Abstract
Background
Brain repair mechanisms fail to promote recovery after stroke, and approaches to induce brain regeneration are scarce. Mesenchymal stem cells (MSC) are thought to be a promising therapeutic option. However, their efficacy is not fully elucidated, and the mechanism underlying their effect is not known.
Methods and Results
The middle cerebral artery occlusion model was utilized to determine the efficacy of interferon‐γ–activated mesenchymal stem cells (aMSCγ) as an acute therapy for stroke. Here we show that treatment with aMSCγ is a more potent therapy for stroke than naive MSC. aMSCγ treatment results in significant functional recovery assessed by the modified neurological severity score and open‐field analysis compared with vehicle‐treated animals. aMSCγ‐treated animals showed significant reductions in infarct size and inhibition of microglial activation. The aMSCγ treatment suppressed the hypoxia‐induced microglial proinflammatory phenotype more effectively than treatment with naive MSC. Importantly, treatment with aMSCγ induced recruitment and differentiation of oligodendrocyte progenitor cells to myelin‐producing oligodendrocytes in vivo. To elucidate the mechanism underlying high efficacy of aMSCγ therapy, we examined the secretome of aMSCγ and compared it to that of naive MSC. Intriguingly, we found that aMSCγ but not nMSC upregulated neuron‐glia antigen 2, an important extracellular signal and a hallmark protein of oligodendrocyte progenitor cells.
Conclusions
These results suggest that activation of MSC with interferon‐γ induces a potent proregenerative, promyelinating, and anti‐inflammatory phenotype of these cells, which increases the potency of aMSCγ as an effective therapy for ischemic stroke.
Keywords: inflammation, ischemia, mesenchymal stem cells, oligodendrogenesis, stroke, therapy
Subject Categories: Ischemic Stroke, Neurogenesis, Neuroprotectants, Cognitive Impairment
Clinical Perspective
What Is New?
Activation of mesenchymal stem cells with interferon‐γ promotes proregenerative characteristics and facilitates the anti‐inflammatory characteristics of naive mesenchymal stem cells, making them more potent therapy following ischemic stroke.
What Are the Clinical Implications?
There is an unmet need to develop proregenerative therapy for the treatment of ischemic stroke.
Most clinical studies using mesenchymal stem cells use them in their naive form, which has limited anti‐inflammatory effect and little proregenerative impact.
Interferon‐γ–activated mesenchymal stem cells injected at the acute phase of the insult exhibit both anti‐inflammatory and proregenerative effects leading to functional recovery.
Nonstandard Abbreviations and Acronyms.
7‐AAD 7‐aminoactinomycin D
aMSCγ interferon‐γ–activated mesenchymal stem cells
BDNF brain‐derived neurotrophic factor
BMP1 bone morphogenetic protein 1
CNPase 2′,3′‐cyclic‐nucleotide 3′‐phosphodiesterase
CSPG4 chondroitin sulfate proteoglycan 4
DCX doublecortin
DIC differential interference contrast
GDNF glial cell‐derived neurotrophic factor
GFAP glial fibrillary acidic protein
IFN‐γ
interferon‐γ
IGF‐1
insulin‐like growth factor 1
IL‐4
interleukin 4
IL‐6
interleukin 6
LDH lactate dehydrogenase
MBP myelin basic protein
MCAO middle cerebral artery occlusion
MG microglia
MRI magnetic resonance imaging
MSC mesenchymal stem cells
MTT 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
N.D. not detected
nMSC naive mesenchymal stem cells
NPC neural progenitor cell(s)
NSC neural stem cell(s)
OGD oxygen‐glucose deprivation
OPC oligodendrocyte progenitor cells
PDGFRα platelet‐derived growth factor receptor α
SVZ subventricular zone
TNF‐α tumor necrosis factor α
Introduction
Stroke is the fifth leading cause of death each year in the United Sates and is the number 1 cause of permanent disability in the world.1, 2 There are ≈800 000 new stroke victims in the United States each year, and almost 130 000 mortalities.3 The vast majority, around 90%, of stroke patients suffer from an ischemic stroke caused by temporary or permanent occlusion of a blood vessel to the brain.4 Current therapy options for ischemic stroke patients in the acute setting are drastically limited, with only 1 FDA‐approved drug, tissue plasminogen activator available. However, because of a number of limitations, mainly a therapeutic window of 3 to 4.5 hours, only 4% to 7% of patients are eligible for tissue plasminogen activator therapy.5 This therapeutic window can be increased to up to 24 hours with the addition of endovascular thrombectomy, but this type of therapy can only be administered in certain hospital settings and only after meeting a number of different treatment criteria.6, 7 Therefore, it is crucial for new therapeutic options to be developed for the treatment of these patients in the acute setting.
Inflammatory responses after stroke involve both peripheral immune cells and microglia. How these processes affect long‐term brain repair is still not fully understood. Many studies have demonstrated both detrimental8, 9 and beneficial10, 11, 12 effects of brain inflammation. Furthermore, these changes, and whether they are good or bad, often depend on the time frame in which they are studied, with the beneficial effects of these cells attributed to the chronic phase of stroke.13, 14, 15 There have been numerous research studies and clinical trials looking into the efficacy of anti‐inflammatory agents as stroke therapies; however, the majority of these studies reported either worse outcomes or no change from placebo,16 and all failed to make it beyond clinical trials.
Endogenous neurogenesis processes, long posited as therapeutic targets for brain repair, have largely been noncontributory in the setting of ischemic stroke. Although endogenous neural stem cells (NSC) do react acutely to ischemic stroke with increases in cell proliferation and cell migration,17, 18, 19, 20, 21, 22 only 10% to 20% of these cells survive long term.18, 23 These few surviving cells, however, do seem to mature into functional cells,18, 24, 25 with the majority of them becoming spiny neostriatal projection neurons18, 24 or calretinin‐positive interneurons.26 But despite these interesting data, it is still largely unknown why the vast majority of these new cells do not mature and survive long term and why they fail to support brain recovery.
Mesenchymal stem cells (MSC) have unique characteristics that make them an interesting tool to study brain repair after ischemic stroke. They have the ability to (1) reduce overall inflammation, thereby eliminating the potentially toxic environment that could be leading to NSC death,27, 28, 29 and (2) help support NSC survival and function via secretion of various neurotrophic factors.29, 30, 31, 32 To date, there have been many preclinical studies as well as clinical trials demonstrating the efficacy of MSC therapy in stroke preclinical studies and safety of MSC treatment in clinical trials.33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Nevertheless, the extent of recovery following MSC treatment is not clear. Importantly, the mechanism by which MSC treatment conveys a functional benefit is not known, which impedes the development of an effective therapy. Additionally, most of the clinical trials using MSC have utilized this treatment in subacute39, 48, 49, 50 and chronic51, 52, 53, 54, 55 stroke patients. Therefore, whether these cells can be an effective therapy acutely is largely unknown. Additionally, previous graft‐versus‐host studies have demonstrated that when MSC are activated by interferon‐γ (IFN‐γ) ex vivo (aMSCγ), their effect is greatly enhanced, and similar effect sizes can be seen using substantially fewer cells.56, 57 However, the efficacy of aMSCγ in brain repair is unknown.
In these studies we examined whether treatment with aMSCγ acutely after ischemic stroke could enhance functional recovery and compared its efficacy and mechanism to treatment with naive MSC (nMSC). In addition, we investigated how microglia and NSC responded to hypoxia and ischemia and how treatment with aMSCγ could regulate these cell types to promote long‐term functional recovery after stroke. Finally, we analyzed the secretome of aMSCγ and nMSC in an attempt to determine the factors underlying the extent of functional recovery that these treatments exert.
Methods
Detailed materials and methods can be found in Data S1. Data sets generated or analyzed for these studies are available from the corresponding author upon reasonable request. Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the MassIVE repository with the dataset identifier HYPERLINK "ftp://massive.ucsd.edu/MSV000082984"MSV000082984. Figure 6 and Supplemental Tables 3 and 4 were generated from these data.
Study Design
For all studies, sample sizes were predetermined using published data and our previous studies using G*Power 3.1 (Department of Psychology, Heinrich Heine Universität, Düsseldorf, Germany)58 assuming a 2‐sided α level of 0.05, power of 80%, and homogeneous variances. Before the beginning of experiments, animals were randomly assigned to groups using a random number generator. Investigators responsible for data collection and analyses were blinded to group allocation throughout the experiments. End points were determined in advance as 24 hours, 1 week, or 3 weeks after reperfusion or for humane end points as determined by our institutional animal care and use committee. No data were excluded from these studies, and no outliers are reported.
Animals
All animal experiments were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee and are reported according to the ARRIVE (Animal Research Reporting of In Vivo Experiments) guidelines. For surgical experiments female Sprague‐Dawley rats (age 14‐16 weeks, weight 225‐275 g) were purchased from Envigo (Huntingdon, Cambridgeshire, UK) and were group housed and maintained in standard housing conditions (14/10 hour light/dark cycle) with access to food and water ad libitum. Postoperatively, the animals were singly housed.
Middle Cerebral Artery Occlusion
Focal cerebral ischemia was induced using the middle cerebral artery occlusion model (MCAO) as detailed previously.59 Briefly, anesthesia was induced and maintained in isoflurane in 100% oxygen for the duration of the procedure, and body temperature was maintained at 37±0.4°C via rectal probe–monitored heating pad. Ventral midline neck dissection was performed, and the right middle cerebral artery was occluded via intraluminal filament for 90 minutes before the filament was removed, and the animal was allowed to recover. The model is described in detail in Data S1. Three hours following the onset of reperfusion after MCAO, the animals were reanesthetized with isoflurane, and MSC were administered intravenously via the retro‐orbital sinus. Cells were given at a dose of 5×106 cells/kg.
Behavioral Testing After MCAO
Animals were scored using the modified neurological severity score obtained at baseline before surgery and on postoperative days 1 to 7, 14, and 21, with higher scores reflecting worse impairment. Animals were also subjected to open‐field testing for 15 minutes on postoperative days 1 and 7 using an open‐field apparatus (Med Associates, Inc, Fairfax, VT). Center/periphery analysis and rotational analysis were done using predetermined configurations in the corresponding Activity Monitor Software package (Med Associates, Inc).
Magnetic Resonance Imaging
Image Acquisition
Forty‐eight hours after surgery, animals were anesthetized using isoflurane, and magnetic resonance imaging was performed using an Agilent 9.4T magnetic resonance system (Agilent Technologies, Santa Clara, CA) with a 600 mT/m gradient insert, a 72‐mm inside diameter active decoupling birdcage radiofrequency coil for transmission, and a 4‐channel phase‐array coil as the receiver (Rapid Biomed, Ripmar, Germany). In each animal T1‐, T2‐, and diffusion‐weighted magnetic resonance images were acquired using spin‐echo multislice sequences. For the T1 sequence, imaging settings were as follows: TR/TE=555/13 ms, field of view=40×40 mm, averages=6, matrix=256×256, slice thickness=1.0 mm, gap=0 mm. For the T2 sequence, imaging settings were as follows: TR/TE=2000/45 ms, field of view=40×40 mm, averages=4, matrix=256×256, slice thickness=1.0 mm, gap=0 mm. For the diffusion‐weighted imaging sequence, imaging settings were as follows: TR/TE=2000/45 ms, field of view=40×40 mm, averages=2, matrix=128×128, slice thickness=1.0 mm, gap=0 mm, 2 b‐values of 0 and 650 s/mm2, diffusion separation Δ=25 ms, diffusion gradient duration δ=5 ms.
Infarct Volume Calculation
Infarct volume and percentage infarction were calculated using the diffusion‐weighted magnetic resonance imaging (MRI) sequences. With correction for edema, these were calculated as follows:
where the individual slice infarct volume is InV, the individual slice ipsilateral hemisphere volume is IpV, individual slice contralateral hemisphere volume is CoV, and n is the slice number.
MSC Isolation and Culture
Female rats <4 weeks of age were euthanized via isoflurane overdose and decapitation. Femurs and tibias were dissected out, and bone marrow plugs were flushed out of the bones. The cell suspension was washed, filtered through a 70‐μm cell strainer, spun, resuspended in warm growth medium, and plated at 37°C plus 5% CO2. Medium was changed after 24 hours and every 3 days thereafter. MSC were purified via magnetic bead negative selection using anti‐CD11b and anti‐CD45 antibodies to remove contaminating macrophages, and phenotype was confirmed via flow cytometry. Detailed methodologies are described in Data S1. For in vitro activation with IFN‐γ, MSC were plated at a density of 3000 cells/cm2 and supplemented with 500 U/mL recombinant rat IFN‐γ on days 0 and 3 of plating. Cells were used on day 6 after plating. To collect MSC‐conditioned medium, MSC were plated at a density of 3000 cells/cm2 and were cultured normally or treated with IFN‐γ as above. On day 6 after plating, media were removed, cells were washed 3 times, and appropriate media were added. After 24 hours, media were collected, spun at 300g for 10 minutes to remove cell debris, and filtered through a 0.22‐μm syringe filter. Media were either used immediately or frozen at −80°C for use at a later time.
Statistical Analyses
All statistical analyses were done in GraphPad Prism (Version 7.03; GraphPad Software Inc, San Diego, CA). All data shown represent mean±SEM, and a probability of <0.05 was considered statistically significant. Data were analyzed with nonparametric testing where appropriate. Individual statistical analyses are described in the appropriate text and figure legends.
Results
MSC Improve Functional Recovery Following MCAO
We set out to determine whether nMSC or aMSCγ could be beneficial in treating ischemic stroke acutely. To do this, rats were subjected to 90 minutes of MCAO, administered vehicle (saline), nMSC, or aMSCγ intravenously 3 hours after reperfusion, assessed using open‐field testing and modified neurological severity score,60 and had MRI performed to assess lesion sizes. We found that vehicle‐treated stroke animals performed worse during open‐field testing compared with sham animals (Figure 1A and 1B) and that both nMSC (Figure 1C) and aMSCγ treatments (Figure 1D) caused complete functional recovery. At 24 hours, we show that vehicle‐treated animals had traveled less distance (Figure 1E), for fewer times (Figure 1F), and for less time (Figure 1G) than did sham animals, and both nMSC and aMSCγ treatment corrected all of these metrics. Furthermore, all animals moved at the same velocity (Figure 1H), suggesting that the impairments in the vehicle‐treated animals are not an inability to initiate movement but rather an inability to sustain ambulation. Further, although not significant, vehicle‐treated animals had a trending preference for turning in a clockwise fashion (Figure 1I; a manifestation of unilateral brain injury) compared with sham animals, with MSC treatment eliminating this preference. These changes were maintained 7 days after surgery (Figure 1J through 1N).
Figure 1. Mesenchymal stem cells (MSC) improve functional recovery and infarct volume following middle cerebral artery occlusion (MCAO).
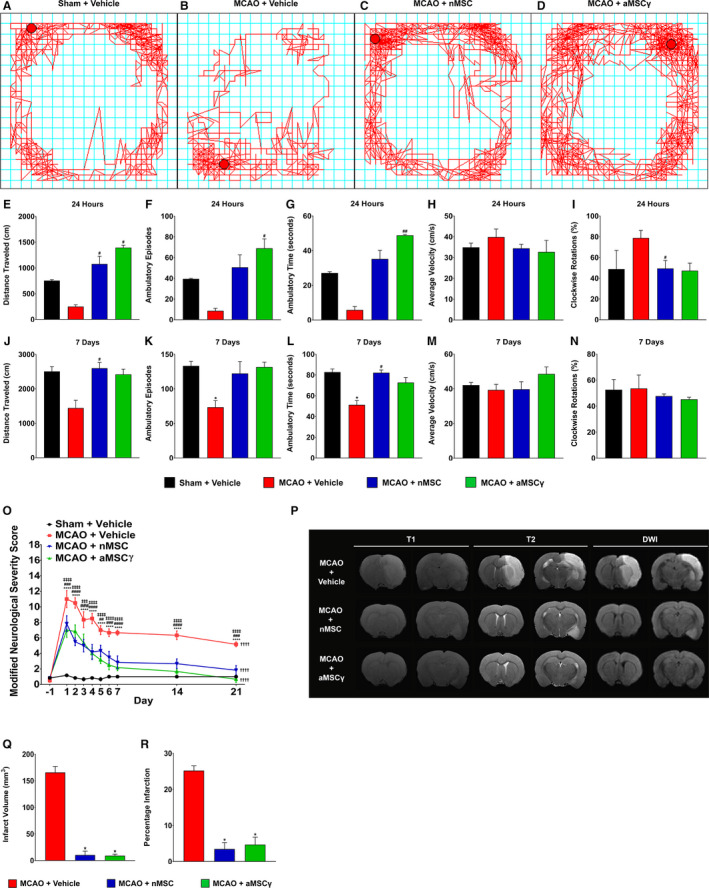
A through D, Representative open field line tracking of sham+vehicle (A), MCAO+vehicle (B), MCAO+nMSC (C), and MCAO+aMSCγ (D). E through I, Open‐field measures from sham+vehicle (black), MCAO+vehicle (red), MCAO+nMSC (blue), and MCAO+aMSCγ (green) animals of total distance traveled (E), ambulatory episodes (F), ambulatory time (G), average velocity (H), and clockwise rotations (I) 24 hours after surgery and treatment. J through N, Open‐field measures of total distance traveled (J), ambulatory episodes (K), ambulatory time (L), average velocity (M), and clockwise rotations (N) 7 days after surgery and treatment. Data are mean±SEM; n=3 animals per group. Data were compared using nonparametric Kruskal‐Wallis 1‐way ANOVA. *Compared with Sham+Vehicle. #Compared with MCAO+Vehicle. *P≤0.05, # P≤0.05, ## P<0.01. O, Modified neurological severity score of sham+vehicle (black), MCAO+vehicle (red), MCAO+nMSC (blue), and MCAO+aMSCγ (green) animals. Data were compared using repeated‐measures 2‐way ANOVA with Holm‐Sidak multiple‐comparison testing. *Sham+Vehicle vs MCAO+Vehicle; #MCAO+Vehicle vs MCAO+nMSC; ‡MCAO+Vehicle vs MCAO+aMSCγ; †Day 1 vs Day 21. **P<0.01, ***P<0.001, ****P<0.0001, ## P<0.01, ### P<0.001, #### P<0.0001, †††† P<0.0001, ‡‡‡ P<0.001, ‡‡‡‡ P<0.0001. P, Representative T1‐weighted, T2‐weighted, and diffusion‐weighted imaging (DWI) from MCAO+vehicle (top), MCAO+nMSC (middle), and MCAO+aMSCγ (bottom) animals 48 hours after surgery. Q, Infarct volume calculations from MCAO+vehicle (red), MCAO+nMSC (blue), and MCAO+aMSCγ (green) animals. R, Percentage infarction calculations from MCAO+vehicle (red), MCAO+nMSC (blue), and MCAO+aMSCγ (green) animals. Data are mean±SEM; n=3 animals per group. Data were compared using nonparametric Kruskal‐Wallis 1‐way ANOVA. *P≤0.05. aMSCγ indicates interferon‐γ–activated mesenchymal stem cells; and nMSC, naive mesenchymal stem cells.
Additionally, when assessed via modified neurological severity score, animals treated with both nMSC and aMSCγ exhibited a more rapid recovery, with animals returning to near baseline functional status by day 21 (Figure 1O). Although vehicle‐treated animals did improve significantly from day 1 to day 21, they still remained significantly more impaired compared with MSC‐treated animals, and there were no differences between nMSC‐ and aMSCγ‐treated animals. Last, MRI imaging was performed (Figure 1P and Video S1) to quantify lesion sizes in these animals. Both nMSC‐ and aMSCγ‐treated animals had significantly smaller infarct volumes (Figure 1Q) and percentage infarction (Figure 1R) compared with vehicle‐treated animals, and sham animals demonstrated no lesion on MRI (data not shown).
MSC Reduce Microglia Activation and Inflammatory Signaling
A large portion of damage done following a stroke is a result of inflammatory mediators released by immune cells, especially activated microglia. This response results in an increase in toxic inflammatory mediators including but not limited to interleukin‐1β (IL‐1β), IL‐6, and tumor necrosis factor‐α (TNF‐α).61 MSC have been demonstrated to preferentially polarize peripheral macrophages to a proregenerative phenotype as well as to reduce secreted levels of IL‐12 and TNF‐α and increase secretion of the proregenerative IL‐10.62 Furthermore, it has been shown that in a mouse model of wound healing, both nMSC and, to a greater extent, aMSCγ MSC enhance regeneration and wound healing demonstrated by increased wound tensile strength, with this effect being abrogated by depletion of macrophages.57 However, how MSC affects microglia following a stroke is not fully elucidated. Microglia are specialized cells originating from a hematopoietic lineage,63, 64 similar to macrophages, and we hypothesized that these interactions between macrophages and MSC would hold. To investigate this, brain sections from vehicle‐treated, nMSC‐treated, and aMSCγ‐treated rats were evaluated for cluster of differentiation 68 (CD68) immunoreactivity 1 week after MCAO (Figure 2A through 2C). CD68 is highly expressed in the monocyte lineage.65 We found that animals treated with both nMSC and aMSCγ had reduced immunoreactivity for CD68 (Figure 2D) compared with vehicle‐treated animals, with sham animals demonstrating no CD68 immunoreactivity (for the latter, data not shown). Furthermore, when assessed at 3 weeks after MCAO, nMSC‐ and aMSCγ‐treated animals maintained reductions in CD68 immunoreactivity (Figure 2F and 2G) compared with vehicle‐treated animals (Figure 2E). Interestingly, at the 3‐week time point, brains from vehicle‐treated animals had undergone liquefactive necrosis with demonstration of a large cavitary lesion (Figure 2E, outlined by dashed white line), which was not present in nMSC‐ and aMSCγ‐treated animals (Figure 2F and 2G). In addition to quantifying total CD68 immunoreactivity, we also analyzed microglia morphology in the peri‐infarct region from vehicle‐treated (Figure 2H,H’), nMSC‐treated (Figure 2I,I’), and aMSCγ‐treated animals (Figure 2J,J’) and found, that in vehicle‐treated animals, microglia had morphologies consistent with activated microglia with large cell bodies and few processes, whereas microglia from MSC‐treated animals exhibited morphology much more consistent with resting‐state microglia with small cell bodies and numerous branching processes.
Figure 2. Mesenchymal stem cells (MSC) reduce microglia activation and inflammatory signaling.
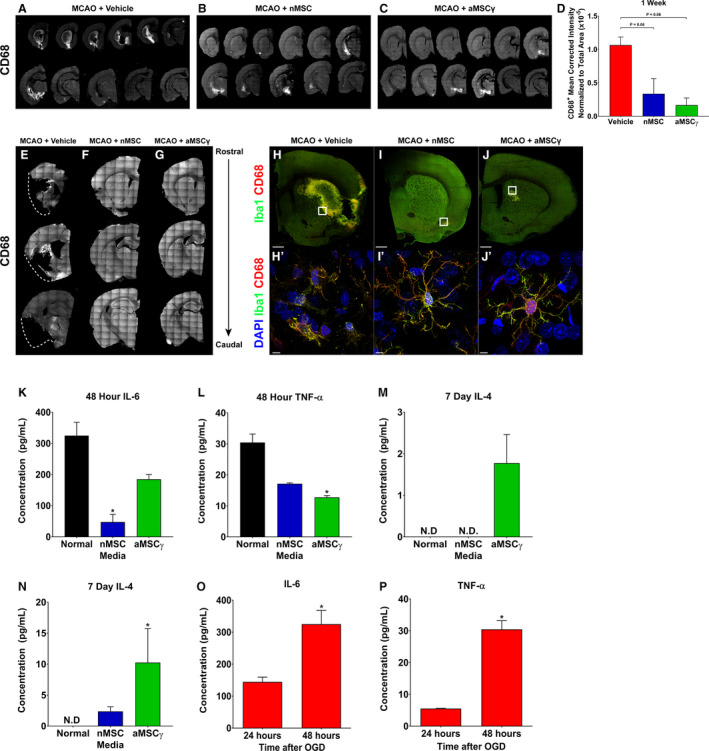
A through C, Representative images of CD68 staining of brain sections spaced 300 μm apart through the whole brain from middle cerebral artery occlusion (MCAO)+vehicle (A), MCAO+nMSC (B), and MCAO+aMSCγ (C) animals 1 week after surgery and treatment. D, Quantitation of CD68 staining 1 week after surgery and treatment. Data are mean±SEM; n=3 to 4 animals per group. Data were analyzed using nonparametric Kruskal‐Wallis 1‐way ANOVA. E through G, Representative images of CD68 staining of brain sections spaced 300 μm apart through the whole brain from MCAO+vehicle (E), MCAO+nMSC (F), and MCAO+aMSCγ (G) animals 3 weeks after surgery and treatment. Dashed lines (E) approximate the location of the lateral border of the tissue in a normal brain. H through J, Representative confocal images of whole brain slices of MCAO+vehicle (H), MCAO+nMSC (I), and MCAO+aMSCγ (J) animals 1 week after surgery and treatment stained for Iba1 (green) and CD68 (red). Scale bar represents 1 mm. H’ through J’, Representative confocal images at high magnification from the white boxes in H through J stained for DAPI (blue), Iba1 (green), and CD68 (red). Scale bar represents 5 μm. K through N, Concentration of interleukin 6 (IL‐6) (K), tumor necrosis factor α (TNF‐α) (L), IL‐4 (M), and IL‐10 (N) secreted by primary microglia after priming in oxygen and glucose deprivation (OGD) and then growth in normal (black), nMSC‐conditioned medium (blue), or aMSCγ conditioned medium (green). IL‐6 and TNF‐α were assayed at 48 hours, and IL‐4 and IL‐10 were assayed at 7 days. Data are mean±SEM; n=3 independent experiments. Data were analyzed using a nonparametric Kruskal‐Wallis 1‐way ANOVA. N.D., not detected, *Compared with Normal, *P≤0.05. O and P, Concentration of IL‐6 (O) and TNF‐α (P) secreted by primary microglia after OGD. Data are mean±SEM; n=3 independent experiments. Data were analyzed using a 2‐tail, unpaired t test. *P≤0.05. aMSCγ indicates interferon‐γ–activated mesenchymal stem cells; and nMSC, naive mesenchymal stem cells.
To further characterize how microglia respond to MSC treatment following hypoxia, primary microglia were primed in oxygen‐ and glucose‐deprivation conditions (OGD) and then cultured in normal medium, conditioned medium from nMSC, or conditioned medium from aMSCγ. We found that when cultured in both nMSC‐ and aMSCγ‐conditioned media, microglia have reduced secretion of proinflammatory cytokines IL‐6 (Figure 2K) and TNF‐α (Figure 2L) 48 hours later. Additionally, although not detectable in cells grown in normal medium, levels of anti‐inflammatory IL‐4 (Figure 2M) and IL‐10 (Figure 2N) were detected at 7 days in cells grown in MSC‐conditioned media, with aMSCγ having the largest effect. These data show that, similar to previous studies with peripheral macrophages, MSC have the ability to reduce microglial activation as well as convert them from a proinflammatory phenotype to a proregenerative phenotype. To validate the effect of hypoxia on cytokine production by microglia, we subjected primary microglial‐cultured cells to OGD. Secreted levels of IL‐6 and TNF‐α (Figure 2O and 2P, respectively) were significantly increased 48 hours after OGD compared with levels at 24 hours. To determine whether alterations in cytokine production following OGD were the result of the increased number of cells, we examined the viability as well as the toxicity levels in culture using MTT [3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide], LDH (lactate dehydrogenase), and terminal deoxynucleotidyl transferase dUTP nick end labeling assays. We observed some reduction in viability based on the MTT assay but no sign of toxicity or apoptosis in the culture (Figures S1 and S2). These results support the notion that MSC reverse the effect of hypoxia on microglial phenotype following stroke.
aMSCγ Induce Oligodendrogenesis Following MCAO
Another cell population that plays a role following stroke are neural progenitor cells (NPC) in the subventricular zone (SVZ). An estimated 10% of NPC survive after stroke.23 Hypotheses demonstrating the detrimental effects of inflammation on neurogenesis have been proposed.8, 9 However, how inflammatory mediators released by immune cells, especially activated microglia, affect NSC and NPC is still largely unknown. To start to address that, we quantified the extent of cell proliferation and lineage markers in both the ipsilateral SVZ and ipsilateral striatum (Figure 3). We found that 24 hours after MCAO, vehicle‐treated animals had a reduced number of proliferating cells, whereas aMSCγ‐treated rats had similar number of proliferating cells to sham animals (Figure 3A through 3E). Further, after MCAO, vehicle‐treated animals had no changes in the number of neuroblasts/immature neurons (Figure 3F). No change was observed in the number of proliferating neuroblasts (Figure 3G). This suggests that the increase in the number of proliferating cells observed in the MSC‐treated animals does not lead to an increased number of cells differentiating into neurons. Thus, we asked whether proliferating cells differentiate into other cell types. For this purpose we examined the number of cells expressing platelet‐derived growth factor receptor α (PDGFRα). These precursors give rise to myelin‐producing oligodendrocytes.66 Interestingly, we observed a marked increase in the number of cells in the oligodendrocytic lineage in the SVZ of aMSCγ‐treated rats compared with sham, vehicle‐, or nMSC‐treated MCAO rats (Figure 3H through 3R). The significant increase in the number of PDGFRα‐positive cells in the SVZ of aMSCγ‐treated rats suggested that aMSCγ induce an oligodendrogenic phenotype (Figure 3R).
Figure 3. Interferon‐γ–activated mesenchymal stem cells (aMSCγ) induce oligodendrogenesis following middle cerebral artery occlusion (MCAO).
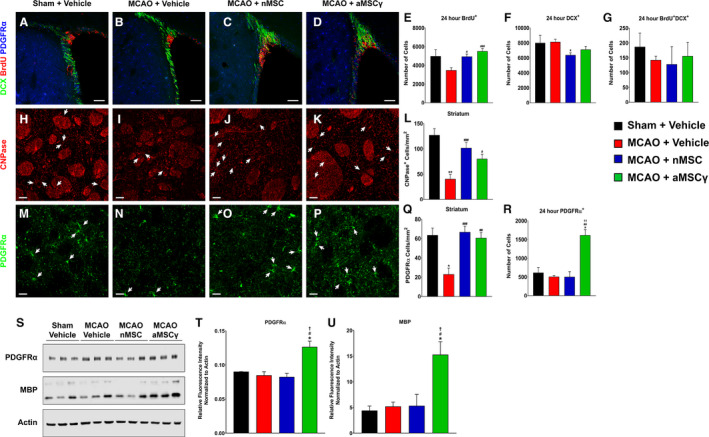
A through D, Representative confocal images of the ipsilateral SVZ from sham+vehicle (A) MCAO+vehicle (B), MCAO+nMSC (C), and MCAO+aMSCγ (D) animals 24 hours after surgery and treatment stained for doublecortin (DCX, green), bromodeoxyuridine (BrdU, red), and platelet‐derived growth factor receptor α (PDGFRα, blue). Scale bar represents 100 μm. E through G, Quantification of total numbers of BrdU+ cells (E), DCX+ cells (F), and BrdU+DCX+ cells (G) in the ipsilateral SVZ 24 hours after MCAO. Data are mean±SEM; n=3 animals for the sham group and n=5 to 6 animals for the remaining groups. Data were analyzed using nonparametric Kruskal‐Wallis 1‐way ANOVA. *Compared with sham+vehicle. #Compared with MCAO+vehicle. *P≤0.05, # P≤0.05. H through K, Representative confocal images of the ipsilateral striatum from sham+vehicle (H), MCAO+vehicle (I), MCAO+nMSC (J), and MCAO+aMSCγ (K) animals 3 weeks after surgery and treatment stained for 2′3′‐cyclic‐nucleotide 3′‐phosphodiesterase (CNPase, red). Scale bar represents 20 μm. L, Quantification of CNPase+ cells in the ipsilateral striatum. Data are mean±SEM; n=3 animals per group. Data were analyzed using nonparametric Kruskal‐Wallis 1‐way ANOVA. *Compared with sham+vehicle. #Compared with MCAO+vehicle. **P<0.01, # P≤0.05, ### P<0.001. M through P, Representative confocal images of the ipsilateral striatum from sham+vehicle (M), MCAO+vehicle (N), MCAO+nMSC (O), and MCAO+aMSCγ (P) animals 3 weeks after surgery and treatment stained for PDGFRα (green). Scale bar represents 20 μm. Q, Quantification of PDGFRα+ cells in the ipsilateral striatum. Data are mean±SEM; n=3 animals per group. Data were analyzed using nonparametric 1‐way Kruskal‐Wallis ANOVA. *Compared with sham+vehicle. #Compared with MCAO+vehicle. *P≤0.05, ## P<0.01, ### P<0.001. R, Quantification of total numbers of PDGFR+ cells in the ipsilateral subventricular zone (SVZ) 24 hours after MCAO. Data are mean±SEM; n=3 animals for the sham group and n=5 to 6 animals for the remaining groups. Data were analyzed using nonparametric Kruskal‐Wallis 1‐way ANOVA. *Compared with sham+vehicle. #Compared with MCAO+vehicle. †Compared with MCAO+nMSC. *P≤0.05, ## P<0.01, †† P<0.01. S through U, Western blots of the oligoprogenitor marker PDGFRα and mature oligodendrocyte marker MBP (myelin basic protein) (S) and their quantification (T and U) from brain homogenate from the ipsilateral hemisphere 1 week after surgery and treatment normalized to actin. Data are mean±SEM; n=3 animals per group. Data were analyzed using nonparametric Kruskal‐Wallis 1‐way ANOVA. *Compared with sham+vehicle. #Compared with MCAO+vehicle. †Compared with MCAO+nMSC. *P≤0.05, # P≤0.05, † P≤0.05. nMSC indicates naive mesenchymal stem cells.
These changes, however, were not sustained 1 week after MCAO (Figure S3). Additionally, when examining SVZ cell counts 3 weeks after MCAO, we found significant decreases in the numbers of neuroblasts/immature neurons (Figure S4A and S4B) with no changes in the number of mature oligodendrocytes in the SVZ (Figure S4C). This suggests that the increase in oligodendrocyte progenitors at 24 hours contributes to cells migrating out of the SVZ into the striatum toward the ischemic lesion. To investigate this, we assessed numbers of mature oligodendrocytes and oligodendrocyte progenitor cells in the ipsilateral striatum. We found that vehicle‐treated animals had significantly fewer mature oligodendrocytes (Figure 3H, 3I, and 3L) based on the number of cells expressing the myelin‐associated enzyme CNPase (2′,3′‐cyclic‐nucleotide 3′‐phosphodiesterase), and oligodendrocyte progenitor cells (PDGFRα+ cells, Figure 3M, 3N, and 3Q) compared with those in sham rats. Further, animals treated with either nMSC or aMSCγ had significantly more mature oligodendrocytes (Figure 3J through 3L) as well as oligodendrocyte progenitor cells (PDGFRα+ cells; Figure 3O through 3Q) compared with vehicle‐treated animals. Last, total protein levels of PDGFRα and myelin basic protein were quantified in tissue lysate of the ipsilateral hemisphere from animals 1 week after MCAO (Figure 3S through 3U). In animals treated with aMSCγ there was a significant increase in the total amount of PDGFRα (Figure 3S and 3T) and myelin basic protein (Figure 3S and 3U), suggesting that aMSCγ treatment not only induces oligodendrocyte differentiation but also causes these cells to become mature, myelinating oligodendrocytes.
Changes in Microglia and NSC After Hypoxia
To further investigate the effect of reduced oxygen on neural progenitor cells, neurosphere cultures were subjected to in vitro OGD. We found that in contrast to the in vivo conditions, OGD induced an increase in NPC proliferation, measured via bromodeoxyuridine uptake, 24 hours after exposure to OGD, but this increase was not sustained beyond 24 hours (Figure 4A). Additionally, mRNA levels of glia‐derived neurotrophic factor (Figure 4B), brain‐derived neurotrophic factor (Figure 4C), and IGF‐1 (insulin‐like growth factor 1; Figure 4D) were all significantly increased in neurosphere extracts 24 hours after OGD, but by 48 hours these mRNA levels were significantly decreased to almost nonexistent levels. To determine whether a change in neurotrophic factor production results from a change in cell number or viability of the culture, we counted the number of NPC at different time points after OGD and performed MTT, LDH, and terminal deoxynucleotidyl transferase dUTP nick end labeling. We observed that the number of NPC was not significantly changed 48 hours after OGD, although the viability of the culture was reduced (Figure S5). No sign of toxicity or apoptosis was observed in culture following OGD (Figures S5 and S6). Thus, increased production of neurotrophic factors 24 hours following OGD is not simply due to an increased number of cells in culture. We next asked whether the effect on NPC proliferation in vivo reflects a combined effect of OGD and microglia. Previous studies have implicated TNF‐α secreted by activated microglia as an inhibitor of NPC function.8, 9 To address this, neurospheres were grown in normal medium, conditioned medium from normoxia‐primed microglia, and conditioned medium from OGD‐primed microglia, and proliferation was measured via bromodeoxyuridine uptake. We found that when grown in medium from OGD‐primed microglia, NPC had a small but significantly reduced proliferation compared with NPC grown in normal medium or medium from normoxia‐primed microglia at both 48 hours (Figure 4E) and 72 hours (Figure 4F). This agrees with our result that secreted levels of IL‐6 and TNF‐α were significantly increased 48 hours after OGD compared with levels at 24 hours (Figure 2O and 2P, respectively).
Figure 4. Changes in microglia (MG) and neural stem cells (NSC) after hypoxia.

A, In vitro proliferation of subventricular zone (SVZ)‐derived NSC after exposure to oxygen and glucose deprivation (OGD) measured via bromodeoxyuridine (BrdU) uptake and represented as percentage of normoxia. Data are mean±SEM; n=5 independent experiments. Normoxia vs OGD data within a given time were compared using a 2‐tail unpaired t test. Data across time were compared using nonparametric Kruskal‐Wallis 1‐way ANOVA. *Compared with normoxia; #Compared with 24 hours OGD. *P≤0.05, # P≤0.05, ## P<0.01. B through D, mRNA fold change levels of glia‐derived neurotrophic factor (GDNF) (B), brain‐derived neurotrophic factor (BDNF) (C), and insulin‐like growth factor (IGF‐1) (D) from SVZ‐derived NSC after exposure to OGD. Data are mean±SEM; n=3 independent experiments. Data were analyzed using a 2‐way ANOVA with Holm‐Sidak multiple‐comparison testing. **P<0.01, ***P<0.001, ****P<0.0001. E and F, In vitro proliferation of SVZ‐derived NSC at 48 hours (E) and 72 hours (F) after exposure to normal medium (black), conditioned medium from normoxia‐primed microglia (blue), or hypoxia‐primed microglia (red) measured via BrdU uptake and represented as percentage of normal. Data are mean±SEM; n=3 independent experiments. Data were analyzed using a nonparametric Kruskal‐Wallis 1‐way ANOVA. *P≤0.05, **P<0.01.
MSC Promote NSC Function and Differentiation
To further investigate the effect of MSC on NPC phenotype, we examined the effect of MSC on trophic factor production level in OGD conditions. As shown earlier, NSC demonstrate trophic factor loss following OGD; however, IFN‐γ activation causes significant increases in MSC production of glia‐derived neurotrophic factor and IGF‐1 (Figure S7, Table S1), suggesting that MSC might aid in sustaining trophic factor levels after OGD. We found that, after OGD‐priming, NPC grown in a coculture system with aMSCγ have significantly increased production of mRNA for glia‐derived neurotrophic factor (Figure 5A), brain‐derived neurotrophic factor (Figure 5B), and IGF‐1 (Figure 5C) after both 24 and 48 hours.
Figure 5. Mesenchymal stem cells (MSC) promote neural stem‐cell (NSC) function and differentiation.

A through C, mRNA fold change levels of glia‐derived neurotrophic factor (GDNF) (A), brain‐derived neurotrophic factor (BDNF) (B), and insulin‐like growth factor 1 (IGF‐1) (C) from oxygen and glucose deprivation (OGD)‐primed subventricular zone (SVZ)‐derived NSC after coculture with aMSCγ in a ratio of aMSCγ:NSC of 1:10. Data are mean±SEM; n=3 independent experiments. Data were analyzed using a 2‐way ANOVA with Holm‐Sidak multiple comparison testing. *P≤0.05. D through F, DIC imaging of NSC grown in normal medium (D), nMSC‐conditioned medium (E), or aMSCγ‐conditioned medium (F). Scale bar represents 20 μm. G through I, Concatenated contour plots from bromodeoxyuridine (BrdU) flow cytometry of NSC grown in normal medium (G), nMSC‐conditioned medium (H), or aMSCγ‐conditioned medium (I). J through L, Quantification of cell cycle stages G0/1 (blue), S (green), and G2/M (pink) from BrdU flow cytometry of NSC grown in normal medium, nMSC‐conditioned medium, or aMSCγ‐conditioned medium at 24 hours (J), 48 hours (K), and 72 hours (L). Data are mean±SEM; n=3 independent experiments. Data were analyzed using 2‐way ANOVA with Holm‐Sidak multiple comparison testing. *Compared with Normal. #Compared with nMSC. **P<0.01, ***P<0.001, ****P<0.0001, #### P<0.0001. M through O, Western blots of lineage‐specific markers GFAP (glial fibrillary acidic protein) (astrocytes) and platelet‐derived growth factor receptor α (PDGFRα) (oligodendrocytes) (M) and their quantification (N and O) from NSC grown in normal medium, nMSC‐conditioned medium, or aMSCγ‐conditioned medium for 6 days normalized to actin. P through R, Western blots of lineage‐specific markers neurofilament L (neurons) and Sox2 (stem cells) (P) and their quantification (Q and R) from NSC grown in normal medium, nMSC‐conditioned medium, or aMSCγ‐conditioned medium for 6 days normalized to actin. Data are mean±SEM; n=3 independent experiments. Data were analyzed using nonparametric Kruskal‐Wallis 1‐way ANOVA. *Compared with Normal. *P≤0.05, **P<0.01. 7‐AAD indicates 7‐aminoactinomycin D; aMSCγ, interferon‐γ–activated mesenchymal stem cells; DIC, differential interference contrast; and nMSC, naive mesenchymal stem cells.
Normally, NSC grow as neurospheres in suspension (Figure 5D). However, after culture in conditioned medium from either nMSC or aMSCγ, these cells adhere to the plate and put out fine processes (Figure 5E and 5F), similar to their morphology while undergoing differentiation. To determine how MSC‐conditioned medium affected the NPC cell cycle, bromodeoxyuridine flow cytometry was performed to determine the percentage of cells in each of the cell cycle stages (Figure 5G through 5L). We found that when grown in nMSC‐conditioned medium and, to a larger extent, aMSCγ‐conditioned medium, NPC had significantly fewer cells in the S phase of the cell cycle and significantly more cells in the G0/1 phase of the cell cycle at 24 hours (Figure 5J), 48 hours (Figure 5K), and 72 hours (Figure 5L), suggesting that these cells are no longer proliferating and are exiting the cell cycle—a process that occurs when these cells undergo differentiation.
To determine what types of cells these NPC were becoming, they were grown for 6 days in either normal medium, nMSC‐conditioned medium, or aMSCγ‐conditioned medium, at which point lineage‐specific markers were analyzed by Western blot (Figure 5M through 5R, Table S2). We found that, when the cells were grown in nMSC‐conditioned medium and, to a significant extent, aMSCγ‐conditioned medium, there was reduced expression of the astrocyte marker glial fibrillary acidic protein (Figure 5N), the neuron marker neurofilament L (Figure 5Q), and the stem‐cell marker Sox2 (Figure 5R), with significantly increased expression of the oligodendrocyte lineage marker PDGFRα (Figure 5O). Together, these data support our in vivo observations and demonstrate that conditioned medium from MSC, with a larger effect from aMSCγ, causes NPC to differentiate toward an oligodendrocyte lineage.
Identification of the MSC Secretome
The protein secretome from MSC (for MSC identification and characterization see Figures S8 and S9) has been described under various conditions67, 68, 69, 70, 71; however, it is unknown what factors secreted by MSC induce NPC oligodendrogenesis and phenotype change in microglia subjected to hypoxic conditions. In addition, the differences in secreted proteins between nMSC and aMSCγ have never been described before. Based on the data presented in this study, aMSCγ exert a more potent effect on microglial phenotype and induce oligodendrogenesis and myelination. Therefore, in order to determine what potential protein factors secreted by the aMSCγ were responsible for the changes we observed in vitro and in vivo, proteomics analyses of the MSC and aMSCγ secretome were performed via mass spectrometry (Figure 6; for the full list of proteins see Tables S3 and S4). From this approach, 244 proteins were identified, with 16 proteins being unique to nMSC, 27 proteins being unique to aMSCγ, and the remaining 201 proteins being secreted by both MSC types (Figure 6A). Additionally, secreted proteins belong to many different biologic processes and protein classes with almost a quarter of proteins (21.6%) belonging to the signaling molecule or extracellular matrix classes (Figure 6B). Further, the secretome between both experimental and biological replicates remains consistent with very little variability between replicates both in the number of secreted proteins and the amount of those proteins (Figure 6C). Furthermore, with ex vivo IFN‐γ activation, there are 18 proteins that are significantly upregulated (>2‐fold increase, P<0.008) and 21 proteins that are significantly downregulated (>2‐fold decrease, P<0.008) compared with nMSC (Figure 6D). Notably, aMSCγ but not nMSC induce upregulation of CSPG4 (chondroitin sulfate proteoglycan 4), an important extracellular signal and a hallmark protein of oligodendrocyte precursor cells (OPC).72 In addition, we observed upregulation of BMP1 (bone morphogenetic protein 1 ), which promotes the generation of mature myelinating oligodendrocytes in vivo.73 MetaCore (Thomson Reuters, Toronto, Ontario, Canada) pathway analysis of these differential proteins resulted in 24 significantly enriched pathways (Tables S3 and S4, false discovery rate, FDR<0.05 ).
Figure 6. Identification of the interferon‐γ–activated and naive mesenchymal stem cell (aMSCγ, nMSC) secretome.
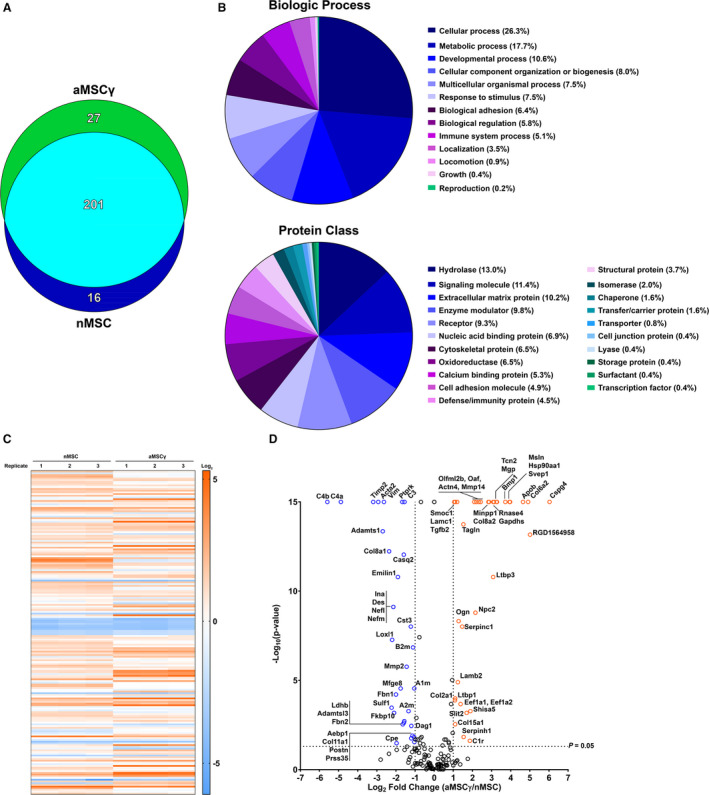
A, Venn diagram illustrating the number of proteins identified in each medium type. B, Biologic process (top chart) and protein class (bottom chart) breakdowns of identified proteins. C, Heat map analysis of biological replicates from both nMSC‐ and aMSCγ‐conditioned media. D, Volcano plot showing differentially secreted proteins between aMSCγ and nMSC. Positive fold changes indicate higher expression in aMSCγ, and negative fold changes indicate higher expression in nMSC. Data are mean±SEM; n=3 independent experiments. Data were analyzed by a permutation test with unadjusted significance level P<0.05 corrected by the Benjamini‐Hochberg procedure (new P<0.008).
Discussion
This study reports several important observations. We first showed that, when administered 3 hours after ischemic injury, MSC treatment leads to a significant functional recovery of treated animals as a direct result of preservation of brain parenchyma. In that regard, for various reasons, including delay to treatment and lack of recognition of stroke symptoms, no stroke therapy can be developed to completely regenerate infarcted tissue or to prevent the formation of an infarct core. Consequently, therapies aimed at limiting the size of the infarct and infarct penumbra are most clinically relevant. Related to this, preservation of the support structure within the penumbra is crucial for long‐term functional recovery after stroke. Thus, the observation that MSC treatment as administered in this study can minimize the infarct and penumbra is highly significant. The feasibility of this treatment is similar to that of tissue plasminogen activator, the only treatment currently available for acute ischemic stroke16 within 3 hours of onset. Because MSC do not induce a host immune response, they could be banked as a blood‐ bank product and should be available for treatment on thawing.
Second, we demonstrated that MSC reverse the proinflammatory phenotype of microglia induced by hypoxia and ischemia. Additionally, treatment of stroke animals by both nMSC and aMSCγ leads to a significant reduction in CD68+ monocytes and microglia, which may contribute to the preservation of brain tissue and the reduction in infarct size. This observation suggests that MSC are effective modifiers of microglial or monocyte phenotype, and their effect may be applicable to other brain insults characterized by increased inflammation. It should be noted that Iba‐1 and CD68 recognize both microglia and infiltrating monocytes, which can present a different phenotype. Thus, we cannot exclude the possibility that the detected effect reflects both cell types.
Third, we showed that aMSCγ are more potent than nMSC. Although both nMSC and aMSCγ lead to equivalent levels of functional recovery, aMSCγ exert 2 main molecular and cellular advantages that we believe make them a better therapeutic option. We showed (1) that, compared with nMSC, aMSCγ cause significant increases in anti‐inflammatory cytokine secretion by microglia with corresponding decreases in proinflammatory cytokines, and (2) that treatment with aMSCγ in vivo more effectively induces oligodendrocyte differentiation and myelination. Thus, we propose that aMSCγ would be a significantly more effective therapeutic agent than nMSC.
Fourth, in contrast to previous reports, we observed that ischemia suppresses the proliferation of neural progenitor cells in the SVZ and that neurogenesis is not induced either by ischemia or by MSC treatment. Therefore, future therapeutic avenues should test the regenerative efficacy of aMSCγ in combination with proneurogenic approaches.
Fifth, we showed that treatment with aMSCγ after stroke induced a significant increase in the number of oligodendrocyte progenitor cells (OPC) in the SVZ, ultimately leading to their differentiation and increased myelin production. We also demonstrated a significant increase in the number of OPC and mature oligodendrocytes in the striatum of stroke animals treated with aMSCγ. To start to address the mechanism underlying aMSCγ‐induced oligodendrogenesis, we compared the secretome of aMSCγ and nMSC. We unraveled a set of proteins that may be involved in the differential effect exerted by aMSCγ, which may underlie the robust pro‐oligodendrogenic response seen with aMSCγ treatment. For example, we observed upregulation of BMP1, which promotes the generation of mature myelinating oligodendrocytes in vivo.73 Further, we observed upregulation of chondroitin sulfate proteoglycan 4 (CSPG4, also known as neuron‐glia antigen 2), an important extracellular signal and a hallmark protein of the OPC. Mutations in CSPG4 cause OPC dysfunction and are implicated in familial schizophrenia.74 Interestingly, CSPG4 is highly expressed by brain pericytes, an important component of the neurovascular unit. Thus, it is possible that aMSCγ affect the composition of the blood‐brain barrier.75 This may have implications for the integrity of the blood‐brain barrier and for brain function.76 In support of this notion, the administration of MSC‐derived extracellular vesicles following spinal cord injury enhanced the integrity of the blood–spinal cord barrier by affecting pericyte signaling.77 Another important extracellular component upregulated in aMSCγ is collagen α‐2(VI), 1 of the 3 α‐chains of type VI collagen, a protein known to have a protective effect in the central nervous system under stress conditions, including enhancement of neural cell viability and reduction of oxidative stress.78 Importantly, an earlier study suggested that collagen VI regulates proper myelination in the peripheral nervous system.79 Interestingly, we observed that Svep1 (sushi, von Willebrand factor type A, EGF, and pentraxin domain containing 1) and SRPX2 (sushi repeat containing protein X‐linked 2) are differentially upregulated in the aMSCγ. SRPX2 is implicated in angiogenesis and is a ligand of the urokinase plasminogen activator surface receptor.80 In addition, it was shown to promote synapse formation.81 On the other hand, we observed downregulation of the immunomodulator SEMA7A (Semaphorin 7A) involved in inflammatory responses. SEMA7A is also known as the John‐Milton‐Hagen blood group antigen, an 80‐kDa glycoprotein expressed on activated lymphocytes and erythrocytes. Interestingly, SEMA5A is an inhibitor of axonal growth expressed by oligodendrocytes and their precursor cells in the developing optic nerve and is thought to contribute to the inhibition of CNS axon growth by oligodendrocyte lineage cells after injury.82 In addition, we observed downregulation of olfm13‐ expressed on microglia.83 Of note, Q6AYQ9 peptidyl‐prolyl cis‐trans isomerase is expressed in MSC, but its role and the significance of its levels are yet to be understood.
One limitation of this study is the small sample size. A prerequisite for the successful translation of this approach would be the examination of this treatment in a large cohort. In addition, further studies are warranted in order to determine whether poststroke treatment with aMSCγ promotes neuronal replacement. In that regard the efficacy of a combined therapy of aMSCγ with other stem cell–based promising treatments that promote neuronal production is an avenue worth exploring. For example, vasculoprotective drugs, such as activated protein C, a serine protease known to promote cytoprotective signaling in the ischemic brain endothelium at the blood‐brain barrier,84 have been shown to stimulate neuronal production by transplanted human NSC, promote circuit restoration, and improve functional recovery.85
In summary, our study reveals important information that advances our understanding of the efficacy and therapeutic potential of MSC for the treatment of ischemic stroke. It provides a potent alternative in the form of aMSCγ and unravels its mechanism of action, which may facilitate drug development.
Sources of Funding
This work was supported by National Institute on Aging R01‐AG033570, R01‐AG060238, R01‐AG062251, R21‐AG061628, University of Illinois at Chicago, Center for Clinical and Translational Science CCTS‐0512‐06 (Lazarov); National Heart, Lung, and Blood Institute/T32‐HL007692, and American Heart Association AHA/15PRE25080088 (Tobin); University of Illinois at Chicago Abraham Lincoln Fellowship (Pergande); National Institute of Allergy and Infectious Diseases/P01‐AI089556 (Bartholomew); University of Illinois at Chicago Department of Chemistry Startup Funds, Ara Parseghian Medical Research Fund (Cologna).
Disclosures
None.
Supporting information
Video S1
Data S1Tables S1–S4Figures S1–S9Reference 60
Acknowledgments
The authors would like to thank Dr Weiguo Li, the director of the Preclinical Imaging Core in the University of Illinois at Chicago Research Resources Center and his graduate student, Jin Gao, for their help with the MRI studies and the University of Illinois at Chicago Core for Research Bioinformatics for their help with analysis of mass spectrometry data. The authors would also like to thank Dr Ernesto Bongarzone for generously providing us with the CNPase antibody.
Author contributions: Tobin designed the research studies, conducted experiments, acquired and analyzed data, and wrote the manuscript. Lopez conducted experiments and analyzed data. Pergande designed the research studies, conducted experiments, acquired and analyzed data, and wrote the manuscript. Stephen wrote the manuscript. Cologna designed the research studies, conducted experiments, and acquired and analyzed data. Bartholomew designed the research studies and analyzed data. Lazarov designed the research studies and wrote the manuscript. All authors critically revised and approved the manuscript.
(J Am Heart Assoc. 2020;9:e013583 DOI: 10.1161/JAHA.119.013583.)
For Sources of Funding and Disclosures see page 14.
References
- 1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. [DOI] [PubMed] [Google Scholar]
- 5. Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, Bhatt DL, Grau‐Sepulveda MV, Peterson ED, Fonarow GC. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines‐Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. [DOI] [PubMed] [Google Scholar]
- 6. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 7. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega‐Gutierrez S, McTaggart RA, Torbey MT, Kim‐Tenser M, Leslie‐Mazwi T, et al. DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, Kokaia Z, Lindvall O. Suppression of stroke‐induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–1587. [DOI] [PubMed] [Google Scholar]
- 9. Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. [DOI] [PubMed] [Google Scholar]
- 11. Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci USA. 2006;103:13174–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation regulates functional integration of neurons born in adult brain. J Neurosci. 2008;28:12477–12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. [DOI] [PubMed] [Google Scholar]
- 14. Denes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppinen RA, Allan SM. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. [DOI] [PubMed] [Google Scholar]
- 15. Thored P, Heldmann U, Gomes‐Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, et al. Long‐term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. [DOI] [PubMed] [Google Scholar]
- 16. Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. [DOI] [PubMed] [Google Scholar]
- 20. Tsai YW, Yang YR, Wang PS, Wang RY. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c‐Fos expression and reverses spatial memory deficits in rats. PLoS One. 2011;6:e24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tonchev AB. Brain ischemia, neurogenesis, and neurotrophic receptor expression in primates. Arch Ital Biol. 2011;149:225–231. [DOI] [PubMed] [Google Scholar]
- 22. Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Neurosci. 2003;23:292–301. [DOI] [PubMed] [Google Scholar]
- 23. Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. [DOI] [PubMed] [Google Scholar]
- 25. Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu X, Xia J, Wang L, Song Y, Yang J, Yan Y, Ren H, Zhao G. Efficacy and safety of ginsenoside‐Rd for acute ischaemic stroke: a randomized, double‐blind, placebo‐controlled, phase II multicenter trial. Eur J Neurol. 2009;16:569–575. [DOI] [PubMed] [Google Scholar]
- 27. Zhao S, Wehner R, Bornhauser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune‐mediated disorders. Stem Cells Dev. 2010;19:607–614. [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell‐based tissue regeneration is governed by recipient T lymphocytes via IFN‐γ and TNF‐α. Nat Med. 2011;17:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castillo‐Melendez M, Yawno T, Jenkin G, Miller SL. Stem cell therapy to protect and repair the developing brain: a review of mechanisms of action of cord blood and amnion epithelial derived cells. Front Neurosci. 2013;7:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moradi F, Haji Ghasem Kashani M, Ghorbanian MT, Lashkarbolouki T. Spontaneous expression of neurotrophic factors and TH, Nurr1, Nestin genes in long‐term culture of bone marrow mesenchymal stem cells. Cell J. 2012;13:243–250. [PMC free article] [PubMed] [Google Scholar]
- 31. Sadan O, Melamed E, Offen D. Intrastriatal transplantation of neurotrophic factor‐secreting human mesenchymal stem cells improves motor function and extends survival in R6/2 transgenic mouse model for Huntington's disease. PLoS Curr. 2012;4:e4f7f6dc013d014e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sadan O, Shemesh N, Barzilay R, Dadon‐Nahum M, Blumenfeld‐Katzir T, Assaf Y, Yeshurun M, Djaldetti R, Cohen Y, Melamed E, et al. Mesenchymal stem cells induced to secrete neurotrophic factors attenuate quinolinic acid toxicity: a potential therapy for Huntington's disease. Exp Neurol. 2012;234:417–427. [DOI] [PubMed] [Google Scholar]
- 33. Chen JR, Cheng GY, Sheu CC, Tseng GF, Wang TJ, Huang YS. Transplanted bone marrow stromal cells migrate, differentiate and improve motor function in rats with experimentally induced cerebral stroke. J Anat. 2008;213:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. [DOI] [PubMed] [Google Scholar]
- 35. Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. IV infusion of brain‐derived neurotrophic factor gene‐modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG, Roh W, Hwang SJ, Ko HJ, Huh YM, Kim HT, et al. Implantation of human umbilical cord‐derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. [DOI] [PubMed] [Google Scholar]
- 38. Lim JY, Jeong CH, Jun JA, Kim SM, Ryu CH, Hou Y, Oh W, Chang JW, Jeun SS. Therapeutic effects of human umbilical cord blood‐derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gutierrez‐Fernandez M, Rodriguez‐Frutos B, Ramos‐Cejudo J, Otero‐Ortega L, Fuentes B, Vallejo‐Cremades MT, Sanz‐Cuesta BE, Diez‐Tejedor E. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: proof of concept in rats. J Transl Med. 2015;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, Liu D, Lin F. Effects of transplantation with bone marrow‐derived mesenchymal stem cells modified by survivin on experimental stroke in rats. J Transl Med. 2011;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lowrance SA, Fink KD, Crane A, Matyas J, Dey ND, Matchynski JJ, Thibo T, Reinke T, Kippe J, Hoffman C, et al. Bone‐marrow‐derived mesenchymal stem cells attenuate cognitive deficits in an endothelin‐1 rat model of stroke. Restor Neurol Neurosci. 2015;33:579–588. [DOI] [PubMed] [Google Scholar]
- 42. Quittet MS, Touzani O, Sindji L, Cayon J, Fillesoye F, Toutain J, Divoux D, Marteau L, Lecocq M, Roussel S, et al. Effects of mesenchymal stem cell therapy, in association with pharmacologically active microcarriers releasing VEGF, in an ischaemic stroke model in the rat. Acta Biomater. 2015;15:77–88. [DOI] [PubMed] [Google Scholar]
- 43. Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T,;Et al. Intra‐arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One. 2015;10:e0127302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR‐133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome‐enriched extracellular particles. Stem Cells. 2013;31:2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamauchi T, Kuroda Y, Morita T, Shichinohe H, Houkin K, Dezawa M, Kuroda S. Therapeutic effects of human multilineage‐differentiating stress enduring (MUSE) cell transplantation into infarct brain of mice. PLoS One. 2015;10:e0116009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang C, Liu H, Liu D. Mutant hypoxia‐inducible factor 1α modified bone marrow mesenchymal stem cells ameliorate cerebral ischemia. Int J Mol Med. 2014;34:1622–1628. [DOI] [PubMed] [Google Scholar]
- 48. Moniche F, Gonzalez A, Gonzalez‐Marcos JR, Carmona M, Pinero P, Espigado I, Garcia‐Solis D, Cayuela A, Montaner J, Boada C, et al. Intra‐arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43:2242–2244. [DOI] [PubMed] [Google Scholar]
- 49. Diez‐Tejedor E, Gutierrez‐Fernandez M, Martinez‐Sanchez P, Rodriguez‐Frutos B, Ruiz‐Ares G, Lara ML, Gimeno BF. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double‐blind, placebo‐controlled, single‐center, pilot clinical trial. J Stroke Cerebrovasc Dis. 2014;23:2694–2700. [DOI] [PubMed] [Google Scholar]
- 50. Moniche F, Rosado‐de‐Castro PH, Escudero I, Zapata E, de la Torre Laviana FJ, Mendez‐Otero R, Carmona M, Pinero P, Bustamante A, Lebrato L, et al. Increasing dose of autologous bone marrow mononuclear cells transplantation is related to stroke outcome: results from a pooled analysis of two clinical trials. Stem Cells Int. 2016;2016:8657173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suarez‐Monteagudo C, Hernandez‐Ramirez P, Alvarez‐Gonzalez L, Garcia‐Maeso I, de la Cuetara‐Bernal K, Castillo‐Diaz L, Bringas‐Vega ML, Martinez‐Aching G, Morales‐Chacon LM, Baez‐Martin MM, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151–161. [DOI] [PubMed] [Google Scholar]
- 52. Battistella V, de Freitas GR, da Fonseca LM, Mercante D, Gutfilen B, Goldenberg RC, Dias JV, Kasai‐Brunswick TH, Wajnberg E, Rosado‐de‐Castro PH, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6:45–52. [DOI] [PubMed] [Google Scholar]
- 53. Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Honmou O. [Phase III clinical trial using autologous mesenchymal stem cells for stroke patients]. Nihon Rinsho. 2016;74:649–654. [PubMed] [Google Scholar]
- 55. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, et al. Clinical outcomes of transplanted modified bone marrow‐derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, et al. IFN‐γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee S, Szilagyi E, Chen L, Premanand K, DiPietro LA, Ennis W, Bartholomew AM. Activated mesenchymal stem cells increase wound tensile strength in aged mouse model via macrophages. J Surg Res. 2013;181:20–24. [DOI] [PubMed] [Google Scholar]
- 58. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 59. Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622; discussion 1623 [DOI] [PubMed] [Google Scholar]
- 60. Wang Y, Zhang Z, Chow N, Davis TP, Griffin JH, Chopp M, Zlokovic BV. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43:2444–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Janardhan V, Qureshi AI. Mechanisms of ischemic brain injury. Curr Cardiol Rep. 2004;6:117–123. [DOI] [PubMed] [Google Scholar]
- 62. Kim J, Hematti P. Mesenchymal stem cell‐educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA. 1997;94:4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- 66. Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Silva NA, Moreira J, Ribeiro‐Samy S, Gomes ED, Tam RY, Shoichet MS, Reis RL, Sousa N, Salgado AJ. Modulation of bone marrow mesenchymal stem cell secretome by ECM‐like hydrogels. Biochimie. 2013;95:2314–2319. [DOI] [PubMed] [Google Scholar]
- 68. Rocha B, Calamia V, Casas V, Carrascal M, Blanco FJ, Ruiz‐Romero C. Secretome analysis of human mesenchymal stem cells undergoing chondrogenic differentiation. J Proteome Res. 2014;13:1045–1054. [DOI] [PubMed] [Google Scholar]
- 69. Xiao B, Rao F, Guo ZY, Sun X, Wang YG, Liu SY, Wang AY, Guo QY, Meng HY, Zhao Q, et al. Extracellular matrix from human umbilical cord‐derived mesenchymal stem cells as a scaffold for peripheral nerve regeneration. Neural Regen Res. 2016;11:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maffioli E, Nonnis S, Angioni R, Santagata F, Cali B, Zanotti L, Negri A, Viola A, Tedeschi G. Proteomic analysis of the secretome of human bone marrow‐derived mesenchymal stem cells primed by pro‐inflammatory cytokines. J Proteomics. 2017;166:115–126. [DOI] [PubMed] [Google Scholar]
- 71. Wang X, Chen G, Huang C, Tu H, Zou J, Yan J. Bone marrow stem cells‐derived extracellular matrix is a promising material. Oncotarget. 2017;8:98336–98347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Birey F, Kokkosis AG, Aguirre A. Oligodendroglia‐lineage cells in brain plasticity, homeostasis and psychiatric disorders. Curr Opin Neurobiol. 2017;47:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. See J, Mamontov P, Ahn K, Wine‐Lee L, Crenshaw EB 3rd, Grinspan JB. BMP signaling mutant mice exhibit glial cell maturation defects. Mol Cell Neurosci. 2007;35:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Vrij FM, Bouwkamp CG, Gunhanlar N, Shpak G, Lendemeijer B, Baghdadi M, Gopalakrishna S, Ghazvini M, Li TM, Quadri M, et al. Candidate CSPG4 mutations and induced pluripotent stem cell modeling implicate oligodendrocyte progenitor cell dysfunction in familial schizophrenia. Mol Psychiatry. 2019;24:757–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood‐brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. Blood‐brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y, Yao Y, Duan R, Jia Y. Bone mesenchymal stem cell‐derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood‐spinal cord barrier. Front Neurosci. 2019;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cescon M, Chen P, Castagnaro S, Gregorio I, Bonaldo P. Lack of collagen VI promotes neurodegeneration by impairing autophagy and inducing apoptosis during aging. Aging (Albany NY). 2016;8:1083–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen P, Cescon M, Megighian A, Bonaldo P. Collagen VI regulates peripheral nerve myelination and function. FASEB J. 2014;28:1145–1156. [DOI] [PubMed] [Google Scholar]
- 80. Eden G, Archinti M, Furlan F, Murphy R, Degryse B. The urokinase receptor interactome. Curr Pharm Des. 2011;17:1874–1889. [DOI] [PubMed] [Google Scholar]
- 81. Schirwani S, McConnell V, Willoughby J, Study DDD, Balasubramanian M. Exploring the association between SRPX2 variants and neurodevelopment: how causal is it? Gene. 2018;685:50–54. [DOI] [PubMed] [Google Scholar]
- 82. Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage‐specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest. 2017;127:3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Y, Zhao Z, Rege SV, Wang M, Si G, Zhou Y, Wang S, Griffin JH, Goldman SA, Zlokovic BV. 3K3A‐activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat Med. 2016;22:1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Data S1Tables S1–S4Figures S1–S9Reference 60


