Abstract
Background
Differences in risk factors for atrial fibrillation (AF) and heart failure (HF) are incompletely understood. Aim of this study was to understand whether risk factors and biomarkers show different associations with incident AF and HF and to investigate predictors of subsequent onset and mortality.
Methods and Results
In N=58 693 individuals free of AF/HF from 5 population‐based European cohorts, Cox regressions were used to find predictors for AF, HF, subsequent onset, and mortality. Differences between associations were estimated using bootstrapping. Median follow‐up time was 13.8 years, with a mortality of 15.7%. AF and HF occurred in 5.0% and 5.4% of the participants, respectively, with 1.8% showing subsequent onset. Age, male sex, myocardial infarction, body mass index, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) showed similar associations with both diseases. Antihypertensive medication and smoking were stronger predictors of HF than AF. Cholesterol, diabetes mellitus, and hsCRP (high‐sensitivity C‐reactive protein) were associated with HF, but not with AF. No variable was exclusively associated with AF. Population‐attributable risks were higher for HF (75.6%) than for AF (30.9%). Age, male sex, body mass index, diabetes mellitus, and NT‐proBNP were associated with subsequent onset, which was associated with the highest all‐cause mortality risk.
Conclusions
Common risk factors and biomarkers showed different associations with AF and HF, and explained a higher proportion of HF than AF risk. As the subsequent onset of both diseases was strongly associated with mortality, prevention needs to be rigorously addressed and remains challenging, as conventional risk factors explained only 31% of AF risk.
Keywords: atrial fibrillation, biomarkers, heart failure, population, risk factors
Subject Categories: Cardiovascular Disease, Risk Factors
Clinical Perspective
What Is New?
Atrial fibrillation and heart failure have distinct risk profiles, as they show different associations with common cardiovascular risk factors.
Common cardiovascular risk factors explain a higher proportion of the risk of incident heart failure compared with atrial fibrillation.
Although both diseases increase mortality risk, co‐occurrence of both diseases carries the strongest association with mortality.
What Are the Clinical Implications?
Modifiable risk factors should be targeted rigorously at the population level to reduce the risk of incident atrial fibrillation and heart failure, in particular in individuals in whom one disease has already occurred.
In particular, overweight/obesity should be addressed as this explains the highest proportion of modifiable attributable risk for both diseases.
Nonstandard Abbreviations and Acronyms.
AF atrial fibrillation
BMI body mass index
HF heart failure
HR hazard ratio
hsCRP high‐sensitivity C‐reactive protein
IQR interquartile range
NT‐proB NPN‐terminal pro‐B‐type natriuretic peptide
PAR population‐attributable risk
Atrial fibrillation (AF) and heart failure (HF) are increasingly prevalent cardiovascular diseases. They frequently occur together or complicate each other. In permanent AF, the coprevalence of HF accounts for >50% of the cases.1 In individuals with HF, AF is observed in >40%.2 Both diseases are more frequently observed in older patients, and outcome seems to be worse if AF and HF coincide.3, 4
AF and HF share common pathophysiological features as the atria and ventricles are interrelated mechanically, structurally, and electrically. For example, increased end‐diastolic left ventricular pressure in HF adversely affects atrial hemodynamics and may lead to AF.5 On the other hand, irregular atrioventricular conduction, in particular high heart rates, can induce left ventricular remodeling and, ultimately, HF.6
Similar conventional cardiovascular risk factors have been reported to be associated with incident AF and HF, such as age, arterial hypertension, smoking, and obesity. Many of the shared risk factors can be easily evaluated in the primary care setting.7, 8, 9, 10
Biomarkers are another important tool for risk assessment. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) is widely used in this setting. It reflects cardiac stress, including end‐diastolic left ventricular pressure. This constitutes a pathophysiological link between AF and HF. It has been shown that NT‐proBNP has a strong predictive value for both diseases in the general population.11, 12 In addition, hsCRP (high‐sensitivity C‐reactive protein) is widely used for cardiovascular risk assessment, as population‐based studies have shown strong associations with cardiovascular and all‐cause mortality events.13
Although the association of cardiovascular risk factors with incident AF and HF has been shown in different studies, no direct comparison of the direction and strength of these associations has been performed across populations with differing levels of absolute HF and AF risk. In addition, biomarkers commonly used in cardiovascular medicine, such as NT‐proBNP and hsCRP, have not been compared in their predictive ability for both diseases.11, 12 Therefore, we systematically evaluated the direction and strength of the association of common cardiovascular risk factors and circulating biomarkers with the long‐term incidence of AF and HF as well as with subsequent disease onset and mortality in European population‐based cohorts.
Methods
The data supporting the findings presented in this study are available from the Biomarker for Cardiovascular Risk Assessment in Europe consortium (http://www.biomarcare.eu) on reasonable request.
Study Sample
This study is based on the Monica Risk, Genetics, Archiving, and Monograph/Biomarker for Cardiovascular Risk Assessment in Europe consortium. This consortium provides harmonization of risk factors, biomarker measurements, and end points from European population‐based cohort studies.14 Information on AF and HF status at baseline and follow‐up was available in 59 913 participants from 5 cohort studies (DanMONICA, FINRISK, Moli‐sani, Northern Sweden, and Scottish Heart Health Extended Cohort). Individuals with self‐reported and/or physician‐diagnosed history of AF or HF and/or prior International Classification of Diseases, Tenth Revision (ICD‐10), coding for AF or HF at baseline were excluded from this analysis (N=1220). A total of 58 693 individuals were included into the analysis. Details on the enrollment and follow‐up procedures for each study separately are provided in Data S1 (Chapter 1–5).
Risk Factors and Follow‐Up
Risk factor information was collected at the baseline visit. Body mass index (BMI), systolic blood pressure, heart rate, and total cholesterol were measured locally according to the World Health Organization Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) protocol (https://thl.fi/publications/monica/manual/index.htm). Obesity was defined as BMI ≥30 kg/m2. Information on diabetes mellitus status, antihypertensive medication use, history of cardiovascular diseases, smoking, and average daily consumption of alcohol was obtained by self‐report and collected locally in the study centers. All data from the cohort studies were harmonized in the Monica Risk, Genetics, Archiving, and Monograph project.15 Creatinine was centrally measured from stored blood samples, and glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula with creatinine.16 Data on missing values are displayed in Table S1.
AF and HF diagnoses were based on self‐reported history of AF/HF and/or national hospital discharge registry data and/or data on ambulatory visits to specialized clinics. In addition, cause of death registry data were screened for incident AF and HF as comorbidities of individuals who died from other causes (https://thl.fi/publications/morgam/cohorts/index.html). Mortality data were derived from central death registries. The periods of baseline examinations of the cohorts varied between 1982 and 2010. The last follow‐up was between 2010 and 2011 in the different cohorts (detailed information by study cohort are provided in Data S1, Chapter 1–5).
All participating cohort studies were approved by local ethics committees and institutional review boards; and all obtained informed consent from participants.
Biomarker Measurement
Biomarker measurements were performed from stored blood samples. hsCRP was measured by latex immunoassay (Abbott, Architect c8000) in 95% of the individuals, with intra‐assay and interassay coefficients of variation of 0.93 and 0.83, respectively. NT‐proBNP was measured on the ELECSYS 2010 platform using an electrochemiluminescence immunoassay (Roche Diagnostics) in 72.8% of the individuals. The analytical range is given as 5 to 35 000 pg/mL. Intra‐assay and interassay coefficients of variation were 2.58 and 1.38, respectively.
Statistical Analysis
Binary variables are given as absolute and relative frequencies, and continuous variables are given as median (25th, 75th percentile).
Heart rate was excluded from the analyses because it was only available in 2 cohorts (FINRISK and Moli‐sani).
Univariable and multivariable‐adjusted Cox proportional hazard regressions with incident AF, incident HF, subsequent incident AF and HF, and mortality as end points were performed, using time since baseline as time scale. For the analysis of subsequent incident AF and HF, the end point was the first co‐occurrence of both diseases and time to event was the time from baseline to this moment. When incident AF, incident HF, and/or subsequent incident AF and HF were covariates, they were included as time‐dependent covariates. Variable selection in the multivariable regression analysis was based on stepwise regression, using only variables that in corresponding univariate Cox proportional hazards regression had a hazard ratio (HR) with P<0.1, with backwards elimination of the least significant variable, when the P value of its HR exceeds 0.05 during a regression step. Regression steps were repeated until no more variables were eliminated. All variables from Table 1 were included into the regression analysis. C‐statistics of the fitted Cox models were calculated using the summary.coxph function from the Survival package in R.
Table 1.
Baseline Characteristics of the Total Study Cohort
| Characteristic | Study Population (N=58 693) |
|---|---|
| Age, y | 50.5 (41.4, 59.2) |
| Sex (men) | 28 920 (49.3) |
| Body mass index, kg/m2 | 26.1 (23.4, 29.2) |
| Total serum cholesterol, mmol/L | 5.8 (5.0, 6.6) |
| Average daily consumption of alcohol, g | 5.0 (0, 15.0) |
| Systolic blood pressure, mm Hg | 131 (118, 145) |
| Heart rate, bpm | 67.0 (60.0, 74.0) |
| Antihypertensive medication | 8570 (14.8) |
| Smoking | 16 611 (28.4) |
| Prevalent diabetes mellitus | 2274 (3.9) |
| Prevalent myocardial infarction | 1536 (2.6) |
| Prevalent stroke | 731 (1.3) |
| NT‐proBNP, pg/mL | 48 (26, 89) |
| hsCRP, mg/L | 1.3 (0.6, 2.8) |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 97.1 (85.8, 106.5) |
Baseline characteristics of the pooled study cohort. Continuous variables are presented as median (25th, 75th percentile), and binary variables are presented as absolute and relative frequencies. Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula with creatinine.16 All shown variables were used for the analyses. Bpm indicates beats per minute; hsCRP, high‐sensitivity C‐reactive protein; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
To be able to compare the models with incident AF and HF, these models must contain the same variables, but with reversed roles of incident AF and HF. This was ensured by parallelizing the stepwise regressions in the following way: the covariates incident AF and HF were treated as if they were the same variable, but in the model with incident AF as end point the covariate incident HF was used and vice versa in the model with incident HF as end point the covariate incident AF was used. Then, the initially included variables were those that had a P value <0.1 in either the univariable analysis for AF or the univariable analysis for HF. During each regression step, each variable has 2 P values (one from the incident AF model and one from the incident HF model) and the variable with the largest minimum of both was eliminated when both P values >0.05. All Cox proportional hazards regressions were adjusted for age, sex, and study area. To avoid nonlinearity in all multivariable Cox proportional hazards models, the statistical significance of all possible second‐order (self [ie, quadratic] and nonself) interactions of the variables in the model was assessed and an interaction was included as additional covariate if its Bonferroni‐corrected P value was <0.05. Interactions with time since baseline were added when needed to avoid violations of the proportional hazard's assumption, which were identified using the R function cox.zph. To add these interactions with time to the model, the analysis data set was further segmented into time intervals of 1 year and the time variable was updated yearly, starting with time=0 during the first time interval. When included in an interaction, numeric variables were centered on their mean. Two‐sided P values for differences between HRs and CIs were estimated using bootstrapping with 1000 replications. The constructed bootstrap populations were formed by replicating each person 10 times. Bootstrap samples were then drawn from this bootstrap population using simple random sampling. Symmetric 95% bootstrap CIs were estimated as m−e and m+e, where m=the mean of the bootstrap estimates and e=the 95% percentile of absolute deviations of bootstrap estimates from m.17, 18
Population‐attributable risks (PARs) were calculated using the fully adjusted estimated HRs to replace the relative risk ratios in the original formula for PAR. Hence, the PAR of each risk category of each risk factor was calculated using pd×(HR−1)/HR, where pd is the proportion of those in the risk category among the cases (incident AF or HF) during a 5‐year follow‐up. The total PAR of a risk factor is the sum of all PARs of its risk categories. For each PAR, bootstrapping with 1000 repetitions was used for estimating its associated 95% CI, the P values for the differences between PARs, and associated 95% CIs. For the calculation of PARs, continuous variables were categorized as follows: BMI using 3 categories (<25, 25–<30, and ≥30 kg/m2) and total cholesterol using 2 categories based on the cutoff value 5.2 mmol/L (as it is an accepted cutoff point for elevated total cholesterol in the general population; eg, this cutoff point is used in the Framingham risk score). The category containing the lowest values was taken as reference level during the PAR calculation.
The Bonferroni correction was used to account for multiple testing in the presented models (eg, dividing the prespecified α of 0.05 by the number of independent tests).
Analyses were performed with R v.3.5.1.19
Results
Baseline Characteristics
The baseline characteristics of study participants are shown in Table 1 for the overall cohort and in Tables S2 through S6 for each individual cohort study. The median age of the study population was 50.5 years (interquartile range [IQR], 41.4–59.2 years), and 49.3% of the individuals were men. During a median follow‐up of 13.8 years (IQR, 4.5–21.9 years), 2959 (5.0%) individuals were diagnosed with incident AF and 3141 (5.4%) individuals were diagnosed with incident HF. Median time to the first AF or HF event was 12.7 (IQR, 7.1–18.1) and 11.1 (IQR, 5.6–16.6) years, respectively. Subsequent disease onset was observed in 1028 (1.8% of the total sample) individuals.
Different Associations of Cardiovascular Risk Factors and Biomarkers With AF and HF
Multivariable‐adjusted Cox regression analysis indicated different associations of common cardiovascular risk factors and circulating biomarkers with incident AF and HF (Table 2): Age, male sex, prevalent myocardial infarction, and log10(NT‐proBNP) showed a similar strength of association with incident AF and HF. BMI, antihypertensive medication, and smoking were observed to be stronger predictors of incident HF than incident AF. Total serum cholesterol, prevalent diabetes mellitus, and hsCRP were associated with an increased risk of incident HF; however, they did not show a statistically significant association with incident AF. No variable was exclusively associated with incident AF. After Bonferroni correction, AF and HF no longer differed in their strength of association with BMI.
Table 2.
Association of Risk Factors and Biomarker With Incident Diseases
| Variable | Disease | Hazard Ratio (95% CI) | P Value | Hazard Ratio Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| Age (per 5‐y increase) | AF | 1.59 (1.52/1.67) | <0.001 | 0.00 (−0.10/0.10) | 0.99 |
| HF | 1.59 (1.52/1.67) | <0.001 | |||
| Sex (men) | AF | 2.92 (2.48/3.41) | <0.001 | 0.58 (−0.01/1.17) | 0.05 |
| HF | 2.34 (1.98/2.75) | <0.001 | |||
| Body mass index (per 5‐kg/m2 increase) | AF | 1.31 (1.25/1.38) | <0.001 | −0.11 (−0.19/−0.02) | 0.02 |
| HF | 1.42 (1.35/1.49) | <0.001 | |||
| Total serum cholesterol (per 1‐mmol/L increase) | AF | 1.01 (0.97/1.05) | 0.55 | −0.06 (−0.12/−0.01) | 0.03 |
| HF | 1.07 (1.03/1.12) | <0.001 | |||
| Antihypertensive medication | AF | 1.24 (1.09/1.39) | <0.001 | −0.29 (−0.50/−0.08) | <0.001 |
| HF | 1.52 (1.35/1.71) | <0.001 | |||
| Smoking | AF | 1.19 (1.06/1.32) | <0.001 | −0.92 (−1.15/−0.69) | <0.001 |
| HF | 2.10 (1.90/2.31) | <0.001 | |||
| Prevalent diabetes mellitus | AF | 1.14 (0.68/1.60) | 0.56 | −2.19 (−3.14/−1.25) | <0.001 |
| HF | 3.33 (2.50/4.17) | <0.001 | |||
| Prevalent myocardial infarction | AF | 1.56 (1.16/1.97) | <0.001 | −0.25 (−0.83/0.33) | 0.39 |
| HF | 1.81 (1.44/2.20) | <0.001 | |||
| Log10(NT‐proBNP) (per 0.3 increase) | AF | 1.54 (1.45/1.63) | <0.001 | 0.07 (−0.05/0.19) | 0.23 |
| HF | 1.46 (1.38/1.55) | <0.001 | |||
| hsCRP (per 5‐mg/L increase) | AF | 1.00 (0.93/1.06) | 0.91 | −0.13 (−0.20/−0.05) | <0.001 |
| HF | 1.13 (1.08/1.17) | <0.001 | |||
| Estimated glomerular filtration rate (per 20‐mL/min per 1.73 m2 increase) | AF | 1.04 (0.95/1.14) | 0.38 | 0.10 (−0.01/0.22) | 0.09 |
| HF | 0.94 (0.86/1.03) | 0.16 | |||
| Incident AF during follow‐up | HF | 6.84 (4.45/9.28) | <0.001 | ||
| Incident HF during follow‐up | AF | 7.05 (4.43/9.80) | <0.001 |
The following variables were also fitted into, but then dropped from, the Cox regression model: cholesterol‐lowering medication, daily consumption of alcohol, systolic blood pressure, heart rate, and prevalent stroke. In addition, cohort stratification was adjusted for. After Bonferroni correction to account for multiple testing, a P value threshold of <0.002 was used. Additional interaction terms were added to improve model fit: sex and age, log10(NT‐proBNP) and prevalent myocardial infarction, AF/HF and age, AF/HF and log10(NT‐proBNP), diabetes mellitus and age, diabetes mellitus and cholesterol, log10(NT‐proBNP) and age, log10(NT‐proBNP) and log10(NT‐proBNP), CRP and age, glomerular filtration rate and sex, AF/HF and antihypertensive medication, FINRISK and sex, age and time, CRP and time, northern Sweden and time, and DanMONICA and time. P values and CIs estimated by bootstrapping with 1000 repetitions. AF indicates atrial fibrillation; HF, heart failure; hsCRP, high‐sensitivity C‐reactive protein; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The C‐statistic for the model for incident AF was 0.846, whereas the C‐statistic for the model for incident HF was 0.87.
Interim incidence of AF was associated with an increased risk of subsequent incident HF and vice versa (HR, 6.84 [95% CI, 4.89–9.59] [P<0.001]; and HR, 7.05 [95% CI, 4.89–10.16] [P<0.001]). Sex‐stratified analyses are provided in Tables S7 and S8.
Predictors of Subsequent Disease Onset
Age, male sex, BMI, total serum cholesterol, prevalent diabetes mellitus, and log10(NT‐proBNP) were significantly associated with an increased risk of subsequent new onset of both diseases (Table 3). Evaluating the subsequent disease onset on the basis of duration between each diagnosis (within 30 days versus AF before HF outside of 30 days versus HF before AF outside of 30 days) did not lead to relevant changes of these associations. After Bonferroni correction, total serum cholesterol was no longer significantly associated with subsequent disease onset.
Table 3.
HRs for Subsequent Disease Onset of Incident AF and Incident HF
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Age (per 5‐y increase) | 1.80 (1.66/1.96) | <0.001 |
| Sex (men) | 4.41 (3.06/6.36) | <0.001 |
| Body mass index (per 5‐kg/m2 increase) | 1.61 (1.49/1.73) | <0.001 |
| Total serum cholesterol (per 1‐mmol/L increase) | 1.09 (1.02/1.16) | 0.01 |
| Prevalent diabetes mellitus | 1.66 (1.27/2.17) | <0.001 |
| Log10(NT‐proBNP) (per 0.3 increase) | 1.96 (1.85/2.08) | <0.001 |
The following variables were also fitted into, but then dropped from, the Cox regression model: cholesterol‐lowering medication, daily consumption of alcohol, systolic blood pressure, heart rate, antihypertensive medication, smoking status, prevalent myocardial infarction, prevalent stroke, hsCRP (high‐sensitivity C‐reactive protein), and estimated glomerular filtration rate. Adjustment for cohort stratification was implemented. After Bonferroni correction to account for multiple testing, a P value threshold of <0.0022 was used. Additional interaction terms were added to improve model fit: age and sex and log10(NT‐proBNP) and time. AF indicates atrial fibrillation; HF, heart failure; HR, hazard ratio; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The C‐statistic for the model for subsequent disease onset was 0.91.
Predictors of All‐Cause Mortality
During the follow‐up, 9221 (15.7%) individuals died with a median time to death of 12.7 (IQR, 7.2–18.1) years. The results of the multivariable‐adjusted Cox regression analysis for the end point of all‐cause mortality are shown in Figure 1. The incidence of either disease was significantly associated with all‐cause mortality, whereas this association was stronger for incident HF than incident AF (AF: HR, 2.29 [95% CI, 2.04–2.58] [P<0.001]; and HF: HR, 10.95 [95% CI, 8.92–13.44] [P<0.001]). Subsequent new onset of both diseases further increased mortality risk (HR, 13.10 [95% CI, 10.13–16.93] [P<0.001] compared with individuals without AF and HF). All associations remained statistically significant after Bonferroni correction.
Figure 1. Hazard ratios of cardiovascular risk factors, circulating biomarkers, incident diseases, and subsequent disease onset for all‐cause mortality.

The following variables were fitted into the Cox regression model as independent predictors: age, sex, body mass index, total serum cholesterol, cholesterol‐lowering medication, daily consumption of alcohol, systolic blood pressure, heart rate, antihypertensive medication, smoking status, diabetes mellitus, prevalent myocardial infarction, prevalent stroke, log10(NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]), hsCRP (high‐sensitivity C‐reactive protein), estimated glomerular filtration rate, incident atrial fibrillation (AF), incident heart failure (HF), subsequent disease onset, and cohort stratification. After Bonferroni correction to account for multiple testing, a P value threshold of <0.0021 was used. Additional interaction terms were added to improve model fit: age and sex, systolic blood pressure and age, diabetes mellitus and age, log10(NT‐proBNP) and age, log10(NT‐proBNP) and log10(NT‐proBNP), hsCRP and age, hsCRP and hsCRP, HF and age, HF and sex, sequential disease onset and sex, FINRISK and AF, age and time, and northern Sweden and time.
The C‐statistic for the model for all‐cause mortality was 0.82.
Population‐Attributable Risks
PAR for 5‐year incidence of AF or HF based on common cardiovascular risk factors and circulating biomarkers is shown in Figure 2 (Table S11). The overall PAR of common cardiovascular risk factors was 30.9% for incident AF and 75.6% for incident HF. After Bonferroni correction, the PAR of BMI for AF and HF was no longer significantly different, whereas the differences between the PAR of other cardiovascular risk factors for AF and HF remained statistically significant.
Figure 2. Bar chart showing the population‐attributable risks (PARs) for 5‐year incidence for atrial fibrillation (AF) or heart failure (HF) on common cardiovascular risk factors.
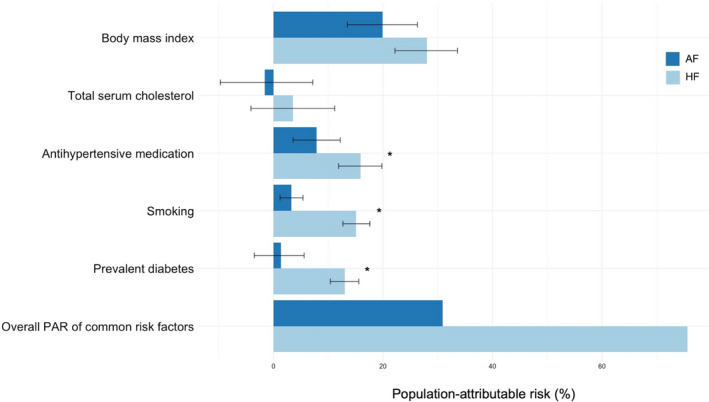
Error bars represent 95% CIs. P values and CIs estimated by bootstrapping with 1000 repetitions. Variables with a statistically significant difference after Bonferroni correction (P<0.01) of the PAR between both diseases are marked with an asterisk (*).
Discussion
In this pooled analysis of European population‐based cohorts, incident AF and HF showed different associations with common cardiovascular risk factors. The association was generally stronger with incident HF, and no risk factor was exclusively associated with incident AF. Obesity demonstrated the highest attributable risk for both diseases. In general, PAR was higher for incident HF, with >70% of the PAR explained for HF. Among the tested biomarkers, NT‐proBNP showed a strong association with both incident diseases. The incidence of both diseases was predictive of mortality. The highest risk of all‐cause death was observed with subsequent onset of both diseases. The present study extends prior knowledge by showing that the associations of common cardiovascular risk factors and incident AF and HF appear to differ in strength.
Association of Common Cardiovascular Risk Factors With Incident AF and HF
Approximately 5% of individuals in our study spanning a large age range developed AF or HF, with ≈2% showing subsequent onset of both diseases. Common cardiovascular risk factors showed different associations with both incident diseases in multivariable‐adjusted Cox regression models. Only 4 cardiovascular risk factors revealed comparable associations with both diseases, whereas no variable was exclusively associated with incident AF. The directions of the associations were in line with previous reports.12, 20, 21 The association of diabetes mellitus with AF has remained inconsistent, whereas a strong association with incident HF is known.7, 22
A possible explanation for these findings could be that risk factors contribute specifically to distinct pathophysiological pathways. This results in a different impact on the genesis of each disease. As the strength of association appeared to be generally higher for incident HF than for incident AF, it can be assumed that classic risk factor related mechanisms have a greater impact on HF than on AF. Misclassification of events, especially in the case of asymptomatic AF, could also have weakened the associations and may help explain the observed differences.
The strength of association translated into greater attributable risks of each individual risk factor for HF than for AF. Consequently, targeting the modifiable risk factors obesity, cholesterol, arterial hypertension, smoking, and diabetes mellitus seems to be associated with a greater risk reduction for HF than for AF (75.6% versus 30.9%). Among all evaluated modifiable risk factors, higher BMI revealed the highest attributable risk for both incident diseases. The presented data indicate that 19.9% of AF cases and 28.0% of HF cases in this population could be attributed to a BMI of ≥25 kg/m2. Our findings are consistent with previous studies reporting a strong association of overweight/obesity with incident AF and HF.23, 24 Consequently, overweight/obesity should be rigorously targeted by prevention strategies for both diseases.
Circulating Biomarkers
Among circulating biomarkers, NT‐proBNP showed a strong association with both diseases. This observation is in line with previous studies consistently reporting a relationship between NT‐proBNP and AF and HF.11, 25, 26, 27 NT‐proBNP is closely related to left ventricular pressure and myocardial stretch.28 Both are increased as a consequence of HF and a pathogenetic factor for AF.29 In the general population, elevated NT‐proBNP might thus serve as an indicator of cardiac stress, which is related to both, subclinical AF, or HF. It may constitute an early warning sign of patients at high risk of disease manifestation. Although direct targeting of NT‐proBNP is a theoretical approach, this conveys the importance of reducing left ventricular filling pressures (eg, via treatment of arterial hypertension) to decrease the risk of subsequent AF and HF.
hsCRP showed a strong association with incident HF, but not with incident AF. Although previous studies have reported an association of hsCRP and incident AF, the association has remained weak and did not improve risk scores based on common cardiovascular risk factors.11 Similarly, in the current analysis, which included several common cardiovascular risk factors in the multivariable analysis, hsCRP did not reach statistical significance. On the basis of these findings, it can be speculated that inflammation is only a minor driver for AF.
For HF, inflammatory pathways are known to be involved in its pathogenesis, whereas the exact direction of the relationship is incompletely understood.30, 31 The strong association of hsCRP with incident HF indicates a potential role of inflammation‐modulating therapies for prevention at the population level.
Risk of Subsequent Disease Onset and All‐Cause Mortality
Although incident HF was associated with a higher risk of all‐cause death than AF, the highest risk was observed with subsequent disease onset. Either disease showed a high risk for subsequent disease onset. The direction and strength of these associations did not differ if accounting for timing of disease onset. Reports from diseased cohorts show similar increases in disease or premature mortality outcomes with subsequent onset of both diseases.3 The subsequent disease onset may be an indicator of disease deterioration. This would explain the substantially higher mortality risk. Modifiable clinical risk factors, in particular BMI and diabetes mellitus, were observed to be significantly associated with subsequent disease onset. Other variables (eg, antihypertensive medication or smoking) were not related to subsequent disease incidence despite their association with both diseases individually. However, medication at baseline may have been modified after disease onset, and smoking cessation often is a reaction to a cardiac event and baseline smoking status may thus have lost its association.32
Although the exact nature of this finding cannot be explained on the basis of the present analysis, the increased risk of subsequent disease onset highlights the importance of preventive therapies in individuals with recent onset of either disease.
Future Implications
In this analysis, classic modifiable risk factors substantially contributed to the risk of incident AF and HF (Figure 3). As both diseases are associated with a higher risk of mortality, modifiable risk factors should be rigorously targeted in the general population and patients with incident disease. Measures to reduce the risk of either disease should particularly focus on overweight/obesity, as overweight/obesity explained the highest proportion of risk for AF and HF. Furthermore, prevention of diabetes mellitus, smoking, and arterial hypertension seems to provide merit in reducing the risk of incident HF, but only to a minor part in reducing the risk of incident AF. Preventive measures should also include individuals with either disease, as subsequent disease onset was associated with the highest mortality risk.
Figure 3. Common cardiovascular risk factors and biomarkers show different associations with incident atrial fibrillation (AF) and heart failure (HF), their subsequent onset, and death.
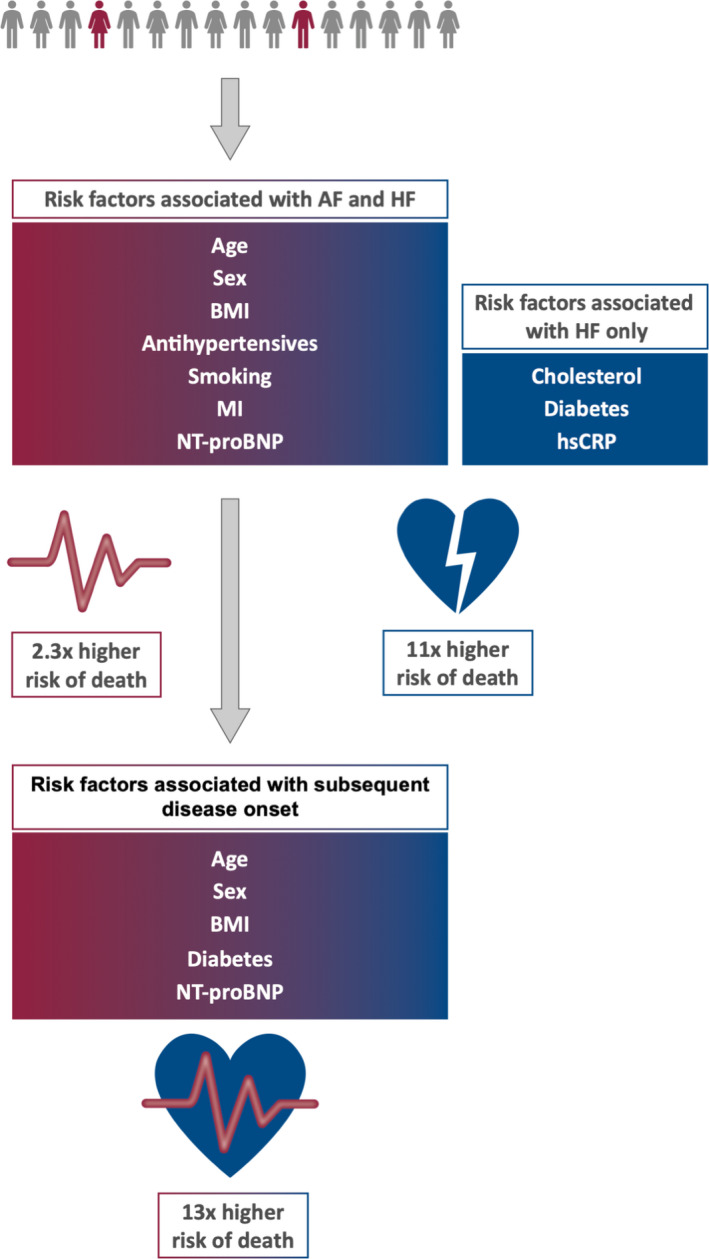
BMI indicates body mass index; hsCRP, high‐sensitivity C‐reactive protein; MI, prevalent myocardial infarction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The strong association of elevated NT‐proBNP with both incident diseases highlights a potential area for future research. Reduction of left ventricular filling pressures, either via treatment of causative diseases (eg, arterial hypertension) or as a direct drug target, could be a promising measure at the general population level to decrease the risk of incident AF or HF. Anti‐inflammatory interventions might have merits as preventive measures for HF because of the strong association of hsCRP with incident HF.
Limitations
Information on distinct AF and HF characteristics during the follow‐up cannot be provided, as data on these outcomes were derived from ICD‐10–based healthcare registries. In addition, these secondary data might have varying quality, which could have impacted the results. Some variables, including baseline AF and HF, were self‐reported. Thus, misclassification may have occurred. Furthermore, asymptomatic cases might have been missed more easily for AF than for HF. A significant bias caused by misclassification would have led to a more homogeneous shift of the associations when comparing AF and HF. However, our results predominantly revealed heterogeneous differences in the associations, which are less likely to be explained by mere misclassification. There are no available data on the treatment after incident manifestation of either disease, which might have influenced the results. Consequently, the present finding cannot be used to draw a causal relationship between both diseases and their relation to mortality.
Conclusions
Although AF and HF share pathophysiological similarities and frequently coexist, our data reveal distinct risk profiles that may be related to specific pathophysiological aspects of both diseases. Overall, classic risk factors explain a higher proportion of risk of HF compared with AF. Both disease entities significantly increase the risk of all‐cause mortality, most strongly if both diseases occur together. Consequently, prevention of AF and HF, in particular in individuals in whom one disease has occurred already, needs to be addressed rigorously. Modifiable traditional risk factors, such as overweight and obesity, hypertension, and diabetes mellitus, provide opportunities for interventions.
Sources of Funding
This project has received funding from the European Research Council under the European Union's Horizon 2020 Research and Innovation Program (grant 648131), German Ministry of Research and Education (BMBF 01ZX1408A), and German Research Foundation Emmy Noether Program (SCHN 1149/3‐1) (to Dr Schnabel). Dr Schrage is funded by the German Research Foundation. Additional funding was provided to Dr Schnabel (81Z1710103) and Dr Zeller (81Z1710101) by the German Center for Cardiovascular Research. The Biomarker for Cardiovascular Risk Assessment in Europe Project was funded by the European Union Seventh Framework Program (FP7/2007–2013) under grant HEALTH‐F2‐2011 to 278913. The Monica Risk, Genetics, Archiving, and Monograph (MORGAM) collaboration was funded by the European Commission Seventh Framework Program, reference FP7/2007–2013 (HEALTH‐F4‐2007‐2014113, European Network for Genetic and Genomic Epidemiology [ENGAGE]; HEALTH‐F3‐2010‐242244, Consortium on Health and Ageing: Network of Cohorts in Europe and United States [CHANCES]). A part of the biomarker determinations in the population cohorts was funded by the Medical Research Council London (G0601463, identification 80983: Biomarkers in the MORGAM Populations). The FINRISK surveys were mainly funded by budgetary funds of the National Institute for Health and Welfare, Finland (THL). Additional funding has been obtained from numerous nonprofit foundations. Dr Salomaa (principal investigator) has been supported by the Finnish Foundation for Cardiovascular Research and the Academy of Finland (grant 139635). Dr Niiranen has been funded by the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Paavo Nurmi Foundation, and the Academy of Finland (grant 321351). The DanMONICA cohorts at the Research Center for Prevention and Health were established over a period of 10 years and have been funded by numerous sources that have been acknowledged, where appropriate, in the original articles. The Moli‐sani study was partially supported by research grants from Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (Rome, Italy)–Programma Triennale di Ricerca, Decreto n.1588 and Instrumentation Laboratory (Milan, Italy). The northern Sweden MONICA project was supported by Norrbotten and Västerbotten County Councils. Dr Söderberg has been supported by the Swedish Heart‐Lung Foundation (grants 20140799, 20120631, and 20100635), the County Council of Västerbotten (VLL‐548791), and Umeå University. The Scottish Heart Health Extended Cohort was funded by the Scottish Health Department Chief Scientist Organization, the British Heart Foundation, and the FP Fleming Trust.
Disclosures
Dr Schrage reports lecture fees from AstraZeneca (unrelated to the present study). Dr Schnabel reports consulting and lecture fees from BMS/Pfizer (unrelated to the present study). Dr Salomaa has participated in a conference trip sponsored by Novo Nordisk and received a honorarium for participating in an advisory board meeting. He also has research collaboration with Bayer Ltd (unrelated to the present study). Dr Söderberg reports consulting and lecture fees from Actelion Pharmaceuticals Ltd (unrelated to the present study). Dr Hughes has recently taken up a position with Novartis RWE but is working on research unrelated to the present study. Dr Koenig reports personal fees (consulting) from AstraZeneca, Novartis, Pfizer, The Medicines Company, DalCor, Kowa, and Amgen; personal fees (honorarium for lectures) from AstraZeneca, Sanofi, and Berlin‐Chemie; grants and nonfinancial support (provision of reagents for biomarker measurements free of charge) from Roche Diagnostics, Beckmann, Singulex, and Abbott, outside the submitted work. Dr Blankenberg reports honoraria from Abbott, Siemens, Thermo Fisher, and Roche, outside of the submitted work. Dr Magnussen has received lecture fees from AstraZeneca, Novartis and Loewenstein Medical Technology (unrelated to the current project). The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S11
Reference 29
(J Am Heart Assoc. 2020;9:e015218 DOI: 10.1161/JAHA.119.015218.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Chiang C‐E, Naditch‐Brûlé L, Murin J, Goethals M, Inoue H, O'Neill J, Silva‐Cardoso J, Zharinov O, Gamra H, Alam S, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real‐life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5:632–639. [DOI] [PubMed] [Google Scholar]
- 2. van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, Maggioni AP, Voors AA. Co‐morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16:103–111. [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 4. Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath‐Ordoubadi F, Neyses L. A meta‐analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676–683. [DOI] [PubMed] [Google Scholar]
- 5. Aronson D, Mutlak D, Bahouth F, Bishara R, Hammerman H, Lessick J, Carasso S, Dabbah S, Reisner S, Agmon Y. Restrictive left ventricular filling pattern and risk of new‐onset atrial fibrillation after acute myocardial infarction. Am J Cardiol. 2011;107:1738–1743. [DOI] [PubMed] [Google Scholar]
- 6. Nerheim P, Birger‐Botkin S, Piracha L, Olshansky B. Heart failure and sudden death in patients with tachycardia‐induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–252. [DOI] [PubMed] [Google Scholar]
- 7. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens ACJW, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF Consortium. J Am Heart Assoc. 2013;2:e000102 DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Kritchevsky SB, Wilson PWF, Newman AB, Harris TB, et al; Cardiovascular Health Study . Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circ Heart Fail. 2010;3:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 10. Wang TJ, Parise H, Levy D, D'Agostino RB, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 11. Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, et al. B‐type natriuretic peptide and C‐reactive protein in the prediction of atrial fibrillation risk: the CHARGE‐AF Consortium of community‐based cohort studies. Europace. 2014;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall‐Pedoe H, Kuulasmaa K, Yarnell J, Schnabel RB, Wild PS, et al. Contribution of 30 biomarkers to 10‐year cardiovascular risk estimation in 2 population cohorts. Circulation. 2010;121:2388–2397. [DOI] [PubMed] [Google Scholar]
- 14. Zeller T, Hughes M, Tuovinen T, Schillert A, Conrads‐Frank A, den Ruijter H, Schnabel RB, Kee F, Salomaa V, Siebert U, et al. BiomarCaRE: rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur J Epidemiol. 2014;29:777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Evans A, Salomaa V, Kulathinal S, Asplund K, Cambien F, Ferrario M, Perola M, Peltonen L, Shields D, Tunstall‐Pedoe H, et al. MORGAM (an international pooling of cardiovascular cohorts). Int J Epidemiol. 2005;34:21–27. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, Zhang Y (Lucy), Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Särndal CE, Swensson B, Wretman J. Model Assisted Survey Sampling. New York: Springer Series in Statistics; 1992:694. [Google Scholar]
- 18. Efron B. Bootstrap methods: another look at the jack knife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 19. R core team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 20. Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang W, Zhang Z, Latini R, Masson S, Agabiti N, Sever P, et al. Risk for incident heart failure: a subject‐level meta‐analysis from the heart “OMics” in AGEing (HOMAGE) Study. J Am Heart Assoc. 2017;6:e005231 DOI: 10.1161/JAHA.116.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 22. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berkovitch A, Kivity S, Klempfner R, Segev S, Milwidsky A, Erez A, Sabbag A, Goldenberg I, Sidi Y, Maor E. Body mass index and the risk of new‐onset atrial fibrillation in middle‐aged adults. Am Heart J. 2016;173:41–48. [DOI] [PubMed] [Google Scholar]
- 24. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. J Am Coll Cardiol. 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 25. Choi E‐Y, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida ALC, Yoneyama K, Opdahl A, Jain A, et al. N‐terminal pro‐B‐type natriuretic peptide, left ventricular mass, and incident heart failure. Circ Heart Fail. 2012;5:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goya Wannamethee S, Welsh P, Whincup PH, Lennon L, Papacosta O, Sattar N. N‐terminal pro brain natriuretic peptide but not copeptin improves prediction of heart failure over other routine clinical risk parameters in older men with and without cardiovascular disease: population‐based study. Eur J Heart Fail. 2014;16:25–32. [DOI] [PubMed] [Google Scholar]
- 27. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation. Circulation. 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mair J, Hammerer‐Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med. 2001;39:571–588. [DOI] [PubMed] [Google Scholar]
- 29. De Jong AM, Van Gelder IC, Vreeswijk‐Baudoin I, Cannon MV, Van Gilst WH, Maass AH. Atrial remodeling is directly related to end‐diastolic left ventricular pressure in a mouse model of ventricular pressure overload. PLoS One. 2013;8:e72651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BHC, Witteman JCM. C‐reactive protein and risk of heart failure: the Rotterdam Study. Am Heart J. 2006;152:514–520. [DOI] [PubMed] [Google Scholar]
- 31. Van Linthout S, Tschöpe C. Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scholte op Reimer W, de Swart E, De Bacquer D, Pyörälä K, Keil U, Heidrich J, Deckers JW, Kotseva K, Wood D, Boersma E. Smoking behaviour in European patients with established coronary heart disease. Eur Heart J. 2006;27:35–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S11
Reference 29


