Abstract
Background
Peripheral microvascular endothelial dysfunction (PMED) has been linked to an increased risk of cardiovascular events, but there is a lack of information characterizing the predictive value of PMED for future risk of ischemic stroke (IS).
Methods and Results
This retrospective observational cohort study enrolled 637 patients who underwent non‐invasive microvascular endothelial function assessment using reactive hyperemia peripheral arterial tonometry. Reactive hyperemia peripheral arterial tonometry index ≤2 was defined as PMED. Of 280 patients with PMED, 12 (4.3%) patients developed IS, compared with only 4 (1.1%) of 357 patients without PMED during a median follow‐up of 5.3 years. Patients with PMED had lower IS‐free survival compared with patients without PMED (log‐rank P=0.03). Cox proportional hazard ratio (HR) analyses showed that PMED predicted the incidence of IS, with a HR of 3.43, 95% CI, 1.10–10.63 (P=0.03); adjusted HR of 3.70, 95% CI, 1.18–11.59 (P=0.02) after adjusting for sex, smoking history, and atrial fibrillation; adjusted HR of 3.45, 95% CI, 1.11–10.72 (P=0.03) after adjusting for CHA2DS2‐VASc score; adjusted HR of 5.70, 95% CI, 1.40–23.29 (P=0.02) after adjusting for revised Framingham Stroke Risk Score. Reactive hyperemia peripheral arterial tonometry index improved discrimination of risk for IS after adding reactive hyperemia peripheral arterial tonometry index to CHA2DS2‐VASc score and revised Framingham Stroke Risk Score.
Conclusions
PMED was associated with a >3‐fold increased risk of IS. These findings underscore the concept of the systemic nature of endothelial dysfunction, which could act as a potential marker to predict future risk of IS.
Keywords: endothelial dysfunction, ischemic stroke, microvascular dysfunction, vascular reactivity
Subject Categories: Endothelium/Vascular Type/Nitric Oxide
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- HR
hazard ratio
- PMED
peripheral microvascular endothelial dysfunction
- RH‐PAT
reactive hyperemia peripheral arterial tonometry
Clinical Perspective
What Is New?
Peripheral microvascular endothelial dysfunction, defined as reactive hyperemia peripheral arterial tonometry index ≤2.0 was associated with a >3‐fold increased risk of ischemic stroke.
Assessment of peripheral microvascular endothelial dysfunction added prognostic value to CHA2DS2‐VASc score or revised Framingham Stroke Risk Score (aged ≥55 years) in predicting ischemic stroke.
What Are the Clinical Implications?
Findings underscore the concept of the systemic nature of endothelial dysfunction, which could act as a potential marker to predict future risk of ischemic stroke.
Introduction
Measurement of peripheral vasomotor response as a measure for endothelial dysfunction has been linked to adverse cardiovascular outcomes.1 Reactive hyperemia peripheral arterial tonometry (RH‐PAT) is a non‐invasive method to measure the vasomotor response using fingertip device. RH‐PAT index correlated well with coronary microvascular endothelial function and can be used to non‐invasively assess peripheral microvascular endothelial dysfunction (PMED), which is associated with increased risk of late cardiovascular adverse events in individuals with minimal cardiovascular risk factors. Thus, PMED could be viewed as an early feature of vascular disease.2, 3, 4
Endothelial cells play a key role in cerebral circulation: (1) endothelial cells are the site of blood brain barrier, thus controlling the movement of ions, molecules, and cells, (2) endothelial cells affect resting cerebral blood flow as well as vasomotor responses to shear stress, neurotransmitters, and metabolic factors, and (3) endothelial cells affect the function of neurons, microglia, and oligodendrocytes.5 Therefore, endothelial dysfunction may predispose brain tissue to be at risk for ischemic injury during decrease in perfusion pressure. However, there is a lack of evidence to show the association between PMED and ischemic stroke.
Atrial fibrillation (AF) is a well‐known cause of left atrial thrombus formation, leading to massive cardioembolic stroke.6 However, only ≈50% to 60% of ischemic strokes in patients with AF were reported to be cardioembolic in etiology, whereas one third of ischemic strokes might be lacunar infarcts caused by small vessel occlusion.7, 8 Increased levels of circulating von Willebrand factor and soluble E‐selectin, both of which are correlated with endothelial dysfunction, were associated with an increased risk of ischemic stroke in real‐world AF patients,9 suggesting the link between endothelial dysfunction and ischemic stroke. CHA2DS2‐VASc score (congestive heart failure, hypertension, aged ≥75 years, diabetes mellitus, stroke or transient ischemic attack, peripheral artery disease, aged 65–74 years, sex category) was created to predict the risk of ischemic stroke in patients with non‐valvular AF, and is widely used to guide antithrombotic therapy in this patient group.10, 11, 12 In contrast, recently published clinical studies have demonstrated that use of the CHA2DS2‐VASc score in predicting ischemic stroke has extended to patients without AF for which it was not initially proposed.13, 14, 15, 16
We hypothesized that PMED could predict the future risk of ischemic stroke. This study aimed to investigate the association between PMED and the incidence of ischemic stroke. Also, we sought to assess the incremental prognostic value provided by PMED in predicting ischemic stroke when combined with the CHA2DS2‐VASc score or revised Framingham Stroke Risk Score.
Methods
The data that supported the findings of this study are available from the corresponding author upon reasonable request.
Study Population
In this observational cohort study, we enrolled 637 patients who visited the Mayo Clinic between January 2006 and February 2014 and underwent endothelial function testing using the EndoPAT 2000 device (Itamar Medical Inc., Caesarea, Israel) for the assessment of cardiovascular risk and/or chest pain. Endothelial function was evaluated at the clinical discretion of the evaluating physician. This study was conducted in accordance with the guidelines of the Declaration of Helsinki, and the Mayo Clinic Institutional Review Board approved the study protocol. All patients provided written informed consent for participation in the current study.
Assessment of Microvascular Endothelial Function
The peripheral microvascular endothelial function was evaluated by RH‐PAT, as previously described.3, 17, 18, 19 Briefly, the study protocol included a 5‐minute baseline measurement, followed by 5‐minute inflation of a blood pressure cuff around the test arm with a pressure of 60 mm Hg above baseline systolic blood pressure up to 200 mm Hg, followed by a 6‐minute PAT measurement after deflation of the cuff. Blood pressure cuff occlusion was not applied to the control arm (contralateral arm). RH‐PAT ratio was determined as the average pulse wave amplitude for a 1‐minute‐period beginning 1 minute after pressure cuff deflation (test arm=A; control arm=C) divided by the average pulse wave amplitude during a 3.5‐minute baseline period (test arm=B; control arm=D). The RH‐PAT index was calculated automatically through a computer algorithm by normalizing baseline signal and indexing the RH‐PAT ratio on the test arm to that of the control arm: RH‐PAT index=(A/B)/(C/D) × baseline correction. All vasoactive medications, including calcium channel blockers, β‐blockers, and long‐acting nitrates, were discontinued for at least 24 hours before endothelial function testing. A calculated RH‐PAT index ≤2.0 was used as a cut‐off value for the diagnosis of peripheral microvascular endothelial dysfunction (PMED) in this study.18, 20, 21, 22
Clinical Assessment
Clinical history, laboratory data, and current medications were collected from a detailed chart review by an investigator masked to RH‐PAT data. Data were collected on the following parameters: (1) sex, age, smoking status, and atrial fibrillation, (2) dyslipidemia, defined by a documented history of hyperlipidemia, treatment with lipid‐lowering therapy, a low‐density lipoprotein cholesterol level above the target (<130 mg/dL for low risk patients, <100 mg/dL for moderate‐high risk patients, <70 mg/dL for high risk, and <55 mg/dL for extremely high risk patients based on 10‐year atherosclerotic cardiovascular disease risk),23 high‐density lipoprotein cholesterol <40 mg/dL in men or <50 mg/dL in women, or triglycerides >150 mg/dL, (3) type 2 diabetes mellitus, defined as a documented history of or treatment for type 2 diabetes mellitus, (4) hypertension, defined as a documented history of or treatment for hypertension, (5) coronary artery disease, defined as a documented history of myocardial infarction, revascularization, or >50% luminal stenosis in any coronary artery diagnosed using coronary angiography or computed tomography coronary angiography, (6) peripheral vascular disease, including intermittent claudication, previous surgery or percutaneous intervention on the abdominal aorta or the lower extremity vessels, abdominal or thoracic surgery, arterial and venous thrombosis, and (7) a diagnosis of an ischemic stroke before and after the first RH‐PAT test. Ischemic stroke events were identified in accordance with the American Heart Association/American Stroke Association definition.24 All ischemic strokes were classified into cardioembolic, lacunar, and large artery disease, using modified TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.8 All diagnoses of ischemic stroke were made by experienced neurologists at Mayo Clinic.
Calculation of CHA2DS2‐VASc Score and Revised Framingham Stroke Risk Score
Based on CHA2DS2‐VSASc score, patients were given 1 point for congestive heart failure, hypertension, aged 65 to 74 years, diabetes mellitus, vascular disease, and female sex, and 2 points for aged ≥75 years and previous thromboembolism.10 Revised Framingham Stroke Risk Score was calculated using the published equations in patients ≥55 years.25
Statistical Analysis
Continuous variables distributed normally were expressed as the mean±SD, and those with a skewed distribution were expressed as the median with interquartile range. Categorical variables were expressed as frequency (percentage). Enrolled patients were divided into 2 groups; those with PMED (RH‐PAT index ≤2.0) and those without PMED (RH‐PAT index >2.0). For between‐groups comparisons, unpaired t test was used for normally distributed continuous variables, Mann–Whitney U test for non‐normally distributed variables, and χ2 test (and Fisher exact test) for categorical variables. Kaplan‐Meier methods were used to estimate ischemic stroke‐free survival rates. The difference between groups was analyzed using the log‐rank test. Univariate logistic regression analyses were performed to estimate the effects of PMED on the risk of ischemic stroke, with additional stratification by age, sex, and the presence of cardiovascular risk factors and atrial fibrillation. P value for interaction was calculated to assess if the effects of PMED on the risk of ischemic stroke differ between the subgroups. Additionally, univariate and multivariate Cox proportional hazard ratio (HR) analyses were performed to estimate the risk for ischemic stroke. In multivariable analyses, 4 covariate sets were investigated: (1) RH‐PAT index ≤2.0, sex, smoking history, atrial fibrillation, (2) RH‐PAT index ≤2.0, age, diabetes mellitus, hypertension, and dyslipidemia, (3) RH‐PAT index ≤2.0 and CHA2DS2‐VASc score, and (4) RH‐PAT index ≤2.0 and revised Framingham Stroke Risk Score. These covariate sets were chosen for clinical relevance. Finally, we evaluated the discriminatory power of the RH‐PAT index for identifying ischemic stroke when adding RH‐PAT index to CHA2DS2‐VASc score or revised Framingham Stroke Risk Score by calculating net reclassification improvement and integrated discrimination improvement. For all tests, a P<0.05 was considered statistically significant. All statistical analyses were performed using JMP Pro software (SAS Institute, Inc., Cary, NC, USA) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
The baseline characteristics of the study population are summarized in Table 1. Of 637 patients, 280 patients (44.0%) had PMED, and 357 patients (56.0%) had normal peripheral microvascular endothelial function at baseline. Patients with PMED were more likely to have cardiovascular risk factors such as diabetes mellitus and dyslipidemia. Coronary artery disease was more prevalent in patients with PMED compared with those with normal peripheral microvascular endothelial function. Atrial fibrillation was detected in 15 patients (5.4%) with PMED and 15 patients (4.2%) with normal peripheral microvascular endothelial function (P=0.49). The frequency of a previous history of ischemic stroke was similar between patients with normal and abnormal peripheral microvascular endothelial function (n=20 [5.6%] versus n=14 [5.0%], respectively; P=0.74). CHA2DS2‐VASc score at baseline was not different between groups (P=0.94).
Table 1.
Baseline Characteristics of Patients With Normal vs Abnormal Peripheral Microvascular Endothelial Function
| Characteristics | Total (N=637) | RH‐PAT Index | |
|---|---|---|---|
| ≤2.0 (n=280) | >2.0 (n=357) | ||
| Age, y | 52.0±13.6 | 51.8±13.5 | 52.1±13.7 |
| Sex, n (%) | |||
| Women | 389 (61.1) | 155 (55.4) | 234 (65.5) |
| Men | 248 (38.9) | 125 (44.6) | 123 (34.5) |
| Race, n (%) | |||
| Whites | 578 (90.7) | 258 (92.1) | 320 (89.6) |
| Non‐Whites | 59 (9.3) | 22 (7.9) | 37 (10.4) |
| Comorbidities, n (%) | |||
| Hypertension | 283 (44.4) | 127 (45.4) | 156 (43.7) |
| Diabetes mellitus | 56 (8.8) | 36 (12.9) | 20 (5.6) |
| Dyslipidemia | 450 (70.6) | 213 (76.1) | 237 (66.4) |
| Chronic kidney disease | 83 (14.6) | 41 (16.2) | 42 (13.3) |
| Coronary artery disease | 144 (22.6) | 76 (27.2) | 68 (19.1) |
| Atrial fibrillation | 30 (4.7) | 15 (5.4) | 15 (4.2) |
| Previous stroke | 34 (5.3) | 14 (5.0) | 20 (5.6) |
| Smoking history, n (%) | 234 (3.7) | 107 (38.2) | 127 (35.6) |
| Laboratory data | |||
| LDL‐C, mg/dL | 103 (80–127) | 101 (78–125) | 103 (83–129) |
| HDL‐C, mg/dL | 54 (44–66) | 50 (41–62) | 58 (46–70) |
| Triglyceride, mg/dL | 109 (77–158) | 121 (80–183) | 102 (74–147) |
| FPG, mg/dL | 96 (90–104) | 97 (92–105) | 95 (89–102) |
| HbA1c, % | 5.5 (5.2–5.9) | 5.6 (5.2–6.0) | 5.4 (5.2–5.9) |
| Creatinine, mg/dL | 0.93±0.22 | 0.94±0.25 | 0.92±0.20 |
| Systolic BP, mm Hg | 122.1±16.7 | 121.5±16.6 | 122.6±16.8 |
| Diastolic BP, mm Hg | 79.4±11.0 | 73.8±9.9 | 75.8±11.8 |
| RH‐PAT index | 2.09 (1.74–2.53) | 1.70 (1.48–1.84) | 2.48 (2.22–2.79) |
| Medications, n (%) | |||
| Anti‐platelet | 337 (52.9) | 158 (56.4) | 179 (50.1) |
| Statins | 269 (42.3) | 130 (46.4) | 139 (39.0) |
| Anti‐hypertensive | 329 (51.7) | 155 (55.4) | 174 (48.7) |
| Anti‐diabetic | 46 (7.3) | 31 (11.2) | 15 (4.2) |
| CHA2DS2‐VASc score | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| Revised FSRS (≥55 y) |
2.2 (1.2–4.5) n=287 |
2.7 (1.2–4.5) n=124 |
1.9 (1.2–4.3) n=163 |
BP indicates blood pressure; FPG, fasting plasma glucose; FSRS, Framingham Stroke Risk Score; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; and RH‐PAT, reactive hyperemia peripheral arterial tonometry.
Impact of PMED on the Incidence of Ischemic Stroke
During the median (interquartile range) follow‐up of 5.3 (0.4–13.6) years, 12 patients with PMED (4.3%) developed ischemic stroke, compared with 4 patients with normal peripheral microvascular endothelial function (1.1%). Among 12 ischemic stroke patients with PMED, 3 patients had AF and 2 of them were diagnosed as cardioembolic stroke. There were no AF‐related strokes in patients without PMED. Stroke‐free survival was significantly lower in patients with PMED compared with those without PMED (log‐rank P=0.03) (Figure 1). The association between PMED and risk of ischemic stroke during follow‐up is shown in Table 2. PMED was significantly associated with incident ischemic stroke in all individuals, patients aged ≥60 years, patients with dyslipidemia, and patients without diabetes mellitus (P=0.02, 0.02, 0.02, and 0.03, respectively). Odds ratio could not be calculated in men, patients without dyslipidemia, and patients with atrial fibrillation, because all patients in each group who developed ischemic stroke during follow‐up had PMED. We did not find significant interaction between these subgroups. Next, we performed univariate Cox proportional HR analyses to estimate the risk of ischemic stroke. PMED, age, atrial fibrillation, diabetes mellitus, hypertension, CHA2DS2‐VASc score, and Revised Framingham Stroke Risk Score were all associated with an increased risk of ischemic stroke during follow‐up (P=0.03, <0.0001, 0.03, 0.04, 0.02, <0.0001, and 0.002, respectively) (Table 3). PMED was an independent predictor of ischemic stroke during follow‐up after adjustment for other cardiovascular risk factors (multivariate model 1 and 2) or stroke risk score (multivariate model 3 and 4) (multivariate 1: adjusted HR, 3.70; 95% CI, 1.18–11.59, P=0.02; multivariate 2: adjusted HR, 3.36; 95% CI, 1.05–10.78, P=0.04; multivariate 3: adjusted HR, 3.45; 95% CI, 1.11–10.72, P=0.03; multivariate 4: adjusted HR 5.70; 95% CI, 1.40–23.29, P=0.02) (Table 4). PMED was a robust predictor of ischemic stroke even after adjustment for other components of the CHA2DS2‐VASc score individually, including congestive heart failure, myocardial infarction, and peripheral vascular diseases (adjusted HR 3.36, 95% CI, 1.05–10.78, P=0.04).
Figure 1. Comparison of ischemic stroke‐free survival between patients with normal vs abnormal peripheral microvascular endothelial function.
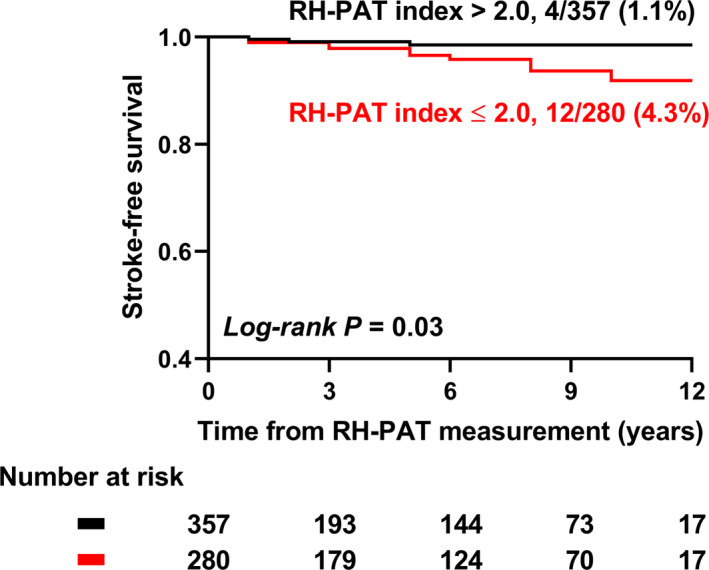
Out of 287 patients with reactive hyperemia peripheral arterial tonometry index ≤2.0, 12 patients (4.3%) developed ischemic stroke during follow‐up, whereas 4 out of 280 (1.1%) ischemic stroke was detected in patients with reactive hyperemia peripheral arterial tonometry index >2.0. Patients with reactive hyperemia peripheral arterial tonometry index ≤2.0 had a lower ischemic stroke‐free survival compared with those with normal peripheral microvascular endothelial function at baseline (log‐rank P=0.03). RH‐PAT indicates reactive hyperemia peripheral arterial tonometry.
Table 2.
Association Between RH‐PAT Index ≤2.0 and Risk of Incident Ischemic Stroke
| Stratified by | No. of Patients With RH‐PAT Index ≤2.0/All Patients (%) | No. of Patients With Incident Ischemic Stroke/All Patients (%) | Odds Ratio | 95% CI | P Value | P for Interaction |
|---|---|---|---|---|---|---|
| All individuals | 280/637 (44.0) | 16/637 (2.5) | 3.91 | 1.26 to 12.39 | 0.02 | |
| Sex | ||||||
| Men | 125/248 (50.4) | 6/248 (2.4) | * | * | 0.99 | 0.07 |
| Women | 155/389 (39.9) | 10/389 (2.6) | 2.32 | 0.64 to 8.34 | 0.20 | |
| Age | ||||||
| <60 y | 147/318 (46.2) | 3/318 (0.9) | 2.34 | 0.21 to 26.12 | 0.48 | 0.60 |
| ≥60 y | 133/319 (41.7) | 13/319 (4.1) | 4.96 | 1.34 to 18.38 | 0.02 | |
| Dyslipidemia | ||||||
| (−) | 67/187 (35.8) | 1/187 (0.5) | * | * | 0.99 | 0.12 |
| (+) | 213/450 (47.3) | 15/450 (3.3) | 4.66 | 1.30 to 16.73 | 0.02 | |
| Diabetes mellitus | ||||||
| (−) | 244/581 (42.0) | 12/581 (2.1) | 4.26 | 1.14 to 15.92 | 0.03 | 0.53 |
| (+) | 36/56 (64.3) | 4/56 (7.1) | 1.73 | 0.17 to 17.80 | 0.65 | |
| CAD | ||||||
| (−) | 203/492 (41.3) | 9/492 (1.8) | 2.90 | 0.72 to 11.75 | 0.14 | 0.59 |
| (+) | 76/144 (52.8) | 7/144 (4.9) | 5.74 | 0.67 to 48.97 | 0.11 | |
| Atrial fibrillation | ||||||
| (−) | 265/607 (43.7) | 13/607 (2.1) | 2.97 | 0.90 to 9.75 | 0.07 | 0.18 |
| (+) | 15/30 (50.0) | 3/30 (10.0) | * | * | 0.99 | |
*Odds ratio could not be calculated in the subgroups.CAD indicates coronary artery disease; and RH‐PAT, reactive hyperemia peripheral arterial tonometry.
Table 3.
Univariate Cox Proportional HR Analysis for the Risk of Ischemic Stroke
| Univariate | |||
|---|---|---|---|
| HR | 95% CI | P Value | |
| RH‐PAT index ≤2.0 | 3.43 | 1.10 to 10.63 | 0.03 |
| Male sex | 0.93 | 0.34 to 2.56 | 0.89 |
| Age, 10‐y increment | 2.44 | 1.54 to 3.99 | <0.0001 |
| Diabetes mellitus | 3.35 | 1.08 to 10.39 | 0.04 |
| Hypertension | 4.73 | 1.35 to 16.60 | 0.02 |
| Dyslipidemia | 5.4 | 0.71 to 40.95 | 0.10 |
| Smoking history | 0.77 | 0.27 to 2.21 | 0.62 |
| Atrial fibrillation | 3.93 | 1.12 to 13.79 | 0.03 |
| CHA2DS2‐VASc score | 1.88 | 1.39 to 2.50 | <0.0001 |
| Revised FSRS (≥55 y) | 1.15 | 1.03 to 1.24 | 0.002 |
FSRS indicates Framingham Stroke Risk Score; HR, hazard ratio; and RH‐PAT, reactive hyperemia peripheral arterial tonometry.
Table 4.
Multivariate Cox Proportional HR Analysis for the Risk of Ischemic Stroke
| Multivariate 1 | Multivariate 2 | Multivariate 3 | Multivariate 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| RH‐PAT index ≤2.0 | 3.70 | 1.18 to 11.59 | 0.02 | 3.36 | 1.05 to 10.78 | 0.04 | 3.45 | 1.11 to 10.72 | 0.03 | 5.70 | 1.40 to 23.29 | 0.02 |
| Male sex | 0.77 | 0.28 to 2.15 | 0.62 | |||||||||
| Age, 10‐y increment | 2.24 | 1.36 to 3.84 | 0.001 | |||||||||
| Diabetes mellitus | 1.46 | 0.44 to 4.80 | 0.53 | |||||||||
| Hypertension | 1.96 | 0.51 to 7.48 | 0.33 | |||||||||
| Dyslipidemia | 2.30 | 0.29 to 18.27 | 0.43 | |||||||||
| Smoking history | 0.78 | 0.27 to 2.26 | 0.65 | |||||||||
| Atrial fibrillation | 4.29 | 1.21 to 15.24 | 0.02 | |||||||||
| CHA2DS2‐VASc score | 1.90 | 1.40 to 2.56 | <0.0001 | |||||||||
| Revised FSRS (≥55 y) | 1.20 | 1.06 to 1.33 | 0.001 | |||||||||
FSRS indicates Framingham Stroke Risk Score; HR, hazard ratio; and RH‐PAT, reactive hyperemia peripheral arterial tonometry.
Discriminatory Power of RH‐PAT Index for Ischemic Stroke
Risk of ischemic stroke increased with CHA2DS2‐VASc score (0, 0/101 [0%]; 1, 1/263 [0.4%]; 2, 5/144 [3.5%]; 3, 4/76 [5.3%]; 4, 5/36 [13.9%]; 5, 1/10 [10.0%]; 6, 0/3 [0%], 7, 0/1 [0%], respectively; P<0.0001) (Figure 2A). Incidence of ischemic stroke based on CHA2DS2‐VASc score and RH‐PAT index is shown in Figure 2B. Finally, we assessed the discriminatory power of RH‐PAT index for ischemic stroke when adding RH‐PAT index to the CHA2DS2‐VASc score or revised Framingham Stroke Risk Score by calculating net reclassification improvement and integrated discrimination improvement. The discriminatory accuracy for ischemic stroke significantly improved after adding RH‐PAT index to CHA2DS2‐VASc score (integrated discrimination improvement 0.02, 95% CI, 0.005–0.04, P=0.02; net reclassification improvement 0.64, 95% CI, 0.20–1.07, P=0.004) and revised Framingham Stroke Risk Score (integrated discrimination improvement 0.03, 95% CI, 0.0001–0.06, P=0.049; net reclassification improvement 0.71, 95% CI, 0.23–1.18, P=0.003) (Table 5).
Figure 2. Incidence of ischemic stroke categorized by CHA2DS2‐VASc score and reactive hyperemia peripheral arterial tonometry index.
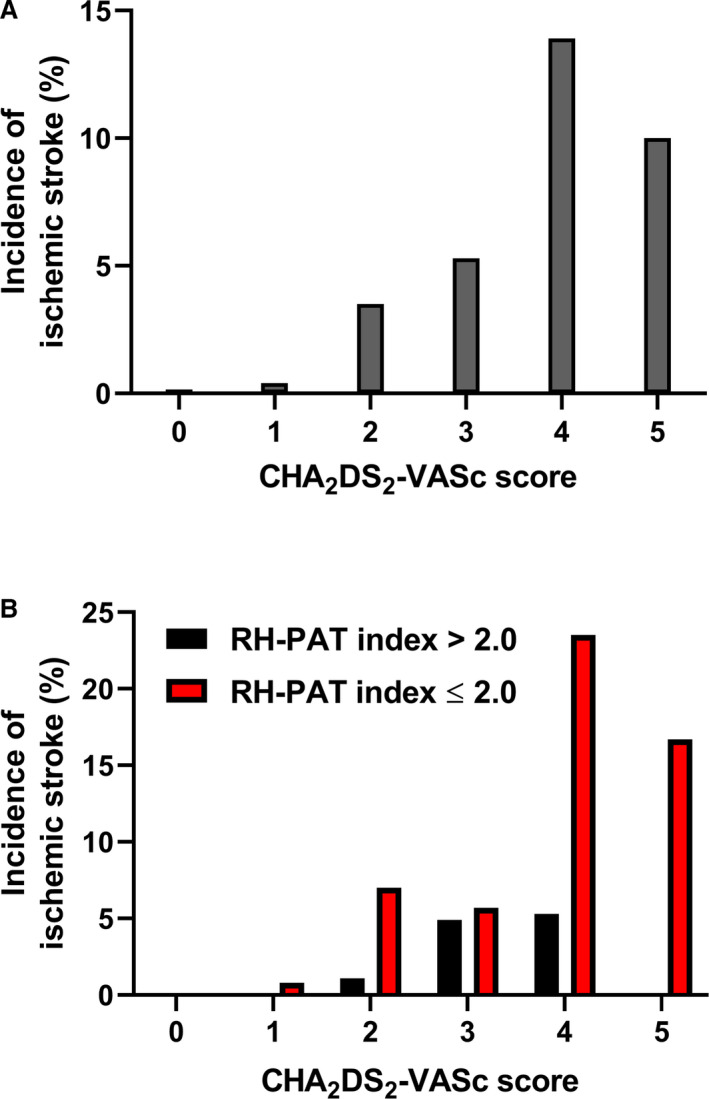
A, Incidence of ischemic stroke according to CHA2DS2‐VASc score (0, 0/101 [0%]; 1, 1/263 [0.4%]; 2, 5/144 [3.5%]; 3, 4/76 [5.3%]; 4, 5/36 [13.9%]; 5, 1/10 [10.0%]; 6, 0/3 [0%], 7, 0/1 [0%], respectively; P<0.0001). B, Comparison of incidence of ischemic stroke according to CHA2DS2‐VASc score between patients with normal vs abnormal microvascular endothelial dysfunction. RH‐PAT indicates reactive hyperemia peripheral arterial tonometry.
Table 5.
Reclassification of Ischemic Stroke After Addition of RH‐PAT Index to CHA2DS2‐VASc Score or Revised Framingham Stroke Risk Score
| Discrimination | CHA2DS2‐VASc Score | CHA2DS2‐VASc Score+RH‐PAT Index ≤2.0 | Revised FSRS (≥55 y) | Revised FSRS (≥55 y)+RH‐PAT Index ≤2.0 |
|---|---|---|---|---|
| C‐statistics | 0.82 | 0.85 | 0.68 | 0.78 |
| IDI (95% CI) | 0.02 (0.005–0.04); P=0.02 | 0.03 (0.0001–0.06); P=0.049 | ||
| NDI (95% CI) | 0.64 (0.20–1.07); P=0.004 | 0.71 (0.23–1.18); P=0.003 | ||
FSRS indicates Framingham Stroke Risk Score; IDI, integrated discrimination improvement; NDI, net reclassification improvement; and RH‐PAT, reactive hyperemia peripheral arterial tonometry.
Discussion
In the current study, we show that individuals with PMED had a >3‐fold increased risk of developing ischemic stroke compared with patients without PMED at baseline, even after adjusting for other cardiovascular risk factors or stroke risk score such as CHA2DS2‐VASc score and revised Framingham Stroke Risk Score. Patients with PMED had a lower ischemic stroke‐free survival rate compared with individuals with normal microvascular endothelial function at baseline. Moreover, the assessment of PMED added prognostic value to the CHAD2DS2‐VASc score and revised Framingham Stroke Risk Score (aged ≥55 years) in predicting ischemic stroke. Thus, the current study supports the concept that PMED may predispose to the development of ischemic stroke and/or may act as a surrogate marker of risk for the development of ischemic stroke in the future.
Microvascular Endothelial Dysfunction and the Development of Cerebral Small Vessel Disease
Endothelial dysfunction has been linked to an increased risk of cerebral small vessel disease.26, 27 One study reported that cerebral vasomotor response to 5‐minute CO2‐enriched (5%) gas mixture inhalation, evaluated by the change of blood flow velocity at the right middle cerebral artery at least in part through endothelial function,28 was associated with symptomatic lacunar infarction, whereas endothelial‐dependent conduit vessel (brachial and carotid artery) reactivity was not.29 The majority of strokes detected in this study (12/16, 75%) were thought to be related to lacunar infarction caused by small vessel occlusion. Given that RH‐PAT index is an indicator of microvascular endothelial function as opposed to flow‐mediated dilatation of the brachial and carotid artery, which is an indicator of macrovascular endothelial function,30 the observed association between PMED and ischemic stroke in this study may suggest the critical role of microvascular function on the progression of cerebral small vessel disease. However, the lack of flow‐mediated dilatation data in the majority of the study population limits our ability to meaningfully evaluate the potentially different roles of macro‐ and microvascular endothelial function on the development of cerebral vascular disease.
Incremental Predictive Value of RH‐PAT Index
In this study population, CHA2DS2‐VASc score predicted incident ischemic stroke with a C‐statistic of 0.82, though only 4.7% of patients had AF. This finding was consistent with previous observations that showed the prognostic value of CHA2DS2‐VASc score in predicting ischemic stroke in patients without AF.13, 14, 15, 16 Interestingly, RH‐PAT index added prognostic value to the CHA2DS2‐VASc score alone when predicting ischemic stroke with a C‐statistic of 0.85. Also, RH‐PAT index added prognostic value to the revised Framingham Stroke Risk Score in patients ≥55 years. The discriminatory difference was small, but significant (Table 5). RH‐PAT index seemed to be able to discriminate the risk of ischemic stroke in patients with lower CHA2DS2‐VASc score (≤2) as well as higher CHA2DS2‐VASc score (>2) (Figure 2B). This observation was not confirmatory, however, and should be validated in different populations.
Limitations
This study has several limitations. First, because of its retrospective observational cohort design, it is challenging to derive causal associations from the current study. The evaluation of RH‐PAT index was performed at the discretion of the evaluating physician. Therefore, some selection bias cannot be excluded. Second, despite collecting clinical data from detailed chart review, misclassification and underdetection of incident ischemic stroke may have occurred. Of note, however, as previously mentioned, clinical data were collected by an investigator masked to the RH‐PAT data. Finally, though we calculated the predictive value of the RH‐PAT index using a multivariable analysis, we could not adjust for all the variables because of the small number of events in our sample. Nevertheless, an RH‐PAT index ≤2.0 remained an independent predictor of incident ischemic stroke after adjusting for variables shown to be relevant to ischemic stroke in previous studies.
Conclusions
PMED, defined by an RH‐PAT index ≤2.0, may predict incident ischemic stroke. These findings underscore the concept of the systemic nature of endothelial dysfunction, which could act as a potential marker to predict future risk of ischemic stroke, though they will require further validation. Whether improvement in PMED translates into a reduced incidence of ischemic stroke remains to be determined. Similarly, the mechanism underlying this association needs to be defined in future studies.
Sources of Funding
This study was partly supported by the National Institute of Health (NIH grants DK120292 and DK122734) and the Mayo Foundation.
Disclosures
Prof. Amir Lerman declared consulting for Itamar Medical (Caesarea, Israel). The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:e015703 DOI: 10.1161/JAHA.119.015703.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. [DOI] [PubMed] [Google Scholar]
- 2. Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 3. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 4. Lerman A, Zeiher AM. Endothelial function. Circulation. 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 5. Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017;120:449–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. [DOI] [PubMed] [Google Scholar]
- 7. Hart RG, Pearce LA, Miller VT, Anderson DC, Rothrock JF, Albers GW, Nasco E. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis. 2000;10:39–43. [DOI] [PubMed] [Google Scholar]
- 8. Perera KS, Sharma M, Connolly SJ, Wang J, Gold MR, Hohnloser SH, Lau CP, Van Gelder IC, Morillo C, Capucci A, et al. Stroke type and severity in patients with subclinical atrial fibrillation: an analysis from the asymptomatic atrial fibrillation and stroke evaluation in pacemaker patients and the atrial fibrillation reduction atrial pacing trial (ASSERT). Am Heart J. 2018;201:160–163. [DOI] [PubMed] [Google Scholar]
- 9. Krishnamoorthy S, Khoo CW, Lim HS, Lane DA, Pignatelli P, Basili S, Violi F, Lip GY. Prognostic role of plasma von Willebrand factor and soluble E‐selectin levels for future cardiovascular events in a ‘real‐world’ community cohort of patients with atrial fibrillation. Eur J Clin Invest. 2013;43:1032–1038. [DOI] [PubMed] [Google Scholar]
- 10. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 11. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 13. Lip GY, Lin HJ, Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Comparative assessment of published atrial fibrillation stroke risk stratification schemes for predicting stroke, in a non‐atrial fibrillation population: the Chin‐Shan Community Cohort Study. Int J Cardiol. 2013;168:414–419. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell LB, Southern DA, Galbraith D, Ghali WA, Knudtson M, Wilton SB. Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2‐VASc scores. Heart. 2014;100:1524–1530. [DOI] [PubMed] [Google Scholar]
- 15. Melgaard L, Gorst‐Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2‐VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314:1030–1038. [DOI] [PubMed] [Google Scholar]
- 16. Parsons C, Patel SI, Cha S, Shen WK, Desai S, Chamberlain AM, Luis SA, Aguilar MI, Demaerschalk BM, Mookadam F, et al. CHA2DS2‐VASc score: a predictor of thromboembolic events and mortality in patients with an implantable monitoring device without atrial fibrillation. Mayo Clin Proc. 2017;92:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. [DOI] [PubMed] [Google Scholar]
- 18. Toya T, Sara JD, Corban MT, Taher R, Godo S, Herrmann J, Lerman LO, Lerman A. Assessment of peripheral endothelial function predicts future risk of solid‐tumor cancer. Eur J Prev Cardiol. 2020;27:608–618. [DOI] [PubMed] [Google Scholar]
- 19. Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]
- 20. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taher R, Sara JD, Prasad M, Kolluri N, Toya T, Lerman LO, Lerman A. Elevated serum uric acid is associated with peripheral endothelial dysfunction in women. Atherosclerosis. 2019;290:37–43. [DOI] [PubMed] [Google Scholar]
- 22. Taher R, Sara JD, Heidari B, Toya T, Lerman LO, Lerman A. Metabolic syndrome is associated with peripheral endothelial dysfunction amongst men. Diabetes Metab Syndr Obes. 2019;12:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease—executive summary. Endocr Pract. 2017;23:479–497. [DOI] [PubMed] [Google Scholar]
- 24. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dufouil C, Beiser A, McLure LA, Wolf PA, Tzourio C, Howard VJ, Westwood AJ, Himali JJ, Sullivan L, Aparicio HJ, et al. Revised Framingham Stroke Risk Profile to reflect temporal trends. Circulation. 2017;135:1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke. 2009;40:e322–e330. [DOI] [PubMed] [Google Scholar]
- 27. Knottnerus IL, Ten Cate H, Lodder J, Kessels F, van Oostenbrugge RJ. Endothelial dysfunction in lacunar stroke: a systematic review. Cerebrovasc Dis. 2009;27:519–526. [DOI] [PubMed] [Google Scholar]
- 28. Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–H1861. [DOI] [PubMed] [Google Scholar]
- 29. Deplanque D, Lavallee PC, Labreuche J, Gongora‐Rivera F, Jaramillo A, Brenner D, Abboud H, Klein IF, Touboul PJ, Vicaut E, et al. Cerebral and extracerebral vasoreactivity in symptomatic lacunar stroke patients: a case‐control study. Int J Stroke. 2013;8:413–421. [DOI] [PubMed] [Google Scholar]
- 30. Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow‐mediated dilation in men and women. Am J Physiol Heart Circ Physiol. 2011;301:H1118–H1126. [DOI] [PMC free article] [PubMed] [Google Scholar]


