Abstract
Background
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) and sodium‐glucose cotransporter‐2 inhibitors (SGLT2is) have shown their beneficial effects on cardiovascular outcomes and multiple cardiovascular risk factors, including hypertension. However, the mechanism of blood pressure (BP)–lowering effects of these agents has not been elucidated. This study aims to evaluate the effect of hemoglobin A1c reduction or body weight reduction with GLP‐1RA treatment and SGLT2i treatment on BP changes in patients with type 2 diabetes mellitus.
Methods and Results
Studies were identified by a search of MEDLINE, EMBASE, and the Cochrane Central Register until June 2019. Meta‐regression analysis was performed to evaluate the association between hemoglobin A1c reduction or body weight reduction and changes of BP. A total of 184 trials were included. Both GLP‐1RA and SGLT2i led to significant reductions in systolic BP (weighted mean difference, −2.856 and −4.331 mm Hg, respectively; P<0.001 for both) and diastolic BP (weighted mean difference, −0.898 and −2.279 mm Hg, respectively; P<0.001 for both). For both drug classes, hemoglobin A1c reduction was not independently associated with systolic BP reduction or diastolic BP reduction. In GLP‐1RA treatment, weight reduction was positively associated with systolic BP reduction and diastolic BP reduction (β=0.821 and β=0.287, respectively; P<0.001 for both). In SGLT2i treatment, weight loss was significantly associated with systolic BP reduction (β=0.820; P=0.001) but was not associated with diastolic BP reduction.
Conclusions
Treatment with GLP‐1RA and SGLT2i led to significant reductions in BP in patients with type 2 diabetes mellitus. Weight reduction was significantly and independently associated with BP reductions in GLP‐1RA treatment and SGLT2i treatment.
Keywords: blood pressure, glucagon‐like peptide‐1 receptor agonists, meta‐analysis, sodium‐glucose cotransporter‐2 inhibitors, type 2 diabetes mellitus
Subject Categories: Blood Pressure; Meta Analysis; Diabetes, Type 2
Nonstandard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- GLP‐1
glucagon‐like peptide‐1
- GLP‐1RA
glucagon‐like peptide‐1 receptor agonist
- SBP
systolic blood pressure
- SGLT2i
sodium‐glucose cotransporter‐2 inhibitor
- T2DM
type 2 diabetes mellitus
- WMD
weighted mean difference
Clinical Perspective
What Is New?
To date, 2 classes of antidiabetic agents, glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) and sodium‐glucose cotransporter‐2 inhibitor (SGLT2i), have been shown to improve cardiovascular outcomes and multiple cardiovascular risk factors, including hypertension; however, the mechanism of blood pressure (BP)–lowering effects of these drugs has not been fully elucidated.
Evidence for the association between glycemic control or weight reduction and the BP changes provided a mix of results.
The effect of hemoglobin A1c reduction or weight reduction on BP changes in GLP‐1RA treatment and SGLT2i treatment is evaluated in this study.
What Are the Clinical Implications?
Weight reduction, not hemoglobin A1c reduction, is positively associated with BP reductions in GLP‐1RA treatment and SGLT2i treatment.
The findings of the present study might offer some insight into the potential mechanism by which GLP‐1RA and SGLT2i reduce BP in patients with diabetes mellitus.
Treatment with GLP‐1RA and SGLT2i results in weight loss and BP reduction in patients with diabetes mellitus, and these effects are attractive therapeutic properties in the management of type 2 diabetes mellitus.
Introduction
Type 2 diabetes mellitus (T2DM) is associated with a high risk of macrovascular events and microvascular disease.1, 2, 3 Hypertension is a common comorbidity that affects more than half of patients with T2DM and contributes to the risk of cardiovascular disease and microvascular complications.4, 5 It was demonstrated that optimal blood pressure (BP) control could reduce the risks of all‐cause mortality, cardiovascular disease, stroke, as well as diabetic retinopathy and albuminuria in patients with T2DM.6 BP control is therefore an important strategy for improving the prognosis of patients with T2DM.
Two classes of antidiabetic agents, glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs)7, 8, 9, 10 and sodium‐glucose cotransporter‐2 inhibitors (SGLT2is),11, 12, 13 showed their beneficial effects on cardiovascular outcomes and multiple cardiovascular risk factors, including hypertension. The BP‐lowering effects of these 2 agents were established recently,14, 15, 16 but the exact mechanisms accounting for their antihypertensive effects were not elucidated yet. It was suggested that the BP reduction of GLP‐1RA treatment and SGLT2i treatment might be in part via weight loss.17, 18, 19 In addition, it was supposed that endothelial dysfunction and arterial stiffness induced by hyperglycemia might be involved in the pathogenesis of hypertension.20, 21 Thus, improvement in glycemic control may indirectly contribute to the BP‐lowering effect of these agents.
Previously, a pooled data analysis demonstrated that improved glycemic control and weight reduction was associated with BP reduction in patients with T2DM treated with exenatide.22 Furthermore, pooled analyses indicated that the weight loss associated with dapagliflozin and canagliflozin contributed to the reduction in systolic BP (SBP).23, 24 However, results from another study found a weak correlation between weight lost and reduction in SBP in exenatide‐treated patients.25 In addition, in a meta‐analysis evaluating the effects of SGLT2i on 24‐hour ambulatory BP, no significant association was observed between 24‐hour ambulatory BP and change in body weight.26 Some researchers indicated that the BP‐lowering effect occurred earlier than any significant weight loss in GLP‐1RA treatment27, 28, 29 and SGLT2i treatment,30 suggesting that the BP reduction may be mediated through mechanisms other than weight loss. To date, evidence for the association between blood glucose changes or weight reduction and the BP changes provided a mix of results.
Therefore, the aim of this meta‐analysis is to evaluate the effect of hemoglobin A1c (HbA1c) reduction or body weight reduction on BP changes in GLP‐1RA treatment and SGLT2i treatment in patients with T2DM.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Search Strategy
This meta‐analysis was conducted according to the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines.31 The study protocol is available in the International Prospective Register of Systematic Reviews, PROSPERO (registration Nos. CRD42018108738 and CRD42018105041).
Studies were identified by a literature search of MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials until June 2019. The overall searching strategy was performed using T2DM separately with the following terms: GLP‐1RA, albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, taspoglutide, SGLT2i, dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, luseogliflozin, ertugliflozin, remogliflozin, and sotagliflozin. Complete details of the search strategy are shown in Data S1.
Study Selection and Data Extraction
Studies were included if they met the following criteria: (1) randomized controlled trials comparing the efficacy and safety of GLP‐1RA or SGLT2i with placebo or other antidiabetic agents in participants with T2DM; (2) studies with duration ≥4 weeks; (3) the primary outcome was change in HbA1c, weight, or BP; cardiovascular outcome trials that reported BP changes from baseline were also included; and (4) studies that reported BP changes from baseline with mean difference.
The exclusion criteria were as follows: (1) non–randomized controlled trials; (2) studies in patients with type 1 diabetes mellitus; (3) studies with duration <4 weeks; (3) studies that did not report BP changes from baseline; and (4) studies that did not report SD, SE, or 95% CI of BP changes. Extension studies were excluded from this meta‐analysis to minimize the variations.
Two review authors (M.H. and S.Z.) independently performed the data extraction from each publication using a standardized form: publication data (study title, first author, publication year, and source of publication), study design, baseline characteristics of the study population (sample size, sex, age, body mass index [BMI], duration of T2DM, and baseline BP), description of the study drugs and dosages, duration of follow‐up, and changes of HbA1c, body weight, SBP, and diastolic BP (DBP) from baseline to study end point. Disagreements or discrepancies were resolved by discussion among the 2 review authors and a third investigator (W.Y.).
Assessment of Methodological Quality
The quality of each study was evaluated according to criteria provided in the Cochrane Handbook.32 Each trial was judged into low, high, or unclear risk of bias for the following aspects: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias.
Statistical Analysis
In this meta‐analysis, weighted mean difference (WMD) and 95% CI were calculated using inverse variance weighted random effect model to evaluate the changes of HbA1c, body weight, SBP, and DBP from baseline in GLP‐1RA and SGLT2i treatments. For placebo‐controlled trials and active‐controlled trials, variables compared with placebo or different classes of comparators were also calculated. Meta‐regression analysis was performed to evaluate the association between HbA1c reduction or body weight reduction and changes of BP. Confounding factors, including age, sex, BMI, and duration of diabetes mellitus, were adjusted by using multivariable meta‐regression analysis. Subgroup analyses were performed by pooling data for each individual GLP‐1RA and SGLT2i separately. If trials with >1 intervention group were identified, we determined which treatment groups in the study are relevant to our meta‐analysis/subgroup analysis, according to the Cochrane Handbook,32 and only these treatment groups were used in analyses. The number of observations refers to the number of treatment group (group of participants who receive GLP‐1RA or SGLT2i treatment) of studies. The heterogeneity among studies was assessed using the Higgins I2 statistics. Publication bias was assessed via a visual inspection of the funnel plot and Egger's test. All statistical analyses were conducted using STATA statistical software package, version 14.0.
Results
Search Selection and Characteristics
Details of the study selection process are presented by a flowchart (Figure 1). Finally, a total of 184 studies were included in the meta‐analysis, including 89 studies with GLP‐1RA treatment and 94 studies with SGLT2i treatment. One study compared the efficacy and safety of coinitiation of the GLP‐1RA and SGLT2i with either drug alone.33 A total of 44 trials compared a GLP‐1RA with a placebo, and 85 trials compared a SGLT2i with a placebo.
Figure 1. Flowchart of trial identification for meta‐analysis.
CENTRAL indicates Cochrane Central Register of Controlled Trials; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; and SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
This meta‐analysis was based on data from 61 299 individuals in the GLP‐1RA treatment and 40 874 individuals in the SGLT2i treatment. Characteristics of the individuals receiving GLP‐1RA treatment and SGLT2i treatment in this meta‐analysis were shown in Table S1. The range of age of the patients who received treatment with GLP‐1RA and SGLT2i was from 46.7 to 68.0 years and from 50.6 to 70.9 years, with the male percentage ranging from 24.8% to 83.0% and from 28.3% to 83.2%, respectively. The mean SBP level at randomization was 132.30 mm Hg (range, 122–138 mm Hg) in GLP‐1RA trials and 131.90 mm Hg (range, 122–151 mm Hg) in SGLT2i trials. Mean DBP level at baseline was 78.66 mm Hg (range, 72.9–84.8 mm Hg) in GLP‐1RA trials and 78.89 mm Hg (range, 73.5–91.2 mm Hg) in SGLT2i trials. Clinical characteristics of included studies with GLP‐1RA treatment and SGLT2i treatment are presented as Tables S2 and S3. The risk of bias was assessed with the Cochrane Handbook. Details of the quality of bias assessment were shown in Tables S4 and S5.
BP Changes in GLP‐1RA Treatment
Analysis of the pooled data across all studies showed that GLP‐1RA led to significant decreases in SBP (WMD, −2.856 mm Hg; 95% CI, −3.017 to −2.695 mm Hg; P<0.001) and DBP (WMD, −0.898 mm Hg; 95% CI, −1.007 to −0.789 mm Hg; P<0.001) from baseline (Figure 2 and Table S6). Compared with placebo, GLP‐1RA resulted in a significantly greater decrease in SBP (WMD, −1.724 mm Hg; 95% CI, −2.043 to −1.404 mm Hg; P<0.001). GLP‐1RA treatment was also associated with a significantly greater reduction in SBP in comparison with insulin treatment (WMD, −2.763 mm Hg; 95% CI, −3.306 to −2.220 mm Hg; P<0.001), sulfonylurea treatment (WMD, −2.721 mm Hg; 95% CI, −3.459 to −1.983 mm Hg; P<0.001), and dipeptidyl‐peptidase‐4 inhibitor treatment (WMD, −1.150 mm Hg; 95% CI, −1.657 to −0.644 mm Hg; P<0.001). No significant difference in DBP was found when GLP‐1RA treatment compared with placebo or active comparator treatment, except for sulfonylureas treatment (WMD, −1.318 mm Hg; 95% CI, −1.944 to −0.693 mm Hg; P<0.001). The changes in SBP and DBP with each individual GLP‐1RA treatment were also shown in Table S6. Subgroup analysis stratified by treatment strategy (monotherapy or combination therapy), study duration, and study primary end point showed that the results were similar as the total group, except for DBP changes in studies with BP change as the primary end point (WMD, 0.187 mm Hg; 95% CI, −0.471–0.845 mm Hg; P=0.577; Table S7), which may be attributed to the limited number of studies included in this subgroup. Statistical tests for the comparisons of the effect sizes among subgroups were shown in Table S8. HbA1c and weight changes in GLP‐1RA treatment were shown in Table S9.
Figure 2. Forest plots of systolic blood pressure (SBP ) changes (A) and diastolic blood pressure (DBP ) changes (B) in glucagon‐like peptide‐1 receptor agonist (GLP‐1RA ) treatment.
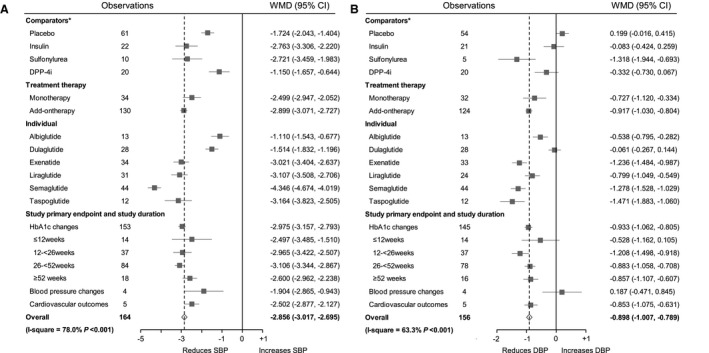
*Weighted mean difference (WMD) and 95% CI were calculated for a change from baseline to the study end point for GLP‐1RA vs placebo or different classes of comparators. DPP‐4i indicates dipeptidyl‐peptidase‐4 inhibitor; and HbA1c, hemoglobin A1c.
BP Changes in SGLT2i Treatment
Treatment with SGLT2i resulted in significant decreases in SBP (WMD, −4.331 mm Hg; 95% CI, −4.476 to −4.185 mm Hg; P<0.001) and DBP (WMD, −2.279 mm Hg; 95% CI, −2.376 to −2.182 mm Hg; P<0.001) from baseline (Figure 3 and Table S6). Compared with placebo, SGLT2i treatment led to a significantly greater reduction in SBP (WMD, −3.612 mm Hg; 95% CI, −3.844 to −3.379 mm Hg; P<0.001) and led to a significantly greater reduction in DBP (WMD, −1.559 mm Hg; 95% CI, −1.713 to −1.406 mm Hg; P<0.001). SGLT2i treatment was also associated with significantly greater decreases in SBP and DBP in comparison with metformin, sulfonylurea, and dipeptidyl‐peptidase‐4 inhibitor treatment. The changes in SBP and DBP with each individual SGLT2i treatment were also shown in Table S6. No significant differences in BP changes were found by subgroup analysis stratified by treatment strategy (monotherapy or combination therapy) and study duration. The effect of SGLT2i on SBP changes was greater in studies in which the primary end point was changes in BP (WMD, −6.331 mm Hg; 95% CI, −6.853 to −5.809 mm Hg; P<0.001; Table S7). The possible reason is that the baseline SBP levels of participants were higher in those studies. Statistical test for the comparisons of the effect sizes among subgroups were shown in Table S8. HbA1c and weight changes in SGLT2i treatment were shown in Table S10.
Figure 3. Forest plots of systolic blood pressure (SBP ) changes (A) and diastolic blood pressure (DBP ) changes (B) in sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) treatment.
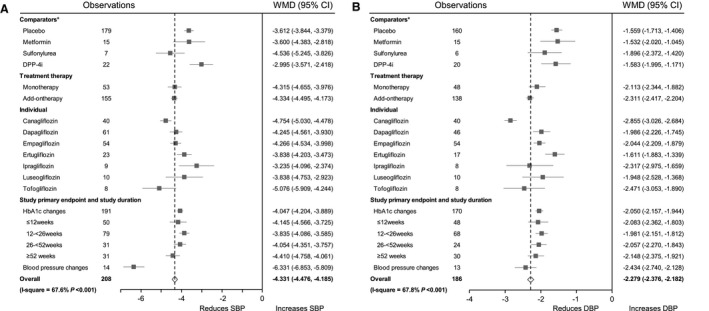
*Weighted mean difference (WMD ) and 95% CI were calculated for a change from baseline to the study end point for SGLT2i vs placebo or different classes of comparators. DPP‐4i indicates dipeptidyl‐peptidase‐4 inhibitor; and HbA1c, hemoglobin A1c.
Effect of HbA1c Change or Weight Reduction on BP Changes in GLP‐1RA Treatment
In terms of absolute BP changes, HbA1c change from baseline was significantly associated with SBP reduction (β=2.538; 95% CI, 1.652–3.425; P<0.001, adjusted for age, sex, BMI, and duration of diabetes mellitus; Figure 4A) and was also significantly associated with DBP reduction (adjusted β=0.727; 95% CI, 0.226–1.227; P=0.005; Figure 4B). In terms of placebo‐corrected BP changes, HbA1c reduction was positively associated with placebo‐corrected reduction in SBP (adjusted β=3.614; 95% CI, 2.107–5.122; P<0.001; Figure S1A) and HbA1c reduction was also positively associated with placebo‐corrected reduction in DBP (adjusted β=1.397; 95% CI, 0.280–2.515; P=0.015; Figure S1B).
Figure 4. Meta‐regression analysis of the associations between hemoglobin A1c (HbA1c) reduction or body weight reduction and blood pressure changes in glucagon‐like peptide‐1 receptor agonist (GLP‐1RA ) treatment.
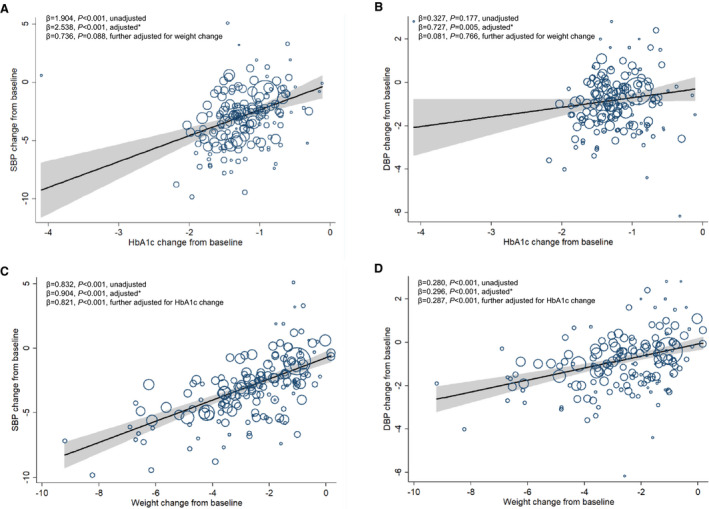
A, Association between HbA1c change from baseline and systolic blood pressure (SBP ) change from baseline. B, Association between HbA1c change from baseline and diastolic blood pressure (DBP ) change from baseline. C, Association between weight change from baseline and SBP change from baseline. D, Association between weight change from baseline and DBP change from baseline. The size of circles is proportional to the weight of each study in the meta‐regression. *Analyses were adjusted for age, sex, body mass index (BMI), and duration of diabetes mellitus.
Weight change from baseline was significantly associated with net changes in SBP and DPB (adjusted β=0.904 [95% CI, 0.739–1.070] and adjusted β=0.296 [95% CI, 0.196–0.396], respectively; P<0.001 for both) in GLP‐1RA treatment (Figure 4C and 4D). In terms of placebo‐corrected BP changes, weight reduction was positively associated with placebo‐corrected SBP reduction with significance (adjusted β=0.523; 95% CI, 0.270–0.776; P<0.001), but was not associated with placebo‐corrected DBP reduction (Figure S1C and S1D). Details were shown in Table 1 and Figures S1 through S3.
Table 1.
Effect of HbA1c Reduction or Weight Reduction on BP Changes in GLP‐1RA Treatment
| Variable | SBP Changes | DBP Changes | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P Value | Coefficient | 95% CI | P Value | |
| HbA1c change from baseline, % | ||||||
| Total | 2.538 | 1.652, 3.425 | <0.001 | 0.727 | 0.226, 1.227 | 0.005 |
| Placebo controlled | 3.614 | 2.107, 5.122 | <0.001 | 1.397 | 0.280, 2.515 | 0.015 |
| Active controlled | 2.392 | 1.341, 3.443 | <0.001 | 0.580 | −0.005, 1.166 | 0.052 |
| Insulin | 1.255 | −0.251, 2.761 | 0.096 | 0.327 | −0.647, 1.302 | 0.484 |
| Sulfonylurea | 2.919 | −9.527, 15.365 | 0.419 | … | … | … |
| DPP‐4i | 3.491 | 0.689, 6.292 | 0.018 | 0.228 | −1.775, 2.232 | 0.810 |
| Monotherapy | 2.144 | −0.414, 4.702 | 0.097 | −0.092 | −1.653, 1.469 | 0.904 |
| Add‐on therapy | 2.598 | 1.595, 3.600 | <0.001 | 0.790 | 0.249, 1.331 | 0.005 |
| Individual | ||||||
| Albiglutide | 4.102 | −6.201, 14.405 | 0.378 | 1.257 | −3.472, 5.987 | 0.550 |
| Dulaglutide | 3.281 | 0.526, 6.037 | 0.022 | 0.570 | −0.476, 1.616 | 0.271 |
| Exenatide | 2.105 | −0.100, 4.309 | 0.061 | 0.362 | −1.073, 1.797 | 0.608 |
| Liraglutide | 0.457 | −1.453, 2.3681 | 0.626 | 0.065 | −1.565, 1.695 | 0.934 |
| Semaglutide | 4.290 | 2.432, 6.148 | <0.001 | 1.186 | 0.176, 2.196 | 0.023 |
| Taspoglutide | −2.503 | −6.628, 1.621 | 0.188 | −0.141 | −2.708, 2.426 | 0.898 |
| Weight change from baseline, kg | ||||||
| Total | 0.904 | 0.739, 1.070 | <0.001 | 0.296 | 0.196, 0.396 | <0.001 |
| Placebo controlled | 0.523 | 0.270, 0.776 | <0.001 | 0.036 | −0.130, 0.203 | 0.661 |
| Active controlled | 0.876 | 0.660, 1.093 | <0.001 | 0.264 | 0.134, 0.395 | <0.001 |
| Insulin | 0.403 | −0.159, 0.965 | 0.147 | 0.109 | −0.244, 0.463 | 0.518 |
| Sulfonylurea | 0.931 | −3.359, 5.221 | 0.449 | … | … | … |
| DPP‐4i | 0.733 | 0.206, 1.259 | 0.010 | 0.087 | −0.292, 0.466 | 0.631 |
| Monotherapy | 1.114 | 0.530, 1.698 | 0.001 | 0.342 | −0.060, 0.744 | 0.092 |
| Add‐on therapy | 0.881 | 0.697, 1.064 | <0.001 | 0.262 | 0.154, 0.371 | <0.001 |
| Individual | ||||||
| Albiglutide | 1.481 | −7.048, 10.010 | 0.694 | 1.621 | −2.208, 5.450 | 0.350 |
| Dulaglutide | 0.710 | −0.081, 1.501 | 0.076 | 0.170 | −0.135, 0.474 | 0.260 |
| Exenatide | 1.811 | 1.155, 2.468 | <0.001 | 0.610 | 0.053, 1.167 | 0.033 |
| Liraglutide | 0.277 | −0.427, 0.981 | 0.425 | 0.151 | −0.392, 0.695 | 0.565 |
| Semaglutide | 0.904 | 0.595, 1.214 | <0.001 | 0.281 | 0.104, 0.457 | 0.003 |
| Taspoglutide | 0.654 | −0.324, 1.632 | 0.153 | 0.160 | −0.449, 0.769 | 0.544 |
Analyses were adjusted for age, sex, body mass index, and duration of diabetes mellitus by meta‐regression. BP indicates blood pressure; DBP, diastolic BP; DPP‐4i, dipeptidyl‐peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, hemoglobin A1c; and SBP, systolic BP.
Effect of HbA1c Change or Weight Reduction on BP Changes in SGLT2i Treatment
HbA1c reduction was not associated with SBP reduction or DBP reduction in SGLT2i treatment (Figure 5A and 5B). In terms of absolute BP changes, weight reduction in SGLT2i was positively associated with SBP reduction with significance (adjusted β=0.771; 95% CI, 0.314–1.228; P=0.001), but was not associated with DBP reduction (Figure 5C and 5D). In addition, weight change from baseline was significantly associated with SBP reduction in SGLT2i monotherapy (adjusted β=1.211; 95% CI, 0.140–2.283; P=0.028), and weight reduction was also significantly associated with SBP reduction in SGLT2i add‐on therapy (adjusted β=0.711; 95% CI, 0.204–1.219; P=0.007). No significant association was observed between weight reduction and DBP reduction, as either monotherapy or add‐on therapy. In terms of placebo‐corrected BP changes, weight reduction was associated with placebo‐corrected reduction in SBP (adjusted β=0.965; 95% CI, 0.456–1.473; P<0.001; Figure S4C) and weight reduction was also associated with placebo‐corrected reduction in DBP (adjusted β=0.385; 95% CI, 0.042–0.728; P=0.028; Figure S4D). Details were shown in Table 2 and Figures S4 through S6.
Figure 5. Meta‐regression analysis of the associations between hemoglobin A1c (HbA1c) reduction or body weight reduction and blood pressure changes in sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) treatment.
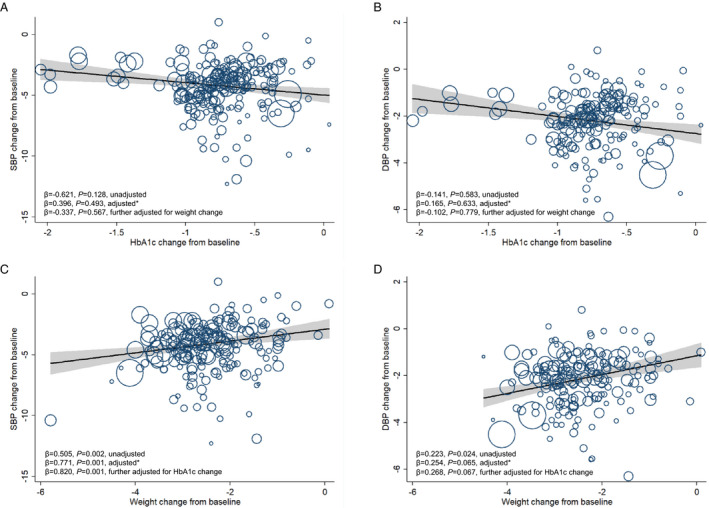
A, Association between HbA1c change from baseline and systolic blood pressure (SBP) change from baseline. B, Association between HbA1c change from baseline and diastolic blood pressure (DBP) change from baseline. C, Association between weight change from baseline and SBP change from baseline. D, Association between weight change from baseline and DBP change from baseline. The size of circles is proportional to the weight of each study in the meta‐regression. *Analyses were adjusted for age, sex, body mass index (BMI), and duration of diabetes mellitus.
Table 2.
Effect of HbA1c Reduction or Weight Reduction on BP Changes in SGLT2i Treatment
| Variable | SBP Changes | DBP Changes | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P Value | Coefficient | 95% CI | P Value | |
| HbA1c change from baseline, % | ||||||
| Total | 0.396 | −0.745, 1.537 | 0.493 | 0.165 | −0.517, 0.846 | 0.633 |
| Placebo controlled | −0.237 | −1.262, 0.788 | 0.647 | −0.296 | −1.005, 0.414 | 0.410 |
| Active controlled | 2.099 | −0.256, 4.455 | 0.078 | 1.593 | −0.036, 3.221 | 0.055 |
| Metformin | … | … | … | … | … | … |
| Sulfonylurea | … | … | … | … | … | … |
| DPP‐4i | 4.235 | −0.735, 9.206 | 0.086 | 1.364 | −3.183, 5.911 | 0.501 |
| Monotherapy | 0.587 | −2.337, 3.510 | 0.684 | 0.597 | −1.273, 2.467 | 0.516 |
| Add‐on therapy | 0.350 | −0.850, 1.550 | 0.564 | 0.198 | −0.546, 0.943 | 0.597 |
| Individual | ||||||
| Canagliflozin | −0.412 | −2.373, 1.549 | 0.669 | −0.277 | −1.275, 0.721 | 0.572 |
| Dapagliflozin | −0.386 | −2.179, 1.407 | 0.665 | −0.303 | −1.614, 1.008 | 0.638 |
| Empagliflozin* | 1.059 | −0.889, 3.007 | 0.280 | 0.574 | −0.667, 1.814 | 0.357 |
| Ertugliflozin | 0.870 | −1.250, 2.990 | 0.394 | 1.891 | −1.489, 5.272 | 0.233 |
| Ipragliflozin | −3.190 | −2.651, 2.173 | 0.773 | −3.958 | −45.880, 37.964 | 0.724 |
| Luseogliflozin | 13.433 | −2.002, 28.868 | 0.073 | 6.465 | −0.086, 13.016 | 0.052 |
| Tofogliflozin | −6.672 | −36.852, 23.509 | 0.442 | −8.437 | −28.661, 11.788 | 0.215 |
| Weight change from baseline, kg | ||||||
| Total | 0.771 | 0.314, 1.228 | 0.001 | 0.254 | −0.016, 0.524 | 0.065 |
| Placebo controlled | 0.965 | 0.456, 1.473 | <0.001 | 0.385 | 0.042, 0.728 | 0.028 |
| Active controlled | 0.924 | 0.052, 1.796 | 0.039 | 0.634 | −0.035, 1.302 | 0.062 |
| Metformin | … | … | … | … | … | … |
| Sulfonylurea | … | … | … | … | … | … |
| DPP‐4i | 0.296 | −1.504, 2.096 | 0.714 | −0.519 | −2.234, 1.196 | 0.487 |
| Monotherapy | 1.211 | 0.140, 2.283 | 0.028 | 0.767 | −0.038, 1.572 | 0.061 |
| Add‐on therapy | 0.711 | 0.204, 1.219 | 0.007 | 0.238 | −0.063, 0.540 | 0.119 |
| Individual | ||||||
| Canagliflozin | 0.763 | −0.226, 1.752 | 0.125 | 0.343 | −0.175, 0.861 | 0.184 |
| Dapagliflozin | 0.818 | −0.054, 1.691 | 0.065 | −0.143 | −0.812, 0.526 | 0.662 |
| Empagliflozin | 0.292 | −0.263, 0.847 | 0.295 | 0.327 | −0.035, 0.689 | 0.076 |
| Ertugliflozin | 0.397 | −0.642, 1.435 | 0.426 | 0.470 | −0.847, 1.788 | 0.434 |
| Ipragliflozin | 1.681 | −7.405, 10.766 | 0.597 | 0.421 | −10.693, 11.536 | 0.885 |
| Luseogliflozin | 3.246 | 0.113, 6.378 | 0.045 | 1.365 | −0.065, 2.795 | 0.057 |
| Tofogliflozin | −0.534 | −8.765, 7.698 | 0.806 | −2.684 | −8.459, 3.092 | 0.184 |
Analyses were adjusted for age, sex, body mass index, and duration of diabetes mellitus by meta‐regression. BP indicates blood pressure; DBP, diastolic BP; DPP‐4i, dipeptidyl‐peptidase‐4 inhibitor; HbA1c, hemoglobin A1c; SBP, systolic BP; and SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
For empagliflozin, analyses were adjusted for age, sex, and body mass index because most of the studies did not report duration of diabetes mellitus.
Effects of HbA1c and Weight Reduction on BP Changes in GLP‐1RA and SGLT2i Treatment
Analyses were conducted to explore the joint effects of HbA1c and weight reduction on BP changes (Table 3). In GLP‐1RA treatment, the associations between HbA1c reduction and SBP or DBP reduction became insignificant after further adjustment for weight change (Figure 4A and 4B). Weight reduction was positively associated with SBP reduction (β=0.821; 95% CI, 0.631–1.011; P<0.001), and weight reduction was also positively associated with DBP reduction (β=0.287; 95% CI, 0.172–0.403; P<0.001), independent of age, sex, BMI, duration of diabetes mellitus, and change in HbA1c (Figure 4C and 4D). In SGLT2i treatment, the effect of weight reduction on SBP change was also significant after adjustment for age, sex, BMI, duration of diabetes mellitus, and HbA1c change from baseline (β=0.820; 95% CI, 0.332–1.307; P=0.001; Figure 5C). Sex and antihypertensive therapy did not affect the association between weight loss and BP reductions in GLP‐1RA treatment and SGLT2i treatment (Tables S11 and S12). When data from GLP‐1RA studies and SGLT2i studies were merged into one data set, weight reduction was also positively and independently associated with SBP reduction and DBP reduction (β=0.903 [95% CI, 0.736–1.070] and β=0.375 [95% CI, 0.269–0.482], respectively; P<0.001 for both; Figure S7). Taken together, weight reduction was significantly and independently associated with BP reductions in GLP‐1RA treatment and SGLT2i treatment.
Table 3.
Effects of HbA1c and Weight Reduction on BP Changes in GLP‐1RA and SGLT2i Treatment
| Change From Baseline | SBP Changes | DBP Changes | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P Value | Coefficient | 95% CI | P Value | |
| GLP‐1RA | ||||||
| HbA1c change, % | 0.736 | −0.111, 1.584 | 0.088 | 0.081 | −0.454, 0.616 | 0.766 |
| Weight change, kg | 0.821 | 0.631, 1.011 | <0.001 | 0.287 | 0.172, 0.403 | <0.001 |
| SGLT2i | ||||||
| HbA1c change, % | −0.337 | −1.501, 0.826 | 0.567 | −0.102 | −0.820, 0.616 | 0.779 |
| Weight change, kg | 0.820 | 0.332, 1.307 | 0.001 | 0.268 | −0.019, 0.556 | 0.067 |
Analysis was performed using meta‐regression, with age, sex, body mass index, duration of diabetes mellitus, HbA1c change from baseline, and weight change from baseline as covariates. BP indicates blood pressure; DBP, diastolic BP; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, hemoglobin A1c; SBP, systolic BP; and SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
Publication Bias
The funnel plots for SBP and DBP analysis in GLP‐1RA studies were symmetry (Figure S8), but Egger's regression analysis suggested the presence of publication bias in the analysis of DBP (Egger's test P=0.044). The funnel plot of SBP changes in SGLT2i studies showed slight asymmetry (Figure S9A), and Egger's regression analysis also detected a potential publication bias (P=0.025). No evidence of publication bias was found for DBP analysis in SGLT2i studies by funnel plot or Egger's test (P=0.682; Figure S9B). Imputation of possibly unpublished negative studies by trim‐and‐fill method34 did not significantly alter the general results, suggesting that the publication bias did not impact the interpretation of the results.
Discussion
To date, among various classes of antihyperglycemic agents, both GLP‐1RA and SGLT2i have been shown to improve cardiovascular outcomes in patients with T2DM.35, 36, 37 The cardiovascular benefits of these drugs may partly be attributable to their BP‐lowering effects. The present meta‐analysis showed that weight reduction, not HbA1c reduction, was significantly and independently associated with BP reductions in GLP‐1RA and SGLT2i treatment. These results indicated that weight loss contributed to the BP‐lowering effects of GLP‐1RA and SGLT2i.
The BP‐lowering effects of GLP‐1RA and SGLT2i have been well documented in clinical trials and previous meta‐analyses.38, 39, 40, 41, 42 It has been reported that GLP‐1RA treatment was associated with significant reductions in SBP and DBP in comparison with placebo or other antidiabetic drugs.16, 43 Similar results of favorable effects of SGLT2i on BP have also been reported in recent meta‐analyses and systematic reviews.26, 44, 45
The exact mechanism responsible for the BP‐lowering effects with these agents has not been fully understood. Weight loss may be one of the important factors because evidence in clinical trials and epidemiologic studies showed that weight loss was associated with reduced BP.46, 47, 48 A pooled analysis of 6 randomized controlled trials reported a weak correlation between weight loss and reduction in SBP for exenatide‐treated subjects.25 Similarly, a weak but statistically significant association between weight reduction and SBP lowering was observed in another pooled analysis of randomized controlled trials.49 Both studies showed that the SBP reduction in GLP‐1RA treatment weakly associated with weight loss, but both studies included only 6 trials and the relationship was calculated by linear correlation without adjusting for possible confounding factors. In addition, in a meta‐analysis of 33 GLP‐1RA trials, the degree of SBP change was not related to weight loss or improvement in HbA1c, but trials of patients without T2DM were also included.50 Paul et al have reported that short‐term dynamics of BP in exenatide treatment were related to concomitant effects on glycemia and body weight, demonstrating that improved glycemic control and weight reduction were associated with BP reduction in treatment with exenatide.22 Meta‐regression analysis in our study found that weight reduction was significantly associated with BP lowering in GLP‐1RA treatment, even after adjusting for possible confounding factors, including age, sex, BMI, duration of diabetes mellitus, and change in HbA1c, indicating that weight loss may contribute to the BP‐lowering effect of GLP‐1RA. However, results of the joint effects of HbA1c reduction and weight reduction on BP showed that HbA1c reduction was not correlated with BP changes after adjusting for weight loss. It is likely that glycemic control can be improved by weight loss; therefore, the effect of HbA1c reduction on BP changes may be dependent on reduction in weight.
Some researchers indicated that the BP reductions observed in the clinical trials occurred earlier than any significant weight loss, suggesting that GLP‐1RA treatment may provide extra benefits independent of weight loss that lead to BP lowering.51, 52 Vasodilatation and natriuresis mediated by activation of glucagon‐like peptide‐1 (GLP‐1) receptor on cardiovascular and renal tissue likely contribute to the antihypertensive effect.19, 53 A study found that infusion of recombinant GLP‐1 improved endothelial function in patients with T2DM and established coronary artery disease.54 Moreover, infusion of GLP‐1 enhanced acetylcholine‐mediated vasodilation.55 In patients with T2DM, the administration of exenatide was associated with increased plasma concentrations of a series of vasodilator and suppression of renin‐angiotensin system.56 These results indicated a potentially direct benefit on vascular factors of GLP‐1 in humans. On the other hand, sustained liraglutide administration increased urinary sodium excretion in hypertensive subjects with T2DM.57 Similarly, another study observed intravenous infusions of GLP‐1 promoted natriuresis in both healthy and insulin‐resistant obese men.58 Therefore, GLP‐1–induced natriuresis may provide another mechanism for antihypertensive effect associated with GLP‐1RA.
Several studies reported the association between weight reduction and BP changes in treatment with SGLT2i. A previous meta‐analysis that involved 6 trials reported SGLT2i significantly reduced 24‐hour ambulatory SBP and DBP. However, no significant association between change in body weight and 24‐hour BP was observed in the study.26 Pooled data from placebo‐controlled studies in patients with T2DM indicated that weight loss contributed to reductions in BP in treatment with dapagliflozin24 or canagliflozin.23 Data from our meta‐analysis also support the evidence that weight reduction was positively associated with BP reduction, independent of age, sex, BMI, duration of diabetes mellitus, and HbA1c reduction. However, reductions in BP in SGLT2i treatment were also observed before body weight reductions,30, 59 suggesting that the BP‐lowering effect of SGLT2i cannot solely be ascribed to weight loss. Osmotic diuresis and mild natriuresis are thought to be the most likely explanations for the antihypertensive effect of SGLT2i.59, 60, 61, 62 The glucose‐based osmotic diuresis leads to an excess urinary output by 110 to 470 mL/d.63 A 7% reduction in plasma volume was observed in patients with T2DM treated with dapagliflozin, indicating the diuretic‐like capacity of dapagliflozin possibly resulted from enhanced sodium excretion or osmotic diuresis.64 In addition, reduction in arterial stiffness induced by SGLT2i might also play a part in BP lowering.65, 66 Further studies are needed to elucidate the underlying mechanism by which GLP‐1RA and SGLT2i reduce BP in patients with T2DM.
In the current analysis, there was a greater effect of GLP‐1RA and SGLT2i on SBP compared with DBP. The differential effects may be attributed to the mechanism for the antihypertensive actions of both drugs. In the present study, we demonstrated that weight loss was associated with BP reductions in GLP‐1RA treatment and SGLT2i treatment. A difference in response in SBP compared with DBP to weight reduction was observed in previous meta‐analysis, in which the effects of weight loss appear to be larger on SBP than on DBP.46, 67 Moreover, the BP‐lowering effects of GLP‐1RA treatment and SGLT2i treatment are thought to be partly mediated through enhanced urinary sodium excretion. The magnitude of the association between serum sodium levels and SBP was greater than DBP.68 Wannamethee et al69 found a positive association between serum sodium and SBP in hypertensive individuals. Although there was also a slight tendency for DBP to increase with increasing serum sodium, the trend was not significant. Another possible explanation of these finding is that the plasma volume reduction resulting from the increase in urinary glucose excretion induced by SGLT2i62, 70 and the relative reduction in intravascular volume resulting from vasodilation induced by GLP‐1RA71, 72 would be more likely to result in reductions in SBP compared with DBP.
Our meta‐analysis involved a substantial number of placebo‐controlled trials and active‐controlled trials for GLP‐1RA or SGLT2i treatment. With data from 61 299 individuals in the GLP‐1RA treatment and 40 874 individuals in the SGLT2i treatment, our analysis provided sufficient power to evaluate the effect of HbA1c reduction or weight reduction on BP changes in patients with T2DM receiving GLP‐1RA treatment and SGLT2i treatment. However, we acknowledge several limitations of our study. First, there was some moderate level of heterogeneity across studies, which may influence the interpretation of the results. Data from separate studies were combined for analysis. Baseline characteristic, agent dosage, and duration of follow‐up varied across studies, which may cause a high level of heterogeneity. Confounding factors, such as the presence or absence of hypertension diagnosis in the study population, the background antihypertensive therapies, and changes in medication during the course of trial, were not available in many of the included studies, which might be another possible explanation for the heterogeneity. Second, most of the included studies were clinical assessment of efficacy of GLP‐1RA and SGLT2i treatment. Therefore, glycemic control was the primary end point in most of the studies and changes of BP were typically reported as safety outcomes or secondary outcomes. Third, the association examined by meta‐regression analysis may not be interpreted as a causal effect. Last, funnel plot analysis suggested the presence of publication bias. Although we used trim‐and‐fill method to further assess the impact of publication bias, our results should be interpreted with caution.
Conclusions
Treatment with GLP‐1RA and SGLT2i led to significant reductions in BP in patients with T2DM. Weight reduction was significantly and independently associated with BP reductions in GLP‐1RA treatment and SGLT2i treatment. These results indicated that weight loss contributed to the BP‐lowering effects of GLP‐1RA and SGLT2i. Further studies are needed to elucidate the underlying mechanism of the BP‐lowering effects of these 2 drugs and its potential impact on cardiovascular outcomes.
Sources of Funding
This meta‐analysis was supported by grants from the National Key Research and Development Program of China (2016YFC1304901) and the National Natural Science Foundation of China (81970698, 81970708). The funding agencies had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Disclosures
Dr Ji has received fees for lecture presentations from AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi‐Aventis, and Takeda. Dr Ji has received consulting fees from companies including AstraZeneca, Merck, Novartis, Lilly, Roche, Sanofi‐Aventis, and Takeda. Dr Ji has received grants/research support from AstraZeneca, Bristol‐Myers Squibb, Merck, Novartis, and Sanofi‐Aventis. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S12
Figures S1–S9
References 9, 11, 13, and 73–240
Acknowledgments
Design of this meta‐analysis: Drs Cai and Ji study selection and data extraction: Drs Hu, Yang, Zhang, and Nie; statistical analyses: Drs Cai and Hu; manuscript writing: Drs Hu, Cai, Zhang, and Ji. All authors contributed to the manuscript drafts and gave final approval for this manuscript.
J Am Heart Assoc. 2020;9:e015323 DOI: 10.1161/JAHA.119.015323
Contributor Information
Xiaoling Cai, Email: dr_junel@sina.com.
Linong Ji, Email: jiln@bjmu.edu.cn.
References
- 1. Woodward M, Zhang X, Barzi F, Pan W, Ueshima H, Rodgers A, MacMahon S; Asia Pacific Cohort Studies Consortium . The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia‐Pacific region. Diabetes Care. 2003;26:360–366. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . 10: Cardiovascular disease and risk management: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S103–S123. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association . 11: Microvascular complications and foot care: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S124–S138. [DOI] [PubMed] [Google Scholar]
- 4. Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, Rossing P, Zoungas S, Bakris G. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273–1284. [DOI] [PubMed] [Google Scholar]
- 6. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2015;313:603–615. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519–1529. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 9. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121–130. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 12. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 13. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 14. Blonde L, Russell‐Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the lead 1‐5 studies. Diabetes Obes Metab. 2009;11(suppl 3):26–34. [DOI] [PubMed] [Google Scholar]
- 15. Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co‐transport‐2 inhibitors in type 2 diabetes: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457–466. [DOI] [PubMed] [Google Scholar]
- 16. Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, Ni Y, Liu D, Zhu Z. Blood pressure‐lowering effects of GLP‐1 receptor agonists exenatide and liraglutide: a meta‐analysis of clinical trials. Diabetes Obes Metab. 2013;15:737–749. [DOI] [PubMed] [Google Scholar]
- 17. Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliva RV, Bakris GL. Blood pressure effects of sodium‐glucose co‐transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 2014;8:330–339. [DOI] [PubMed] [Google Scholar]
- 19. Petrie JR. The cardiovascular safety of incretin‐based therapies: a review of the evidence. Cardiovasc Diabetol. 2013;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferreira MT, Leite NC, Cardoso CR, Salles GF. Correlates of aortic stiffness progression in patients with type 2 diabetes: importance of glycemic control: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care. 2015;38:897–904. [DOI] [PubMed] [Google Scholar]
- 21. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–270. [DOI] [PubMed] [Google Scholar]
- 22. Paul S, Best J, Klein K, Han J, Maggs D. Effects of HBA1C and weight reduction on blood pressure in patients with type 2 diabetes mellitus treated with exenatide*. Diabetes Obes Metab. 2012;14:826–834. [DOI] [PubMed] [Google Scholar]
- 23. Cefalu WT, Stenlof K, Leiter LA, Wilding JP, Blonde L, Polidori D, Xie J, Sullivan D, Usiskin K, Canovatchel W, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58:1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sjostrom CD, Hashemi M, Sugg J, Ptaszynska A, Johnsson E. Dapagliflozin‐induced weight loss affects 24‐week glycated haemoglobin and blood pressure levels. Diabetes Obes Metab. 2015;17:809–812. [DOI] [PubMed] [Google Scholar]
- 25. Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23:334–339. [DOI] [PubMed] [Google Scholar]
- 26. Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, Carbone S, Abbate A, Dixon DL. Effects of sodium‐glucose cotransporter 2 inhibitors on 24‐hour ambulatory blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6:e005686 DOI: 10.1161/JAHA.117.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumarathurai P, Anholm C, Fabricius‐Bjerre A, Nielsen OW, Kristiansen O, Madsbad S, Haugaard SB, Sajadieh A. Effects of the glucagon‐like peptide‐1 receptor agonist liraglutide on 24‐h ambulatory blood pressure in patients with type 2 diabetes and stable coronary artery disease: a randomized, double‐blind, placebo‐controlled, crossover study. J Hypertens. 2017;35:1070–1078. [DOI] [PubMed] [Google Scholar]
- 28. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, During M, Matthews DR; Group L‐S . Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the lead (Liraglutide Effect and Action in Diabetes)‐2 study. Diabetes Care. 2009;32:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russell‐Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R, Liraglutide E, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Majewski C, Bakris GL. Blood pressure reduction: an added benefit of sodium‐glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38:429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the prisma statement. Ann Intern Med. 2009;151:264–269, W264. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JPT, Green S; Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England; Hoboken, NJ: Wiley‐Blackwell; 2008. [Google Scholar]
- 33. Frias JP, Guja C, Hardy E, Ahmed A, Dong F, Ohman P, Jabbour SA. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION‐8): a 28 week, multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. [DOI] [PubMed] [Google Scholar]
- 34. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 35. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, et al. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. [DOI] [PubMed] [Google Scholar]
- 36. Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundstrom J, Neal B. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4:411–419. [DOI] [PubMed] [Google Scholar]
- 37. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 38. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. [DOI] [PubMed] [Google Scholar]
- 39. Ferdinand KC, White WB, Calhoun DA, Lonn EM, Sager PT, Brunelle R, Jiang HH, Threlkeld RJ, Robertson KE, Geiger MJ. Effects of the once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–737. [DOI] [PubMed] [Google Scholar]
- 40. Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, Uchiyama K, Niijima Y, Katsuya T, Urata H, et al. 24‐Hour blood pressure‐lowering effect of an SGLT‐2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo‐controlled sacra study. Circulation. 2018;139:2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liakos A, Lambadiari V, Bargiota A, Kitsios K, Avramidis I, Kotsa K, Gerou S, Boura P, Tentolouris N, Dimitriadis G, et al. Effect of liraglutide on ambulatory blood pressure in patients with hypertension and type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2019;21:517–524. [DOI] [PubMed] [Google Scholar]
- 42. Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, Woerle HJ, Broedl UC, Johansen OE. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension. 2016;68:1355–1364. [DOI] [PubMed] [Google Scholar]
- 43. Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, Zhang Y, Quan X, Ji L, Zhan S. Impact of GLP‐1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Res Clin Pract. 2015;110:26–37. [DOI] [PubMed] [Google Scholar]
- 44. Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium‐glucose cotransport‐2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta‐analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6:e004007 DOI: 10.1161/JAHA.116.004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med. 2013;159:262–274. [DOI] [PubMed] [Google Scholar]
- 46. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2003;42:878–884. [DOI] [PubMed] [Google Scholar]
- 47. Semlitsch T, Jeitler K, Berghold A, Horvath K, Posch N, Poggenburg S, Siebenhofer A. Long‐term effects of weight‐reducing diets in people with hypertension. Cochrane Database Syst Rev. 2016;3:CD008274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siebenhofer A, Jeitler K, Horvath K, Berghold A, Posch N, Meschik J, Semlitsch T. Long‐term effects of weight‐reducing drugs in people with hypertension. Cochrane Database Syst Rev. 2016;3:CD007654. [DOI] [PubMed] [Google Scholar]
- 49. Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient‐level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014;28:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Katout M, Zhu H, Rutsky J, Shah P, Brook RD, Zhong J, Rajagopalan S. Effect of GLP‐1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta‐analysis and meta‐regression. Am J Hypertens. 2014;27:130–139. [DOI] [PubMed] [Google Scholar]
- 51. Wijkman MO, Dena M, Dahlqvist S, Sofizadeh S, Hirsch I, Tuomilehto J, Martensson J, Torffvit O, Imberg H, Saeed A, et al. Predictors and correlates of systolic blood pressure reduction with liraglutide treatment in patients with type 2 diabetes. J Clin Hypertens. 2019;21:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vilsboll T, Zdravkovic M, Le‐Thi T, Krarup T, Schmitz O, Courreges JP, Verhoeven R, Buganova I, Madsbad S. Liraglutide, a long‐acting human glucagon‐like peptide‐1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610. [DOI] [PubMed] [Google Scholar]
- 53. Vilsboll T, Garber AJ. Non‐glycaemic effects mediated via GLP‐1 receptor agonists and the potential for exploiting these for therapeutic benefit: focus on liraglutide. Diabetes Obes Metab. 2012;14(suppl 2):41–49. [DOI] [PubMed] [Google Scholar]
- 54. Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon‐like peptide‐1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. [DOI] [PubMed] [Google Scholar]
- 55. Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP‐1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–E1295. [DOI] [PubMed] [Google Scholar]
- 56. Chaudhuri A, Ghanim H, Makdissi A, Green K, Abuaysheh S, Batra M, N DK, Dandona P. Exenatide induces an increase in vasodilatory and a decrease in vasoconstrictive mediators. Diabetes Obes Metab. 2017;19:729–733. [DOI] [PubMed] [Google Scholar]
- 57. Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38:132–139. [DOI] [PubMed] [Google Scholar]
- 58. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, et al. Glucagon‐like peptide 1 induces natriuresis in healthy subjects and in insulin‐resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–3061. [DOI] [PubMed] [Google Scholar]
- 59. Muskiet MHA, van Bommel EJ, van Raalte DH. Antihypertensive effects of SGLT2 inhibitors in type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4:188–189. [DOI] [PubMed] [Google Scholar]
- 60. Brown E, Rajeev SP, Cuthbertson DJ, Wilding JPH. A review of the mechanism of action, metabolic profile and haemodynamic effects of sodium‐glucose co‐transporter‐2 inhibitors. Diabetes Obes Metab. 2019;21(suppl 2):9–18. [DOI] [PubMed] [Google Scholar]
- 61. Reed JW. Impact of sodium‐glucose cotransporter 2 inhibitors on blood pressure. Vasc Health Risk Manag. 2016;12:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, Kubozono T, Ohishi M. Mechanism of the blood pressure‐lowering effect of sodium‐glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol. 2017;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium‐glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long‐term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035–1041. [DOI] [PubMed] [Google Scholar]
- 68. Gao S, Cui X, Wang X, Burg MB, Dmitrieva NI. Cross‐sectional positive association of serum lipids and blood pressure with serum sodium within the normal reference range of 135‐145 mmol/l. Arterioscler Thromb Vasc Biol. 2017;37:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wannamethee G, Whincup PH, Shaper AG, Lever AF. Serum sodium concentration and risk of stroke in middle‐aged males. J Hypertens. 1994;12:971–979. [PubMed] [Google Scholar]
- 70. Weir MR, Januszewicz A, Gilbert RE, Vijapurkar U, Kline I, Fung A, Meininger G. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens. 2014;16:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clarke SJ, Giblett JP, Yang LL, Hubsch A, Zhao T, Aetesam‐Ur‐Rahman M, West NEJ, O'Sullivan M, Figg N, Bennett M, et al. GLP‐1 is a coronary artery vasodilator in humans. J Am Heart Assoc. 2018;7:e010321 DOI: 10.1161/JAHA.118.010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP‐1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. [DOI] [PubMed] [Google Scholar]
- 73. Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long‐acting GLP‐1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nauck MA, Stewart MW, Perkins C, Jones‐Leone A, Yang F, Perry C, Reinhardt RR, Rendell M. Efficacy and safety of once‐weekly GLP‐1 receptor agonist albiglutide (Harmony 2): 52 week primary endpoint results from a randomised, placebo‐controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia. 2016;59:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once‐weekly GLP‐1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose‐dependent effects on glycaemic control in a randomized, double‐blind, placebo‐controlled study. Diabetic Med. 2012;29:1260–1267. [DOI] [PubMed] [Google Scholar]
- 76. Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide is non‐inferior to once‐daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26‐week randomized phase III study. Diabetes Obes Metab. 2015;17:974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, Brodows RG. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug‐naive patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, parallel‐group study. Clin Ther. 2008;30:1448–1460. [DOI] [PubMed] [Google Scholar]
- 78. Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzik M. Pioneer 1: randomized clinical trial comparing the efficacy and safety of oral semaglutide monotherapy with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–1732. [DOI] [PubMed] [Google Scholar]
- 79. Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbol JD, Hansen T, Bain SC. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251–260. [DOI] [PubMed] [Google Scholar]
- 80. Raz I, Fonseca V, Kipnes M, Durrwell L, Hoekstra J, Boldrin M, Balena R. Efficacy and safety of taspoglutide monotherapy in drug‐naive type 2 diabetic patients after 24 weeks of treatment: results of a randomized, double‐blind, placebo‐controlled phase 3 study (T‐emerge 1). Diabetes Care. 2012;35:485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ahren B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, Feinglos MN. Harmony 3: 104‐week randomized, double‐blind, placebo‐ and active‐controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–2148. [DOI] [PubMed] [Google Scholar]
- 82. Dungan KM, Weitgasser R, Perez Manghi F, Pintilei E, Fahrbach JL, Jiang HH, Shell J, Robertson KE. A 24‐week study to evaluate the efficacy and safety of once‐weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD‐8). Diabetes Obes Metab. 2016;18:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ludvik B, Frias JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, Garcia‐Perez LE, Woodward DB, Milicevic Z. Dulaglutide as add‐on therapy to sglt2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD‐10): a 24‐week, randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. [DOI] [PubMed] [Google Scholar]
- 84. Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, Kuhstoss D, Lakshmanan M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37:2159–2167. [DOI] [PubMed] [Google Scholar]
- 85. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care. 2014;37:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, Braun D, Giaconia J, Malone J. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double‐blind, placebo‐controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Apovian CM, Bergenstal RM, Cuddihy RM, Qu Y, Lenox S, Lewis MS, Glass LC. Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes. Am J Med. 2010;123:468.e9–468.e7. [DOI] [PubMed] [Google Scholar]
- 88. Liutkus J, Rosas Guzman J, Norwood P, Pop L, Northrup J, Cao D, Trautmann M. A placebo‐controlled trial of exenatide twice‐daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab. 2010;12:1058–1065. [DOI] [PubMed] [Google Scholar]
- 89. Gadde KM, Vetter ML, Iqbal N, Hardy E, Ohman P. Efficacy and safety of autoinjected exenatide once‐weekly suspension versus sitagliptin or placebo with metformin in patients with type 2 diabetes: the duration‐neo‐2 randomized clinical study. Diabetes Obes Metab. 2017;19:979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guja C, Frias JP, Somogyi A, Jabbour S, Wang H, Hardy E, Rosenstock J. Effect of exenatide qw or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: the duration‐7 randomized study. Diabetes Obes Metab. 2018;20:1602–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, Hoogwerf BJ, Rosenstock J. Use of twice‐daily exenatide in basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103–112. [DOI] [PubMed] [Google Scholar]
- 92. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Ahren B, Torffvit O, Attvall S, Ekelund M, Filipsson K, Tengmark BO, et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomised clinical trial (MDI liraglutide trial). BMJ. 2015;351:h5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vanderheiden A, Harrison L, Warshauer J, Li X, Adams‐Huet B, Lingvay I. Effect of adding liraglutide vs placebo to a high‐dose insulin regimen in patients with type 2 diabetes: a randomized clinical trial. JAMA Intern Med. 2016;176:939–947. [DOI] [PubMed] [Google Scholar]
- 95. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care. 2009;32:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ahmann A, Rodbard HW, Rosenstock J, Lahtela JT, de Loredo L, Tornoe K, Boopalan A, Nauck MA. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Davies MJ, Bain SC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, Bosch‐Traberg H, Syren A, Umpierrez GE. Efficacy and safety of liraglutide versus placebo as add‐on to glucose‐lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA‐RENAL): a randomized clinical trial. Diabetes Care. 2016;39:222–230. [DOI] [PubMed] [Google Scholar]
- 98. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, Andreasen AH, Jensen CB, DeFronzo RA. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the scale diabetes randomized clinical trial. JAMA. 2015;314:687–699. [DOI] [PubMed] [Google Scholar]
- 99. Nauck MA, Petrie JR, Sesti G, Mannucci E, Courreges JP, Lindegaard ML, Jensen CB, Atkin SL. A phase 2, randomized, dose‐finding study of the novel once‐weekly human GLP‐1 analog, semaglutide, compared with placebo and open‐label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231–241. [DOI] [PubMed] [Google Scholar]
- 100. Davies M, Pieber TR, Hartoft‐Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318:1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lingvay I, Desouza CV, Lalic KS, Rose L, Hansen T, Zacho J, Pieber TR. A 26‐week randomized controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care. 2018;41:1926–1937. [DOI] [PubMed] [Google Scholar]
- 102. Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet. 2019;394:39–50. [DOI] [PubMed] [Google Scholar]
- 103. Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, Araki E, Chu PL, Wijayasinghe N, Norwood P. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, Thrasher J, Woo V, Philis‐Tsimikas A. Semaglutide once weekly as add‐on to sglt‐2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–367. [DOI] [PubMed] [Google Scholar]
- 105. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. [DOI] [PubMed] [Google Scholar]
- 106. Bergenstal RM, Forti A, Chiasson JL, Woloschak M, Boldrin M, Balena R. Efficacy and safety of taspoglutide versus sitagliptin for type 2 diabetes mellitus (T‐emerge 4 trial). Diabetes Ther. 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Henry RR, Mudaliar S, Kanitra L, Woloschak M, Balena R. Efficacy and safety of taspoglutide in patients with type 2 diabetes inadequately controlled with metformin plus pioglitazone over 24 weeks: T‐emerge 3 trial. J Clin Endocrinol Metab. 2012;97:2370–2379. [DOI] [PubMed] [Google Scholar]
- 108. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37:2168–2176. [DOI] [PubMed] [Google Scholar]
- 109. Chen YH, Huang CN, Cho YM, Li P, Gu L, Wang F, Yang J, Wang WQ. Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East‐Asian patients with type 2 diabetes in a multicentre, double‐blind, randomized, parallel‐arm, active comparator, phase III trial. Diabetes Obes Metab. 2018;20:2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Russell‐Jones D, Cuddihy RM, Hanefeld M, Kumar A, Gonzalez JG, Chan M, Wolka AM, Boardman MK. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug‐naive patients with type 2 diabetes (DURATION‐4): a 26‐week double‐blind study. Diabetes Care. 2012;35:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Xu W, Bi Y, Sun Z, Li J, Guo L, Yang T, Wu G, Shi L, Feng Z, Qiu L, et al. Comparison of the effects on glycaemic control and beta‐cell function in newly diagnosed type 2 diabetes patients of treatment with exenatide, insulin or pioglitazone: a multicentre randomized parallel‐group trial (the CONFIDENCE Study). J Intern Med. 2015;277:137–150. [DOI] [PubMed] [Google Scholar]
- 112. Garber A, Henry R, Ratner R, Garcia‐Hernandez PA, Rodriguez‐Pattzi H, Olvera‐Alvarez I, Hale PM, Zdravkovic M, Bode B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet. 2009;373:473–481. [DOI] [PubMed] [Google Scholar]
- 113. Seino Y, Terauchi Y, Osonoi T, Yabe D, Abe N, Nishida T, Zacho J, Kaneko S. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab. 2018;20:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Weissman PN, Carr MC, Ye J, Cirkel DT, Stewart M, Perry C, Pratley R. Harmony 4: randomised clinical trial comparing once‐weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57:2475–2484. [DOI] [PubMed] [Google Scholar]
- 115. Dungan KM, Povedano ST, Forst T, Gonzalez JG, Atisso C, Sealls W, Fahrbach JL. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349–1357. [DOI] [PubMed] [Google Scholar]
- 116. Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once‐weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once‐daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open‐label, phase III, non‐inferiority study. Diabetes Obes Metab. 2015;17:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, Milicevic Z. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet. 2015;385:2057–2066. [DOI] [PubMed] [Google Scholar]
- 118. Wang W, Nevarez L, Filippova E, Song KH, Tao B, Gu L, Wang F, Li P, Yang J. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52‐week open‐label, randomized phase III trial. Diabetes Obes Metab. 2019;21:234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pratley RE, Aroda VR, Lingvay I, Ludemann J, Andreassen C, Navarria A, Viljoen A; SUSTAIN 7 investigators . Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3B trial. Lancet Diabetes Endocrinol. 2018;6:275–286. [DOI] [PubMed] [Google Scholar]
- 120. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38:2241–2249. [DOI] [PubMed] [Google Scholar]
- 121. Wysham CH, MacConell L, Hardy E. Efficacy and safety of multiple doses of exenatide once‐monthly suspension in patients with type 2 diabetes: a phase II randomized clinical trial. Diabetes Care. 2016;39:1768–1776. [DOI] [PubMed] [Google Scholar]
- 122. Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, Trautmann M, Porter L. Duration‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–1310. [DOI] [PubMed] [Google Scholar]
- 123. Rosenstock J, Balas B, Charbonnel B, Bolli GB, Boldrin M, Ratner R, Balena R. The fate of taspoglutide, a weekly GLP‐1 receptor agonist, versus twice‐daily exenatide for type 2 diabetes: the T‐emerge 2 trial. Diabetes Care. 2013;36:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374:39–47. [DOI] [PubMed] [Google Scholar]
- 125. Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long‐acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the helping evaluate exenatide in patients with diabetes compared with long‐acting insulin (HEELA) study. Diabetes Obes Metab. 2009;11:1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION‐2): a randomised trial. Lancet. 2010;376:431–439. [DOI] [PubMed] [Google Scholar]
- 127. Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, Trautmann M. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION‐3): an open‐label randomised trial. Lancet. 2010;375:2234–2243. [DOI] [PubMed] [Google Scholar]
- 128. Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet. 2013;381:117–124. [DOI] [PubMed] [Google Scholar]
- 129. Davies M, Heller S, Sreenan S, Sapin H, Adetunji O, Tahbaz A, Vora J. Once‐weekly exenatide versus once‐ or twice‐daily insulin detemir: randomized, open‐label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care. 2013;36:1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ji L, Onishi Y, Ahn CW, Agarwal P, Chou CW, Haber H, Guerrettaz K, Boardman MK. Efficacy and safety of exenatide once‐weekly vs exenatide twice‐daily in Asian patients with type 2 diabetes mellitus. J Diabetes Invest. 2013;4:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wysham CH, Rosenstock J, Vetter ML, Dong F, Ohman P, Iqbal N. Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Diamant M, Nauck MA, Shaginian R, Malone JK, Cleall S, Reaney M, de Vries D, Hoogwerf BJ, MacConell L, Wolffenbuttel BH. Glucagon‐like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37:2763–2773. [DOI] [PubMed] [Google Scholar]
- 133. Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet. 2008;372:1240–1250. [DOI] [PubMed] [Google Scholar]
- 134. Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M. A comparison of twice‐daily exenatide and biphasic insulin as part in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non‐inferiority study. Diabetologia. 2007;50:259–267. [DOI] [PubMed] [Google Scholar]
- 135. Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and safety of once‐weekly semaglutide versus exenatide er in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41:258–266. [DOI] [PubMed] [Google Scholar]
- 136. Simo R, Guerci B, Schernthaner G, Gallwitz B, Rosas‐Guzman J, Dotta F, Festa A, Zhou M, Kiljanski J. Long‐term changes in cardiovascular risk markers during administration of exenatide twice daily or glimepiride: results from the European exenatide study. Cardiovasc Diabetol. 2015;14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Meier JJ, Rosenstock J, Hincelin‐Mery A, Roy‐Duval C, Delfolie A, Coester HV, Menge BA, Forst T, Kapitza C. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38:1263–1273. [DOI] [PubMed] [Google Scholar]
- 138. Brady EM, Davies MJ, Gray LJ, Saeed MA, Smith D, Hanif W, Khunti K. A randomized controlled trial comparing the GLP‐1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during ramadan: the treat 4 ramadan trial. Diabetes Obes Metab. 2014;16:527–536. [DOI] [PubMed] [Google Scholar]
- 139. D'Alessio D, Haring HU, Charbonnel B, de Pablos‐Velasco P, Candelas C, Dain MP, Vincent M, Pilorget V, Yki‐Jarvinen H. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17:170–178. [DOI] [PubMed] [Google Scholar]
- 140. Abreu M, Tumyan A, Elhassan A, Peicher K, Papacostea O, Dimachkie P, Siddiqui MS, Pop LM, Gunasekaran U, Meneghini LF, et al. A randomized trial comparing the efficacy and safety of treating patients with type 2 diabetes and highly elevated HbA1c levels with basal‐bolus insulin or a glucagon‐like peptide‐1 receptor agonist plus basal insulin: the SIMPLE study. Diabetes Obes Metab. 2019;1211:2133‐2141. [DOI] [PubMed] [Google Scholar]
- 141. Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Thomsen AB, Sondergaard RE, Davies M. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet. 2010;375:1447–1456. [DOI] [PubMed] [Google Scholar]
- 142. Charbonnel B, Steinberg H, Eymard E, Xu L, Thakkar P, Prabhu V, Davies MJ, Engel SS. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56:1503–1511. [DOI] [PubMed] [Google Scholar]
- 143. de Wit HM, Vervoort GM, Jansen HJ, de Grauw WJ, de Galan BE, Tack CJ. Liraglutide reverses pronounced insulin‐associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia. 2014;57:1812–1819. [DOI] [PubMed] [Google Scholar]
- 144. Bailey TS, Takacs R, Tinahones FJ, Rao PV, Tsoukas GM, Thomsen AB, Kaltoft MS, Maislos M. Efficacy and safety of switching from sitagliptin to liraglutide in subjects with type 2 diabetes (LIRA‐SWITCH): a randomized, double‐blind, double‐dummy, active‐controlled 26‐week trial. Diabetes Obes Metab. 2016;18:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Nauck M, Rizzo M, Johnson A, Bosch‐Traberg H, Madsen J, Cariou B. Once‐daily liraglutide versus lixisenatide as add‐on to metformin in type 2 diabetes: a 26‐week randomized controlled clinical trial. Diabetes Care. 2016;39:1501–1509. [DOI] [PubMed] [Google Scholar]
- 146. Zang L, Liu Y, Geng J, Luo Y, Bian F, Lv X, Yang J, Liu J, Peng Y, Li Y, et al. Efficacy and safety of liraglutide versus sitagliptin, both in combination with metformin, in Chinese patients with type 2 diabetes: a 26‐week, open‐label, randomized, active comparator clinical trial. Diabetes Obes Metab. 2016;18:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kaku K, Kiyosue A, Ono Y, Shiraiwa T, Kaneko S, Nishijima K, Bosch‐Traberg H, Seino Y. Liraglutide is effective and well tolerated in combination with an oral antidiabetic drug in Japanese patients with type 2 diabetes: a randomized, 52‐week, open‐label, parallel‐group trial. J Diabetes Invest. 2016;7:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, Serusclat P, Violante R, Watada H, Davies M, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Aroda VR, Bain SC, Cariou B, Piletic M, Rose L, Axelsen M, Rowe E, DeVries JH. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355–366. [DOI] [PubMed] [Google Scholar]
- 150. Ahren B, Masmiquel L, Kumar H, Sargin M, Karsbol JD, Jacobsen SH, Chow F. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341–354. [DOI] [PubMed] [Google Scholar]
- 151. Kaku K, Yamada Y, Watada H, Abiko A, Nishida T, Zacho J, Kiyosue A. Safety and efficacy of once‐weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes Metab. 2018;20:1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nauck M, Horton E, Andjelkovic M, Ampudia‐Blasco FJ, Parusel CT, Boldrin M, Balena R. Taspoglutide, a once‐weekly glucagon‐like peptide 1 analogue, vs. insulin glargine titrated to target in patients with type 2 diabetes: an open‐label randomized trial. Diabetic Med. 2013;30:109–113. [DOI] [PubMed] [Google Scholar]
- 153. Pratley RE, Urosevic D, Boldrin M, Balena R. Efficacy and tolerability of taspoglutide versus pioglitazone in subjects with type 2 diabetes uncontrolled with sulphonylurea or sulphonylurea‐metformin therapy: a randomized, double‐blind study (T‐emerge 6). Diabetes Obes Metab. 2013;15:234–240. [DOI] [PubMed] [Google Scholar]
- 154. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, 12‐week study. Diabetes Obes Metab. 2013;15:1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24‐week, randomized, double‐blind, placebo‐controlled, phase III study. Expert Opin Pharmacother. 2014;15:1501–1515. [DOI] [PubMed] [Google Scholar]
- 156. Stenlof K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N, Tokudome T, Langkilde AM, Parikh S. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2013;15:432–440. [DOI] [PubMed] [Google Scholar]
- 158. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bailey CJ, Iqbal N, T'Joen C, List JF. Dapagliflozin monotherapy in drug‐naive patients with diabetes: a randomized‐controlled trial of low‐dose range. Diabetes Obes Metab. 2012;14:951–959. [DOI] [PubMed] [Google Scholar]
- 160. Ji L, Ma J, Li H, Mansfield TA, T'Joen CL, Iqbal N, Ptaszynska A, List JF. Dapagliflozin as monotherapy in drug‐naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther. 2014;36:84–100.e109. [DOI] [PubMed] [Google Scholar]
- 161. Kaku K, Kiyosue A, Inoue S, Ueda N, Tokudome T, Yang J, Langkilde AM. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab. 2014;16:1102–1110. [DOI] [PubMed] [Google Scholar]
- 162. Kadowaki T, Haneda M, Inagaki N, Terauchi Y, Taniguchi A, Koiwai K, Rattunde H, Woerle HJ, Broedl UC. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12‐week, double‐blind, placebo‐controlled, phase II trial. Adv Ther. 2014;31:621–638. [DOI] [PubMed] [Google Scholar]
- 163. Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. [DOI] [PubMed] [Google Scholar]
- 164. Terra SG, Focht K, Davies M, Frias J, Derosa G, Darekar A, Golm G, Johnson J, Saur D, Lauring B, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19:721–728. [DOI] [PubMed] [Google Scholar]
- 165. Fonseca VA, Ferrannini E, Wilding JP, Wilpshaar W, Dhanjal P, Ball G, Klasen S. Active‐ and placebo‐controlled dose‐finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:268–273. [DOI] [PubMed] [Google Scholar]
- 166. Seino Y, Sasaki T, Fukatsu A, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12‐week, randomized, placebo‐controlled, phase II study. Curr Med Res Opin. 2014;30:1219–1230. [DOI] [PubMed] [Google Scholar]
- 167. Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Dose‐finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12‐week, randomized, double‐blind, placebo‐controlled, phase II study. Curr Med Res Opin. 2014;30:1231–1244. [DOI] [PubMed] [Google Scholar]
- 168. Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled, phase 3 study. Curr Med Res Opin. 2014;30:1245–1255. [DOI] [PubMed] [Google Scholar]
- 169. Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, Tanizawa Y, Araki E, Ueda M, Suganami H, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter‐2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo‐controlled, double‐blind, parallel‐group comparative study. Cardiovasc Diabetol. 2014;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Townsend RR, Machin I, Ren J, Trujillo A, Kawaguchi M, Vijapurkar U, Damaraju CV, Pfeifer M. Reductions in mean 24‐hour ambulatory blood pressure after 6‐week treatment with canagliflozin in patients with type 2 diabetes mellitus and hypertension. J Clin Hypertens. 2016;18:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W. Dose‐ranging effects of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Inagaki N, Harashima S, Maruyama N, Kawaguchi Y, Goda M, Iijima H. Efficacy and safety of canagliflozin in combination with insulin: a double‐blind, randomized, placebo‐controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Qiu R, Capuano G, Meininger G. Efficacy and safety of twice‐daily treatment with canagliflozin, a sodium glucose co‐transporter 2 inhibitor, added on to metformin monotherapy in patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2014;1:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ji L, Han P, Liu Y, Yang G, Van Dieu NK, Vijapurkar U, Qiu R, Meininger G. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17:23–31. [DOI] [PubMed] [Google Scholar]
- 175. Bode B, Stenlof K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract (1995). 2013;41:72–84. [DOI] [PubMed] [Google Scholar]
- 176. Lavalle‐Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yale JF, Bakris G, Cariou B, Yue D, David‐Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wilding JP, Charpentier G, Hollander P, Gonzalez‐Galvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rosenstock J, Chuck L, Gonzalez‐Ortiz M, Merton K, Craig J, Capuano G, Qiu R. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug‐naive type 2 diabetes. Diabetes Care. 2016;39:353–362. [DOI] [PubMed] [Google Scholar]
- 181. Wilding JP, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care. 2009;32:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin‐angiotensin system blockade. Blood Press. 2016;25:93–103. [DOI] [PubMed] [Google Scholar]
- 183. Schumm‐Draeger PM, Burgess L, Koranyi L, Hruba V, Hamer‐Maansson JE, de Bruin TW. Twice‐daily dapagliflozin co‐administered with metformin in type 2 diabetes: a 16‐week randomized, placebo‐controlled clinical trial. Diabetes Obes Metab. 2015;17:42–51. [DOI] [PubMed] [Google Scholar]
- 184. Araki E, Onishi Y, Asano M, Kim H, Yajima T. Efficacy and safety of dapagliflozin over 1 year as add‐on to insulin therapy in Japanese patients with type 2 diabetes: the daisy (Dapagliflozin Added to Patients Under InSulin therapY) trial. Diabetes Obes Metab. 2017;19:562–570. [DOI] [PubMed] [Google Scholar]
- 185. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375:2223–2233. [DOI] [PubMed] [Google Scholar]
- 186. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2011;13:928–938. [DOI] [PubMed] [Google Scholar]
- 187. Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. [DOI] [PubMed] [Google Scholar]
- 188. Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin xr, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66:446–456. [DOI] [PubMed] [Google Scholar]
- 189. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S; Dapagliflozin 006 Study Group . Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–415. [DOI] [PubMed] [Google Scholar]
- 191. Jabbour SA, Hardy E, Sugg J, Parikh S. Dapagliflozin is effective as add‐on therapy to sitagliptin with or without metformin: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study. Diabetes Care. 2014;37:740–750. [DOI] [PubMed] [Google Scholar]
- 192. Leiter LA, Cefalu WT, de Bruin TW, Gause‐Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. J Am Geriatr Soc. 2014;62:1252–1262. [DOI] [PubMed] [Google Scholar]
- 193. Cefalu WT, Leiter LA, de Bruin TW, Gause‐Nilsson I, Sugg J, Parikh SJ. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high‐risk patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. Diabetes Care. 2015;38:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, Chen H, Hansen L, Iqbal N. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009–2017. [DOI] [PubMed] [Google Scholar]
- 195. Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S. Dapagliflozin improves glycemic control and reduces body weight as add‐on therapy to metformin plus sulfonylurea: a 24‐week randomized, double‐blind clinical trial. Diabetes Care. 2015;38:365–372. [DOI] [PubMed] [Google Scholar]
- 196. Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, Iqbal N. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376–383. [DOI] [PubMed] [Google Scholar]
- 197. Yang W, Han P, Min KW, Wang B, Mansfield T, T'Joen C, Iqbal N, Johnsson E, Ptaszynska A. Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: a randomized controlled trial. J Diabetes. 2016;8:796–808. [DOI] [PubMed] [Google Scholar]
- 198. Fioretto P, Del Prato S, Buse JB, Goldenberg R, Giorgino F, Reyner D, Langkilde AM, Sjostrom CD, Sartipy P, Investigators DS. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE study. Diabetes Obes Metab. 2018;20:2532–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yang W, Ma J, Li Y, Li Y, Zhou Z, Kim JH, Zhao J, Ptaszynska A. Dapagliflozin as add‐on therapy in Asian patients with type 2 diabetes inadequately controlled on insulin with or without oral antihyperglycemic drugs: a randomized controlled trial. J Diabetes. 2018;10:589–599. [DOI] [PubMed] [Google Scholar]
- 200. Pollock C, Stefansson B, Reyner D, Rossing P, Sjostrom CD, Wheeler DC, Langkilde AM, Heerspink HJL. Albuminuria‐lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7:429–441. [DOI] [PubMed] [Google Scholar]
- 201. Rosenstock J, Perl S, Johnsson E, Garcia‐Sanchez R, Jacob S. Triple therapy with low‐dose dapagliflozin plus saxagliptin versus dual therapy with each monocomponent, all added to metformin, in uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21:2152–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, Woerle HJ. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add‐on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–1160. [DOI] [PubMed] [Google Scholar]
- 203. Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. [DOI] [PubMed] [Google Scholar]
- 204. Ross S, Thamer C, Cescutti J, Meinicke T, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:699–702. [DOI] [PubMed] [Google Scholar]
- 205. Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, Broedl UC. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–1823. [DOI] [PubMed] [Google Scholar]
- 206. Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Haring HU, Merker L, Seewaldt‐Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC. Empagliflozin as add‐on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2013;36:3396–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Haring HU, Merker L, Seewaldt‐Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ. Empagliflozin as add‐on to metformin in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2014;37:1650–1659. [DOI] [PubMed] [Google Scholar]
- 209. Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC. Empagliflozin improves glycaemic and weight control as add‐on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab. 2014;16:147–158. [DOI] [PubMed] [Google Scholar]
- 210. Kawamori R, Haneda M, Suzaki K, Cheng G, Shiki K, Miyamoto Y, Solimando F, Lee C, Lee J, George J. Empagliflozin as add‐on to linagliptin in a fixed‐dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab. 2018;20:2200–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Ferdinand KC, Izzo JL, Lee J, Meng L, George J, Salsali A, Seman L. Antihyperglycemic and blood pressure effects of empagliflozin in black patients with type 2 diabetes mellitus and hypertension. Circulation. 2019;139:2098–2109. [DOI] [PubMed] [Google Scholar]
- 212. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. [DOI] [PubMed] [Google Scholar]
- 213. Softeland E, Meier JJ, Vangen B, Toorawa R, Maldonado‐Lutomirsky M, Broedl UC. Empagliflozin as add‐on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24‐week randomized, double‐blind, parallel‐group trial. Diabetes Care. 2017;40:201–209. [DOI] [PubMed] [Google Scholar]
- 214. Amin NB, Wang X, Mitchell JR, Lee DS, Nucci G, Rusnak JM. Blood pressure‐lowering effect of the sodium glucose co‐transporter‐2 inhibitor ertugliflozin, assessed via ambulatory blood pressure monitoring in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2015;17:805–808. [DOI] [PubMed] [Google Scholar]
- 215. Amin NB, Wang X, Jain SM, Lee DS, Nucci G, Rusnak JM. Dose‐ranging efficacy and safety study of ertugliflozin, a sodium‐glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes on a background of metformin. Diabetes Obes Metab. 2015;17:591–598. [DOI] [PubMed] [Google Scholar]
- 216. Dagogo‐Jack S, Liu J, Eldor R, Amorin G, Johnson J, Hille D, Liao Y, Huyck S, Golm G, Terra SG, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the vertis sita2 placebo‐controlled randomized study. Diabetes Obes Metab. 2018;20:530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Grunberger G, Camp S, Johnson J, Huyck S, Terra SG, Mancuso JP, Jiang ZW, Golm G, Engel SS, Lauring B. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the vertis renal randomized study. Diabetes Ther. 2018;9:49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Pratley RE, Eldor R, Raji A, Golm G, Huyck SB, Qiu Y, Sunga S, Johnson J, Terra SG, Mancuso JP, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the vertis factorial randomized trial. Diabetes Obes Metab. 2018;20:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Rosenstock J, Frias J, Pall D, Charbonnel B, Pascu R, Saur D, Darekar A, Huyck S, Shi H, Lauring B, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. 2018;20:520–529. [DOI] [PubMed] [Google Scholar]
- 220. Ji L, Liu Y, Miao H, Xie Y, Yang M, Wang W, Mu Y, Yan P, Pan S, Lauring B, et al. Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: vertis asia. Diabetes Obes Metab. 2019;21:1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose‐finding study. Diabetes Obes Metab. 2013;15:403–409. [DOI] [PubMed] [Google Scholar]
- 222. Shestakova MV, Wilding JPH, Wilpshaar W, Tretter R, Orlova VL, Verbovoy AF. A phase 3 randomized placebo‐controlled trial to assess the efficacy and safety of ipragliflozin as an add‐on therapy to metformin in Russian patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;146:240–250. [DOI] [PubMed] [Google Scholar]
- 223. Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, Ueyama E. A randomized, double‐blind, placebo‐controlled study on long‐term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long‐term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: illuminate, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab. 2015;17:304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Lu CH, Min KW, Chuang LM, Kokubo S, Yoshida S, Cha BS. Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: results of a phase 3 randomized, placebo‐controlled, double‐blind, multicenter trial. J Diabetes Invest. 2016;7:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Han KA, Chon S, Chung CH, Lim S, Lee KW, Baik S, Jung CH, Kim DS, Park KS, Yoon KH, et al. Efficacy and safety of ipragliflozin as an add‐on therapy to sitagliptin and metformin in Korean patients with inadequately controlled type 2 diabetes mellitus: a randomized controlled trial. Diabetes Obes Metab. 2018;20:2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Seino Y, Sasaki T, Fukatsu A, Imazeki H, Ochiai H, Sakai S. Efficacy and safety of luseogliflozin added to insulin therapy in Japanese patients with type 2 diabetes: a multicenter, 52‐week, clinical study with a 16‐week, double‐blind period and a 36‐week, open‐label period. Curr Med Res Opin. 2018;34:981–994. [DOI] [PubMed] [Google Scholar]
- 228. Haneda M, Seino Y, Inagaki N, Kaku K, Sasaki T, Fukatsu A, Kakiuchi H, Sato Y, Sakai S, Samukawa Y. Influence of renal function on the 52‐week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther. 2016;38:66–88.e20. [DOI] [PubMed] [Google Scholar]
- 229. Rosenstock J, Cefalu WT, Lapuerta P, Zambrowicz B, Ogbaa I, Banks P, Sands A. Greater dose‐ranging effects on A1c levels than on glucosuria with LX4211, a dual inhibitor of SGLT1 and SGLT2, in patients with type 2 diabetes on metformin monotherapy. Diabetes Care. 2015;38:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Terauchi Y, Tamura M, Senda M, Gunji R, Kaku K. Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J‐STEP/INS): results of a 16‐week randomized, double‐blind, placebo‐controlled multicentre trial. Diabetes Obes Metab. 2017;19:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Tanizawa Y, Kaku K, Araki E, Tobe K, Terauchi Y, Utsunomiya K, Iwamoto Y, Watada H, Ohtsuka W, Watanabe D, et al. Long‐term safety and efficacy of tofogliflozin, a selective inhibitor of sodium‐glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open‐label, randomized controlled trials. Expert Opin Pharmacother. 2014;15:749–766. [DOI] [PubMed] [Google Scholar]
- 232. Kario K, Hoshide S, Okawara Y, Tomitani N, Yamauchi K, Ohbayashi H, Itabashi N, Matsumoto Y, Kanegae H. Effect of canagliflozin on nocturnal home blood pressure in Japanese patients with type 2 diabetes mellitus: the SHIFT‐J study. J Clin Hypertens. 2018;20:1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet. 2013;382:941–950. [DOI] [PubMed] [Google Scholar]
- 234. Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care. 2013;36:2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Nauck MA, Del Prato S, Meier JJ, Duran‐Garcia S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care. 2011;34:2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Muller‐Wieland D, Kellerer M, Cypryk K, Skripova D, Rohwedder K, Johnsson E, Garcia‐Sanchez R, Kurlyandskaya R, Sjostrom CD, Jacob S, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add‐on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:2598–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Araki E, Tanizawa Y, Tanaka Y, Taniguchi A, Koiwai K, Kim G, Salsali A, Woerle HJ, Broedl UC. Long‐term treatment with empagliflozin as add‐on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:665–674. [DOI] [PubMed] [Google Scholar]
- 238. Terauchi Y, Utsunomiya K, Yasui A, Seki T, Cheng G, Shiki K, Lee J. Safety and efficacy of empagliflozin as add‐on therapy to GLP‐1 receptor agonist (LIRAGLUTIDE) in Japanese patients with type 2 diabetes mellitus: a randomised, double‐blind, parallel‐group phase 4 study. Diabetes Ther. 2019;10:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add‐on to metformin in patients with type 2 diabetes: a 104‐week randomised, active‐controlled, double‐blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. [DOI] [PubMed] [Google Scholar]
- 240. Hollander P, Liu J, Hill J, Johnson J, Jiang ZW, Golm G, Huyck S, Terra SG, Mancuso JP, Engel SS, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the vertis su randomized study. Diabetes Ther. 2018;9:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S12
Figures S1–S9
References 9, 11, 13, and 73–240


