Abstract
Background
The incidence of conduction abnormalities requiring permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) with early and later generation prostheses remains debated.
Methods and Results
Based on the administrative hospital‐discharge database, we collected information for all patients treated with TAVR between 2010 and 2019 in France. We compared the incidence of PPI after TAVR according to the type and generation of valve implanted. A total of 49 201 patients with aortic stenosis treated with TAVR using the balloon‐expandable (BE) Edwards SAPIEN valve (early Sapien XT and latest Sapien 3) or the self‐expanding (SE) Medtronic CoreValve (early CoreValve and latest Evolut R) were found in the database. Mean (SD) follow‐up was 1.2 (1.5 years) (median [interquartile range] 0.6 [0.1–2.0] years). PPI after the procedure was reported in 13 289 patients, among whom 11 010 (22.4%) had implantation during the first 30 days. In multivariable analysis, using early BE TAVR as reference, adjusted odds ratio (95% CI) for PPI during the first 30 days was 0.88 (0.81–0.95) for latest BE TAVR, 1.40 (1.27–1.55) for early SE TAVR, and 1.17 (1.07–1.27) for latest SE TAVR. Compared with early BE TAVR, the adjusted hazard ratio for PPI during the whole follow‐up was 1.01 (0.95–1.08) for latest BE TAVR, 1.30 (1.21–1.40) for early SE TAVR, and 1.25 (1.18–1.34) for latest SE TAVR.
Conclusions
In patients with aortic stenosis treated with TAVR, our systematic analysis at a nationwide level found higher rates of PPI than previously reported. BE technology was independently associated with lower incidence rates of PPI both at the acute and chronic phases than SE technology. Recent generations of TAVR were not independently associated with different rates of PPI than early generations during the overall follow‐up.
Keywords: aortic stenosis, pacemaker, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Pacemaker
The number of transcatheter aortic valve replacement (TAVR) procedures has risen worldwide in recent years for treating patients with aortic stenosis, and is expected to continue growing.1 The incidence of conduction abnormalities requiring permanent pacemaker implantation (PPI) after TAVR with different devices available in recent years remains a matter of debate. So far in France, 2 different technologies are available: the balloon‐expandable (BE) Edwards SAPIEN valve (Edwards lifesciences Inc., Irvine, CA) and the self‐expanding (SE) Medtronic CoreValve (Medtronic Inc., Minneapolis, MN). In France in 2018, the latest available generations of each valve (Edwards Sapien 3 and Medtronic CoreValve Evolut R) were widely introduced in 2014 and 2015, respectively, with the aim to reduce the incidence of paravalvular leak. A patient‐tailored transcatheter heart valve therapy would need to be evaluated, and specific recommendations for implantation of each prosthesis taking into consideration some clinical characteristics may be needed to reduce the risk of PPI. We compared the incidence of PPI after TAVR according to the type and generation of valve implanted.
Methods
The data and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Because this study used data from human subjects, the data and everything pertaining to the data are governed by the French Health Agencies and cannot be made available to other researchers.
This retrospective cohort study was based on the national hospitalization database covering hospital care from the entire French population. The data for all patients admitted with aortic stenosis in France from January 2010 to June 2019 were collected from the national administrative Programme de Médicalisation des Systèmes d'Information (PMSI) database. Through this program, medical activity is recorded in a database and rendered anonymous. It includes >98% of the French population (67 million people) from birth (or immigration) to death (or emigration), even if a person changes occupation or retires. This process allows the determination of each hospital's budget, in the 1546 French healthcare facilities for both public and private hospitals. Each hospitalization is encoded in a standardized data set, which includes information about the patient (age and sex), hospital, stay (date of admission, date of discharge, and mode of discharge), pathologies, and procedures. Routinely collected medical information includes the principal diagnosis and secondary diagnoses. In the PMSI system, identified diagnoses are coded according to the International Classification of Diseases, Tenth Revision (ICD‐10). All medical procedures are recorded according to the national nomenclature, Classification Commune des Actes Medicaux. The PMSI contains individual pseudoanymized information on each hospitalization that are linked to create a longitudinal record of hospital stays and diagnoses for each patient. The reliability of PMSI data has already been assessed, and this database has previously been used to study patients with cardiovascular conditions, including those with aortic stenosis treated with TAVR.1, 2
The study was conducted retrospectively and, as patients were not involved in its conduct, there was no impact on their care. Ethical approval was not required, because all data were anonymized. The French Data Protection Authority granted access to the PMSI data. Procedures for data collection and management were approved by the Commission Nationale de l'Informatique et des Libertés, the independent National Ethical Committee protecting human rights in France, which ensures that all information is kept confidential and anonymous, in compliance with the Declaration of Helsinki (authorization number 1897139).
Study Population
From January 1, 2010 to June 30, 2019, 520 662 adults (age ≥18 years) were hospitalized with a diagnosis of aortic stenosis (I350, I352, I060, and I062 using ICD‐10 codes) as the principal diagnosis (ie, the health problem that justified admission to hospital), the related diagnosis (ie, potential chronic disease or health state during the hospital stay), or a significantly associated diagnosis (ie, comorbidity or associated complication). For the analysis of TAVR procedures, we included all adults with a single percutaneous procedure (Classification Commune des Actes Medicaux code: DBLF001). Early and more recent generation balloon‐expandable (BE) and self‐expandable (SE) TAVR were differentiated using their codes used for pricing. Patient information (demographics, comorbidities, medical history, and events during hospitalization or follow‐up) was described using data collected in the hospital records. For each hospital stay, combined diagnoses at discharge were obtained. Each variable was identified using ICD‐10 codes. Based on the database, we were able to estimate a proxy of the EuroSCORE II (Data S1 and Figure S1). We also used the Charlson Comorbidity Index and the Claims‐based Frailty Indicator to assess patient clinical status. Exclusion criteria were age <18 years.
Statistical Analysis
Qualitative variables are described as frequency and percentages and quantitative variable as means (SDs). Comparisons were made using χ2 tests for categorical variables and the Student t test or nonparametric Kruskal–Wallis test, as appropriate, for continuous variables.
For the analysis in the cohort, PPI was identified using its several specific codes from the Classification Commune des Actes Medicaux and we report outcomes at 30 days and during whole follow‐up. The logistic regression model was used for the specific outcome of pacemaker implantation at 30 days and odds ratios are reported. The cut‐off at 30 days has been used in many reference analyses.3, 4, 5 The evaluation during a “hospital phase” may be less standardized because the duration and pathway across several departments (or hospitals) during the initial hospital phase may markedly differ in different patients treated with TAVR. For longer‐term follow‐up, the incidence rates for outcomes (%/y) in each group were estimated. Analyses were performed using a Cox regression model and the hazard ratios are reported. Multivariable analysis included all parameters listed in Table 1. All comparisons with P<0.05 were considered statistically significant. All analyses were performed using Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC), USA and STATA version 12.0 (Stata Corp, College Station, TX).
Table 1.
Characteristics and Outcomes of Patients Treated With TAVR
| Early BE TAVR | Latest BE TAVR | Early SE TAVR | Latest SE TAVR | |
|---|---|---|---|---|
| (n=4262) | (n=25 174) | (n=5319) | (n=14 446) | |
| Age, y | 82.9±7.1 (84, 8) | 82.4±6.9 (84, 8) | 82.8±6.8 (84, 7) | 82.7±6.8 (84, 7) |
| EuroSCORE II | 3.8±1.0 (3.7, 1.3) | 3.7±1.0 (3.5, 1.3) | 3.9±1.0 (3.8, 1.4) | 3.8±1.0 (3.6, 1.4) |
| Charlson comorbidity index | 4.8±2.8 (5, 4) | 4.0±2.9 (3, 4) | 4.9±2.9 (5, 4) | 3.8±2.8 (3, 3) |
| Frailty index | 11.3±9.3 (9, 12) | 8.7±8.7 (6.1, 10.5) | 11.6±9.5 (9.5, 12.9) | 8.7±8.7 (6.1, 10.4) |
| Sex (male) | 2014 (47.3) | 13 413 (53.3) | 2800 (52.6) | 5866 (40.6) |
| Hypertension | 3709 (87.0) | 21 312 (84.7) | 4643 (87.3) | 12 214 (84.5) |
| Diabetes mellitus | 1394 (32.7) | 7831 (31.1) | 1727 (32.5) | 4370 (30.3) |
| Heart failure | 2722 (63.9) | 13 697 (54.4) | 3435 (64.6) | 7574 (52.4) |
| Coronary artery disease | 2867 (67.3) | 16 218 (64.4) | 3677 (69.1) | 9098 (63.0) |
| Previous myocardial infarction | 554 (13.0) | 3518 (14.0) | 905 (17.0) | 1985 (13.7) |
| Previous PCI | 1074 (25.2) | 7539 (29.9) | 1500 (28.2) | 4194 (29.0) |
| Previous CABG | 0.1±0.3 | 0.1±0.3 | 0.1±0.3 | 0.1±0.3 |
| Vascular disease | 1473 (34.6) | 9144 (36.3) | 2137 (40.2) | 5150 (35.7) |
| Mitral regurgitation | 813 (19.1) | 4767 (18.9) | 1052 (19.8) | 2787 (19.3) |
| Aortic regurgitation | 619 (14.5) | 2544 (10.1) | 742 (13.9) | 1890 (13.1) |
| Tricuspid regurgitation | 138 (3.2) | 1110 (4.4) | 185 (3.5) | 719 (5.0) |
| Atrial fibrillation | 2028 (47.6) | 11 359 (45.1) | 2480 (46.6) | 6345 (43.9) |
| Left bundle branch block | 512 (12.0) | 4379 (17.4) | 883 (16.6) | 2895 (20.0) |
| Right bundle branch block | 285 (6.7) | 1968 (7.8) | 395 (7.4) | 1142 (7.9) |
| Previous pacemaker or defibrillator | 833 (19.5) | 4766 (18.9) | 1408 (26.5) | 3012 (20.9) |
| Ischemic stroke | 176 (4.1) | 1370 (5.4) | 300 (5.6) | 893 (6.2) |
| Smoker | 345 (8.1) | 2390 (9.5) | 493 (9.3) | 1293 (9.0) |
| Dyslipidemia | 2225 (52.2) | 12 636 (50.2) | 2765 (52.0) | 7323 (50.7) |
| Obesity | 1247 (29.3) | 7327 (29.1) | 1452 (27.3) | 3946 (27.3) |
| Alcohol‐related diagnoses | 224 (5.3) | 1395 (5.5) | 322 (6.1) | 652 (4.5) |
| Abnormal renal function | 757 (17.8) | 4242 (16.9) | 1066 (20.0) | 2473 (17.1) |
| Lung disease | 1128 (26.5) | 5668 (22.5) | 1529 (28.7) | 3192 (22.1) |
| Sleep apnea syndrome | 292 (6.9) | 2403 (9.5) | 437 (8.2) | 1315 (9.1) |
| Liver disease | 189 (4.4) | 1257 (5.0) | 298 (5.6) | 689 (4.8) |
| Thyroid diseases | 564 (13.2) | 3340 (13.3) | 657 (12.4) | 2285 (15.8) |
| Anemia | 1130 (26.5) | 6802 (27.0) | 1485 (27.9) | 4191 (29.0) |
| Previous cancer | 634 (14.9) | 5095 (20.2) | 916 (17.2) | 2627 (18.2) |
| Outcomes | ||||
| Death during follow‐up | 1525 (35.8) | 3486 (13.8) | 1807 (34.0) | 1666 (11.5) |
| Death at day 30 | 233 (5.5) | 621 (2.5) | 312 (5.9) | 453 (3.1) |
| Death beyond day 30a | 1292 (11.1) | 2865 (11.5) | 1495 (11.8) | 1213 (10.1) |
| Pacemaker implantation during follow‐up | 1267 (29.7) | 6191 (24.6) | 1739 (32.7) | 4092 (28.3) |
| Pacemaker implantation at day 30 | 934 (21.9) | 5203 (20.7) | 1359 (25.5) | 3514 (24.3) |
| Pacemaker implantation beyond day 30a | 333 (3.9) | 988 (5.2) | 380 (4.2) | 578 (6.3) |
Values are mean±SD (median, interquartile range) for continuous variables, N (%) for categorical variables. BE indicates balloon‐expandable; CABG, coronary artery bypass graft; Early BE, Edwards Sapien XT; Early SE, Medtronic Corevalve; Latest BE, Edwards Sapien 3; Latest SE, Medtronic Evolut; PCI, percutaneous coronary intervention; SE, self‐expandable; and TAVR, transcatheter aortic valve replacement.
% are yearly incidence.
Results
Among 49 201 patients, patients treated with early BE and SE valves had higher Charlson comorbidity and frailty indexes than those treated with second generation, and slightly higher EuroSCORE II. Patients treated with SE valves had higher rates of previous pacemaker or defibrillator than those treated with BE valves (Table 1). Mean (SD) follow‐up was 1.2 (1.5 years) (median [interquartile range] 0.6 [0.1–2.0] years).
PPI at the time of or after the procedure was reported in 13 289 patients, among whom 11 010 had implantation in during the first 30 days (ranging from 20.7% for latest BE to 25.5% for early SE TAVR) (Table 1) (Figure).
Figure 1. Incidence of permanent pacemaker implantation in patients treated with TAVR, according to type and generation of device.
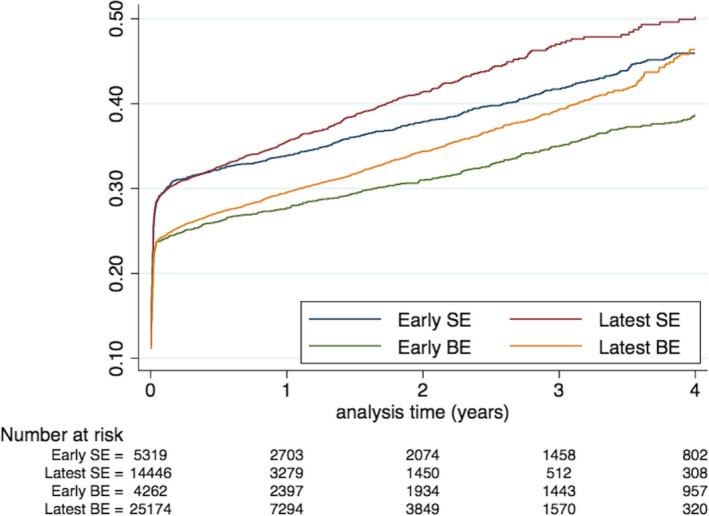
BE indicates balloon‐expandable; Early BE, Edwards Sapien XT; Early SE, Medtronic Corevalve; Latest BE, Edwards Sapien 3; Latest SE, Medtronic Evolut; SE, self‐expandable; and TAVR, transcatheter aortic valve replacement.
In multivariable analysis, using early BE TAVR as reference, adjusted odds ratios (95% CI) for PPI during the first 30 days was 0.88 (0.81–0.95) for latest BE TAVR, 1.40 (1.27–1.55) for early SE TAVR, and 1.17 (1.07–1.27) for latest SE TAVR. Other independent predictors of PPI during the first 30 days were older age, male sex, history of hypertension, obesity, diabetes mellitus, myocardial infarction, history of pulmonary edema, atrial fibrillation, left bundle branch block, right bundle branch block, and abnormal renal function (Table 2).
Table 2.
Independent predictors of PPI During the First 30 Days and During the Whole Follow‐Up in Patients Treated With TAVR
| Whole Follow‐Up | First 30 Days | Beyond 30 Days | ||||
|---|---|---|---|---|---|---|
| HR, 95% CI | P Value | OR, 95% CI | P Value | HR, 95% CI | P Value | |
| Age, per 10 y | 1.09 (1.06–1.12) | <0.0001 | 1.14 (1.10–1.18) | <0.0001 | 0.97 (0.90–1.04) | 0.34 |
| EuroSCORE II | 1.02 (0.99–1.04) | 0.28 | 1.01 (0.98–1.05) | 0.45 | 1.04 (0.98–1.11) | 0.23 |
| Charlson comorbidity index | 0.98 (0.97–0.99) | 0.001 | 0.98 (0.95–1.01) | 0.40 | 0.98 (0.96–1.01) | 0.14 |
| Frailty index, per 10 units | 0.99 (0.97–1.01) | 0.35 | 0.98 (0.95–1.00) | 0.18 | 1.13 (1.08–1.18) | <0.0001 |
| Sex (male) | 1.24 (1.20–1.29) | <0.0001 | 1.29 (1.23–1.36) | <0.0001 | 1.45 (1.33–1.59) | <0.0001 |
| Hypertension | 1.09 (1.04–1.14) | <0.0001 | 1.16 (1.09–1.23) | <0.0001 | 0.95 (0.85–1.06) | 0.35 |
| Diabetes mellitus | 1.09 (1.05–1.14) | <0.0001 | 1.08 (1.02–1.14) | 0.01 | 1.17 (1.06–1.30) | 0.002 |
| Heart failure | 1.03 (0.99–1.08) | 0.12 | 1.01 (0.96–1.07) | 0.75 | 1.15 (1.05–1.27) | 0.004 |
| History of pulmonary edema | 1.12 (1.03–1.21) | 0.01 | 1.13 (1.01–1.27) | 0.03 | 1.03 (0.84–1.27) | 0.76 |
| Aortic regurgitation | 1.06 (1.00–1.12) | 0.04 | 1.07 (1.00–1.15) | 0.06 | 1.05 (0.93–1.19) | 0.45 |
| Mitral regurgitation | 0.91 (0.87–0.96) | <0.0001 | 0.87 (0.82–0.92) | <0.0001 | 1.00 (0.90–1.12) | 0.94 |
| Coronary artery disease | 0.96 (0.92–1.00) | 0.06 | 0.93 (0.88–0.99) | 0.01 | 1.01 (0.91–1.12) | 0.90 |
| Previous myocardial infarction | 1.13 (1.06–1.20) | <0.0001 | 1.15 (1.06–1.24) | 0.001 | 1.16 (1.01–1.34) | 0.04 |
| Previous PCI | 1.01 (0.97–1.06) | 0.54 | 1.04 (0.98–1.01) | 0.25 | 0.95 (0.86–1.06) | 0.38 |
| Previous CABG | 0.92 (0.86–0.99) | 0.04 | 0.92 (0.83–1.01) | 0.09 | 0.90 (0.77–1.05) | 0.17 |
| Vascular disease | 0.90 (0.86–0.94) | <0.0001 | 0.85 (0.80–0.90) | <0.0001 | 1.01 (0.91–1.13) | 0.81 |
| Atrial fibrillation | 1.06 (1.02–1.10) | 0.003 | 1.05 (1.01–1.11) | 0.03 | 1.26 (1.16–1.37) | <0.0001 |
| Left bundle branch block | 1.29 (1.23–1.34) | <0.0001 | 1.35 (1.27–1.42) | <0.0001 | 1.75 (1.58–1.93) | <0.0001 |
| Right bundle branch block | 1.71 (1.61–1.81) | <0.0001 | 2.21 (2.03–2.40) | <0.0001 | 1.34 (1.14–1.58) | <0.0001 |
| Ischemic stroke | 1.01 (0.94–1.09) | 0.82 | 0.98 (0.89–1.09) | 0.73 | 1.07 (0.89–1.28) | 0.50 |
| Smoker | 1.01 (0.94–1.07) | 0.89 | 1.01 (0.92–1.10) | 0.87 | 1.03 (0.89–1.20) | 0.68 |
| Dyslipidemia | 1.01 (0.98–1.05) | 0.45 | 1.03 (0.98–1.08) | 0.33 | 0.98 (0.90–1.07) | 0.69 |
| Obesity | 1.16 (1.11–1.21) | <0.0001 | 1.24 (1.17–1.31) | <0.0001 | 1.00 (0.90–1.11) | 0.95 |
| Alcohol‐related diagnoses | 1.02 (0.93–1.12) | 0.72 | 1.10 (0.97–1.24) | 0.14 | 0.74 (0.58–0.93) | 0.009 |
| Abnormal renal function | 1.07 (1.02–1.12) | 0.009 | 1.09 (1.02–1.17) | 0.01 | 0.99 (0.87–1.11) | 0.81 |
| Lung disease | 0.95 (0.91–0.99) | 0.03 | 0.95 (0.90–1.01) | 0.08 | 0.89 (0.81–0.99) | 0.03 |
| Sleep apnea syndrome | 0.99 (0.93–1.05) | 0.72 | 0.96 (0.88–1.05) | 0.35 | 1.12 (0.97–1.30) | 0.13 |
| Liver disease | 0.97 (0.88–1.06) | 0.47 | 0.91 (0.81–1.09) | 0.12 | 1.07 (0.87–1.32) | 0.53 |
| Thyroid diseases | 1.01 (0.96–1.07) | 0.63 | 1.02 (0.95–1.09) | 0.63 | 1.02 (0.90–1.16) | 0.75 |
| Inflammatory disease | 1.02 (0.96–1.08) | 0.60 | 1.03 (0.95–1.11) | 0.49 | 0.99 (0.86–1.14) | 0.89 |
| Anemia | 0.96 (0.93–1.00) | 0.07 | 0.96 (0.91–1.02) | 0.17 | 0.95 (0.86–1.05) | 0.33 |
| Previous cancer | 1.02 (0.97–1.07) | 0.54 | 0.99 (0.93–1.06) | 0.71 | 1.06 (0.95–1.19) | 0.31 |
| Edwards Sapien XT | 1.00 | ··· | 1.00 | ··· | 1.00 | ··· |
| Edwards Sapien 3 | 1.01 (0.95–1.08) | 0.75 | 0.88 (0.81–0.95) | 0.002 | 1.35 (1.18–1.53) | <0.0001 |
| Medtronic Corevalve | 1.30 (1.21–1.40) | <0.0001 | 1.40 (1.27–1.55) | <0.0001 | 1.27 (1.10–1.48) | 0.001 |
| Medtronic Evolut | 1.25 (1.17–1.34) | <0.0001 | 1.16 (1.07–1.27) | 0.001 | 1.59 (1.38–1.83) | <0.0001 |
BE indicates balloon‐expandable; CABG, coronary artery bypass graft; Early BE, Edwards Sapien XT; Early SE, Medtronic Corevalve; HR, hazard ratio; Latest BE, Edwards Sapien 3; Latest SE, Medtronic Evolut; PCI, percutaneous coronary intervention; PPI, permanent pacemaker implantation; SE, self‐expandable; and TAVR, transcatheter aortic valve replacement.
Compared with early BE TAVR, adjusted hazard ratio for PPI during the whole follow‐up was 1.01 (0.95–1.08) for latest BE TAVR, 1.30 (1.21–1.40) for early SE TAVR, and 1.25 (1.18–1.34) for latest SE TAVR (Table 2). Other independent predictors of PPI during the whole follow‐up as well as predictors for PPI for the specific period beyond the 30th day post TAVR are in Table 2.
While latest BE TAVR was associated with a lower risk of PPI than early BE TAVR during the first 30 days, it was associated with a higher risk of PPI than early BE TAVR in the subsequent period beyond the 30th day (adjusted hazard ratio 1.35 [1.18–1.53]). Similarly, latest SE TAVR was associated with a lower risk of PPI than early SE TAVR during the first 30 days, but was associated with a higher risk of PPI than early SE TAVR on the subsequent period beyond the 30th day.
Discussion
Our large adjusted analysis showed that BE TAVR technology was associated with lower incidence rates of PPI compared with SE TAVR. Latest generations of TAVR had slightly lower adjusted odds ratio for PPI at day 30, but higher adjusted hazard ratios for PPI on a longer‐term follow‐up, resulting in a similar rate of PPI for the overall follow‐up.
Since TAVR was introduced, the BE Sapien technology has evolved from Sapien XT to Sapien 3 (in 2012) and the SE CoreValve technology, which has evolved to the Evolut R system (in 2013). International guidelines recommend that severe aortic stenosis be treated with TAVR in eligible patients without recommendation regarding the type of TAVR technology. There is considerable heterogeneity in the average PPI rates in the literature, ranging from 5.9% to 20.7% for BE‐bioprosthesis.6 A higher rate of 30‐day PPI has been reported with SE valves.7 Our analysis at a nationwide level has the main advantage of being exhaustive, avoiding selection and reporting biases for a reliable picture of PPI after TAVR. We also found a higher rate of 30‐day pacemaker implantation with SE valves, although the difference was less marked than in the CENTER (Cerebrovascular Events in Patients Undergoing Transcatheter Aortic Valve Implantation) analysis.7 This difference persisted in the longer term. The second‐generation BE prosthesis has an outer skirt to minimize paravalvular leakage and has been associated with higher rates of PPI,8 which was not clearly seen in our study (similar for overall follow‐up, being lower in the early phase and higher on a longer term in the multivariable analysis). Our “real life” incidences of pacemaker implantation at 30 days are slightly higher than in the randomized SOLVE TAVR (Comparison of Second‐Generation Self‐Expandable vs. Balloon‐Expandable Valves and General vs. Local Anaesthesia in Transcatheter Aortic Valve Implantation) trial.9 They were also higher than in an earlier French observational study, considering the hospital phase (16–18%) and using a declarative method that may underestimate the true rates of PPI.10 Higher rates of in‐hospital PPI (up to 22%) were, however, reported in the most recent findings from this declarative registry.11 This contrasts in part with the findings at day 30 in our systematic analysis at a nationwide level from a mandatory administrative database, including centers where patients may be transferred after the TAVR procedure.
This overall suggests that, in daily practice, physicians may have a relatively aggressive approach towards pacemaker implantation for patients treated with TAVR, regardless of the type of valve implanted. Targeting shorter hospital stay may play a role in these results, favoring more frequent and earlier PPI, and avoiding a too long ECG monitoring.12, 13, 14 It has also been reported that delayed atrioventricular block may be an underappreciated complication of TAVR among patients without preprocedure pacing devices,15 and this may lead to a more aggressive approach at a nationwide level. Predictors for PPI at the acute phase post TAVR and on a longer term were broadly similar to those previously reported by others in far smaller series, particularly for older age and right bundle branch block.15, 16, 17 Our results also suggest that the best strategy for PPI in these patients, including the appropriate role of electrophysiological study, may need to be more properly defined. A few randomized studies are ongoing, 2 of them comparing incidences of PPI with 2 different TAVR devices and 1 comparing an electrophysiology‐based algorithmic approach to standard clinical follow‐up with clinical events at the 12th month.18
Limitations
A main limitation of our work is inherent to the retrospective, observational nature of the study and its potential biases. Furthermore, the study was based on administrative data, with limitations inherent to such methodology. The PMSI database contains diagnoses coded using ICD‐10, which are obtained at hospital discharge and are the physician's responsibility. Data were not systematically externally checked, and this could have caused information bias. However, the large scale of the database is likely to partly compensate this bias and, as coding of complications is linked to reimbursement and is regularly controlled, it is expected to be of good quality.
Our large population of patients treated with the TAVR procedure likely represents a heterogeneous group of patients admitted with various kinds of illnesses and severities. The nonrandomized design of the analysis leaves a risk of residual confounding factors. We have been able to estimate the EuroSCORE II, which in our cohort showed a satisfying correlation with early clinical outcomes. Moreover, the Charlson comorbidity index and Frailty index were used as risk predictors of all‐cause death over a longer term. Our analysis was restricted to the variables present in the database, which meant that characteristics such as mean gradient, valve area, calcifications, and paravalvular leak were not available for analysis. We had information for diagnoses of left or right bundle branch block or atrioventricular block on ICD‐10 codes, but precise QRS durations in ms on surface ECG and results obtained during electrophysiological study were not available. Left anterior fascicular block was not included in our analysis because it is generally not reliably indicated in administrative medical records. Definite conclusions for comparisons between groups may not be fully appropriate, as multivariable analysis cannot fully eradicate the possible confounding related to some of these (or other) variables between these groups. There were multiple independent risk factors (>10) predictive for PPI outside of TAVI type, but it should be acknowledged that this could be a result of the large study size because the effect size of many independent variables was relatively small. Finally, the latest generation of Sapien BE valve is the Sapien Ultra. However, despite CE (European Conformity) mark in November 2018, this valve was not available in France during the study and still has not been launched in 2019.
Conclusions
In patients with aortic stenosis treated with TAVR, our systematic analysis at a nationwide level found higher rates of PPI than previously reported. BE technology was independently associated with lower incidence rates of PPI both at the acute and chronic phases than SE technology. However, this was less apparent than previously reported in this large analysis of unselected patients seen in “real life” practice. Recent generations of TAVR were not independently associated with a different rate of PPI than early generations.
Sources of Funding
None.
Disclosures
Pierre reports personal fees from Abbott, Biotronik, Boston Scientific, and Microport. Saint Etienne reports honoraria from Abbott and Biotronik. Fauchier reports consultant or speaker activities for Bayer, BMS/Pfizer, Boehringer Ingelheim, Medtronic, and Novartis. Clementy reports consultant activities for Medtronic and Boston Scientific. The remaining authors have no disclosures to report.
Supporting information
Data S1
Figure S1
References 19 and 20
(J Am Heart Assoc. 2020;9:e015896 DOI: 10.1161/JAHA.120.015896.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.015896
For Sources of Funding and Disclosures, see page 7.
See Editorial by Huang and Mansour
References
- 1. Nguyen V, Michel M, Eltchaninoff H, Gilard M, Dindorf C, Iung B, Mossialos E, Cribier A, Vahanian A, Chevreul K, et al. Implementation of transcatheter aortic valve replacement in France. J Am Coll Cardiol. 2018;71:1614–1627. [DOI] [PubMed] [Google Scholar]
- 2. Deharo P, Bisson A, Herbert J, Lacour T, Saint Etienne C, Grammatico‐Guillon L, Porto A, Collart F, Bourguignon T, Cuisset T, et al. Impact of Sapien 3 balloon‐expandable versus evolut R self‐expandable transcatheter aortic valve implantation in patients with aortic stenosis: data from a nationwide analysis. Circulation. 2020;141:260–268. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 4. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al.; PARTNER 3 Investigators . Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 5. Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB Jr, Chetcuti S, et al. 2‐year outcomes in patients undergoing surgical or self‐expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113–121. [DOI] [PubMed] [Google Scholar]
- 6. Maeno Y, Abramowitz Y, Kawamori H, Kazuno Y, Kubo S, Takahashi N, Mangat G, Okuyama K, Kashif M, Chakravarty T, et al. A highly predictive risk model for pacemaker implantation after TAVR. JACC Cardiovasc Imaging. 2017;10:1139–1147. [DOI] [PubMed] [Google Scholar]
- 7. Vlastra W, Chandrasekhar J, Munoz‐Garcia AJ, Tchetche D, de Brito FS Jr, Barbanti M, Kornowski R, Latib A, D'Onofrio A, Ribichini F, et al. Comparison of balloon‐expandable vs. self‐expandable valves in patients undergoing transfemoral transcatheter aortic valve implantation: from the CENTER‐collaboration. Eur Heart J. 2019;40:456–465. [DOI] [PubMed] [Google Scholar]
- 8. De Torres‐Alba F, Kaleschke G, Diller GP, Vormbrock J, Orwat S, Radke R, Reinke F, Fischer D, Reinecke H, Baumgartner H. Changes in the pacemaker rate after transition from Edwards SAPIEN XT to SAPIEN 3 transcatheter aortic valve implantation: the critical role of valve implantation height. JACC Cardiovasc Interv. 2016;9:805–813. [DOI] [PubMed] [Google Scholar]
- 9. Thiele H. A 2x2 randomized trial of self‐expandable vs balloon‐expandable valves and general vs local anesthesia in patients undergoing transcatheter aortic valve implantation. Presented at tct, 23 September, San Diego, CA; 2018. [Google Scholar]
- 10. Auffret V, Lefevre T, Van Belle E, Eltchaninoff H, Iung B, Koning R, Motreff P, Leprince P, Verhoye JP, Manigold T, et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to FRANCE TAVI. J Am Coll Cardiol. 2017;70:42–55. [DOI] [PubMed] [Google Scholar]
- 11. Van Belle E, Vincent F, Labreuche J, Auffret V, Debry N, Lefevre T, Eltchaninoff H, Manigold T, Gilard M, Verhoye JP, et al. Balloon‐expandable versus self‐expanding transcatheter aortic valve replacement: a propensity‐matched comparison from the FRANCE‐TAVI registry. Circulation. 2020;141:243–259. [DOI] [PubMed] [Google Scholar]
- 12. Sheng SP, Strassle PD, Arora S, Kolte D, Ramm CJ, Sitammagari K, Guha A, Paladugu MB, Cavender MA, Vavalle JP. In‐hospital outcomes after transcatheter versus surgical aortic valve replacement in octogenarians. J Am Heart Assoc. 2019;8:e011206 DOI: 10.1161/JAHA.118.011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, El‐Chami MF, Herrmann HC, Mack M, Makkar RR, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (placement of aortic transcatheter valves) trial and registry. JACC Cardiovasc Interv. 2015;8:60–69. [DOI] [PubMed] [Google Scholar]
- 14. Alkhalil A, Lamba H, Deo S, Bezerra HG, Patel SM, Markowitz A, Simon DI, Costa MA, Davis AC, Attizzani GF. Safety of shorter length of hospital stay for patients undergoing minimalist transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2018;91:345–353. [DOI] [PubMed] [Google Scholar]
- 15. Ream K, Sandhu A, Valle J, Weber R, Kaizer A, Wiktor DM, Borne RT, Tumolo AZ, Kunkel M, Zipse MM, et al. Ambulatory rhythm monitoring to detect late high‐grade atrioventricular block following transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;73:2538–2547. [DOI] [PubMed] [Google Scholar]
- 16. Kiani S, Kamioka N, Black GB, Lu MLR, Lisko JC, Rao B, Mengistu A, Gleason PT, Stewart JP, Caughron H, et al. Development of a risk score to predict new pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:2133–2142. [DOI] [PubMed] [Google Scholar]
- 17. Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli S, Elgin E, Donato A. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2016;9:2189–2199. [DOI] [PubMed] [Google Scholar]
- 18. Rodes‐Cabau J, Ellenbogen KA, Krahn AD, Latib A, Mack M, Mittal S, Muntane‐Carol G, Nazif TM, Sondergaard L, Urena M, et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74:1086–1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Figure S1
References 19 and 20


