or MI − >45 years old−18–44 years included with DM or 2 or more cardiac risk factors | Age, DM | |
| Culic et al, 20028 | Croatia | Cross‐sectional | Questionnaire | MI | ≥2 of the following: ECG changes suggestive of MI, symptoms indicating MI, increase in 1 or more cardiac enzymes | 1990–1995 | 1996 (1395/601) | 57/63 | – First‐time MI | – Previous infarctionUnable to answer questions | Age, risk factors, cardiac enzyme level |
| Grace et al, 200327 | Canada | Cross‐sectional | Patient survey | MI | Diagnosis of MI at CCU | Not described | 482 (347/135) | 59.2/66.3 | – 18 years or older | – Too ill or confused to give informed consent | None |
| Løvlien, Schei and Gjengedal, 200628 | Norway | Cross‐sectional | Questionnaire | MI | Diagnosis of MI at CCU | March–October 1999 | 82 (44/38) | Divided into age groups | −Patients up to age 65−First time MI | None | |
| Hirakawa et al, 200629 | Japan | Cross‐sectional | Medical records | MI | Diagnosis of MI in medical chart | 2001–2003 | 2221 (1712/509) | Divided into absence/presence of chest pain | – Presence or absence of chest pain unknown | None | |
| Arslanian‐Engoren et al, 200630 | United States | Cross‐sectional | Medical records | ACS | ≥2 of the following: ECG changes, increases in serum enzymes or documentation of coronary artery disease | 1999– 2004 | 1941 (1238/683) | 61/67 | – Admitted to hospital alive | – Non‐ACS presentation | Age |
| Løvlien, Schei and Hole, 20069 | Norway | Cross‐sectional | Questionnaire | MI | Elevated cardiac troponin, ECG changes and the presence of clinically appropriate symptoms | 2003–2004 | 533 (384/149) | 58.5/61.2 | −2 weeks after hospital discharge−First time MI−<76 years old | ‐ Hospitalized patients | Age |
| Dey et al, 200831 | Multinational (14 countries) | Cross‐sectional | Medical records | ACS | Clinical history of ACS accompanied by at least 1 of the following: ECG changes, increase in biochemical markers or documented coronary artery disease | 1999–2006 |
a. 43 393 (29 213/14 180) b. 1026 (682/344) |
Divided into age groups | Chest pain No chest pain | – Non‐cardiovascular cause for ACS (trauma, surgery) | None |
| Kirchberger et al, 201132 | Germany | Cross‐sectional | Patient interview | MI | According to criteria of the ESC and American College of Cardiology | 2001–2006 | 2278 (1710/568) | 59.2/62.9 | −Age 25‐74 years −Survived >24 hours with MI−First time MI | Age, hypertension, DM, comorbidity | |
| Angerud et al, 201133 | Sweden | Cross‐sectional | Medical records | MI | Typical chest pain and biomarkers. If only one of the 2 parameters was positive, ECG analysis was used. | 2000–2006 | 4028 (2805/1223) | Divided into age groups | – Age 25–74 years | ‐ Previous MI‐ Patients who were dead by the time they reached medical help | None |
| Canto et al, 201234 | United States | Cross‐sectional | Medical records | MI | Clinical presentation (ischemic symptoms) and elevated cardiac biomarker level, ECG evidence or autopsy evidence | 1994–2006 | 1 143 513 (661 932/481 581) | Divided into age groups | ‐ Secondary diagnosis of MI‐ Patients with missing information on age, sex or symptoms | Age | |
| Pelter et al, 201235 | Multinational (United States, Australia, New Zealand) | Cross‐sectional | Patient interview | ACS | Discharge diagnosis of ACS in medical record | 2001–2004 | 331 (211/110) | Divided into age groups | −Prior diagnosis of coronary artery disease | ‐ Serious comorbidity‐Untreated malignancy or neurologic disorder‐Major hearing loss | None |
| O'Donnell et al, 201236 | Ireland | Cross‐sectional | ACS response to symptoms index | ACS | Discharge diagnosis of ACS | 2007–2009 | 1947 (1402/545) | Divided into age groups | −Admitted through ED−Clinically stable | – Cognitive impairment | Age, BMI, DM, comorbidity, smoking |
| Zevallos et al, 201237 | Puerto Rico | Cross‐sectional | Medical records | MI | Clinical history suggestive of AMI, serum enzyme elevations, and serial ECG findings during hospitalization. | 2007 | 1415 (778/637) | 63.2/68.6 | −Hispanic residents−First time MI | – MI secondary to interventional procedure or surgery | None |
| Coventry et al, 201338 | Australia | Cross‐sectional | Voice recordings of emergency telephone calls | MI | As defined by ICD‐10 | January 2008‐ October 2009 | 1681 (1060/621) | 69.1/77.6 | – Arrival at ED by ambulance | – Arrival by private transport or helicopter | Age |
| Melberg et al, 201339 | Norway | Cross‐sectional | Voice recordings of emergency telephone calls | STEMI | Documented ST elevation on presenting ECG, ischemic symptoms and a typical rise in serum troponin levels | 2004–2007 | 244 (179/65) | Median: 62/67 | – First contact with healthcare system by 113 phone call | None | |
| Khan et al, 201340 | Multinational (Canada, United States, Switzerland) | Cross‐sectional | McSweeney symptom survey | ACS |
1. Signs and symptoms 2. One of the following: a) ECG changes or b) increase in cardiac enzyme levels (troponin I or T, or CK‐MB, or CPK) |
2009–2012 | 1015 (710/305) | Median: 49.0/49.0 |
‐ 55 years or younger ‐ Admitted to CCU, ICU or cardiology ward |
None | |
| Asgar Pour et al, 201541 | Iran | Cross‐sectional | ACS symptom checklist | ACS | ECG changes (ST‐segment and T‐wave changes) and cardiac enzyme (CK‐MB) | Not mentioned | 320 (183/137) | 60.92/63.29 | – Admitted with at least 1 typical symptom(chest pain/pressure/heaviness/tightness, diaphoresis, dyspnea, arm pain) or atypical symptom (palpitation, vomiting, dizziness, fatigue, indigestion) | – History of stroke, neurologic disorders, COPD, pneumonia or pulmonary embolism | None |
| DeVon et al, 201742 | United States | Cross‐sectional | ACS symptom checklist | ACS | Evidence of ischemia on ECG or elevated troponin level | 2011–2014 | 474 (343/131) | 59.5/61.3 | – Admitted through ED | – Cognitive impairment | Age, African American race, comorbidity |
| Lichtman et al, 201843 | United States | Cross‐sectional | Patient interview | MI |
1. Increased cardiac biomarker levels 2. Symptoms of ischemia or ECG changes |
2008–2012 | 2985 (976/2009) | 47.2/47.1 |
– Between 18 and 55 years old –<24 hours since event –2:1 female enrollment |
None | |
| Sederholm Lawesson et al, 201810 | Sweden | Cross‐sectional | Questionnaire | STEMI | ST elevation on ECG and diagnosis of acute MI at discharge | 2012–2014 | 532 (406/126) | 64.3/69.7 |
– Patients with STEMI –Clinically stable –<24 hours since event |
Age, level of education, smoking status, comorbidity | |
| Allana et al, 201844 | Pakistan | Cross‐sectional | Response to Symptoms Questionnaire + interview | ACS | Troponin values and ECG changes | 3‐mo period | 249 (133/116) | 56.5/55.8 |
– Clinically stable – <72 hours since event |
– Cognitive or mental impairment | None |
| An et al, 20183 | China | Cross‐sectional | McSweeney symptom survey + interview | ACS | As defined by ICD‐10 | 2013–2014 | 806 (323/483) | 59.2/63.9 | –First ACS event | – Cognitive or mental impairment | Age, DM, smoking |
| Plaza‐Martin et al, 201945 | Spain | Cross‐sectional | Medical record | ACS | According to ESC guidelines | January–August 2017 | 1056 (749/307) | 64.0/71.0 | – Above 18 years of age |
– Type 2 or type 4 MI – Evident secondary cause of myocardial ischemia |
None |
ACS indicates acute coronary syndrome; ACI, acute coronary ischemia; BMI, body mass index; CCU, coronary care unit; CK‐MB, creatinine kinase‐MB; COPD, chronic obstructive pulmonary disorder; CPK, creatinine phosphokinase; DM, diabetes mellitus; ED, emergency department; ESC, European Society of Cardiology; ICD‐10, International Classification of Diseases, Tenth Revision; ICU, intensive care unit; LDH, lactate dehydrogenase; AMI, acute myocardial infarction; MI, myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Characteristics of Patient Population
Ten studies included a patient population with ACS (either myocardial infarction [STEMI or NSTEMI] or UA), and 17 studies included patients with myocardial infarction (STEMI or NSTEMI) only. Most studies (n=25) included more men than women, in total 60% and 40%, respectively, and women were generally older than men when presenting with ACS. In most studies women more often had comorbidities such as diabetes mellitus or hypertension at presentation, and less often smoked compared with men.
Symptoms
Figure 2 and Table 2 provide the pooled crude and adjusted odds ratios for sex differences in each examined symptom of patients with confirmed ACS. Study‐specific results are provided in Table S4. Forest plots for all symptoms from the aggregated meta‐analysis and the cumulative meta‐analysis can be found in Figures S12–S26 and S27–S41, respectively.
Figure 2.
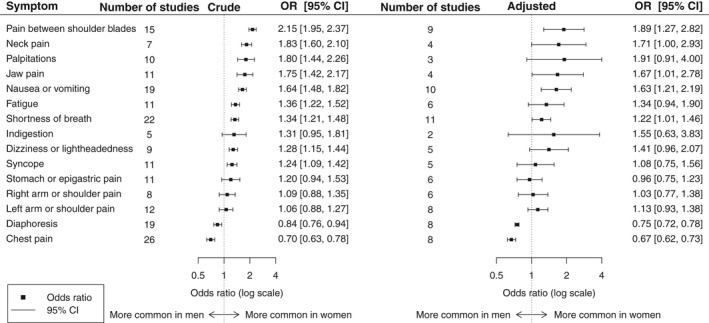
Pooled crude and adjusted odds ratios of symptoms experienced by women relative to men. OR indicates odds ratio.
Table 2.
Crude and Adjusted Results of the Aggregated Meta‐Analysis for All Symptoms
| Symptom | Analysis of Crude Odds Ratio | Stable Results From Cumulative Analysis (y) | Analysis of Adjusted Odds Ratio | ||||
|---|---|---|---|---|---|---|---|
| No. Studies | Pooled Odds Ratio (95% CI) | I2 | No. Studies | Pooled Odds Ratio (95% CI) | I2 | ||
| Pain between the shoulder blades | 15 | 2.15 (1.95–2.37) | 0% | Early 2000s | 9 | 1.89 (1.27–2.82) | 0% |
| Neck pain | 7 | 1.83 (1.60–2.10) | 0% | 2003 | 4 | 1.71 (1.00–2.93) | 0% |
| Palpitations | 10 | 1.80 (1.44–2.26) | 56% | 2015 | 3 | 1.91 (0.91–4.00) | 0% |
| Jaw pain | 11 | 1.75 (1.42–2.17) | 56% | Early 2000s | 4 | 1.67 (1.01–2.78) | 0% |
| Nausea or vomiting | 19 | 1.64 (1.48–1.82) | 53% | 2011 | 10 | 1.63 (1.21–2.19) | 0% |
| Fatigue | 11 | 1.36 (1.22–1.52) | 23% | Early 2000s | 6 | 1.34 (0.94–1.90) | 0% |
| Shortness of breath | 22 | 1.34 (1.21–1.48) | 63% | Early 2000s | 11 | 1.22 (1.01–1.46) | 0% |
| Indigestion | 5 | 1.31 (0.95–1.81) | 37% | NA | 2 | 1.55 (0.63–3.83) | 0% |
| Dizziness or lightheadedness | 9 | 1.28 (1.15–1.44) | 17% | 2003 | 5 | 1.41 (0.96–2.07) | 0% |
| Syncope | 11 | 1.24 (1.09–1.42) | 0% | Early 2000s | 5 | 1.08 (0.75–1.56) | 0% |
| Stomach or epigastric pain | 11 | 1.20 (0.94–1.53) | 69% | NA | 6 | 0.96 (0.75–1.23) | 0% |
| Right arm or right shoulder pain | 8 | 1.09 (0.88–1.35) | 74% | NA | 6 | 1.03 (0.77–1.38) | 47% |
| Left arm or left shoulder pain | 12 | 1.06 (0.88–1.27) | 80% | NA | 8 | 1.13 (0.93–1.38) | 7% |
| Diaphoresis | 19 | 0.84 (0.76–0.94) | 59% | 2003 | 8 | 0.75 (0.72–0.78) | 28% |
| Chest pain | 26 | 0.70 (0.63–0.78) | 85% | 2006 | 8 | 0.67 (0.62–0.73) | 72% |
Chest pain
Women with ACS had lower odds of presenting with chest pain compared with men with ACS (OR 0.70; CI, 0.63–0.78, I2=84.5%). This OR remained virtually unchanged from 2006 onwards (Figure 3, 3, 8, 9, 10, 18, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45) and was similar for studies that provided adjusted results (OR 0.67; CI, 0.62–0.79, I2=72%).
Figure 3.
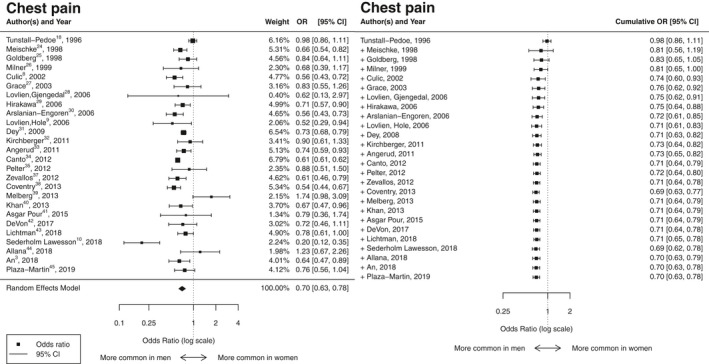
Results of the aggregated and cumulative meta‐analysis for chest pain as a symptom of ACS in women relative to men summarized in a forest plot. ACS indicates acute coronary syndromes; OR indicates odds ratio.
Pain between the shoulder blades
Women with ACS had higher odds of presenting with pain between the shoulder blades compared with men with ACS (2.15 [1.95–2.37], I2=0%). This OR remained virtually unchanged from the early 2000s and was similar for studies that provided adjusted results (1.89 [1.27–2.82], I2=0%).
Neck pain
Women with ACS had higher odds of presenting with neck pain (1.83 [1.60–2.10], I2=0%) and this remained in studies that provided adjusted results (1.71 [1.00–2.93], I2=0%). Studies published after 2003 hardly changed the point estimate.
Palpitations
Women with ACS had higher odds of presenting with palpitations (1.80 [1.44–2.26], I2=56%), which can be seen from 2015 onwards. The direction of effect remained after adjustment, but the OR became non‐significant (1.91 [0.91–4.00], I2=0%).
Jaw pain
Women had higher odds of presenting with jaw pain (1.75 [1.42–2.17], I2=56%). This OR has changed minimally since the early 2000s and was similar in studies that provided adjusted results (1.67 [1.01–2.78], I2=0%).
Nausea or vomiting
Women had higher odds of presenting with nausea or vomiting (1.64 [1.48–1.82], I2=52%), and this remained in studies that provided adjusted results (1.63 [1.21–2.19], I2=0%). Studies published after 2011 hardly changed the point estimate.
Fatigue
Women had higher odds of presenting with fatigue (1.36 [1.22–1.52], I2=23%), which has changed minimally since the early 2000s. The direction of effect remained after adjustment, but the OR became non‐significant (1.34 [0.94–1.90], I2=0%).
Shortness of breath
Women had higher odds of presenting with shortness of breath (1.34 [1.21–1.48], I2=63%). This OR has changed minimally since 2000 and was similar for studies that provided adjusted results (1.22 [1.01–1.46], I2=0%).
Indigestion
There was no significant sex difference in presentation with indigestion (1.31 [0.95–1.81]), and this remained non‐significant in studies that provided adjusted results (1.55 [0.63–3.83], I2=0%).
Dizziness or light‐headedness
Women had higher odds of presenting with dizziness (1.28 [1.15–1.44], I2=17%), which has changed minimally since 2003. The direction of effect remained after adjustment, but the OR became non‐significant (1.41 [0.96–2.07], I2=0%).
Syncope
Women had higher odds of presenting with syncope (1.24 [1.09–1.42], I2=0%), which has changed minimally since the early 2000s. The direction of effect remained after adjustment, but the OR became non‐significant (1.08 [0.75–1.56], I2=0%).
Stomach or epigastric pain
There was no significant sex difference in presentation with stomach or epigastric pain (1.20 [0.94–1.53]), and this remained non‐significant in studies that provided adjusted results (0.96 [0.75–1.23], I2=0%).
Right arm or right shoulder pain
There was no significant sex difference in presentation with right arm or shoulder pain (1.09 [0.88–1.35]), and this remained non‐significant after adjustment (1.02 [0.77–1.38], I2=47%).
Left arm or left shoulder pain
There was no significant sex difference in presentation with left arm or shoulder pain (1.06 [0.88–1.27]), and this remained non‐significant after adjustment (1.13 [0.93–1.38], I2=7%).
Diaphoresis
Women with ACS had a lower odds of presenting with diaphoresis than men (0.84 [0.76–0.94], I2=59%). This OR did not change materially since 2003 and was similar for studies that provided adjusted results (0.75 [0.72–0.78], I2=28%).
Meta‐regression
Differences in symptom presentation between men and women did not differ significantly by the mean age of study participants. Results of the meta‐regression analysis are presented in Table S5.
Symptom prevalence
Both men and women with confirmed ACS presented most often with chest pain (pooled prevalence men 79% [72–85]; women 74% [67–81]), diaphoresis (men 47% [38–55]; women 44% [35–53]), shortness of breath (men 40% [35–46]; women 48% [42–53]), left arm and left shoulder pain (men 37% [28–46]; women 38% [27–48]) and nausea or vomiting (men 28% [24–31]; women 39% [33–45]) (Figure 4). Overall, men and women with confirmed ACS showed considerable overlap in symptoms at presentation.
Figure 4.
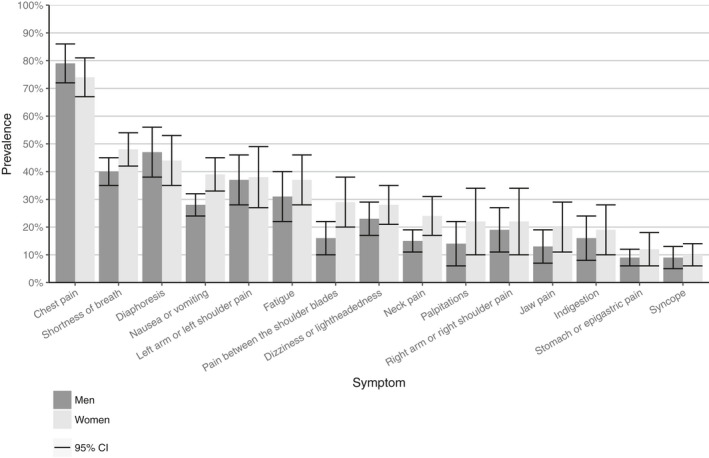
Results of the meta‐analysis of the pooled prevalence and corresponding 95% CI for all symptoms for ACS in women and men. ACS indicates acute coronary syndromes.
Discussion
This systematic review and meta‐analysis of 27 studies including >1 million patients shows that sex differences exist in the symptom presentation in patients with confirmed ACS, while at the same time overlap in symptoms between men and women with confirmed ACS is substantial. Women with ACS have higher odds of presenting with pain between the shoulder blades, nausea or vomiting and shortness of breath compared with men. In contrast, women with ACS have lower odds of presenting with chest pain and diaphoresis compared with their male counterparts. No significant sex differences were found in presentation with left or right arm and shoulder pain, stomach or epigastric pain and indigestion. The cumulative meta‐analyses show that more recent studies did not add materially to the available evidence. For both sexes, chest pain, diaphoresis, shortness of breath, left arm and left shoulder pain, and nausea or vomiting were found to be the most prevalent symptoms.
This present meta‐analyses updates and importantly extends earlier systematic reviews of sex differences in ACS symptom presentation,12, 13, 15 by including more recently published studies, with larger sample sizes and more standardized methods of data collection such as the McSweeney Acute and Prodromal Myocardial Infarction Symptom Survey14 and 13‐item Acute Coronary Syndrome checklist11 and by investigating a broader range of symptoms. Through cumulative meta‐analyses, we demonstrated that sex differences in symptoms for patients with established ACS have been evident since the early 2000s and hardly changed over time since then. Altogether, our results are consistent with previous systematic reviews, which also concluded that women with ACS are less likely to report chest pain and more likely to report a variety of symptoms than men with ACS.12, 13, 15
Over the past 2 decades, considerable research has been done on sex differences in the pathophysiology, symptom presentation, and outcomes of IHD.46 Studies have shown that younger women with ACS present more often with type II ACS,47, 48 characterized by coronary artery spasms and vascular dysfunction,16, 47, 48 whereas younger men present more often with type I ACS, caused by coronary artery obstruction.47, 48 In addition, at all ages, women with ACS less often have plaque ruptures and present more frequently with plaque erosions than men with ACS.47, 49 Whilst progress in the understanding of sex differences has been made, a recent AHA scientific statement identified several gaps in our understanding of mechanisms for symptoms and pathophysiology of ACS in women.46 Future research should address these gaps and evaluate sex differences in symptom presentation of type I and type II ACS.
Implications for Future Research and Clinical Practice
This review shows that sex differences in symptoms among patients with confirmed ACS have been established in the literature for more than a decade. To address the remaining uncertainties, such as how sex differences in symptom presentation may differ by age or other patient characteristics, future research should focus on standardized data collection and reporting. Our findings suggest that researchers and medical professionals should refrain from labeling symptoms of ACS as “typical” and “atypical”, and, instead, consider the established differences and overlap in symptom presentation between men and women in future studies and clinical practice. Consequently, we need to educate medical professionals more to be familiar with the existing sex differences and overlap. Moreover, studies comparing the symptoms of women and men with a suspicion of ACS, but before the clinical diagnosis, are needed to improve the diagnosis of ACS in routine care. The studies included in the present review were conducted among patients with confirmed ACS. As such, our findings alone cannot be used in the development of diagnostic tools, as a comparison of symptoms among women and men with and without confirmed ACS is required.
Strengths and Limitations
This systematic review has several strengths. A comprehensive and systematic approach of the literature search including the use of multiple databases minimalized the possibility of missing relevant evidence. Quality assessment was performed and studies that required the presence of one specific symptom for the diagnosis of ACS were excluded to limit the risk of selective results. As well as aggregated meta‐analysis, cumulative meta‐analysis was performed to analyze the direction of the effects over time.
Limitations of this study are inherent to its design and include heterogeneity between studies in terms of sample size, inclusion criteria, data collection methods, and adjustment for covariables. Data collection by medical record retrieval could induce information bias, while the use of patient interviews makes results liable to recall bias, in particular since most studies were conducted in those with confirmed ACS. Although our meta‐regression analysis did not imply that the sex differences of symptom presentation were different between studies including relatively younger versus relatively older patients, this should be more thoroughly explored by individual patient data meta‐analysis. Statistical heterogeneity for the analyses of chest pain was considerable and sensitivity analyses showed that this was partly driven by the inclusion of 1 large study.34 In addition, 5 studies were excluded because they were written in Polish or Persian, thus it is possible that potentially relevant studies could have been missed. Moreover, some symptoms had to be combined because of their low prevalence. Finally, the present study was restricted to those with confirmed ACS and did not compare sex differences in symptom presentation of individuals with suspected ACS. The symptoms that women with ACS more often present with than men with ACS are highly common for other conditions and may complicate timely diagnosis. Future research should focus on the development and validation of a diagnostic tool to take sex differences in symptom presentation into account.
Conclusions
This systematic review with meta‐analysis shows that there are sex differences in symptom presentation in patients with confirmed ACS. Whilst there is also substantial overlap in symptoms of ACS, women with ACS have higher odds of presenting with pain between the shoulder blades, nausea or vomiting and shortness of breath, and lower odds of presenting with chest pain and diaphoresis compared with men with ACS. Sex differences in symptom presentation seem to be well‐established, meaning that the terms “atypical” and “typical” to label symptoms of ACS are outdated.
Sources of Funding
Dr de Boer is supported by the grant “Facts and Figures” from the Dutch Heart Foundation. Dr Peters is supported by a UK Medical Research Council Skills Development Fellowship (MR/P014550/1).
Disclosures
None.
Supporting information
Data S1. Search string ‘sex differences in symptom presentation in acute coronary syndrome: a systematic review and meta‐analysis’.
Data S2. Adapted Newcastle‐Ottawa quality assessment scale1 (maximum 7 stars) .
Table S1. Reported symptoms in each included study.
Table S2. Subgroups for meta‐regression.
Table S3. Results of the quality assessment using the Newcastle‐Ottawa Scale.
Table S4. Odds ratios of symptoms experienced when presenting with ACS in women relative to men.
Table S5. Meta‐regression showing effects of age on the odds ratio of the symptom being present in women relative to men.
Figure S1. Funnel plot for pooled odds ratio of chest pain.
Figure S2. Funnel plot for pooled odds ratio of fatigue.
Figure S3. Funnel plot for pooled odds ratio of jaw pain.
Figure S4. Funnel plot for pooled odds ratio of left arm or left shoulder pain.
Figure S5. Funnel plot for pooled odds ratio of nausea or vomiting.
Figure S6. Funnel plot for pooled odds ratio of pain between shoulder blades.
Figure S7. Funnel plot for pooled odds ratio of palpitations.
Figure S8. Funnel plot for pooled odds ratio of shortness of breath.
Figure S9. Funnel plot for pooled odds ratio of stomach or epigastric pain.
Figure S10. Funnel plot for pooled odds ratio of syncope.
Figure S11. Funnel plot for pooled odds ratio of diaphoresis.
Figure S12. Results of the aggregated meta‐analysis for chest pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S13. Results of the aggregated meta‐analysis for diaphoresis as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S14. Results of the aggregated meta‐analysis for dizziness or light‐headedness as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S15. Results of the aggregated meta‐analysis for fatigue as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S16. Results of the aggregated meta‐analysis for indigestion as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S17. Results of the aggregated meta‐analysis for jaw pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S18. Results of the aggregated meta‐analysis for left arm pain or left shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S19. Results of the aggregated meta‐analysis for nausea or vomiting as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S20. Results of the aggregated meta‐analysis for neck pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S21. Results of the aggregated meta‐analysis for pain between shoulder blades as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S22. Results of the aggregated meta‐analysis for palpitations as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S23. Results of the aggregated meta‐analysis for right arm pain or right shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S24. Results of the aggregated meta‐analysis for shortness of breath as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S25. Results of the aggregated meta‐analysis for stomach or epigastric pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S26. Results of the aggregated meta‐analysis for syncope as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S27. Results of the cumulative meta‐analysis for chest pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S28. Results of the cumulative meta‐analysis for diaphoresis as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S29. Results of the cumulative meta‐analysis for dizziness or light‐headedness as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S30. Results of the cumulative meta‐analysis for fatigue as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S31. Results of the cumulative meta‐analysis for indigestion as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S32. Results of the cumulative meta‐analysis for jaw pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S33. Results of the cumulative meta‐analysis for left arm pain or left shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S34. Results of the cumulative meta‐analysis for nausea or vomiting as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S35. Results of the cumulative meta‐analysis for neck pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S36. Results of the cumulative meta‐analysis for pain between shoulder blades as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S37. Results of the cumulative meta‐analysis for palpitations as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S38. Results of the cumulative meta‐analysis for right arm pain or right shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S39. Results of the cumulative meta‐analysis for shortness of breath as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S40. Results of the cumulative meta‐analysis for stomach and epigastric pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S41. Results of the cumulative meta‐analysis for syncope as a symptom of ACS in women relative to men summarized in a forest plot.
(J Am Heart Assoc. 2020;9:e014733 DOI: 10.1161/JAHA.119.014733.)
This work was presented on the European Society of Cardiology (ESC) scientific platform EAPC Essentials 4 You on May 4, 2020.
References
- 1. Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis‐Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila‐Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda‐Orjuela CA, Castillo‐Rivas J, Catalá‐López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi‐Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés‐Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor‐Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Health Estimates 2016: Deaths by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization; 2018. [Google Scholar]
- 3. An L, Li W, Shi H, Zhou X, Liu X, Wang H, Liu J, Fan S. Gender difference of symptoms of acute coronary syndrome among Chinese patients: a cross‐sectional study. Eur J Cardiovasc Nurs. 2019;18:179–184. [DOI] [PubMed] [Google Scholar]
- 4. Koopman C, Vaartjes I, Heintjes EM, Spiering W, van Dis I, Herings RMC, Bots ML. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur Heart J. 2013;34:3198–3205. [DOI] [PubMed] [Google Scholar]
- 5. Johansson I, Strömberg A, Swahn E. Factors related to delay times in patients with suspected acute myocardial infarction. Heart Lung. 2004;33:291–300. [DOI] [PubMed] [Google Scholar]
- 6. Kaul P, Armstrong PW, Sookram S, Leung BK, Brass N, Welsh RC. Temporal trends in patient and treatment delay among men and women presenting with ST‐elevation myocardial infarction. Am Heart J. 2011;161:91–97. [DOI] [PubMed] [Google Scholar]
- 7. Diercks DB, Owen KP, Kontos MC, Blomkalns A, Chen AY, Miller C, Wiviott S, Peterson ED. Gender differences in time to presentation for myocardial infarction before and after a national women's cardiovascular awareness campaign: a temporal analysis from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation (CRUSADE) and the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network‐Get with the Guidelines (NCDR ACTION Registry‐GWTG). Am Heart J. 2010;160:80–87. [DOI] [PubMed] [Google Scholar]
- 8. Čulić V, Eterović D, Mirić D, Silić N. Symptom presentation of acute myocardial infarction: influence of sex, age, and risk factors. Am Heart J. 2002;144:1012–1017. [DOI] [PubMed] [Google Scholar]
- 9. Løvlien M, Schei B, Hole T. Women with myocardial infarction are less likely than men to experience chest symptoms. Scand Cardiovasc J. 2006;40:342–347. [DOI] [PubMed] [Google Scholar]
- 10. Sederholm Lawesson S, Isaksson RM, Thylén I, Ericsson M, Ängerud K, Swahn E. Gender differences in symptom presentation of ST‐elevation myocardial infarction – an observational multicenter survey study. Int J Cardiol. 2018;264:7–11. [DOI] [PubMed] [Google Scholar]
- 11. Devon HA, Rosenfeld A, Steffen AD, Daya M. Sensitivity, specificity, and sex differences in symptoms reported on the 13‐item acute coronary syndrome checklist. J Am Heart Assoc. 2014;3 DOI: 10.1161/JAHA.113.000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coventry LL, Finn J, Bremner AP. Sex differences in symptom presentation in acute myocardial infarction: a systematic review and meta‐analysis. Heart Lung. 2011;40:477–491. [DOI] [PubMed] [Google Scholar]
- 13. Canto JG, Goldberg RJ, Hand MM, Bonow RO, Sopko G, Pepine CJ, Long T. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med. 2007;167:2405–2413. [DOI] [PubMed] [Google Scholar]
- 14. McSweeney JC, Cleves MA, Fischer EP, Rojo MO, Armbya N, Moser DK. Reliability of the McSweeney acute and prodromal myocardial infarction symptom survey among black and white women. Eur J Cardiovasc Nurs. 2013;12:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin JY, Martin R, Suls J. Meta‐analytic evaluation of gender differences and symptom measurement strategies in acute coronary syndromes. Heart Lung. 2010;39:283–295. [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardialinfarction (2018). Circulation. 2018; 138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 17. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 18, 2019.
- 18. Tunstall‐Pedoe H, Morrison C, Woodward M, Fitzpatrick B, Watt G. Sex differences in myocardial infarction and coronary deaths in the Scottish MONICA population of Glasgow 1985 to 1991: presentation, diagnosis, treatment, and 28‐day case fatality of 3991 events in men and 1551 events in women. Circulation. 1996;93:1981–1992. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane; 2019. Available at: www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeVon HA, Zerwic JJ. The symptoms of unstable angina: do women and men differ? Nurs Res. 2003;52:108–118. [DOI] [PubMed] [Google Scholar]
- 21. DeVon HA, Ryan CJ, Ochs AL, Moshe S. Symptoms across the continuum of acute coronary syndromes: differences between women and men. Am J Crit Care. 2008;17:14–24. [PMC free article] [PubMed] [Google Scholar]
- 22. Omran S, Al‐Hassan M. Gender differences in signs and symptoms presentation and treatment of Jordanian myocardial infarction patients. Int J Nurs Pract. 2006;12:198–204. [DOI] [PubMed] [Google Scholar]
- 23. Chen W, Woods SL, Wilkie DJ, Puntillo KA. Gender differences in symptom experiences of patients with acute coronary syndromes. J Pain Symptom Manage. 2005;30:553–562. [DOI] [PubMed] [Google Scholar]
- 24. Meischke H, Larsen MP, Eisenberg MS. Gender differences in reported symptoms for acute myocardial infarction: impact on prehospital delay time interval. Am J Emerg Med. 1998;16:363–366. [DOI] [PubMed] [Google Scholar]
- 25. Goldberg RJ, O'Donnell C, Yarzebski J, Bigelow C, Savageau J, Gore JM. Sex differences in symptom presentation associated with acute myocardial infarction: a population‐based perspective. Am Heart J. 1998;136189–195.26. [DOI] [PubMed] [Google Scholar]
- 26. Milner KA, Funk M, Richards S, Wilmes RM, Vaccarino V, Krumholz HM. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396–399. [DOI] [PubMed] [Google Scholar]
- 27. Grace SL, Abbey SE, Bisaillon S, Shnek ZM, Irvine J, Stewart DE. Presentation, delay, and contraindication to thrombolytic treatment in females and males with myocardial infarction. Women's Health Issues. 2003;13:214–221. [DOI] [PubMed] [Google Scholar]
- 28. Løvlien M, Schei B, Gjengedal E. Are there gender differences related to symptoms of acute myocardial infarction? A Norwegian perspective Prog Cardiovasc Nurs. 2006;21:14–19. [DOI] [PubMed] [Google Scholar]
- 29. Hirakawa Y, Masuda Y, Kuzuya M, Iguchi A, Uemura K. Japanese patients with acute myocardial infarction who present without chest pain: a high‐risk group. Int Heart J. 2006;47:483–490. [DOI] [PubMed] [Google Scholar]
- 30. Arslanian‐Engoren C, Patel A, Fang J, Armstrong D, Kline‐Rogers E, Duvernoy CS, Eagle KA. Symptoms of men and women presenting with acute coronary syndromes. Am J Cardiol. 2006;98:1177–1181. [DOI] [PubMed] [Google Scholar]
- 31. Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA, Eagle KA; Global Registry of Acute Coronary Events investigators . Sex‐related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The Global Registry of Acute Coronary Events. Heart. 2009;95:20–26. [DOI] [PubMed] [Google Scholar]
- 32. Kirchberger I, Heier M, Kuch B, Wende R, Meisinger C. Sex differences in patient‐reported symptoms associated with myocardial infarction (from the population‐based MONICA/KORA myocardial infarction registry). Am J Cardiol. 2011;107:1585–1589. [DOI] [PubMed] [Google Scholar]
- 33. Ängerud KH, Brulin C, Näslund U, Eliasson M. Patients with diabetes are not more likely to have atypical symptoms when seeking care of a first myocardial infarction. An analysis of 4028 patients in the Northern Sweden MONICA Study. Diabet Med. 2012;29:e82–e87. [DOI] [PubMed] [Google Scholar]
- 34. Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng ZJ; NRMI Investigators . Association of age and sex with myocardial infarction symptom presentation and in‐hospital mortality. JAMA. 2012;307:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pelter MM, Riegel B, McKinley S, Moser DK, Doering LV, Meischke H, Davidson P, Baker H, Yang W, Dracup K. Are there symptom differences in patients with coronary artery disease presenting to the ED ultimately diagnosed with or without ACS? Am J Emerg Med. 2012;30:1822–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Donnell S, McKee G, O'Brien F, Mooney M, Moser DK. Gendered symptom presentation in acute coronary syndrome: a cross sectional analysis. Int J Nurs Stud. 2012;49:1325–1332. [DOI] [PubMed] [Google Scholar]
- 37. Zevallos JC, Yarzebski J, Banchs H, González‐Sánchez JA, Mattei H, Goldberg RJ, González Mdel C, Quevedo J, Mojica G, Pericchi LR, García‐Palmieri MR. Gender disparities in Puerto Ricans hospitalized with an initial acute myocardial infarction: a population‐based perspective. P R Health Sci J. 2012;31:192–198. [PubMed] [Google Scholar]
- 38. Coventry LL, Bremner AP, Jacobs IG, Finn J. Myocardial infarction: sex differences in symptoms reported to emergency dispatch. Prehospital Emerg Care. 2013;17:193–202. [DOI] [PubMed] [Google Scholar]
- 39. Melberg T, Kindervaag B, Rosland J. Gender‐specific ambulance priority and delays to primary percutaneous coronary intervention: a consequence of the patients’ presentation or the management at the emergency medical communications center? Am Heart J. 2013;166:839–845. [DOI] [PubMed] [Google Scholar]
- 40. Khan NA, Daskalopoulou SS, Karp I, Eisenberg MJ, Pelletier R, Tsadok MA, Dasgupta K, Norris CM, Pilote L; GENESIS PRAXY Team . Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern Med. 2013;173:1863–1871. [DOI] [PubMed] [Google Scholar]
- 41. Asgar Pour H, Norouzzadeh R, Heidari MR. Gender differences in symptom predictors associated with acute coronary syndrome: a prospective observational study. Int Emerg Nurs. 2016;25:13–18. [DOI] [PubMed] [Google Scholar]
- 42. DeVon HA, Burke LA, Vuckovic KM, Haugland T, Eckhardt AL, Patmon F, Rosenfeld AG. Symptoms suggestive of acute coronary syndrome when is sex important? J Cardiovasc Nurs. 2017;32:383–392. [DOI] [PubMed] [Google Scholar]
- 43. Lichtman JH, Leifheit EC, Safdar B, Bao H, Krumholz HM, Lorenze NP, Daneshvar M, Spertus JA, D'Onofrio G. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the VIRGO study (variation in recovery: role of gender on outcomes of young AMI patients). Circulation. 2018;137:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allana S, Moser DK, Ali TS, Khan AH. Sex differences in symptoms experienced, knowledge about symptoms, symptom attribution, and perceived urgency for treatment seeking among acute coronary syndrome patients in Karachi Pakistan. Heart Lung. 2018;47:584–590. [DOI] [PubMed] [Google Scholar]
- 45. Plaza‐Martín M, Sanmartin‐Fernandez M, Álvarez‐Álvarez B, Andrea R, Seoane‐García T, González‐D'Gregorio J, Hernández‐Betancor I, Rozado J, Carrasco‐Ávalos F, Del Mar Alameda‐Ortiz M, Gómez‐Talavera S, Sanchís J, Anguita Sánchez M, Peral‐Disdier V, Ibáňez B, Del Prado Díaz S, Zamorano Gómez JL. Contemporary differences between men and women with acute coronary syndromes: CIAM multicenter registry. J Cardiovasc Med. 2019;20:525–530. [DOI] [PubMed] [Google Scholar]
- 46. McSweeney J, Rosenfeld AG, Abel W, Braun L, Burke L, Daugherty S, Fletcher GF, Gulati M, Mehta LS, Pettey C, Reckelhoff JF; on behalf of the American Heart Association Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Hypertension, Council on Lifestyle and cardiometabolic Health, and Council on Quality of Care and Outcomes Research. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation. 2016;133:1302–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–267. [DOI] [PubMed] [Google Scholar]
- 48. Chandrasekhar J, Mehran R. Sex‐based differences in acute coronary syndromes insights from invasive and noninvasive coronary technologies. JACC Cardiovasc Imaging. 2016;9:451–464. [DOI] [PubMed] [Google Scholar]
- 49. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search string ‘sex differences in symptom presentation in acute coronary syndrome: a systematic review and meta‐analysis’.
Data S2. Adapted Newcastle‐Ottawa quality assessment scale1 (maximum 7 stars) .
Table S1. Reported symptoms in each included study.
Table S2. Subgroups for meta‐regression.
Table S3. Results of the quality assessment using the Newcastle‐Ottawa Scale.
Table S4. Odds ratios of symptoms experienced when presenting with ACS in women relative to men.
Table S5. Meta‐regression showing effects of age on the odds ratio of the symptom being present in women relative to men.
Figure S1. Funnel plot for pooled odds ratio of chest pain.
Figure S2. Funnel plot for pooled odds ratio of fatigue.
Figure S3. Funnel plot for pooled odds ratio of jaw pain.
Figure S4. Funnel plot for pooled odds ratio of left arm or left shoulder pain.
Figure S5. Funnel plot for pooled odds ratio of nausea or vomiting.
Figure S6. Funnel plot for pooled odds ratio of pain between shoulder blades.
Figure S7. Funnel plot for pooled odds ratio of palpitations.
Figure S8. Funnel plot for pooled odds ratio of shortness of breath.
Figure S9. Funnel plot for pooled odds ratio of stomach or epigastric pain.
Figure S10. Funnel plot for pooled odds ratio of syncope.
Figure S11. Funnel plot for pooled odds ratio of diaphoresis.
Figure S12. Results of the aggregated meta‐analysis for chest pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S13. Results of the aggregated meta‐analysis for diaphoresis as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S14. Results of the aggregated meta‐analysis for dizziness or light‐headedness as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S15. Results of the aggregated meta‐analysis for fatigue as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S16. Results of the aggregated meta‐analysis for indigestion as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S17. Results of the aggregated meta‐analysis for jaw pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S18. Results of the aggregated meta‐analysis for left arm pain or left shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S19. Results of the aggregated meta‐analysis for nausea or vomiting as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S20. Results of the aggregated meta‐analysis for neck pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S21. Results of the aggregated meta‐analysis for pain between shoulder blades as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S22. Results of the aggregated meta‐analysis for palpitations as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S23. Results of the aggregated meta‐analysis for right arm pain or right shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S24. Results of the aggregated meta‐analysis for shortness of breath as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S25. Results of the aggregated meta‐analysis for stomach or epigastric pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S26. Results of the aggregated meta‐analysis for syncope as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S27. Results of the cumulative meta‐analysis for chest pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S28. Results of the cumulative meta‐analysis for diaphoresis as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S29. Results of the cumulative meta‐analysis for dizziness or light‐headedness as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S30. Results of the cumulative meta‐analysis for fatigue as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S31. Results of the cumulative meta‐analysis for indigestion as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S32. Results of the cumulative meta‐analysis for jaw pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S33. Results of the cumulative meta‐analysis for left arm pain or left shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S34. Results of the cumulative meta‐analysis for nausea or vomiting as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S35. Results of the cumulative meta‐analysis for neck pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S36. Results of the cumulative meta‐analysis for pain between shoulder blades as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S37. Results of the cumulative meta‐analysis for palpitations as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S38. Results of the cumulative meta‐analysis for right arm pain or right shoulder pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S39. Results of the cumulative meta‐analysis for shortness of breath as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S40. Results of the cumulative meta‐analysis for stomach and epigastric pain as a symptom of ACS in women relative to men summarized in a forest plot.
Figure S41. Results of the cumulative meta‐analysis for syncope as a symptom of ACS in women relative to men summarized in a forest plot.


