Abstract
Background
There is little knowledge about the significance of extremely high values (>655) for the ratio of sFlt‐1 (soluble fms‐like tyrosine kinase 1) to PlGF (placental growth factor). We aim to describe the time‐to‐delivery interval and maternal and perinatal outcomes when such values are demonstrated while assessing suspected or confirmed placental dysfunction based on clinical or sonographic criteria.
Methods and Results
A multicenter retrospective cohort study was performed on 237 singleton gestations between 20+0 and 37+0 weeks included at the time of first demonstrating a sFlt‐1/PlGF ratio >655. Clinicians were aware of this result, but standard protocols were followed for delivery indication. Main outcomes were compared for women with and without preeclampsia at inclusion. In those with preeclampsia (n=185, of whom 77.3% had fetal growth restriction), severe preeclampsia features and fetal growth restriction in stages III or IV were present in 49.2% and 13.5% cases, respectively, at inclusion and in 77.3% and 28.6% cases, respectively, at delivery. In the group without preeclampsia (n=52, 82.7% had fetal growth restriction), these figures were 0% and 30.8%, respectively, at inclusion and 21.2% and 50%, respectively, at delivery. Interestingly, 28% of women without initial preeclampsia developed it later. The median time to delivery was 4 days (interquartile range: 1–6 days) and 7 days (interquartile range: 3–12 days), respectively (P<0.01). Overall, perinatal mortality was 62.1% before 24 weeks; severe morbidity surpassed 50% before 29 weeks but became absent from 34 weeks. Maternal serious morbidity was high at any gestational age.
Conclusions
An sFlt‐1/PlGF ratio >655 is almost invariably associated with preeclampsia or fetal growth restriction that progresses rapidly. In our tertiary care settings, we observed that maternal adverse outcomes were high throughout gestation, whereas perinatal adverse outcomes diminished as pregnancy advanced.
Keywords: fetal growth restriction, placental dysfunction, placental growth factor, preeclampsia, sFlt1
Subject Categories: Pregnancy, Angiogenesis, Biomarkers
Nonstandard Abbreviations and Acronyms
- EFW
estimated fetal weight
- FGR
fetal growth restriction
- GA
gestational age
- HELLP
hemolysis, elevated liver enzymes and low platelet count
- NICU
neonatal intensive care unit
- PD
placental dysfunction
- PE
preeclampsia
- PlGF
placental growth factor
- REDCap
Research Electronic Data Capture tool
- sFlt‐1
soluble fms‐like tyrosine kinase‐1
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- UA
umbilical artery
Clinical Perspective
What Is New?
This cohort of pregnant women with extremely high values for the sFlt‐1/PlGF ratio (>655) is the largest in which maternal and perinatal outcomes are described.
What Are the Clinical Implications?
Median time to delivery was short after an sFlt‐1/PlGF ratio >655 was demonstrated, varying from 4 to 7 days depending on the initial presence or not of preeclampsia.
Maternal serious morbidity was high (>30%) at any gestational age, whereas perinatal severe morbidity and mortality surpassed 50% before 29 to 30 weeks and significantly diminished to about 15% between 30 and 34 weeks and was null thereafter in our cohort.
introduction
A high ratio of sFlt‐1 (soluble fms‐like tyrosine kinase‐1) to PlGF (placental growth factor) in maternal serum during the second half of pregnancy is related to placental dysfunction (PD),1 including preeclampsia,2 fetal growth restriction (FGR),3 and abruptio placentae.4 The higher the value of the sFlt‐1/PlGF ratio, the greater the underlying placental disease and the shorter time‐to‐delivery interval.2
The sFlt‐1/PlGF ratio threshold of >655 has been described as the third quartile of the sFlt‐1/PlGF values in singleton pregnancies diagnosed with early onset preeclampsia and has been associated with a 2.7 increased risk of expeditious delivery (<48 hours) in those with <655.5 These high values are also found in ≈1% to 2% of women tested for suspicion of preeclampsia.6 Recently, Stolz et al7 compared the perinatal outcomes in 30 preeclampsia cases with sFlt‐1/PlGF >655 and 30 preeclampsia controls with <655 and matched by gestational age (GA). This small study could not find significant differences in the perinatal outcomes between groups; however, maternal complications were not assessed, and cases without preeclampsia were not included. There is little prognostic information about the significance of exceeding the threshold of 655 in pregnancies complicated with preeclampsia in a preterm phase. Even less is known about pregnancies without preeclampsia. When PD debuts at early GAs, expectant management is pursued, but it is often difficult to predict the course of the disease, and both patients and physicians are usually concerned about the threats of prolonging the pregnancy.8 Therefore, it may be of interest to recognize the implications for the mother and the fetus of facing extremely high sFlt‐1/PlGF ratios at different stages of pregnancy to better balance the benefits and risks of an expectant attitude.
Our aim is to analyze maternal and perinatal outcomes in pregnancies with or without preeclampsia in which an sFlt‐1/PlGF ratio has been requested during the second half of pregnancy in the context of a clinical suspicion of PD and a result of >655 has been obtained.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design and Population
This multicenter observational retrospective cohort study was conducted in 9 hospitals in Spain that have incorporated use of the sFlt‐1/PlGF ratio into clinical practice as an aid to rule out suspected PD and to support diagnosis and follow‐up. All consecutive singleton pregnancies with live and structurally healthy fetuses at these centers from February 2014 to May 2019 with at least 1 sFlt‐1/PlGF ratio determination >655 between 20+0 and 37+0 weeks were included. We excluded cases with incomplete perinatal outcome. The study was approved by every hospital's local ethics committee without requiring informed consent, given that the use of angiogenic biomarkers to “aid in diagnosis” is standard practice in Spain,9 in addition to the study's retrospective character and use of encoded and anonymized data.
Data Collection, Follow‐Up, and Outcome Measures
Maternal characteristics including age, height, weight, smoking status, race, method of conception, low‐dose aspirin intake, heparin prophylaxis, and risk factors for preeclampsia and other PD‐related disorders, according to the National Institute for Health and Care Excellence (NICE) guidelines,10 were collected from the medical records. GA was estimated according to the American College of Obstetricians and Gynecologists: reliable last menstrual period was corrected by the crown–rump length before 14+0 weeks or biparietal diameter from 14+0 to 21+6 weeks, when a significant discrepancy of >7 or >10 days was found, respectively.11
Reasons for assessing the sFlt‐1/PlGF ratio were (1) clinical suspicion of PD, including the presence of signs and symptoms suggestive of preeclampsia,5 abnormal uterine artery Doppler at 24+0 to 28+6 weeks,12 and early onset FGR (diagnosed <32+0 weeks),13 and (2) serial follow‐up in cases of diagnosed preeclampsia and FGR.14 The first sample in which an sFlt‐1/PlGF ratio >655 was demonstrated was the one selected for analysis. The sFlt‐1 and PlGF concentrations (in pg/mL) were determined using an automated assay system (Cobas 6000 e701 module; Roche Diagnostics). The sFlt‐1/PlGF ratio was expressed in absolute values. The obstetricians involved in the management of these cases were aware of the results of the sFlt‐1/PlGF ratio. They knew that the sFlt‐1/PlGF ratio cutoff of ≤38 helps to rule out preeclampsia5 and that ≥85 aids in diagnosis of preeclampsia,15 and >655 is associated with a high risk of the need to deliver within 48 hours.6 This information was used for tailored management including risk stratification, planning follow‐up, and allocation of patients. However, the indications for delivery were intended to be guided by current protocols, regardless of the results of the sFlt‐1/PlGF ratio. To evaluate the potential influence of the knowledge of the sFlt‐1/PlGF ratio in clinical management, an independent expert at each center was designated to assess the appropriateness of clinical decisions. Those included hospitalization, fetal maturation, indication of iatrogenic delivery, and overall adequacy of clinical management.
Preeclampsia assessment
In cases of suspected PD, the presence of preeclampsia was evaluated at inclusion and at every visit afterward by measuring blood pressure and determining proteinuria. For the purpose of this study, a case was classified as preeclampsia when both hypertension and proteinuria were demonstrated, according to the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy.16 However, in the clinical setting, patients were managed as having preeclampsia even in the absence of significant proteinuria when other severity criteria were met and indications for delivery followed current recommendations.17 Expectant management was initially attempted for fetal interest whenever the GA was ≥24 weeks and was discussed with the parents at earlier weeks. During expectant management, maternal complications were assessed, including pulmonary edema; refractory hypertension; complete or incomplete HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome; oliguria <500 mL/24 hours; abruptio placentae (defined as placental detachment before delivery of the fetus, including the identification of a retroplacental hematoma or evidence of blood clot in at least 20% of its surface based on clinical data provided by the attending obstetrician at delivery18); neurological deficits; and eclampsia. If any complication was identified, immediate delivery was indicated regardless of GA. Delivery was also recommended after 34+0 weeks in the presence of severe features of preeclampsia and in any preeclampsia case after 37+0 weeks.
FGR assessment
Diagnosis, fetal surveillance, timing, and route of delivery followed a stage‐based protocol.19 Fetal weight was estimated using the Hadlock formula,20 and centiles were calculated according to the references established in each center; for the purpose of presenting the results in this study, estimated fetal weight (EFW) centiles were homogenized by customization using the GROW software.21 Whenever anterograde flow in the umbilical artery was present, weekly monitoring (fetal Doppler including interrogation of the ductus venosus plus conventional cardiotocography) was planned, and vaginal delivery (in the absence of other contraindications) was recommended after 37 weeks. If absent end‐diastolic umbilical artery flow was detected, subsequent follow‐up controls were performed every 48 to 72 hours, and elective cesarean section was indicated at 34 weeks. When reverse end‐diastolic umbilical artery flow or ductus venosus pulsatility index >95th centile was found, hospitalization and daily monitoring were carried out until elective cesarean section at 30 weeks. Whenever a reverse a‐wave flow in the ductus venosus or spontaneous decelerations in the cardiotocography were noted, elective cesarean section was indicated provided that the GA was ≥26+0 weeks and estimated fetal weight ≥500 g (if any of these thresholds were not reached, the decision to deliver was agreed with the parents after detailed neonatal counseling).
Corticoids for fetal maturity (betamethasone 12 mg/day for 2 days) were administered before 34+6 weeks if fetal viability was reached and short‐term delivery was expected, including the presence of severe features of preeclampsia and the loss of anterograde end‐diastolic umbilical artery flow and after the demonstration of sFlt‐1/PlGF ratio >655 (if not indicated previously). A repeated cycle of corticoids was considered after 1 week of the administration of the first cycle if the GA remained below 34+6 weeks. Magnesium sulfate for fetal neuroprotection was indicated when there was a risk of imminent delivery at 31+6 weeks or less and was not being administered for maternal indication.
Perinatal data including date and mode of delivery, birth weight, Apgar test at 5 minutes, arterial cord pH, neonatal intensive care unit admission, severe morbidity at discharge home (bronchopulmonary dysplasia, hypoxic‐ischemic encephalopathy, intraventricular hemorrhage grade ≥3, cystic periventricular leukomalacia, necrotizing enterocolitis, sepsis, and retinopathy of prematurity), and mortality were recorded. Postnatal follow‐up for at least 6 months was available for all survivors.
Outcome data were recorded by all centers in a common database created on the Research Electronic Data Capture (REDCap) tool22 hosted at the “imas12” research institute.
Statistical Analysis
Sample size was estimated for a 5% significance level and 80% power, basing our calculations in a prior study that determined cases with suspected preeclampsia before 34 weeks and sFlt‐1/PlGF higher than the third tertile had a 78% chance of developing an adverse outcome.23 We calculated that 104 cases (52 with preeclampsia and 52 without preeclampsia at inclusion) were required to identify a difference >25% in severe maternal and perinatal outcomes.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was followed for reporting the results. Continuous variables were expressed as mean (SD) or median (interquartile range) when nonnormally distributed. Categorical variables were expressed as percentage. Univariate comparisons between cases with and without preeclampsia at diagnosis were performed using the Student t test or the Mann–Whitney U test for continuous variables and the χ2 or Fisher exact test for categorical variables. Kaplan–Meier survival curves were generated for the analysis of the time to delivery after the demonstration of sFlt‐1/PlGF >655. A 2‐sided P<0.05 was considered statistically significant. Data were carefully entered and analyzed after data cleansing, using the STATA statistical package, version 14.1 (StataCorp).
Results
An sFlt‐1/PlGF ratio >655 was identified in 242 singleton pregnancies during the study period, among which 5 neonates were lost to follow‐up and were excluded from further analysis, leaving a study population of 237 cases. At the time of the first sFlt‐1/PlGF ratio >655, 185 women had preeclampsia (78.1%). Of them, 49.2% had severe features of preeclampsia, 77.3% had FGR, and 13% had FGR in stage III or IV. At delivery, these values were 77.3%, 84.9%, and 28.6%, respectively. The remaining 52 cases without preeclampsia at inclusion had mainly FGR (82.7%), 21.2% in stage III or IV. At delivery, these values were 90.4% and 50%, respectively. Moreover, 23 of these women developed preeclampsia (44.2%), 16 with severe features (30.8%; Figure 1).
Figure 1. Progression of fetal growth restriction (FGR) from inclusion with sFlt‐1 (soluble fms‐like tyrosine kinase 1)/PlGF (placental growth factor) ratio >655 to delivery in cases with preeclampsia (A) and without preeclampsia (B) at inclusion.
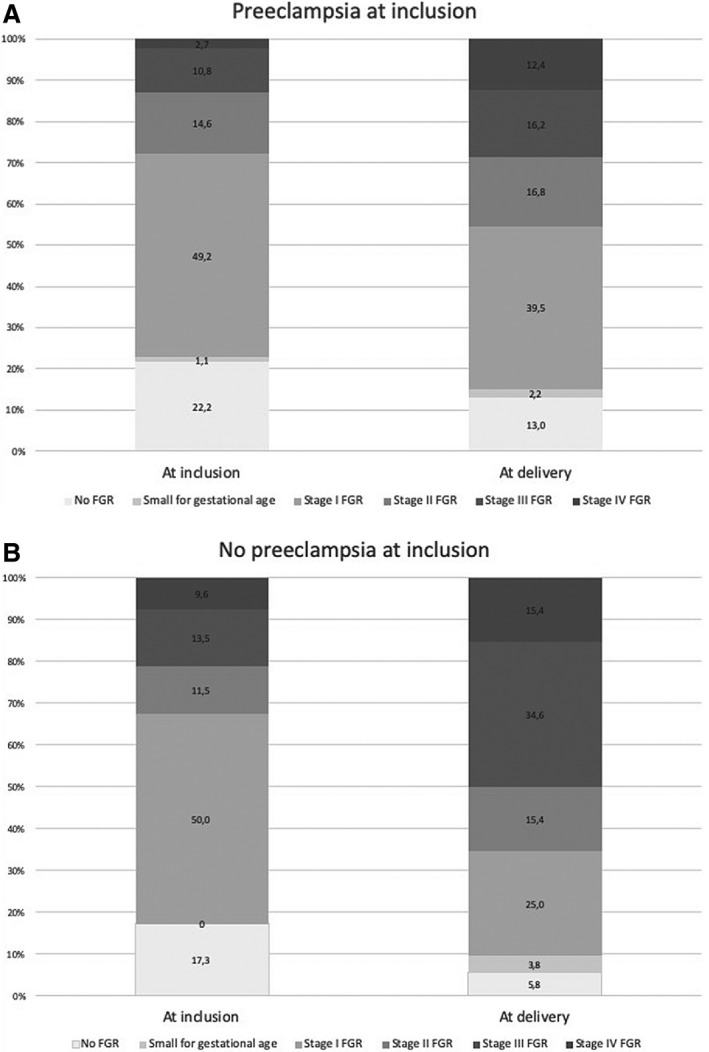
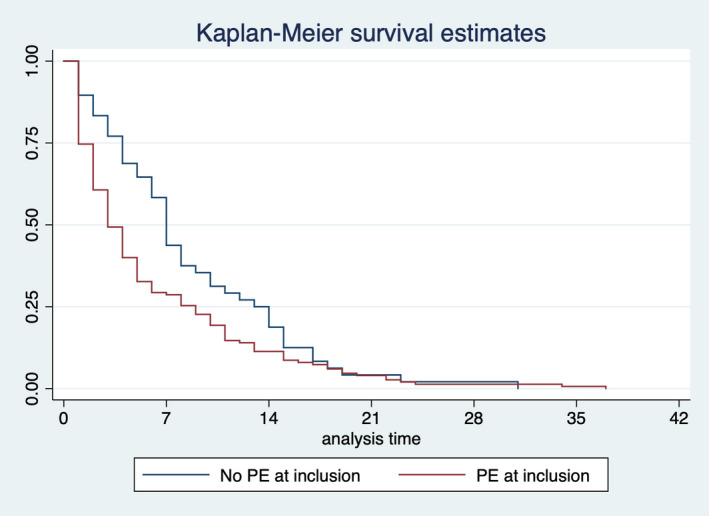
There were no differences in the baseline characteristics between women with and without preeclampsia at inclusion, as shown in Table 1, except for a higher proportion of prior preeclampsia in the preeclampsia group. Main perinatal characteristics are depicted in Table 2. Most cases (82.7%) classified as without preeclampsia at inclusion were normotensive at the sFlt‐1/PlGF determination. Women with preeclampsia at the time of obtaining an sFlt‐1/PlGF ratio >655 had higher GA of 29.4 versus 27.7 weeks (P<0.01) but lower median latency time until delivery of 4 versus 7 days (P<0.01) than those without preeclampsia at inclusion. At 48 hours, 60.5% and 82.7% women remained pregnant, respectively (Figure 2). This still resulted in a higher GA at delivery (30.1 versus 28.9 weeks) and higher birth weight (1079 versus 828 g) in the cases that already had preeclampsia. Reasons for delivery varied between groups: in the group with preeclampsia at inclusion (versus no preeclampsia), a higher proportion of deliveries due to maternal complications (50.8% versus 21.2%, P<0.01) and a lower proportion due to fetal deterioration (41.6% versus 59.6%, P<0.01) were found. We made a further analysis of the time until delivery in the group of women without preeclampsia at inclusion, dividing them in 2 subgroups depending on the final presence or absence of preeclampsia, and we did not observe any difference in this latency period between them (7 days [interquartile range: 4–14] and 7 days [interquartile range: 3–13], respectively), as shown in Figure S1.
Table 1.
Baseline Characteristics of the Study Population Stratified by the Presence of Preeclampsia at the Time of Demonstration of sFlt‐1/PlGF >655
| Characteristics | Total (N=237) | Preeclampsia at Inclusion | ||
|---|---|---|---|---|
| No (n=52) | Yes (n=185) | P Value | ||
| Maternal age, y | 33.7±5.8 | 32.0±6.1 | 31.6±5.8 | 0.49 |
| Height, cm | 161.1±9.1 | 162.2±7.3 | 159.7±8.5 | 0.09 |
| Prepregnancy weight, kg | 69.1±15.2 | 71.0±17.0 | 68.9±14.8 | 0.59 |
| Prepregnancy BMI, kg/m2 | 26.7±6.4 | 26.5±6.0 | 26.6±6.0 | 0.96 |
| Smoking status | ||||
| Current | 7 (3.0) | 1 (1.9) | 6 (3.3) | 0.16 |
| Cigarettes/d, median (range) | 9 (3–20) | 8 (3–24) | 10 (4–20) | |
| Race or ethnic groupb | ||||
| White | 169 (71.3) | 42 (80.8) | 127 (68.7) | |
| Hispanic | 35 (14.8) | 7 (13.5) | 28 (15.1) | |
| Asian | 5 (2.1) | 0 (0) | 5 (2.7) | 0.60 |
| Black | 11 (4.6) | 1 (1.9) | 10 (5.4) | |
| Arab | 14 (5.9) | 2 (3.8) | 12 (6.5) | |
| Other | 3 (0.4) | 0 (0) | 3 (1.6) | |
| Risk factors for PD | ||||
| High | ||||
| Previous preeclampsia | 25 (10.5) | 0 (0) | 25 (13.5) | 0.01 |
| Chronic hypertension | 30 (12.7) | 4 (7.7) | 26 (14.1) | 0.22 |
| Prepregnancy diabetes mellitus | 1 (0.4) | 0 (0) | 1 (0.54) | 0.60 |
| Chronic kidney disease | 4 (1.7) | 0 (0) | 4 (2.2) | 0.49 |
| Thrombophilia | 5 (2.1) | 1 (1.9) | 4 (2.2) | 0.92 |
| Systemic lupus erythematosus | 3 (1.3) | 0 (0) | 3 (1.6) | 0.11 |
| Moderate | ||||
| Nulliparity | 159 (67.1) | 34 (65.4) | 125 (67.6) | 0.77 |
| Age ≥40 y | 13 (16.7) | 3 (5.8) | 10 (5.4) | 0.92 |
| Prepregnancy BMI ≥35 | 23 (10.0) | 5 (9.6) | 18 (9.7) | 0.98 |
| Family history of preeclampsiaa | 7 (3.0) | 0 (0) | 7 (3.8) | 0.35 |
| ≥1 High‐risk or 2 moderate‐risk factors | 74 (31.2) | 11 (21.2) | 64 (34.6) | 0.07 |
| Mode of conception | ||||
| Spontaneous | 215 (90.7) | 46 (88.5) | 165 (89.2) | |
| In vitro fertilization | 16 (6.8) | 6 (11.5) | 10 (5.4) | 0.06 |
| Oocyte donation | 6 (2.5) | 0 (0) | 6 (3.2) | |
| Low‐dose aspirin intake (100 mg/d) | ||||
| No | 204 (86.4) | 45 (86.5) | 158 (85.4) | 0.68 |
| Starting at or before 16 wk | 29 (12.3) | 7 (13.5) | 22 (11.9) | |
| Starting after 16 wk | 3 (1.3) | 0 (0) | 3 (1.6) | |
| Low‐dose heparin prophylaxis | ||||
| No | 210 (88.6) | 47 (90.4) | 163 (88.1) | |
| Starting at or before 16 wk | 6 (2.5) | 1 (1.9) | 5 (2.7) | 0.89 |
| Starting after 16 wk | 21 (8.9) | 4 (7.7) | 17 (9.2) | |
Data are mean±SD or n (%), unless otherwise stated. BMI indicates body mass index; PD, placental dysfunction; PlGF, placental growth factor; and sFlt‐1, soluble fms‐like tyrosine kinase 1.
First‐degree relative (mother or sister) with a history of preeclampsia.
Evaluated after Bonferroni adjustment.
Table 2.
Main Perinatal Outcomes of the Study Population Stratified by the Presence of Preeclampsia at the Time of Demonstration of sFlt‐1/PlGF >655
| Perinatal Outcome | Total (N=237) | Preeclampsia at Inclusion | ||
|---|---|---|---|---|
| No (n=52) | Yes (n=185) | P Value | ||
| GA at diagnosis, median (Q1–Q3) | 29.0 (3.3) | 27.7 (25.6–30.4) | 29.4 (27.1–31.9) | <0.01 |
| GA at delivery, median (Q1–Q3) | 29.8 (3.3) | 28.9 (27.1–31.5) | 30.1 (27.8–32.5) | 0.03 |
| Time to delivery, median (IQR) | 4 (2–10) | 7 (4–13) | 4 (1–6) | <0.01 |
| sFlt‐1/PlGF, median (IQR) | 823 (718–1051) | 818 (725–1064) | 823 (712–1048) | 0.92 |
| Mean arterial pressure, mm Hg | 110±13 | 101±9 | 112±12 | <0.01 |
| Hypertension (SBP ≥140 or DBP >90 mm Hg) | 195 (78.1) | 10 (19.5) | 185 (100) | <0.01 |
| Reason for delivery | ||||
| Spontaneous onset | 1 (0.4) | 0 (0) | 1 (0.5) | |
| PPROM | 2 (0.8) | 1 (1.9) | 1 (0.5) | |
| Maternal indication due to preeclampsia | 105 (44.4) | 11 (21.2) | 94 (50.8) | <0.01 |
| Fetal indication due to FGR | 108 (45.4) | 31 (59.6) | 77 (41.6) | |
| Abruptio placentae | 15 (6.3) | 6 (11.5) | 9 (4.8) | |
| Other | 6 (2.5) | 3 (5.8) | 3 (1.6) | |
| Intrauterine demise | 21 (8.9) | 10 (19.2) | 11 (6.9) | <0.01 |
| Neonatal mortality | 15 (6.3) | 5 (9.6) | 10 (5.4) | 0.28 |
| Overall perinatal mortality | 36 (15.2) | 15 (28.8) | 21 (11.4) | <0.01 |
| Neonatal weight | 1024±445 | 828±438 | 1079±432 | <0.01 |
| NICU admission | 193 (81.4) | 39 (75.0) | 154 (83.2) | 0.27 |
| Days at NICU | 37.6±29.2 | 47.8±32.8 | 36.1±28.2 | 0.21 |
| Neonatal severe morbiditya | ||||
| Any | 74 (34.3) | 18 (42.9) | 56 (32.2) | 0.19 |
| Sepsis | 47 (21.8) | 13 (31.0) | 34 (19.5) | 0.11 |
| Bronchopulmonary dysplasia | 32 (14.8) | 7 (16.7) | 25 (14.4) | 0.61 |
| Necrotizing enterocolitis | 11 (5.1) | 3 (7.1) | 8 (4.6) | 0.50 |
| Periventricular leukomalacia | 5 (2.3) | 1 (2.4) | 4 (2.3) | 0.97 |
| Retinopathy (stage III–V) | 12 (5.6) | 4 (9.5) | 8 (4.6) | 0.21 |
| IVH grade III or IV | 6 (2.8) | 1 (2.4) | 5 (2.9) | 0.86 |
| Maternal severe morbidity | ||||
| Any | 99 (41.8) | 19 (36.5) | 80 (43.2) | 0.39 |
| Severe hypertension | 39 (16.5) | 3 (5.8) | 36 (19.5) | 0.02 |
| HELLP syndrome | 28 (11.8) | 3 (5.8) | 25 (13.5) | 0.13 |
| Abruptio placentae | 27 (11.4) | 11 (21.2) | 16 (8.7) | 0.01 |
| Oliguria (<500 mL/24 h) | 15 (6.3) | 0 (0) | 15 (8.1) | 0.03 |
| Pulmonary edema | 7 (3.0) | 1 (1.9) | 6 (3.2) | 0.62 |
| IDC | 2 (0.8) | 0 (0) | 2 (1.1) | 0.45 |
| Eclampsia | 3 (1.3) | 0 (0) | 3 (1.6) | 0.36 |
| Myocardial infarction | 1 (0.4) | 1 (1.9) | 0 (0) | 0.06 |
Data are mean±SD or n (%), unless otherwise stated. DBP indicates diastolic blood pressure; FGR, fetal growth restriction; GA, gestational age; HELLP, hemolysis, elevated liver enzymes, and low platelet count; IDC, intravascular disseminated coagulopathy; IQR, interquartile range; IVH, intraventricular hemorrhage; NICU, neonatal intensive care unit; PlGF, placental growth factor; PPROM, preterm premature rupture of membranes; Q, quartile; SBP, systolic blood pressure; and sFlt‐1, soluble fms‐like tyrosine kinase 1.
Calculated among neonatal survivors.
Figure 2. Kaplan–Meier survival estimates of the time to delivery from inclusion with sFlt‐1 (soluble fms‐like tyrosine kinase 1)/PlGF (placental growth factor) ratio >655, stratified by the presence of preeclampsia (PE).
Severe maternal morbidity was observed in 99 cases (44.1%). As depicted in Table 3, a high percentage of maternal complications remained present even after 34 weeks. Women with preeclampsia at inclusion frequently developed severe hypertension (19.5% versus 5.8%, P=0.02) and oliguria (8.1% versus 0%, P=0.03), whereas those without initial preeclampsia more often had abruptio placentae (21.2% versus 8.7%, P=0.01).
Table 3.
Maternal and Perinatal Complications Stratified by GA at Inclusion With sFlt‐1/PlGF >655
| GA et Inclusion, wks | No. of Cases | Maternal Morbidity | Neonatal Severe Adverse Outcome | |||
|---|---|---|---|---|---|---|
| Any | Intrauterine Demise | Neonatal Demisea | Severe Morbidityb | |||
| ≤24 | 29 | 13 (44.8) | 26 (89.7) | 12 (41.3) | 6 (26.1) | 8 (72.7) |
| 25–26 | 38 | 23 (60.5) | 31 (81.6) | 7 (18.4) | 7 (22.5) | 17 (70.8) |
| 27–28 | 51 | 20 (39.2) | 33 (64.7) | 1 (2.0) | 2 (0.4) | 30 (62.5) |
| 29–30 | 50 | 24 (48.0) | 10 (20.0) | 0 (0) | 0 (0) | 10 (38.5) |
| 31–32 | 42 | 14 (33.3) | 7 (16.6) | 1 (2.4) | 0 (0) | 6 (14.6) |
| 33–34 | 17 | 6 (35.3) | 3 (17.6) | 0 (0) | 0 (0) | 3 (17.6) |
| 35–37 | 10 | 3 (30.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
GA, gestational age; PlGF, placental growth factor; and sFlt‐1, soluble fms‐like tyrosine kinase 1.Among liveborn children.
Among perinatal survivors.
There were 21 cases (8.9%) of intrauterine demise, more common in women without preeclampsia at inclusion and most related to previable severe FGR. Only 6 of 21 cases (28.6%) reached criteria for active management. There were also 15 of 216 cases (6.9%) of neonatal mortality among live births, and 74 of 216 cases (34.3%) of overall severe neonatal morbidity. There were no differences regarding neonatal mortality or morbidity when stratifying by the presence of preeclampsia at inclusion. As shown in Table 3, neonatal complications were most frequent when a sFlt‐1/PlGF value >655 was obtained before 28 weeks. From 29 weeks onward, there were no cases of neonatal death, and severe morbidity decreased to 19.6% and became absent at 34 weeks. Maternal morbidity remained high even after 34 weeks. In our series, we found 1 case of both refractory severe hypertension and eclampsia and 2 cases of refractory severe hypertension and pulmonary edema.
External analysis of the clinical management showed that most decisions (86.5%) were made according to current protocols. However, fetal maturation was anticipated in 14 cases (5.9%), hospital admission in 14 (5.9%), and iatrogenic delivery in 23 (9.7%) because of the knowledge of the sFlt‐1/PlGF ratio. When overall management was evaluated, the independent obstetrician expert deemed modified actions to be correct in 8.4% and incorrect in 3% (Table S1).
Discussion
Main Findings
Extremely high values of the sFlt‐1/PlGF ratio (>655) are invariably associated with PD, usually of early onset and quickly worsening. The median time to delivery is 4 or 7 days, depending on the presence or absence, respectively, of preeclampsia when the result is obtained. The risk for maternal serious morbidity is high at any GA (>30%), as is the risk for perinatal severe morbidity and mortality before 29 to 30 weeks (>50%). However, the latter diminishes thereafter without finding any severe morbidity after 34 weeks. These outcomes do not differ significantly between cases with and without preeclampsia at the time of the first sFlt‐1/PlGF ratio >655.
Interpretation of Results
The main clinical application of the sFlt‐1/PlGF ratio is ruling out preterm preeclampsia.5 However, less evidence suggests how to interpret the detection of pathologic values and what they entail for clinical management. It is known that abnormal values of sFlt‐1/PlGF are good indicators of PD, increasing not only in preeclampsia but also in FGR and abruptio placentae. Furthermore, the magnitude of the elevation correlates inversely with the time‐to‐delivery interval. The original study that evaluated the cutoff of 655 in 24 cases of early onset preeclampsia in which the physicians were blinded to this result showed that after 48 hours, only 29.4% women remained pregnant.6 Our larger series, in which the physicians were aware of the results, demonstrates that this percentage is higher than expected (60.5% remained pregnant after 48 hours). Our study also shows that preeclampsia may be absent in about 1 in 5 cases of sFlt‐1/PlGF >655, and in these cases, latency until delivery is longer (50% remained pregnant after 1 week). In any case, the interval to delivery is crucially shortened when extremely high values are obtained, usually occurring at very early GAs. Fetal deterioration was quick in both groups, and 36.5% of cases that did not initially present with FGR developed it before delivery. Among those with FGR at inclusion, 38.3% progressed to a poorer stage and 45.4% of pregnancies required immediate delivery for fetal deterioration. We believe that these findings also support the assertion that the classical conceptions of preeclampsia and FGR as separate conditions is artificial and that, according to current knowledge, both entities should be considered as different manifestations of the same PD. We have shown that an extreme angiogenic imbalance reflects a status of severe PD, in which there is a high risk of developing a broad spectrum of maternal and fetal complications that must be assessed comprehensively, regardless of whether or not a reliable diagnosis of preeclampsia has been reached.
Given abrupt fetal worsening leading to short latency until delivery, GA at sFlt‐1/PlGF >655 shows great correlation with perinatal results. It is important that both clinicians and parents are aware that if a value >655 is detected before 24 weeks, perinatal mortality and severe perinatal morbidity can be as high as 62% and 90%, respectively. Conversely, perinatal results improve drastically when an sFlt‐1/PlGF >655 is detected from 29 weeks, with much lower rates of perinatal mortality and severe morbidity.
GA is not so crucial when considering maternal outcomes; severe maternal morbidity was found in 41.8% of cases and remained high even when the sFlt‐1/PlGF ratio >655 was found after 34 weeks. Notably, 12% of cases developed a HELLP syndrome and 11% had abruptio placentae. Therefore, these complications should be particularly monitored by increasing the follow‐up controls for their early detection before they become clinically manifest.
The highest incidence of our cases was found between 28 and 30 weeks, similar to Stolz et al. 7 At these early GAs, expectant management is usually pursued in the absence of features of imminent complications to favor fetal prognosis. In light of our results, this aim can be seriously compromised when extremely high values for the sFlt‐1/PlGF ratio are detected. Although Stolz and colleagues proposed that levels >1000 were more reliable to predict perinatal adverse outcomes than 655, they did not consider the substantial maternal adverse outcomes underlying a sFlt‐1/PlGF ratio >655 that we found in our study. Thus, our data further ratify experts’ recommendations regarding immediate referral to a tertiary care hospital for these patients, admission for close surveillance, and administration of corticosteroids for fetal maturation, which should be strongly considered when fetal viability has been reached. Moreover, from the clinical point of view, in light of our results, we believe that future research should take into account these extreme values of the sFlt‐1/PlGF ratio in the decision of delivery, especially when reaching 34 weeks, given the crossover of maternal and perinatal major complications seen beyond this time point.
Strengths and Limitations
The main potential bias in this study is that clinicians were not blinded to the sFlt‐1/PlGF ratio; therefore, management could have been influenced by its result and by the experience of the group using biomarkers. Nevertheless, the inclusion of different hospitals may better reflect the influence of this tool in the real world compared with the experience of a single center. To approximate the magnitude of the bias due to knowledge of the results, we carried out an external evaluation of each case, observing that certainly only in a minority of the cases the clinical decisions were influenced by the use of biomarkers. This accounted for an additional 6% of hospitalized cases, 5.9% of administering corticosteroids for fetal maturation, and 9% of anticipating delivery, compared to what it was expected if only conventional criteria had been followed. This result is in accordance with another study24 that evaluated the impact of the awareness of angiogenic biomarkers in suspected preeclampsia regarding additional hospitalizations (5.9%) and fetal maturation (7.7%). The anticipated delivery was lower (1.7%) than ours but was conducted in the setting of suspected preeclampsia, where the values of the sFlt‐1/PlGF ratio were far from 655 in most cases. We have not compared our cases with others that did not reach such high values for the sFlt‐1/PlGF ratio because that would have implied a different study design. In addition, this retrospective study is subject to information, selection, and confusion biases. Caution should be taken not to generalize our findings to more diverse populations; our sample was mainly composed of white and Hispanic women. Similarly, we acknowledge that the results reflect the performance of highly specialized hospitals and thus may not be extrapolated to other types of centers. Furthermore, this study was based only in the first determination >655 and did not consider serial measurements to evaluate the progression of angiogenic biomarkers; however, the short time to delivery in most of these cases precludes such longitudinal follow‐up. Even considering these limitations, we must remark that this cohort is the largest to date describing the course of patients with these extremely high values for the sFlt‐1/PlGF ratio. Even though our results lack the evidence to support changes in current clinical practice, they could be of interest in inspiring the design of further clinical trials to test whether revealing biomarker levels would affect clinical outcome and support decision‐making based on the use of biomarkers, such as the recently published stepped‐wedge trial.25
In conclusion a sFlt‐1/PlGF value >655 must alert clinicians who should closely monitor both mothers and fetuses and consider fetal maturation and maternal hospitalization given the short time to delivery due to the high risk of imminent severe adverse outcomes, including HELLP syndrome, abruptio placentae, and rapid progression of the FGR sequence of deterioration.
Sources of Funding
None.
Disclosures
Herraiz, Galindo, Mendoza, and Valle have received lecture fees and consultancy payments from Roche Diagnostics. The remaining authors have no disclosures to report.
Supporting information
Table S1 Figure S1
(J Am Heart Assoc. 2020;9:e015548 DOI: 10.1161/JAHA.119.015548.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Friedman AM, Cleary KL. Prediction and prevention of ischemic placental disease. Semin Perinatol. 2014;38:177–182. [DOI] [PubMed] [Google Scholar]
- 2. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 3. Herraiz I, Dröge LA, Gómez‐Montes E, Henrich W, Galindo A, Verlohren S. Characterization of the soluble fms‐like tyrosine kinase‐1 to placental growth factor ratio in pregnancies complicated by fetal growth restriction. Obstet Gynecol. 2014;124:265–273. [DOI] [PubMed] [Google Scholar]
- 4. Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol. 2006;108:338–344. [DOI] [PubMed] [Google Scholar]
- 5. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, Calda P, Holzgreve W, Galindo A, Engels T, et al. The sFlt‐1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1–58.e8. [DOI] [PubMed] [Google Scholar]
- 6. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, et al. Predictive value of the sFlt‐1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. [DOI] [PubMed] [Google Scholar]
- 7. Stolz M, Zeisler H, Heinzl F, Binder J, Farr A. An sFlt‐1:PlGF ratio of 655 is not a reliable cut‐off value for predicting perinatal outcomes in women with preeclampsia. Pregnancy Hypertens. 2018;11:54–60. [DOI] [PubMed] [Google Scholar]
- 8. Haddad B, Sibai BM. Expectant management in pregnancies with severe preeclampsia. Semin Perinatol. 2009;33:143–151. [DOI] [PubMed] [Google Scholar]
- 9. Lapaire O, Shennan A, Stepan H. The preeclampsia biomarkers soluble fms‐like tyrosine kinase‐1 and placental growth factor: current knowledge, clinical implications and future application. Eur J Obstet Gynecol Reprod Biol. 2010;151:122–129. [DOI] [PubMed] [Google Scholar]
- 10. The National Institute for Health and Care Excellence (NICE) . Hypertension in pregnancy: diagnosis and management. NICE Guidelines (NG133). 2019. www.nice.org.uk. Available at: https://www.nice.org.uk/guidance/ng133. Accessed March 15, 2020. [PubMed]
- 11. Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal‐Fetal Medicine . Committee opinion No 700: methods for estimating the due date. Obstet Gynecol. 2017;129:e150–e154. [DOI] [PubMed] [Google Scholar]
- 12. Herraiz I, Simón E, Gómez‐Arriaga PI, Quezada MS, García‐Burguillo A, López‐Jiménez EA, Galindo A. Clinical implementation of the sFlt‐1/PlGF ratio to identify preeclampsia and fetal growth restriction: a prospective cohort study. Pregnancy Hypertens. 2018;13:279–285. [DOI] [PubMed] [Google Scholar]
- 13. Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, Silver RM, Wynia K, Ganzevoort W. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48:333–339. [DOI] [PubMed] [Google Scholar]
- 14. Herraiz I, Quezada MS, Rodriguez‐Calvo J, Gómez‐Montes E, Villalaín C, Galindo A. Longitudinal change of sFlt‐1/PlGF ratio in singleton pregnancy with early‐onset fetal growth restriction. Ultrasound Obstet Gynecol. 2018;52:631–638. [DOI] [PubMed] [Google Scholar]
- 15. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, Sabria J, Markfeld‐Erol F, Galindo A, Schoofs K, et al. New gestational phase‐specific cutoff values for the use of the soluble fms‐like tyrosine kinase‐1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352. [DOI] [PubMed] [Google Scholar]
- 16. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 17. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 18. Ananth CV, Berkowitz GS, Savitz DA, Lapinski RH. Placental abruption and adverse perinatal outcomes. JAMA. 1999;282:1646–1651. [DOI] [PubMed] [Google Scholar]
- 19. Figueras F, Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage‐based management protocol. Fetal Diagn Ther. 2014;36:86–98. [DOI] [PubMed] [Google Scholar]
- 20. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements a prospective study. Am J Obstet Gynecol. 1985;151:333–337. [DOI] [PubMed] [Google Scholar]
- 21. Gardosi J, Francis A. Customized weight centile calculator. GROW version 8.0.1, 2018. Gestation Network, www.gestation.net. Available at: https://www.gestation.net/cc/6/884259.htm. Accessed March 15, 2020.
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klein E, Schlembach D, Ramoni A, Langer E, Bahlmann F, Grill S, Grill S, Schaffenrath H, van der Does R, Messinger D, et al. Influence of the sFlt‐1/PlGF ratio on clinical decision‐making in women with suspected preeclampsia. PLoS One. 2016;11:e0156013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, Shennan AH, Chappell LC; PARROT trial group . Placental growth factor testing to assess women with suspected pre‐eclampsia: a multicentre, pragmatic, stepped‐wedge cluster‐randomised controlled trial. Lancet. 2019;393:1807–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Figure S1


