Abstract
BACKGROUND
Outcomes from extracorporeal cardiopulmonary resuscitation (ECPR) are felt to be influenced by selective use, but the characteristics of those receiving ECPR are undefined. We demonstrate the relationship between individual patient and hospital characteristics and the probability of ECPR use.
METHODS AND RESULTS
We performed an observational analysis of adult inpatient cardiac arrests in the United States from 2000 to 2018 reported to the American Heart Association's Get With The Guidelines—Resuscitation registry restricted to hospitals that provided ECPR. We calculated case mix adjusted relative risk (RR) of receiving ECPR for individual characteristics. From 2000 to 2018, 129 736 patients had a cardiac arrest (128 654 conventional cardiopulmonary resuscitation and 1082 ECPR) in 224 hospitals that offered ECPR. ECPR use was associated with younger age (RR, 1.5 for <40 vs. 40–59 years; 95% CI, 1.2–1.8), no pre‐existing comorbidities (RR, 1.4; 95% CI, 1.1–1.8) or cardiac‐specific comorbidities (congestive heart failure [RR, 1.3; 95% CI, 1.2–1.5], prior myocardial infarction [RR, 1.4; 95% CI, 1.2–1.6], or current myocardial infarction [RR, 1.5; 95% CI, 1.3–1.8]), and in locations of procedural areas at the times of cardiac arrest (RR, 12.0; 95% CI, 9.5–15.1). ECPR decreased after hours (3–11 pm [RR, 0.8; 95% CI, 0.7–1.0] and 11 pm–7 am [RR, 0.6; 95% CI, 0.5–0.7]) and on weekends (RR, 0.7; 95% CI, 0.6–0.9).
CONCLUSIONS
Less than 1% of in‐hospital cardiac arrest patients are treated with ECPR. ECPR use is influenced by patient age, comorbidities, and hospital system factors. Randomized controlled trials are needed to better define the patients in whom ECPR may provide a benefit.
Keywords: cardiopulmonary resuscitation, extracorporeal cardiopulmonary resuscitation, extracorporeal life support, extracorporeal membrane oxygenation, in‐hospital cardiac arrest, resuscitation
Nonstandard Abbreviations and Acronyms
- ECMO
extracorporeal membrane oxygenation
- CCPR
conventional cardiopulmonary resuscitation
- ECPR
extracorporeal cardiopulmonary resuscitation
- GWTG‐R
Get With The Guidelines—Resuscitation
- IHCA
in‐hospital cardiac arrest
- IQR
interquartile range
- OR
odds ratio
- RR
relative risk
Clinical Perspective
What Is New?
Extracorporeal cardiopulmonary resuscitation is used in <1% of all US in‐hospital cardiac arrests.
After case mix adjustment, the decision to use extracorporeal cardiopulmonary resuscitation remains strongly influenced by patient age, sex, race, comorbidities, type, and arrest location.
Extracorporeal cardiopulmonary resuscitation is used significantly more often in a generally younger, healthier, male cohort undergoing cardiac interventions and during the daytime.
What Are the Clinical Implications?
Conclusions about extracorporeal cardiopulmonary resuscitation benefits and harms in observational studies are strongly influenced by this observed selective use.
Survival after extracorporeal cardiopulmonary resuscitation (ECPR) ranges widely from <15% to >50%, although most studies report ≈30%.1, 2, 3, 4, 5 Some individuals have claimed that this high survival rate of ECPR patients compared with 10% to 20% for conventional cardiopulmonary resuscitation (CCPR)6, 7 has eliminated clinical equipoise.8 However, survival rate variance between CCPR and ECPR likely reflects confounding by indication with those thought to have a high likelihood of survival preferentially receiving ECPR. Propensity‐matched cases of ECPR to CCPR have shown discordant results among patients with in‐hospital cardiac arrest (IHCA).4, 9 In 2 propensity‐matched studies, investigators were unable to match 50% and 25% of the ECPR patients to CCPR patients, suggesting that patients receiving ECPR differ substantively from those receiving CCPR. If significant differences among patients being offered ECPR versus CCPR exist, this limits the generalizability of observational descriptions of ECPR use and survival and suggests barriers to clinical trial enrollment and randomization equipoise.
ECPR remains an uncommon therapy, even in high‐volume centers.10, 11 Most previous ECPR studies of adults have been limited by sample sizes of <100 to 300 patients,2, 3, 4, 5, 9, 10, 11, 12, 13, 14 which limits comparisons across patients and hospitals. The large population studies of ECPR come from Asia and may not be reflective of US practice.3, 4, 11, 15 To define the characteristics that bias toward the use of ECPR, we used a large national sample spanning nearly 20 years of US IHCA patients treated at ECPR‐available hospitals. The American Heart Association GWTG‐R (Get With The Guidelines—Resuscitation) registry is a nationally representative, prospective, multicenter, hospital‐based registry containing granular cardiac arrest details of patients with IHCA.16 Using this data set, we sought to define hospital‐level and patient‐level characteristics that increase the probability of treatment with ECPR, rather than CCPR alone, for cardiac arrest. By defining the features that bias toward ECPR use, we aim to better understand which patients physicians feel may benefit from ECPR and inform future clinical trial design.
METHODS
Our analysis is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology Guidelines.17
Data Sharing
To facilitate research reproducibility, replicability, accuracy, and transparency, our analytic code is available in the Open Science Foundation repository (DOI: 10.17605/osf.io/u9pae; https://osf.io/U9PAE). The data that support the findings of this study are available from the American Heart Association GWTG‐R investigators, which were used under license for the current study and can be requested from the American Heart Association. The code was deidentified in accordance with section 164.514 of the Health Insurance Portability and Accountability Act.
Data Source
Data were obtained from the American Heart Association GWTG‐R registry.16 Patients are identified as having an IHCA if they have lack of pulse, apnea, and unresponsiveness, without do‐not‐resuscitate orders, and subsequently receive chest compressions/cardiopulmonary resuscitation or defibrillation. Complete data capture is ensured through multiple case finding approaches, including a review of hospital paging system logs, centralized collection of cardiac arrest flowsheets, routine checks of code‐carts, and a review of pharmacy drug‐tracing records and hospital billing charges for medications.18, 19, 20 The registry uses standard Utstein‐style variable definitions.18, 21 Data are voluntarily submitted and include baseline, comorbid, prearrest, intra‐arrest, and outcome characteristics. The processes ensuring case ascertainment, data quality, and accuracy have previously been described.22 Hospital data are available within the data set, come from the American Hospital Association Annual Survey, and were analyzed consistent with previous studies.8, 23, 24, 25 IQVIA is the data collection coordination center for the American Heart Association/American Stroke Association Get With The Guidelines programs.
Study Population
We identified cardiac arrest events from 2000 to 2018. We excluded patients younger than 18 years of age, those with out‐of‐hospital cardiac arrest preceding admission, those for whom the arrest occurred >4 hours before the date/time of admission, those with missing date/time values, visitors, and those whose hospital identification could not be matched to a hospital within the data set. To control for availability of ECPR, we excluded patients from hospitals with no record of ECPR use in the registry. Among hospitals that reported ECPR use for IHCA patients, we only included patients who were enrolled after the date of the first ECPR patient in the registry (Figure S1). We excluded all nonindex cardiac arrest events for each patient and all patients who were coded as brain dead on the admission Cerebral Performance Category variable. Finally, we excluded hospitals that had submitted <6 months of data or fewer than 5 cardiac arrest events (Data S1).
Study Variables and Outcomes
Our primary outcome is the receipt of ECPR during IHCA, defined within the GWTG‐R as receipt of ECPR/cardiopulmonary bypass as an adjunctive therapy during resuscitation. Patient‐level data available from the GWTG‐R included demographics (age, sex, race), initial rhythm (ventricular fibrillation, pulseless ventricular tachycardia, pulseless electrical activity, and asystole), location of cardiac arrest (intensive care unit, nonmonitored inpatient, ambulatory, outpatient, rehabilitiation, cardiac/coronary, catheterization laboratory, operating room), time of day (work hours, 7:00 am–2:59 pm vs. after hours, 3:00 pm–10:59 pm, 11:00 pm–6:59 am) and day of week (weekday vs. weekend) of cardiac arrest, and use of a hospital‐wide emergency response (ie, “Code Blue”). Information was obtained on comorbid conditions, including myocardial infarction; congestive heart failure; diabetes mellitus; hepatic, renal, or respiratory insufficiency; neurological status prearrest (as determined by admission Cerebral Performance Category scores)26; baseline evidence of cognitive, motor, or functional deficits; pneumonia; arrhythmia; acute stroke; hypotension; trauma; sepsis; metabolic or electrolyte abnormality; cancer; and therapeutic interventions in place at the time of cardiac arrest (mechanical ventilation, arterial catheters, endotracheal tubes). Moreover, information was obtained on intra‐arrest characteristics, including return of spontaneous circulation, duration of arrest, and arrest treatments, including defibrillation, medication administration, and use of adjunctive therapies including the use of cardiopulmonary bypass/ECPR or induced hypothermia.
Statistical Analysis
Patient demographic, prearrest, and intra‐arrest characteristics and hospital characteristics were summarized descriptively stratified by receipt of ECPR versus CCPR. Continuous variables were summarized as mean (SD) or median (interquartile range [IQR]), and categorical variables were summarized as frequency and percentage. Patient characteristics were compared with ECPR versus CCPR using mixed effects regression models to account for clustering within hospitals.
Our primary analytic goal was to measure the strength of association between individual patient and hospital characteristics and ECPR use. To account for the national sample and differences in patient complexity across hospitals, we adjusted our analyses for patient case‐mix and hospital characteristics associated with differing levels of hospital care.27 Each analysis presents an unadjusted estimate of the associations with ECPR use and 2 adjusted estimates: one adjusted for patient variables and the second adjusted for patient and hospital variables.
Univariable association with receiving ECPR was assessed for each demographic, prearrest, intra‐arrest, and hospital characteristic variable using a mixed effects log‐binomial regression model with a log link and included a random effect for hospital. This modeling framework was used rather than simple tests because of the potential correlation of patient characteristics within hospitals. For the adjusted analysis, we selected a subset of patient and hospital characteristics based on previous associations with survival after cardiac arrest,24, 28, 29, 30, 31 minimal missingness, and an absence of collinearity. Selected patient characteristics included age, initial pulseless rhythm, sex, race, illness category (medical vs. surgical, cardiac vs. noncardiac), event location,23, 32, 33 and ethnicity. Selected hospital‐level characteristics included number of cardiac intensive care unit beds, region of the country, and teaching status.23, 32 Era, categorized in 5‐year increments, was included in the models as our data set spanned nearly 20 years. All variables, except ethnicity, achieved statistical significance in univariable analyses, had minimal missingness (≤5.3%), and had no collinearity among them (variance inflation factors were all <2.5). All univariable associations with receiving ECPR were assessed for each candidate patient and hospital variable in our data set. We repeated these comparisons adjusting for both the subset of patient and hospital characteristics described previously (primary results) and adjusting for the patient characteristics alone (descriptive results) using the same modeling approach. The exponentiated model coefficients yielded relative risks (RRs) reported with their 95% CIs and P values. The log‐binomial mixed effects model was chosen over a logistic mixed effects model for analyses reporting model coefficients because RRs are often more intuitive than odds ratios (ORs).34 However, because ECPR cases represent about 0.8% of our data set, ORs provide a close approximation to RRs. For analyses where only P values were reported or where the log‐binomial model could not converge, we used mixed effects logistic regression reporting ORs. These exceptions have been noted via footnotes in the tables.
Hospital characteristics were compared between ECPR‐available and non‐ECPR‐available hospitals using chi‐squared tests. Patient characteristics were compared between ECPR‐available and non‐ECPR‐available hospitals using the log‐binomial mixed effects regression modeling framework described previously.
All statistical analyses were conducted in R version 3.4.35 Statistical significance was assessed at the 0.05 level, and all tests were 2‐tailed. This study was approved by the institutional review board under No. 00091962 on September 8, 2016, with a waiver of informed consent.
RESULTS
Patient Characteristics
The final cohort included 129 736 patients (128 654 CCPR and 1082 ECPR) from 219 hospitals that offered ECPR (Table S1). Unadjusted patient characteristics are presented in Table 1. Patients were predominately 60 years of age or older (66%), male (58%), white (68%), and cardiac surgical or cardiac medical patients (40%). Arrest characteristics included 39% located in the intensive care unit, 86% witnessed, and 93% were found without a pulse, of whom 18% presented with a shockable rhythm. Patients received 2 defibrillations (IQR, 1–4) and 3 boluses (IQR, 1–5) of epinephrine after becoming pulseless and underwent a median of 15 minutes (IQR, 6–26) of resuscitation, and 70% achieved return of spontaneous circulation at some point.
Table 1.
Descriptive Summary of Admission, Prearrest, and Arrest Characteristics
| Variable | CCPR (N=127 537) | ECPR (N=1082) | All (N=128 619) | P Valuea |
|---|---|---|---|---|
| Age, y, n(%) | <0.001 | |||
| <40 | 9802 (7.7) | 166 (15.3) | 9968 (7.8) | ··· |
| 40–59 | 33 189 (26) | 355 (32.8) | 33 544 (26.1) | ··· |
| ≥60 | 84 546 (66.3) | 561 (51.8) | 85 107 (66.2) | ··· |
| Male sex, n (%) | 73 928 (58) | 666 (61.6) | 74 594 (58) | 0.035 |
| Race, n (%) | <0.001 | |||
| White | 86 822 (68.2) | 799 (74.1) | 87 621 (68.3) | ··· |
| Black | 29 351 (23.1) | 168 (15.6) | 29 519 (23) | ··· |
| Other | 11 050 (8.7) | 112 (10.4) | 11 162 (8.7) | ··· |
| Hispanic ethnicity, n (%) | 6088 (4.8) | 55 (5.1) | 6143 (4.8) | 0.98 |
| Weight, median (IQR), kg | 78.9 (65–95.5) | 80 (67–95.7) | 78.9 (65–95.5) | 0.34 |
| Pre‐existing condition, n (%) | ||||
| None | 6315 (5) | 86 (8) | 6401 (5) | 0.001 |
| Preceding hypoperfusion | 37 205 (29.3) | 470 (43.6) | 37 675 (29.4) | <0.001 |
| CVA or neurologic disorder | 27 086 (21.3) | 100 (9.3) | 27 186 (21.2) | <0.001 |
| CHF | 37 902 (29.8) | 366 (33.9) | 38 268 (29.8) | <0.001 |
| Diabetes mellitus | 41 897 (32.9) | 246 (22.8) | 42 143 (32.9) | <0.001 |
| Hepatic insufficiency | 10 531 (8.3) | 53 (4.9) | 10 584 (8.3) | <0.001 |
| Major trauma | 6674 (5.2) | 40 (3.7) | 6714 (5.25) | 0.12 |
| Cancer | 15 395 (12.1) | 42 (3.9) | 15 437 (12) | <0.001 |
| History of MI | 19 823 (15.6) | 229 (21.2) | 20 052 (15.6) | <0.001 |
| MI this hospitalization | 19 981 (15.7) | 289 (26.8) | 20 270 (15.8) | <0.001 |
| Renal insufficiency | 45 494 (35.8) | 244 (22.6) | 45 738 (35.7) | <0.001 |
| Respiratory insufficiency | 59 229 (46.6) | 443 (41.1) | 59 672 (46.5) | 0.002 |
| Sepsis | 22 446 (17.6) | 87 (8.1) | 22 533 (17.6) | <0.001 |
| Admission CPC, n (%)b | <0.001 | |||
| CPC 1: good cerebral performance | 59 580 (60.8) | 667 (78.1) | 60 247 (60.9) | ··· |
| CPC 2: moderate cerebral disability | 20 289 (20.7) | 88 (10.3) | 20 377 (20.6) | ··· |
| CPC 3: severe cerebral disability | 10 859 (11.1) | 39 (4.6) | 10 898 (11) | ··· |
| CPC 4: coma or vegetative state | 7306 (7.5) | 60 (7) | 7366 (7.4) | ··· |
| Duration between admission and arrest, n (%) | 0.005 | |||
| <24 h | 120 495 (94.5) | 1044 (96.5) | 121 539 (94.5) | ··· |
| 24 to <48 h | 5268 (4.1) | 29 (2.7) | 5297 (4.1) | ··· |
| 48 h to <1 wk | 1583 (1.2) | 6 (0.6) | 1589 (1.2) | ··· |
| ≥1 wk | 191 (0.1) | 3 (0.3) | 194 (0.2) | ··· |
| Devices, n (%) | ||||
| Mechanical ventilation | 37 626 (29.5) | 556 (51.4) | 38 182 (29.7) | <0.001 |
| Invasive airway | 36 726 (28.8) | 531 (49.1) | 37 257 (29) | <0.001 |
| Arterial line | 17 329 (13.6) | 478 (44.2) | 17 807 (13.9) | <0.001 |
| Time of day of arrest, n (%) | <0.001 | |||
| 7 am to 2:59 pm | 44 729 (35.5) | 500 (48.3) | 45 229 (35.6) | ··· |
| 3 pm to 10:59 pm | 42 165 (33.5) | 358 (34.6) | 42 523 (33.5) | ··· |
| 11 pm to 6:59 am | 39 052 (31) | 177 (17.1) | 39 229 (30.9) | ··· |
| Day of week, n (%) | ||||
| Weekday | 94 290 (73.9) | 896 (82.8) | 95 186 (74) | <0.001 |
| Weekend | 33 247 (26.1) | 186 (17.2) | 33 433 (26) | ··· |
| Arrest location, n (%) | <0.001 | |||
| General inpatientc | 41 134 (32.3) | 100 (9.2) | 41 234 (32.1) | ··· |
| Ambulatory/outpatientd | 2150 (1.7) | 16 (1.5) | 2166 (1.7) | ··· |
| Cardiac/coronary unit | 9933 (7.8) | 103 (9.5) | 10 036 (7.8) | ··· |
| ICU | 50 229 (39.4) | 402 (37.2) | 50 631 (39.4) | ··· |
| Operating room/procedural/cath lab | 11 160 (8.8) | 421 (38.9) | 11 581 (9) | ··· |
| Emergency department | 12 817 (10.1) | 40 (3.7) | 12 857 (10) | ··· |
| Illness category, n (%) | ||||
| Medical—noncardiac | 61 058 (47.9) | 134 (12.4) | 61 192 (47.6) | <0.001 |
| Medical—cardiac | 40 578 (31.9) | 334 (30.9) | 40 912 (31.8) | ··· |
| Surgical—cardiac | 9731 (7.6) | 528 (48.8) | 10 259 (8) | ··· |
| Surgical—noncardiac | 16 032 (12.6) | 86 (7.9) | 16 118 (12.5) | ··· |
| Witnessed, n (%) | 109 519 (85.9) | 1049 (97) | 110 568 (86) | <0.001 |
| Hospital resuscitation activated, n (%) | 87 174 (68.4) | 483 (44.6) | 87 657 (68.2) | <0.001 |
| Condition of first assessment, n (%) | 0.09 | |||
| Poor perfusion, lost pulses | 7195 (5.6) | 90 (8.3) | 7285 (5.7) | ··· |
| Poor perfusion, never pulseless | 2674 (2.1) | 24 (2.2) | 2698 (2.1) | ··· |
| Pulseless | 117 603 (92.3) | 968 (89.5) | 118 571 (92.2) | ··· |
| Presenting rhythm status, n (%) | <0.001 | |||
| Asystole | 32 372 (26.8) | 214 (21.2) | 32 586 (26.8) | ··· |
| PEA | 57 397 (47.5) | 403 (40) | 57 800 (47.5) | ··· |
| Pulseless ventricular tachycardia | 8923 (7.4) | 93 (9.2) | 9016 (7.4) | ··· |
| Ventricular fibrillation | 12 210 (10.1) | 184 (18.3) | 12 394 (10.2) | ··· |
| Palpable pulse initially | 9869 (8.2) | 114 (11.3) | 9983 (8.2) | ··· |
| First rhythm, n (%) | <0.001 | |||
| Accelerated idioventricular rhythm | 201 (2) | 2 (1.8) | 203 (2) | ··· |
| Bradycardia | 6727 (68.4) | 55 (48.2) | 6782 (68.1) | ··· |
| Pacemaker | 300 (3) | 16 (14) | 316 (3.2) | ··· |
| Sinus (including sinus tachycardia) | 753 (7.7) | 13 (11.4) | 766 (7.7) | ··· |
| Supraventricular tachyarrhythmia | 249 (2.5) | 1 (0.9) | 250 (2.5) | ··· |
| Unknown | 919 (9.3) | 15 (13.2) | 934 (9.4) | ··· |
| Ventricular tachycardia with pulse | 692 (7) | 12 (10.5) | 704 (7.1) | ··· |
| Any VF/VT, n (%) | 44 634 (35) | 588 (54.3) | 45 222 (35.2) | <0.001 |
| Number of defibrillations, median (IQR) | 2 (1–4) | 3 (2–6) | 2 (1–4) | <0.001 |
| Received compressions, n (%) | 125 408 (98.4) | 1054 (97.4) | 126 462 (98.4) | 0.049 |
| Compression method, n (%) | ||||
| Manual | 100 687 (99.3) | 755 (92.5) | 101 442 (99.2) | <0.001 |
| Mechanical | 25 131 (24.8) | 198 (24.3) | 25 329 (24.8) | 0.023 |
| Open cardiac massage | 722 (0.7) | 154 (18.9) | 876 (0.9) | <0.001 |
| Epi boluses before pulseless, median (IQR) | 3 (2–5) | 4 (2–6) | 3 (2–5) | 0.003 |
| Epi boluses after pulseless, median (IQR) | 3 (1–5) | 4 (2–8) | 3 (1–5) | <0.001e |
| Any ROSC, n (%) | 88 686 (70.1) | 861 (79.9) | 89 547 (70.2) | <0.001 |
| Total duration before durable ROSC, median (IQR) | 14 (6–26) | 36 (17–69.2) | 15 (6–26) | <0.001 |
| Induced hypothermia, n (%) | 3477 (3.5) | 114 (12.7) | 3591 (3.6) | <0.001 |
Missing values by group: sex=6/0, race=314/3, weight (kg)=68 934/559; pre‐existing condition: none=347/3, preceding hypoperfusion=347/3, CVA or neurologic disorder=347/3, CHF=347/3, diabetes mellitus=347/3, hepatic insufficiency=347/3, major trauma=347/3, cancer =347/3, history of MI=347/3, MI this hospitalization=347/3, renal insufficiency=347/3, respiratory insufficiency=347/3, sepsis=347/3, admission CPC=29 503/228; devices: mechanical ventilation=144/1, invasive airway=144/1, arterial line=144/1; time of day of arrest=1591/47; patient type=121/0; arrest location=114/0; illness category=138/0; witnessed=89/0; hospital resuscitation activated=95/0; condition of first assessment=65/0; presenting rhythm status=6766/74; first rhythm=117 696/968; any VF/VT=148/0; number of defibrillations=87 038/568; received compression?=73/0; compression method: manual=26 116/266, mechanical=26 116/266, open cardiac massage=26 116/266; Epi boluses before pulseless=123 160/1025; Epi boluses after pulseless=30 943/341; any ROSC=1079/5; total duration before durable ROSC=4353/162; induced hypothermia=27 928/184. cath lab indicates cardiac catheterization laboratory; CCPR, conventional cardiopulmonary resuscitation; CHF, congestive heart failure; CPC, cerebral performance category; CVA, cerebrovascular accident; ECPR, extracorporeal cardiopulmonary resuscitation; Epi, epinephrine; ICU, intensive care unit; IQR, interquartile range; MI, myocardial infarction; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; and VF/VT, ventricular fibrillation/ventricular tachycardia.
Type 3 P values from mixed effects logistic regression model, with hospitals included as a random effect.
CPC is defined as follows: CPC 1, good cerebral performance; CPC 2, moderate cerebral disability; CPC 3, severe cerebral disability; CPC 4, coma or vegetative state.
Includes adults arresting in the newborn unit.
Includes rehabilitation and other.
Type 3 P value from linear mixed effects model regressing the variable on ECPR indicator, with hospitals included as a random effect due to a convergence issue.
Patient and Hospital Characteristics by Hospital
Hospitals offering ECPR differed from hospitals not offering ECPR (Table S2), as did the characteristics of patients treated at them (Table S3). Patients treated at non‐ECPR‐available hospitals were more likely to be younger than 40 years of age (7.6% vs. 5.7%; P<0.001) and black (22.6% vs. 19.8%; P<0.001) and have more comorbidities (P≤0.002 for all) and better neurologic function at admission (Cerebral Performance Category 1, 59.5% vs. 57.1%; P<0.001). Hospitals offering ECPR were larger metropolitan teaching hospitals and had higher cardiac and overall volumes (Table S2).
Adjusted Probability of Receiving ECPR
Demographic Characteristics
After adjusting for patient and hospital characteristics, patients were more likely to receive ECPR if they were younger than 40 years of age (RR, 1.5; 95% CI, 1.2–1.8; P<0.001), had a preceding period of hypoperfusion before their arrest (RR, 1.6; 95% CI, 1.4–1.8; P<0.001), and had congestive heart failure (RR, 1.3; 95% CI, 1.2–1.5; P<0.001) or a prior or current history of myocardial infarction (RR, 1.3; 95% CI, 1.2–1.6; P<0.001; and RR, 1.5; 95% CI, 1.3–1.7; P<0.001; respectively) (Table 2). Patients with no comorbidities were more likely to receive ECPR (RR, 1.4; 95% CI, 1.1–1.8; P=0.004). Correspondingly, older patients, those with 1 or more comorbidities, and patients with decreased neurologic function upon admission were all significantly less likely to be treated with ECPR. Patients of black race and women were significantly less likely to receive ECPR (RR, 0.8; 95% CI, 0.7–1.0; P<0.001; and RR, 0.9; 95% CI, 0.8–1.0; P=0.04; respectively).
Table 2.
Probability of ECPR Use for Cardiac Arrest
| Variable | Risk Ratio (95% CI)a | P Value | Risk Ratio (95% CI)b | P Value | Risk Ratio (95% CI)c | P Value |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| 40–59 | Reference | ··· | Reference | ··· | Reference | ··· |
| <40 | 1.5 (1.3–1.8) | <0.001 | 1.5 (1.3–1.9) | <0.001 | 1.5 (1.2–1.8) | <0.001 |
| ≥60 | 0.6 (0.5–0.7) | <0.001 | 0.6 (0.5–0.7) | <0.001 | 0.6 (0.5–0.7) | <0.001 |
| Sex | ||||||
| Male | Reference | ··· | Reference | ··· | Reference | ··· |
| Female | 0.9 (0.8–1.0) | 0.034 | 0.9 (0.8–1.0) | 0.022 | 0.9 (0.8–1.0) | 0.044 |
| Race | ||||||
| White | Reference | ··· | Reference | ··· | Reference | ··· |
| Black | 0.7 (0.6–0.8) | <0.001 | 0.8 (0.7–1.0) | 0.013 | 0.8 (0.7–1.0) | 0.020 |
| Other | 1.0 (0.8–1.2) | 0.96 | 1.0 (0.8–1.3) | 0.74 | 1.0 (0.8–1.3) | 0.84 |
| Hispanic ethnicity | 1.0 (0.7–1.3) | 0.96 | 1.0 (0.8–1.3) | 0.94 | 1.0 (0.7–1.3) | 1.00 |
| Weight, kg | 1.0 (1.0–1.0) | 0.31 | 1.0 (1.0–1.0) | 0.66 | 1.0 (1.0–1.0) | 0.65 |
| Pre‐existing conditions | ||||||
| None | 1.5 (1.2–1.9) | <0.001 | 1.5 (1.2–1.8) | 0.002 | 1.4 (1.1–1.8) | 0.004 |
| Preceding hypoperfusion | 1.8 (1.6–2.1) | <0.001 | 1.6 (1.4–1.8) | <0.001 | 1.6 (1.4–1.8) | <0.001 |
| CVA or neurologic disorder | 0.4 (0.3–0.5) | <0.001 | 0.5 (0.4–0.6) | <0.001 | 0.5 (0.4–0.6) | <0.001 |
| CHF | 1.2 (1.1–1.4) | <0.001 | 1.3 (1.1–1.5) | <0.001 | 1.3 (1.2–1.5) | <0.001 |
| Diabetes mellitus | 0.6 (0.5–0.7) | <0.001 | 0.7 (0.6–0.8) | <0.001 | 0.7 (0.6–0.8) | <0.001 |
| Hepatic insufficiency | 0.5 (0.4–0.7) | <0.001 | 0.6 (0.5–0.8) | <0.001 | 0.6 (0.5–0.8) | 0.001 |
| Major trauma | 0.8 (0.6–1.1) | 0.12 | 0.7 (0.5–1.0) | 0.06 | 0.7 (0.5–1.0) | 0.025 |
| Cancer | 0.3 (0.2–0.4) | <0.001 | 0.3 (0.2–0.5) | <0.001 | 0.3 (0.2–0.5) | <0.001 |
| History of MI | 1.5 (1.3–1.8) | <0.001 | 1.4 (1.2–1.6) | <0.001 | 1.3 (1.2–1.6) | <0.001 |
| MI this hospitalization | 2.1 (1.8–2.4) | <0.001 | 1.6 (1.3–1.8) | <0.001 | 1.5 (1.3–1.7) | <0.001 |
| Renal insufficiency | 0.5 (0.5–0.6) | <0.001 | 0.6 (0.5–0.7) | <0.001 | 0.6 (0.5–0.7) | <0.001 |
| Respiratory insufficiency | 0.8 (0.7–0.9) | 0.002 | 0.8 (0.7–0.9) | 0.004 | 0.8 (0.7–0.9) | 0.006 |
| Sepsis | 0.4 (0.3–0.5) | <0.001 | 0.5 (0.4–0.6) | <0.001 | 0.5 (0.4–0.6) | <0.001 |
| Admission CPCd | ||||||
| CPC 1 | Reference | ··· | Reference | ··· | Reference | ··· |
| CPC 2 | 0.4 (0.3–0.5) | <0.001 | 0.5 (0.4–0.6) | <0.001 | 0.5 (0.4–0.6) | <0.001 |
| CPC 3 | 0.3 (0.2–0.4) | <0.001 | 0.4 (0.3–0.5) | <0.001 | 0.4 (0.3–0.5) | <0.001 |
| CPC 4 | 0.7 (0.6–1.0) | 0.027 | 0.9 (0.7–1.2) | 0.34 | 0.9 (0.7–1.2) | 0.35 |
| Hospital beds | ||||||
| 1–100 | Reference | ··· | Reference | ··· | Reference | ··· |
| 101–199 | 1.1 (0.3–3.9) | 0.87 | 1.3 (0.4–4.6) | 0.64 | 1.3 (0.4–4.4) | 0.67 |
| 201–249 | 0.8 (0.2–2.7) | 0.67 | 0.9 (0.3–3.2) | 0.91 | 1.0 (0.3–3.5) | 0.95 |
| 251–299 | 0.5 (0.2–1.7) | 0.28 | 0.6 (0.2–2.1) | 0.44 | 0.5 (0.2–1.7) | 0.31 |
| 301–349 | 0.4 (0.1–1.5) | 0.19 | 0.5 (0.1–1.5) | 0.20 | 0.4 (0.1–1.5) | 0.19 |
| 351–499 | 0.4 (0.1–1.3) | 0.12 | 0.5 (0.1–1.5) | 0.19 | 0.4 (0.1–1.3) | 0.12 |
| ≥500 | 0.4 (0.1–1.4) | 0.16 | 0.5 (0.2–1.6) | 0.25 | 0.4 (0.1–1.2) | 0.11 |
| Annual admissions | ||||||
| 100–2499 | Reference | ··· | Reference | ··· | Reference | ··· |
| 2500–4999 | 1.8 (0.3–10.9) | 0.51 | 2.0 (0.3–12.3) | 0.44 | 0.9 (0.1–5.1) | 0.88 |
| 5000–7499 | 2.7 (0.6–13.2) | 0.22 | 3.4 (0.7–17.3) | 0.13 | 2.6 (0.5–12.7) | 0.23 |
| 7500–9999 | 0.7 (0.1–3.9) | 0.72 | 0.9 (0.2–4.8) | 0.89 | 0.6 (0.1–3.1) | 0.54 |
| 10 000–14 999 | 0.8 (0.2–3.2) | 0.72 | 0.9 (0.2–4.1) | 0.92 | 0.7 (0.2–2.9) | 0.60 |
| 15 000–19 999 | 0.7 (0.2–2.9) | 0.61 | 0.8 (0.2–3.5) | 0.76 | 0.5 (0.2–2.2) | 0.38 |
| 20 000–29 999 | 0.6 (0.1–2.5) | 0.48 | 0.7 (0.2–3.0) | 0.62 | 0.4 (0.1–1.9) | 0.27 |
| 30 000–39 999 | 0.5 (0.1–2.0) | 0.31 | 0.6 (0.1–2.6) | 0.48 | 0.3 (0.1–1.4) | 0.12 |
| ≥40 000 | 0.7 (0.2–2.8) | 0.56 | 0.8 (0.2–3.6) | 0.76 | 0.4 (0.1–1.9) | 0.26 |
| Cardiac ICU beds | ||||||
| ≥31 | Reference | ··· | Reference | ··· | Reference | ··· |
| 0 | 1.6 (1.0–2.7) | 0.054 | 1.7 (1.0–2.7) | 0.032 | 1.7 (1.1–2.8) | 0.027 |
| 1–5 | 1.4 (0.5–4.2) | 0.50 | 1.2 (0.4–3.8) | 0.72 | 1.0 (0.3–3.3) | 1.00 |
| 6–10 | 1.2 (0.7–2.0) | 0.57 | 1.1 (0.6–1.8) | 0.77 | 1.1 (0.7–1.8) | 0.73 |
| 11–15 | 0.9 (0.5–1.5) | 0.56 | 0.9 (0.5–1.5) | 0.64 | 0.9 (0.5–1.4) | 0.56 |
| 16–20 | 1.4 (0.8–2.4) | 0.21 | 1.3 (0.8–2.1) | 0.35 | 1.1 (0.7–1.8) | 0.78 |
| 21–30 | 1.7 (1.0–2.8) | 0.06 | 1.6 (1.0–2.6) | 0.07 | 1.6 (1.0–2.6) | 0.053 |
| Cardiac ICU beds/total beds (%) | ||||||
| 0.1 to <2.5 | Reference | ··· | Reference | ··· | Reference | ··· |
| 0 | 1.7 (1.1–2.8) | 0.026 | 1.8 (1.1–2.8) | 0.013 | 2.0 (1.3–3.2) | 0.003 |
| 2.5 to <5 | 1.2 (0.8–1.8) | 0.40 | 1.1 (0.8–1.7) | 0.52 | 1.1 (0.8–1.7) | 0.50 |
| 5 to <7.5 | 1.5 (0.9–2.5) | 0.09 | 1.5 (0.9–2.3) | 0.11 | 1.6 (1.0–2.5) | 0.052 |
| 7.5 to <10 | 2.7 (1.1–6.5) | 0.025 | 2.8 (1.2–6.3) | 0.017 | 3.1 (1.3–7.0) | 0.008 |
| ≥10 | 1.3 (0.5–3.4) | 0.61 | 1.2 (0.5–3.0) | 0.74 | 1.3 (0.5–3.3) | 0.58 |
| Geographic region of United States | ||||||
| North/Mid Atlantic | Reference | ··· | Reference | ··· | Reference | ··· |
| South Atlantic and Puerto Rico | 0.6 (0.4–0.9) | 0.008 | 0.6 (0.4–1.0) | 0.035 | 0.7 (0.4–1.0) | 0.06 |
| North Central | 0.9 (0.6–1.3) | 0.49 | 0.9 (0.6–1.4) | 0.75 | 1.0 (0.6–1.5) | 0.86 |
| South Central | 0.8 (0.5–1.2) | 0.27 | 0.9 (0.6–1.5) | 0.79 | 1.1 (0.7–1.7) | 0.78 |
| Mountain/Pacific | 1.1 (0.7–1.8) | 0.61 | 1.2 (0.8–1.9) | 0.41 | 1.3 (0.8–2.2) | 0.25 |
| Intensivist services on site | 1.5 (0.8–2.6) | 0.19 | 1.3 (0.7–2.3) | 0.35 | 1.1 (0.6–2.0) | 0.65 |
| Urban/rural location | ||||||
| Urban | Reference | ··· | Reference | ··· | Reference | ··· |
| Rural | 0.7 (0.3–1.7) | 0.49 | 0.8 (0.4–1.8) | 0.59 | 0.8 (0.4–1.7) | 0.55 |
| Teaching status | ||||||
| Major teaching | Reference | ··· | Reference | ··· | Reference | ··· |
| Minor teaching | 0.7 (0.5–1.0) | 0.050 | 0.8 (0.6–1.0) | 0.07 | 0.7 (0.5–1.0) | 0.025 |
| Nonteaching | 0.9 (0.5–1.5) | 0.65 | 0.9 (0.6–1.5) | 0.75 | 0.8 (0.5–1.3) | 0.36 |
| Medicare days | ||||||
| 0 | Reference | ··· | Reference | ··· | Reference | ··· |
| 1–1500 | 12.4 (3.5–43.4) | <0.001 | 8.6 (2.6–28.5) | <0.001 | 8.2 (2.4–27.4) | <0.001 |
| 1501–5000 | 6.8 (0.9–50.8) | 0.06 | 2.7 (0.4–17.9) | 0.31 | 2.7 (0.4–18.7) | 0.32 |
| 5001–20 000 | 1.3 (0.4–4.3) | 0.64 | 1.1 (0.3–3.3) | 0.93 | 0.9 (0.3–2.7) | 0.80 |
| >20 000 | 0.7 (0.2–1.8) | 0.42 | 0.5 (0.2–1.4) | 0.21 | 0.5 (0.2–1.2) | 0.12 |
Risk ratio and 95% CI were estimated using mixed effects log‐binomial model with hospital included as a random effect. Because ECPR is a rare event (0.8%), the odds ratio is an approximation to the risk ratio. We reported the unadjusted risk ratio (column 1), with the risk ratio adjusting for patient variables (age, sex, race, Hispanic origin, presenting rhythm status, subject type, and event location; column 2) and the risk ratio adjusting for patient and hospital variables (cardiac ICU beds, area type, teaching status, and admission period [2000–2004, 2005–2009, etc]; column 3). For the variables hospital beds, annual admission, and cardiac ICU beds/total beds (%), we did not adjust for cardiac ICU beds. CHF indicates congestive heart failure; CPC, cerebral performance category; CVA, cerebrovascular accident; ECPR, extracorporeal cardiopulmonary resuscitation; ICU, intensive care unit; and MI, myocardial infarction.
Unadjusted risk ratio.
Risk ratio adjusting for patient variables.
Risk ratio adjusting for patient and hospital variables.
CPC is defined as follows: CPC 1, good cerebral performance; CPC 2, moderate cerebral disability; CPC 3, severe cerebral disability; CPC 4, coma or vegetative state.
Hospital Characteristics
Across hospitals offering ECPR, hospitals with a higher strata ratio of cardiac intensive care unit beds/total beds (percentage) were more likely to use ECPR (RR, 3.1; 95% CI, 1.3–7.0; P=0.008; for stratum 7.5 to <10%) (Table 2). At hospitals that had ECPR programs, the ECPR/CCPR ratio did not significantly change over time (Figure S2, Tables S4 and S5).
Illness Characteristics
After controlling for both patient and hospital characteristics, the presence of arterial catheters (RR, 3.1; 95% CI, 2.7–3.5; P<0.001), mechanical ventilation (RR, 2.0; 95% CI, 1.7–2.3; P<0.001), and invasive airways (RR, 1.7; 95% CI, 1.5–2.0; P<0.001) each increased the probability of ECPR receipt during the cardiac arrest (Table 3). The probability of ECPR treatment increased in ambulatory/outpatient settings such as same‐day procedural areas (RR, 3.1; 95% CI, 1.8–5.2; P<0.001) and inpatient cardiac units (RR, 4.0; 95% CI, 3.0–5.3; P<0.001), intensive care units (RR, 2.8; 95% CI, 2.2–3.5; P<0.001), and operating rooms (RR, 12.0; 95% CI, 9.6–15.2; P<0.001) compared with general inpatient units (Figure 1). Correspondingly, cardiac medical and cardiac surgical patients were more likely to be treated with ECPR (RR, 4.3; 95% CI, 3.5–5.4; P<0.001; and RR, 24.1; 95% CI, 19.5–29.6; P<0.001; respectively).
Table 3.
Risk Ratio of Getting ECPR for Prearrest Characteristics
| Variable | Risk Ratio (95% CI)a | P Value | Risk Ratio (95% CI)b | P Value | Risk Ratio (95% CI)c | P Value |
|---|---|---|---|---|---|---|
| Duration between admission and arrest | ||||||
| <24 h | Reference | ··· | Reference | ··· | Reference | ··· |
| 24 to <48 h | 0.6 (0.4–0.9) | 0.007 | 0.7 (0.5–1.0) | 0.07 | 0.7 (0.5–1.0) | 0.06 |
| 48 h to <1 wk | 0.4 (0.2–0.8) | 0.018 | 0.5 (0.2–1.0) | 0.07 | 0.5 (0.2–1.1) | 0.08 |
| ≥1 wk | 1.4 (0.5–4.3) | 0.57 | 1.3 (0.4–4.1) | 0.62 | 1.4 (0.4–4.1) | 0.59 |
| Devices | ||||||
| Mechanical ventilation | 2.6 (2.3–3.0) | <0.001 | 2.0 (1.7–2.3) | <0.001 | 2.0 (1.7–2.3) | <0.001 |
| Invasive airway | 2.4 (2.2–2.7) | <0.001 | 1.8 (1.5–2.0) | <0.001 | 1.7 (1.5–2.0) | <0.001 |
| Arterial line | 4.8 (4.2–5.4) | <0.001 | 3.2 (2.8–3.6) | <0.001 | 3.1 (2.7–3.5) | <0.001 |
| Time of day of arrest | ||||||
| 7 am to 2:59 pm | Reference | ··· | Reference | ··· | Reference | ··· |
| 3 pm to 10:59 pm | 0.8 (0.7–0.9) | <0.001 | 0.9 (0.7–1.0) | 0.021 | 0.8 (0.7–1.0) | 0.020 |
| 11 pm to 6:59 am | 0.4 (0.3–0.5) | <0.001 | 0.6 (0.5–0.7) | <0.001 | 0.6 (0.5–0.7) | <0.001 |
| Day of week | ||||||
| Weekday | Reference | ··· | Reference | ··· | Reference | ··· |
| Weekend | 0.6 (0.5–0.7) | <0.001 | 0.7 (0.6–0.9) | <0.001 | 0.7 (0.6–0.9) | <0.001 |
| Arrest location | ||||||
| General inpatientd | Reference | ··· | Reference | ··· | Reference | ··· |
| Ambulatory/outpatiente | 3.0 (1.7–5.0) | <0.001 | 3.0 (1.8–5.1) | <0.001 | 3.1 (1.8–5.2) | <0.001 |
| Cardiac/coronary unit | 4.0 (3.0–5.3) | <0.001 | 3.7 (2.8–5.0) | <0.001 | 4.0 (3.0–5.3) | <0.001 |
| ICU | 3.2 (2.6–4.0) | <0.001 | 2.9 (2.3–3.6) | <0.001 | 2.8 (2.2–3.5) | <0.001 |
| Operating room/procedural/cath lab | 14.0 (11.3–17.4) | <0.001 | 12.1 (9.7–15.2) | <0.001 | 12.0 (9.6–15.2) | <0.001 |
| Emergency department | 1.4 (0.9–2.0) | 0.09 | 1.3 (0.9–1.8) | 0.23 | 1.2 (0.8–1.8) | 0.37 |
| Illness category | ||||||
| Medical—noncardiac | Reference | ··· | Reference | ··· | Reference | ··· |
| Medical—cardiac | 3.8 (3.1–4.6) | <0.001 | 4.3 (3.5–5.3) | <0.001 | 4.3 (3.5–5.4) | <0.001 |
| Surgical—cardiac | 21.5 (17.7–26.0) | <0.001 | 23.8 (19.5–29.2) | <0.001 | 24.0 (19.5–29.6) | <0.001 |
| Surgical—noncardiac | 2.3 (1.8–3.0) | <0.001 | 2.5 (1.9–3.3) | <0.001 | 2.6 (1.9–3.4) | <0.001 |
| Witnessed | 4.7 (3.4–6.7) | <0.001 | 4.3 (3.0–6.2) | <0.001 | 4.5 (3.1–6.5) | <0.001 |
cath lab indicates cardiac catheterization laboratory; ECPR, extracorporeal cardiopulmonary resuscitation; and ICU, intensive care unit.
Unadjusted risk ratio.
Risk ratio adjusting for patient variables.
Risk ratio adjusting for patient and hospital variables.
Includes adults arresting in the newborn unit.
“Ambulatory/outpatient” includes rehabilitation unit.
Figure 1. Comparison of CCPR vs ECPR use within hospitals (patient type and physical location).
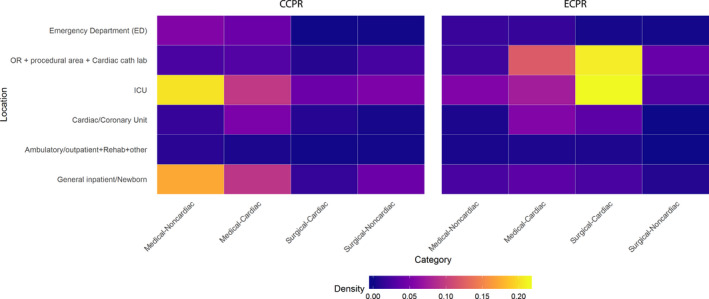
CCPR indicates conventional cardiopulmonary resuscitation; ECPR, extracorporeal cardiopulmonary resuscitation; and ICU, intensive care unit.
Arrest Characteristics
Arrest characteristics associated with the use of ECPR included the presence of ventricular fibrillation (RR, 1.5; 95% CI, 1.2–1.8; P<0.001), witnessed arrest (RR, 4.5; 95% CI, 3.1–6.5; P<0.001), or any return of spontaneous circulation (RR, 1.5; 95% CI, 1.3–1.8; P<0.001) during the arrest (Tables 3 and 4). Features associated with decreased ECPR use included arrests occurring after 3 pm or before 7 am (RR, 0.8; 95% CI, 0.7–1.0; P=0.02; and RR, 0.6; 95% CI, 0.5–0.7; P<0.001; respectively) and on weekends (RR, 0.7; 95% CI, 0.6–0.9; P<0.001) (Table 3, Figure 2). Increased duration of resuscitation (RR, 1.02 per minute; 95% CI, 1.02–1.03; P<0.001) and use of post‐arrest‐induced hypothermia (RR, 3.2; 95% CI, 2.7–3.9; P<0.001) were both associated with ECPR use.
Table 4.
Risk Ratio of Getting ECPR for Arrest Characteristics/Management
| Variable | Risk Ratio (95% CI)a | P Value | Risk Ratio (95% CI)b | P Value | Risk Ratio (95% CI)c | P Value |
|---|---|---|---|---|---|---|
| Hospital resuscitation activated | 0.3 (0.3–0.4) | <0.001 | 0.5 (0.4–0.6) | <0.001 | 0.5 (0.4–0.6) | <0.001 |
| Presenting rhythm status | ||||||
| Asystole | Reference | ··· | Reference | ··· | Reference | ··· |
| PEA | 1.0 (0.8–1.2) | 0.83 | 0.9 (0.8–1.1) | 0.43 | 0.9 (0.8–1.1) | 0.45 |
| Pulseless VT | 1.4 (1.1–1.7) | 0.012 | 1.2 (1.0–1.6) | 0.11 | 1.2 (0.9–1.5) | 0.15 |
| VF | 2.0 (1.6–2.4) | <0.001 | 1.6 (1.3–1.9) | <0.001 | 1.5 (1.2–1.8) | <0.001 |
| Palpable pulse initially | 1.4 (1.1–1.8) | 0.005 | 1.2 (0.9–1.5) | 0.14 | 1.2 (0.9–1.5) | 0.23 |
| Any VF/VT | 2.1 (1.9–2.4) | <0.001 | 2.0 (1.7–2.2) | <0.001 | 1.9 (1.7–2.2) | <0.001 |
| Compression method | ||||||
| Manual | 0.073 (0.054–0.098)d | <0.001 | 0.139 (0.101–0.191)d | <0.001 | 0.137 (0.099–0.189)d | <0.001 |
| Mechanical | 1.3 (1.0–1.5) | 0.023 | 1.3 (1.0–1.6) | 0.017 | 1.5 (1.1–2.1) | 0.006 |
| Open cardiac massage | 20.1 (17.1–23.8) | <0.001 | 18.349 (14.718–22.876)d | <0.001 | 18.026 (14.399–22.566)d | <0.001 |
| Any ROSC | 1.6 (1.4–1.9) | <0.001 | 1.5 (1.3–1.7) | <0.001 | 1.5 (1.3–1.8) | <0.001 |
| Total duration before durable ROSC | 1.024 (1.023–1.026)d | <0.001 | 1.024 (1.023–1.026)d | <0.001 | 1.024 (1.023–1.026)d | <0.001 |
| Induced hypothermia | 3.5 (2.9–4.3) | <0.001 | 3.2 (2.7–3.9) | <0.001 | 3.2 (2.7–3.9) | <0.001 |
ECPR indicates extracorporeal cardiopulmonary resuscitation; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Unadjusted risk ratio.
Risk ratio adjusting for patient variables.
Risk ratio adjusting for patient and hospital variables.
A mixed effects logistic model was used because of convergence issues, and the reported odds ratio is an approximation to the risk ratio.
Figure 2. Temporal relationship of CCPR vs ECPR use.
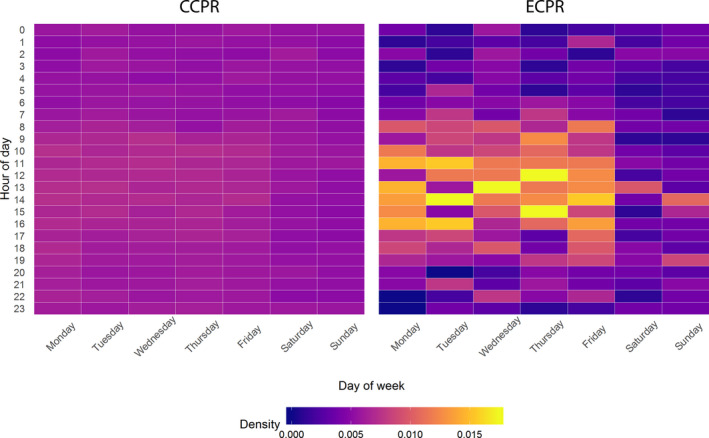
CCPR indicates conventional cardiopulmonary resuscitation; and ECPR, extracorporeal cardiopulmonary resuscitation.
DISCUSSION
In this large, multicenter, observational study of ECPR as an adjunctive treatment of IHCA, we determined that the decision to offer ECPR is highly influenced by patient and arrest characteristics. Patients who received ECPR were younger, more likely to have cardiac‐specific conditions, and less likely to have other pre‐existing conditions. Patients whose arrest occurred during daytime and weekdays and in procedural areas (operating room, coronary catheterization laboratory) were more likely to be offered ECPR. This likely reflects the schedule of planned procedures, familiarity with the patient, and an increased numbers of proceduralists required to provide ECPR in house. As an example of this, nonshockable rhythms are not traditionally considered an inclusion criteria for ECPR programs given their lower observed survival2, 10, 36, 37; despite this, >60% of ECPR patients had a nonshockable rhythm initially. Given 48.9% of ECPR patients were located in a procedural area at the time of arrest, the inclusion of nonshockable rhythms likely reflects the proceduralist's familiarity with the patient at the time of the arrest and therefore willingness to use ECPR. ECPR increased if patients had witnessed arrests, shockable initial rhythms, or intermittent/temporary return of spontaneous circulation. Overall, the 1% of patients who receive ECPR for IHCA are characteristically different than CCPR patients, which likely effects the survival and quality of survival for these patients with “favorable” arrest characteristics.38
ECPR series have shown heterogenous survival, underscoring the critical importance of randomized clinical trials for this invasive and expensive therapy1, 2, 3, 4, 5; yet ECPR use has dramatically increased during the previous 2 decades.39, 40 Some have opined that the growing clinical use of ECPR may reflect a lack of equipoise among some providers for certain patients.41 Others have stated that extracorporeal membrane oxygenation (ECMO) “is a heroic measure that involves high cost, invasive procedures, and exposes the patients to a series of potential complications.”42 Both of these opinions are likely correct. Unequivocally, the use of ECMO is an ethically complex topic that has generated significant discussion and controversy for more than 30 years.41, 42, 43, 44, 45, 46 The addition of patients in acute cardiopulmonary arrest to the pool of potential ECMO candidates has only added to this controversy. We believe this simultaneously emphasizes the need for randomized controlled trials of ECPR to define outcomes from unbiased patient selection and the difficulty in their design and recruitment. To adequately enroll patients, trials will need to select patients for whom there is a willingness to perform ECPR yet for whom there remains equipoise across diverse regions, hospitals, providers, and patients. Our study fills a previous knowledge gap in that it defines the features that favor ECPR use for IHCA. By identifying these patients, clinical trials may have a target population for enrollment.
Limitations
The data set is voluntary and captures fewer than 10% of US hospitals. Despite this, the use of this large data set enabled us to perform the largest published analysis of ECPR arrest characteristics contributing to generalizability. Some variables, such as admission Cerebral Performance Category, had a high degree of missingness (>15%) (Table 1), which may influence the findings for these variables. Previous analyses have demonstrated that the ECPR variable within the data set may not capture all instances of ECPR.47 As the ratio of ECPR to CCPR was <1%, we feel the amount of miscoded ECPR cases within the CCPR cohort is below a meaningful level. Finally, ECMO support details were not collected.
CONCLUSIONS
ECPR is increasing in use for IHCA and out‐of‐hospital cardiac arrest; however, reported outcomes are heterogenous and influenced by favorable patient selection. Randomized controlled trials are needed to define the best use of this technology to rescue patients after cardiac arrest. As enrollment in randomized controlled trials is a major barrier to trial feasibility,48 the success of future randomized controlled trials of ECPR depends on defining patients, providers, and clinical illnesses for whom there is both equipoise and a low barrier to trial implementation.49 Our findings identify and define the characteristics that bias toward use of ECPR. Physicians are willing to place younger patients who have primarily a cardiac history with few other comorbidities on ECPR. This may be a group to target for enrollment in future trials.
Sources of Funding
Dr Tonna was supported by a career development award (K23HL141596) from the National Heart, Lung, and Blood Institute of the National Institutes of Health. This study was also indirectly supported in part by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067‐02 (formerly 8UL1TR000105 and UL1RR025764). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the funding sources were involved in the design or conduct of the study, collection, management, analysis or interpretation of the data, or preparation, review, or approval of the manuscript.
Disclosures
Dr Tonna received modest support from LivaNova as a speaking honorarium relevant to cardiac arrest. The remaining authors have no disclosures to report.
Supporting information
Appendix S1 Data S1 Tables S1–S5 Figures S1–S2
Acknowledgments
Author contributions: J.E. Tonna had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and the integrity of the submission as a whole, from inception to published article. J.E. Tonna, R.R. Thiagarajan, A.P. Presson, and H.T. Keenan conceived study design; J.E. Tonna, A.P. Presson, C. Zhang, R.R. Thiagarajan, S. Girotra, L.B. Becker, C.H. Selzman, and H.T. Keenan contributed to data analysis; J.E. Tonna, C. Zhang, A.P. Presson, and H.T. Keenan drafted the work. All authors revised the article for important intellectual content, had final approval of the work to be published, and agreed to be accountable for all aspects of the work.
(J Am Heart Assoc. 2020;9:e015522 DOI: 10.1161/JAHA.119.015522.)
This article was handled independently by Daniel Edmundowicz, MD, as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
Presented in part at the American Heart Association's Resuscitation Science Symposium, Chicago, IL, November 10–11, 2018.
For Sources of Funding and Disclosures, see page 12.
References
- 1. Yannopoulos D, Bartos JA, Martin C, Raveendran G, Missov E, Conterato M, Frascone RJ, Trembley A, Sipprell K, John R, et al. Minnesota Resuscitation Consortium's advanced perfusion and reperfusion cardiac life support strategy for out‐of‐hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5:e003732 DOI: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stub D, Bernard S, Pellegrino V, Smith K, Walker T, Sheldrake J, Hockings L, Shaw J, Duffy SJ, Burrell A, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94. [DOI] [PubMed] [Google Scholar]
- 3. Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, Hase M, Tahara Y, Atsumi T, SAVE‐J Study Group . Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out‐of‐hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. [DOI] [PubMed] [Google Scholar]
- 4. Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. [DOI] [PubMed] [Google Scholar]
- 5. Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out‐of‐hospital cardiac arrest of cardiac origin. Crit Care Med. 2013;41:1186–1196. [DOI] [PubMed] [Google Scholar]
- 6. Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS, American Heart Association Get with the Guidelines‐Resuscitation Investigators . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goto Y, Funada A, Goto Y. Relationship between the duration of cardiopulmonary resuscitation and favorable neurological outcomes after out‐of‐hospital cardiac arrest: a prospective, nationwide, population‐based cohort study. J Am Heart Assoc. 2016;5:e002819 DOI: 10.1161/JAHA.115.002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersen LW, Granfeldt A, Callaway CW, Bradley SM, Soar J, Nolan JP, Kurth T, Donnino MW, American Heart Association's Get With The Guidelines‐Resuscitation Investigators . Association between tracheal intubation during adult in‐hospital cardiac arrest and survival. JAMA. 2017;317:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin JW, Wang MJ, Yu HY, Wang CH, Chang WT, Jerng JS, Huang SC, Chou NK, Chi NH, Ko WJ, et al. Comparing the survival between extracorporeal rescue and conventional resuscitation in adult in‐hospital cardiac arrests: propensity analysis of three‐year data. Resuscitation. 2010;81:796–803. [DOI] [PubMed] [Google Scholar]
- 10. Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A, John R, Connett J, Benditt DG, Lurie KG, et al. Coronary artery disease in patients with out‐of‐hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–1117. [DOI] [PubMed] [Google Scholar]
- 11. Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, Song YB, Hahn JY, Choi SH, Gwon HC, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011;39:1–7. [DOI] [PubMed] [Google Scholar]
- 12. Lee SW, Han KS, Park JS, Lee JS, Kim SJ. Prognostic indicators of survival and survival prediction model following extracorporeal cardiopulmonary resuscitation in patients with sudden refractory cardiac arrest. Ann Intensive Care. 2017;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wengenmayer T, Rombach S, Ramshorn F, Biever P, Bode C, Duerschmied D, Staudacher DL. Influence of low‐flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care. 2017;21:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang C‐H, Chou N‐K, Becker LB, Lin J‐W, Yu H‐Y, Chi N‐H, Hunag S‐C, Ko W‐J, Wang S‐S, Tseng L‐J, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest—a comparison with that for extracorporeal rescue for in‐hospital cardiac arrest. Resuscitation. 2014;85:1219–1224. [DOI] [PubMed] [Google Scholar]
- 15. Wang GN, Chen XF, Qiao L, Mei Y, Lv JR, Huang XH, Shen B, Zhang JS. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: a meta‐analysis of 2 260 patients with cardiac arrest. World J Emerg Med. 2017;8:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane‐Trultt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14 720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cummins RO, Chamberlain D, Hazinski MF, Nadkarni V, Kloeck W, Kramer E, Becker L, Robertson C, Koster R, Zaritsky A, et al. Recommended guidelines for reviewing, reporting, and conducting research on in‐hospital resuscitation: the in‐hospital ‘Utstein style’. American Heart Association. Circulation. 1997;95:2213–2239. [DOI] [PubMed] [Google Scholar]
- 19. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane‐Truitt T, Potts J, Ornato JP, et al. First documented rhythm and clinical outcome from in‐hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. [DOI] [PubMed] [Google Scholar]
- 20. Peberdy MA, Ornato JP, Larkin GL, Braithwaite RS, Kashner TM, Carey SM, Meaney PA, Cen L, Nadkarni VM, Praestgaard AH, et al. Survival from in‐hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–792. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D'Este K, Finn J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, Interamerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004;110:3385–3397. [DOI] [PubMed] [Google Scholar]
- 22. Girotra S, Cram P, Spertus JA, Nallamothu BK, Li Y, Jones PG, Chan PS, American Heart Association's Get With the Guidelines‐Resuscitation Investigators . Hospital variation in survival trends for in‐hospital cardiac arrest. J Am Heart Assoc. 2014;3:e000871 DOI: 10.1161/JAHA.114.000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, Rathore SS, Nallamothu BK, American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators . Racial differences in survival after in‐hospital cardiac arrest. JAMA. 2009;302:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merchant RM, Berg RA, Yang L, Becker LB, Groeneveld PW, Chan PS, American Heart Association's Get With the Guidelines‐Resuscitation Investigators . Hospital variation in survival after in‐hospital cardiac arrest. J Am Heart Assoc. 2014;3:e000400 DOI: 10.1161/JAHA.113.000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ofoma UR, Basnet S, Berger A, Kirchner HL, Girotra S, American Heart Association Get With the Guidelines‐Resuscitation Investigators . Trends in survival after in‐hospital cardiac arrest during nights and weekends. J Am Coll Cardiol. 2018;71:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. [DOI] [PubMed] [Google Scholar]
- 27. Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, et al. Variability in case‐mix adjusted in‐hospital cardiac arrest rates. Med Care. 2012;50:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engdahl J, Abrahamsson P, Bang A, Lindqvist J, Karlsson T, Herlitz J. Is hospital care of major importance for outcome after out‐of‐hospital cardiac arrest? Experience acquired from patients with out‐of‐hospital cardiac arrest resuscitated by the same Emergency Medical Service and admitted to one of two hospitals over a 16‐year period in the municipality of Goteborg. Resuscitation. 2000;43:201–211. [DOI] [PubMed] [Google Scholar]
- 29. Keenan SP, Dodek P, Martin C, Priestap F, Norena M, Wong H. Variation in length of intensive care unit stay after cardiac arrest: where you are is as important as who you are. Crit Care Med. 2007;35:836–841. [DOI] [PubMed] [Google Scholar]
- 30. Carr BG, Goyal M, Band RA, Gaieski DF, Abella BS, Merchant RM, Branas CC, Becker LB, Neumar RW. A national analysis of the relationship between hospital factors and post‐cardiac arrest mortality. Intensive Care Med. 2009;35:505–511. [DOI] [PubMed] [Google Scholar]
- 31. Carr BG, Kahn JM, Merchant RM, Kramer AA, Neumar RW. Inter‐hospital variability in post‐cardiac arrest mortality. Resuscitation. 2009;80:30–34. [DOI] [PubMed] [Google Scholar]
- 32. Joseph L, Chan PS, Bradley SM, Zhou Y, Graham G, Jones PG, Vaughan‐Sarrazin M, Girotra S, American Heart Association Get With the Guidelines‐Resuscitation Investigators . Temporal changes in the racial gap in survival after in‐hospital cardiac arrest. JAMA Cardiol. 2017;2:976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stiell IG, Wells GA, DeMaio VJ, Spaite DW, Field BJ III, Munkley DP, Lyver MB, Luinstra LG, Ward R. Modifiable factors associated with improved cardiac arrest survival in a multicenter basic life support/defibrillation system: OPALS Study Phase I results. Ontario Prehospital Advanced Life Support. Ann Emerg Med. 1999;33:44–50. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? Int J Public Health. 2008;53:165–167. [DOI] [PubMed] [Google Scholar]
- 35. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
- 36. Grunau B, Hornby L, Singal RK, Christenson J, Ortega‐Deballon I, Shemie SD, Bashir J, Brooks SC, Callaway CW, Guadagno E, et al. Extracorporeal cardiopulmonary resuscitation for refractory out‐of‐hospital cardiac arrest: the state of the evidence and framework for application. Can J Cardiol. 2018;34:146–155. [DOI] [PubMed] [Google Scholar]
- 37. Tonna JE, Johnson NJ, Greenwood J, Gaieski DF, Shinar Z, Bellezo JM, Becker L, Shah AP, Youngquist ST, Mallin MP, et al. Practice characteristics of Emergency Department extracorporeal cardiopulmonary resuscitation (ECPR) programs in the United States: the current state of the art of Emergency Department extracorporeal membrane oxygenation (ED ECMO). Resuscitation. 2016;107:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Panchal AR, Berg KM, Hirsch KG, Kudenchuk PJ, Del Rios M, Cabanas JG, Link MS, Kurz MC, Chan PS, Morley PT, et al. 2019 American Heart Association focused update on advanced cardiovascular life support: use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2019;140:e881–e894. [DOI] [PubMed] [Google Scholar]
- 39. Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, Paden ML, for the ELSO Member Centers . Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63:60–67. [DOI] [PubMed] [Google Scholar]
- 40. Richardson AS, Schmidt M, Bailey M, Pellegrino VA, Rycus PT, Pilcher DV. ECMO cardio‐pulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12‐years. Resuscitation. 2017;112:34–40. [DOI] [PubMed] [Google Scholar]
- 41. Burns B, Reid C, Scott R, Bernard S, Lamhaut L, Bellezzo J, Shinar Z, Dennis M, Forrest P. Authors’ response: extracorporeal cardiopulmonary resuscitation probably good, but adoption should not be too fast and furious!. Emerg Med J. 2017;34:557. [DOI] [PubMed] [Google Scholar]
- 42. Ramanathan K, Cove ME, Caleb MG, Teoh KL, Maclaren G. Ethical dilemmas of adult ECMO: emerging conceptual challenges. J Cardiothorac Vasc Anesth. 2015;29:229–233. [DOI] [PubMed] [Google Scholar]
- 43. Riggs KR, Becker LB, Sugarman J. Ethics in the use of extracorporeal cardiopulmonary resuscitation in adults. Resuscitation. 2015;91:73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bein T, Weber‐Carstens S, Herridge M. Extracorporeal life support, ethics, and questions at the bedside: how does the end of the pathway look? Intensive Care Med. 2015;41:1714–1715. [DOI] [PubMed] [Google Scholar]
- 45. Frank HA. Ethics of randomization: use of extracorporeal membrane oxygenation. N Engl J Med. 1977;296:397–398. [PubMed] [Google Scholar]
- 46. Abrams DC, Prager K, Blinderman CD, Burkart KM, Brodie D. Ethical dilemmas encountered with the use of extracorporeal membrane oxygenation in adults. Chest. 2014;145:876–882. [DOI] [PubMed] [Google Scholar]
- 47. Bembea MM, Ng DK, Rizkalla N, Rycus P, Lasa JJ, Dalton H, Topjian AA, Thiagarajan RR, Nadkarni VM, Hunt EA, et al. Outcomes after extracorporeal cardiopulmonary resuscitation of pediatric in‐hospital cardiac arrest: a report from the get with the guidelines‐resuscitation and the extracorporeal life support organization registries. Crit Care Med. 2019;47:e278–e285. [DOI] [PubMed] [Google Scholar]
- 48. Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated trials in the clinicaltrials.Gov results database: evaluation of availability of primary outcome data and reasons for termination. PLoS One. 2015;10:e0127242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, Wilson C, Beard D, Blazeby JM, Birtle A, et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLoS Med. 2016;13:e1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Data S1 Tables S1–S5 Figures S1–S2


