Abstract
Background
Women have been associated with higher rates of recurrent events after percutaneous coronary intervention than men, possibly attributable to advanced age at presentation and greater comorbidities. These factors also put women at higher risk of bleeding, which may influence therapeutic strategies and clinical outcomes.
Methods and Results
We performed a patient‐level pooled analysis of 4 postapproval registries to evaluate sex‐related differences in patients at high bleeding risk (HBR) undergoing percutaneous coronary intervention. HBR required fulfillment of at least 1 major or 2 minor criteria of the Academic Research Consortium definition. Outcomes of interest were major bleeding and major adverse cardiac events (composite of cardiac death, myocardial infarction, or definite/probable stent thrombosis). Of the total 10 502 patients, 2832 (27.0%) were women. The prevalence of HBR was higher in women compared with men (29.0% versus 20.5%, P<0.0001). Women at HBR were older and had more comorbidities, while men at HBR were more often smokers, with prior myocardial infarction and more complex coronary lesions. At 4 years, women at HBR had significantly higher major bleeding compared with men at HBR (10.8% versus 6.2%, P<0.0001); however, this difference was attenuated after multivariable adjustment (hazard ratio, 0.92; 95% CI, 0.41–2.08). Major adverse cardiac event rates between groups were similar (12.2% versus 12.6%, P=0.82) and remained consistent after adjustment (hazard ratio, 0.64; 95% CI, 0.32–1.28).
Conclusions
The prevalence of HBR was higher in women compared with men, with considerable differences in the distribution of criteria. Women at HBR experienced higher rates of major bleeding but similar major adverse cardiac event rates compared with men at HBR at 4 years.
Keywords: everolimus‐eluting stent, high bleeding risk, major bleeding, percutaneous coronary intervention, sex
Subject Categories: Percutaneous Coronary Intervention, Stent
Clinical Perspective
What Is New?
Among patients undergoing percutaneous coronary intervention, the prevalence of high bleeding risk (HBR) was significantly higher in women compared with men, with considerable differences in the distribution of qualifying criteria.
Women at HBR experienced higher rates of major bleeding compared with men at HBR, a finding that could be partly ascribed to differing baseline clinical conditions.
Rates of major adverse cardiac events in patients at HBR were comparable across sexes.
What Are the Clinical Implications?
The Academic Research Consortium definition represents a useful tool for bleeding risk assessment in both men and women undergoing percutaneous coronary intervention.
Sex‐related differences in HBR features should be taken into consideration when optimizing revascularization strategies and subsequent antithrombotic therapy.
Nonstandard Abbreviations and Acronyms.
ARC Academic Research Consortium
CKD chronic kidney disease
CoCr‐EES cobalt‐chromium everolimus‐eluting stent
DAPT dual antiplatelet therapy
DES drug‐eluting stent
HBR high bleeding risk
ID‐TLR ischemia‐driven target lesion revascularization
LVEF left ventricular ejection fraction
MACE major adverse cardiac events
MB major bleeding
MI myocardial infarction
OAC oral anticoagulation
PCI percutaneous coronary intervention
ST stent thrombosis
Lack of awareness regarding the prevalence of cardiovascular disease in women makes them less likely to undergo diagnostic catheterization and subsequent coronary stenting compared with men.1 In addition, as women are often considered to be at higher risk of bleeding after percutaneous coronary intervention (PCI),2, 3 they tend to receive a shorter dual antiplatelet therapy (DAPT) regimen4 as well as less potent antiplatelet agents.5 This conundrum is further amplified in women who are considered to be at high bleeding risk (HBR) as a result of advanced age or multiple comorbid conditions. Concurrently, many of the conditions associated with HBR have also been identified as risk factors for ischemic events.6 Whether this risk perception influences treatment strategies and eventually impacts clinical outcomes after PCI differently in men and women is poorly understood.
Recent randomized trials have demonstrated the superiority of certain second‐generation drug‐eluting stents (DES) over bare metal stents in patients at HBR undergoing PCI followed by a shortened DAPT duration.7, 8, 9 However, there are currently no available data on sex‐based long‐term outcomes for patients at HBR undergoing PCI with second‐generation DES in general or with the cobalt‐chromium everolimus‐eluting stent (CoCr‐EES) specifically.
Therefore, in this study, we aimed to: (1) examine the baseline risk profile of patients at HBR undergoing PCI according to sex, (2) compare the long‐term bleeding and ischemic outcomes by sex in patients at HBR undergoing PCI with CoCr‐EES implantation, and (3) identify the predictors of bleeding and ischemic events in men and women at HBR.
Methods
Study Design and Population
The data that support the findings of this study will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. We pooled patient‐level data from 4 postapproval, prospective, open‐label, multicenter, single‐arm registries with up to 4‐year follow‐ups. These registries were designed to provide further information on the safety profile of the CoCr‐EES XIENCE V stent (Abbott Vascular) during commercial use in real‐world settings in the United States (NCT00676520), Japan (NCT01086228), India (NCT00631228), and China (NCT01178268), as previously detailed.10, 11, 12 All studies complied with the Declaration of Helsinki and were approved by an institutional review board at each study site. Only patients who could provide written informed consent and were treated exclusively with CoCr‐EES were included. There were no protocol‐mandated exclusions on the basis of clinical descriptors or angiographic criteria. For this analysis, only patients at HBR were included from the overall pooled database. HBR was defined according to the recently published Academic Research Consortium for High Bleeding Risk (ARC‐HBR) definition adapted to the variables available in all the studies.13 Patients were considered to be at HBR if at least 1 major criterion or 2 minor criteria were met. Major ARC‐HBR criteria included moderate or severe anemia (hemoglobin <11 g/dL), use of long‐term oral anticoagulation, severe or end‐stage chronic kidney disease (CKD) (estimated glomerular filtration rate, <30 mL/min), thrombocytopenia (platelet count <100×109/L), spontaneous bleeding requiring hospitalization or transfusion, and active malignancy. Minor ARC‐HBR criteria included age 75 years and older, mild anemia (hemoglobin 11–12.9 g/dL for men or 11–11.9 g/dL for women), moderate CKD (estimated glomerular filtration rate, 30–59 mL/min), and moderate or severe stroke >6 months ago. Treatment strategies, including stent implantation techniques and periprocedural pharmacotherapy, were determined by site‐based clinical practice. The antithrombotic management for all patients was ultimately determined by the treating physicians. However, protocol recommendations for antiplatelet therapy included an indefinite duration of aspirin, along with a required minimum of 6 months of P2Y12 inhibitors.
End Point Definitions
The primary end points were major bleeding (MB) and major adverse cardiac events (MACE). MB was defined according to the Thrombolysis In Myocardial Infarction (TIMI) or Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries (GUSTO) scales depending on the registry, and included bleeding events categorized as TIMI minor or major (Xience V United States and Xience V India) or GUSTO moderate or severe (Xience V China and Xience V Japan). MACE was defined as occurrence of cardiac death, myocardial infarction (MI) according to the World Health Organization (WHO) definition14 or definite/probable stent thrombosis (ST) according to the Academic Research Consortium (ARC) definition.15 Other end points of interest were all‐cause death, cardiac death, MI, ST, and ischemia‐driven target lesion revascularization. End points adjudicated by an independent clinical events committee are reported in Table S1. To summarize, all cardiac and majority of bleeding events were adjudicated. Patients were clinically followed up by either telephone contact or office visits at 30 days, 180 days, and 1 year, and then annually up to 4 years after index PCI. The median duration of follow‐up for the study patients was 1430 days (interquartile range, 1083–1460).
Statistical Analysis
Continuous variables were reported as mean±SD or median±interquartile range and compared using Student t test or Wilcoxon rank sum test. Categorical data were reported as proportions and compared by chi‐square test or Fisher exact test. Four‐year event rates were estimated using Kaplan–Meier time‐to‐event methodology and compared using log‐rank test. Independent predictors of MB and MACE were evaluated using stepwise Cox proportional hazard regression. Two separate multivariable models were created for men and women at HBR. Variables were entered into the model either through clinical judgement or at the 0.05 significance level and removed at the 0.05 level (from the Wald chi‐square statistic). Variables were eligible for inclusion in the multivariable model‐building process if the variable was present for 90% of the patients in the analyses, had a univariate P<0.05, and had the higher level of significance, if highly correlated with another variable (r>0.5 and P<0.05). The following variables were included: age 75 years and older, history of MB, history of stroke, chronic oral anticoagulation, CKD, anemia, thrombocytopenia, sex, multivessel disease, diabetes mellitus, current smoker, hypertension, hyperlipidemia, acute MI at admission, left ventricular ejection fraction <30%, prior cardiac intervention, prior MI, minimum reference vessel diameter, maximum lesion length, left main lesion, graft lesion, B2/C lesion, bifurcation lesion, restenotic lesion, ostial lesion, number of lesions treated, number of vessels treated, and number of stents implanted. Results are reported as hazard ratios (HRs) with 95% CIs. We reported 2‐sided P values and considered P<0.05 to be significant. All analyses were performed using SAS version 9.4 software (SAS Institute Inc).
Results
Baseline and Procedural Characteristics
Of the total 10 502 patients included in this pooled analysis, 2832 (27.0%) patients were women and 7670 (73.0%) patients were men. The prevalence of HBR was significantly higher in women compared with men (29.0% versus 20.5%, respectively; P<0.0001). Baseline and procedural characteristics according to sex are detailed in Table 1. Women at HBR were older and had more comorbidities such as diabetes mellitus, hypertension, and hyperlipidemia. Men at HBR were more often active smokers and had a higher prevalence of prior MI. With regard to the procedural characteristics, women at HBR were more likely to have a smaller reference vessel diameter and shorter lesion length, while men at HBR had a higher prevalence of B2/C lesions. Comparison of the prevalence of various criteria in patients at HBR by sex is illustrated in Figure 1. The most common major ARC‐HBR criterion was moderate/severe anemia in women at HBR and use of long‐term oral anticoagulation in men at HBR. Age 75 years and older and mild anemia were the most common minor ARC‐HBR criteria in women and men at HBR, respectively.
Table 1.
Clinical and Procedural Characteristics
| Women at HBR (n=821) | Men at HBR (n=1576) | P Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 72.7±10.5 (821) | 70.6±11.0 (1576) | <0.0001 |
| Current smoker | 9.6% (75/785) | 19.5% (295/1515) | <0.0001 |
| Diabetes mellitus | 48.7% (399/820) | 42.9% (673/1570) | 0.007 |
| Hypertension | 89.6% (735/820) | 85.1% (1335/1568) | 0.002 |
| Hyperlipidemia | 79.4% (639/805) | 71.0% (1079/1520) | <0.0001 |
| LVEF <30% | 4.4% (27/608) | 4.8% (55/1145) | 0.73 |
| Multivessel disease | 40.7% (334/821) | 45.3% (711/1571) | 0.03 |
| Prior cardiac intervention | 48.1% (379/788) | 49.1% (741/1510) | 0.66 |
| Prior MI | 26.7% (198/741) | 32.4% (471/1453) | 0.006 |
| Clinical presentation | |||
| Acute MI | 17.1% (122/712) | 18.6% (267/1434) | 0.40 |
| Procedural characteristics | |||
| No. of treated lesions per patient | 1.3±0.6 (821) | 1.4±0.7 (1576) | 0.82 |
| No. of treated vessels per patient | 1.1±0.4 (787) | 1.1±0.4 (1483) | 0.21 |
| No. of stents implanted per patient | 1.6±0.8 (821) | 1.6±0.9 (1576) | 0.46 |
| RVD, mm | 2.92±0.48 (983) | 3.00±0.58 (1896) | <0.0001 |
| Lesion length, mm | 17.1±10.5 (972) | 18.7±11.5 (1897) | 0.0003 |
| B2/C lesion | 55.5% (501/902) | 60.0% (1035/1724) | 0.03 |
| Left main | 2.2% (24/1104) | 3.0% (64/2132) | 0.17 |
| Graft | 3.6% (40/1104) | 5.3% (112/2132) | 0.04 |
| Restenosis lesion | 10.8% (119/1103) | 10.4% (221/2123) | 0.74 |
| Bifurcation | 9.7% (105/1085) | 9.6% (198/2073) | 0.91 |
| Ostial lesion | 14.4% (145/1009) | 15.9% (296/1861) | 0.28 |
| No. of HBR criteria | |||
| Major ARC‐HBR | 0.7±0.6 | 0.6±0.6 | |
| Minor ARC‐HBR | 1.4±1.0 | 1.4±1.0 | |
| LEADERS FREE | 1.4±0.6 | 1.3±0.6 | |
Data are reported as percentage and number of patients as well as mean and SD as appropriate. ARC indicates Academic Research Consortium; HBR, high bleeding risk; LEADERS FREE, Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug‐Coated Stent versus the Gazelle Bare‐Metal Stent in Patients at High Bleeding Risk trial; LVEF, left ventricular ejection fraction; MI, myocardial infarction; and RVD, reference vessel diameter.
Figure 1. Comparison of the prevalence of various Academic Research Consortium High Bleeding Risk (ARC‐HBR) criteria by sex.
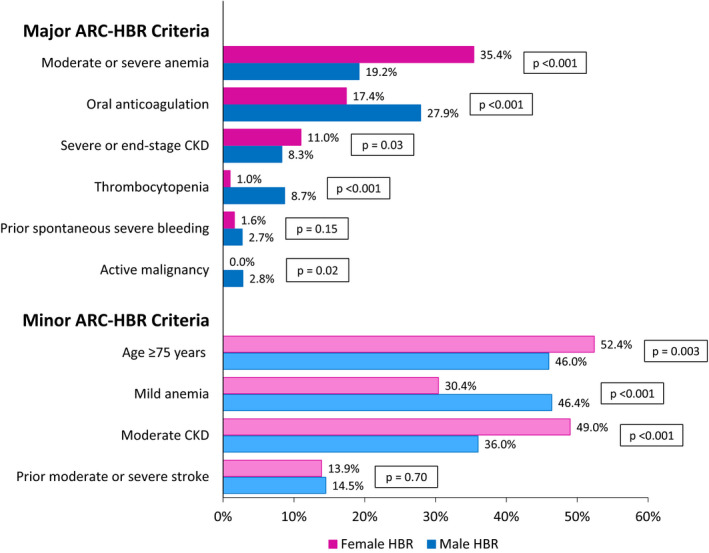
CKD indicates chronic kidney disease; HBR, high bleeding risk; and OAC, oral anticoagulation.
DAPT Management
Comparison of DAPT management between patients at HBR and those not at HBR according to sex is illustrated in Figure 2. A total of 75.4% of women at HBR were on DAPT 1 year after index procedure, while 82.3% of women not at HBR were on DAPT at the same time point (P<0.0001). At 4 years post‐index procedure, 38.7% of women at HBR and 44.1% of women not at HBR were on DAPT (P=0.01).
Figure 2. Sex‐wise dual antiplatelet therapy (DAPT) management up to 4‐year follow‐up.
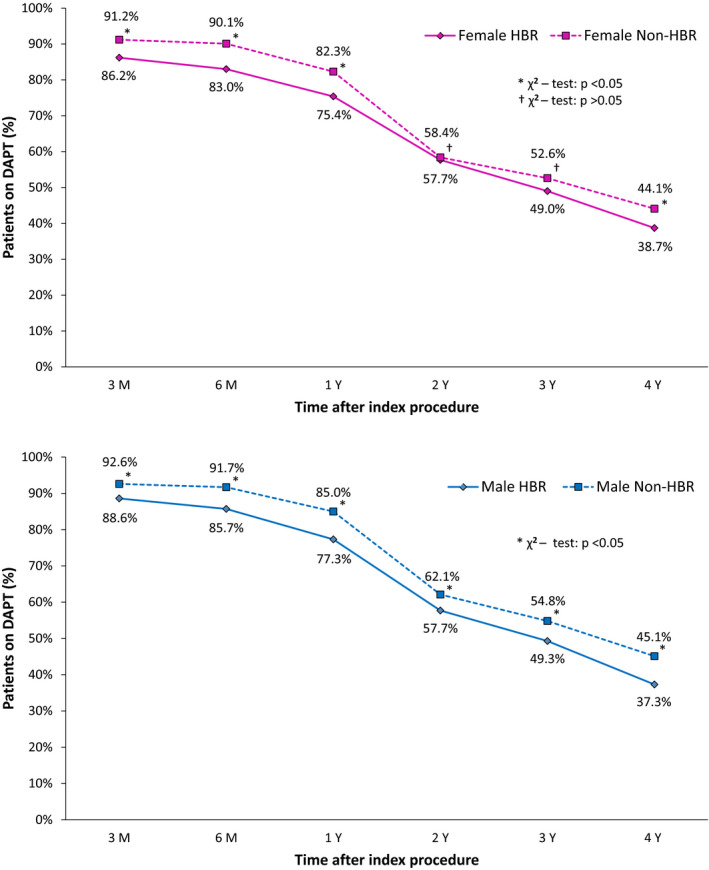
HBR indicates high bleeding risk.
In men, 77.3% of patients at HBR were on DAPT 1 year post‐PCI compared with 85.0% of patients not at HBR (P<0.0001). The percentage of patients on DAPT at 4 years post‐PCI was also significantly lower in men at HBR compared with men not at HBR (37.3% versus 45.1%, respectively; P<0.0001).
Tables S2 and S3 compare the DAPT rates between men and women at HBR and those not at HBR, respectively. Within the HBR population, we observed comparable DAPT rates between women and men up to 4 years. In the non‐HBR population, however, DAPT rates were significantly lower in women compared with men up to 2 years post‐PCI, with no significant differences at 3 and 4 years.
Clinical Outcomes
Long‐term clinical outcomes for HBR patients by sex are detailed in Table 2 and Figure 3. Women at HBR had significantly higher rates of MB at 4 years compared with men at HBR (10.8% versus 6.2%, P<0.0001; respectively). Rates of 4‐year MACE were similar between the 2 groups (12.2% versus 12.6%, P=0.82; respectively). There were no significant differences in the incidence of individual end points such as all‐cause mortality, cardiac death, MI, and ST between men and women at HBR. After adjusting for possible baseline confounders, the differences in MB were attenuated and no longer significant between men and women at HBR (HR, 0.92; 95% CI, 0.41–2.08). The findings for MACE remained consistent after multivariable adjustment (HR, 0.64; 95% CI, 0.32–1.28).
Table 2.
Sex‐Wise 4‐Year Outcomes in Patients at HBR
| Women at HBR (n=821) | Men at HBR (n=1576) | Log‐Rank P Value | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|
| MACE | 12.2% | 12.6% | 0.82 | 0.97 (0.75–1.25) | 0.82 | 0.64 (0.32–1.28) | 0.20 |
| All‐cause death | 18.4% | 18.4% | 0.90 | 0.99 (0.80–1.21) | 0.90 | 0.55 (0.30–1.03) | 0.06 |
| Cardiac death | 9.7% | 9.8% | 0.88 | 0.98 (0.73–1.30) | 0.88 | 0.52 (0.23–1.20) | 0.13 |
| Noncardiac death | 9.7% | 9.6% | 0.98 | 1.00 (0.74–1.34) | 0.98 | 0.63 (0.24–1.63) | 0.34 |
| MI | 4.3% | 3.9% | 0.51 | 1.16 (0.75–1.80) | 0.51 | 1.61 (0.55–4.72) | 0.38 |
| Definite/Probable ST | 1.5% | 2.0% | 0.41 | 0.75 (0.37–1.50) | 0.41 | 0.64 (0.10–3.93) | 0.63 |
| Major bleeding | 10.8% | 6.2% | <0.0001 | 1.81 (1.34–2.43) | <0.0001 | 0.92 (0.41–2.08) | 0.84 |
| ID‐TLR | 11.1% | 7.4% | 0.004 | 1.55 (1.16–2.08) | 0.003 | 2.24 (1.07–4.68) | 0.03 |
Adjusted hazard ratio (HR) adjusted for age 75 years and older, diabetes mellitus, smoker, hypertension, hyperlipidemia, prior myocardial infarction (MI), prior cardiac intervention, left ventricular ejection fraction <30%, acute coronary syndrome, multivessel disease, B2/C lesion. Major adverse cardiac event (MACE) is a composite of cardiac death, MI, or definite/probable stent thrombosis (ST). HBR indicates high bleeding risk; and ID‐TLR, ischemia‐driven target lesion revascularization.
Figure 3. Kaplan–Meier curves for 4‐year clinical outcomes.
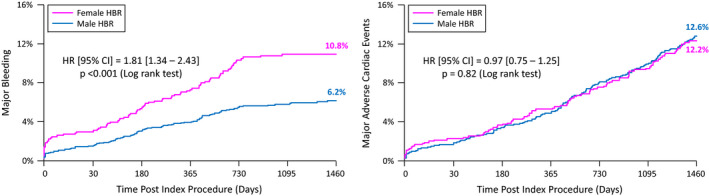
Major adverse cardiac events: composite of cardiac death, myocardial infarction, or definite/probable stent thrombosis.
Table S4 illustrates the sex‐wise outcomes in patients at HBR up to 3 years. In addition, rates of MB in women, men, and the overall non‐HBR population are reported in Table S5. At 1 year, women not at HBR had a 1.8% rate of MB compared with 1.5% for men not at HBR. At 4 years, rates of MB were 6.9% and 4.6% in women and men not at HBR, respectively.
Sex‐wise 4‐year clinical outcomes in the overall population are detailed in Table S6. While the event rates in the overall population were much lower than in the HBR cohort, the unadjusted and adjusted HRs between sexes in the overall study population were qualitatively similar to the HBR population.
To evaluate the impact of clinical presentation on sex‐based long‐term outcomes, women and men at HBR were further stratified into ST‐segment–elevation MI and non–ST‐segment–elevation acute coronary syndrome groups. Outcomes at 4 years are reported in Tables S7 and S8 for ST‐segment–elevation MI and non–ST‐segment–elevation acute coronary syndrome, respectively. In line with the primary results of the analysis, MACE rates were comparable between sexes, with numerically higher rates of MB observed in women at HBR for both ST‐segment–elevation MI and non–ST‐segment–elevation acute coronary syndrome presentations.
Predictors of 4‐Year MB and MACE
Table 3 reports the independent predictors of MB and MACE (cardiac death, MI, or ST) at 4 years in women and men at HBR. The strongest predictors of MB were prior MI (HR, 1.75; P=0.02) in women at HBR, and multivessel disease (HR, 1.93; P=0.003) and age (HR, 1.03; P=0.02) in men at HBR. In regard to MACE, the strongest predictors for women at HBR were prior MI (HR, 1.77; P=0.01) and diabetes mellitus (HR, 1.58; P=0.04), while for men at HBR they were multivessel disease (HR, 1.72; P=0.002), prior MI (HR, 1.67; P=0.003), and age (HR, 1.02; P=0.03).
Table 3.
Sex‐Wise Predictors of 4‐Year MB and MACE
| Variable | Coefficient (SE) | HR (95% CI) | P Value |
|---|---|---|---|
| MB | |||
| Women at HBR | |||
| Prior MI | 0.56 (0.24) | 1.75 (1.09–2.80) | 0.02 |
| Men at HBR | |||
| Multivessel disease | 0.66 (0.22) | 1.93 (1.26–2.95) | 0.003 |
| Age | 0.03 (0.01) | 1.03 (1.00–1.05) | 0.02 |
| MACE | |||
| Women at HBR | |||
| Prior MI | 0.57 (0.23) | 1.77 (1.13–2.77) | 0.01 |
| Diabetes mellitus | 0.46 (0.22) | 1.58 (1.02–2.45) | 0.04 |
| Men at HBR | |||
| Multivessel disease | 0.54 (0.17) | 1.72 (1.23–2.41) | 0.002 |
| Prior MI | 0.51 (0.17) | 1.67 (1.19–2.34) | 0.003 |
| Age | 0.02 (0.01) | 1.02 (1.00–1.04) | 0.03 |
The multivariable model was created using stepwise regression, where variables were entered into the model either through clinical judgement or at the 0.05 significance level and removed at the 0.05 level (from the Wald chi‐square statistic). Variables were eligible for inclusion in the multivariable model‐building process if the variable was present for 90% of the patients in the analyses, had a univariate P<0.05, and had the higher level of significance if highly correlated with another variable (r>0.5 and P<0.05). Major bleeding (MB) was defined according to the Thrombolysis In Myocardial Infarction (TIMI) or Global Utilization of Streptokinase and TPA for Occluded arteries (GUSTO) scales depending on the registry, and included bleeding events categorized as TIMI minor/major (Xience V US and Xience V India) or GUSTO moderate/severe (Xience V China and Xience V Japan). Major adverse cardiac event (MACE) is a composite of cardiac death, myocardial infarction (MI), or definite/probable stent thrombosis. HBR indicates high bleeding risk; HR, hazard ratio; and SE, standard error.
Table S9 reports the bleeding risk (HR and 95% CI) associated with each major and minor ARC‐HBR criterion considered individually, when compared with the absence of any of these criteria (non‐HBR group). Every criterion included in our adapted ARC‐HBR definition was independently associated with an increased risk of MB compared with patients who were not at HBR.
Discussion
The main findings of this large patient‐level pooled analysis of 4 postapproval registries are as follows: (1) the prevalence of HBR was significantly higher in women compared with men; (2) clinical characteristics qualifying patients as HBR significantly differ between women and men at HBR; (3) at 4 years, women at HBR had significantly higher rates of MB compared with men at HBR; however, this difference was attenuated after adjustment for possible baseline confounders; (4) MACE rates between women and men at HBR were similar and remained consistent after multivariable adjustment.
Previous studies have shown significant differences between the risk profiles of women and men undergoing PCI, with women being older and having more associated comorbidities.16, 17 Because of these factors, it was observed that women were at higher risk for both ischemic3, 16, 18 and bleeding events after PCI.2, 19 Sex‐related differences in pharmacodynamic and pharmacokinetic response to antithrombotic medications may partly explain the trend towards increased bleeding risk observed among women in clinical trials.20 Despite the continuous advancements in DES technology and their association with improved outcomes compared with bare metal stents,7, 8 physicians still tend to exercise caution when using DES for patients at HBR because of the alleged need for a longer duration of DAPT. This risk may be further amplified in women given their higher prevalence of HBR factors.
The HBR population constitutes a substantial portion of patients undergoing PCI, with its prevalence varying based on definitions and inclusion criteria used for patient selection.21 We used the recently published consensus‐based definition of the ARC consisting of major and minor criteria to identify our HBR population.13 Although the precise characterization of 20 clinical variables is primarily intended for prospective use in clinical trials, we adapted the definition according to the variables available in all of the included registries. In our analysis, we observed that the prevalence of HBR in women was significantly higher than in men (29.0% versus 20.5%, respectively). This reflects the differences noted in the sex‐wise HBR characteristics where we found that women at HBR had a higher prevalence of moderate or severe anemia and CKD, 2 conditions that are often concomitant. Indeed, since erythropoietin production mainly takes place in the kidneys, it is hypothesized that a lack of this circulating factor is responsible for the occurrence of anemia in patients with CKD.22 Conversely, HBR‐related conditions such as use of long‐term oral anticoagulation, thrombocytopenia, and active malignancy were more frequent in men at HBR. Finally, comorbidities such as diabetes mellitus, hypertension, and hyperlipidemia were more frequent in women at HBR, whereas men, although younger, were more likely to present with a history of prior MI and to be active smokers.
Importantly, we found that event rates for men and women at HBR were higher than both the patients not at HBR in our cohort as well as previously reported all‐comer contemporary PCI populations.23, 24 There are no prior data on sex‐based long‐term outcomes following PCI specifically in the HBR population with which the present results may be compared. However, it has been previously reported in an all‐comer population study that despite the more advanced age and greater prevalence of comorbid conditions, women undergoing PCI showed similar rates of mortality and ischemic outcomes as men.25 We extend this evidence to the frail HBR population where we observed similar rates of MACE and its individual components between men and women at HBR; findings that remained consistent even after multivariable adjustment. Of note, the rates of MI and definite/probable ST in our study were lower compared with those in the LEADERS FREE (Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug‐Coated Stent versus the Gazelle Bare‐Metal Stent in Patients at High Bleeding Risk) trial.7 This could be attributed to the differences in the prescribed DAPT duration, stent platforms, as well as complexity of coronary artery disease between the 2 studies. While the protocol of the LEADERS FREE trial mandated only 1 month of DAPT after PCI, the registries included in our study had a longer DAPT duration. Contrary to the ischemic outcomes, we found that women at HBR experienced higher crude rates of MB compared with men at HBR. However, these differences were attenuated after adjusting for baseline clinical and procedural variables. This finding suggests that female sex, when considered in isolation, does not increase the risk for bleeding complications. It is rather the prevalence of specific clinical conditions associated with bleeding risk that vary according to sex, which determine the difference in outcomes between men and women. As such, women at HBR constitute a vulnerable subset of patients undergoing PCI and should therefore not be denied the benefits of coronary revascularization with DES implantation despite concerns regarding their worse baseline clinical characteristics.
At 4‐year follow‐up, the percentage of women at HBR on DAPT, although significantly lower than women not at HBR (38.7% versus 44.1%, respectively), was still much higher than expected. The high rates of DAPT prescription in women at HBR can be explained not only by the high prevalence of traditional risk factors such as diabetes mellitus but also by the fact that bleeding determinants such as CKD and prior stroke are also associated with ischemic risk,6 which physicians tend to perceive and prioritize more compared with bleeding risk. Similarly, we observed that 37.3% of men at HBR were on DAPT at 4‐year follow‐up.
As patients at HBR tend to experience higher rates of both bleeding and ischemic events, we investigated the predictors of 4‐year MB as well as MACE in patients at HBR according to sex. Notably, in women at HBR, prior MI was a strong predictor for both MB and MACE, while diabetes mellitus was an independent predictor only for MACE. These findings are concordant with what was observed in the PARIS (Patterns of Non‐Adherence to Anti‐Platelet Regimen in Stented Patients) study, which included both prior revascularization and diabetes mellitus in the risk score for coronary thrombotic events in the general population.6 For men at HBR, we found that multivessel disease and age were independent predictors for both MB and MACE, while prior MI was a strong predictor only for MACE. All of the above findings reinforce the need to not only consider the crucial features specific to sex when approaching this frail patient population but to also customize the treatment modalities and DAPT duration according to the individual risk profile of each patient.
Limitations
As a post hoc analysis, the study findings should be considered exploratory. Since the current analysis was performed in a pooled data set exclusively evaluating the Xience V CoCr‐EES, the findings cannot be extrapolated to other DES. Being a retrospective analysis of prospectively collected registries, unmeasurable confounders may have remained in spite of the multivariable adjustment. HBR was defined according to the recently published ARC‐HBR criteria and adapted to the available data collected in the data set; however, we cannot exclude the possibility that the use of other HBR definitions could have altered the results. MI was defined according to the ARC definition in the Xience V China study and according to the WHO definition in the other registries, which may have potentially led to imprecision. Spontaneous bleeding requiring hospitalization or transfusion and thrombocytopenia were not collected for the Xience V Japan and China registries, which may have led to an underestimation of the prevalence of HBR.
Conclusions
In this large patient‐level pooled analysis of patients receiving CoCr‐EES, the prevalence of HBR was significantly higher in women compared with men. There were also important differences in the distribution of HBR characteristics according to sex. Women at HBR experienced higher rates of MB but similar rates of MACE compared with men at HBR at 4 years.
Sources of Funding
None.
Disclosures
Mehran has received institutional research grant support from AstraZeneca, Bayer, Beth Israel Deaconess Medical Center, Bristol‐Myers Squibb, CSL Behring, Eli Lilly/Daiichi Sankyo, Medtronic, Novartis Pharmaceuticals, and OrbusNeich; has served on the executive committee of Janssen Pharmaceuticals and Osprey Medical Inc.; has served on the data safety monitoring board of Watermark Research Partners; has served as a consultant for Abbott Laboratories, Abiomed (Spouse), Boston Scientific, Cardiovascular Systems, Inc., Medscape, Siemens Medical Solutions, The Medicines Company (Spouse), Roivant Sciences, Inc, Volcano Corporation and Sanofi; and has equity in Claret Medical Inc. and Elixir Medical Corporation. Krucoff is a consultant and has received research grants from Abbott, Medtronic, OrbusNeich, Biosensors, and Boston Scientific. Angiolillo reports receiving payments as an individual for: consulting fees or honorarium from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; participation in review activities from CeloNova and St. Jude Medical; and institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli‐Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions. Bangalore serves on the advisory board/received honoraria from Abbott Vascular, Biotronik, Amgen, Pfizer, Reata; has received research grants from Abbott Vascular, and the National Heart, Lung, and Blood Institute. Seth serves as a consultant/speaker's bureau for Abbott Vascular. Bhatt discloses the following relationships—advisory board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, and Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; chair: American Heart Association Quality Oversight Committee; data monitoring committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED [CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis Requiring Aortic Valve Replacement] trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE [Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation] trial, funded by Daiichi Sankyo), and Population Health Research Institute; honoraria: American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; vice chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI (Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting] clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II [ApoA‐I Event Reducing in Ischemic Syndromes II] executive committee funded by CSL Behring), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (CME steering committees); other: Clinical Cardiology (deputy editor), NCDR's [National Cardiovascular Data Registry's] ACTION Registry Steering Committee (chair), and VA CART Research and Publications Committee (chair); research funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, and The Medicines Company; royalties: Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); site co‐investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; trustee: American College of Cardiology; unfunded research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda. Hermiller is a consultant for Abbott. Neumann reports that his institution has received research grants, consultancy fees, and speaker honoraria from Daiichi Sankyo, Astra Zeneca, Sanofi‐Aventis, Bayer, The Medicines Company, Bristol, Novartis, Roche, Boston Scientific, Biotronik, Medtronic, and Edwards. Kozuma serves as an advisory board member for Abbott Vascular Japan and receives honorarium for lectures. Ruster and Wang are employees of Abbott Vascular. All 4 registries included for this pooled analysis were funded by Abbott. The remaining authors have no disclosures to report.
Supporting information
Tables S1‐S9
(J Am Heart Assoc. 2020;9:e014611 DOI: 10.1161/JAHA.119.014611.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.014611
For Sources of Funding and Disclosures, see page 9.
References
- 1. Chieffo A, Hoye A, Mauri F, Mikhail GW, Ammerer M, Grines C, Grinfeld L, Madan M, Presbitero P, Skelding KA. Gender‐based issues in interventional cardiology: a consensus statement from the Women in Innovations (WIN) Initiative. Catheter Cardiovasc Interv. 2010;75:145–152. [DOI] [PubMed] [Google Scholar]
- 2. Yu J, Mehran R, Grinfeld L, Xu K, Nikolsky E, Brodie BR, Witzenbichler B, Kornowski R, Dangas GD, Lansky AJ, et al. Sex‐based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS‐AMI trial. Catheter Cardiovasc Interv. 2015;85:359–368. [DOI] [PubMed] [Google Scholar]
- 3. Hess CN, McCoy LA, Duggirala HJ, Tavris DR, O'Callaghan K, Douglas PS, Peterson ED, Wang TY. Sex‐based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE‐ACS. J Am Heart Assoc. 2014;3:000523 DOI: 10.1161/JAHA.113.000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batchelor W, Kandzari DE, Davis S, Tami L, Wang JC, Othman I, Gigliotti OS, Haghighat A, Singh S, Lopez M, et al. Outcomes in women and minorities compared with white men 1 year after everolimus‐eluting stent implantation: insights and results from the PLATINUM diversity and PROMUS element plus post‐approval study pooled analysis. JAMA Cardiol. 2017;2:1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandrasekhar J, Baber U, Sartori S, Faggioni M, Aquino M, Kini A, Weintraub W, Rao S, Kapadia S, Weiss S. Sex‐related differences in outcomes among men and women under 55 years of age with acute coronary syndrome undergoing percutaneous coronary intervention: results from the PROMETHEUS study. Catheter Cardiovasc Interv. 2017;89:629–637. [DOI] [PubMed] [Google Scholar]
- 6. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, et al. Coronary thrombosis and major bleeding after PCI with drug‐eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
- 7. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrie D, Naber C, Lipiecki J, Richardt G, Iniguez A, Brunel P, et al. Polymer‐free drug‐coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–2047. [DOI] [PubMed] [Google Scholar]
- 8. Ariotti S, Adamo M, Costa F, Patialiakas A, Briguori C, Thury A, Colangelo S, Campo G, Tebaldi M, Ungi I. Is bare‐metal stent implantation still justifiable in high bleeding risk patients undergoing percutaneous coronary intervention?: a pre‐specified analysis from the ZEUS trial. JACC Cardiovasc Interv. 2016;9:426–436. [DOI] [PubMed] [Google Scholar]
- 9. Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrie D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, et al. Drug‐eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single‐blind trial. Lancet. 2018;391:41–50. [DOI] [PubMed] [Google Scholar]
- 10. Krucoff MW, Rutledge DR, Gruberg L, Jonnavithula L, Katopodis JN, Lombardi W, Mao VW, Sharma SK, Simonton CA, Tamboli HP, et al. A new era of prospective real‐world safety evaluation primary report of XIENCE V USA (XIENCE V Everolimus Eluting Coronary Stent System condition‐of‐approval post‐market study). JACC Cardiovasc Interv. 2011;4:1298–1309. [DOI] [PubMed] [Google Scholar]
- 11. Seth A, Patel TM, Stuteville M, Kumar R, Mullasari AS, Kaul U, Mathew R, Kumar AS, Ying SW, Sudhir K. Three‐year data from the XIENCE V® INDIA study: safety and efficacy of XIENCE V® in 1000 real world Indian patients. Indian Heart J. 2014;66:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aoki J, Kozuma K, Awata M, Nanasato M, Shiode N, Tanabe K, Yamaguchi J, Kusano H, Nie H, Kimura T, et al. Five‐year clinical outcomes of everolimus‐eluting stents from the post marketing study of CoCr‐EES (XIENCE V/PROMUS) in Japan. Cardiovasc Interv Ther. 2019;34:40–46. [DOI] [PubMed] [Google Scholar]
- 13. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40:2632–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, Ngu Blackett K, Lisheng L; infarction WgobotpeotWcfroWdom . World Health Organization definition of myocardial infarction: 2008–09 revision. Int J Epidemiol. 2010;40:139–146. [DOI] [PubMed] [Google Scholar]
- 15. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 16. Kovacic JC, Mehran R, Karajgikar R, Baber U, Suleman J, Kim MC, Krishnan P, Dangas G, Sharma SK, Kini A. Female gender and mortality after percutaneous coronary intervention: results from a large registry. Catheter Cardiovasc Interv. 2012;80:514–521. [DOI] [PubMed] [Google Scholar]
- 17. Lansky AJ, Mehran R, Cristea E, Parise H, Feit F, Ohman EM, White HD, Alexander KP, Bertrand ME, Desmet W. Impact of gender and antithrombin strategy on early and late clinical outcomes in patients with non–ST‐elevation acute coronary syndromes (from the ACUITY trial). Am J Cardiol. 2009;103:1196–1203. [DOI] [PubMed] [Google Scholar]
- 18. Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient‐level pooled analysis of the REPLACE‐2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS‐AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654–664. [DOI] [PubMed] [Google Scholar]
- 20. Romano S, Buccheri S, Mehran R, Angiolillo DJ, Capodanno D. Gender differences on benefits and risks associated with oral antithrombotic medications for coronary artery disease. Expert Opin Drug Saf. 2018;17:1041–1052. [DOI] [PubMed] [Google Scholar]
- 21. Sorrentino S, Baber U, Claessen BE, Camaj A, Vogel B, Sartori S, Guedeney P, Chandrasekhar J, Farhan S, Barman N, et al. Determinants of significant out‐of‐hospital bleeding in patients undergoing percutaneous coronary intervention. Thromb Haemost. 2018;118:1997–2005. [DOI] [PubMed] [Google Scholar]
- 22. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, et al. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Birgelen C, Kok MM, van der Heijden LC, Danse PW, Schotborgh CE, Scholte M, Gin RMTJ, Somi S, van Houwelingen K, Stoel Mh. Very thin strut biodegradable polymer everolimus‐eluting and sirolimus‐eluting stents versus durable polymer zotarolimus‐eluting stents in allcomers with coronary artery disease (BIO‐RESORT): a three‐arm, randomised, non‐inferiority trial. The Lancet. 2016;388:2607–2617. [DOI] [PubMed] [Google Scholar]
- 25. Mehilli J, Kastrati A, Dirschinger J, Pache J, Seyfarth M, Blasini R, Hall D, Neumann FJ, Schomig A. Sex‐based analysis of outcome in patients with acute myocardial infarction treated predominantly with percutaneous coronary intervention. JAMA. 2002;287:210–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S9


