Abstract
Background
Job strain is implicated in many atherosclerotic diseases, but its role in peripheral artery disease (PAD) is unclear. We investigated the association of job strain with hospital records of PAD, using individual‐level data from 11 prospective cohort studies from Finland, Sweden, Denmark, and the United Kingdom.
Methods and Results
Job strain (high demands and low control at work) was self‐reported at baseline (1985–2008). PAD records were ascertained from national hospitalization data. We used Cox regression to examine the associations of job strain with PAD in each study, and combined the study‐specific estimates in random effects meta‐analyses. We used τ2, I2, and subgroup analyses to examine heterogeneity. Of the 139 132 participants with no previous hospitalization with PAD, 32 489 (23.4%) reported job strain at baseline. During 1 718 132 person‐years at risk (mean follow‐up 12.8 years), 667 individuals had a hospital record of PAD (3.88 per 10 000 person‐years). Job strain was associated with a 1.41‐fold (95% CI, 1.11–1.80) increased average risk of hospitalization with PAD. The study‐specific estimates were moderately heterogeneous (τ2=0.0427, I2: 26.9%). Despite variation in their magnitude, the estimates were consistent in both sexes, across the socioeconomic hierarchy and by baseline smoking status. Additional adjustment for baseline diabetes mellitus did not change the direction or magnitude of the observed associations.
Conclusions
Job strain was associated with small but consistent increase in the risk of hospitalization with PAD, with the relative risks on par with those for coronary heart disease and ischemic stroke.
Keywords: epidemiology, job strain, meta‐analysis, peripheral artery disease, risk factors
Subject Categories: Peripheral Vascular Disease, Atherosclerosis, Cardiovascular Disease, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
Job strain, a marker of psychosocial stress at work, was associated with small but consistent increase in the risk of hospitalization with peripheral artery disease.
The strength of the association was similar to that of job strain with coronary heart disease and ischemic stroke.
What Are the Clinical Implications?
Physicians in occupational health and primary care need to recognize work‐related stress as a risk factor for many cardiovascular disease outcomes, including peripheral artery disease.
Nonstandard Abbreviations and Acronyms
Danish Work Environment Cohort Study
Finnish Public Sector study
- HHS
Helsinki Health Study
Individual‐Participant Data Meta‐Analysis in Working Populations
- PAD
peripheral artery disease
- SHR
sub‐distribution hazard ratio
- WOLF S
Work, Lipids and Fibrinogen Stockholm
Introduction
Peripheral artery disease (PAD) is a manifestation of atherosclerotic cardiovascular disease, characterized by intermittent claudication or atypical leg pain.1 In 2010, this disease affected >200 million people worldwide, reflecting a 13.1% increase in its prevalence in high income countries between 2000 and 2010.1 With the population agiing, larger numbers of people are living with PAD for longer, a trend which is reflected by the wider uptake of secondary preventive treatments, such as statins, antiplatelets, angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers.2 Given the scale of the disease and the effort of keeping PAD at bay by means of secondary preventive measures, it is not surprising that the costs of PAD to patients (in terms of decreased quality of life and years of life lost, disability, sickness absence, and loss of income) and healthcare systems (in terms of medical, endovascular, and surgical management) are now comparable with those incurred by coronary heart disease and stroke.3, 4
Despite the considerable burden of PAD, the evidence on specific risk factors, including potential primary preventive targets, for this disease is scarce.5 Advanced age, type 2 diabetes mellitus and elevated blood pressure, circulating lipids, and clotting are important risk factors for all atherosclerotic cardiovascular diseases, including PAD.1, 5 In addition, recent large‐scale observational “mega‐studies” have shown that stress is associated with many cardiovascular outcomes, most strongly as a trigger or a prognostic factor for major cardiac events in high‐risk populations and in those with pre‐existing cardiovascular disease.6 Reflecting this evidence, European clinical guidelines now recognize psychosocial stress as an important clinical target in the management of heart disease and stroke.5 However, in contrast to the extensive research into the associations of various stress exposures with myocardial infarction, stroke, atrial fibrillation, and venous thromboembolism,7, 8 few studies have examined the relationship between stress and PAD.
The Individual‐Participant Data Meta‐Analysis in Working Populations (IPD‐Work) Consortium is among the world's largest collaborations using harmonized individual‐participant data on work stress and health outcomes in adults.9 Here we have used data from >139 000 men and women from the Consortium's studies to investigate the association between work‐related stress, operationalized as job strain, and hospital‐treated PAD.
Methods
Data Availability
This study used data from 11 independent studies, which all have different data sharing policies. Our data protection agreements with the participating cohort studies do not allow IPD‐Work Consortium to share individual‐level data from these studies to third parties. Requests for individual study data can be addressed to each study's executive committee. Syntax for the main analyses is provided in Data S2.
Studies and Participants
The analyses presented here are based on data from 11 prospective cohort studies, which had available data on job strain and hospital‐treated PAD. Eight of the 19 Consortium studies were not included in the analyses because of missing exposure or outcome data. The analyses were based on data from Copenhagen Psychosocial Questionnaire versions I and II,10, 11 DWECS (Danish Work Environment Cohort Study),12, 13 FPS (Finnish Public Sector) study,14 Health and Social Support,15 HHS (Helsinki Health Study),16 Intervention Project on Absence and Well‐Being,17, 18 Swedish Longitudinal Occupational Survey of Health,19 Still Working,20, 21 Whitehall II,22 and Work, Lipids and Fibrinogen Stockholm (WOLF S).23 All studies were approved by local and/or national ethics committees and participants gave informed consent to take part. Details of the studies have been published previously and are provided in Data S1. Participants were included in the analyses if they had baseline data available on job strain, age, sex, and socioeconomic position, and follow‐up data on hospitalizations. Those with a hospital record of PAD at or before baseline were excluded.
Measurements
The main exposure in our analyses was job strain, the most extensively used operationalization of work‐related psychosocial stress, was ascertained from baseline questionnaires in all studies.24, 25 A detailed description of the harmonization of job strain has been published previously.26 Briefly, participants were asked to rate statements describing psychosocial aspects of their job on a Likert‐type scale. Mean response scores were calculated for job demands items (eg, “my job requires working very fast”) and job control items or (eg, “my job allows me to learn new things”) for each participant. Using the original and most commonly used categorization, we defined high demands as having a job demand score above the study‐specific median and low control as having a job control score below the study‐specific median. According to the original model, a combination of high demands and low control was defined as job strain, and all other demand‐control combinations as no strain.25 To minimize investigator bias, we validated the job strain measure before linking exposure and covariate data to outcome data.
Covariates in our analyses were participant age, sex, socioeconomic position (harmonized into 3 categories: low, intermediate and high), body mass index (BMI: weight in kilograms divided by height in meters squared, harmonized into underweight [<18.5 kg/m2], normal weight [18.5 to <25 kg/m2], overweight [25 to <30 kg/m2] and obese [≥30 kg/m2]), smoking (harmonized into never, former and current), alcohol consumption (harmonized into none, moderate and heavy), leisure‐time physical activity (sedentary or active) and baseline diabetes mellitus (yes or no). Details of ascertainment of these covariates are provided in Data S1.
PAD outcomes were ascertained by linking participants’ study records (with the participants’ consent, using national identification numbers in the Nordic studies and the National Health Service number in Whitehall II) to national hospitalization registers (Nordic studies) and administrative hospitalization data (Whitehall II). Any episode of hospital care with a record of an International Classification of Diseases, Eighth Revision (ICD‐8), Ninth Revision (ICD‐9), or Tenth Revision (ICD‐10) code indicating PAD either as primary or secondary diagnostic code27 (Table S1) was counted as a PAD event. Deaths from any cause were ascertained by linking participants’ study records to national death registers.
Statistical Analyses
We used Cox proportional hazards regression to examine the associations between job strain and hospital‐treated PAD events during follow‐up. Time to the outcome of interest was defined as time from the baseline assessment to the first hospital record of PAD, death of the participant or the end of study‐specific follow‐up, whichever occurred first. Examination of log(−log) plots and Schoenfeld test provided no evidence for violation of the proportional hazards assumption.
First, we examined the associations of job strain with hospital‐treated PAD in each study, using harmonized individual‐participant data. This approach was chosen because of ethical and data protection regulations, only study‐level results from the studies conducted in Sweden and Denmark could be used in the combined analyses. Second, we combined the study‐specific hazard ratios (HRs) and their 95% CIs in random effects meta‐analyses, using empirical Bayes (EB) estimator for between‐study variance. Sensitivity analyses were conducted using DerSimonian and Laird and restricted maximum likelihood estimators for between‐studies variance. Random effects approach was chosen to estimate the mean of the associations between job strain and PAD, which are likely to differ in different countries and work settings. We calculated I2 and τ2 to estimate relative and absolute heterogeneity, respectively, among the study‐specific estimates. In addition to the random effects, overall HR and its 95% CI (which estimate the average association between job strain and PAD and uncertainty about this average), we calculated a 95% prediction interval to estimate the range of associations of job strain with PAD across different study settings. The calculation of the prediction interval is based on the assumptions that the study‐specific estimates in a meta‐analysis represent a random, normally distributed sample from an underlying distribution of estimates.28, 29 Whilst these assumptions cannot be formally checked in the available data, our use of previously unpublished, harmonized data reassures us that the studies included in our analyses are unlikely to be severely biased by publication or other reporting biases. To help meet the normality assumption of the study‐specific estimates, the calculations for the prediction interval were performed on the log‐scale and results back‐transformed to ratio‐scale for ease of interpretation. Stratified meta‐analyses and random effects meta‐regression were used to explore potential sources for heterogeneity. Analyses in the Nordic studies were conducted using SAS 9.4 (Cary, NC, USA) and in Whitehall II using Stata IC 15 (Stata Corporation, College Station, TX, USA), with user‐written Stata packages ipdmetan 30 and metareg.31
Results
In all, 139 132 men and women had baseline data available on job strain, age, sex, and socioeconomic position, and had no previous hospital record relating to PAD (Table).10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 The study‐specific mean age ranged from 38.6 to 49.2 years. Overall, 50 583 (36.4%) of the participants were men, with the study‐specific proportions of men ranging from 20% in FPS to 77% in Still Working. Just under a quarter of participants reported job strain at baseline (n=32 489, 23.4%). The study‐specific distributions of the baseline characteristics are shown in Table S2. The number of PAD patients included in the unadjusted, age‐ and sex‐adjusted and multivariable‐adjusted models was different in HHS, Swedish Longitudinal Occupational Survey of Health, and Whitehall II because a small proportion of participants in these studies had incomplete data on relevant covariates.
Table 1.
Study and Participant Characteristics
| Study | Country | Baseline, y | Setting (Occupational vs Population‐Based) | n, Participants | Mean (Rangea) Baseline Age (y) | n (%) Men | n (%) Job Strain at Baseline | Mean (SD) Follow‐Up (y) | n With PAD (Incidence Per 10 000 Person‐Years) |
|---|---|---|---|---|---|---|---|---|---|
| COPSOQ‐I10 | Denmark | 1997 | Population | 1769 | 40.7 (23–57) | 908 (51.3) | 363 (20.5) | 11.7 (1.6) | 16 (7.71) |
| COPSOQ‐II11 | Denmark | 2004 to 2005 | Population | 3424 | 42.8 (25–58) | 1630 (47.6) | 486 (14.2) | 5.0 (0.4) | 12 (7.71) |
| DWECS12, 13 | Denmark | 2000 | Population | 5563 | 41.8 (23–59) | 2963 (53.3) | 1239 (22.3) | 8.8 (1.2) | 37 (7.53) |
| FPS14 | Finland | 2000 to 2004 | Occupational | 65 495 | 43.7 (17–69) | 13 104 (20.0) | 17 859 (27.3) | 9.8 (1.8) | 110 (1.72) |
| HeSSup15 | Finland | 1998 and 2003 | Population | 18 465 | 38.6 (20–59) | 7760 (42.0) | 5203 (28.2) | 13.1 (2.2) | 55 (2.28) |
| HHS16 | Finland | 2000 to 2002 | Occupational | 6448 | 49.2 (39–60) | 1397 (21.7) | 1001 (15.5) | 14.4 (1.8) | 41 (4.41) |
| IPAW17, 18 | Denmark | 1996 to 1997 | Occupational | 2025 | 41.2 (24–57) | 672 (33.1) | 355 (17.5) | 12.8 (1.9) | 25 (9.66) |
| SLOSH19 | Sweden | 2006 to 2008 | Population | 10 974 | 47.7 (19–68) | 5063 (46.1) | 2223 (20.3) | 6.5 (1.2) | 16 (2.23) |
| Still Working20, 21 | Finland | 1986 | Occupational | 9154 | 40.9 (18–65) | 7071 (77.2) | 1424 (15.6) | 21.7 (4.0) | 161 (8.11) |
| Whitehall II22 | United Kingdom | 1985 to 1988 | Occupational | 10 153 | 44.4 (34–56) | 6799 (70,0) | 1417 (14.0) | 26.8 (4.6) | 159 (5.82) |
| WOLF S23 | Sweden | 1992 | Occupational | 5662 | 41.5 (19–70) | 3216 (56.8) | 919 (16.2) | 15.5 (2.2) | 35 (3.98) |
COPSOQ‐I and –II indicates Copenhagen Psychosocial Questionnaire versions I and II; DWECS, Danish Work Environment Cohort Study; FPS, Finnish Public Sector study; HeSSup, Health and Social Support; HHS, Helsinki Health Study; IPAW, Intervention Project on Absence and Well‐being; PAD, peripheral artery disease; SLOSH, Swedish Longitudinal Occupational Survey of Health; and WOLF S, Work, Lipids and Fibrinogen Stockholm.
5th and 95th percentiles are presented for COPSOQ‐I, COPSOQ‐II, DWECS, and IPAW for data security reasons.
During 1 718 132 person‐years at risk, 667 men and women (0.2%–1.8% of participants, depending on the study) had a hospital record of PAD during the follow‐up. The overall incidence of PAD per 10 000 person‐years of follow‐up was 3.88, ranging from 1.72 (FPS) to 8.11 (Still Working) (Table).
The unadjusted associations between job strain and hospital‐treated PAD, calculated using empirical Bayes between‐study variance estimator, suggested that the average risk of hospitalization with PAD was higher in participants reporting job strain compared with those with no strain (HR: 1.25, 95% CI, 1.04–1.50) (Figure 1).10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Adjustment for age and sex increased the point estimate and widened its CI (HR: 1.46, 95% CI, 1.17–1.83) and further adjustment for lifestyle‐related covariates decreased it only slightly (HR: 1.41, 95% CI, 1.11–1.80). Additional adjustment for baseline diabetes mellitus attenuated the overall point estimate and narrowed its CI (HR: 1.31, 95% CI, 1.07–1.59).
Figure 1.
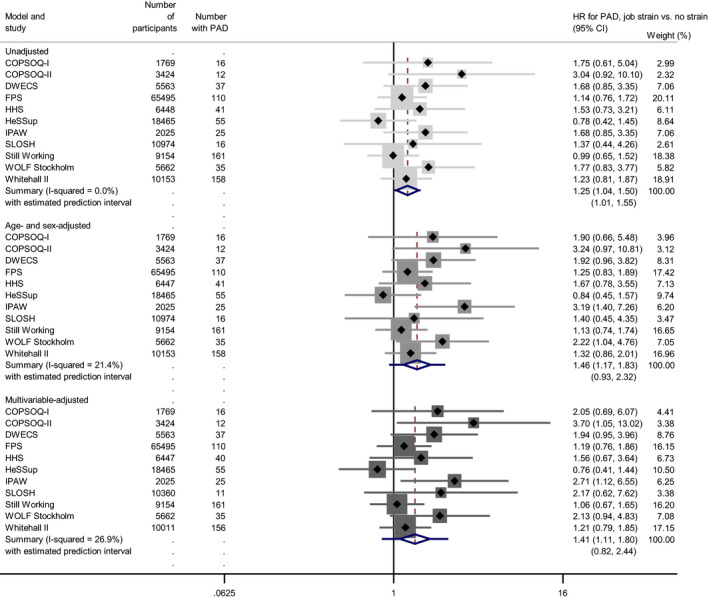
Job strain and hospital record of peripheral artery disease.10 11 12 13 14 15 16 17 18 19 20 21 22 23
COPSOQ‐I and –II indicates Copenhagen Psychosocial Questionnaire versions I and II; DWECS, Danish Work Environment Cohort Study; FPS, Finnish Public Sector study; HeSSup, Health and Social Support; HHS, Helsinki Health Study; IPAW, Intervention Project on Absence and Well‐Being; PAD, peripheral artery disease; SLOSH, Swedish Longitudinal Occupational Survey of Health; and WOLF S, Work, Lipids and Fibrinogen Stockholm.
All unadjusted study‐specific estimates were consistent with each other (all I2 <0.1%) but the covariate‐adjusted estimates were moderately heterogeneous. In the multivariable‐adjusted meta‐analyses, τ2 of 0.0427 indicated that the study‐specific estimates were somewhat dispersed around their mean (ie, the overall random‐effects HR). The corresponding I2 denoted that 26.9% of this variation was attributable to differences beyond chance variation in the association of job strain with PAD in different cohort studies (Table S3). Accordingly, the 95% prediction interval from the multivariable‐adjusted meta‐analyses (0.82–2.44) crossed the null‐value, suggesting that though the average association of job strain with hospitalization for PAD was firmly positive, in some contexts job strain can be associated with over 2‐fold increase in this risk and in others with a decreased risk (Figure 1).
We explored sex, socioeconomic position, and smoking as potential sources for the observed heterogeneity (Figure 2). The subgroup associations were consistent in direction, all indicating an increased risk, but the sizes of the estimated average associations varied. Job strain was associated with an increased average risk of hospitalization with PAD in men (HR: 1.59, 95% CI, 1.12–2.28), individuals with a high socioeconomic position (HR: 2.77, 95% CI, 1.35–5.71), and baseline smokers (HR: 1.52, 95% CI, 1.10–2.09). The estimates were directionally consistent but imprecise in women, people from low or intermediate socioeconomic positions, ex‐smokers, and those who had never smoked. Neither the 95% prediction intervals from the stratified meta‐analyses nor the meta‐regression analyses provided evidence for differences by sex (P=0.3) or trend by baseline smoking status (P=0.7) beyond chance variation. There was some indication of the association between job strain and hospital‐treated PAD being stronger in the high socioeconomic group than in the low socioeconomic group (P=0.046) but no evidence for a linear trend across the socioeconomic groups (P=0.3). Because of overall low numbers of PAD cases in the subgroups the power in the subgroup analyses, however, was limited.
Figure 2.
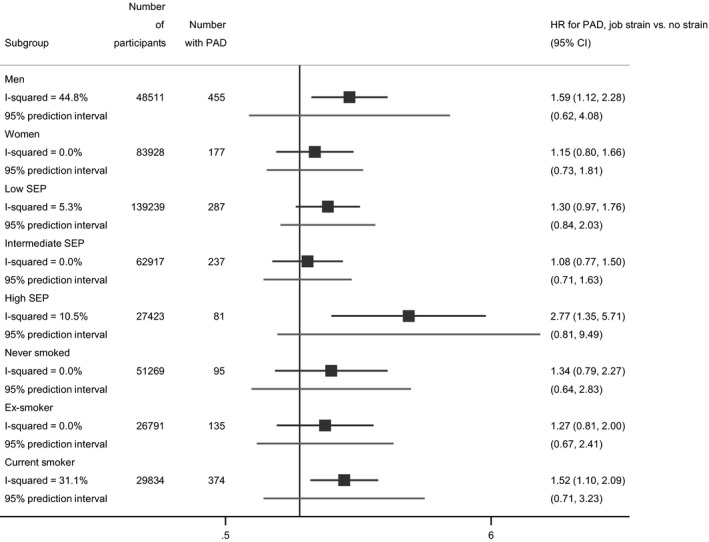
Job strain and hospital record of peripheral artery disease, by subgroup.
PAD indicates peripheral artery disease; and SEP, socioeconomic position.
The findings from sensitivity analyses excluding men and women with a hospital record of PAD during the first year of follow‐up, as well as from those using DerSimonian and Laird and restricted maximum likelihood variance estimators in the meta‐analyses, were similar in direction and magnitude to our main findings (Tables S3 and S4).
Analysis of absolute risks showed that in our study population of working‐age men and women, the incidence of PAD per 10 000 person‐years ranged from 1.72 (FPS) to 9.66 (Intervention Project on Absence and Well‐Being) (Table). The average difference in the absolute risks between the job strain and no strain groups was firmly positive (3.28, 95% CI, 0.78–5.78) but the study‐specific estimates varied (Table S5).
Discussion
Our analysis of individual‐participant data from >139 000 men and women suggest that job strain is associated with an ≈1.4‐fold average increase in the risk of having a hospital record of PAD. This association was observed in all participant subgroups, and the findings were robust to additional adjustment for baseline diabetes mellitus and uncertainty deriving from different ways of estimating between‐study variation.
A large and increasing evidence shows that psychosocial stress is implicated in the development of various forms of atherosclerotic cardiovascular disease.6 However, we are unaware of previous investigations of work‐related stress and the risk of PAD, and must discuss our findings in relationship to previous studies of other stress measures and other cardiovascular disease outcomes. Our findings support those of previous studies, pointing to a role of life stress in atherosclerotic disease. A pooled analysis of the Health Survey for England and Scottish Health Survey, for example, suggests that psychological distress is associated with some 3‐fold increase in the risk of peripheral vascular disease during an average follow‐up of 9.5 years.32 Meta‐analyses of large, prospective individual‐level data sets, have also shown that the general population of adults who reported stress at work or in private life had an 1.1‐ to 1.6‐fold increased risk of coronary heart disease or stroke.6
One possible explanation for the elevated risk of hospitalization with PAD among individuals reporting job strain is that stress has a role in the development of PAD, independently of the known risk factors of age, male sex, low socioeconomic position, smoking, heavy alcohol intake, obesity, and physical inactivity. The associations observed in our investigation were in line with those observed for other atherosclerotic cardiovascular diseases in the IPD‐Work Consortium and other studies: job strain has shown robust associations with hospitalization for ischemic stroke (average relative risk: 1.18, 95% CI, 1.00–1.39),33 coronary heart disease overall (average relative risk: 1.23, 95% CI, 1.10–1.27)9 as well as among those with pre‐existing cardiovascular disease (average relative risk: 1.61, 95% CI, 1.14–2.28).34 While there is limited evidence directly linking stress to atherosclerosis per se, stress response is associated with increased systemic inflammation and elevated blood glucose, which may contribute to exacerbations and complications of PAD.6 In addition to this worsening effect of work‐related stress on pre‐existing artery disease, our findings could reflect other mechanisms, such as stress symptoms lowering the threshold for visiting a physician and subsequently delaying referral and diagnosis.
We conducted sensitivity analyses excluding individuals with a hospital record of PAD during the first year of follow‐up, but we cannot completely eliminate the possibility of early stage, undiagnosed, or subclinical PAD influencing our findings. As less severe manifestations of PAD can be managed medically in primary care, it is possible that the group of participants with no record of hospital‐treated PAD includes individuals with subclinical, early stage, or mild PAD. If this is the case, the association between job strain and hospitalization for PAD may reflect work‐related stress triggering a PAD event among those with existing peripheral artery atherosclerosis. Previous research in high‐risk populations and in adults who already have some form of cardiovascular disease suggests that stress incurs an ≈2‐ to 5.6‐fold increased risks of death.6 Results of a small case‐control study, in which women with coronary vascular dysfunction experienced more peripheral vasoconstriction after a mental stress test than control women,35 may provide a mechanistic explanation for the ability of stress to induce cardiovascular events in general and PAD events specifically in individuals with pre‐existing cardiovascular disease.
PAD is a multifactorial disease, with a large number of risk factors making a relatively modest contribution to its pathogenesis. Smoking, hypertension and type 2 diabetes mellitus have been consistently shown to be associated with an increased risk of developing PAD.1, 2 For instance, a meta‐analysis of 22 published studies showed that current smoking was associated with a 2.72 ‐fold (95% CI, 2.39–3.09), history of previous cardiovascular disease with 2.55‐fold (95% CI, 2.16–3.02) and diabetes mellitus with 1.88‐fold average odds (95% CI, 1.66–2.14) of PAD.1 The odds ratios were lower for hypertension (1.55, 95% CI, 1.42–1.71) and hypercholesterolemia (1.19, 95% CI, 1.07–1.33).1 The hazard ratios from our meta‐analyses suggest that the risk associated with job strain is not as large as that deriving from smoking or history of cardiovascular disease but is on par with the relative risks associated with hypertension and hypercholesterolemia.
The I2 and τ2 pointed to moderate heterogeneity among the study‐specific hazard ratios in our meta‐analyses. The 95% prediction interval from the multivariable‐adjusted meta‐analysis (0.82–2.44) suggests that though on average, job strain is associated with an increase in the relative risk of hospitalization with PAD, true relative risks vary from about one fifth decrease to >2‐fold increase in different study settings. This variation could reflect differences in diagnostic and referral practices over time and across healthcare systems. The absolute risk differences varied between studies, pointing to different baseline risks of PAD in the study populations. However, the 95% prediction interval should be interpreted with caution: although the studies in our analyses had a low risk of publication or reporting biases, it is possible that the prediction interval reflects heterogeneity derived from other, unknown sources of bias.
The point‐estimates for the subgroup associations between job strain and PAD were consistent in direction, all indicating an increased risk in individuals reporting job strain; however, their magnitude varied by sex, socioeconomic position, and baseline smoking status and the subgroup differences did not conclusively explain the observed heterogeneity among the study‐specific findings.
The main strength of our analyses is that they were based on previously unpublished, harmonized, prospective data (including pre‐defined job strain exposure and objectively assessed PAD outcomes) from 3 Nordic and 1 Western European countries. The analytical strategies we used to pool their results aimed to reduce the risk of biases arising from publication preferences, differential exposure, or outcome reporting, and data dredging. We ascertained PAD events from routinely collected hospitalization data, which cover a range of severities of this disease, from intermittent claudication to gangrene and tissue loss. However, early stages of PAD can often be managed in primary care, and although participants with a previous hospital record of PAD were excluded from our analyses, some PAD patients who were treated in primary care may have been included in the comparison group. Thus, the hospital‐treated PAD in our analyses represents the severe end of the disease spectrum and the findings reported here are possibly not generalizable to less severe manifestations of PAD. Unfortunately, we had no access to primary care data and were unable to explore this further.
Data on lipids and blood pressure were not available in all the cohorts included in our analyses, and we were thus unable to examine their roles in the association between job strain and PAD. However, previous research suggests that additional adjustment for lipids and blood pressure is unlikely to have a major effect on the association between job strain and PAD. Our previous work in the IPD‐Work Consortium data has shown that job strain is not associated with either systolic or diastolic blood pressure or circulating cholesterol,36 and that the association between job strain and coronary heart disease (another atherosclerotic outcome) was robust to adjustment for the Framingham Cardiovascular Risk Score, including conventional biological risk factors (eg, diabetes mellitus, lipids, and blood pressure).9
Ours was a sample of studies from an existing research collaboration, and it is possible that other studies, particularly from parts of the world other than Northern Europe, would produce different estimates of the association between job strain and PAD. We also recognize that although well‐conducted, large prospective observational epidemiological studies can indicate temporal relationships between risk factors and disease outcomes, such as PAD, no judgement on the causality of such associations can be made based on longitudinal observational findings alone. Furthermore, although all study‐specific analyses were adjusted for a number of harmonized covariates, we cannot exclude the possibility that residual confounding from imprecisely measured, unmeasured, or unknown confounders has impacted on our estimates. For instance, we were unable to adjust the analyses for sedentary work (eg, large proportion of working time spent sitting), which might confound the association between job strain and PAD.
Conclusions
Findings of this multi‐national multi‐cohort study show that that job strain is associated with a small but consistent increase in the risks of hospitalization with PAD. The strength of the observed association is approximately the same as that of job strain with other atherosclerotic diseases, such as coronary heart disease and ischemic stroke.
Sources of Funding
The IPD‐Work Consortium was supported by NordForsk (the Nordic Research Programme on Health and Welfare), the United Kingdom Medical Research Council (K013351, R024227), the Academy of Finland (311492) and Helsinki Institute of Life Sciences. Professor Lallukka is supported by the Academy of Finland (Grants #287488 and #319200). The funders had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit.
Disclosures
None.
Supporting information
Data S1–S2Tables S1–S5References 10‐23
Acknowledgments
The authors thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The Whitehall II study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants, and data entry staff, who make the study possible.
(J Am Heart Assoc. 2020;9:e013538 DOI: 10.1161/JAHA.119.013538.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 2. Cea‐Soriano L, Fowkes FGR, Johansson S, Allum AM, Garcia Rodriguez LA. Time trends in peripheral artery disease incidence, prevalence and secondary preventive therapy: a cohort study in The Health Improvement Network in the UK. BMJ Open. 2018;8:e018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–215. [DOI] [PubMed] [Google Scholar]
- 4. Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT; Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators . Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. [DOI] [PubMed] [Google Scholar]
- 5. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kivimaki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15:215–229. [DOI] [PubMed] [Google Scholar]
- 7. Fransson EI, Stadin M, Nordin M, Malm D, Knutsson A, Alfredsson L, Westerholm PJM. The association between job strain and atrial fibrillation: results from the Swedish WOLF Study. Biomed Res Int. 2015;2015:371905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kivimaki M, Nyberg ST, Batty GD, Madsen IEH, Tabak AG; IPD‐Work Consortium . Long working hours and risk of venous thromboembolism. Epidemiology. 2018;29:e42–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kivimaki M, Nyberg ST, Batty GD, Fransson E, Heikkila K, Alfredsson L, Bjorner JB, Borritz M, Burr H, Casini A, et al. Job strain as a risk factor for future coronary heart disease: collaborative meta‐analysis of 2358 events in 197,473 men and women. Lancet. 2012;380:1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kristensen TS, Hannerz H, Hogh A, Borg V. The Copenhagen Psychosocial Questionnaire—a tool for the assessment and improvement of the psychosocial work environment. Scand J Work Environ Health. 2005;31:438–449. [DOI] [PubMed] [Google Scholar]
- 11. Pejtersen JH, Kristensen TS, Borg V, Bjorner JB. The second version of the Copenhagen Psychosocial Questionnaire. Scand J Public Health. 2010;38:8–24. [DOI] [PubMed] [Google Scholar]
- 12. Burr H, Bjorner JB, Kristensen TS, Tüchsen F, Bach E. Trends in the Danish work environment in 1990–2000 and their associations with labor‐force changes. Scand J Work Environ Health. 2003;29:270–279. [DOI] [PubMed] [Google Scholar]
- 13. Feveile H, Olsen O, Burr H, Bach E. Danish work environment cohort study 2005: from idea to sampling design. Stat Transit. 2007;8:441–458. [Google Scholar]
- 14. Kivimaki M, Lawlor DA, Smith GD, Kouvonen A, Virtanen M, Elovainio M, Vahtera J. Socioeconomic position, co‐occurrence of behavior‐related risk factors, and coronary heart disease: the Finnish Public Sector study. Am J Public Health. 2007;97:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korkeila K, Suominen S, Ahvenainen J, Ojanlatva A, Rautava P, Helenius H, Koskenvuo M. Non‐response and related factors in a nation‐wide health survey. Eur J Epidemiol. 2001;17:991–999. [DOI] [PubMed] [Google Scholar]
- 16. Lahelma E, Aittomaki A, Laaksonen M, Lallukka T, Martikainen P, Piha K, Rahkonen O, Saastamoinen P. Cohort profile: the Helsinki health study. Int J Epidemiol. 2013;42:722–730. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen M, Kristensen T, Smith‐Hansen L. The Intervention Project on Absence and Well‐Being (IPAW): design and results from the baseline of a 5‐year study. Work Stress. 2002;16:191–206. [Google Scholar]
- 18. Nielsen ML, Rugulies R, Christensen KB, Smith‐Hansen L, Bjorner JB, Kristensen T. Impact of the psychosocial work environment on registered absence from work: a two‐year longitudinal study using the IPAW cohort. Work Stress. 2004;18:323–335. [Google Scholar]
- 19. Magnusson Hanson LL, Leineweber C, Persson V, Hyde M, Theorell T, Westerlund H. Cohort profile: the Swedish Longitudinal Occupational Survey of Health (SLOSH). Int J Epidemiol. 2018;47:691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalimo R, Toppinen S. Organizational well‐being: ten years of research and development: in a forest industry corporation In: Kompier M, Cooper C, eds. Preventing Stress, Improving Productivity: European Case Studies in the Workplace. London: Routledge; 1999:52–85. [Google Scholar]
- 21. Vaananen A, Murray M, Koskinen A, Vahtera J, Kouvonen A, Kivimaki M. Engagement in cultural activities and cause‐specific mortality: prospective cohort study. Prev Med. 2009;49:142–147. [DOI] [PubMed] [Google Scholar]
- 22. Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–256. [DOI] [PubMed] [Google Scholar]
- 23. Peter R, Alfredsson L, Hammar N, Siegrist J, Theorell T, Westerholm P. High effort, low reward, and cardiovascular risk factors in employed Swedish men and women: baseline results from the WOLF Study. J Epidemiol Community Health. 1998;52:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karasek R, Brisson C, Kawakami N, Houtman I, Bongers P, Amick B. The Job Content Questionnaire (JCQ): an instrument for internationally comparative assessments of psychosocial job characteristics. J Occup Health Psychol. 1998;3:322–355. [DOI] [PubMed] [Google Scholar]
- 25. Karasek R, Theorell T. Healthy Work: Stress, Productivity, and the Reconstruction of Working Life. New York: Basic Books; 1990. [Google Scholar]
- 26. Fransson EI, Nyberg ST, Heikkila K, Alfredsson L, de Bacquer D, Batty GD, Bonenfant S, Casini A, Clays E, Goldberg M, et al. Comparison of alternative versions of the job demand‐control scales in 17 European cohort studies: the IPD‐Work Consortium. BMC Public Health. 2012;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batty GD, Gale CR, Kivimaki M, Bell S. Aetiological utility of different placements of cause of mortality on death certificates in multiple cohort studies comprising 700,000 individuals. JAMA Netw Open. 2019;2:e198024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 29. Serghiou S, Goodman SN. Random‐effects meta‐analysis: summarizing evidence with caveats. JAMA. 2019;321:301–302. [DOI] [PubMed] [Google Scholar]
- 30. Fisher DJ. Two‐stage individual participant data meta‐analysis and generalized forest plots. Stata J. 2015;15:369–396. [Google Scholar]
- 31. Harbord RM, Higgins JPT. Meta–regression in Stata. Stata J. 2008;8:493–519. [Google Scholar]
- 32. Batty GD, Russ TC, Stamatakis E, Kivimaki M. Psychological distress and risk of peripheral vascular disease, abdominal aortic aneurysm, and heart failure: pooling of sixteen cohort studies. Atherosclerosis. 2014;236:385–388. [DOI] [PubMed] [Google Scholar]
- 33. Fransson EI, Nyberg ST, Heikkila K, Alfredsson L, Bjorner JB, Borritz M, Burr H, Dragano N, Geuskens GA, Goldberg M, et al. Job strain and the risk of stroke: an individual‐participant data meta‐analysis. Stroke. 2015;46:557–559. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Zhang M, Loerbroks A, Angerer P, Siegrist J. Work stress and the risk of recurrent coronary heart disease events: a systematic review and meta‐analysis. Int J Occup Med Environ Health. 2015;28:8–19. [DOI] [PubMed] [Google Scholar]
- 35. Mehta PK, Hermel M, Nelson MD, Cook‐Wiens G, Martin EA, Alkhoder AA, Wei J, Minissian M, Shufelt CL, Marpuri S, et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: results from the NHLBI‐sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol. 2018;251:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nyberg ST, Fransson EI, Heikkila K, Alfredsson L, Casini A, Clays E, De Bacquer D, Dragano N, Erbel R, Ferrie JE, et al. Job strain and cardiovascular disease risk factors: meta‐analysis of individual‐participant data from 47,000 men and women. PLoS One. 2013;8:e67323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1–S2Tables S1–S5References 10‐23
Data Availability Statement
This study used data from 11 independent studies, which all have different data sharing policies. Our data protection agreements with the participating cohort studies do not allow IPD‐Work Consortium to share individual‐level data from these studies to third parties. Requests for individual study data can be addressed to each study's executive committee. Syntax for the main analyses is provided in Data S2.


