Abstract
Background
We investigated the effects of insulin, glucagon‐like peptide‐1 receptor agonists (GLP‐1RA), sodium‐glucose cotransporter‐2 inhibitors (SGLT‐2i), and their combination on vascular and cardiac function of patients with type 2 diabetes mellitus.
Methods and Results
A total of 160 patients with type 2 diabetes mellitus were randomized to insulin (n=40), liraglutide (n=40), empagliflozin (n=40), or their combination (GLP‐1RA+SGLT‐2i) (n=40) as add‐on to metformin. We measured at baseline and 4 and 12 months posttreatment: (a) perfused boundary region of the sublingual arterial microvessels (marker of endothelial glycocalyx thickness), (b) pulse wave velocity (PWV) and central systolic blood pressure, (c) global left ventricular longitudinal, circumferential, and radial strain, (d) myocardial work index (global work index) derived by pressure‐myocardial strain loops using speckle tracking imaging. Twelve months posttreatment, all patients improved perfused boundary region, PWV, global longitudinal strain, global circumferential strain, and global radial strain (P<0.05). GLP‐1RA, SGLT‐2i, and their combination showed a greater reduction of perfused boundary region, PWV, and central systolic blood pressure than insulin, despite a similar glycosylated hemoglobin reduction (P<0.05). GLP‐1RA or GLP‐1RA+SGLT‐2i provided a greater increase of global work index (12.7% and 17.4%) compared with insulin or SGLT‐2i (3.1% and 2%). SGLT‐2i or GLP‐1RA and SGLT‐2i showed a greater decrease of PWV (10.1% and 13%) and central and brachial systolic blood pressure than insulin or GLP‐1RA (PWV, 3.6% and 8.6%) (P<0.05 for all comparisons). The dual therapy showed the greatest effect on measured markers in patients with left ventricular ejection fraction <55% (P<0.05).
Conclusions
Twelve‐month treatment with GLP‐1RA, SGLT‐2i, and their combination showed a greater improvement of vascular markers and effective cardiac work than insulin treatment in type 2 diabetes mellitus. The combined therapy as second line was superior to either insulin or GLP‐1RA and SGLT‐2i separately.
Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03878706.
Keywords: arterial stiffness, endothelial glycocalyx, glucagon‐like peptide‐1 receptor agonists, left ventricular function, sodium‐glucose cotransporter‐2 inhibitors
Subject Categories: Endothelium/Vascular Type/Nitric Oxide; Imaging; Vascular Disease; Diabetes, Type 2; Atherosclerosis
Nonstandard Abbreviations and Acronyms
- AI
augmentation index
- CAD
coronary artery disease
- GCS
global circumferential strain
- GCW
global constructive work
- GLP‐1RA
glucagon like peptide‐1 receptor agonists
- GLS
global longitudinal strain
- GRS
global radial strain
- GWI
global work index
- GWW
global wasted work
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- PBR
perfused boundary region
- PP
pulse pressure
- PWV
pulse wave velocity
- SGLT‐2i
sodium‐glucose cotransporter‐2 inhibitors
- T2DM
type 2 diabetes mellitus
1.
Clinical Perspective
What Is New?
Twelve‐month treatment with glucagon‐like peptide‐1 receptor agonists, sodium‐glucose cotransporter‐2 inhibitors, and their combination showed a greater improvement of vascular markers and endothelial glycocalyx thickness and a more effective cardiac work than insulin treatment in patients with type 2 diabetes mellitus.
The combined therapy as second line was superior to either insulin or glucagon‐like peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors separately.
The dual treatment had the most favorable effect on metabolic parameters and vascular and myocardial function, particularly in patients with an impaired left ventricular function.
What Are the Clinical Implications?
A combined treatment of glucagon‐like peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors is a promising second‐line therapeutic option in subjects with high or very high cardiovascular risk and poor glycemic control.
2. Introduction
Type 2 diabetes mellitus (T2DM) exacerbates mechanisms of atherosclerosis and heart failure. Thus, patients with T2DM are most often at high and very high cardiovascular disease risk.1 To date, 2 drug classes, glucagon‐like peptide‐1 receptor agonists (GLP‐1RA) and sodium‐glucose cotransporter‐2 inhibitors (SGLT‐2i), have been shown to reduce remarkably the risk of cardiovascular complications, such as myocardial infarction, stroke, and cardiovascular mortality.2 GLP‐1RA exert their beneficial action predominantly through antiatherogenic and anti‐inflammatory mechanisms, whereas SGLT‐2i have an important effect on vascular hemodynamics, raising the possibility that combined therapy with these 2 classes may produce additive or synergistic cardiovascular benefits.3 For that reason, these newer drug classes surpass even metformin as first‐line antidiabetic treatment in latest guidelines.4
However, their effects on endothelial glycocalyx, central blood pressure hemodynamics, and myocardial work have not been fully investigated in humans. Endothelial glycocalyx, a mesh of glycoproteins, proteoglycans, and associated glycosaminoglycans, covers the endothelium and prevents the direct contact of blood cells to the endothelial surface. Damage of glycocalyx integrity has been shown to occur during hyperglycemia.5, 6 Novel techniques have permitted the noninvasive assessment of the sublingual microvascular glycocalyx thickness using dedicated cameras.7
Arterial stiffness, as assessed by pulse wave velocity (PWV), has been found to be elevated in T2DM8 and is associated with impaired left ventricular (LV) myocardial deformation.9 Moreover, myocardial work index is a novel marker that is used to provide additional information about ventricular‐arterial interaction and is derived by pressure–LV longitudinal myocardial strain curves acquired by speckle tracking echocardiography.10
However, it is not clear whether combination of GLP‐1RA and SGLT‐2i has a synergistic favorable effect on endothelial, vascular, and cardiac function.
In the present study of patients with T2DM and high or very high cardiovascular risk, we hypothesized that endothelial glycocalyx, arterial stiffness, and LV myocardial deformation are improved after treatment with combination of GLP‐1RA and SGLT‐2i as a second‐line step, compared with either each one agent or with treatment with insulin. We also hypothesized that despite a similar glycemic control, GLP‐1RA, SGLT‐2i, or their combination will improve the markers of vascular and cardiac function to greater extent than the traditional insulin plus metformin regimen.
Thus, we examined the changes of endothelial glycocalyx thickness, central arterial hemodynamics, PWV, LV global longitudinal strain, and myocardial work index, assessed by speckle tracking echocardiography, before and after 4 and 12 months of treatment in 4 parallel groups of patients with T2DM treated with insulin, GLP‐1RA, SGLT‐2i, and the combination GLP‐1RA and SGLT‐2i for 1 year.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
We examined 180 subjects with T2DM (72% men; mean age, 58 years; range, 27–78 years) who were at high or very high cardiovascular risk. All patients underwent a clinical, vascular, and echocardiography examination at 4 and 12 months after inclusion in the study. All studies were analyzed by 2 observers (G.P. and D.B. for echocardiography studies and J.T. and A.K. for vascular studies), blinded to clinical and laboratory data. High cardiovascular risk patients were considered all subjects with T2DM and a calculated systematic coronary risk estimation ≥5% and <10%, whereas very high cardiovascular risk patients were considered subjects with T2DM and target organ damage or with a major risk factor, such as smoking, marked hypercholesterolemia, or marked hypertension (a calculated systematic coronary risk estimation ≥10%).11 Patients were recruited from the Cardiometabolic outpatient clinic of Attikon hospital outpatient clinic, and they were randomized to receive, as a second‐line treatment after metformin, basal insulin, as previously published,12, 13 1.8 mg of liraglutide once daily (with a weekly dose escalation as instructed by the Summary of Product Characteristics [SPC]) as a subcutaneous injection, 25 mg oral empagliflozin once daily, or their combination (liraglutide plus empagliflozin) for 12 months. In the present study, total daily basal insulin dose ranged between 10 and 50 IU. Basal insulin was titrated according to the standard of the American Diabetes Association and the European Association for the Study of Diabetes to reach fasting plasma glucose target without hypoglycemia.14
Of the 180 participants, 20 did not complete the study protocol. More specifically, in the insulin group, 3 subjects discontinued because of fear of needle puncture and 2 subjects were lost to follow‐up; in GLP‐1RA group, 4 participants discontinued because of nausea, vomiting, and diarrhea, and 1 subject was lost to follow‐up; in SGLT‐2i group, 4 participants discontinued because of urinary tract infections and 1 subject was lost to follow‐up; finally, in combined therapy group, 2 subjects discontinued because of prolonged gastrointestinal symptoms, 2 subjects stopped because of urinary tract infection, and 1 participant was lost to follow‐up.
Exclusion criteria were malignancies, chronic inflammatory disease, chronic kidney disease (estimated glomerular filtration rate <60 mL/min per 1.73 m2 for a period of at least 90 days), liver failure, peripheral vascular disease, and retinopathy. None of the female patients was on hormone replacement treatment. All patients had dyslipidemia treated with statins. Hypertension was defined as clinic brachial blood pressure >140/90 mm Hg or use of antihypertensive medication.
The investigation conforms to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the University General Hospital “Attikon” Institutional Review Board. In addition, all participants gave their written informed consent.
Primary and Secondary End Points
The primary end point was changes in global LV longitudinal, circumferential, radial strain, and myocardial work index after 12‐month treatment with combination of GLP‐1RA and SGLT‐2i compared with either each one agent or with treatment with insulin.
Secondary end points were changes in endothelial glycocalyx thickness, PWV, LV twisting, and untwisting posttreatment with combination of GLP‐1RA and SGLT‐2i compared with either each one agent or treatment with insulin.
Patients and clinicians prescribing the treatment were blinded to the results of the vascular and cardiac function tests.
Blood Pressure Measurement
Each patient rested in a supine position for 10 minutes in a quiet room at 23°C. We measured the brachial blood pressure and the heart rate in the right arm using an automated digital oscillometric sphygmomanometer (TensioMed, Budapest, Hungary). Two sequential measurements separated by 2‐minute interval were obtained, and the mean value was used for statistical analysis.
Endothelial Glycocalyx
The perfused boundary region (PBR) of the sublingual arterial microvessels with a diameter that ranged from 5 to 25 μm was measured using Sidestream Dark Field imaging (Microscan, Glycocheck, Microvascular Health Solutions Inc, Salt Lake City, UT). This technique provides a fast and noninvasive assessment of the endothelial glycocalyx thickness.15 The PBR is the cell‐poor layer that results from the separation between the flowing red blood cell column and plasma on the surface of the vascular lumen. An increased PBR value indicates a deeper penetration of blood cells into the luminal part of the glycocalyx and is a precise marker of reduced glycocalyx thickness.15 The assessment of glycocalyx thickness using dedicated cameras provides measurements of multiple sample sites (>3000 vascular segments of sublingual microvessels) within 3 minutes and has good reproducibility.15 Thus, this technique was proposed as a valid technique to assess endothelial integrity by the European Society of Cardiology Working Group on Peripheral Circulation.7
Central Hemodynamics
We measured the carotid‐femoral PWV, augmentation index (AI), and central aortic pressures (central systolic and diastolic) using tonometry by Complior (Alam Medical, Vincennes, France). Normal values were PWV <10 m/s.16 AI was calculated using the formula: 100×(P2−P1)/PP, where P2 is the late backward systolic wave, P1 is the early forward systolic wave, and PP is the pulse pressure.17
Echocardiography
Studies were performed using a Vivid Ε95 (GE Medical Systems, Horten, Norway) ultrasound system and were digitally stored in a computerized station (EchoPac GE 202, Horten, Norway). All studies were analyzed by 2 observers (G.P. and D.B.), blinded to clinical and laboratory data.
Two‐Dimensional Strain and Strain Rate Analysis
We measured LV global longitudinal strain (GLS; %) and peak diastolic strain rate (global longitudinal strain rate E; 1/s) from 2‐dimensional echocardiography images obtained with a frame rate of 70/s to 80/s, from the apical 4‐, 2‐, and 3‐chamber views using 17 LV segment model and using dedicated software (EchoPac PC 203; GE Healthcare, Horten, Norway).17, 18 The normal value for GLS is reported to be −22.5±2.7%.19 In addition, we measured global circumferential strain (GCS; %) and global radial strain (GRS; %) for the 6 mid‐LV segments of parasternal short axis at the level of papillary muscles, as previously published.18, 19 The normal values for GCS and GRS are considered to be −31.9±4.5% and 37.4±8.4%, respectively.19 The intraobserver and interobserver reproducibility values for LV strain and strain rate parameters were 8% and 9%, respectively.
We also calculated the ratio of carotid‐femoral PWV/global longitudinal strain (m/s%) as an index of ventricular‐arterial interaction, as previously published.10, 20 The ratio had negative values because of negative GLS values. Hence, the more negative the value, the more normal.
Myocardial Work Index
Dedicated software (EchoPac PC 203; GE Healthcare, Horten, Norway) was used to construct pressure–LV longitudinal myocardial strain curves by speckle tracking echocardiography and measure the myocardial global work index (GWI; area under the curve from mitral valve closure to mitral valve opening; mm Hg%).10, 21 The software calculated the constructive (work performed during shortening in systole adding negative work during lengthening in isovolumetric relaxation; global constructive work [GCW]; mm Hg%) and the wasted myocardial work (negative work performed during lengthening in systole adding work performed during shortening in isovolumetric relaxation; global wasted work [GWW]; mm Hg%). The constructive work divided by the sum of constructive and wasted work provides the myocardial global work efficiency (%).21 The interobserver and intraobserver reproducibility values for GWI were ≤8% and ≤9%, respectively. The normal value for GWI is considered to be 1896±308 mm Hg%.21
1. Twisting and Untwisting
LV twisting and untwisting were assessed using parasternal short axis views at basal and apical level.18 Subsequently, twisting‐untwisting rotation and velocity curves along time were constructed (EchoPac PC 203; GE Healthcare). We measured peak twisting (°), peak twisting velocity (°/s), and peak untwisting velocity (°/s) by the respective rotation curves. The interobserver and intraobserver reproducibility values for LV twisting‐untwisting were ≤8% and ≤10%, respectively.
Statistical Analysis
A power analysis was conducted in an initial pilot study to define the minimum sample size as the effects of each 1 of the 4 studied medications on myocardial deformation and LV twisting have not been previously published. The pilot sample included 40 patients recruited in the study and randomized into 4 treatment groups (insulin, liraglutide [GLP‐1RA], empagliflozin [SGLT‐2i], or liraglutide and empagliflozin [GLP‐1RA+SGLT‐2i]) at a ratio of 1:1:1:1. A repeated measures design was conducted with one within‐subject 2‐level factor (time points at 4 and 12 months) and one between‐subject 4‐level factor (treatment groups). Among the primary end points, the percentage change of GLS (ΔGLS%) at 4 and 12 months was the one selected for the purpose of the power analysis (insulin, 5.123% and 5.910%; GLP‐1RA, 5.309% and 11.284%; SGLT‐2i, 4.616% and 2.521%; and GLP‐1RA+SGLT‐2i, 8.07% and 13.822% at 4 and 12 months, respectively).
A 4×2 means matrix with the means of ΔGLS% for the 4 treatment groups in 2 time points was used to compute the effect size, which was 0.27 (SD=1.71). The within‐subject SD and autocorrelation (Rho) were 10.1% and 0.21, respectively, and were calculated from the mean squares of a repeated measures ANOVA table, assuming constant autocorrelation model. To achieve 80% power to test the treatment×time interaction, the required sample size was 160 cases (40 cases in each treatment group) if a Geisser‐Greenhouse corrected F test was used with a 5 significance level.22 The analysis was conducted with the dedicated software PASS v.11 (©2011 NCSS, LLC, http://www.ncss.com).
Statistical analysis was performed using the SPSS 22.0 statistical software package (SPSS Inc, Chicago, IL). All variables are expressed as mean±SD. Categorical variables are expressed as percentages of the population. Continuous variables were tested by the Kolmogorov‐Smirnov test to assess the normality of distribution. Variables with a nonnormal distribution were analyzed after transformation into ranks. Categorical data were analyzed using the χ2 test.
All analyses were intention to treat. ANOVA (general linear model; SPSS 22; SPSS Inc) for repeated measurements was applied (1) for measurements of the examined markers at baseline and 4 and 12 months after treatment used as a within‐subject factor and (2) for the effects of treatment, as a between‐subject factor (insulin, GLP‐1RA, SGLT‐2i, and combination GLP‐1RA and SGLT‐2i). The F and P values of the interaction between time of measurement of the examined markers and the examined covariates were calculated. The F and P values of the comparison between treatments were calculated. The Greenhouse‐Geisser correction was used when the sphericity assumption, as assessed by Mauchly's test, was not met. Post hoc comparisons were performed with Bonferroni's correction. Age, sex, smoking, body mass index (BMI), ΔBMI, Δcholesterol, Δmean blood pressure, and Δhematocrit were included as covariates. The inclusion of Δhematocrit was decided because of the previously reported association of its change with clinical outcome in patients treated with SGLT‐2i.23 The percentage changes of the examined variables posttreatment between the study groups were also analyzed by ANOVA. All statistical tests were 2 tailed, and P<0.05 was considered to be the level of statistical significance.
Results
Study Population
The baseline characteristics of the study population are shown in Table 1. All patients had similar age, sex, risk factors, cardiovascular medications, glycosylated hemoglobin (HbA1c), weight, and BMI at inclusion (Table 2).
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | All Patients (n=160) | Insulin (n=40) | GLP‐1RA (n=40) | SGLT‐2i (n=40) | GLP‐1RA+SGLT‐2i (n=40) | P Value |
|---|---|---|---|---|---|---|
| Duration of diabetes mellitus, y | 6.5 (2 to 10) | 6.7 (1 to 9) | 5.9 (1 to 8) | 6.6 (1 to 11) | 6.8 (2 to 12) | 0.446 |
| CAD, n (%) | 54 (34) | 14 (35) | 13 (32.5) | 13 (32.5) | 14 (35) | 0.869 |
| LVEF <55%, n (%) | 74 (46) | 18 (45) | 19 (47.5) | 18 (45) | 19 (47.5) | 0.379 |
| Age, y | 58±10 | 57±10 | 57±9 | 58±10 | 58±9 | 0.518 |
| Sex (male/female), n (%) | 115/45 (72/28) | 28/12 (70/30) | 27/13 (67.5/32.5) | 30/10 (75/25) | 30/10 (75/25) | 0.151 |
| Creatinine, mg/dL | 1.1±0.3 | 1.0±0.3 | 1.1±0.2 | 1.1±0.2 | 1.1±0.3 | 0.833 |
| eGFR, mL/min per 1.73 m2 | 85±10 | 86±9 | 85±8 | 85±10 | 83±11 | 0.315 |
| Risk factors, n (%) | ||||||
| Current smoking | 64 (40) | 15 (37.5) | 17 (42.5) | 16 (40) | 16 (40) | 0.837 |
| Hypertension | 97 (61) | 24 (60) | 24 (60) | 24 (60) | 25 (62.5) | 0.789 |
| Dyslipidemia | 160 (100) | 40 (100) | 40 (100) | 40 (100) | 40 (100) | 1.000 |
| Family history of CAD | 51 (32) | 12 (30) | 11 (27.5) | 15 (37.5) | 13 (32.5) | 0.392 |
| Cardiovascular medications, n (%) | ||||||
| Antiplatelet | 57 (36) | 14 (35) | 13 (32.5) | 15 (37.5) | 15 (37.5) | 0.898 |
| β Blockers | 78 (49) | 18 (45) | 19 (47.5) | 21 (52.5) | 20 (50) | 0.753 |
| Calcium channel blocker | 40 (25) | 10 (25) | 9 (22.5) | 10 (25) | 11 (27.5) | 0.512 |
| ACEI or ARB | 80 (50) | 20 (50) | 19 (47.5) | 21 (52.5) | 20 (50) | 0.734 |
| Diuretics | 28 (17.5) | 6 (15) | 5 (12.5) | 8 (20) | 9 (22.5) | 0.969 |
| Aldosterone antagonists | 7 (4) | 1 (2.5) | 2 (5) | 1 (2.5) | 3 (7.5) | 0.827 |
| Statins | 160 (100) | 40 (100) | 40 (100) | 40 (100) | 40 (100) | 1.000 |
| Fibrate | 10 (6) | 2 (5) | 2 (5) | 3 (7.5) | 3 (7.5) | 0.787 |
| Antidiabetic medications, n (%) | ||||||
| Metformin | 109 (68) | 29 (72.5) | 25 (62.5) | 27 (67.5) | 28 (70) | 0.192 |
Data are expressed as number (percentage), mean±SD, or median (first quartile to third quartile). Continuous variables were compared with the paired Student t test. Binary variables were compared with the χ2 test. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; LVEF, left ventricular ejection fraction; P, P of model of the ANOVA for comparisons between groups; and SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitors.
Table 2.
Changes in Metabolic and Hematologic Parameters in the Study Population During the Study Period
| Variable | All Patients (n=160) | Insulin (n=40) | GLP‐1RA (n=40) | SGLT‐2i (n=40) | GLP‐1RA+SGLT‐2i (n=40) |
|---|---|---|---|---|---|
| Fasting glucose, mg/dL | |||||
| Baseline | 152±42 | 158±48 | 152±45 | 145±34 | 151±40 |
| 4 mo | 127±30‖ | 134±44‖ | 122±27‡ | 126±20‡ | 125±29‡ |
| Δ% | −19.7 | −17.9 | −24.6 | −15.1 | −20.8 |
| 12 mo | 120±31§ | 121±40‡ | 118±24‡ | 124±30‡ | 116±30‡ |
| Δ% | −26.7 | −30.6 | −28.8 | −16.9 | −30.2 |
| HbA1c, % | |||||
| Baseline | 8.1±1.1 | 8.2±1.2 | 8±1.1 | 7.8±0.9 | 8.2±1.2 |
| 4 mo | 6.9±1.1‖ | 7±1.1§ | 6.7±1‡ | 7±1‖ | 6.7±0.8‖ |
| Δ% | −17.4 | −17.1 | −19.4 | −11.4 | −22.4 |
| 12 mo | 6.8±1.1‖ | 7.1±1.2‖ | 6.7±0.9‡ | 7.1±1.1§ | 6.4±0.8§ |
| Δ% | −19.1 | −15.5 | −19.5 | −9.8 | −28.1 |
| Weight, kg | |||||
| Baseline | 86.3±10 | 85.5±8 | 87.1±12 | 84.6±9 | 87.9±10 |
| 4 mo | 83.6±10‖ | 83±9 | 84.2±11‖ | 82±10‖ | 85±9‖ |
| Δ% | −3.2 | −3 | −3.4 | −3.2 | −3.4 |
| 12 mo | 81.8±11‖ | 83.5±9 | 81.3±12§ | 80.6±10‖ | 81.7±9* , ‖ |
| Δ% | −5.5 | −2.4 | −7.1 | −4.9 | −7.6 |
| BMI, kg/m2 | |||||
| Baseline | 30±3 | 29.7±3 | 30±4 | 29.8±3 | 30.5±3 |
| 4 mo | 28.5±3‖ | 28.8±3 | 28±3‖ | 28.8±2‖ | 28.5±3‖ |
| Δ% | −5.3 | −3.1 | −7.1 | −3.5 | −7 |
| 12 mo | 28.2±3‖ | 29.6±3 | 27.6±3§ | 28.4±2 | 27.3±4† , ‖ |
| Δ% | −6.4 | −0.3 | −8.7 | −4.9 | −11.7 |
| TC, mg/dL | |||||
| Baseline | 172±42 | 178±43 | 172±38 | 163±37 | 175±50 |
| 4 mo | 152±44§ | 160±48‡ | 154±47 | 140±30‡ | 154±51‡ |
| Δ% | −13.2 | −11.3 | −11.7 | −16.4 | −13.6 |
| 12 mo | 147±31§ | 154±25‡ | 145±28‡ | 147±32 | 144±35§ |
| Δ% | −17 | −15.5 | −18.6 | −10.9 | −21.5 |
| LDL‐C, mg/dL | |||||
| Baseline | 99±26 | 104±27 | 101±26 | 92±21 | 100±30 |
| 4 mo | 85±31§ | 90±35 | 91±33 | 78±24‡ | 79±29‡ |
| Δ% | −16.5 | −15.5 | −11 | −17.9 | −26.6 |
| 12 mo | 80±24§ | 82±23 | 80±21‡ | 81±25 | 75±24‡ |
| Δ% | −23.7 | −26.8 | −26.3 | −13.5 | −33.4 |
| HDL‐C, mg/dL | |||||
| Baseline | 42±12 | 46±12 | 41±11 | 39±9 | 43±14 |
| 4 mo | 43±11 | 46±13 | 39±7 | 41±10 | 45±12 |
| Δ% | 2.3 | 0.4 | −5.1 | 4.8 | 4.4 |
| 12 mo | 44±10 | 46±7 | 42±11 | 43±8 | 46±13 |
| Δ% | 4.5 | 0.6 | 2.4 | 9.3 | 6.5 |
| Triglycerides, mg/dL | |||||
| Baseline | 155±44 | 143±42 | 158±41 | 157±44 | 161±48 |
| 4 mo | 139±37‡ | 123±28‡ | 140±38 | 146±43‡ | 147±40 |
| Δ% | −11.5 | −16.2 | −12.9 | −7.5 | −10.9 |
| 12 mo | 122±27§ | 118±31 | 124±22‡ | 125±29‡ | 123±24‡ |
| Δ% | −27 | −21.1 | −27.4 | −25.6 | −30.9 |
| Hemoglobin, g/dL | |||||
| Baseline | 14.2±1.3 | 13.8±1 | 14.3±1.2 | 14.4±1.4 | 14.1±1.5 |
| 4 mo | 14.2±1.2 | 13.9±1.3 | 14.2±0.8 | 14.5±1.3 | 14.1±1.4 |
| Δ% | 0.2 | 0.7 | −0.7 | 0.7 | 0.3 |
| 12 mo | 14.3±1.2 | 13.9±1.1 | 14.4±1.1 | 14.6±1.3 | 14.3±1.4 |
| Δ% | 0.7 | 0.7 | 0.7 | 1.4 | 1.4 |
| Hematocrit, % | |||||
| Baseline | 42.4±4 | 41.4±3 | 42.8±3.8 | 43.1±4.2 | 42.3±4.6 |
| 4 mo | 42.5±3.7 | 41.7±4 | 42.6±2.4 | 43.4±3.9 | 42.4±4.4 |
| Δ% | 0.2 | 0.7 | −0.5 | 0.7 | 0.2 |
| 12 mo | 42.9±3.8 | 41.7±3.4 | 43.2±3.3 | 43.7±3.9 | 43.0±4.5 |
| Δ% | 1.2 | 0.7 | 0.9 | 1.5 | 1.7 |
| MCV, fl | |||||
| Baseline | 86.8±4.4 | 84.8±3.6 | 87.7±4.2 | 88.1±5.3 | 86.7±4.4 |
| 4 mo | 87.2±4.3 | 85.5±3.8 | 87.5±4.3 | 88.7±4.7 | 86.9±4.5 |
| Δ% | 0.5 | 0.8 | −0.2 | 0.7 | 0.2 |
| 12 mo | 87.6±4.4 | 85.4±4 | 88.2±4.3 | 89±5.1 | 87.7±4.2 |
| Δ% | 0.9 | 0.7 | 0.6 | 1 | 1.1 |
Data are presented as mean±SD. Δ% indicates percentage change from baseline; BMI, body mass index; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MCV, mean corpuscular volume of red blood cells; SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitors; and TC, total cholesterol.
*P<0.05, † P<0.01 for time×treatment interaction obtained by repeated‐measures ANOVA. ‡ P<0.05, § P<0.01, || P<0.001 for comparisons of 4 or 12 months vs baseline by ANOVA using post hoc analysis with Bonferroni correction.
At 12 months, antihypertensive medication was down titrated in 20% of the patients under combined therapy with GLP‐1RA and SGLT‐2i, in 15% of patients under SGLT‐2i, and in 10% of patients treated with GLP‐1RA because of significant decrease of central and brachial blood pressure. In insulin group, the use of antihypertensive medications remained unchanged.
Interrelation Between Metabolic, Endothelial, Vascular, and LV Function Markers at Baseline
In all study patients, HbA1c was associated with PBR (b=0.34, P=0.04), PWV (b=0.35, P=0.016), GLS (b=0.30, P=0.001), and GCS (b=0.32, P=0.024). PBR was associated with central systolic blood pressure (SBP) (b=0.31, P<0.001), PWV (b=0.30, P=0.031), GLS (b=0.29, P=0.035), and GCS (b=0.33, P=0.028).
Changes in Metabolic and Hematologic Parameters Posttreatment
All patients had significantly improved plasma glucose levels and HbA1c at 4 months (P<0.001) and 12 months (P=0.013 and P<0.001, respectively). Νo significant differences were observed in hematocrit levels, hemoglobin concentration, or mean corpuscular volume of red blood cells among the study groups throughout the follow‐up period (P>0.05; Table 2). Weight and BMI were decreased in the overall population after 4 and 12 months (P<0.001; Table 2). However, there was a significant interaction between the type of treatment and the change of BMI posttreatment (F=5.939, P for interaction=0.002; Table 2). Patients treated with GLP‐1RA, SGLT‐2i, and combination of GLP‐1RA+SGLT‐2i showed a reduction in BMI at 4 and 12 months (P<0.05 for all changes; Table 2), whereas those under insulin showed no significant reduction of BMI at either 4 months (P=0.155) or 12 months (P=0.755; Table 2). Patients treated with combination of GLP‐1RA+SGLT‐2i presented a greater percentage reduction of BMI compared with GLP‐1RA (P=0.042) or SGLT‐2i (P=0.009; Table 2) alone during 12 months. Moreover, total cholesterol, low‐density lipoprotein cholesterol, and triglycerides were reduced remarkably in all groups at 4 and 12 months posttreatment (P<0.01; Table 2).
Effect of Treatment in Vascular Function
Endothelial Glycocalyx and Wave Reflection
There was a significant interaction between the type of treatment and the change of PBR (F=3.513, P for interaction=0.026) in a model including age, sex, smoking, BMI, ΔBMI, Δcholesterol, Δmean blood pressure, and Δhematocrit. At 4 months, no change of PBR was observed in all patients (P>0.05). Conversely, at 12 months, all patients reduced PBR (P=0.042) with the combination of GLP‐1RA+SGLT‐2i, showing a 3‐fold higher reduction compared with the other 3 treatment groups (‐9.1% versus −3.4% in SGLT‐2i, −2.9% in GLP‐1RA, and −2.4% in insulin; Table 3, Figure 1A; P<0.05 for all comparisons).
Table 3.
Changes in Endothelial Glycocalyx Thickness and Arterial Stiffness Markers in the Study Population During the Study Period
| Variable | All Patients (n=160) | Insulin (n=40) | GLP‐1RA (n=40) | SGLT‐2i (n=40) | GLP‐1RA+SGLT‐2i (n=40) |
|---|---|---|---|---|---|
| PBR, 5 to 25 μm | |||||
| Baseline | 2.13±0.3 | 2.13±0.3 | 2.1±0.29 | 2.15±0.3 | 2.16±0.29 |
| 4 mo | 2.14±0.3 | 2.15±0.3 | 2.07±0.3 | 2.19±0.3 | 2.12±0.32 |
| Δ% | 0.5 | 0.9 | −1.4 | 1.8 | −1.9 |
| 12 mo | 2.05±0.3† | 2.10±0.3 | 2.04±0.2† | 2.08±0.2† | 1.98±0.3* , ‡ |
| Δ% | −3.9 | −2.4 | −2.9 | −3.4 | −9.1 |
| PWV, m/s | |||||
| Baseline | 11.8±2.7 | 11.5±2.7 | 11.6±2.8 | 12±2.8 | 12.3±2.6 |
| 4 mo | 11.5±2.6 | 11.5±2.7 | 11.4±2.5 | 11.4±2.4 | 11.5±2.6† |
| Δ% | −2.6 | −0.1 | −1.8 | −5.3 | −6.1 |
| 12 mo | 10.8±2† | 11.1±2.3 | 10.5±1.9† | 10.9±2.1† | 10.8±2* , † |
| Δ% | −9.3 | −3.6 | −8.6 | −10.1 | −13 |
| AI, % | |||||
| Baseline | 12 (2 to 23) | 13.6 (1 to 25) | 12.7 (3 to 25) | 10.8 (2 to 19) | 11 (1 to 21) |
| 4 mo | 10.8 (0 to 21)§ | 14.1 (4 to 23) | 10.8 (0 to 21)‡ | 10 (2 to 19) | 8.3 (0 to 18)§ |
| Δ% | −11.1 | 3.5 | −17.6 | −8 | −32.5 |
| 12 mo | 10.3 (1 to 22)§ | 14.5 (3 to 24) | 8.9 (−2 to 18)§ | 10.4 (3 to 24) | 7.4 (0 to 19)* , § |
| Δ% | −16.5 | 6.2 | −42.7 | −3.8 | −48.6 |
| SBP, mm Hg | |||||
| Baseline | 137±17 | 137±16 | 137±18 | 134±17 | 139±18 |
| 4 mo | 135±16 | 138±15 | 134±17 | 130±16† | 135±17† |
| Δ% | −1.5 | 0.7 | −2.2 | −3.1 | −3 |
| 12 mo | 133±15§ | 134±15‡ | 135±15 | 130±15† | 132±14* , ‡ |
| Δ% | −3 | −2.2 | −0.7 | −3.1 | −5.3 |
| DBP, mm Hg | |||||
| Baseline | 83±10 | 83±10 | 84±11 | 82±9 | 83±9 |
| 4 mo | 81±9 | 82±10 | 84±10 | 79±8† | 80±10† |
| Δ% | −2.5 | −1.2 | −0.2 | −3.8 | −3.7 |
| 12 mo | 79±9‡ | 80±10† | 82±9 | 77±8† | 78±7† |
| Δ% | −5.1 | −3.8 | −2.4 | −6.5 | −6.4 |
| Central SBP, mm Hg | |||||
| Baseline | 134±19 | 133±20 | 134±21 | 134±18 | 135±17 |
| 4 mo | 132±19 | 135±19 | 131±19† | 131±19† | 132±20† |
| Δ% | −1.5 | 1.5 | −2.3 | −2.2 | −2.3 |
| 12 mo | 130±19† | 132±21 | 132±20† | 130±20† | 128±17* , ‡ |
| Δ% | −3.1 | −0.8 | −1.5 | −3 | −5.5 |
| HR, bpm | |||||
| Baseline | 71±10 | 70±10 | 73±10 | 69±10 | 72±11 |
| 4 mo | 73±11† | 69±10 | 78±11† | 68±11 | 76±10‡ |
| Δ% | 2.7 | −1.4 | 6.4 | −1.5 | 5.3 |
| 12 mo | 73±11† | 71±10 | 77±10† | 67±11 | 75±11* , † |
| Δ% | 2.7 | 1.4 | 5.2 | −3 | 4 |
Data are presented as mean±SD or median (first quartile to third quartile). Δ% indicates percentage change from baseline; AI, augmentation index; bpm, beats per minute; DBP, diastolic blood pressure; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; HR, heart rate; PBR, perfused boundary region; PWV, pulse wave velocity; SBP, systolic blood pressure; and SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitors.
*P<0.05 for time×treatment interaction obtained by repeated‐measures ANOVA. † P<0.05, ‡ P<0.01, § P<0.001 for comparisons of 4 or 12 months vs baseline by ANOVA using post hoc analysis with Bonferroni correction.
Figure 1. Percentage changes (Δs) from baseline in perfused boundary region (PBR) (A), pulse wave velocity (PWV) (B), central systolic blood pressure (cSBP) (C), left ventricular global longitudinal strain (GLS) (D), PWV/GLS ratio (E), and myocardial global work index (GWI) (F) at 4 and 12 months in the 4 study groups.
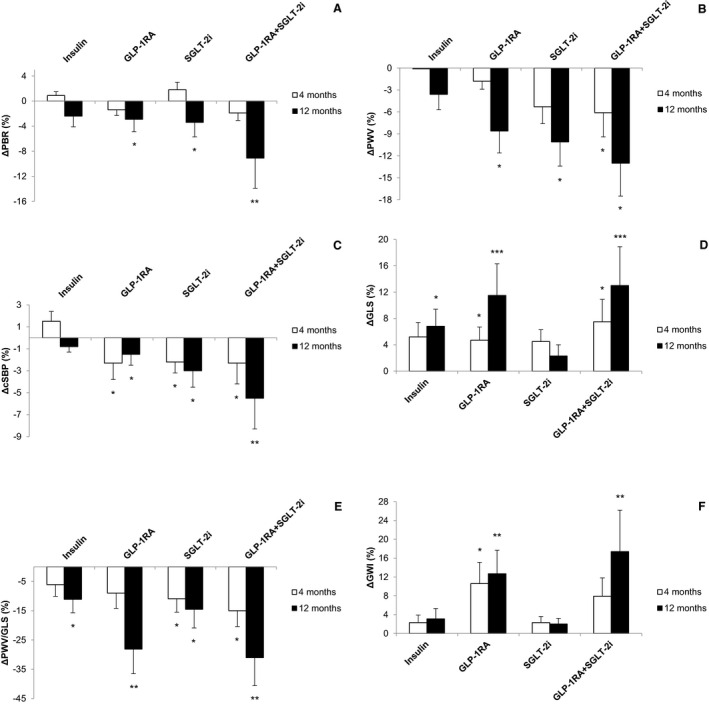
Data shown are means±SD. *P<0.05, **P<0.01, ***P<0.001 vs baseline. GLP‐1RA indicates glucagon‐like peptide‐1 receptor agonists; and SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitors.
After 4 and 12 months of treatment, all patients showed decreased AI (P<0.001 and P<0.001) and increased heart rate compared with baseline (P=0.035 and P=0.031). There was a significant interaction between the type of treatment and the change of AI and heart rate (F=3.734 [P for interaction=0.019] and F=3.913 [P=0.014], respectively) in a model including age, sex, smoking, BMI, ΔBMI, Δcholesterol, Δmean blood pressure, and Δhematocrit.
At 4 months, treatment with GLP‐1RA and combination with GLP‐1RA+SGLT‐2i showed a greater reduction of AI (−17.6% and −32.5%) compared with insulin (3.5%; P=0.024, P=0.030) or SGLT‐2i treatment (−8%; P=0.022, P=0.001, respectively) and a greater increase of heart rate (6.4% and 5.3% versus 1.4% and 1.5%, P=0.033, P=0.002 and P=0.039, P=0.041, respectively; Table 3).
At 12 months, treatment with GLP‐1RA and the combination with GLP‐1RA+SGLT‐2i resulted in a further decrease of AI (−42.7% and −48.6%) compared with insulin or SGLT‐2i treatment (6.2% and −3.8%; P<0.001 for all comparisons) and a similar to 4 months increase of heart rate compared with baseline (P=0.033 and P=0.023, respectively). No significant change of AI and heart rate was observed post–SGLT‐2i or insulin treatment at 12 months (P=0.629, P=0.277 and P=0.345, P=0.267, respectively; Table 3).
Arterial Elasticity
A significant interaction between the type of treatment and the change of PWV, central SBP, and brachial SBP posttreatment was observed (F=4.018 [P for interaction=0.011], F=3.424 [P=0.032], and F=3.576 [P=0.030], respectively) in a model including age, sex, smoking, BMI, ΔBMI, Δcholesterol, Δmean blood pressure, and Δhematocrit.
At 4 months, SGLT‐2i and the combined GLP‐1RA+SGLT‐2i treatment resulted in reduction of brachial systolic (P=0.021, P=0.020) and diastolic blood pressure (P=0.020, P=0.022), whereas no significant differences were observed in the above markers in the other 2 treatment groups (P>0.05; Table 3). Central SBP was reduced in all 3 treatment groups (P<0.05) but not in the insulin regimen (P=0.09).
After 12‐month treatment, all patients had improved PWV, brachial systolic and diastolic, and central blood pressure (P<0.001). Patients under GLP‐1RA, SGLT‐2i, and their combination achieved a greater reduction of PWV, central SBP, and brachial SBP than those under insulin (P<0.05, for comparisons; Table 3, Figure 1B and 1C), despite a similar reduction of HbA1c (F=2.073, P for interaction for treatment =0.237) at 12 months. Furthermore, SGLT‐2i and the combination GLP‐1RA+SGLT‐2i resulted in a greater reduction of PWV (−10.1% and −13%) compared with insulin (−3.6%; P=0.028 and P=0.018) and GLP‐1RA (−8.6%; P=0.040 and P=0.016, respectively), as well as a greater reduction of brachial SBP (−3.1% and −5.3%) compared with insulin (−2.2%; P=0.037, P=0.015) or GLP‐1RA (−0.7%; P=0.027, P=0.010, respectively) at 12 months. Finally, the combination of GLP‐1RA+SGLT‐2i showed a 2‐fold higher reduction of central SBP compared with the other 3 treatment groups (P<0.01; Table 3).
In general, the combined therapy with GLP‐1RA+SGLT‐2i presented a greater improvement of the measured markers than each one treatment (P<0.05; Table 3). There were no statistically significant differences in the above‐mentioned measured markers between patients with coronary artery disease (CAD) and patients without CAD in the 4 study groups 4 and 12 months posttreatment (P>0.05, data not shown).
Effect of Treatment in LV Myocardial Deformation
Baseline echocardiographic parameters are presented in Table 4. Compared with baseline, there was no significant change in LV ejection fraction (LVEF) after 4 and 12 months of treatment in the overall study population. No interaction was observed between change of LVEF and presence of CAD (P=0.49).
Table 4.
Changes in Echocardiographic Markers of LV Myocardial Function in the Study Population During the Study Period
| Variable | All Patients (n=160) | Insulin (n=40) | GLP‐1RA (n=40) | SGLT‐2i (n=40) | GLP‐1RA+SGLT‐2i (n=40) |
|---|---|---|---|---|---|
| LVEF, % | |||||
| Baseline | 53±8 | 54±7 | 52±8 | 54±8 | 51±8 |
| 4 mo | 54±8 | 53±9 | 54±9 | 55±8 | 52±7 |
| Δ% | 1.8 | −1.9 | 3.7 | 1.8 | 1.9 |
| 12 mo | 54±8 | 55±10 | 54±8 | 54±7 | 53±8 |
| Δ% | 1.9 | 1.8 | 3.7 | 0.1 | 3.8 |
| GLS, % | |||||
| Baseline | −16.4±3.7 | −16.4±3.5 | −16.2±3.5 | −17±4 | −16±4 |
| 4 mo | −17.3±3.9‖ | −17.3±4 | −17±3.8§ | −17.8±3.6 | −17.3±4.2§ |
| Δ% | 5.2 | 5.2 | 4.7 | 4.5 | 7.5 |
| 12 mo | −17.9±3.9¶ | −17.6±4.2§ | −18.3±3.5¶ | −17.4±3.4 | −18.4±4.7† , ¶ |
| Δ% | 8.4 | 6.8 | 11.5 | 2.3 | 13 |
| GLSR E, 1/s | |||||
| Baseline | 0.84±0.32 | 0.92±0.34 | 0.81±0.3 | 0.83±0.28 | 0.8±0.33 |
| 4 mo | 0.9±0.37 | 0.95±0.33 | 0.89±0.45 | 0.88±0.26 | 0.88±0.4§ |
| Δ% | 6.7 | 3.2 | 9 | 5.7 | 9.1 |
| 12 mo | 0.96±0.35‖ | 1±0.34 | 0.97±0.42‖ | 0.88±0.33 | 0.97±0.33§ |
| Δ% | 12.5 | 8 | 16.5 | 5.7 | 17.5 |
| GCS, % | |||||
| Baseline | −17.4±5 | −17.9±6 | −17.7±6 | −17.1±4 | −17±5 |
| 4 mo | −18.4±5‖ | −18.9±5 | −18.6±6§ | −17.9±4 | −18.5±5§ |
| Δ% | 5.4 | 5.3 | 4.8 | 4.5 | 6.3 |
| 12 mo | 19.3±7¶ | −19.3±7§ | −20.1±7¶ | −17.7±7 | −9.9±6† , ¶ |
| Δ% | 9.8 | 7.3 | 11.9 | 3.4 | 14.6 |
| GRS, % | |||||
| Baseline | 35.8±8.6 | 36±8.5 | 35.7±8.1 | 36.2±9 | 35.3±8.8 |
| 4 mo | 36.6±9§ | 36.7±8.8 | 36.5±8.9§ | 36.9±8.8 | 36.3±9.4§ |
| Δ% | 2.2 | 1.9 | 2.0 | 1.9 | 2.9 |
| 12 mo | 36.9±9.4‖ | 36.8±9.2 | 37.1±9.3§ | 36.8±9.2 | 37.3±9.7* , ‖ |
| Δ% | 2.9 | 2.2 | 3.8 | 1.6 | 5.1 |
| PWV/GLS | |||||
| Baseline | −0.73±0.34 | −0.7±0.31 | −0.73±0.33 | −0.71±0.35 | −0.76±0.36 |
| 4 mo | −0.66±0.26‖ | −0.66±0.28 | −0.67±0.27 | −0.64±0.21§ | −0.66±0.3§ |
| Δ% | −11 | −6.1 | −9 | −10.9 | −15 |
| 12 mo | −0.6±0.2¶ | −0.63±0.21§ | −0.57±0.16‖ | −0.62±0.16§ | −0.58±0.28*,‖ |
| Δ% | −22 | −11.1 | −28.1 | −14.5 | −31 |
| GWI, mm Hg% | |||||
| Baseline | 1538±430 | 1644±416 | 1510±403 | 1536±535 | 1463±362 |
| 4 mo | 1633±423§ | 1682±377 | 1689±406§ | 1572±468 | 1589±441 |
| Δ% | 5.8 | 2.3 | 10.6 | 2.3 | 7.9 |
| 12 mo | 1692±412¶ | 1696±377 | 1730±318‖ | 1568±456 | 1772±499† , ‖ |
| Δ% | 9.1 | 3.1 | 12.7 | 2 | 17.4 |
| GCW, mm Hg% | |||||
| Baseline | 1908±515 | 1971±478 | 1872±427 | 1943±654 | 1847±504 |
| 4 mo | 1998±461 | 2096±407 | 1995±433 | 1972±544 | 1929±461 |
| Δ% | 4.5 | 6 | 6.2 | 1.5 | 4.3 |
| 12 mo | 2108±484‖ | 2016±493 | 2134±451‖ | 2108±509§ | 2173±482* , ‖ |
| Δ% | 9.5 | 2.2 | 12.3 | 7.8 | 15 |
| GWW, mm Hg% | |||||
| Baseline | 196±105 | 176±119 | 197±86 | 192±102 | 218±114 |
| 4 mo | 171±82¶ | 162±82§ | 160±77§ | 171±71§ | 192±97§ |
| Δ% | −14.6 | −8.6 | −23.1 | −12.3 | −13.5 |
| 12 mo | 159±79¶ | 155±95§ | 142±71§ | 183±86‖ | 154±63‡ , ‖ |
| Δ% | −23.3 | −13.5 | −38.7 | −4.9 | −41.6 |
| pTw, ° | |||||
| Baseline | 15.7±6 | 16±5.1 | 15.6±5 | 15.2±6 | 16.1±8 |
| 4 mo | 15.3±5.3 | 15.9±5.5 | 15±6 | 16±4.8 | 14.5±5§ |
| Δ% | −2.6 | −0.6 | −4 | 5 | −11 |
| 12 mo | 14.6±5.1 | 15.4±5.4 | 14.4±5.4§ | 14.7±4.6 | 14±5* , ‖ |
| Δ% | −7.5 | −3.9 | −8.3 | −3.4 | −15 |
| pUtwVel, °/s | |||||
| Baseline | −104±42 | −100±44 | −107±41 | −101±28 | −111±54 |
| 4 mo | −114±45§ | −108±43 | −118±47§ | −109±43§ | −120±47§ |
| Δ% | 8.8 | 7.4 | 9.3 | 7.3 | 7.5 |
| 12 mo | −116±49§ | −107±55§ | −114±45§ | −108±38§ | −134±61*,§ |
| Δ% | 10.3 | 6.5 | 6.1 | 6.5 | 17.2 |
Data are presented as mean±SD. Δ% indicates percentage change from baseline; GCS, global circumferential strain; GCW, global constructive work; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; GLS, global longitudinal strain; GLSR E, global longitudinal early diastolic strain rate; GRS, global radial strain; GWI, global work index; GWW, global wasted work; LV, left ventricular; LVEF, LV ejection fraction; pTw, peak twisting; pUtwVel, peak untwisting velocity; PWV, pulse wave velocity; and SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitors.
*P<0.05, † P<0.01, ‡ P<0.001 for time×treatment interaction obtained by repeated‐measures ANOVA. § P<0.05, || P<0.01, ¶ P<0.001 for comparisons of 4 or 12 months vs baseline by ANOVA using post hoc analysis with Bonferroni correction.
All patients had improved GLS, GCS, GRS, and PWV/GLS ratio at 4 months (P=0.008, P=0.006, P=0.034, and P=0.007, respectively) and 12 months posttreatment (P<0.001, P<0.001, P=0.009, and P<0.001, respectively; Table 4, Figure 1D and 1E). A significant interaction between changes of GLS, GCS, GRS, and PWV/GLS ratio and type of treatment was observed (F=3.627 [P for interaction=0.002], F=3.899 [P=0.001], F=3.243 [P=0.036], and F=3.265 [P=0.033], respectively).
At 4 months, compared with the other 3 treatment groups, the combined GLP‐1RA+SGLT‐2i resulted in a greater improvement of GLS (7.5% versus 5.2% in insulin, 4.7% in GLP‐1RA, and 4.5% in SGLT‐2i group; P=0.04, P=0.035, and P=0.032), GCS (6.3% versus 5.3% in insulin, 4.8% in GLP‐1RA, and 4.5% in SGLT‐2i group; P=0.047, P=0.039, and P=0.031) and GRS (2.9% versus 1.9% in insulin, 2% in GLP‐1RA, and 1.9% in SGLT‐2i group; P=0.029, P=0.049, and P=0.032). Similar results were observed for global longitudinal strain rate E (P<0.05; Table 4).
At 12 months, patients under GLP‐1RA and combination of GLP‐1RA+SGLT‐2i presented a greater percentage improvement of the GLS (11.5% and 13% versus 6.8% and 2.3%; P<0.05 for all comparisons), GCS (11.9% and 14.6% versus 7.3% and 3.4%; P<0.05 for all comparisons), and GRS (3.8% and 5.1% versus 2.2% and 1.6%; P<0.05 for all comparisons; Table 4), than those under insulin or SGLT‐2i alone. Similar results were observed for global longitudinal strain rate E and PWV/GLS ratio (P<0.05; Table 4). No statistically significant differences were observed in the changes of GLS, GCS, GRS, and PWV/GLS ratio 12 months posttreatment between CAD and non‐CAD patients (P for interaction=0.297, P=0.318, P=0.466, and P=0.459, respectively).
Myocardial Work Index
Compared with baseline, all patients had increased myocardial work index and reduced myocardial wasted work (P=0.041 and P<0.001, respectively) at 4 months posttreatment. After 12 months, all patients had an increase of myocardial work index because of an increase in constructive and reduction of wasted myocardial work (P<0.001, P=0.003, and P<0.001, respectively; Table 4). A significant interaction between type of treatment and changes of GWI, GCW, and GWW was observed (F=5.179 [P for interaction=0.003], F=4.624 [P=0.016], and F=6.011 [P<0.001], respectively).
At 12 months, patients treated with GLP‐1RA and combination of GLP‐1RA and SGLT‐2i showed a greater increase of GWI (12.7% and 17.4% versus 3.1% and 2%, respectively; Figure 1F) and of GCW (12.3% and 15% versus 2.2% and 7.8%, respectively), and reduction of GWW (38.7% and 41.6% versus 13.5% and 4.9%, respectively) compared with those under insulin or SGLT‐2i (for insulin, P=0.042, P=0.036, P=0.038, P=0.007, P<0.001, and P=0.019, respectively; and for SGLT‐2i, P=0.039, P=0.015, P=0.031, P=0.032, P=0.044, and P=0.037, respectively) (Figures 2 and 3). No significant differences were found in GWI, GCW, and GWW changes between CAD and non‐CAD patients in the 4 study groups after 12 months treatment (P for interaction=0.296, P=0.340, and P=0.441, respectively).
Figure 2. Representative images of myocardial work index derived by pressure–left ventricular (LV) longitudinal myocardial strain loop during one cardiac cycle by speckle tracking echocardiography in 2 patients under combination therapy with glucagon‐like peptide‐1 receptor agonists+sodium‐glucose cotransporter‐2 inhibitors.
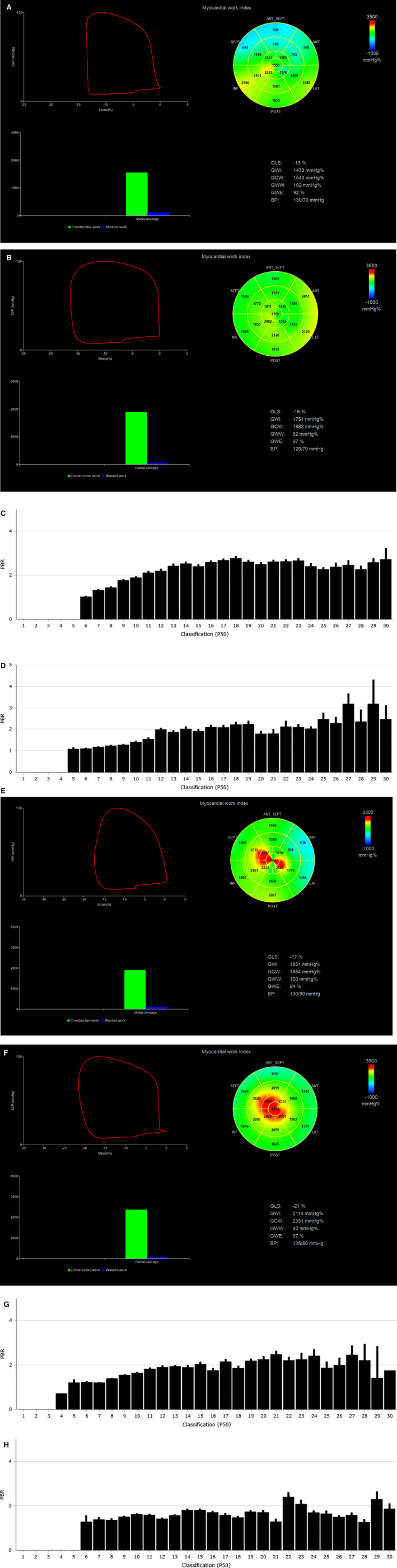
The bull's eye shows the myocardial work index in each one of the 17 LV myocardial segments. The perfused boundary region of sublingual microvessels ranging from 5 to 25 μm diameter (PBR 5‐25), reflecting glycocalyx thickness (higher PBR indicates thinner glycolaxyx), is shown as bars by Glycocheck software. The first patient had an LV ejection fraction (LVEF) of 45% and impaired global longitundinal strain (GLS), myocardial work index, and endothelial glycocalyx thickness (a GLS of −13%, a global work index [GWI] of 1433 mm Hg%, and a PBR 5‐25 of 2.24 μm) at baseline and showed a significant improvement (a GLS of −16%, a GWI of 1751 mm Hg%, and a PBR 5‐25 of 1.79 μm) after 12 months treatment (A through D). The second patient with an LVEF of 60%, a GLS of −17%, a GWI of 1851 mm Hg%, and a PBR 5‐25 of 1.87 μm at baseline showed a further improvement to a GLS of −21%, a GWI of 2114 mm Hg%, and a PBR 5‐25 of 1.64 μm after 12 months treatment (E through H). Images provided as courtesy by Dr I. Ikonomidis, Laboratory of Preventive Cardiology and Echocardiography Department, Attikon Hospital, National and Kapodistrian University of Athens (NKUA), Athens, Greece. ANT indicates anterior segments; BP, blood pressure; GCW, global constructive work; GWW, global wasted work; GWE, global work efficiency; INF, inferior segments; LAT, lateral segments; LVP, left ventricular pressure; P50, Median width of red blood cell column; POST, posterior segments; and SEPT, septal segments.
Figure 3. Representative images of myocardial work index derived by pressure–left ventricular (LV) longitudinal myocardial strain loop during one cardiac cycle by speckle tracking echocardiography in a patient under treatment with insulin at baseline and 12 months posttreatment (A and B).
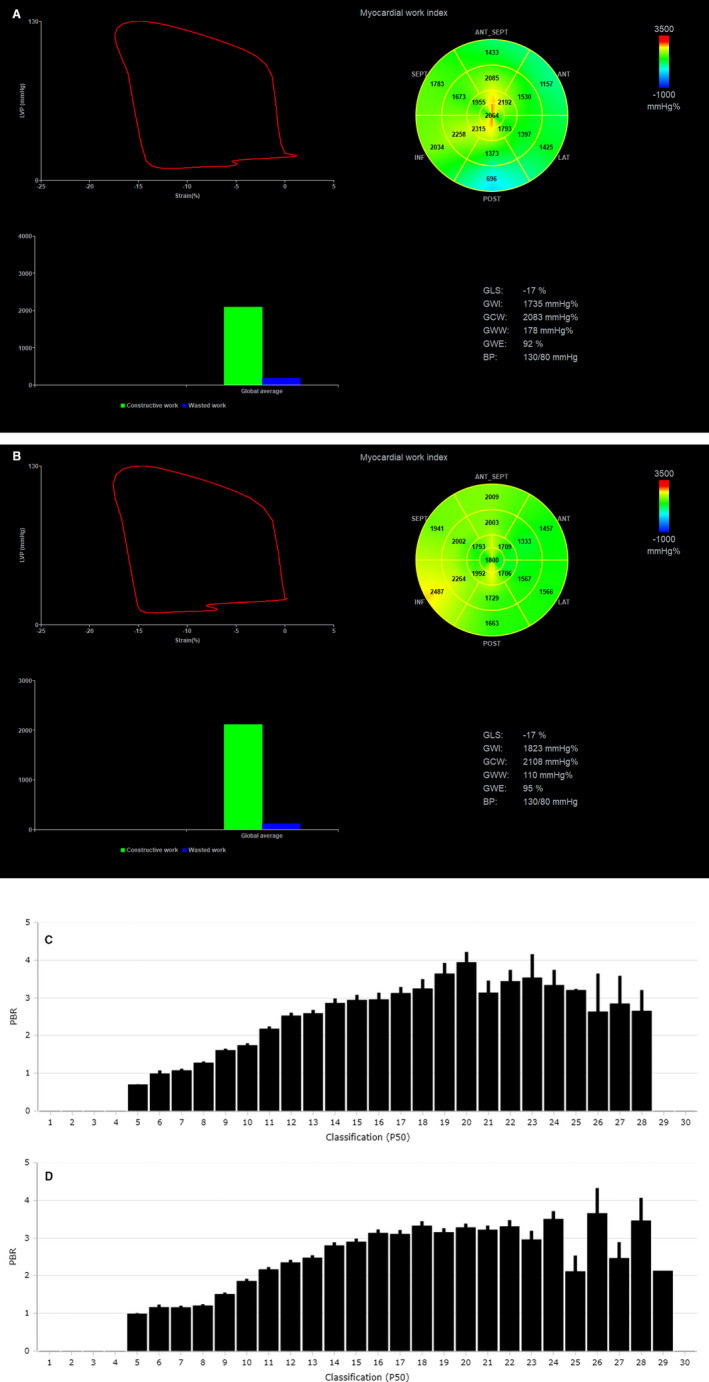
The bull's eye shows the myocardial work index in each one of the 17 LV myocardial segments. The perfused boundary region of sublingual microvessels ranging from 5 to 25 μm diameter (PBR 5‐25), reflecting glycocalyx thickness (higher PBR indicates thinner glycolaxyx), is shown as bars by Glycocheck software (C and D). The patient at baseline had an LV ejection fraction of 50%, a global longitundinal strain (GLS) of −17%, a global work index (GWI) of 1735 mm Hg%, and a PBR 5‐25 of 2.58 μm; and 12 months posttreatment, the patient maintained a GLS of −17%, a GWI of 1823 mm Hg%, and a PBR 5‐25 of 2.46 μm. Images provided as courtesy by Dr I. Ikonomidis, Laboratory of Preventive Cardiology and Echocardiography Department, Attikon Hospital, National and Kapodistrian University of Athens (NKUA), Athens, Greece. ANT indicates anterior segments; BP, blood pressure; GCW, global constructive work; GWW, global wasted work; GWE, global work efficiency; INF, inferior segments; LAT, lateral segments; LVP, left ventricular pressure; P50, Median width of red blood cell column; POST, posterior segments; and SEPT, septal segments.
At 12 months posttreatment, patients under combined therapy with GLP‐1RA+SGLT‐2i who had baseline LVEF <55% (n=19) showed a greater improvement in GLS (15% versus 10%; P=0.045), global longitudinal strain rate E (19% versus 12%; P=0.042), GCS (17% versus 11%; P=0.038), GRS (6% versus 4%; P=0.047), GWI (23% versus 12%; P=0.037), GCW (19% versus 12%; P=0.025) and GWW (−64% versus −32%; P=0.047) compared with patients with LVEF ≥55% (n=21). In patients treated with GLP‐1RA, SGLT‐2i, or insulin, no significant differences were observed in the above‐mentioned LV myocardial markers between those with LVEF <55% and those with LVEF ≥55% (P>0.05 for all comparisons; data not shown).
1. Twisting and Untwisting Velocity
All patients had increased LV untwisting velocity, after 4‐ and 12‐month treatment (P=0.048 and P=0.042; Table 4). There was a significant interaction between type of treatment and changes of peak twisting and peak untwisting velocity (F=3.469 [P for interaction=0.027] and F=3.516 [P=0.036], respectively; Table 4). Only patients under combination of GLP‐1RA+SGLT‐2i showed a statistically significant reduction of LV twisting at 4 and 12 months posttreatment (P=0.043 and P=0.036).
At 12 months, patients under combined treatment with GLP‐1RA+SGLT‐2i achieved a 2‐fold reduction of peak twisting and 2‐fold increase of peak untwisting velocity than those under each one regimen (P=0.043, P=0.02 and P=0.045, P=0.039, respectively) or insulin (P=0.04 and P=0.036, respectively; Table 4). There were no differences in the changes of peak twisting and peak untwisting velocity posttreatment between CAD and non‐CAD patients in the study groups (P for interaction=1.273 and P=0.478, respectively).
Discussion
In the present study, we have shown that patients treated with GLP‐1RA, SGLT‐2i, and their combination achieved a greater reduction of BMI, central SBP, and PWV and greater improvement of endothelial glycocalyx thickness compared with patients treated with insulin after 12 months of antidiabetic treatment. Moreover, patients treated with GLP‐1RA or combination of GLP‐1RA and SGLT‐2i showed a greater increase of myocardial work index attributed to an increase in constructive and decrease of wasted myocardial work than those treated with insulin or SGLT‐2i at 12 months, despite a similar improvement of glycemic burden, as assessed by the reduction of HbA1c value. On the other hand, patients treated with SGLT‐2i or combination of GLP‐1RA and SGLT‐2i showed a greater reduction of PWV and systolic brachial blood pressure compared with those treated with insulin or GLP‐1RA, respectively. The combined treatment of GLP‐1RA and SGLT‐2i showed a greater improvement of the measured vascular and myocardial markers than each one treatment.
Several studies have shown that GLP‐1RA modulate the cardiovascular system and present favorable effects on blood pressure, body weight, HbA1c, and lipid status.24 However, the extent of effect on traditional risk factors overall is modest. Especially, the reduction of SBP is considerably less that presented by SGLT‐2 inhibitors,24 as also shown in the present study. Moreover, GLP‐1RA have been shown to improve endothelial function and decrease inflammation, atherosclerosis, and myocardial ischemia.25 In patients with ST‐segment–elevation myocardial infarction, the use of GLP‐1RA has been shown to reduce infarct size and improve regional and global LV function.26, 27 Furthermore, GLP‐1RA augment ventricular contractility, enhance myocardial glucose uptake, and improve LV performance in conscious dogs with pacing‐induced dilated cardiomyopathy, whereas GLP‐1RA exert cytoprotective and metabolic actions on cardiomyocytes.28, 29 In a previous study, we have shown that 6‐month treatment with the GLP‐1 receptor agonist liraglutide resulted in a greater improvement of LV longitudinal deformation compared with metformin in newly diagnosed patients with T2DM.30 In line with the above findings, in the current study, patients under GLP‐1RA had improved LV myocardial strain (longitudinal, circumferential, and radial) and showed more effective cardiac work, as estimated by the increase of global myocardial work index, related to an increase in constructive and decrease in wasted myocardial work, compared with the group of insulin or SGLT‐2i, despite a similar improvement of glycemic burden. These findings may be interpreted by both the direct cardiac effects of GLP‐1RA and the reduction of arterial stiffness and central arterial hemodynamics, as observed in this study post–GLP‐1RA treatment.
Previous studies have shown that the use of GLP‐1RA is associated with elevated heart rate.31, 32 This effect was also observed in our study. GLP‐1 receptors have been localized to all 4 cardiac chambers, including the sinoatrial node,33 which may provide an explanation of the chronotropic effect of GLP‐1 receptor agonists.
SGLT‐2 blockade in the proximal convoluted tubule leads to osmotic diuresis, caused by glycosuria, and natriuresis.24 In addition, SGLT‐2i promote a greater decrease in interstitial fluid relative to intravascular volume.34 Thus, through the above mechanisms, SGLT‐2i are able to reduce preload and LV filling pressures in patients with heart failure. Moreover, SGLT‐2i have an important role in modulation of afterload through decrease of arterial stiffness and vascular resistance, in addition to reduction in blood pressure,35 leading to improvement of subendocardial blood flow in patients with heart failure.36 SGLT‐2i are being studied as heart failure treatment independently of the diabetic status, and the results of studies on the effects of SGLT‐2i treatment on cardiovascular events37 and markers of LV function38 in patients with heart failure and no diabetes mellitus are expected. In a previous study, treatment with dapagliflozin versus hydrochlorothiazide significantly improved arterial stiffness and systemic endothelial function, as assessed by the aortic PWV, brachial flow‐mediated dilation, and shear rates, independent of changes in blood pressure, suggesting that SGLT‐2i improve systemic vascular function.39 Indeed, in our study, patients treated with SGLT‐2i had greater reduction of brachial and central SBP, and PWV already evident by the fourth month of treatment than patients under insulin or GLP‐1RA.
Current data also suggest that SGLT‐2i may inhibit myocardial Na+/H+ exchange and lead to lower intracellular Na+ and Ca2+ while increasing mitochondrial Ca2+ concentrations in isolated ventricular myocytes from rats and rabbits.40 Treatment with SGLT‐2i improved longitudinal, circumferential, and radial LV strain and ameliorated adverse cardiac remodeling and heart failure in a nondiabetic porcine model,41 as also confirmed in the present study of patients with T2DM. In addition, SGLT‐2i increase both ketonemia and cardiomyocyte use of ketone bodies.41 This is important as exogenous infusion of ketone bodies improves myocardial contractility in patients with heart failure and reduced ejection fraction.42 Moreover, animal models have shown that SGLT‐2i reduce myocardial extracellular matrix accumulation and cardiac fibrosis, activate the signal transducer and activator of transcription 3 signaling pathway and reduce myocardial interleukin‐6 and inducible NO synthase expressions regulating inflammatory responses and oxidation‐reduction signaling in the ischemic myocardium.43, 44 Recent studies suggest that SGLT‐2i have favorable effect on LV mass and diastolic function in patients with T2DM and CAD.45, 46 However, SGLT‐2i are primarily localized in the kidney and are not found in the heart, making a direct cardiac effect unlikely compared with GLP‐1RA.3, 47 In the present study, we observed that patients treated with SGLT‐2i had a smaller increase of LV myocardial strain (longitudinal, circumferential, and radial strain) and global work index compared with those treated with GLP‐1RA at 12 months, but this increase remained higher than those treated with insulin.
Because the cardiovascular benefit of SGLT‐2i is most likely related to the agent's hemodynamic benefit, whereas those of the GLP‐1RA are also related to their direct cardiac actions, the 2 classes of antidiabetic medications may produce an additive cardiovascular benefit, as we demonstrated in the present study. Indeed, in our study, patients under combination of GLP‐1RA and SGLT‐2i had a remarkable increase of endothelial glycocalyx thickness, as assessed by PBR. The glycocalyx is a gel‐like layer of proteoglycans, glycoproteins, and adsorbed plasma proteins, lining the luminal surface of the endothelium. It serves as a barrier that protects vessel wall from the circulating inflammatory cells.7 In addition, experimental studies have shown that glycocalyx has a crucial role in vascular homeostasis as it acts as a transducer of fluid shearing stress, mediating shear‐induced release of NO by endothelial cells. Insulin resistance is related with impaired glycocalyx, resulting in abnormal myocardial deformation in first‐degree relatives of diabetic patients.48 Acute‐ and long‐term hyperglycemia also results in glycocalyx damage with a concomitant increase of vascular permeability and activation of coagulation, leading to endothelial dysfunction.6 Indeed, in a recent study, we have shown that HbA1c is positively correlated with PBR, a noninvasive marker of glycocalyx thickness, suggesting that excessive hyperglycemia may contribute to the loss of glycocalyx integrity.49 On the contrary, the endothelial glycocalyx thickness improved after successful glycemic control or treatment with incretin‐based agents at the 1‐year follow‐up.49 Using invasive techniques, endothelial glycocalyx volume is estimated using the differences in the intravascular volumes of glycocalyx‐permeable tracers, such as dextran (40 kDa) or fluorescein‐labeled erythrocytes.6 Nevertheless, the invasive nature and the time‐consuming preparations limit its use in clinical settings. In the current study, a noninvasive, semiautomated imaging method is performed to measure glycocalyx by side‐view dark‐field imaging of the sublingual vasculature. Glycocalyx limits the proximity of red blood cells to the endothelial surface. Thus, the red blood cell–endothelium gap of the capillaries, as assessed by the PBR using side‐view dark‐field imaging, quantifies glycocalyx thickness.7 Smoking cessation, use of incretin‐based medication, and statins have been shown to improve PBR.7, 49, 50
In this study, the greater endothelial glycocalyx improvement in patients under combination of GLP‐1RA and SGLT‐2i was associated with a higher reduction of arterial stiffness and a greater improvement on LV myocardial function markers (namely, GLS, GCS, GRS, and myocardial work index) than each one the other 3 treatment regimens alone. Consequently, the combination GLP‐1RA and SGLT‐2i showed the greatest improvement in the ventricular‐arterial interaction, as assessed by PWV/GLS ratio, and is in agreement with the proposals of recent consensus article to select the appropriate medications that improve the ventricular‐arterial coupling in diabetes mellitus to improve prognosis.10 Interestingly, in the subset of patients under combined therapy with GLP‐1RA and SGLT‐2i and an LVEF <55%, GLS, GCS, and effective myocardial work presented greater improvement than patients under the same therapy and LVEF ≥55%. This finding suggests a favorable effect of the dual therapy in patients with impaired LV myocardial function.
In addition, recent study has shown that SGLT‐2i added to GLP‐1RA improved glycemic control with notable reduction in body weight and fat mass in patients with T2DM.51 Moreover, GLP‐1 receptor agonist and SGLT‐2i dual therapy produced sustained reduction in body weight, prediabetes mellitus prevalence, and SBP in obese adults without diabetes mellitus after 12‐month treatment.52 In our study, the combined therapy with GLP‐1RA and SGLT‐2i was also associated with a greater reduction in BMI than GLP‐1RA monotherapy in subjects with T2DM. The combination of appetite suppression by GLP‐1RA and calorie loss as a result of glucosuria induced by SGLT‐2i may be a possible causative mechanism. Thus, our findings suggest that the dual treatment with GLP‐1RA and SGLT‐2i has the most favorable effect on metabolic parameters and vascular and myocardial function, particularly in patients with an already impaired LV function.
Limitations
The limitations of the current study were the modest number of subjects for the treatment groups tested. Prospective large‐scale studies are needed to investigate the long‐term cardioprotective properties of GLP‐1RA and SGLT‐2i and expand our findings.
Conclusions
Twelve‐month treatment with GLP‐1RA, SGLT‐2i, and their combination confers a greater improvement of vascular markers, endothelial glycocalyx thickness, and ventricular‐arterial interaction and a more effective cardiac work than insulin treatment in patients with T2DM. These benefits are probably attributable not only to effective glycemic control but also the direct and indirect vascular and cardiac effects of the newer antidiabetic agents. The results of our study suggest that a combined treatment of GLP‐1RA and SGLT‐2i is a promising second‐line therapeutic option in subjects with high or very high cardiovascular risk with poor glycemic control.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e015716 DOI: 10.1161/JAHA.119.015716.)
For Sources of Funding and Disclosures, see page 19.
References
- 1. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus ‐ mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. [DOI] [PubMed] [Google Scholar]
- 3. DeFronzo RA. Combination therapy with GLP‐1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 5. Van Teeffelen JW, Brands J, Stroes ES, Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med. 2007;17:101–105. [DOI] [PubMed] [Google Scholar]
- 6. Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. [DOI] [PubMed] [Google Scholar]
- 7. Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775–789. [DOI] [PubMed] [Google Scholar]
- 8. Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Westerhof N, Stehouwer CD. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–181. [DOI] [PubMed] [Google Scholar]
- 9. Tritakis V, Tzortzis S, Ikonomidis I, Dima K, Pavlidis G, Trivilou P, Paraskevaidis I, Katsimaglis G, Parissis J, Lekakis J. Association of arterial stiffness with coronary flow reserve in revascularized coronary artery disease patients. World J Cardiol. 2016;8:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, De Carlo M, Delgado V, Lancellotti P, Lekakis J, et al. The role of ventricular‐arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions: a consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail. 2019;21:402–424. [DOI] [PubMed] [Google Scholar]
- 11. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, et al; ESC Scientific Document Group . 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther. 2016;7:187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerstein HC, Jung H, Rydén L, Diaz R, Gilbert RE, Yusuf S; ORIGIN Investigators . Effect of basal insulin glargine on first and recurrent episodes of heart failure hospitalization: the ORIGIN trial (Outcome Reduction With Initial Glargine Intervention). Circulation. 2018;137:88–90. [DOI] [PubMed] [Google Scholar]
- 14. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23:1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al; ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 17. Ikonomidis I, Makavos G, Papadavid E, Varoudi M, Andreadou I, Gravanis K, Theodoropoulos K, Pavlidis G, Triantafyllidi H, Parissis J, et al. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: the role of oxidative stress and inflammation. Can J Cardiol. 2015;31:287–295. [DOI] [PubMed] [Google Scholar]
- 18. Ikonomidis I, Tzortzis S, Andreadou I, Paraskevaidis I, Katseli C, Katsimbri P, Pavlidis G, Parissis J, Kremastinos D, Anastasiou‐Nana M, et al. Increased benefit of interleukin‐1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7:619–628. [DOI] [PubMed] [Google Scholar]
- 19. Sugimoto T, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Akhaladze N, Athanassopoulos GD, Barone D, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18:833–840. [DOI] [PubMed] [Google Scholar]
- 20. Ikonomidis I, Katsanos S, Triantafyllidi H, Parissis J, Tzortzis S, Pavlidis G, Trivilou P, Makavos G, Varoudi M, Frogoudaki A, et al. Pulse wave velocity to global longitudinal strain ratio in hypertension. Eur J Clin Invest. 2019;49:e13049. [DOI] [PubMed] [Google Scholar]
- 21. Manganaro R, Marchetta S, Dulgheru R, Sugimoto T, Tsugu T, Ilardi F, Cicenia M, Ancion A, Postolache A, Martinez C, et al. Correlation between non‐invasive myocardial work indices and main parameters of systolic and diastolic function: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2019;pii:jez203 DOI: 10.1093/ehjci/jez203. [DOI] [PubMed] [Google Scholar]
- 22. Muller KE, Lavange LM, Ramey SL, Ramey CT. Power calculations for general linear multivariate models including repeated measures applications. J Am Stat Assoc. 1992;87:1209–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41:356–363. [DOI] [PubMed] [Google Scholar]
- 24. Garg V, Verma S, Connelly K. Mechanistic insights regarding the role of SGLT2 inhibitors and GLP1 agonist drugs on cardiovascular disease in diabetes. Prog Cardiovasc Dis. 2019;62:349–357. [DOI] [PubMed] [Google Scholar]
- 25. Del Olmo‐Garcia MI, Merino‐Torres JF. GLP‐1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. J Diabetes Res. 2018;2018:4020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, et al. Exenatide reduces reperfusion injury in patients with ST‐segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. [DOI] [PubMed] [Google Scholar]
- 27. Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon‐like peptide‐1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. [DOI] [PubMed] [Google Scholar]
- 28. Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon‐like peptide‐1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing‐induced dilated cardiomyopathy. Circulation. 2004;110:955–961. [DOI] [PubMed] [Google Scholar]
- 29. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Circulation. 2017;136:849–870. [DOI] [PubMed] [Google Scholar]
- 30. Lambadiari V, Pavlidis G, Kousathana F, Varoudi M, Vlastos D, Maratou E, Georgiou D, Andreadou I, Parissis J, Triantafyllidi H, et al. Effects of 6‐month treatment with the glucagon like peptide‐1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta‐analysis. BMJ Open. 2013;3:e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baggio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, Butany J, Cappola TP, Margulies KB, Drucker DJ. GLP‐1 receptor expression within the human heart. Endocrinology. 2018;159:1570–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drucker DJ. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab. 2016;24:15–30. [DOI] [PubMed] [Google Scholar]
- 34. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. [DOI] [PubMed] [Google Scholar]
- 35. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter‐2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, et al. EMPEROR‐reduced trial committees and investigators. Eur J Heart Fail. 2019;21:1270–1278. [DOI] [PubMed] [Google Scholar]
- 38. Santos‐Gallego CG, Garcia‐Ropero A, Mancini D, Pinney SP, Contreras JP, Fergus I, Abascal V, Moreno P, Atallah‐Lajam F, Tamler R, et al. Rationale and design of the EMPA‐TROPISM Trial (ATRU‐4): are the “cardiac benefits” of empagliflozin independent of its hypoglycemic activity? Cardiovasc Drugs Ther. 2019;33:87–95. [DOI] [PubMed] [Google Scholar]
- 39. Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, Coronel R, Zuurbier CJ. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Santos‐Gallego CG, Requena‐Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia‐Ropero A, Sanz J, Hajjar RJ, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. [DOI] [PubMed] [Google Scholar]
- 42. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, et al. Cardiovascular effects of treatment with the ketone body 3‐hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. [DOI] [PubMed] [Google Scholar]
- 44. Andreadou I, Efentakis P, Balafas E, Togliatto G, Davos CH, Varela A, Dimitriou CA, Nikolaou PE, Maratou E, Lambadiari V, et al. Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front Physiol. 2017;8:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verma S, Garg A, Yan AT, Gupta AK, Al‐Omran M, Sabongui A, Teoh H, Mazer CD, Connelly KA. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA‐REG OUTCOME trial? Diabetes Care. 2016;39:e212–e213. [DOI] [PubMed] [Google Scholar]
- 46. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, et al; EMPA‐HEART CardioLink‐6 Investigators . Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes and coronary artery disease: the EMPA‐HEART CardioLink‐6 randomized clinical trial. Circulation. 2019;140:1693–1702. [DOI] [PubMed] [Google Scholar]
- 47. Di Franco A, Cantini G, Tani A, Coppini R, Zecchi‐Orlandini S, Raimondi L, Luconi M, Mannucci E. Sodium‐dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol. 2017;243:86–90. [DOI] [PubMed] [Google Scholar]
- 48. Ikonomidis I, Pavlidis G, Lambadiari V, Kousathana F, Varoudi M, Spanoudi F, Maratou E, Parissis J, Triantafyllidi H, Dimitriadis G, et al. Early detection of left ventricular dysfunction in first‐degree relatives of diabetic patients by myocardial deformation imaging: the role of endothelial glycocalyx damage. Int J Cardiol. 2017;233:105–112. [DOI] [PubMed] [Google Scholar]
- 49. Lambadiari V, Pavlidis G, Kousathana F, Maratou E, Georgiou D, Andreadou I, Kountouri A, Varoudi M, Balampanis K, Parissis J, et al. Effects of different antidiabetic medications on endothelial glycocalyx, myocardial function, and vascular function in type 2 diabetic patients: one year follow‐up study. J Clin Med. 2019;8:E983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ikonomidis I, Marinou M, Vlastos D, Kourea K, Andreadou I, Liarakos N, Triantafyllidi H, Pavlidis G, Tsougos E, Parissis J, et al. Effects of varenicline and nicotine replacement therapy on arterial elasticity, endothelial glycocalyx and oxidative stress during a 3‐month smoking cessation program. Atherosclerosis. 2017;262:123–130. [DOI] [PubMed] [Google Scholar]
- 51. Seino Y, Yabe D, Sasaki T, Fukatsu A, Imazeki H, Ochiai H, Sakai S. Sodium‐glucose cotransporter‐2 inhibitor luseogliflozin added to glucagon‐like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52‐week, open‐label, single‐arm study. J Diabetes Invest. 2018;9:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lundkvist P, Pereira MJ, Katsogiannos P, Sjöström CD, Johnsson E, Eriksson JW. Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: sustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes Metab. 2017;19:1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]


