Abstract
Background
Childhood adversity and trauma have been shown to be associated with poorer cardiovascular disease (CVD) outcomes in adulthood. However, longitudinal studies of this association are rare.
Methods and Results
Our study used the CARDIA (Coronary Artery Risk Development in Young Adults) Study, a longitudinal cohort that has followed participants from recruitment in 1985–1986 through 2018, to determine how childhood psychosocial environment relates to CVD incidence and all‐cause mortality in middle age. Participants (n=3646) completed the Childhood Family Environment (CFE) questionnaire at the year 15 (2000–2001) CARDIA examination and were grouped by high, moderate, or low relative CFE adversity scores. We used sequential multivariable regression models to estimate hazard ratios of incident (CVD) and all‐cause mortality. Participants were 25.1±3.6 years old, 47% black, and 56% female at baseline and 198 participants developed CVD (17.9 per 10 000 person‐years) during follow‐up. CVD incidence was >50% higher for those in the high CFE adversity group compared with those in the low CFE adversity group. In fully adjusted models, CVD hazard ratios (95% CI) for participants who reported high and moderate CFE adversity versus those reporting low CFE adversity were 1.40 (0.98–2.11) and 1.25 (0.89–1.75), respectively. The adjusted hazard ratios for all‐cause mortality was 1.68 (1.17–2.41) for those with high CFE adversity scores and 1.55 (1.11–2.17) for those with moderate CFE adversity scores.
Conclusions
Adverse CFE was associated with CVD incidence and all‐cause mortality later in life, even after controlling for CVD risk factors in young adulthood.
Keywords: adverse childhood experiences, cardiovascular events, lifetime risk, longitudinal cohort study, mortality, stress
Subject Categories: Cardiovascular Disease, Risk Factors, Primary Prevention, Epidemiology, Mortality/Survival
Clinical Perspective
What Is New?
Our study demonstrates that adverse childhood family environment is associated with increased risk of cardiovascular disease over extended follow‐up through middle adulthood in the CARDIA (Coronary Artery Risk Development in Young Adults) Study.
Exposure to even moderate adversity in childhood is associated with significantly increased risk for all‐cause mortality in adulthood.
What Are the Clinical Implications?
Childhood family environment has a significant impact on risk for cardiovascular events and mortality throughout an individual's life course.
Traditional cardiovascular disease risk factors present in young adulthood may partially mediate the relationship between adverse childhood environment and cardiovascular disease events.
Nonstandard Abbreviations and Acronyms.
ACE adverse childhood experience
CARDIA Coronary Artery Risk Development in Young Adults
CES‐D Center for Epidemiological Studies ‐ Depression
CFE childhood family environment
CRP C‐reactive protein
CVD cardiovascular disease
HR hazard ratio
Introduction
Exposure to adverse emotional or traumatic experiences during childhood and adolescence is increasingly recognized as having a profound impact on cardiometabolic disease throughout the life course.1, 2, 3 These experiences are thought to affect emotional and behavioral regulation, predisposing individuals to higher rates of behavioral cardiovascular disease (CVD) risk factors that persist into adulthood such as smoking, anxiety, depression, and sedentary lifestyle.2, 4, 5, 6, 7, 8 Exposure to adverse childhood experiences is associated with myriad known clinical CVD risk factors including increased body mass index, diabetes mellitus,9, 10, 11 increased blood pressure,12 vascular dysfunction,13, 14 and inflammation.15, 16, 17, 18 However, studies of the association between childhood adversity and CVD morbidity and mortality outcomes have largely been limited to cross‐sectional and retrospective studies, with few longitudinal studies reporting CVD outcomes.1
The purpose of this study was to investigate whether exposure to an adverse childhood family environment (CFE) is associated with increased incidence of CVD events in a diverse population of men and women in the CARDIA (Coronary Artery Risk Development in Young Adults) cohort. To our knowledge, this is the first large longitudinal cohort study to examine the effect of adverse childhood environment on CVD events and mortality in a population with extended follow‐up through early and middle adulthood.
Methods
Study Population
The CARDIA Study is a population‐based epidemiological study that enrolled 5115 participants in 1985–1986 (year 0). The original cohort was 18 to 30 years old at the time of enrollment and was designed to achieve a balance of demographic variables including race (black and white), sex, age, and education level at each participating center. The cohort was recruited from 4 urban areas in the United States: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. Further details of study design and cohort recruitment have been previously described elsewhere.19 One participant dropped out of CARDIA. Participants were excluded from our analyses if data were missing or incomplete for mortality, CVD outcome, or CFE questionnaire, or if participants were pregnant at the baseline examination (3646 participants of 3672 participants [99.3%] at the year 15 examination were included). All study participants provided written informed consent. Institutional review boards at each participating institution approved study protocols (University of Alabama Birmingham, Northwestern University, University of Minnesota, and Kaiser Permanente). The data, methods, and study materials used in this analysis are available to investigators upon request at the CARDIA Coordinating Center for the purposes of reproducing the results or replicating the procedure.20
Assessment of CFE
CFE adversity was assessed at the 2000–2001 CARDIA examination by in‐person administration of the Risky Families questionnaire.21 The Risky Families questionnaire was adapted from the Adverse Childhood Experiences (ACEs) questionnaire2 and assesses how often respondents experienced 7 elements of family environment: parental love and support, verbal abuse, physical affection, physical abuse, presence of alcohol/drug abuser in the home, how well‐organized and well‐managed the household was, and parental/guardian knowledge of what participants were up to during childhood (specific item wording available in Data S1).17 Participants indicated the frequency at which they experienced each questionnaire item on a 4‐point Likert scale. Previous studies using the Risky Families questionnaire in the CARDIA cohort have polarized questionnaire items (score 0–3 for each item) and summed all responses to create an overall score with each response option weighted equally (range 0–21).13, 22 However, because of the diversity in the nature of each questionnaire item and the distribution of Likert scale responses across items, we did not assume that each incremental increase on the Likert scale reflected a proportional exposure to trauma or adversity in childhood. We therefore empirically dichotomized each item of the 7‐item questionnaire individually on the basis of the severity of adversity in the questionnaire item. Items similar to those included in the original ACEs questionnaire including those reflecting physical, verbal, or emotional abuse; neglect; or exposure to drug or alcohol abusers were dichotomized if respondents reported any level of exposure. All other survey items were dichotomized at the midpoint of the 4‐point Likert scale (see Supplementary Materials). Cronbach's α for the 4‐point survey responses and the dichotomized responses were 0.76 and 0.73, respectively.
To measure overall CFE adversity, we grouped participants by the sum of dichotomized questions that constituted an adverse CFE (range 0–7) into low (0–1; 49% of sample), moderate (2–3; 29% of sample), or high (4+; 22% of sample) relative CFE adversity scores. This distribution mirrors population‐based ACEs scores with an almost identical proportion of respondents reporting high CFE adversity scores as those reporting 3 or more ACEs, and the low CFE adversity group reflecting the approximate proportion of individuals reporting zero ACEs.23
Covariates
Covariates used in multivariable analyses were assessed at the baseline (year 0) examination in 1985–1986 with the exception of depressive symptoms, which was first assessed in 1990–1991 (year 5). To better investigate the longitudinal effect of adverse CFE on CVD and mortality outcomes throughout adulthood, covariates from the baseline examination were included in multivariable analyses to adjust for adverse CVD risk factors at the examination in closest proximity to participants’ exposure to adverse CFE. Age, sex, race, recent unemployment, smoking status, participant education, and highest reported parental education (in years of school) were self‐reported. The highest level of parental education achieved by either parent was used in multivariable analyses. Trained examiners collected data according to a standardized protocol across examination sites including height and weight used to calculate body mass index, waist circumference, and blood pressure, plus a venous blood sample for analysis of total cholesterol, high‐density lipoprotein, triglycerides, and fasting glucose. Depressive symptoms were assessed using the Center for Epidemiological Studies‐Depression (CES‐D) scale, using the validated cutoff of CES‐D ≥16 to define depression.24
Assessment of Clinical Outcomes
Participants were contacted by telephone every year and periodically completed an in‐person CARDIA examination (conducted in years 0, 2, 5, 7, 10, 15, 20, 25, and 30) to inquire about vital status, recent hospitalizations, outpatient medical procedures, and other pertinent medical history items. CVD events and mortality end points were reviewed and adjudicated by 2 physician CARDIA investigators using participant death certificates and medical records with a committee of physicians providing final adjudication in case of disagreement. The primary outcome of our investigation was a composite of CVD events that included fatal and nonfatal myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, heart failure, carotid artery disease, peripheral arterial disease, and other fatal atherosclerotic or CVD events. Because the Risky Family questionnaire refers to participants’ childhood experiences, we included nonfatal CVD event outcomes that occurred before the administration of the questionnaire at the 2000–2001 CARDIA examination. All‐cause mortality was used as a secondary outcome in this investigation. Participants who did not have events during the follow‐up period were censored on the date of last contact or the last CARDIA examination they attended.
Statistical Analysis
We used descriptive statistics and nonparametric tests for trend to compare participants across different CFE adversity groups at baseline following imputation of missing covariates using multiple imputation with chained equations.25, 26 Because CFE has been shown to be associated with many of these covariates at baseline, we report results of both unadjusted and adjusted analyses, noting that because these baseline covariates are potential mediators of the association between adverse CFE and CVD outcomes, full adjustment for these baseline covariates may dilute the true association between CFE and the outcome measures. As a result, the fully adjusted results may provide a conservative estimate of true effects.
A series of Cox proportional‐hazards models was then estimated to analyze the effect of CFE adversity group and time to CVD events or mortality outcomes independent of several known CVD risk factors. Model 1 examined the association between CFE adversity groups and the outcome of interest. Model 2 was adjusted for demographic covariates: race, age, sex, and examination center, which were then included in all subsequent models. Models 3 through 5 were each adjusted for a set of baseline characteristics related to CFE and CVD/all‐cause mortality: socioeconomic status (model 3; recent unemployment, personal education, and parental education), clinical health status (model 4; smoking status, body mass index, waist circumference, systolic and diastolic blood pressure, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, and fasting glucose), and year 5 psychological status (model 5; depressive symptoms). Model 6 was adjusted for all covariates included in all previous regression models. The Cox proportional hazards assumption was tested and found to be appropriate for all models.
Post hoc analysis of CVD events was performed to assess the frequency of different CVD events that were included in the composite CVD outcome. A nonparametric test for trend was used to test for significance across CFE adversity groups. Post hoc analysis was also conducted on individual CFE survey items to evaluate whether any 1 item had a disproportionate impact on CVD event risk. Additional sensitivity analyses were performed using complete‐case analysis for all Cox‐proportional hazards regression models. STATA/IC software version 15.1 (College Station, TX) was used for all statistical analyses.
Results
Of the 3672 participants who attended the year 15 examination, 26 (0.7%) were excluded as described in Methods. Differences between excluded participants and the study population on any other baseline characteristics utilized in our analyses are shown in Table S1, and for the 3646 study participants, imputed observations for missing socioeconomic, clinical health status, and psychological characteristics are enumerated in Table S2. Participants (25.1±3.6 years old at year 0 [1985–1986], 47% black, 56% female) had a mean (SD) CFE adversity score of 1.7 (0.8). Across CFE adversity groups, there was a significant trend towards lower socioeconomic status (higher rates of recent unemployment and lower personal and parental education levels), higher rates of smoking, lower systolic blood pressure, and higher rates of depressive symptoms for individuals with higher CFE adversity scores (Table 1). Individuals with high versus low CFE adversity scores had higher body mass index and waist circumference (both P<0.0001 by pairwise testing; data not shown).
Table 1.
Characteristics of Study Sample in 1985–1986 by Childhood Family Adversity Score, Ascertained in 2000–2001: CARDIA, 1985–2001
| Study Population (n=3646) | CFE Adversity Group | P for Trend | |||
|---|---|---|---|---|---|
| Low CFE Range: 0–1 (n=1781) | Medium CFE Range: 2–3 (n=1043) | High CFE Range: 4–7 (n=822) | |||
| Demographic characteristics | |||||
| Age, y, mean (SD) | 25.1 (3.6) | 25.0 (3.6) | 25.1 (3.7) | 25.2 (3.6) | 0.05 |
| Black race, no. (%) | 1716 (47.1) | 806 (45.3) | 527 (50.5) | 383 (46.8) | 0.24 |
| Female sex, no. (%) | 2036 (55.8) | 977 (54.9) | 575 (55.1) | 484 (58.9) | 0.08 |
| Socioeconomic characteristics | |||||
| Recent unemployment, No. (%) | 1011 (27.7) | 452 (25.4) | 313 (30.0) | 246 (29.9) | <0.01 |
| Participant education, no. (%) | |||||
| ≤12 y | 1297 (35.6) | 527 (29.6) | 397 (38.1) | 373 (45.4) | <0.001 |
| 13–15 y | 1969 (54.0) | 1025 (57.4) | 552 (53.0) | 391 (47.6) | <0.001 |
| ≥16 y | 380 (10.4) | 229 (12.9) | 93 (8.9) | 58 (7.1) | <0.001 |
| Parental education, mean (SD), ya | 13.7 (3.1) | 14.0 (3.1) | 13.4 (3.0) | 13.2 (3.1) | <0.001 |
| Clinical characteristics | |||||
| Current smoker, no. (%) | 990 (27.2) | 406 (22.8) | 297 (28.5) | 287 (34.9) | <0.001 |
| Body mass index, mean (SD), kg/mb | 24.9 (5.3) | 24.6 (5.2) | 24.7 (5.1) | 25.5 (5.8) | 0.08 |
| Waist circumference, mean (SD), cm | 78.5 (12.0) | 78.1 (11.7) | 78.3 (11.9) | 79.4 (12.6) | 0.29 |
| Blood pressure, mean (SD), mm Hg | |||||
| Systolic blood pressure | 110 (10.8) | 111 (10.2) | 111 (11.7) | 109 (10.8) | 0.03 |
| Diastolic blood pressure | 69 (9.7) | 69 (9.6) | 69 (10.3) | 68 (9.3) | 0.13 |
| Serum biomarkers | |||||
| Total cholesterol, mean (SD), mg/dL | 177.5 (33.7) | 176.8 (33.7) | 176.4 (32.9) | 179.9 (34.2) | 0.39 |
| HDL cholesterol, mean (SD), mg/dL | 53.0 (13.3) | 53.1 (13.3) | 53.0 (13.3) | 53.0 (13.2) | 0.43 |
| Triglycerides, median (SD), mg/dL | 74.5 (52.6) | 73.8 (50.0) | 74.3 (57.1) | 76.1 (51.8) | 0.87 |
| Fasting glucose, mean (SD), mg/dL | 82.6 (16.6) | 82.7 (18.7) | 82.5 (15.4) | 82.6 (13.6) | 0.12 |
| Psychological characteristics | |||||
| Depressive symptoms, no. (%)b | 858 (23.5) | 303 (17.0) | 279 (26.7) | 276 (33.6) | <0.001 |
CARDIA indicates Coronary Artery Risk Development in Young Adults Study; CES‐D, Center for Epidemiological Studies‐Depression; CFE, childhood family environment; and HDL, high‐density lipoprotein.
Highest number of years achieved by any parental figure.
Participants were classified as having depressive symptoms if CES‐D score reached the validated cutoff of 16 or greater (range 0–60). CES‐D was first measured during the year 5 examination (1990–1991).
Over a median follow‐up period of 30.9 years since the Y0 examination, 198 participants developed CVD (17.9 per 10 000 person‐years). Those with moderate and high CFE adversity scores had higher incidence of CVD over the follow‐up period (20.1 and 22.2 events/10 000 person‐years, respectively) compared with those with low CFE adversity scores (14.7 events/10 000 person‐years; Table 2). Kaplan–Meier analysis for both CVD and all‐cause mortality during the respective follow‐up periods demonstrated significant separation in unadjusted survival curves (P=0.03 and P=0.001, respectively; Figure). In unadjusted Cox regression analysis, those with high CFE adversity scores were more likely to experience CVD than those with a low CFE adversity score (hazard ratio [HR]=1.52; 95% CI, 1.08 to 2.14; Table 2). This relationship remained significant in multivariable adjusted models including those adjusted for demographic (HR=1.58; 95% CI, 1.12–2.24), socioeconomic (HR=1.46; 95% CI, 1.03–2.07), clinical (HR=1.31; 95% CI, 1.06, 2.14), and psychological (HR=1.53; 95% CI, 1.08–2.18) characteristics. The fully adjusted model, however, was not statistically significant (HR=1.40; 95% CI, 0.98–2.01). All demographic characteristics (black race, male sex, examination center, and examination age) were significantly associated with increased CVD risk in model 2 (data not shown). In the fully adjusted model, black race, examination center, older age, lower participant education, smoking status, higher systolic blood pressure, and higher fasting glucose at the baseline (Y0) examination were significantly associated with greater risk of CVD. Interestingly, male sex was not significantly associated with CVD incidence in fully adjusted models. Depressive symptoms at year 5 were not significantly associated with CVD incidence despite significantly higher rates of depressive symptoms in higher CFE adversity groups at baseline. Those with moderate CFE adversity scores were not shown to have greater risk for CVD events than those with low CFE adversity scores.
Table 2.
Incidence Rates and Unadjusted and Multivariable Adjusted Hazard Ratios (95% CIs) for Cardiovascular Disease and All‐Cause Mortality Regressed on CFE Adversity Score Group: CARDIA 1985–2018
| CFE Score | Events/10 000 Person‐Yearsa | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | Race, Age, Sex, and Center | Model 2+Socioeconomicb | Model 2+Clinicalc | Model 2+Psychologicald | Model 2+Socioeconomic,b Clinical,c and Psychologicald | ||||||||
| Incident CVD | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Low | 14.7 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Moderate | 20.1 | 1.38 (0.99, 1.92) | 0.06 | 1.35 (0.97, 1.88) | 0.08 | 1.28 (0.91, 1.78) | 0.15 | 1.31 (0.94, 1.83) | 0.11 | 1.32 (0.95, 1.85) | 0.10 | 1.25 (0.89, 1.75) | 0.19 |
| High | 22.2 | 1.52 (1.08, 2.14) | 0.02 | 1.58 (1.12, 2.24) | <0.01 | 1.46 (1.03, 2.07) | 0.04 | 1.50 (1.06, 2.14) | 0.02 | 1.53 (1.08, 2.18) | 0.02 | 1.40 (0.98, 2.01) | 0.06 |
| All‐cause mortality | |||||||||||||
| Low | 25.8 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Moderate | 43.9 | 1.65 (1.19, 2.30) | <0.01 | 1.65 (1.18, 2.30) | <0.01 | 1.54 (1.11, 2.15) | <0.01 | 1.60 (1.15, 2.24) | <0.01 | 1.63 (1.17, 2.27) | <0.01 | 1.55 (1.11, 2.17) | 0.01 |
| High | 45.5 | 1.77 (1.25, 2.50) | <0.01 | 1.89 (1.33, 2.68) | <0.01 | 1.70 (1.19, 2.41) | <0.01 | 1.80 (1.26, 2.57) | <0.01 | 1.84 (1.30, 2.63) | <0.01 | 1.68 (1.17, 2.41) | <0.01 |
CARDIA indicates Coronary Artery Risk Development in Young Adults Study; CFE, childhood family environment; CVD, cardiovascular disease; and HR, hazard ratio.
Incident CVD events were followed from baseline (year 0 [1985–1986]) to year 30 (2015–2016) examinations. All‐cause mortality was followed from year 15 (2000–2001) to year 30 (2015–2016) examinations because of CFE being assessed at year 15 examination.
Adjusted for recent unemployment, participant education, and parental education.
Adjusted for smoking status, body mass index, waist circumference, systolic and diastolic blood pressure, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, and fasting glucose.
Adjusted for depressive symptoms as measured by Center for Epidemiological Studies‐Depression ≥16.
Figure 1.
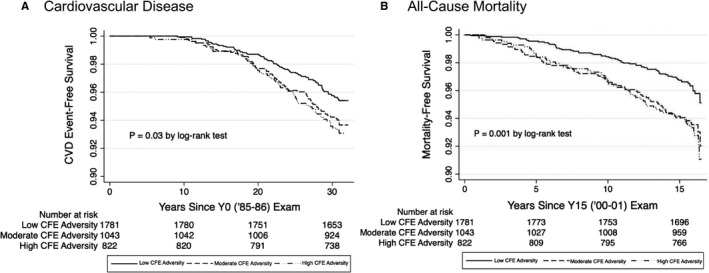
Kaplan–Meier curves for the unadjusted association between CFE adversity group and (A) incident cardiovascular disease and (B) all‐cause mortality: CARDIA 1985–2018. CARDIA indicates Coronary Artery Risk Development in Young Adults Study; CFE, childhood family environment; CVD, cardiovascular disease; Y0, year 0; and Y15, year 15.
Post hoc analysis of individual events that were included in the composite CVD outcome demonstrated that rates of coronary artery disease were significantly higher as CFE adversity score increased; 3.8% of participants with high CFE adversity scores experienced coronary artery disease during the follow‐up period compared with 2.7% and 1.9% of those in the moderate and low CFE adversity score groups, respectively (P<0.01; Table 3). Stroke, heart failure, carotid artery disease, and peripheral artery disease each occurred less frequently than coronary artery disease and were not statistically significantly higher across CFE adversity score groups. Participants who did not participate in the year 15 CARDIA examination experienced similar rates of CVD events over the follow‐up period despite a higher mortality rate (Table S3). Sensitivity analysis demonstrated that only 1 CFE survey item (“Did your family know what you were up to?”) was independently significantly related to increased CVD hazard in unadjusted and fully adjusted analyses.
Table 3.
Frequencies of CVD Events in Each CFE Adversity Group: CARDIA, 1985–2018
| Outcome, No (%) | Study Population (n=3646) | CFE Adversity Group | P for Trend | ||
|---|---|---|---|---|---|
| Low Range: 0–1 (n=1781) | Moderate Range: 2–3 (n=1043) | High Range: 4–7 (n=822) | |||
| All‐cause mortality | 201 (5.5) | 72 (4.0) | 71 (6.8) | 58 (7.1) | <0.001 |
| All CVD eventsa | 198 (5.4) | 80 (4.5) | 63 (6.0) | 55 (6.7) | 0.01 |
| Coronary artery disease | 93 (2.6) | 34 (1.9) | 28 (2.7) | 31 (3.8) | <0.01 |
| MI | 80 (2.2) | 31 (1.7) | 24 (2.3) | 25 (3.0) | 0.03 |
| Non‐MI acute coronary syndrome | 13 (0.4) | 3 (0.2) | 4 (0.4) | 6 (0.7) | 0.03 |
| Stroke | 72 (2.0) | 27 (1.5) | 28 (2.7) | 17 (2.1) | 0.19 |
| Heart failure | 60 (1.7) | 31 (1.7) | 17 (1.6) | 12 (1.5) | 0.60 |
| Carotid artery disease | 1 (0.03) | 1 (0.06) | 0 | 0 | 0.36 |
| Peripheral artery disease | 7 (0.2) | 4 (0.2) | 1 (0.1) | 2 (0.2) | 0.94 |
CFE indicates Childhood Family Environment; CVD, cardiovascular disease; and MI, myocardial infarction.
Participants with more than 1 CVD event were included in all categories for which CVD events occurred.
Over the 15‐year follow‐up period since the questionnaire was administered at the Y15 examination, 201 participants died across all CFE adversity groups (Table 3). Participants with moderate or high CFE adversity scores also had higher rates of all‐cause mortality (43.9 and 45.5 deaths/10 000 person‐years, respectively) compared with participants with low CFE adversity scores (25.8 deaths/10 000 person‐years; Table 2). Unadjusted regression analysis demonstrated that moderate and high CFE adversity groups were at greater risk for mortality when compared with the low CFE adversity group (HR=1.65; 95% CI, 1.19–2.30 and HR=1.77; 95% CI, 1.25–2.50, respectively). This finding was consistent across all analyses, with the fully adjusted model demonstrating a greater risk of mortality for the moderate (HR=1.55; 95% CI, 1.11–2.17) and high CFE adversity group (HR 1.68; 95% CI, 1.17–2.41) when compared with the low CFE adversity group. No notable differences were found in the mortality regression models for the alternative CFE questionnaire cutoffs or in complete case analyses. Similar to CVD incidence, all demographic characteristics were significantly associated with mortality in model 2. In fully adjusted analysis, examination center, older age, smoking status, higher systolic blood pressure, and higher fasting glucose at the baseline (Y0) examination were significantly associated with greater mortality (data not shown). Both race and sex were not significantly associated with mortality in fully adjusted models.
Discussion
We demonstrated that adults at an average age of 40 years who report high levels of childhood psychosocial adversity are at increased risk for both CVD events and death from any cause in early middle adulthood. It is well established that childhood adversity and trauma affect a broad array of cardiovascular disease risk factors in young adulthood. After adjusting for known CVD risk factors and multiple socioeconomic and psychosocial factors in young adulthood, sequential regression models demonstrated that higher reported CFE adversity scores were consistently associated with higher risk of both mortality and CVD events, primarily coronary artery disease over a follow‐up period of nearly 30 years. The relationship between CFE adversity and CVD outcomes was no longer statistically significant in the fully adjusted analysis, indicating that demographic, socioeconomic, clinical, and psychological factors may collectively partially mediate this relationship. These results suggest that unfavorable childhood psychosocial environment not only affects baseline health in young adulthood, but also continues to increase risk well into middle age. This is particularly concerning given the remarkable prevalence of childhood adversity; >20% of our sample reported 4 or more out of 7 indicators of adverse childhood environment.
Our results agree with previously published data that investigated the relationship between adverse CFE (measured as a 4‐point Likert score) and prevalence of traditional CVD risk factors in the CARDIA cohort. Loucks and colleagues demonstrated that CFE was associated with increased carotid intima media thickness in adulthood among white participants as well as an increased 10‐year Framingham CVD risk score in the CARDIA cohort.13, 22 Several other studies have used pathway analyses to relate adverse CFE to poorer metabolic health status, higher blood pressure, and increased serum CRP (C‐reactive protein) in adulthood.12, 17, 21 This study extends these findings by demonstrating the relationship between negative CFE on 30‐year CARDIA outcomes.
Our results are also consistent with retrospective and cross‐sectional survey studies conducted in other adult cohorts.27, 28 Adult self‐reported childhood adversity, measured using ACEs questions, was also found to be associated with higher odds of CVD prevalence. Using a different scale, the original ACEs study by Felitti and colleagues found adjusted odds ratios of 2.2 for ischemic heart disease and 2.4 for stroke among all age adult participants who reported 4 or more ACEs as compared with those reporting none.2, 11 One other longitudinal study investigated the relationship between childhood adversity and incident CVD and mortality; in a Finnish population with median follow‐up of 6.9 years, Korkeila and colleagues demonstrated mixed results based on the type of adversity and sex of study participants.29 With significantly longer follow‐up, our results demonstrate a strong association between high levels of childhood adversity and incident CVD even among middle‐age adults. Strikingly, our results suggest that even moderate exposure to childhood adversity is associated with increased risk of mortality by >50% in middle age as compared with low adversity individuals. Stated simply, regardless of health status in young adulthood, exposure to childhood adversity poses a significant lifelong risk for cardiovascular disease and death. Our data are consistent with the hypothesis that high CFE adversity score specifically affects the cardiovascular system.
Several mechanisms may contribute to the greater risk for CVD events in those with high CFE adversity scores. Childhood adversity is known to cause behavioral dysregulation related to several known CVD risk factors both in childhood and adulthood.5 For example, childhood trauma disrupts ability for children to appropriately cope with and respond to emotionally stressful experiences. As a result, individuals often utilize calorie‐dense foods as a mechanism to cope with psychosocial stress, which contributes to the development of obesity.7, 8 Neuroendocrine and immune pathways have also been shown to contribute to the association between childhood adversity and CVD outcomes. Toxic stress, abuse, and neglect in childhood is thought to alter hypothalamic‐pituitary‐adrenal axis function30, 31, 32 and cause an increase in the volume and activity of the amygdala, the center of the brain responsible for fear and emotional regulation.33 Individuals subsequently experience increases in the stress hormone cortisol and are predisposed to increased levels of inflammation and autonomic dysfunction.15, 17, 34, 35 Analyses of participants in both the CARDIA and MESA (Multi‐Ethnic Study of Atherosclerosis) cohorts demonstrate that lower socioeconomic status and black race are associated with negative changes in cortisol levels, likely at least in part because of psychosocial stress and experiences of discrimination across an individual's life‐course.36, 37 DeSantis and colleagues also showed that the same factors are associated with negative cortisol changes in adolescents.38 A recent prospective study by Tawakol and colleagues demonstrated that amygdalar activity as a result of emotional stress in adults was associated with arterial inflammation, and as a result, individuals with high amygdalar activity experienced higher rates of CVD events over 5 years of follow‐up (HR=4.2, P=0.001).39 This association was found to be substantially mediated by increased bone‐marrow activity and inflammation,39 which are known to be upregulated as a result of emotional stress in both children and adults.15, 16, 17, 18, 40 A recent meta‐analysis demonstrated that individuals who experienced high levels of childhood trauma had significantly higher levels of several serum inflammatory markers including CRP and IL‐6,41 which have been shown to be associated with increased cortisol42 and higher incidence of myocardial infarction and stroke in recent large clinical trials.43, 44 It is likely that a portion of the greater CVD risk in high CFE adversity groups is because of increased levels of cortisol and inflammation over the life course in addition to behavioral risk factors.
The results of our study demonstrate that the prevention and treatment of childhood adversity is an important aspect of reducing adult cardiovascular disease, and despite the original ACEs study being published >20 years ago, little attention has been paid to the consequences of childhood adversity outside of the pediatrics and mental health communities. To our knowledge, no interventions have yet been developed to explicitly address cardiovascular disease risk in individuals with remote histories of childhood trauma. Further research is needed to elucidate the behavioral and physiologic mechanisms pathways involved with the response to childhood adversity. Additionally, public assistance programs such as the Supplemental Nutrition Assistance Program (SNAP) and Women, Infants, and Children (WIC) are important in reducing experiences of adversity among low income children in whom childhood adversity is more prevalent.45 For older children and adolescents, many effective psychosocial intervention programs exist that focus on the development of coping strategies and normalization of their experiences.46 These interventions may be effective ways to reduce CVD risk in those exposed to adverse experiences in childhood, yet funding for these programs remains tenuous and long‐term effects of psychosocial interventions on cardiovascular risk are not well studied.
The present study was limited to those CARDIA participants who survived to undergo the year 15 examination. As such, mortality events before year 15 examination could not be included in the analysis. Participants who did not complete the Y15 CARDIA examination likely represent participants with higher CFE given their lower socioeconomic status. Because they were excluded from the present study, our results likely underestimate the true association between adverse CFE and CVD and mortality outcomes. Additionally, retrospective self‐reported childhood environment may be subject to recall bias by survey respondents, and a recent meta‐analysis has found poor agreement between prospectively and retrospectively measured childhood maltreatment.47 However, it is unclear whether prospectively or retrospectively measured childhood adversity is a more accurate reflection of childhood adversity. Additionally, adults may be more likely to under‐report major adverse events in childhood rather than over‐report, which may dilute the true effect of childhood adversity on CVD incidence and mortality presented in this study.48 Because our results are likely a conservative estimate of the true risk associated with adverse CFE, these results should be interpreted as hypothesis generating. Finally, the present study is not intended to justify the use of the Risky Families questionnaire for screening patients for childhood adversity and trauma.
Overall, this study demonstrates that exposure to adversity and trauma during childhood—including child abuse, neglect, and household dysfunction—is associated with greater risk of incident cardiovascular disease, primarily coronary artery disease. This association was not statistically significant in the fully adjusted model, suggesting that it may be partially mediated by participants’ collective demographic, socioeconomic, clinical, and psychological risk factors. By contrast, the association between childhood adversity and all‐cause mortality remained statistically significant in the fully adjusted model, and individuals exposed to even moderate adversity in childhood psychosocial adversity are at greater risk for all‐cause mortality. This study highlights the importance of this critical developmental period on cardiometabolic disease and risk of death over the entire life course.
Sources of Funding
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I).
Disclosures
None.
Supporting information
Data S1 Tables S1–S3
Acknowledgments
This article has been reviewed by CARDIA for scientific content.
(J Am Heart Assoc. 2020;9:e015326 DOI: 10.1161/JAHA.119.015326.)
See Editorial by Barr
For Sources of Funding and Disclosures, see page 9.
References
- 1. Suglia SF, Koenen KC, Boynton‐Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the american heart association. Circulation. 2018;137:e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 4. Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. [DOI] [PubMed] [Google Scholar]
- 5. Su S, Jimenez MP, Roberts CT, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellis MA, Lowey H, Leckenby N, Hughes K, Harrison D. Adverse childhood experiences: retrospective study to determine their impact on adult health behaviours and health outcomes in a uk population. J Public Health. 2013;36:81–91. [DOI] [PubMed] [Google Scholar]
- 7. Greenfield EA, Marks NF. Violence from parents in childhood and obesity in adulthood: using food in response to stress as a mediator of risk. Soc Sci Med. 2009;68:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrepf A, Markon K, Lutgendorf SK. From childhood trauma to elevated c‐reactive protein in adulthood: the role of anxiety and emotional eating. Psychosom Med. 2014;76:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basu A, McLaughlin KA, Misra S, Koenen KC. Childhood maltreatment and health impact: the examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clin Psychol Sci Pract. 2017;24:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, Gao H, Hao L, Liu L. Adverse childhood experiences and risk of type 2 diabetes: a systematic review and meta‐analysis. Metabolism. 2015;64:1408–1418. [DOI] [PubMed] [Google Scholar]
- 11. Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease. Circulation. 2004;110:1761–1766. [DOI] [PubMed] [Google Scholar]
- 12. Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the cardia study. Health Psychol. 2009;28:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loucks EB, Taylor SE, Polak JF, Wilhelm A, Kalra P, Matthews KA. Childhood family psychosocial environment and carotid intima media thickness: the cardia study. Soc Sci Med. 2014;104:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klassen SA, Chirico D, O'Leary DD, Cairney J, Wade TJ. Linking systemic arterial stiffness among adolescents to adverse childhood experiences. Child Abuse Negl. 2016;56:1–10. [DOI] [PubMed] [Google Scholar]
- 15. Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life‐course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain Behav Immun. 2012;26:239–250. [DOI] [PubMed] [Google Scholar]
- 17. Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C‐reactive protein in the coronary artery risk development in young adults study. Biol Psychiat. 2006;60:819–824. [DOI] [PubMed] [Google Scholar]
- 18. Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, Miller GE. Additive contributions of childhood adversity and recent stressors to inflammation at midlife: findings from the midus study. Dev Psychol. 2015;51:1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. Cardia: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 20. University of Alabama at Birmingham . Cardia study contact information. 2020. Available at: https://www.cardia.dopm.uab.edu/contact-cardia. Accessed January 20, 2020.
- 21. Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the cardia study. Psychosom Med. 2005;67:846–854. [DOI] [PubMed] [Google Scholar]
- 22. Loucks EB, Almeida ND, Taylor SE, Matthews KA. Childhood family psychosocial environment and coronary heart disease risk. Psychosom Med. 2011;73:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of adverse childhood experiences from the 2011–2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatr. 2018;172:1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. [DOI] [PubMed] [Google Scholar]
- 25. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 26. Cuzick J. A Wilcoxon‐type test for trend. Stat Med. 1985;4:543–547. [DOI] [PubMed] [Google Scholar]
- 27. Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the georgia stress and heart study. Circulation. 2015;131:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson EL, Caleyachetty R, Stafford M, Kuh D, Hardy R, Lawlor DA, Fraser A, Howe LD. Prospective associations of psychosocial adversity in childhood with risk factors for cardiovascular disease in adulthood: the MRC national survey of health and development. Int J Equity Health. 2017;16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korkeila J, Vahtera J, Korkeila K, Kivimäki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96:298–303. [DOI] [PubMed] [Google Scholar]
- 30. Coelho R, Viola T, Walss‐Bass C, Brietzke E, Grassi‐Oliveira R. Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr Scand. 2014;129:180–192. [DOI] [PubMed] [Google Scholar]
- 31. Bick J, Naumova O, Hunter S, Barbot B, Lee M, Luthar SS, Raefski A, Grigorenko EL. Childhood adversity and DNA methylation of genes involved in the hypothalamus–pituitary–adrenal axis and immune system: whole‐genome and candidate‐gene associations. Dev Psychopathol. 2012;24:1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA‐axis reactivity in women free of lifetime psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:889–894. [DOI] [PubMed] [Google Scholar]
- 33. Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. [DOI] [PubMed] [Google Scholar]
- 34. Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor‐evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barr DA. The childhood roots of cardiovascular disease disparities. Mayo Clin Proc. 2017;92:1415–1421. [DOI] [PubMed] [Google Scholar]
- 36. Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosom Med. 2006;68:41–50. [DOI] [PubMed] [Google Scholar]
- 37. Hajat A, Diez‐Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi‐ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41:3–13. [DOI] [PubMed] [Google Scholar]
- 39. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang T, Chen Y, Liu H, Zhou Z, Zhai Y, Yang J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein e knockout mice. Thromb Res. 2010;126:386–392. [DOI] [PubMed] [Google Scholar]
- 41. Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta‐analysis of peripheral C‐reactive protein, interleukin‐6 and tumour necrosis factor‐α. Mol Psychiatry. 2016;21:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeSantis A, DiezRoux A, Hajat A, Aiello A, Golden SH, Jenny N, Seeman T, Shea S. Associations of salivary cortisol levels with inflammatory markers: the multi‐ethnic study of atherosclerosis. Psychoneuroendocrinology. 2012;37:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 44. Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 45. Lee BJ, Mackey‐Bilaver L. Effects of WIC and food stamp program participation on child outcomes. Child Youth Ser Rev. 2007;29:501–517. [Google Scholar]
- 46. Cook A, Spinazzola J, Ford J, Lanktree C, Blaustein M, Cloitre M, DeRosa R, Hubbard R, Kagan R, Liautaud J. Complex trauma in children and adolescents. Psychiatr Ann. 2017;35:390–398. [Google Scholar]
- 47. Baldwin JR, Reuben A, Newbury JB, Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta‐analysis. JAMA Psychiatr. 2019;76:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45:260–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Tables S1–S3


