Abstract
Background
The association between mitral valve prolapse (MVP) and sudden death remains controversial. We aimed to describe histopathological changes in individuals with autopsy‐determined isolated MVP (iMVP) and sudden death and document cardiac arrest rhythm.
Methods and Results
The Australian National Coronial Information System database was used to identify cases of iMVP between 2000 and 2018. Histopathological changes in iMVP and sudden death were compared with 2 control cohorts matched for age, sex, height, and weight (1 group with noncardiac death and 1 group with cardiac death). Data linkage with ambulance services provided cardiac arrest rhythm for iMVP cases. From 77 221 cardiovascular deaths in the National Coronial Information System database, there were 376 cases with MVP. Individual case review yielded 71 cases of iMVP. Mean age was 49±18 years, and 51% were women. Individuals with iMVP had higher cardiac mass (447 g versus 355 g; P<0.001) compared with noncardiac death, but similar cardiac mass (447 g versus 438 g; P=0.64) compared with cardiac death. Individuals with iMVP had larger mitral valve annulus compared with noncardiac death (121 versus 108 mm; P<0.001) and cardiac death (121 versus 110 mm; P=0.002), and more left ventricular fibrosis (79% versus 38%; P<0.001) compared with noncardiac death controls. In those with iMVP and witnessed cardiac arrest, 94% had ventricular fibrillation.
Conclusions
Individuals with iMVP and sudden death have increased cardiac mass, mitral annulus size, and left ventricular fibrosis compared with a matched cohort, with cardiac arrest caused by ventricular fibrillation. The histopathological changes in iMVP may provide the substrate necessary for development of malignant ventricular arrhythmias.
Keywords: sudden death, valvular heart disease, ventricular arrhythmia
Subject Categories: Sudden Cardiac Death, Ventricular Fibrillation, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- AMI
acute myocardial infarction
- COD
cause of death
- GERD
gastro‐esophageal reflux disease
- ICD
International Classification of Diseases
- iMVP
isolated mitral valve prolapse
- IQR
inter‐quartile range
- LV
left ventricle
- MR
mitral regurgitation
- MVA
motor vehicle accident
- MVP
mitral valve prolapse
- NCIS
National Coronial Information System
- PM
papillary muscle
- PVC
premature ventricular complex
- RV
right ventricle
- TV
tricuspid valve
- VF
ventricular fibrillation
Clinical Perspective
What Is New?
To our knowledge, this is the first study to compare histopathological findings in individuals with isolated mitral valve prolapse (iMVP; whereby other potential causes of death are excluded) with matched control groups (for age, sex, height, and weight) and to systematically document cardiac arrest rhythm in those with autopsy‐confirmed iMVP.
Individuals with iMVP have significantly increased cardiac mass, mitral annulus size, and left ventricular fibrosis located within the endocardial‐midmural aspect of the left ventricle.
In individuals with iMVP and witnessed cardiac arrest, 94% had ventricular fibrillation.
What Are the Clinical Implications?
Increased cardiac mass, mitral annulus size, and left ventricular fibrosis in patients with iMVP may provide the substrate necessary for the development of malignant ventricular arrhythmias.
Cardiac imaging studies focusing on these findings may allow for the application of risk stratification parameters for living patients with iMVP.
Introduction
Mitral valve prolapse (MVP) is characterized by the atrial displacement of the mitral valve leaflet(s) during ventricular systole, with an estimated prevalence of 2.4%.1 Reported complications of MVP include severe mitral regurgitation (MR) requiring mitral valve surgery, infective endocarditis, stroke, and sudden death.2, 3
The postulated mechanism of sudden death in individuals with MVP has been ventricular arrhythmias,4, 5, 6, 7 although this association remains controversial.1, 3, 8 Although studies have reported the presence of significant ventricular arrhythmias in patients with MVP,9, 10, 11 only 6 isolated case reports in the scientific literature have documented cardiac rhythm at time of death in patients with autopsy‐determined isolated MVP (iMVP; whereby other potential causes of death [CODs] are excluded).7
Pathological characterization may provide clues about the link between iMVP, sudden death, and possible underlying mechanisms. Since Barlow's original description of MVP at autopsy,12 studies have identified various cardiac anatomical changes in MVP, such as increased annulus size or cardiac mass,5, 13, 14 which may contribute to development of sudden death. However, these studies may not have controlled for influencing factors, such as age, sex, height, and weight.15
In this study, we examined our Australian coronial database over a consecutive period of 17 years, with the aim to:
Describe clinical characteristics of individuals with iMVP and sudden death;
Describe underlying histopathological changes in individuals with iMVP and sudden death compared with matched control cohorts;
Describe underlying histopathological changes in individuals with iMVP and sudden death compared with individuals with non‐iMVP and sudden death;
Document cardiac arrest rhythm in individuals with autopsy‐determined iMVP and sudden death.
Methods
Source data used for this study were obtained from the National Coronial Information System (NCIS) database, administered by the Department of Justice and Community Safety, Victoria, Australia. The data that support the findings of this study are available from the corresponding author upon reasonable request subject to approval from the Research and Ethics Committees.
Data Source
Beginning July 2000 (and January 2001 for the state of Queensland), all reportable deaths determined by an Australian coroner are prospectively recorded in the NCIS database. The database classifies antecedent CODs using the International Classification of Diseases, Tenth Revision (ICD‐10) system; and where available, contains individual autopsy reports and police reports on circumstances of death.
The NCIS database was used to identify cases of iMVP and sudden death. Once identified, data linkage with Australian state‐wide ambulance services was performed to retrieve initial cardiac rhythm during attempted resuscitation.
Inclusion Criteria
An NCIS database search was performed in May 2018, and 329 106 cases were available for analysis. All individuals in whom MVP was coded (ICD‐10 code I34.1) as a COD (either underlying or contributing) between July 2000 and May 2018 were screened and autopsy records were examined. Inclusion criterion was the presence of MVP on autopsy examination (evidence of myxomatous leaflets on gross examination and/or histological examination). After individual case review (including circumstances of death, demographics, clinical information, and detailed pathological evaluation), the MVP cohort was then separated into 2 groups: an iMVP group with presumed sudden cardiac death caused by MVP; and a non‐iMVP group with death caused by a possible combination of MVP and other cardiac or systemic illnesses (Figure 1). Patients were adjudicated to have iMVP on the basis of the presence of MVP at autopsy without another possible COD. Definition of sudden cardiac death included witnessed cases in a previously stable individual and unwitnessed cases where an individual was found dead who at time of last witnessed contact was in a usual state of health.16
Figure 1.
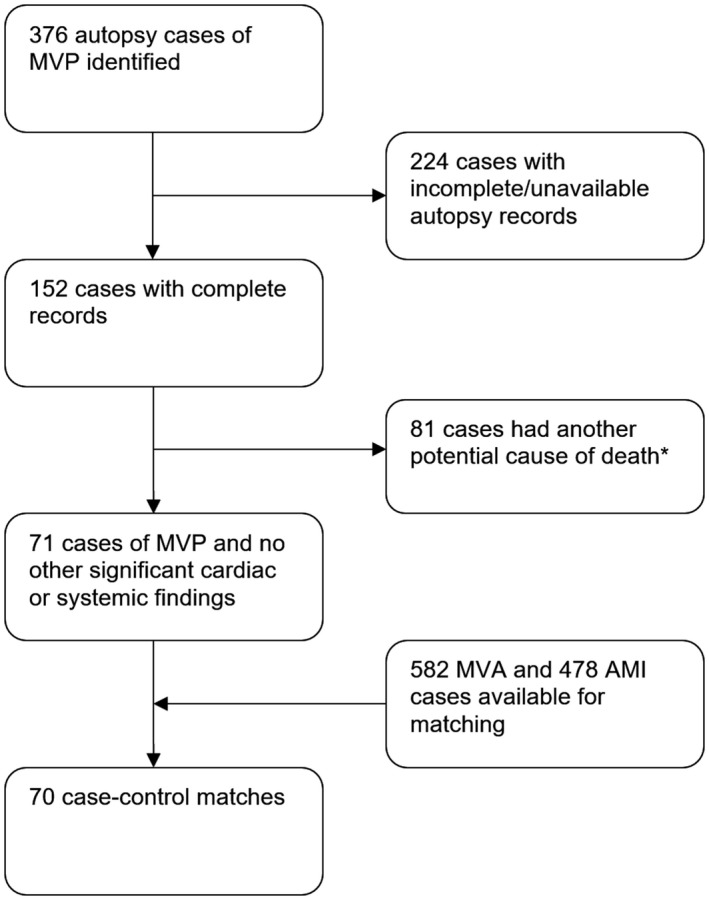
Case identification. *Other significant findings include: ischemic heart disease (36), previous cardiac surgery (8), histological myocarditis (4), significant left ventricular hypertrophy (3), dilated cardiomyopathy (2), severe mitral regurgitation (1), arrhythmogenic right ventricular cardiomyopathy (1), bicuspid aortic valve with aortic coarctation (1), infection (7), respiratory (6), drug overdose (3), cirrhosis (3), head injury (3), metastatic carcinoma (1), hyponatremia (1), and suicide (1). AMI indicates acute myocardial infarction; MVA, motor vehicle accident; and MVP, mitral valve prolapse.
For those with iMVP, 2 control cohorts were randomly extracted from the NCIS database. The first cohort (presumed noncardiac death) included individuals who died because of a motor vehicle accident (MVA; as categorized in the NCIS database) from 2013 to 2014. The second cohort (presumed cardiac death) included all individuals who died because of acute myocardial infarction (AMI; using ICD‐10 code I21) from 2013 to 2014 with an additional search for individuals dying because of AMI aged between 18 and 35 years from 2000 to 2018.
Exclusion Criteria
Exclusion criteria in both the iMVP and MVA groups were the presence of another significant cardiac finding (clinical or autopsy), including ischemic heart disease (≥70% stenosis in any coronary artery, presence of coronary stent, or documented previous history), known cardiomyopathy, severe MR (who may have experienced nonarrhythmic sudden death), previous cardiac surgery, myocarditis (on histology), hypertrophic cardiomyopathy (on histology), or left ventricular wall thickness ≥25 mm. In addition, for the MVA control group, cases were excluded where MVP was found on autopsy or if individuals experienced burns because of the MVA.
Cases with incomplete autopsy records that did not document age, sex, height, weight, or cardiac mass were excluded from all groups.
Data Collection
Data collected for all groups included age, sex, height, weight, and cardiac mass. Using these parameters, a cardiac mass predictor tool was used to determine whether the cardiac mass was >95% predicted.15 Internal organ mass, left and right ventricular wall thickness, and mitral and tricuspid annulus circumference were also recorded. For subjects with iMVP and non‐iMVP, information on leaflet involvement was also collected.
For Victorian cases of iMVP with sudden death (n=17), stored left ventricular histology slides were reviewed by an expert cardiac pathologist (S.P.) for qualitative assessment of fibrosis after hematoxylin and eosin staining. Standardized sampling of the left ventricle includes obtaining transmural biopsies from the mid portion of the anterior, lateral, posterior, and septal walls. Multi‐segment fibrosis was noted if fibrosis was present in ≥2 sections.
State and territory ambulance services from Australian Capital Territory, New South Wales, Northern Territory, Queensland, Tasmania, and Victoria were contacted to provide initial cardiac rhythm and witness status of cardiac arrest. Two remaining states, South Australia and Western Australia (accounting for ≈18% of the Australian population), did not have autopsy reports available on NCIS at the time of the study.
Ethical Approval
Access to the NCIS database and subsequent data linkage were granted by the Justice Human Research and Ethics Committee (CF/16/4998), South Eastern Sydney Local Health District Human Research Ethics Committee (HREC/17/POWH/632), and the Victorian Institute of Forensic Medicine Research Advisory Committee and Ethics Committee (RAC 009‐18). Because of the nature of this study, individual informed consent was waived as per the Ethics Committees listed above.
Statistical Analysis
Continuous data are reported as means with SDs or medians with interquartile range, and the Shapiro‐Wilk test was used to evaluate normality. To determine differences between the iMVP cohort and matched controls, the paired t test or the Wilcoxon signed ranks test was used to compare continuous variables where appropriate. To determine differences between the iMVP cohort and unmatched non‐iMVP group, the Student t test or the Mann‐Whitney U test was used to compare continuous variables where appropriate. Categorical data are presented as absolute figures with percentages and compared using the McNemar's test and Fisher's exact test for matched and unmatched groups, respectively. A 2‐sided P<0.05 was considered significant. Cases were matched with control cohorts in a 1:1 ratio for parameters of age, sex, height, and weight using SPSS Version 24 “Case Control Matching” command. All data analyses were performed using SPSS Version 24 (IBM Corp, Armonk, NY).
Results
Patient Population
Between July 2000 and May 2018, there were 77 221 deaths attributable to a cardiovascular cause (ICD‐10 codes I00–I25 and I30–I52), with 64 734 cases attributable to ischemic heart disease (ICD‐10 codes I20‐I25). In total, there were 376 cases of MVP identified (Figure 1), with complete autopsy records available for 152 cases. There were 71 cases with sudden cardiac death attributable to iMVP and 81 cases with non‐iMVP. For the 81 cases with non‐iMVP, there were 56 cases with suspected cardiac COD and 25 with suspected noncardiac COD. Suspected CODs for those with non‐iMVP are presented in Figure 1 (footnote).
For cases of iMVP, a pool of 582 MVA cases and 478 AMI cases were obtained for the control samples. After 1:1 matching, 70 cases in each group were used for case‐control analysis. A suitable match was unable to be obtained for one morbidly obese iMVP case (weight, 220 kg).
Clinical Characteristics
Baseline clinical characteristics for all 71 cases of iMVP and sudden death are shown in Table 1.
Table 1.
Baseline Clinical Characteristics and Circumstances of Death in Individuals With iMVP
| Clinical Characteristics (n=71) | |||
|---|---|---|---|
| Age range, y | 16–87 | Medications | 38 |
| Female sex | 36 (51) | Cardiac | 11 (29) |
| Medical history | 58 | Aspirin | 2 |
| Cardiac | 25 (43) | Warfarin | 1 |
| Obesity | 12 | β Blockeri | 3 |
| Hypertension | 9 | Digoxin | 2 |
| Dyslipidemia | 9 | Antihypertensivej | 7 |
| Endocarditis (healed) | 1 | Lipid lowering | 5 |
| Atrial fibrillation | 2 | Otherk | 14 (37) |
| Possible long‐QT syndromea | 1 | No medications | 16 (42) |
| PVC ablationb | 1 | Activity at time of death | 66 |
| Pericarditis | 1 | Normal daily activityl | 21 (32) |
| Marfanoidc | 1 | Sitting/resting | 15 (23) |
| Symptomsd | 6 | Sleeping | 12 (18) |
| Other | 21 (36) | Exertion (or soon after) | 9 (14) |
| Chronic respiratory diseasee | 4 | Using toilet | 6 (9) |
| Cancerf | 4 | Physical pain | 2 (3) |
| Psychiatricg | 8 | Emotional stress | 1 (2) |
| Alcoholism | 1 | Approximate time of death | 53 |
| Endocrineh | 2 | 6 am‐2 pm | 20 (38) |
| GERD | 1 | 2 pm‐10 pm | 17 (32) |
| No other medical history | 14 (24) | 10 pm‐6 am | 16 (30) |
Data are given as number or number (percentage), unless otherwise indicated. GERD indicates gastroesophageal reflux disease; iMVP, isolated mitral valve prolapse; and PVC, premature ventricular complex.
ECG unavailable.
For left ventricular origin, PVC possibly related to MVP.
Normal aorta at autopsy.
Includes syncope (2), palpitations (3), and dizziness (1).
Includes asthma (2), chronic obstructive pulmonary disease (1), and obstructive sleep apnea (1).
Includes nonmetastatic prostate cancer (3) and previously undiagnosed non‐Hodgkin lymphoma (1).
Includes depression alone (2), anxiety alone (1), depression and anxiety (3), and schizophrenia (2).
Includes hypothyroidism (1) and hypopituitarism (1).
Includes 1 patient taking sotalol.
Includes 2 patients taking loop diuretics.
Includes inhaled bronchodilators (5), nonsteroidal anti‐inflammatory drugs (1), thyroxine (1), prednisolone (1), benzodiazepines (3), antidepressants (5), olanzapine (1), antacid (3), and sulfasalazine (1).
Includes cases where individuals were found at home, at work performing routine (nonexertional) tasks, or walking.
Of the 71 cases of iMVP and sudden death, 36 (51%) were women. Age ranged from 16 to 87 years, with mean age of 49±18 years.
Additional cardiovascular history (including the presence of hypertension, dyslipidemia, or obesity) was present in 43% of individuals. In 23%, no other medical history was noted. At least one cardiac medication was being taken by 29% of individuals, whereas 42% of individuals were not taking any prescribed medications.
Of the 71 cases with autopsy‐confirmed iMVP, whether there was a premortem diagnosis of MVP was able to be ascertained in 64 cases, and of these, 34 cases (53%) were previously undiagnosed. More important, in those with a premortem diagnosis of MVP, there were no cases with documented severe MR or significant cardiomyopathy.
In total, 41% of deaths occurred in individuals who were either resting (22%) or sleeping (17%), whereas 32% of deaths occurred during daily (nonexertional) activity. Death during (or soon after) physical exertion occurred in 14%, and death while using the toilet occurred in 9%. Approximate time of death was evenly distributed over a 24‐hour period.
Internal Organ Masses
Data about cardiac and other internal organ masses are shown in Tables 2 and 3.
Table 2.
Histopathological Findings in 70 iMVP Cases Compared With Control Groups
| Variable | iMVP Cases | MVA Cases | P Valuea | AMI Cases | P Valuea |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age, y | 49±18 | 49±18 | 0.28 | 50±17 | 0.55 |
| Women | 35 (50) | 35 (50) | 1 | 35 (50) | 1 |
| Weight, kg | 77±18 | 78±16 | 0.52 | 78±17 | 0.16 |
| Height, cm | 172±11 | 172±10 | 0.49 | 172±9 | 0.36 |
| Internal organ masses, g | |||||
| Cardiac | 447±107 | 355±78 | <0.001 | 438±117 | 0.48 |
| Left lung | 617±175 | 454±167 | <0.001 | 667±250 | 0.12 |
| Right lung | 728±214 | 522±170 | <0.001 | 772±271 | 0.21 |
| Brain (n=64) | 1414±147 | 1390±149 | 0.27 | 1383±164 | 0.16 |
| Liver (n=67) | 1846±512 | 1599±386 | <0.001 | 1894±428 | 0.31 |
| Kidneys (n=67) | 326±110 | 274±60 | <0.001 | 345±86 | 0.20 |
| Spleen (n=67) | 223±111 | 140±58 | <0.001 | 211±107 | 0.46 |
| Gross pathological changes | |||||
| Cardiac mass | |||||
| >95% Predicted15 | 25 (36) | 4 (6) | <0.001 | 16 (23) | 0.09 |
| LV thickness, mm | n=49 | n=42 | 0.10 | n=49 | 0.69 |
| Median (IQR) | 15 (13–19) | 14 (12–15) | 15 (12–20) | ||
| RV thickness, mm | n=40 | n=36 | 0.67 | n=43 | 0.23 |
| Median (IQR) | 4 (3–5) | 4 (3–5) | 4 (3–5) | ||
| MV circumference, mm | n=26 | n=16 | <0.001 | n=13 | 0.002 |
| Median (IQR) | 121 (115–139) | 108 (91–115) | 110 (98–115) | ||
| TV circumference, mm | n=21 | n=15 | 0.18 | n=12 | 0.91 |
| Median (IQR) | 130 (120–140) | 120 (120–130) | 125 (116–148) | ||
| Leaflet involvement | |||||
| Reportedb | 54 | ||||
| Bileaflet | 47 (87) | ||||
| Posterior leaflet | 5 (9) | ||||
| Anterior leaflet | 2 (4) | ||||
| Left ventricular histological changes | |||||
| Abnormal | 55 (79)c | 25 (36)d | <0.001 | 61 (87)e | 0.14 |
| Fibrosis or scarring | 52 | 20 | 37 | ||
| Myocyte hypertrophy | 5 | 3 | 5 | ||
| Contraction band | 3 | 6 | |||
| PM fibrosisf | 2 | ||||
| PM calcification | 1 | ||||
| No abnormalities found | 15 (21) | 45 (64) | 9 (13) | ||
Data are given as mean±SD, number, or number (percentage), unless otherwise indicated. AMI indicates acute myocardial infarction; iMVP, isolated mitral valve prolapse; IQR, interquartile range; LV, left ventricle; MV, mitral valve; MVA, motor vehicle accident; PM, papillary muscle; RV, right ventricle; and TV, tricuspid valve.
Compared with iMVP cases.
All 70 cases reported abnormal valve morphological features with descriptors such as redundant, thickened, ballooned, hooded, prolapsed, floppy, pendulous, voluminous, myxomatous, or billowing.
More than one abnormality in some cases.
Other descriptors (>1 in some cases) include interfiber edema (1), inflammatory cells (2), and hemorrhage (1).
Other descriptors (>1 in some cases) include acute/subacute infarct (16), mural infarct (6), healed infarct (4), myocyte necrosis (7), coagulative necrosis (4), myocardial rupture (2), inflammatory cells (4), interstitial hemorrhage (1), and amyloid (1).
One case had previous premature ventricular complex ablation.
Table 3.
Histopathological Findings in 70 iMVP Cases Compared With 81 Non‐iMVP Cases
| Variable | iMVP Cases | Non‐iMVP Cases | |||||
|---|---|---|---|---|---|---|---|
| Total | P Valuea | Cardiac COD | P Valuea | Other COD | P Valuea | ||
| (n=70) | (n=81) | (n=56) | (n=25) | ||||
| Baseline characteristics | |||||||
| Age, y | 49±18 | 62±17 | <0.001 | 61±17 | <0.001 | 62±19 | 0.002 |
| Women | 35 (50) | 38 (47) | 0.77 | 25 (45) | 0.60 | 13 (52) | 0.82 |
| Weight, kg | 77±18 | 70±21 | 0.03 | 76±21 | 0.78 | 56±15 | <0.001 |
| Height, cm | 172±11 | 168±12 | 0.048 | 169±12 | 0.17 | 166±12 | 0.03 |
| Internal organ masses, g | |||||||
| Cardiac | 447±107 | 440±127 | 0.72 | 472±129 | 0.23 | 368±92 | 0.001 |
| Left lung | 617±175 | 589±194 | 0.37 | 598±200 | 0.58 | 570±183 | 0.26 |
| Right lung | 728±214 | 682±198 | 0.18 | 696±207 | 0.40 | 652±174 | 0.11 |
| Brain | 1414±147 | 1363±189 | 0.09 | 1359±186 | 0.09 | 1370±201 | 0.30 |
| Liver | 1846±512 | 1567±504 | 0.001 | 1676±545 | 0.09 | 1349±320 | <0.001 |
| Kidneys | 326±110 | 279±75 | 0.003 | 291±72 | 0.053 | 255±78 | 0.004 |
| Spleen | 223±111 | 175±90 | 0.006 | 179±71 | 0.02 | 167±122 | 0.04 |
| Gross pathological changes | |||||||
| Cardiac mass | |||||||
| >95% Predicted | 25 (36) | 19 (23) | 0.10 | 14 (25) | 0.20 | 5 (20) | 0.15 |
| LV thickness, mm | 15 (13–19) | 15 (12–20) | 0.45 | 15 (12–20) | 0.29 | 14 (13–17) | 0.80 |
| RV thickness, mm | 4 (3–5) | 4 (3–5) | 0.47 | 4 (3–5) | 0.54 | 4 (3–5) | 0.56 |
| MV circumference, mm | 121 (115–139) | 120 (105–139) | 0.60 | 125 (110–140) | 1.0 | 103 (100–130) | 0.13 |
| TV circumference, mm | 130 (120–140) | 130 (114–144) | 0.73 | 133 (116–149) | 0.41 | 120 (113–131) | 0.30 |
| Leaflet involvement | |||||||
| Reported | 54 | 53 | 35 | 18 | |||
| Bileaflet | 47 (87) | 42 (79) | 0.41 | 29 (83) | 0.37 | 13 (72) | 0.28 |
| Posterior leaflet | 5 (9) | 10 (19) | 6 (17) | 4 (22) | |||
| Anterior leaflet | 2 (4) | 1 (2) | 0 | 1 (6) | |||
| Left ventricular histological changes | |||||||
| Abnormal | 55 (79) | 67 (89) | 0.08 | 46 (87) | 0.24 | 21 (95) | 0.07 |
| Fibrosis or scarring | 52 | 63 | 43 | 20 | |||
| Myocyte hypertrophy | 5 | 10 | 7 | 3 | |||
| Contraction band | 3 | 2 | 2 | 0 | |||
| PM fibrosis | 2 | 2 | 2 | 0 | |||
| PM calcification | 1 | 0 | 0 | 0 | |||
| No abnormalities found | 15 (21) | 8 (11) | 7 (13) | 1 (5) | |||
Data are given as mean±SD, number, number (percentage), or median (interquartile range). COD indicates cause of death; iMVP, isolated mitral valve prolapse; LV, left ventricle; MV, mitral valve; PM, papillary muscle; RV, right ventricle; and TV, tricuspid valve.
Compared with iMVP cases.
Compared with those dying from MVA, individuals with iMVP had significantly greater cardiac mass (447 g versus 355 g; P<0.001), but also significantly greater lung (P<0.001), liver (P=0.002), kidney (P=0.002), and spleen (P<0.001) masses. Brain mass was not significantly different.
Compared with those dying as a result of an AMI, individuals with iMVP had similar cardiac (447 g versus 438 g; P=0.64) and other internal organ masses, although there was a trend toward higher combined kidney mass in the AMI group (P=0.07).
Compared with those with non‐iMVP, individuals with iMVP had similar cardiac (447 g versus 440 g; P=0.72), lung, and brain masses. Individuals with iMVP had significantly greater liver (P=0.001), kidney (P=0.003), and spleen (P=0.006) masses.
Histopathological Findings
Histopathological data are shown in Tables 2 and 3. Using a cardiac mass predictor tool,15 cardiac mass was >95% predicted in 36% of iMVP cases, and this was significantly greater than the MVA cohort (6%; P<0.001) but not significantly different to the AMI cohort (23%; P=0.09) and non‐iMVP cohort (23%; P=0.10). Left and right ventricular thickness was not significantly different between the iMVP group, both control groups, and the non‐iMVP group.
Median mitral valve circumference was 121 mm (interquartile range, 115–139 mm), which was significantly greater than both the MVA (P<0.001) and AMI (P=0.002) control groups but similar to the non‐iMVP group (P=0.60). There was no significant difference between the groups with respect to tricuspid valve circumference. Leaflet involvement in iMVP was predominantly bileaflet (87%), followed by posterior leaflet (9%) and then anterior leaflet (4%), whereas 3 cases described concomitant tricuspid valve prolapse. The proportion of cases with bileaflet prolapse was similar between those with iMVP and those with non‐iMVP (79%; P=0.41), including those with suspected cardiac and noncardiac COD.
Abnormal left ventricular histological features were present in 79% of iMVP cases, which was significantly higher than the MVA group (P<0.001) but similar to the AMI group (P=0.14) and non‐iMVP group (P=0.08), including those with suspected cardiac and noncardiac COD. Reported patterns of fibrosis in those with iMVP were predominantly interstitial and/or perivascular. In 4 cases, histological abnormalities involving the papillary muscles were specifically documented.
On the basis of individual histology review for 17 cases, fibrosis distribution patterns were multisegment (n=4; eg, fibrosis affecting ≥2 segments in the subendocardial‐midmural layer of the left ventricle), focal (n=5; eg, interstitial fibrosis in 1 sampled section), or a combination of both (n=4; eg, multisegment fibrosis in the subendocardial‐midmural layer with one section showing focal midmural fibrosis). No fibrosis was detected in 4 cases. In addition, fibrosis involved the subendocardial‐midmural layer in 85% (11/13) of cases, with isolated midmural myocardial fibrosis in the other 2 cases. Transmural fibrosis was present in 2 cases (15%).
Cardiac Arrest Rhythm
Resuscitation by emergency medical services was documented in 40 iMVP cases (Figure 2). Initial cardiac arrest rhythm was ventricular fibrillation in 94% (17/18) of witnessed cases and 32% (7/22) of unwitnessed cases. The remaining cases had documented asystole, and no cases had ventricular tachycardia or pulseless electrical activity as the initial cardiac arrest rhythm.
Figure 2.

Initial cardiac rhythm in cases of autopsy‐determined isolated mitral valve prolapse (iMVP). VF indicates ventricular fibrillation.
Clinical data with cardiac arrest rhythm strip and histological findings for 5 representative cases are shown in Figure 3.
Figure 3.

Histological analysis with initial cardiac rhythm for representative cases of isolated mitral valve prolapse (iMVP) and sudden cardiac death. A, A 31‐year‐old woman with witnessed cardiac arrest while resting in bed and ventricular fibrillation (VF). Histopathological examination showed myxomatous change in both mitral valve leaflets with focal left ventricular fibrosis in a subendocardial‐midmural distribution and papillary muscle fibrosis. B, A 45‐year‐old woman found on the toilet with VF after an unwitnessed cardiac arrest. Histopathological examination showed thickening and billowing of both mitral valve leaflets with multisegment left ventricular fibrosis in a subendocardial‐midmural distribution. C, A 34‐year‐old woman found collapsed in the bathroom with asystole after an unwitnessed cardiac arrest. Histopathological examination showed myxomatous change in both mitral valve leaflets with multisegment left ventricular fibrosis in a midmural distribution. D, A 25‐year‐old woman with witnessed cardiac arrest while washing dishes and VF. Histopathological examination showed myxomatous change in both mitral valve leaflets with multisegment left ventricular fibrosis in a subendocardial‐midmural distribution. E, A 47‐year‐old woman found collapsed in the bathroom with VF after an unwitnessed cardiac arrest. Histopathological examination showed thickened and floppy mitral valve leaflets with no evidence of left ventricular fibrosis.
Discussion
MVP is a common cardiac condition in clinical practice, yet its association with (and potential mechanism of) sudden death has been difficult to elucidate. To our knowledge, this 17‐year nationwide study is the largest case‐control study to investigate histopathological findings in iMVP and sudden death, and the first study to systematically document cardiac arrest rhythm in cases of autopsy‐determined iMVP. The key findings are as follows:
In cases of iMVP and sudden death, the mean age was 49 years, half (51%) were women, and 87% of cases had bileaflet prolapse;
Mitral valve annulus circumference was significantly larger in cases of iMVP with sudden death compared with matched control cohorts;
Individuals with iMVP and sudden death had increased cardiac mass compared to matched individuals with noncardiac death; but similar cardiac mass compared to matched individuals with cardiac death;
Left ventricular fibrosis in cases of iMVP and sudden death predominantly (85%) involved the subendocardial‐midmural aspect of the ventricle;
Ventricular fibrillation was the predominant (94%) cardiac arrest rhythm in individuals with iMVP and witnessed cardiac arrest.
Clinical Characteristics
Mean age in our cohort was 49 years, which is similar to previous autopsy series,13 but higher than previous case series of MVP and sudden death,17 possibly reflecting reporting bias in nonautopsy studies.
Our study found an even distribution between men and women. Previous autopsy studies have found somewhat conflicting results with either equal distribution14 or female predominance,5, 13 although prevalence of redundant MVP appears equally distributed in population screening studies.1 Nonautopsy studies have suggested that female sex may carry greater risk of malignant MVP,4, 7, 18, 19 although the reasons for this are unexplained. Sex differences in seeking medical attention for symptoms20 may account for differences in premortem detection. Results from this study indicate that male patients with iMVP are as susceptible to sudden death events as female patients.
Our study found that 73% of sudden death episodes in iMVP occurred during sleep, rest, or regular daily activity, with an equal distribution of events over a 24‐hour period. Despite historical studies implicating increased adrenergic drive in patients with MVP and ventricular arrhythmias,21 our study indicates that sudden death in patients with iMVP does not necessarily require a short‐term precipitating event, such as physical or emotional stress.
After application of prespecified exclusion criteria, one case had possible long‐QT syndrome, which may have caused sudden death, although the reported association between MVP and repolarization abnormalities may confound matters.22
Cardiac and Other Organ Masses
Previous studies have noted increased cardiac mass in individuals with MVP and sudden death.5, 14 In our study, 36% of cases had cardiac mass >95% predicted. More important, in the groups where influencing factors (age, sex, height, and weight) were controlled for (iMVP, MVA, and AMI), those with iMVP had similar cardiac mass compared with those with AMI and significantly higher cardiac mass compared with those with MVA. Interestingly, those with iMVP and AMI related sudden death also had significantly increased intrathoracic and intra‐abdominal organ mass when compared to those with noncardiac death.
Although increased kidney and spleen mass has been reported in patients with sudden cardiac death compared with noncardiac death,23 the reasons for this are unexplored. One possibility is that increased intrathoracic and intra‐abdominal organ mass may reflect edema from acute biventricular failure in sudden cardiac death, hence implicating a common terminal process (ie, cardiovascular cause of sudden death) in those with iMVP and AMI related sudden death.
Histopathological Findings
In addition to increased cardiac mass, median left ventricular thickness in cases of iMVP and sudden death was 15 mm, which is considered the upper limit of normal in autopsy cases.24 In combination, these gross pathological changes suggest that underlying structural abnormalities involving the left ventricle may be an important factor in cases of iMVP and the development of sudden cardiac death.
Most of our cohort with iMVP had bileaflet prolapse, which is consistent with previous reports indicating a predominantly bileaflet subset of malignant MVP.4 Furthermore, median mitral valve annulus circumferences in those with iMVP were greater than expected on the basis of both our current control cohorts and previous population control data.25 Previous studies have reported interlinking associations between the presence of bileaflet prolapse, abnormal mitral annular physiological features, and ventricular arrhythmias.26, 27, 28 These findings suggest that disorganized mitral annular function may represent an anatomical cause of electrical instability, and hence be as important as the presence of myxomatous prolapse in the development of sudden death in MVP.29
In addition, 79% of iMVP cases had abnormal histological features of the left ventricle. The subendorcardial‐midmural distribution of fibrosis is consistent with other conditions that result in left ventricular remodeling, such as hypertrophic cardiomyopathy, dilated cardiomyopathy, and severe aortic stenosis,30, 31, 32 as well as a previous autopsy study of MVP.5 On the basis of standardized sampling, fibrosis affecting the level of the midventricle, as opposed to previously reported focal changes,5 along with increased overall cardiac mass indicate that a diffuse remodeling process may occur in those with iMVP and sudden death. Recent studies involving cardiac magnetic resonance imaging have linked the presence of fibrosis with ventricular arrhythmias.5, 33, 34 Taken together, the presence of cardiac fibrosis in conjunction with left ventricular remodeling may provide further necessary substrate for malignant ventricular arrhythmias in the pathogenesis of iMVP and sudden death.
Histopathological findings of increased cardiac mass, mitral annular dilatation, and left ventricular fibrosis from our study suggest that patients with iMVP have antemortem changes in their cardiac and mitral valve structure that predispose them to sudden death. Future cardiac imaging studies focusing on these findings may allow the application of risk stratification parameters for living patients with iMVP.
Findings in Non‐iMVP
Individuals with non‐iMVP (including those with suspected cardiac and noncardiac COD) differed significantly from those with iMVP with regard to age, height, and weight. In conjunction with the documented effects of these parameters on various internal organ masses,15, 35 the interpretation of internal organ mass data between those with iMVP and non‐iMVP is confounded.
Direct comparisons between the iMVP group and the non‐iMVP group did not yield any significant differences in terms of proportion with bileaflet prolapse, mitral annulus size, or proportion with left ventricular histological changes. More important, we implemented strict selection criteria for the iMVP cohort to select MVP cases in which there was a high likelihood that sudden death was as a result of MVP. Within the 81 non‐iMVP cases, patients may still have had MVP as a precipitating mechanism for their death, but it was unclear whether this was the likely COD because of existence of potential confounders. These patients did not qualify as having iMVP on the basis of our exclusion criteria.
Consequently, the attributable role of MVP with regard to sudden cardiac death in the setting of other coexistent conditions requires refining.
Cardiac Arrest Rhythm
This study provides the largest collection of cases of autopsy‐determined iMVP and their corresponding cardiac arrest rhythm. In those with witnessed cardiac arrest, 94% had initial ventricular fibrillation (as documented by emergency medical services), indicating that a malignant ventricular arrhythmia is the likely initiating event in patients with iMVP and sudden death.
Limitations
In this autopsy study, we are unable to report on certain premortem characteristics in iMVP, such as the degree of MR (although cases with known severe MR were excluded). More important, most of the cases with iMVP were previously undiagnosed. This study may be subject to referral bias on coronial deaths; however, unexpected deaths in Australia are mandated to be reported to a state coroner. Pathological examination confirmed redundant MVP in all cases. Hence, these results are only applicable to cases of redundant leaflet MVP, although this appears to be the population most at risk of sudden death events.2 Findings from histopathological analysis do not routinely include papillary muscles or inferobasal wall, precluding our ability to comment on fibrosis in those specific regions. However, the presence of fibrosis in the mid left ventricle suggests a diffuse remodeling process in addition to previously described focal changes. We are unable to comment on the importance of MVP in the pathogenesis of death for patients with non‐iMVP. Further work investigating the attributable risk of MVP for sudden cardiac death in the setting of coexistent conditions is warranted.
Conclusions
This nationwide autopsy study indicates that most cases of iMVP and sudden death had bileaflet prolapse, significantly enlarged mitral valve annulus, increased cardiac mass, and histopathological findings of cardiac fibrosis, with ventricular fibrillation being the most common presenting rhythm in cases of witnessed cardiac arrest. The histopathological changes in iMVP may provide the substrate necessary for development of ventricular arrhythmias leading to sudden cardiac death.
Sources of Funding
Dr Han and Dr Lim report having received funding from Austin Medical Research Foundation.
Disclosures
Dr Sanders reports having served on the advisory board of Biosense‐Webster, Medtronic, CathRx, and St. Jude Medical (now Abbott). Dr Sanders reports having received lecture and/or consulting fees from Biosense‐Webster, Medtronic, St. Jude Medical (now Abbott), and Boston Scientific. Dr Sanders reports having received research funding from Medtronic, St. Jude Medical (now Abbott), Boston Scientific, Biotronik, and Sorin. Dr Kalman reports having received research funding from St. Jude Medical (now Abbott), Biosense‐Webster, Medtronic, and Boston Scientific. Dr Lim reports having received research support from St. Jude Medical (now Abbott). The remaining authors have no disclosures to report.
Acknowledgments
The authors acknowledge Jessica Bryan, National Coronial Information System as administered by the Department of Justice and Community Safety, Australia; Kathryn Tumini and Brendan Schultz, Queensland Ambulance Service, Australia; Toby Keene, Australian Capital Territory Ambulance Service, Australia; Con Georgakas, Ambulance Tasmania, Australia; Rosemary Carney, New South Wales Ambulance, Australia; and Malcolm Johnston‐Leek, St John's Ambulance, Northern Territory, Australia.
(J Am Heart Assoc. 2020;9:e015587 DOI: 10.1161/JAHA.119.015587.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral‐valve prolapse. N Engl J Med. 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, McGoon MD, Shub C, Miller FA Jr, Ilstrup DM, Tajik AJ. Echocardiographically documented mitral‐valve prolapse: long‐term follow‐up of 237 patients. N Engl J Med. 1985;313:1305–1309. [DOI] [PubMed] [Google Scholar]
- 3. Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. 2005;365:507–518. [DOI] [PubMed] [Google Scholar]
- 4. Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez‐Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out‐of‐hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. [DOI] [PubMed] [Google Scholar]
- 5. Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, Frigo AC, Rigato I, Migliore F, Pilichou K, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. [DOI] [PubMed] [Google Scholar]
- 6. Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, Vohra JK, Morton JB, Semsarian C, Marwick T, Kalman JM, et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta‐analysis. Heart. 2019;105:144–151. DOI: 10.1136/heartjnl-2017-312932. [DOI] [PubMed] [Google Scholar]
- 7. Han HC, Ha FJ, Teh AW, Calafiore P, Jones EF, Johns J, Koshy AN, O'Donnell D, Hare DL, Farouque O, et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. 2018;7:e010584 DOI: 10.1161/JAHA.118.010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kligfield P, Hochreiter C, Kramer H, Devereux RB, Niles N, Kramer‐Fox R, Borer JS. Complex arrhythmias in mitral regurgitation with and without mitral valve prolapse: contrast to arrhythmias in mitral valve prolapse without mitral regurgitation. Am J Cardiol. 1985;55:1545–1549. [DOI] [PubMed] [Google Scholar]
- 9. Wei JY, Bulkley BH, Schaeffer AH, Greene HL, Reid PR. Mitral‐valve prolapse syndrome and recurrent ventricular tachyarrhythmias: a malignant variant refractory to conventional drug therapy. Ann Intern Med. 1978;89:6–9. [DOI] [PubMed] [Google Scholar]
- 10. Vohra J, Sathe S, Warren R, Tatoulis J, Hunt D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol. 1993;16:387–393. [DOI] [PubMed] [Google Scholar]
- 11. Syed FF, Ackerman MJ, McLeod CJ, Kapa S, Mulpuru SK, Sriram CS, Cannon BC, Asirvatham SJ, Noseworthy PA. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. 2016;9:e004005. [DOI] [PubMed] [Google Scholar]
- 12. Barlow J, Bosman C, Pocock W, Marchand P. Late systolic murmurs and non‐ejection (“mid‐late”) systolic clicks: an analysis of 90 patients. Br Heart J. 1968;30:203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dollar AL, Roberts WC. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. J Am Coll Cardiol. 1991;17:921–931. [DOI] [PubMed] [Google Scholar]
- 14. Farb A, Tang AL, Atkinson JB, McCarthy WF, Virmani R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenly to those who have congestive heart failure from mitral regurgitation and to those with fatal noncardiac conditions. Am J Cardiol. 1992;70:234–239. [DOI] [PubMed] [Google Scholar]
- 15. Wingren CJ, Ottosson A. Postmortem heart weight modelled using piecewise linear regression in 27,645 medicolegal autopsy cases. Forensic Sci Int. 2015;252:157–162. [DOI] [PubMed] [Google Scholar]
- 16. Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, et al. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation. 2006;114:2534–2570. [DOI] [PubMed] [Google Scholar]
- 17. Pocock WA, Bosman CK, Chesler E, Barlow JB, Edwards JE. Sudden death in primary mitral valve prolapse. Am Heart J. 1984;107:378–382. [DOI] [PubMed] [Google Scholar]
- 18. Zuppiroli A, Mori F, Favilli S, Barchielli A, Corti G, Montereggi A, Dolara A. Arrhythmias in mitral valve prolapse: relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am Heart J. 1994;128:919–927. [DOI] [PubMed] [Google Scholar]
- 19. Fulton BL, Liang JJ, Enriquez A, Garcia FC, Supple GE, Riley MP, Schaller RD, Dixit S, Callans DJ, Marchlinski FE, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. 2018;29:146–153. [DOI] [PubMed] [Google Scholar]
- 20. Gerritsen AA, Devillé WL. Gender differences in health and health care utilisation in various ethnic groups in the Netherlands: a cross‐sectional study. BMC Public Health. 2009;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Śniez̊ek‐Maciejewska M, Dubiel J, Piwowarska W, Mroczek‐Czernecka D, Mazurek S, Jaśkiewicz J, Kitliński M. Ventricular arrhythmias and the autonomic tone in patients with mitral valve prolapse. Clin Cardiol. 1992;15:720–724. [DOI] [PubMed] [Google Scholar]
- 22. Akcay M, Yuce M, Pala S, Akcakoyun M, Ergelen M, Kargin R, Emiroglu Y, Ozdemir N, Kaymaz C, Ozkan M. Anterior mitral valve length is associated with ventricular tachycardia in patients with classical mitral valve prolapse. Pacing Clin Electrophysiol. 2010;33:1224–1230. [DOI] [PubMed] [Google Scholar]
- 23. Edston E. The earlobe crease, coronary artery disease, and sudden cardiac death: an autopsy study of 520 individuals. Am J Forensic Med Pathol. 2006;27:129–133. [DOI] [PubMed] [Google Scholar]
- 24. Ho SY. Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. Eur J Echocardiogr. 2009;10:iii3–iii7. [DOI] [PubMed] [Google Scholar]
- 25. Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age‐related changes in normal human hearts during the first 10 decades of life, part II (maturity): a quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. [DOI] [PubMed] [Google Scholar]
- 26. Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound. 2010;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perazzolo Marra M, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi B, Lacognata C, Rigato I, Migliore F, Pilichou K, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hourdain J, Clavel MA, Deharo J‐C, Asirvatham S, Avierinos JF, Habib G, Franceschi F, Probst V, Sadoul N, Martins R, et al. Common phenotype in patients with mitral valve prolapse who experienced sudden cardiac death. Circulation. 2018;138:1067–1069. [DOI] [PubMed] [Google Scholar]
- 29. Dejgaard LA, Skjølsvik ET, Lie ØH, Ribe M, Stokke MK, Hegbom F, Scheirlynck ES, Gjertsen E, Andresen K, Helle‐Valle TM, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72:1600–1609. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka M, Fujiwara H, Onodera T, Wu D, Hamashima Y, Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J. 1986;55:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Unverferth DV, Baker PB, Swift SE, Chaffee R, Fetters JK, Uretsky BF, Thompson ME, Leier CV. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. Am J Cardiol. 1986;57:816–820. [DOI] [PubMed] [Google Scholar]
- 32. Treibel TA, López B, González A, Menacho K, Schofield RS, Ravassa S, Fontana M, White SK, DiSalvo C, Roberts N, et al. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non‐invasive study in 133 patients. Eur Heart J. 2017;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. [DOI] [PubMed] [Google Scholar]
- 34. Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, Little SH, Quinones MA, Lawrie GM, Zoghbi WA, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72:823–834. [DOI] [PubMed] [Google Scholar]
- 35. He Q, Heshka S, Albu J, Boxt L, Krasnow N, Elia M, Gallagher D. Smaller organ mass with greater age, except for heart. J Appl Physiol. 2009;106:1780–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]


