Abstract
Background
Cardiovascular health (CVH) disparities between blacks and whites have persisted in the United States for some time, and although there have been remarkable improvements in addressing cardiovascular disease, it still remains the leading cause of death in the United States. In addition, well‐documented disparities are unfortunately widening incidence gaps across certain regions of the United States. Our focus was on answering the following questions: (1) How much spatial heterogeneity exists in the racial differences in CVH between blacks and whites across this country? and (2) Is the spatial heterogeneity in the racial differences significantly explained by living in the Stroke Belt?
Methods and Results
To explore the spatial patterning in the racial differences in CVH between blacks and whites across the country, we used geographically weighted regression methods, which result in local estimates of the racial differences in CVH. Using data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study, we found significant spatial patterning in these racial differences, even beyond the well‐known Stroke Belt and Stroke Buckle. All of the estimated differences indicated blacks consistently having diminishing CVH compared with whites, where this difference was largely noted in pockets of the Stroke Belt and Stroke Buckle, in addition to moderate to large disparities noted in the Great Lakes region, portions of the Northeast, and along the West coast.
Conclusions
Efforts to improve CVH and ultimately reduce disparities between blacks and whites require culturally competent methods, with a strong focus on geography‐based interventions and policies.
Keywords: biostatistics, cardiovascular outcomes, disparities, geographically weighted regression, regression, spatial patterning
Subject Categories: Race and Ethnicity, Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- CVD
cardiovascular disease
- CVH
cardiovascular health
- GWR
geographically weighted regression
- MESA
Multi‐Ethnic Study of Atherosclerosis
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
Clinical Perspective
What Is New?
Geographic patterning of black‐white differences in cardiovascular health (CVH) in this country was examined using the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study.
Spatial heterogeneity between blacks and whites and their CVH is prevalent, even beyond the well‐known Stroke Belt and Stroke Buckle.
Large racial differences in CVH persist in this country even after considering residency in the Stroke Belt and Stroke Buckle, and across various regions of the country, blacks consistently have diminishing CVH compared with whites.
What Are the Clinical Implications?
Efforts to improve CVH and reduce disparities between blacks and whites require culturally competent, geographically based interventions and policies.
Noted regions of the country that are beyond the well‐known Stroke Belt and Stroke Buckle with disparities in CVH should be examined further.
Cardiovascular health (CVH) disparities between blacks and whites have persisted in the United States for some time.1 And, although there have been remarkable improvements in addressing cardiovascular disease (CVD), it still remains the leading cause of death in the United States, affecting 85.6 million Americans and accounting for 1 in every 6 healthcare dollars spent, with well‐documented disparities unfortunately widening incidence gaps across certain regions of the United States. The idea of CVH, as defined by the American Heart Association, relies on the foundation of primordial prevention, evidence that CVD and risk factors for it often develop early in life, and the appropriate balance between population‐level approaches for health promotion and disease prevention and individualized high‐risk approaches.2 Although only 17% of adults in this country meet the concept of ideal CVH, there remains a disproportionate burden in the prevalence and control of CVH health factors and risks for certain racial/ethnic groups, specifically non‐Hispanic black adults.3 And, in addition to these racial disparities in CVH, geographic disparities in CVH persist across this country.
Previous research has shown the significant racial/ethnic differences for many of the indicators of ideal CVH. Focusing on adults in the ARIC (Atherosclerosis Risk in Communities) study, findings suggested that the prevalence of ideal health behaviors was lower in blacks than whites for smoking (68.5% versus 73.5%); physical activity (22.0% versus 42.8%); and diet (4.4% versus 5.6%).4 Another study, the MESA (Multi‐Ethnic Study of Atherosclerosis), found that black‐white differences were significant for all ideal health factors, most behaviors, and all ideal CVH summary measures examined.5 Although these studies produced the evidence necessary to understand the black‐white differences in CVH, there was a lack of consideration as to the geographical patterning of these differences across the United States.
Some evidence of regional disparities has been documented, with, again, a focus on the individual components of CVH, with differences between blacks and whites highlighted. For instance, there exists within‐group geographic variations in hypertension prevalence among blacks and whites,6 where the associated findings also suggested evidence of substantial heterogeneity in black‐white differences, depending on which geographic groups were compared. Regions of the country like the Stroke Belt, which includes the 8 states of Alabama, Arkansas, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee, and the Stroke Buckle, which includes the coastal plains of North Carolina, South Carolina, and Georgia, experience unusually high incidence of stroke and other forms of CVD,7, 8, 9 leading to poor overall CVH. Other studies have focused on geographical differences in, say, the prevalence of obesity, metabolic syndrome, and diabetes mellitus10; obesity and physical activity11; and even smoking.12 Although these studies highlight the importance of geography and how it can help to often explain some of the racial disparities between blacks and whites, these studies examine these geographic disparities solely on the basis of the individual components of CVH.
Furthermore, to fully understand the geography of the racial differences in CVH, both exploratory and inferential spatial data analysis methods are required.13 These methods range from the use of choropleth maps and autocorrelation statistics to visualize and objectively assess patterns, respectively, to regression‐based approaches, like geographically weighted regression (GWR) methods14 and hierarchical modeling,15 in both traditional frequentist and Bayesian statistical frameworks. Many of these approaches blend both subjective and objective measures of spatial patterning to gain an understanding of varying relationships, and their validity has been proved in many different contexts.
Our goal was to explore the geographic patterning in the black‐white disparities in CVH, using a national, population‐based cohort found in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. This approach drills down below the common state‐ and county‐level based studies, which may mask the true underlying differences between blacks and whites and how these differences vary by region. In addition, this research focuses on overall CVH, as opposed to the individual metrics for CVH or even simply the presence/absence of CVD. Our focus was on answering the following questions: (1) How much spatial heterogeneity exists in the racial differences in CVH between blacks and whites across this country? and (2) Is the spatial heterogeneity in the racial differences significantly explained by living in the Stroke Belt? In answering these questions, we hope to further understand these differences, find where the disparities in CVH are the greatest in this country, and ultimately aid in creating more evidence‐based policies and interventions targeted to eliminating these disparities and improving overall health.
Methods
The data underlying the findings from this research include potentially identifying participant information and cannot be made publicly available because of ethical/legal restrictions. However, data, including statistical code, from this article are available to researchers through the first author's GitHub page.16 Data can be obtained on request through the University of Alabama at Birmingham at regardsadmin@uab.edu.
Study Population
The REGARDS Study is a national, population‐based, longitudinal study of 30 239 black and white adults aged ≥45 years. The primary objective of the REGARDS Study is to determine the causes for the excess stroke mortality in the southeastern United States compared with the rest of the United States, and among blacks compared with whites. Participants were recruited between 2003 and 2007 by mail, then underwent an extensive telephone interview, during which data on stroke risk factors and sociodemographic, lifestyle, and psychosocial characteristics were collected. Written informed consent, physical and physiological measures, and fasting blood samples were collected during a subsequent in‐home visit; furthermore, data on dietary habits were collected via self‐report on a form left with participants during the in‐home visit, and subsequently returned to the coordinating center via mail. Participants are followed up via telephone at 6‐month intervals for identification of stroke events and myocardial infarctions. Details of the study objectives and design are provided elsewhere.17 The sample for our research will consist of all REGARDS Study participants for whom geocoding was successful. Our final analytic data set included 17 889 participants with complete data on CVH, as well as individual‐level characteristics. Although REGARDS Study participants resided in each of the 48 contiguous United States and the District of Columbia, the number of REGARDS Study participants in each state ranged from 6 participants residing in Vermont to 2216 participants residing in South Carolina.
Study Variables
Cardiovascular Health
We used the American Heart Association's definition of ideal CVH, which is defined as the presence of both ideal health behaviors (nonsmoking, body mass index <25 kg/m2, physical activity at goal levels, and pursuit of a diet consistent with current guideline recommendations) and ideal health factors (untreated total cholesterol <200 mg/dL, untreated blood pressure <120/<80 mm Hg, and fasting blood glucose <100 mg/dL). Smoking status was self‐reported by participants and characterized as never, former, or current smoker; body mass index was computed via clinically measured height and weight during the in‐home study visit. Physical activity was self‐reported by participants, who answered the following question, “How many times per week do you engage in intense physical activity, enough to work up a sweat?”; participants answered the question with response options of 0, 1 to 3, or 4 or more times per week. Diet was measured using a self‐administered Block 98 Food Frequency Questionnaire,18 where food intake was recorded. Cholesterol and fasting blood glucose were measured using blood samples collected during the in‐home study visits. Blood pressure was assessed during the in‐home study visit, and the average of 2 readings was used. Self‐reported medication was also considered for the use of antihypertensive, glucose‐lowering, and lipid‐lowering medications.
Each health behavior and factor was assigned a score of 1, 2, or 3 to denote the behavior/factor as poor, intermediate, or ideal, respectively (Table 1). The total CVH score, our outcome of interest, was computed for each participant as the sum of all health behavior and factor scores and ranged from 7 to 21. A total score of 7 to 11 indicates overall inadequate CVH, 12 to 16 indicates average CVH, and 17 to 21 indicates optimal CVH, similar to previous studies that have examined the total CVH score within the REGARDS Study cohort.19, 20
Table 1.
CVH Score Components for all REGARDS Study Participants and by Race/Ethnicity
| Variable | % of All REGARDS Study Participants (N=17 889) | % of Black REGARDS Study Participants (N=5811) | % of White REGARDS Study Participants (N=12 078) |
|---|---|---|---|
| Demographic | |||
| Age, mean (SD), y | 64.6 (9.2) | 63.3 (8.9) | 65.3 (9.31) |
| Sex, n (%) | |||
| Men | 7827 (43.6) | 1934 (33.3) | 5893 (48.8) |
| Women | 10 062 (56.2) | 3877 (66.7) | 6185 (51.2) |
| Region | |||
| Stroke Belt | 10 160 (56.8) | 3052 (52.5) | 7108 (58.9) |
| Non–Stroke Belt | 7729 (43.2) | 2759 (47.5) | 4970 (41.1) |
| CVH Component | Score | Definition | |||
|---|---|---|---|---|---|
| Physical activity | 1 | No exercise | 32.3 | 35.9 | 30.5 |
| 2 | 1–149 min of moderate exercise or 1–74 min of vigorous exercise/week | 37.1 | 37.8 | 36.7 | |
| 3 | ≥150 min of moderate exercise or ≥75 min of vigorous exercise/week | 30.7 | 26.4 | 32.7 | |
| Diet | 1 | 0–1 components of healthy diet | 80.1 | 83 | 78.7 |
| 2 | 2–3 components of healthy diet | 19.8 | 16.9 | 21.2 | |
| 3 | 4–5 components of healthy diet | 0 | 0 | 0 | |
| Blood sugar | 1 | ≥126 mg/dL fasting | 9.7 | 13.9 | 7.7 |
| 2 | 100–125 mg/dL fasting or treated to <100 mg/dL | 26.8 | 31.6 | 24.5 | |
| 3 | <100 mg/dL fasting, unmedicated | 63.5 | 54.5 | 67.8 | |
| Blood pressure | 1 | SBP ≥140 mm Hg or DBP ≥ mm Hg | 20.9 | 27.4 | 17.8 |
| 2 | SBP 120–139 mm Hg or DBP 80–89 mm Hg or treated to <120/80 mm Hg | 59.6 | 62.2 | 58.4 | |
| 3 | <120/80 mm Hg, unmedicated | 19.5 | 10.5 | 23.8 | |
| BMI | 1 | ≥30 kg/m2 | 36.1 | 48.9 | 30 |
| 2 | 25.0–29.99 kg/m2 | 37.9 | 33.8 | 39.9 | |
| 3 | <25.0 kg/m2 | 26 | 17.3 | 30.2 | |
| Cholesterol | 1 | ≥240 mg/dL | 11.7 | 12.4 | 11.4 |
| 2 | 200–239 mg/dL or treated to <200 mg/dL | 53.7 | 50.8 | 55.1 | |
| 3 | <200 mg/dL, unmedicated | 34.5 | 36.8 | 33.4 | |
| Smoking | 1 | Current smoker | 13.7 | 17.3 | 11.9 |
| 2 | Former smoker, quit ≤12 mo ago | 1.8 | 2.2 | 1.6 | |
| 3 | Never smoker or quit >12 mo ago | 84.6 | 80.5 | 86.5 | |
| CVH score | 7–11 | Inadequate | 7.3 | 11.5 | 5.3 |
| 12–16 | Average | 75.1 | 79.5 | 73 | |
| 17–21 | Optimal | 17.6 | 9.1 | 21.7 |
Both races are non‐Hispanic; all comparisons between black and white REGARDS Study participants are statistically significant (P<0.05). BMI indicates body mass index; CVH, cardiovascular health; DBP, diastolic blood pressure; REGARDS, Reasons for Geographic and Racial Differences in Stroke; and SBP, systolic blood pressure.
Statistical Analysis
Descriptive statistics of the CVH behaviors and factors were examined for all REGARDS Study participants (N=17 889), and by race/ethnicity, via counts with percentages. In addition, we examined the distribution of CVH by region of the country, with a focus on participants residing in the Stroke Belt (N=10 160) versus the remainder of the United States (N=7729).
To explore the spatial patterning in the racial differences in CVH within and between blacks and whites across the country, we used GWR methods.21 GWR allows the estimated difference in CVH between blacks and whites to vary spatially, and provides a local approximation, as opposed to a global, for examination of these disparities. For each REGARDS Study participant (i=1, …, N), we assumed the following model for their total CVH score:
where (ui, vi) denotes the coordinates of participant i's home address, β0i is the intercept, and βpi is the estimated effect of explanatory variable Xpi. First, we considered race‐stratified GWR models, to simply examine the variation of CVH in blacks and whites separately. These models included an adjustment for age (years) and sex (male, female). Next, to assess the spatial heterogeneity in the black‐white differences in CVH, we fit a GWR model that included the main effect of race, with an additional adjustment for age and sex, model 1. To additionally assess the impact of living in the Stroke Belt compared with the remainder of the United States, we fitted another model that extended model 1 to also include a binary measure that captured this effect. Furthermore, to obtain the GWR estimates, we used adaptive bandwidths, as opposed to a fixed bandwidth. Because the locations of the REGARDS Study participants are not regularly spaced in a grid‐like manner across the United States, but are clustered in certain regions, even within a given state, the adaptive bandwidth has the ability to increase when the sample points are sparser and decrease when the sample points are denser22 thus, this approach makes sense in the context of our data.
The GWR approach results in local estimates of the racial and geographic differences in CVH; therefore, we assessed the minimum, maximum, median, and first and third quartiles of the differences, as well as the count and percentage of statistically significant negative black‐white differences across the United States, indicative of blacks having unfavorable CVH compared with whites. To determine model fit, we conducted an ANOVA F test to examine if the GWR model fit significantly improved over the traditional ordinary least squares model, which assumes a global association between CVH and race across the United States.21, 23 We assessed the Moran's I statistic to test for residual spatial autocorrelation, and we also compared models 1 and 2 to determine the improvement in model fit when additionally adjusting for the effect of living in the Stroke Belt via the corrected Akaike information criterion.24 All analyses were conducted in R25 using the package spgwr.26
To graphically display the spatial heterogeneity in blacks, whites, and the black‐white differences in CVH, we present maps of the estimated GWR differences across the United States. Race‐specific maps show the spatial patterning of CVH within black and white REGARDS Study participants, with a focus on the geographic variability in the range of CVH. In addition, the patterning of the racial differences between total CVH scores for blacks and whites, before (model 1) and after (model 2) adjusting for the effect of living in the Stroke Belt, is presented as well. These 2 maps allow for a visual assessment of the spatial heterogeneity in the black‐white differences in CVH, but also how these differences change once residence in the Stroke Belt is taken into consideration. The smoothed maps were produced via inverse distance weighting interpolation, which is a deterministic, local, exact method used to predict local values identical to the estimated GWR values at the point locations corresponding to participants’ home addresses.27 We also present maps of the GWR‐associated standard errors (SEs) to further visualize the change in the variation in the GWR estimates, specifically how the variability either increases or decreases when model 2 adjusts for living in the Stroke Belt. The resulting maps were produced using the ArcGISPro Geostatistical Wizard tool.28
The REGARDS Study was approved by Institutional Review Boards of each participating institution, and the present study was approved by the Institutional Review Board for Drexel University.
Results
Although a total of 30 239 participants were included in the original REGARDS Study, Figure 1 shows the various exclusions for our analysis to explore the spatial patterning in racial differences in CVH. Specifically, after excluding participants on the basis of anomalies in informed consent (N=56), missing at least one CVH component (N=12 285), and missing address and latitude/longitude measures (N=9), our final analytic data set included 17 889 participants. Among the participants in the final analytic data set, the average age at baseline was 64.6 years (SD=9 years), and 56.2% of the participants were women. Table 1 displays the CVH components and total score categories for the 17 889 REGARDS Study participants included in the analysis and by race. Overall, most participants had an average total CVH score (75.1%), followed by a smaller portion having optimal CVH (17.6%). Although the majority of black and white participants have an average total CVH score, there are significantly larger proportions of whites (21.7%) with an optimal CVH score compared with blacks (9.1%), evidence of racial differences at even the most crude comparison. Similarly, there are significantly larger proportions of white participants with ideal physical activity, blood sugar, blood pressure, body mass index, and smoking, whereas larger proportions of black participants have ideal cholesterol. There are no black or white participants with ideal diet; however, there are significantly greater proportions of black participants (83%) with poor diet compared with whites (78.7%). Because of the purposeful oversampling design of the REGARDS Study, there are greater proportions of participants residing in the 8 states that make up the Stroke Belt (56.8%) compared with the rest of the United States (43.2%).
Figure 1. Flowchart displaying inclusion and exclusion criteria for the examination of the spatial heterogeneity in racial differences in cardiovascular health (CVH) in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study, 2003 to 2007.

To formally assess the spatial patterning in the racial differences in CVH between blacks and whites across the United States, we examined 2 GWR models for the 17 889 REGARDS Study participants. Table 2 displays the results of the estimated differences in total CVH score per unit increase in the independent variables considered, which vary across all of the United States, with a specific focus on race, although our models were adjusted for age and sex, and then additionally for residence in the Stroke Belt. The initial model that did not consider Stroke Belt residence (model 1) resulted in race effects that ranged from as large as −0.846 (minimum GWR estimate) to as small as −0.165 (maximum GWR estimate), which remained indicative of a persistent and significant black‐white difference in total CVH scores. However, when Stroke Belt residence is considered, the range of these disparities reduces, such that the largest difference was noted as −0.552 (minimum GWR estimate) and the smallest difference was −0.239 (maximum GWR estimate). The noticeable shift in the range can be largely attributed to the adjustment of Stroke Belt residency. Of note, the smallest difference between blacks and whites in total CVH scores went from −0.165 in model 1 to −0.239 in model 2, which actually speaks to the underestimation of the true difference between blacks and whites when residency is ignored. Overall, the race effect shows a reduction in total CVH score for blacks compared with whites (model 2, median GWR estimate=−0.455). The region effect displayed differences that also varied, where, although the median estimate was −0.038, suggestive of poorer CVH, the minimum and maximum GWR estimates resulted in both negative and positive effects, respectively. Model 2 (corrected Akaike information criterion=49776.81) showed slight improvements in model fit in capturing the spatial heterogeneity in overall CVH compared with model 1 (corrected Akaike information criterion=49778.12). And, although both Moran's I test statistics indicate residual spatial heterogeneity in models 1 and 2, both models are significant improvements over their corresponding global ordinary least squares models, as indicated by the significant ANOVA F test statistics.
Table 2.
Estimated Differences From Models 1 and 2 in Total CVH Score per Unit Increase in Independent Variables for GWR Models, as Well as Model Fit Statistics for the REGARDS Study Participants (N=17 889)
| Variable | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | First Quartile | Median | Third Quartile | Maximum | Minimum | First Quartile | Median | Third Quartile | Maximum | |
| Intercept | −0.104 | 0.040 | 0.116 | 0.202 | 0.490 | −0.132 | 0.067 | 0.140 | 0.150 | 0.220 |
| Race (black vs white) | −0.846 | −0.524 | −0.462 | −0.403 | −0.165 | −0.552 | −0.493 | −0.455 | −0.453 | −0.239 |
| Age (y) | −0.123 | −0.011 | 0.011 | 0.029 | 0.114 | −0.046 | −0.009 | 0.010 | 0.006 | 0.019 |
| Sex (male vs female) | −0.220 | −0.005 | 0.053 | 0.100 | 0.259 | −0.099 | −0.001 | 0.043 | 0.039 | 0.079 |
| Region (Stroke Belt vs remainder of the United States) | −0.424 | −0.097 | −0.038 | −0.050 | 0.014 | |||||
| Fit statistics | ||||||||||
| ANOVA F | 1.786* | 2.214* | ||||||||
| Moran's I | 0.016* | 0.023* | ||||||||
| AICc | 49778.12 | 49776.81 | ||||||||
| Locations with negative, significant race effects, n (%) | 17 881 (99.96) | 17 889 (100) | ||||||||
AICc indicates corrected Akaike information criterion; CVH, cardiovascular health; GWR, geographically weighted regression; and REGARDS, Reasons for Geographic and Racial Differences in Stroke.
*Statistically significant estimates, P<0.05, where GWR estimates are tested for significant spatial variation as determined by Leung's F3 test.
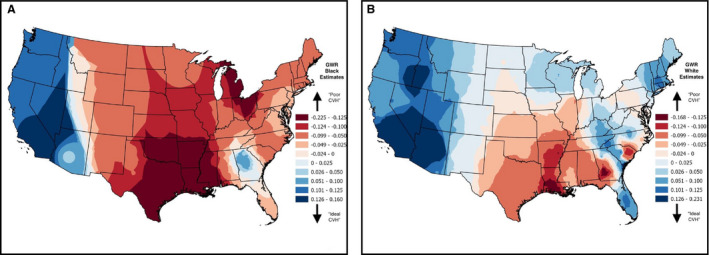
In addition to measuring the racial differences in CVH, we mapped the spatial patterning in total CVH scores for black and white REGARDS Study participants separately in Figure 2, where ideal and poor CVH is indicated by blue and red shades, respectively. Although ideal CVH for blacks is noticeably located in the West in Figure 2A, with particularly improved CVH scores in southern California and Nevada, there is a visible cluster of improved CVH in Georgia, most likely because of the Atlanta metropolitan area in the north‐central part of the state. The central region of the United States, primarily in the Stroke Belt states of Arkansas, Mississippi, and Louisiana, in addition to a significant portion of Texas and Oklahoma have poor CVH for blacks compared with other regions of the country. Parts of Wisconsin, Michigan, and Ohio also display evidence of poor CVH for blacks. Figure 2B shows evidence of improved CVH scores apparent in the West for whites, with improved scores in California, Nevada, Utah, and Arizona, in addition to clusters in the shared border between Oregon and Idaho. The central portion of the United States, including the Midwest, shows evidence of improved CVH for whites. And although the Great Lakes region displayed poor CVH scores for blacks, scores in this region of the country for whites were not as extremely poor, although some states like Illinois, Indiana, and even parts of Pennsylvania still display decreasing CVH scores for whites. Additional clusters of ideal CVH for whites are apparent in the northeast, and parts of Florida. Stroke Belt states of Louisiana, Arkansas, southern Georgia, and the coastal plains of North and South Carolina display the poorest CVH scores for whites in this country. Poorer scores are also noted in other regions and pockets of the South.
Figure 2. Geographically weighted regression (GWR) results of total cardiovascular health (CVH) scores for blacks (A) and whites (B) for the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study participants residing across the United States (NBlack=5811; NWhite=12 078).
To characterize the spatial patterning in these racial differences, Figure 3 displays the black‐white differences for REGARDS Study participants across the country before (model 1, Figure 3A) and after (model 2, Figure 3B) adjusting for residency in the Stroke Belt. For both maps, blue shades indicate smaller differences between blacks and whites, but red shades indicate larger differences. When considering the spatial patterning as a result of model 1 (not including region of residence), there are noticeable clusters of larger differences in the Stroke Belt and Stroke Buckle regions, as expected; however, in some of the same states that are part of the Stroke Belt and Buckle regions, there are also noticeable clusters of smaller differences between blacks and whites. For example, along the coast of the Carolinas, where North and South Carolina intersect, there are relatively smaller racial differences (blue clusters of GWR estimates) compared with the more northern coastal plain of North Carolina and the coastal plain where the borders of Georgia and South Carolina meet (red/orange clusters of GWR estimates). Overall, there are noticeable larger differences between blacks and whites in the eastern portion of the country, outside of the Stroke Belt and Stroke Buckle (eg, Northeast corridor and surrounding the Great Lakes). Once Stroke Belt residency is considered in measuring the racial differences in CVH (model 2), the patterns of black‐white differences in CVH change, as noted in Figure 2B. The variation in these differences is reduced but remains indicative of persistent and significant black‐white differences in total CVH across the country. In the Stroke Belt region, there is a noticeable cluster of disparities for blacks, ranging from −0.2 to −0.4 where Georgia and Alabama intersect (blue clusters of GWR estimates). These smaller differences are also apparent in parts of Texas and the western portion of the country in states like Arizona, Utah, and parts of Idaho and Oregon. In the Stroke Belt, where Louisiana and Mississippi intersect, and the Stroke Buckle, in the Carolinas, the disparities noticeably increase to between −0.5 and −0.6 (red/orange clusters of GWR estimates). There are also patterns of these larger disparities (between −0.5 and −0.6) in the Great Lakes region of the country, with trends extending toward the Midwest, once accounting for region in model 2.
Figure 3. Geographically weighted regression (GWR) results for the effect of race (blacks vs whites) on total cardiovascular health score for the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study participants residing across the United States (N=17 889).
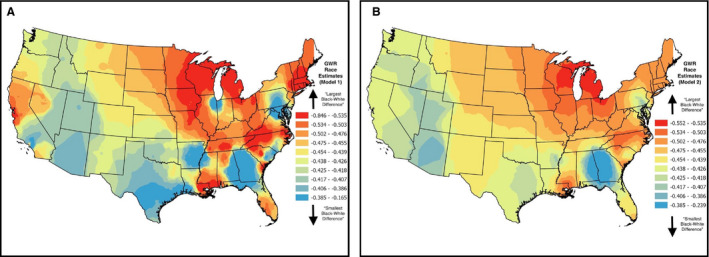
Maps represent model results before (A) vs after (B) controlling for the Stroke Belt region.
We also examined the variation in the GWR race estimates before and after adjusting for residency in the Stroke Belt, and maps of the standard errors (SEs) of these estimates are presented in Figure S1, where larger SEs are shaded pink and smaller SEs are shaded blue. Model 1 resulted in SEs that varied highly within the eastern portion of the country as well as along the western border (Figure S1A). Once we adjusted for Stroke Belt residency in model 2, there were noticeable reductions in SEs across the entire country (Figure S1B). Most of the largest SEs are clustered in the west, specifically in regions of California and Nevada; however, these were still substantially smaller than the SEs resulting from model 1. The smallest SEs are surprisingly located in the Midwest, where the number of REGARDS Study participants are the fewest (North Dakota=18 participants; South Dakota=22; Wyoming=12; and Montana=12); however, the GWR estimates of the race effect in this region are aligned with the overall median GWR of the race effect across the entire country (median GWR estimate=−0.455). Although there were a small number (N=8, all located in Georgia) of the 17 889 GWR estimates that were not statistically significant according to their corresponding t test statistic in model 1, all of the estimates from model 2 were statistically significant.
Discussion
Our study aimed to explore the spatial patterning in the racial differences in CVH between blacks and whites across the United States, and we found significant spatial patterning in these racial differences. All of the estimated differences indicated blacks consistently having diminishing CVH compared with whites, where this difference was largely noted in pockets of the Stroke Belt and Stroke Buckle, in addition to moderate to large disparities noted in the Great Lakes region, portions of the Northeast, and along the West coast. Even after adjusting for Stroke Belt residency, spatial patterning in the racial differences between blacks and whites is persistent across this country. In addition, the spatial heterogeneity present in these racial differences indicates that an overall global measure of racial differences will likely misrepresent the true geographically varying impact of race on CVH.
Similar to previous studies, we found geographic patterning in CVH that highlighted regions of the Southeast, with noted racial differences in the Stroke Belt; however, our findings are specific to overall CVH and not simply the risk factors for CVD. In addition, we found noted racial differences across different regions of the country outside of the Stroke Belt and Stroke Buckle, similar to a recent study that focused on reassessing the Stroke Belt, because evidence of high stroke mortality is extending beyond the 8 states that currently make up the Stroke Belt.29 Our spatial regression method, GWR, allowed for exploration of the racial differences in CVH between blacks and whites, but in a geographically varying, more flexible, approach. Instead of the common methods that result in measuring global racial differences, GWR allows for more informed measurements of this relationship, ultimately aiding in helping to tailor more evidenced‐based interventions and policies related to improving CVH and its associated disparities, with a focus on the local and regional patterns of these differences. Not only did the GWR approach allow for measuring the varying relationship between race and CVH, but use of GWR allowed us to produce maps that visually displayed the varying relationship on the basis of our regression results. These maps highlight geographic trends in the racial differences in CVH, with the added use of maps of the SEs of the GWR estimates, which speak to the statistical uncertainty in our GWR estimates. To fully understand not just the geographically varying racial differences present in CVH between blacks and whites, the SE maps allow for a more objective approach toward determining if the differences estimated themselves are meaningful.
Although our study explores the geographic patterning in the racial differences in CVH between blacks and whites using the national cohort found in the REGARDS Study, there are some noted limitations worth mentioning. Regions of the country outside of the Stroke Belt, particularly in the Midwest and West, have the fewest of REGARDS Study participants; however, even with the sparsity in the REGARDS Study participants living in these regions of the country, there remained significant black‐white differences in CVH, with blacks consistently having lower total CVH scores compared with whites, with varying degrees of these differences noted across these regions. The GWR method we used is powerful in exploring spatial patterning and nonstationarity,21 but has some limitations, including that this method is highly susceptible to the effects of multicollinearity.30 More recently, however, this approach has actually been found to be robust to multicollinearity among explanatory variables, except in the most extreme settings.31
Despite the limitations, our findings have implications in further guiding both local and regional efforts in not only improving overall CVH across the country, but specifically trying to eliminate the racial differences that exist. Recently, evidence suggests that 3 public health interventions that focus on components of overall CVH could save 94 million lives in 25 years. Specifically, these interventions included scaling up treatment of high blood pressure to 70%, reducing sodium intake by 30%, and eliminating the intake of artificial trans fatty acids. The combined effect of these 3 interventions, for example, has been projected to delay 94.3 million deaths during a 25‐year period (2015–2040).32 Although effective interventions like these exist for addressing major CVD risk factors, comprehensive CVH programs are rare. Our findings can help in identifying regions of the country with the greatest needs of similar policies or interventions targeted toward improving overall CVH in this country, all while focusing on the racial and geographic disparities that exist between blacks and whites.
Although our study focused primarily on the geographic patterning of the racial differences in CVH between blacks and whites in the country, we also realize the importance of both individual‐ and neighborhood‐level risk factors that would contribute to, and perhaps help explain, these differences. Not only does spatial patterning potentially exist in either of these types of risk factors, but their relationship with CVH and how these factors contribute to the differences in CVH may also vary across the United States. Because of that, thoroughly examining racial differences in CVH should also include an assessment of the impact of important individual‐ and neighborhood‐level risk factors, all while looking at the varying racial differences to fully disentangle this often‐complex relationship. For example, beyond race and residency, we believe factors like age, income, education, socioeconomic status, and even family history of CVD are important factors to further consider when attempting to explain the geographic patterning of CVH disparities between blacks and whites in this country. In addition, the impact of racism on CVH is vital in completely understanding this complex connection between race and health in this country. Institutional, perceived, and/or internalized racism plays a critical role and should be considered in the context of explaining these disparities in CVH across the United States.33 Evidence suggests that racism, by way of discrimination, leads to unfavorable CVH components, like hypertension,34, 35 coronary artery calcification,36 and obesity.37 One recent study focused on the relationship between reported interpersonal discrimination and CVH in the CARDIA (Coronary Artery Risk Development in Young Adults) study.38 Their findings indicated that black men and women, for example, who experienced discrimination while receiving medical care were associated with lower CVH scores, indicative of poorer CVH. At the neighborhood level, racism through the lens of residential segregation has also been linked to health. Another study that focused on CVD using the MESA cohort found that, among blacks, increases in black segregated neighborhoods were associated with an increased risk of developing CVD.39 Further work is needed to better grasp how racism at both the individual and neighborhood level plays into the broader understanding of the geographic patterning of these black‐white differences in CVH across this country.
Overall, our findings have highlighted the racial and geographic disparities in CVH in this country between blacks and whites, on the basis of a national, population‐based study found in the REGARDS Study. Our research shows that blacks consistently have significantly reduced odds in having ideal CVH, in the Stroke Belt, Stroke Buckle, and beyond. With a focus on CVH, as opposed to the individual metrics of CVH or even simply the presence/absence of CVD, our novel approach provides evidence of a more comprehensive assessment of disparities across this country. In addition, with the Healthy People 2020 goal of improving CVH and quality of life, our findings help to identify regions of the country that would best benefit from prevention, detection, and treatment of risk factors for heart attack and stroke. Efforts to improve CVH and ultimately reduce disparities between blacks and whites require culturally competent methods, with a strong focus on geography‐based interventions and policies.
Sources of Funding
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Additional funding was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award K01HL133515. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Figure S1
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study for their valuable contributions. A full list of participating REGARDS Study investigators and institutions can be found at http://www.regardsstudy.org. We also thank Steve Melly for the production of the maps.
(J Am Heart Assoc. 2020;9:e016556 DOI: 10.1161/JAHA.120.016556.)
For Sources of Funding and Disclosures, see pages 10, 11.
References
- 1. Roth GA, Johnson CO, Abate KH, Abd‐Allah F, Ahmed M, Alam K, Alam T, Alvis‐Guzman N, Ansari H, Ärnlöv J. The burden of cardiovascular diseases among US states, 1990–2016. JAMA Cardiol. 2018;3:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF. Defining and setting national goals for cardiovascular health promotion and disease reduction the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mujahid MS, Moore LV, Petito LC, Kershaw KN, Watson K, Roux AVD. Neighborhoods and racial/ethnic differences in ideal cardiovascular health (the multi‐ethnic study of atherosclerosis). Health Place. 2017;44:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kershaw KN, Roux AVD, Carnethon M, Darwin C, Goff DC, Post W, Schreiner PJ, Watson K. Geographic variation in hypertension prevalence among blacks and whites: the multi‐ethnic study of atherosclerosis. Am J Hypertens. 2010;23:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borhani NO. Changes and geographic distribution of mortality from cerebrovascular disease. Am J Public Health Nations Health. 1965;55:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci. 1999;317:160–167. [DOI] [PubMed] [Google Scholar]
- 9. Lanska DJ, Kuller LH. The geography of stroke mortality in the United States and the concept of a stroke belt. Stroke. 1995;26:1145–1149. [DOI] [PubMed] [Google Scholar]
- 10. Gurka MJ, Filipp SL, DeBoer MD. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutri Diabetes. 2018;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gordon‐Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:417–424. [DOI] [PubMed] [Google Scholar]
- 12. Loop MS, Howard G, de los Campos G, Al‐Hamdan MZ, Safford MM, Levitan EB, McClure LA. Heat maps of hypertension, diabetes mellitus, and smoking in the continental United States. Circ Cardiovasc Qual Outcomes. 2017;10:e003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bivand RS, Pebesma EJ, Gomez‐Rubio V, Pebesma EJ. Applied Spatial Data Analysis With R. New York: Springer; 2008. [Google Scholar]
- 14. Brunsdon C, Fotheringham S, Charlton M. Geographically weighted regression. J R Stat Soc Series D. 1998;47:431–443. [Google Scholar]
- 15. Banerjee S, Carlin BP, Gelfand AE. Hierarchical Modeling and Analysis for Spatial Data. Boca Raton: CRC Press; 2014. [Google Scholar]
- 16. Tabb LP. GitHub. REGARDS.GWR. US. 2020. Available at: https://zenodo.org/badge/latestdoi/254461326.
- 17. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 18. Block G, Woods M, Potosky A, Clifford C. Validation of a self‐administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 19. Garg PK, O'Neal WT, Ogunsua A, Thacker EL, Howard G, Soliman EZ, Cushman M. Usefulness of the American Heart Association's life simple 7 to predict the risk of atrial fibrillation (from the reasons for geographic and racial differences in stroke [REGARDS] study). Am J Cardiol. 2018;121:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fotheringham AS, Brunsdon C, Charlton M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships. England: John Wiley & Sons; 2003. [Google Scholar]
- 22. Charlton M, Fotheringham S, Brunsdon C. Geographically weighted regression. White paper. National Centre for Geocomputation. National University of Ireland Maynooth. 2009.
- 23. Brunsdon C, Fotheringham AS, Charlton M. Some notes on parametric significance tests for geographically weighted regression. J Regional Sci. 1999;39:497–524. [Google Scholar]
- 24. Sakamoto Y, Ishiguro M, Kitagawa G. Akaike Information Criterion Statistics. Dordrecht, the Netherlands: D. Reidel; 1986:81. [Google Scholar]
- 25. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. 2019. Available at: https://www.R-project.org/. [Google Scholar]
- 26. Bivand R, Yu D. spgwr: Geographically Weighted Regression. R package version 0.6‐33. Vienna, Austria. Available at: https://CRAN.R-project.org/package=spgwr.
- 27. ESRI . How inverse distance weighted interpolation works. ESRI; California, US. 2018. Available at: https://www.esri.com/en-us/home. [Google Scholar]
- 28. ArcGIS [ArcGISPro Geostatistical Wizard] . Version 2.3. Redlands, CA: Environmental Systems Research Institute, Inc., 2010.
- 29. Karp DN, Wolff CS, Wiebe DJ, Branas CC, Carr BG, Mullen MT. Reassessing the stroke belt: using small area spatial statistics to identify clusters of high stroke mortality in the United States. Stroke. 2016;47:1939–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wheeler D, Tiefelsdorf M. Multicollinearity and correlation among local regression coefficients in geographically weighted regression. J Geogr Syst. 2005;7:161–187. [Google Scholar]
- 31. Fotheringham AS, Oshan TM. Geographically weighted regression and multicollinearity: dispelling the myth. J Geogr Syst. 2016;18:303–329. [Google Scholar]
- 32. Kontis V, Cobb LK, Mathers CD, Frieden TR, Ezzati M, Danaei G. Three public health interventions could save 94 million lives in 25 years: global impact assessment analysis. Circulation. 2019;140:715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325:315–331. [DOI] [PubMed] [Google Scholar]
- 34. Peters RM. Racism and hypertension among African Americans. West J Nurs Res. 2004;26:612–631. [DOI] [PubMed] [Google Scholar]
- 35. Williams DR, Neighbors H. Racism, discrimination and hypertension: evidence and needed research. Ethn Dis. 2001;11:800–816. [PubMed] [Google Scholar]
- 36. Lewis TT, Everson‐Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton‐Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African‐American women: the Swan Heart Study. Psychosom Med. 2006;68:362–368. [DOI] [PubMed] [Google Scholar]
- 37. Tull S, Wickramasuriya T, Taylor J, Smith‐Burns V, Brown M, Champagnie G, Daye K, Donaldson K, Solomon N, Walker S. Relationship of internalized racism to abdominal obesity and blood pressure in afro‐caribbean women. J Natl Med Assoc. 1999;91:447. [PMC free article] [PubMed] [Google Scholar]
- 38. Bey GS, Person SD, Kiefe C. Gendered Race and Setting Matter: Sources of Complexity in the Relationships Between Reported Interpersonal Discrimination and Cardiovascular Health in the CARDIA Study. J. Racial and Ethnic Health Disparities. 2020;1–11. DOI: 10.1007/s40615-020-00699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Roux AVD. Neighborhood‐level racial/ethnic residential segregation and incident cardiovascular disease: the multi‐ethnic study of atherosclerosis. Circulation. 2015;131:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


