Abstract
Background
The American Heart Association and American College of Cardiology guidelines defined patient‐management groups that would benefit from lowering of low‐density lipoprotein cholesterol (LDL‐C). We assessed gaps in dyslipidemia care among employees and spouses with health benefits.
Methods and Results
We studied 17 889 employees and spouses who were covered by an employer‐sponsored health plan and participated in an annual health assessment. Using medical claims, laboratory tests, and risk assessment questionnaires, we found that 43% of participants were in one of 4 patient‐management groups: secondary prevention, severe hypercholesterolemia (LDL‐C ≥190 mg/dL at least once in the preceding 5 years), diabetes mellitus, or elevated 10‐year risk of cardiovascular disease. To assess gaps in dyslipidemia care, we used LDL‐C ≤70 mg/dL as the goal for both the secondary prevention group and those in the elevated 10‐year risk group with >20% risk; LDL‐C ≤100 mg/dL was used for the other groups. Among those in patient‐management groups, 27.3% were in the secondary prevention group, 7.4% were in the severe hypercholesterolemia group, 29.9% were in the diabetes mellitus group, and 35.4% were in the elevated 10‐year risk group. About 74% of those in patient‐management groups had above‐goal LDL‐C levels, whereas only 31% had evidence of a lipid‐lowering therapy in the past 6 months: 45% in the secondary prevention group, 31% in the severe hypercholesterolemia group, 36% in the diabetes mellitus group, and 17% in the elevated 10‐year risk group.
Conclusions
The substantial gaps in LDL‐C treatment and goal attainment among members of an employer‐sponsored medical plan who were mostly aware of their LDL‐C levels indicate the need for gap‐closure initiatives.
Keywords: cholesterol reduction, epidemiology, guideline adherence
Subject Categories: Cardiovascular Disease, Primary Prevention, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- ACC
American College of Cardiology
- CVD
cardiovascular disease
- NHANES
National Health and Nutrition Examination Survey
Clinical Perspective
What Is New?
Gaps in dyslipidemia care were assessed in 17 889 employees and spouses of a US employer with employees in all 50 states.
Study participants were covered by an employer‐sponsored health plan and participated in an annual health assessment offered by the employer.
Substantial gaps in dyslipidemia care were observed in all 4 American Heart Association and American College of Cardiology–defined patient‐management groups. Many patients were not treated with lipid‐lowering therapies and did not achieve low‐density lipoprotein cholesterol goals.
What Are the Clinical Implications?
Dyslipidemia care is not appropriately managed even among individuals with access to medical care and who are aware of their cardiovascular health.
Programs designed to improve dyslipidemia care targeting both patients and care providers seem warranted.
Introduction
Multiple clinical trials have found that reducing low‐density lipoprotein cholesterol (LDL‐C) levels effectively prevents both primary and secondary cardiovascular disease (CVD) events. Relative risk reduction in these trials was ≈20% for each 40‐mg/dL reduction of LDL‐C.1, 2 Therefore, achieving and maintaining LDL‐C at or below goal has been a major emphasis of CVD prevention guidelines.3, 4
Despite the well‐recognized benefit of maintaining LDL‐C levels at or below goal, elevated LDL‐C levels remain a population health problem for a variety of reasons. Many adults who do not regularly visit a primary‐care provider5 or may not be aware of their high LDL‐C levels or elevated CVD risk.6 Even if LDL‐C–lowering therapy is initiated, it may not be adjusted to achieve LDL‐C at or below goal levels.7 Lack of periodic feedback from primary‐care providers may also result in poor adherence to lipid‐lowering therapy, and poor adherence has been shown to be associated with greater risk of dying.8
Gaps in dyslipidemia care have been reported among patients with established CVD,9, 10, 11, 12, 13 stroke,14 and peripheral artery disease15; in those with greater numbers of CVD risk factors11, 16; and in those eligible for treatment according to guidelines.17, 18 These studies were based on analyses of information limited to patients already under a physician's care, such as patient discharge records,12 patient registries,13, 16medical insurance claims10, 11 or physician surveys.17 In addition, analyses based on national survey data18 do not consider the effect of the participants’ medical insurance availability on lipid‐lowering therapy utilization. We set out to assess gaps in dyslipidemia care in those who are members of a group health plan (who may or may not have a relationship with a healthcare provider) and who are likely to be aware of their cardiovascular health.
Many employers in the US offer annual employer‐sponsored population health assessment programs that include CVD risk assessment and lipid‐level laboratory tests.19 Information collected in health assessment programs can be used to measure gaps in care for working‐aged populations with employer‐provided health benefits.
We investigated the prevalence of above‐goal LDL‐C levels and the prevalence of lipid‐lowering therapy among employees and spouses who were covered by an employer‐sponsored health plan and participated in an annual health assessment offered by a US nationwide employer.
Methods
The population of the study was drawn from 35 276 individuals who participated in an annual health assessment program between September 2017 and June 2018. The health assessment program was offered by a major US clinical diagnostics provider with employees in all 50 states to all its employees and their spouses. A majority of the workforce held jobs related to laboratory testing, phlebotomy, or sample‐handling logistics. We excluded those who did not participate in the employer‐sponsored group health plan for at least 12 consecutive months immediately before participating in the health screening program (n=10 709), those who were aged >75 or <40 years (n=6433), and those with missing data (n=245). The remaining 17 889 participants were included in the analysis (Figure 1). An institutional review board waived the requirement for informed consent by determining that this research was conducted according to the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, 45 CFR 164.514(e), which allows the use of retrospective limited data sets from which direct patient identifiers have been removed. The code developed for the statistical analysis in this article will be provided on request sent to the corresponding author. The data will not be available because distribution of limited data sets is limited by the HIPAA Privacy Rule.
Figure 1. Study participants flow diagram. Horizontal arrows indicate exclusion from the study.
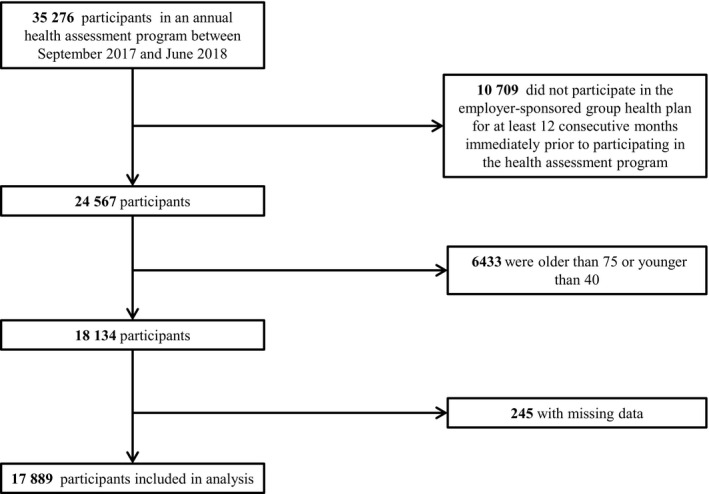
Vertical arrows indicate flow of participants leading to final study population.
The health assessment program included (1) measurement of blood pressure, height, and weight; (2) a health assessment questionnaire, including questions about smoking, diabetes mellitus status, and family history of myocardial infarction; and (3) a panel of laboratory tests performed on freshly drawn fasting blood samples including high‐density lipoprotein cholesterol (HDL‐C), LDL‐C, total cholesterol, triglycerides, high‐sensitivity C‐reactive protein, glucose, hemoglobin A1C, and cotinine. Secondary prevention patients were identified based on the International Classification of Diseases, Ninth Revision (ICD‐9) and ICD‐10 codes in medical claims filed in the 12 months before health assessment program enrollment and up to 5 years before, if available. The ICD codes used to identify secondary prevention patients are listed in Table S1. The use of antihypertensive therapy and lipid‐lowering therapy was defined as a pharmacy claim for a relevant therapy category (Tables S2 and S3) within the past 6 months or a self‐reported use of therapy. Lipid‐lowering discontinuation was defined as a pharmacy claim for lipid‐lowering therapy filed 7 to 12 months before health assessment program enrollment and up to 5 years before, if available, among those not using lipid‐lowering therapy. Statin intensity was defined according to the 2018 American Heart Association and American College of Cardiology (AHA/ACC) guideline on the management of blood cholesterol.3, 4 Diabetes mellitus was defined as having a fasting blood glucose level >125 mg/dL, hemoglobin A1c >6.4%, a prescription for a diabetic medication in the past 6 months, or a self‐reported physician diagnosis of diabetes mellitus. Hypertension was defined as having systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, antihypertensive medication prescription in the past 6 months, or a self‐reported physician diagnosis of hypertension. Smoking status was defined as a positive cotinine test (>2 ng/mL) or self‐reported smoking. Metabolic syndrome was defined using criteria reported in the 2018 AHA/ACC guideline on the management of blood cholesterol.3 The 10‐year risk of CVD for participants without prevalent CVD was calculated using the pooled cohort equations.20
Patient‐management groups were defined according to the criteria in the 2013 and 2018 AHA/ACC guideline on the management of blood cholesterol.3, 4 For this analysis, we placed patients in only 1 patient‐management group. Patients who met the criteria for >1 group were placed in the first group for which they qualified in the following order: (1) the secondary prevention group (those with prevalent CVD), (2) the severe hypercholesterolemia group (those with LDL‐C ≥190 mg/dL at least once in the preceding 5 years), (3) the diabetes mellitus group, and (4) the elevated 10‐year risk of CVD group (>7.5% 10‐year risk or >5% for those with at least 1 risk enhancer). In this study, risk enhancers were defined as LDL‐C ≥160 mg/dL, estimated glomerular filtration rate <60 mL/min per 1.73 m2, triglycerides ≥175 mg/dL, high‐sensitivity C‐reactive protein ≥2 ng/L, or having a metabolic syndrome. LDL‐C goals were defined as ≤70 mg/dL for the secondary prevention group and for those in the elevated 10‐year risk group with >20% 10‐year risk; LDL‐C ≤100 mg/dL was used for the other groups, consistent with the AHA/ACC guideline on the management of blood cholesterol3 and the 2015 National Lipid Association Recommendations.21
Statistical Analysis
Continuous baseline variables were summarized as mean±SD for normally distributed variables and as median and interquartile range otherwise. Categorical variables were summarized as count and percentage. Comparisons of continuous variables between groups were assessed by t test for normally distributed variables and by Wilcoxon rank sum test otherwise. Categorical variables were compared by χ2 test.
The 10‐year risk of CVD at baseline was estimated using the pooled cohort equations.20 The 10‐year survival, S(t), where t=10, is 1−(10‐year risk of CVD). Assuming a constant hazard, the baseline hazard (hb) was estimated as hb=[−logeS(10)]/10. The 10‐year risk of CVD after LDL‐C lowering was estimated by considering the reported hazard ratio for LDL‐C lowering as 0.79 for each 39 mg/dL (1.0 mmol/L) lowered.1, 2 Therefore, the hazards after LDL‐C lowering (ha) were estimated as , where x is the difference in LDL‐C at baseline and LDL‐C level after lowering. The 10‐year risk of CVD after LDL‐C lowering was estimated from the hazard as . The projected CVD events after 10 years of follow‐up at baseline and after LDL‐C lowering were calculated from the means of the estimated 10‐year risks at baseline and after LDL‐C lowering, assuming exponential distribution of time to event with a constant hazard, S(t)=e (−h×t), where t=10. Comparison of 10‐year risk of CVD before and after LDL‐C lowering was assessed by t test of the natural log–transformed difference in 10‐year risk.
The difference between LDL‐C levels in those with and without lipid‐lowering therapy was evaluated by the Wilcoxon rank sum test. Comparisons among LDL‐C goal achievement, lipid‐lowering therapy, and lipid‐lowering therapy discontinuation between women and men were assessed in logistic regression models adjusted for age. All analyses were performed in R software.22
Results
This cross‐sectional study included 17 889 participants, of whom 7628 (43%) were in 1 of 4 patient‐management groups: the secondary prevention group (27.3%, n=2082), the severe hypercholesterolemia group (LDL‐C ≥190 mg/dL at least once in the preceding 5 years; 7.4%, n=567), the diabetes mellitus group (29.9%, n=2277), and the elevated 10‐year risk of CVD group (35.4%, n=2702). The remaining 10 261 participants were not in any patient‐management group and, as expected, had lower levels of CVD risk factors than those in patient management groups (Table).
Table 1.
Characteristics of Study Population According to Patient‐Management Group
| Not in Patient‐Management Group | Primary Prevention Groups | Secondary Prevention Group | P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Severe Hypercholesterolemiab | P Valuea | Diabetes Mellitus | P Valuea | Elevated 10‐y Risk of CVD | P Valuea | ||||
| n | 10 261 | 567 | NA | 2277 | NA | 2702 | NA | 2082 | NA |
| Achieve LDL‐C goal (n) | NA | 41 | NA | 1075 | NA | 563 | NA | 337 | NA |
| Age, y, mean (SD) | 50 (7) | 54 (7) | 9×10−26 | 54 (7) | <1×10−100 | 59 (7) | <1×10−100 | 57 (8) | <1×10−100 |
| Women, n (%) | 7553 (74) | 369 (65) | 1×10−5 | 1319 (58) | 6×10−50 | 882 (33) | <1×10−100 | 1140 (55) | 5×10−66 |
| HDL‐C, mg/dL, mean (SD) | 60 (18) | 54 (14) | 9×10−17 | 49 (14) | <1×10−100 | 50 (15) | <1×10−100 | 53 (17) | 4×10−65 |
| LDL‐C, mg/dL, mean (SD) | 110 (28) | 171 (43) | <1×10−100 | 101 (30) | 3×10−37 | 123 (26) | <1×10−100 | 103 (34) | 3×10−18 |
| TC, mg/dL, mean (SD) | 191 (33) | 253 (48) | 3×10−124 | 175 (36) | 1×10−77 | 198 (32) | 2×10−27 | 179 (40) | 3×10−35 |
| Triglycerides, mg/dL, median (IQR) | 93 (69–129) | 134 (99–184) | 3×10−63 | 126 (92–176) | <1×10−100 | 126 (91–178) | <1×10−100 | 111 (79–161) | 7×10−45 |
| CRP, mg/L, mean (SD) | 3.2 (5.3) | 3.5 (4.7) | 0.1 | 5.1 (8.2) | 3×10−25 | 3.5 (4.9) | 2×10−2 | 4.0 (7.7) | 6×10−6 |
| Fasting glucose, mg/dL, mean (SD) | 92 (9) | 104 (36) | 5×10−14 | 137 (51) | <1×10−100 | 97 (10) | <1×10−100 | 108 (36) | 7×10−78 |
| HbA1c, %, mean (SD) | 5.3 (0.3) | 5.7 (1.2) | 5×10−16 | 7.0 (1.5) | <1×10−100 | 5.4 (0.3) | 8×10−87 | 5.9 (1.2) | 2×10−99 |
| SBP, mm Hg, mean (SD) | 120 (14) | 126 (16) | 6×10−16 | 128 (16) | 8×10−96 | 134 (15) | <1×10−100 | 127 (16) | 3×10−69 |
| DBP, mm Hg, mean (SD) | 76 (10) | 77 (11) | 3×10−4 | 78 (10) | 2×10−29 | 81 (10) | <1×10−100 | 76 (10) | 2×10−4 |
| BMI, kg/m2, mean (SD) | 28 (6) | 29 (6) | 0.04 | 33 (8) | <1×10−100 | 30 (6) | 7×10−21 | 30 (7) | 1×10−28 |
| Hypertension, n (%) | 3241 (32) | 250 (44) | 8×10−10 | 1688 (74) | <1×10−100 | 1822 (67) | <1×10−100 | 1475 (71) | <1×10−100 |
| Smoking, n (%) | 816 (8) | 78 (14) | 2×10−6 | 250 (11) | 3×10−6 | 603 (22) | 4×10−100 | 278 (13) | 4x10−15 |
| Diabetes mellitus, n (%) | 0 (0) | 79 (14) | NA | 2277 (100) | NA | 0 (0) | NA | 593 (28) | NA |
| FH of MI, n (%) | 1007 (10) | 65 (11) | 0.2 | 324 (14) | 8×10−10 | 342 (13) | 2×10−5 | 369 (18) | 2x10−25 |
Between‐group differences in continuous variables were assessed by Student t test, except for triglycerides for which Wilcoxon rank sum test was used. Differences in categorical variables were assessed by the χ2 test. Continuous variables are presented as mean (SD), except for triglycerides, which are presented as median (IQR). Categorical variables are summarized by counts (percentage). BMI indicates body mass index; CRP, C‐reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; FH, family history; HbA1C, hemoglobin A1C; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; NA, not assessed; SBP, systolic blood pressure; and TC, total cholesterol.
P values are for comparison to the first column (not in patient‐management group).
LDL‐C ≥190 mg/dL at least once in the preceding 5 y.
Only 26% of the participants in patient‐management groups had LDL‐C levels at or below goal. Of particular note, only 16% of those in the secondary prevention group were at or below LDL‐C goal. A smaller fraction of women (11%) than men (22%) achieved LDL‐C goals in this secondary prevention group (P=5×10−9; Figure 2A). The highest level of goal achievement (47% in women, 48% in men) was observed for those in the diabetes mellitus group. In both the primary and the secondary prevention groups, many participants with LDL‐C levels above goal were young to middle‐aged (Figure 3). In those above goal, 36% of men were younger than 55 years, and 60% of women were younger than 60.
Figure 2. Fraction of patients achieving low‐density lipoprotein cholesterol (LDL‐C) goal, treated with lipid lowering therapy, and discontinuing lipid‐lowering therapy.
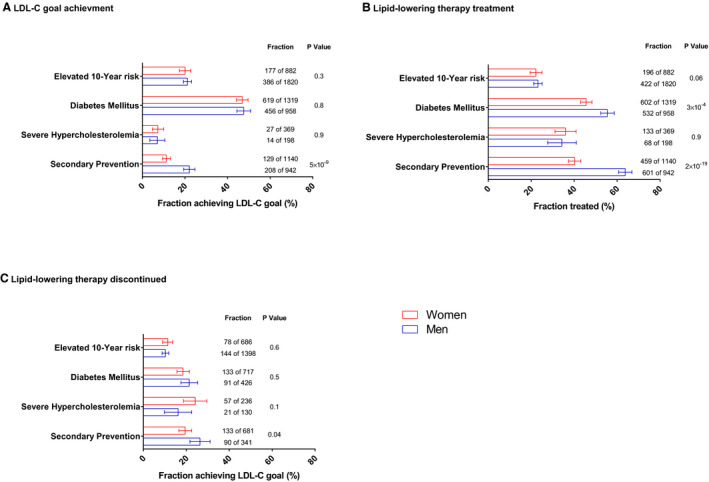
The percentages of patients in each patient‐management group who have achieved LDL‐C goal (A), who are receiving lipid‐lowering therapy (B), and who have discontinued lipid‐lowering therapy (of those not receiving therapy) (C) are presented for women (red) and men (blue). Error bars are 95% CIs. The number of patients in each fraction as well as the total number of patients is indicated. P values are age‐adjusted for the difference between fractions in women and men.
Figure 3. Age distribution in primary and secondary prevention groups.
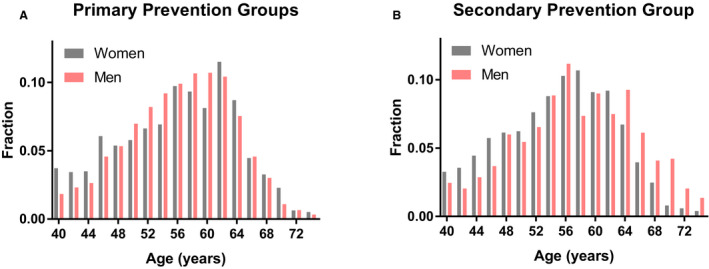
Histograms of age for patients with low‐density lipoprotein cholesterol (LDL‐C) above goal in the primary prevention groups (A) and the secondary prevention group (B). Histograms are plotted for women (gray) and men (pink).
Among women, the highest lipid‐lowering therapy use was 46% in the diabetes mellitus group (Figure 2B). Among men, the highest lipid‐lowering therapy use was observed in the secondary prevention group (64%). Men received lipid‐lowering therapy more commonly than did women in the diabetes mellitus group and in the secondary prevention group (P≤3×10−4). The lipid‐lowering therapy discontinuation rate ranged from a high of 26% among men in the secondary prevention group to 10% in men with elevated 10‐year risk of CVD (Figure 2C). Those with self‐reported family history of myocardial infarction were more likely to have evidence of lipid‐lowering therapy (50.5%; 95% CI, 47.5–53.4%) than did those without family history (37.7%; 95% CI, 36.5–38.8%). In all patient‐management groups, lipid‐lowering therapy was more common in those with LDL‐C level at or below goal than in those above goal (Figure 4). High‐intensity statin therapy, which is recommended for all secondary‐prevention patients, was evident in only 16% of those in the secondary‐prevention patient‐management group, and its use was even lower (ranging from 1% to 11%) in other patient‐management groups.
Figure 4. Lipid‐lowering therapy type by patient‐management group.
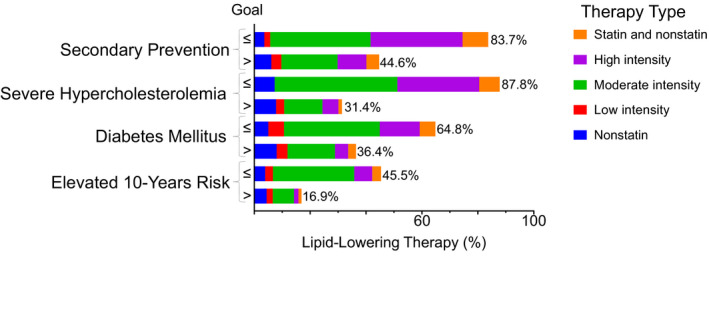
Fraction of patients in each patient‐management group receiving high‐, moderate‐, or low‐intensity statin therapy; other lipid‐lowering therapy; or both statin and nonstatin therapy, according to low‐density lipoprotein cholesterol (LDL‐C) goal achievement.
In both the primary and secondary prevention groups, those using lipid‐lowering therapy had lower LDL‐C levels than did those who did not use therapy (P<0.0001; Figure 5). For the primary prevention groups, the median LDL‐C level was 97 mg/dL (interquartile range: 79–120 mg/dL) among those who used lipid‐lowering therapy (84% with a statin prescription) and 125 mg/dL among those who did not. Similarly, in the secondary prevention group, median LDL‐C was 87 mg/dL (interquartile range: 69–109 mg/dL) among those who used lipid‐lowering‐therapy and 114 mg/dL among those who did not.
Figure 5. Low‐density lipoprotein cholesterol (LDL‐C) distribution.
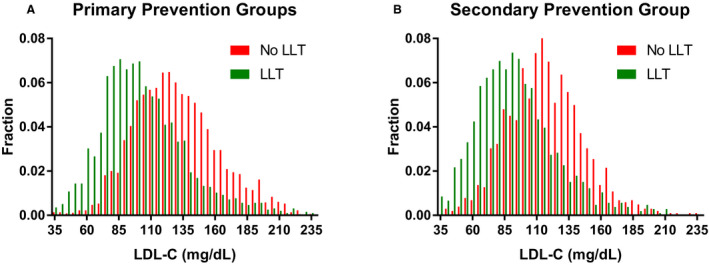
Histograms of LDL‐C levels among patients in the primary prevention groups (A) and among patients in the secondary prevention group (B). Histograms are plotted for patients not receiving lipid‐lowering therapy (LLT; red) and patients receiving LLT (green).
Lowering LDL‐C levels to goal in the 3867 participants in the primary prevention groups with LDL‐C levels above goal could almost double the fraction of those with 10‐year risk of CVD <5% from 16.2% to 30.2% (Figure 6) and could increase, by 36%, the fraction of those with 10‐year risk <7.5% (from 39.5% to 53.7%). Overall, lowering LDL‐C to goal would reduce the mean 10‐year CVD risk to 8.6% from 10.6% (P<0.0001). We estimate that reducing LDL‐C to goal levels in the primary prevention groups would prevent about 20% of the CVD events projected to occur over the following 10 years (77 of the 408 projected CVD events).
Figure 6. Ten‐year risk of cardiovascular disease (CVD) in primary prevention groups.
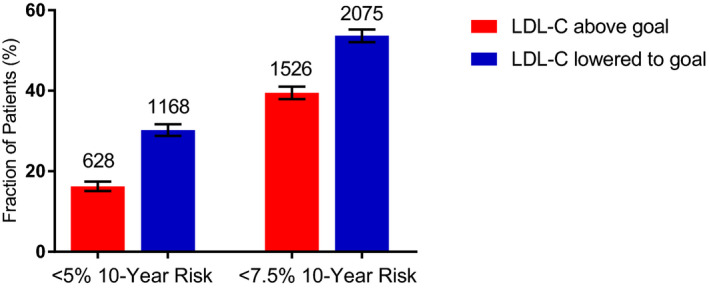
Fraction of patients with low (<5%) or moderately low (<7.5%) 10‐year risk of CVD among those in the primary prevention groups who were above low‐density lipoprotein cholesterol (LDL‐C) goal (red) and aspirational fraction of patients in these groups (blue). The aspirational fractions were calculated by assuming LDL‐C was lowered to goal. Error bars are 95% CIs. Number of patients in each fraction is indicated above bars.
Discussion
In a working‐aged population that was covered by a group health plan and participated in an annual health screen program, we found that LDL‐C levels were above goal in about 74% of those in patient‐management groups that could benefit from lipid‐lowering therapy. Failure to reach goal was particularly common in the secondary prevention group (84%) and in the severe hypercholesterolemia group (93%; those with LDL‐C ≥190 mg/dL at least once in any of the preceding 5 years). These gaps in care are surprising when we consider that 93% of the study population also participated in a health screening program in the prior year and thus were aware of their LDL‐C levels and overall cardiovascular health status for at least 2 consecutive years and should have had an opportunity to address their elevated LDL‐C levels.
More than 40% of participants were in patient‐management groups—groups that would benefit from LDL‐C lowering. The fraction of those in patient‐management groups was similar to the 39% found in the offspring and third‐generation cohorts of the Framingham Heart Study23 but somewhat lower than the 49% found among US adults between the ages of 40 and 75 years in the 2005–2010 National Health and Nutrition Examination Survey (NHANES).24 The higher fraction found in NHANES may reflect the roughly 10‐year difference in the timing of the NHANES analysis and the current study, or it may be that the population of actively working employees with full medical benefits in the current study are simply healthier than the general population represented in NHANES.
About half of those in the secondary prevention and diabetes mellitus groups did not receive guideline‐recommended lipid‐lowering therapy. The fraction using lipid‐lowering therapy was even lower in the severe hypercholesterolemia group and the elevated 10‐year risk of CVD group—only 35% and 23%, respectively, of these groups were on lipid‐lowering therapy (Figure 3). Our findings differ from an analysis of a cardiology practices registry (PINNACLE; National Cardiovascular Data Registry Practice Innovation and Clinical Excellence)25 that found most (about 68%) eligible patients receive lipid‐lowering therapy; perhaps this difference is because all patients in the PINNACLE study were drawn from cardiology clinics and might have been more likely to receive cardiovascular care.
In the secondary prevention group, we found that the fraction receiving lipid‐lowering therapy was substantially smaller for women than for men; perhaps consequently, a smaller fraction of women achieved the LDL‐C goal for secondary prevention patients. However, lipid‐lowering therapy discontinuation was less common among women than among men in the secondary prevention group; therefore, this discontinuation is unlikely to explain the lower therapy and goal achievement in women. This observation is consistent with an analysis of the 2011–2012 NHANES data that found a smaller fraction of women than men achieved LDL‐C goals.18 The discontinuation rates we observed (10–26%) are consistent with the published discontinuation rate among 75‐year‐old primary prevention patients in France (14%)26 but lower than that reported in Japan.27, 28 Statin discontinuation might be detrimental beyond its effect of LDL‐C,29 and discontinuation has been reported to be associated with 33% increased risk of cardiovascular events in 75‐year old primary prevention patients.26 Similarly, in patients of the Veterans Administration System with CVD, low adherence to statin therapy was associated with mortality.8 Therefore, reduction or elimination of lipid‐lowering therapy discontinuation in this population could improve health outcomes.
Our analyses also highlighted a potential underuse of nonstatin lipid‐lowering therapy in those above goal, particularly in those in the secondary prevention group and in the severe hypercholesterolemia group (LDL‐C ≥190 mg/dL at least once in the preceding 5 years). In these groups, >60% of the participants receiving high‐intensity statin had LDL‐C levels above goal. Although guidelines3 suggest considering the addition of ezetimibe to statin in these patients, only 3.8% had prescription for both statin and nonstatin therapy in those above goal in these 2 groups.
We estimated that reducing LDL‐C to goal levels in the primary prevention groups would prevent about 20% of CVD events over the next 10 years in these groups. And given that many in these groups were young to middle‐aged, lowering risk in this group should add a substantial number of quality‐adjusted life‐years to this population.
Although the study population was covered by an employer‐sponsored group health plan and thus was less likely to have financial reasons for not receiving therapy than would the general population, we found gaps in LDL‐C goal attainment and low rates of appropriate lipid‐lowering therapy. This might be explained by the multiple steps that are required to effectively treat dyslipidemia: (1) the patient has to visit a healthcare provider, (2) the healthcare provider has to recognize the need for lipid‐lowering therapy and provide a prescription, (3) the patient has to fill the prescription and begin to use the medication as prescribed, (4) the healthcare provider has to reevaluate the patient after initial prescription and adjust the prescription if needed, and (5) the patient has to continue to use the prescribed medication. Failure to continue to use statin therapy has been ascribed to side effects,30, 31 costs,32, 33 perceived lack effectiveness, and negative news stories about effectiveness.33, 34
Nevertheless, statin discontinuation is only one of the potential causes of gaps in care—a breakdown at any of the multiple steps could create a gap in dyslipidemia care. More research that might identify groups of patients who are less likely to achieve LDL‐C goals might be considered. Wong et al,18 for example, reported low LDL‐C goal achievement in Hispanics and in those with history of stroke. Addressing gaps in dyslipidemia care will require programs that appropriately target steps that have the greatest impact on generating these gaps. To design effective programs, further investigation is needed to understand the causes and relative impact of failure at each step.
This study has several limitations related to potential incompleteness of medical claims data. For example, we likely underestimated the number of secondary prevention patients and the fraction who have discontinued their lipid‐lowering therapy because, although we had access to medical claims from at least the 12 months before study initiation (and from up to the preceding 5 years for some), a longer record of claims for all participants would have likely identified more secondary prevention patients and more evidence of lipid‐lowering therapy discontinuation. Similarly, the record of medication prescriptions in the 6 months before annual health screening participation could be incomplete if, for example, a patient obtained prescription medication outside the employer‐sponsored program (eg, using a spouse insurance plan). We also did not have LDL‐C test results if tests were not performed as part of the annual health screening program. Therefore, we might have underestimated the number of participants who had had severe hypercholesterolemia. Another limitation relates to the use of LDL‐C goals in this study. Although the 2018 AHA/ACC guideline on the management of blood cholesterol set LDL‐C goals for the secondary prevention and severe hypercholesterolemia groups, LDL‐C reduction goals were set for other patient‐management groups. Because this study is based on a single LDL‐C assessment, we were unable to assess percentage of LDL‐C reduction, and instead used clinically reasonable goals to assess goal attainment for all patient‐management groups.
Conclusions
We have found substantial gaps in LDL‐C treatment and goal attainment in working‐aged employees and spouses with employer‐sponsored medical plan and who were mostly aware of their LDL‐C levels. Investigation into the causes of these gaps would help inform the design of gap‐closure programs.
Sources of Funding
Knowles is supported by the Stanford Diabetes Research Center (P30DK116074), the Doris Duke Foundation, and the NIH through grant U41HG009649.
Disclosures
Shiffman, Louie, Devlin, and McPhaul are employees of Quest Diagnostics. Knowles has no disclosures to report.
Supporting information
Tables S1–S3
(J Am Heart Assoc. 2020;9:e015807 DOI: 10.1161/JAHA.119.015807.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Cholesterol Treatment Trialists’ (CTT) Collaboration . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of LDL‐lowering therapy among men and women: meta‐analysis of individual data from 174 000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 3. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al.; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 5. O'hara B, Caswell K. Health status, health insurance, and medical services utilization. Current Population Reports. 2010. Available at: www.census.gov/prod/2012pubs/p70-133.pdf. Accessed January 17, 2019. [Google Scholar]
- 6. Kibler JL, Ma M, Hrzich J, Roas RA. Public knowledge of cardiovascular risk numbers: contextual factors affecting knowledge and health behavior, and the impact of public health campaigns In: Watson RR, Zibadi S, eds. Lifestyle in Heart Health and Disease. Academic Press: Elsevier; 2018:11–20. [Google Scholar]
- 7. Goldberg KC, Melnyk SD, Simel DL. Overcoming inertia: improvement in achieving target low‐density lipoprotein cholesterol. Am J Manag Care. 2007;13:530–535. [PubMed] [Google Scholar]
- 8. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okerson T, Patel J, DiMario S, Burton T, Seare J, Harrison DJ. Effect of 2013 ACC/AHA blood cholesterol guidelines on statin treatment patterns and low‐density lipoprotein cholesterol in atherosclerotic cardiovascular disease patients. J Am Heart Assoc. 2017;6:e004909 DOI: 10.1161/JAHA.116.004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenson RS, Kent ST, Brown TM, Farkouh ME, Levitan EB, Yun H, Sharma P, Safford MM, Kilgore M, Muntner P, et al. Underutilization of high‐intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol. 2015;65:270–277. [DOI] [PubMed] [Google Scholar]
- 11. Johansen ME, Green LA, Sen A, Kircher S, Richardson CR. Cardiovascular risk and statin use in the United States. Ann Fam Med. 2014;12:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valentino M, Al Danaf J, Panakos A, Ragupathi L, Duffy D, Whellan D. Impact of the 2013 American College of Cardiology/American Heart Association cholesterol guidelines on the prescription of high‐intensity statins in patients hospitalized for acute coronary syndrome or stroke. Am Heart J. 2016;181:130–136. [DOI] [PubMed] [Google Scholar]
- 13. Arnold SV, Spertus JA, Tang F, Krumholz HM, Borden WB, Farmer SA, Ting HH, Chan PS. Statin use in outpatients with obstructive coronary artery disease. Circulation. 2011;124:2405–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ovbiagele B, Schwamm LH, Smith EE, Hernandez AF, Olson DM, Pan W, Fonarow GC, Saver JL. Recent nationwide trends in discharge statin treatment of hospitalized patients with stroke. Stroke. 2010;41:1508–1513. [DOI] [PubMed] [Google Scholar]
- 15. Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 17. Kelly KE, Jiroutek MR, Lewis K, Zagar B. Assessing changes in statin prescribing patterns surrounding the 2013 American College of Cardiology/American Heart Association lipid guidelines. Clin Ther. 2019;41:314–321. [DOI] [PubMed] [Google Scholar]
- 18. Wong ND, Young D, Zhao Y, Nguyen H, Caballes J, Khan I, Sanchez RJ. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low‐density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011–2012. J Clin Lipidol. 2016;10:1109–1118. [DOI] [PubMed] [Google Scholar]
- 19. Claxton G, Rae M, Long M, Panchal N, Damico A. Employer Health Benefits: 2016 Annual Survey. Menlo Park, CA: Henry J. Kaiser Family Foundation; 2016. [Google Scholar]
- 20. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al.; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 21. Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA. National lipid association recommendations for patient‐centered management of dyslipidemia: part 1—full report. J Clin Lipidol. 2015;9:129–169. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for statistical computing: Vienna; 2012. [Google Scholar]
- 23. Pursnani A, Massaro JM, D'Agostino RB Sr, O'Donnell CJ, Hoffmann U. Guideline‐based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pencina MJ, Navar‐Boggan AM, D'Agostino RB Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population‐based sample. N Engl J Med. 2014;370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 25. Maddox TM, Borden WB, Tang F, Virani SS, Oetgen WJ, Mullen JB, Chan PS, Casale PN, Douglas PS, Masoudi FA, et al. Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice: insights from the NCDR PINNACLE Registry. J Am Coll Cardiol. 2014;64:2183–2192. [DOI] [PubMed] [Google Scholar]
- 26. Giral P, Neumann A, Weill A, Coste J. Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: a nationwide population‐based cohort study in France. Eur Heart J. 2019;40:3516–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagar SP, Rane PP, Fox KM, Meyers J, Davis K, Beaubrun A, Inomata H, Qian Y, Kajinami K. Treatment patterns, statin intolerance, and subsequent cardiovascular events among Japanese patients with high cardiovascular risk initiating statin therapy. Circ J. 2018;82:1008–1016. [DOI] [PubMed] [Google Scholar]
- 28. Wake M, Oh A, Onishi Y, Guelfucci F, Shimasaki Y, Teramoto T. Adherence and persistence to hyperlipidemia medications in patients with atherosclerotic cardiovascular disease and those with diabetes mellitus based on administrative claims data in Japan. Atherosclerosis. 2019;282:19–28. [DOI] [PubMed] [Google Scholar]
- 29. Jasińska‐Stroschein M, Owczarek J, Wejman I, Orszulak‐Michalak D. Novel mechanistic and clinical implications concerning the safety of statin discontinuation. Pharmacol Rep. 2011;63:867–879. [DOI] [PubMed] [Google Scholar]
- 30. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet‐based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6:208–215. [DOI] [PubMed] [Google Scholar]
- 31. Rosenson RS, Baker S, Banach M, Borow KM, Braun LT, Bruckert E, Brunham LR, Catapano AL, Elam MB, Mancini GBJ, et al. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol. 2017;70:1290–1301. [DOI] [PubMed] [Google Scholar]
- 32. Lewey J, Gagne JJ, Franklin J, Lauffenburger JC, Brill G, Choudhry NK. Impact of high deductible health plans on cardiovascular medication adherence and health disparities. Circ Cardiovasc Qual Outcomes. 2018;11:e004632. [DOI] [PubMed] [Google Scholar]
- 33. Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7:472–483. [DOI] [PubMed] [Google Scholar]
- 34. Nielsen SF, Nordestgaard BG. Negative statin‐related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J. 2016;37:908–916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


