Abstract
Background
Previous studies reported that early‐life exposure to undernutrition is associated with the risk of diabetes mellitus and metabolic syndrome in adulthood, but the association with risk of cardiovascular disease (CVD) later in life remains unclear. The current study aimed to investigate whether exposure to Chinese famine in early life is associated with risk of CVD.
Methods and Results
We used data from REACTION (Risk Evaluation of Cancers in Chinese Diabetic Individuals: A Longitudinal Study), which recruited a total of 259 657 community‐dwelling adults aged 40 years or older from 25 centers across mainland China between 2011 and 2012. Compared with the nonexposed participants, those who had been exposed to famine in early life had a significantly increased risk of total CVD, myocardial infarction, stroke, and coronary heart disease. In the multivariable‐adjusted logistic regression model, the odds ratios (95% CI) for total CVD, myocardial infarction, stroke, and coronary heart disease in fetal famine exposure were 1.35 (1.20–1.52), 1.59 (1.08–2.35), 1.40 (1.11–1.78), and 1.44 (1.26–1.65), respectively; those odds ratios in childhood famine exposure were 1.59 (1.40–1.81), 2.20 (1.52–3.20), 1.82 (1.45–2.28), and 1.80 (1.56–2.09), respectively; and those in adolescent famine exposure were 1.52 (1.27–1.81), 2.07 (1.28–3.35), 1.92 (1.42–2.58), and 1.83 (1.50–2.24), respectively. The main finding of our study is that, compared with those who lived in the less severely affected famine area, individuals in the severely affected famine area had significantly increased risk of total CVD in all 3 exposed groups.
Conclusions
Early‐life exposure to undernutrition is associated with significantly increased risk of CVD in later life, especially among those who were in the severely affected famine area.
Keywords: association, cardiovascular diseases, early‐life exposure, famine
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
This large population‐based study found that undernutrition in early life is associated with increased risk of developing total cardiovascular disease, myocardial infarction, stroke, and coronary heart disease.
These findings have important epidemiological and clinical implications.
To our knowledge, this is the largest population‐based study ever done investigating the association between early‐life famine exposure and cardiovascular disease risks in adulthood.
What Are the Clinical Implications?
Early‐life exposure to undernutrition might have significant effects on the risk of cardiovascular disease in the adulthood.
Our findings highlight the importance of early‐life nutrition in the prevention of adult cardiovascular diseases.
Nonstandard Abbreviations and Acronyms.
BMI body mass index
CHD coronary heart disease
CVD cardiovascular disease
DBP diastolic blood pressure
HDL‐C high‐density lipoprotein cholesterol
LDL‐C low‐density lipoprotein cholesterol
MetS metabolic syndrome
MI myocardial infarction
OR odds ratio
SBP systolic blood pressure
TC total cholesterol
TG triglycerides
Introduction
In the past decades, cardiovascular disease (CVD) has surpassed infectious diseases to become the leading cause of death in China.1 Thus, investigation into the risk factors for CVD is imperative and essential for the improvement of human health. Obesity and lifestyle factors such as smoking, drinking, diet, and lack of physical activity as well as metabolic syndrome (MetS)2 and diabetes mellitus are established risk factors for CVD.3 Experimental studies using animal models have provided evidence for an association of undernutrition exposure and metabolic disorders.4 However, such experiments cannot be performed on humans. Accidentally, however, historical famine cohorts have given us similar conditions to explore the influence of early‐life undernutrition on common noncommunicable diseases in adulthood. The Chinese famine of 1959‐1962 had a long duration, and most of the Chinese mainland was affected.5
Previous studies have indicated that severe malnutrition exposure in early life may influence the risk of common noncommunicable diseases such as diabetes mellitus and MetS later in life.6, 7, 8, 9, 10, 11 However, the association between early‐life exposure to famine and risk of CVD later in life remains unclear. The study conducted by van Abeelen et al found that stronger famine exposure was associated with higher coronary heart disease (CHD) risk and a lower risk of stroke in a Dutch famine cohort.12 Results from a cohort of the Chinese famine in 1959‐1962 reported that early‐life exposure to the Chinese famine exacerbated the association between hypertension and CVD.13 However, results from a cohort of the siege of Leningrad did not observe a direct effect of early‐life famine on the prevalence of CVD.14 Ekamper et al did not found a significant association between prenatal famine exposure and CVD risk.15 Nevertheless, the Chinese famine affected a much wider area and larger population than the Dutch famine or the siege of Leningrad, which provided us an opportunity to conduct a study with greater statistical power on this issue.
Accordingly, we took advantage of the large representative sample of Chinese adults who participated in REACTION (Risk Evaluation of Cancers in Chinese Diabetic Individuals: A Longitudinal) Study to examine the association between early‐life exposure to famine and the risk of CVD later in life among different areas of China. To our knowledge, this is the largest epidemiological study ever done concerning this issue and covered the largest areas of China and the affected population.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of ethical and data‐protective legislation. The REACTION study has a website (http://www.rjh.com.cn/pages/neifenmike/REACTION).15 The data can only be accessed by members of the REACTION group. It is our goal ultimately to share the REACTION study data; however, at this time, we are unable to do so.
Study Population
The REACTION study is a nationwide prospective observational study. The design and methods of the REACTION study have been described previously.16, 17 Briefly, a total of 259 657 community‐dwelling adults, aged 40 years or older from 25 centers across mainland China were recruited to participate in the baseline survey during 2011 to 2012. Eligible study participants were identified according to the local residence registration records and aged 40 years or older. There was no participation restriction on sex or ethnicity. Each eligible participant was approached by trained local community workers using a door‐to‐door invitation method. Those who agreed to participate and signed their informed consent were scheduled for a personal interview and a clinic visit within a week after the recruitment. The clinic visit was performed at a local health station or community clinic in the participant's residential area. The current study presented the baseline data. After exclusion of participants born before January 1, 1941 (n=25 438), a total of 234 219 participants remained in the current analysis. The study was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao‐Tong University. Written informed consent was obtained from all participants.
Participants were categorized into 4 groups: nonexposed or exposed at a fetal stage, childhood, or adolescence, according to birth date as shown in Figure S1.7, 18, 19, 20 To control the confounding effect of age, we further conducted sensitivity analysis using finer age‐group categories.
Data Collection
All questionnaire data collection and anthropometric measurements were performed by trained staff according to a standard protocol at local health stations or community clinics at each study center. Using a detailed questionnaire, we collected information on sociodemographic characteristics and lifestyle factors and medical histories through personal interviews. Educational levels were divided into high school or above versus less than high school. Participants were classified as never, former, or current smokers according to cigarette‐smoking habits. The type and frequency of alcohol consumption were recorded. Information on intensity, duration, and frequency of physical activity was gathered using the short form of the International Physical Activity Questionnaire, and the metabolic equivalent time per week was used to estimate physical activities (1 metabolic equivalent hour per week represents the energy expenditure for an individual at rest). In the dietary section of the questionnaire, data were obtained regarding usual dietary intake over the past 12 months. The questionnaire was designed to capture information on frequency and quantity of major food items such as red meat, fruits and vegetables, dairy, and Chinese traditional food such as pickles and salty vegetables. The dietary questionnaire has previously been evaluated and validated in other cohort studies.21
Height and weight were measured to the nearest 0.1 kg and 0.1 cm separately with participants wearing light‐weight clothes and no shoes. Body mass index (BMI) was calculated by dividing weight (in kilograms) by weight (in meters) squared. Waist circumference was determined using the measuring tape positioned midway between the lowest rib and the superior border of the iliac crest as the patient exhaled normally. Blood pressure was the average of 3 measurements separated by 1‐minute intervals after at least a 5‐minute rest performed on the nondominant arm using an automated electronic device (Omron Model HEM‐725 FUZZY; Omron Co, Dalian, China).
Biochemical Evaluation
A blood draw was administered in the morning after an overnight fast of at least 10 hours. Sera were aliquoted into 0.5‐mL Eppendorf tubes within 2 hours after blood collection and shipped in dry ice at −80°C to the central laboratory located at the Shanghai Institute of Endocrine and Metabolic Diseases, which is certified by the College of American Pathologists. Levels of serum creatinine, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglycerides were measured on an autoanalyzer (c16000 system, ARCHITECT ci16200 analyzer; Abbott Laboratories, Lake Bluff, IL) in the central laboratory. Fasting insulin was measured with chemiluminescent immunoassay (i2000SR system, Architect ci16200 analyzer; Abbott Laboratories). The levels of glycated hemoglobin were assayed by means of high‐performance liquid chromatography method (Variant II and D‐10 Systems; Bio‐Rad, Hercules, CA).
Definitions
With an interviewer‐assisted questionnaire, we collected information on CVDs. The question was open‐ended: “Has a doctor or other health professional ever told you that you have CHD, stroke, or myocardial infarction (MI)?” We grouped CVDs (reported CHD, stroke, or MI) in the analysis. Validation of the self‐reported CVD was performed in Shanghai Youyi Community, 1 of the 25 communities. Two physicians who were blinded to the self‐reported data reviewed the medical records from the relevant hospitals and classified the cases as definite, questionable, or misdiagnosed. The validation rate of the self‐reported CVD was 91.1%.22, 23
The severity of the Chinese famine of the 1950s was regionally different. The excess death rate was calculated as the percentage change in mortality rate from the average level in 1956‐1958 to the highest value over the period of 1959‐1961.24 As described in previous studies,6, 25 an excess mortality rate of 100%24 was used as a threshold value to define the severely or less severely affected famine area in the current study.
MetS was defined according to the National Cholesterol Education Program Adult Treatment Panel III criteria.26 Individuals with any 3 or more of the following conditions were defined as having MetS: (1) systolic/diastolic blood pressure ≥130/85 mm Hg or taking antihypertensive drugs; (2) waist circumference ≥102 cm in men or ≥88 cm in women; (3) triglycerides ≥1.69 mmol/L; (4) high‐density lipoprotein cholesterol <1.03 mmol/L in men or <1.29 mmol/L in women; and/or (5) fasting blood glucose ≥5.6 mmol/L or taking glucose‐lowering medications.26
Statistical Analysis
Data on the basic characteristics were presented as mean±SD or median (interquartile range) for continuous variables and proportion for categorical variables, respectively. Multivariable adjusted logistic regression analysis was used to evaluate the odds ratio and 95% CI of famine exposure in early life and risk of CVD as an adult. The multivariable model was adjusted for age and sex in model 1 and further adjusted for BMI, educational status (high school and above, yes/no), smoking and drinking status (never/former/current), physical activity (moderate to vigorous/none to mild), famine severity (severely affected famine area/less severely affected famine area), healthy diet (yes/no) in model 2. In model 3, MetS (yes/no) was further adjusted based on model 2. In sensitivity analysis we added other social economic factors such as area (rural/urban), economic status (high/low), marriage status (married/unmarried/cohabitation/divorced/separated/widowed/other), occupation (worker/farmer/soldier/medical personnel/self‐employed/business person/government official/housewife/retired/other) in the adjusted model. The economic status was assessed by the mean annual income in the year of our baseline survey (2010), which was treated as a dichotomous variable. The mean level of the current sample (41 890 Chinese yuan per individual per year) was used as a cutoff.27 A 2‐sided P<0.05 was considered statistically significant. The P value for interaction was calculated by a likelihood ratio test comparing models with and without the interaction terms.
Because age is a significant risk factor for CVD and there is a significant difference in age distribution between the famine‐exposed and nonexposed groups, we conducted several sensitivity tests to overcome the confounding effect of age. First, we evaluated the association of famine exposure severity with CVD risk within the same age groups using individuals from the less‐affected area as the reference group. Second, we performed an analysis using the relatively older and younger age‐group combination as the reference to test whether fetal and childhood exposure to famine truly affected the risk of CVD later in life, which neutralized the age gap between the nonexposed group and the exposed groups in the main analysis. Third, we performed an analysis using finer classification of the exposure period to reduce the age differences among groups.
All statistical analyses were performed using SAS (version 9.4, SAS Institute Inc, Cary, NC).
Results
At baseline, among the 234 219 middle‐aged and elderly Chinese participants, a total of 12 561 (5.36%) participants had total CVD, including 884 (0.38%) with MI, 2913 (1.24%) with stroke, and 9606 (4.10%) with CHD. The mean age of the study population was 55.5±7.9 years, and 66.2% of participants were women. The basic characteristics of the included participants are shown in Table 1. There are significant differences in age, sex, BMI, current smoking and drinking status, physical activity, educational status, blood pressure, and lipid profiles between groups (all P<0.05). The study population selection and classification are presented in Figure S1.
Table 1.
Association Between Famine Exposure and Cardiovascular Disease Risk
| All Participants (N=234 219) | Nonexposed (1963–1974) N=54 525 | Fetal‐Exposed Cohorts (1959–1962) N=29 387 | Childhood‐Exposed Cohorts (1949–1958) N=102 370 | Adolescent‐Exposed Cohorts (1941–1948) N=47 937 | P Value |
|---|---|---|---|---|---|
| Age, y | 44.78±2.82 | 50.67±1.42 | 57.56±2.92 | 66.24±2.40 | <0.0001 |
| Male, n (%) | 17 514 (32.1) | 8604 (29.3) | 34 117 (33.3) | 18 886 (39.4) | <0.0001 |
| BMI, kg/m2 | 24.41±3.61 | 24.67±3.49 | 24.74±3.58 | 24.82±3.62 | <0.0001 |
| Current smoker, n (%) | 8416 (15.4) | 4359 (14.8) | 14 826 (14.5) | 5927 (12.4) | <0.0001 |
| Current drinker, n (%) | 5557 (10.2) | 2834 (9.6) | 10 103 (9.9) | 4417 (9.2) | <0.0001 |
| Physical activity (moderate to vigorous), n (%) | 5781 (10.6) | 3514 (12.0) | 13 428 (13.1) | 6427 (13.4) | <0.0001 |
| Educational status (high school or above), n (%) | 22 910 (42.0) | 15 810 (53.8) | 32 432 (31.7) | 13 978 (29.2) | <0.0001 |
| SBP, mm Hg | 124±18 | 128±19 | 133±20 | 140±21 | <0.0001 |
| DBP, mm Hg | 77±12 | 79±11 | 79±11 | 78±11 | <0.0001 |
| TG, mmol/L | 1.17 (0.83–1.75) | 1.28 (0.91–1.89) | 1.36 (0.97–1.96) | 1.35 (0.98–1.92) | <0.0001 |
| HDL‐C, mmol/L | 1.32±0.35 | 1.35±0.36 | 1.34±0.36 | 1.32±0.36 | 0.01 |
| LDL‐C, mmol/L | 2.64±0.80 | 2.88±0.85 | 2.95±0.87 | 2.91±0.88 | <0.0001 |
| TC, mmol/L | 4.64±1.07 | 4.96±1.11 | 5.06±1.12 | 5.00±1.13 | <0.0001 |
Data were shown as mean±SD or median (interquartile range). BMI indicates body mass index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; and TG, triglycerides.
Basically, compared with the nonexposed participants, those who were exposed fetally, in childhood, or as adolescents had significantly increased risk of prevalent total CVD, MI, stroke, and CHD. After adjustment for age and other important related confounders, the odds ratios (95% CIs) for total CVD, MI, stroke, and CHD following fetal famine exposure were, respectively, 1.35 (1.20–1.52), 1.59 (1.08–2.35), 1.40 (1.11–1.78), and 1.44 (1.26–1.65); following childhood famine exposure, these were 1.59 (1.40–1.81), 2.20 (1.52–3.20), 1.82 (1.45–2.28), and 1.80 (1.56–2.09); and after adolescent famine exposure these were 1.52 (1.27–1.81), 2.07 (1.28–3.35), 1.92 (1.42–2.58), and 1.83 (1.50–2.24), respectively (Table 2). Further adjustment for other socioeconomic factors such as area, marriage status, occupation, and economic status did not significantly change the results (Table S1).
Table 2.
Odds Ratio (95% CI) for Cardiovascular Disease Risks According to Famine Exposure
| All Participants (N=234 219) | Nonexposed (1963–1974) N=54 525 | Fetal‐Exposed Cohorts (1959–1962) N=29 387 | Childhood‐Exposed Cohorts (1949–1958) N=102 370 | Adolescent‐Exposed Cohorts (1941–1948) N=47 937 |
|---|---|---|---|---|
| Total CVD, cases (%) | 593 (1.09) | 750 (2.55) | 5836 (5.70) | 5382 (11.23) |
| Model 1* | 1.00 (ref.) | 1.43 (1.27–1.60) | 1.72 (1.52–1.95) | 1.65 (1.39–1.97) |
| Model 2† | 1.00 (ref.) | 1.34 (1.20–1.51) | 1.60 (1.41–1.82) | 1.51 (1.26–1.80) |
| Model 3‡ | 1.00 (ref.) | 1.35 (1.20–1.52) | 1.59 (1.40–1.81) | 1.52 (1.27–1.81) |
| MI, cases (%) | 55 (0.10) | 62 (0.21) | 442 (0.43) | 325 (0.68) |
| Model 1* | 1.00 (ref.) | 1.66 (1.14–2.41) | 2.31 (1.63–3.26) | 2.26 (1.46–3.51) |
| Model 2† | 1.00 (ref.) | 1.65 (1.13–2.41) | 2.23 (1.56–3.18) | 2.13 (1.35–3.34) |
| Model 3‡ | 1.00 (ref.) | 1.59 (1.08–2.35) | 2.20 (1.52–3.20) | 2.07 (1.28–3.35) |
| Stroke, cases (%) | 145 (0.27) | 170 (0.58) | 1335 (1.30) | 1263 (2.63) |
| Model 1* | 1.00 (ref.) | 1.49 (1.19–1.88) | 2.02 (1.63–2.51) | 2.22 (1.68–2.95) |
| Model 2† | 1.00 (ref.) | 1.42 (1.12–1.79) | 1.88 (1.50–2.36) | 1.99 (1.47–2.68) |
| Model 3‡ | 1.00 (ref.) | 1.40 (1.11–1.78) | 1.82 (1.45–2.28) | 1.92 (1.42–2.58) |
| CHD, cases (%) | 422 (0.77) | 561 (1.91) | 4452 (4.35) | 4171 (8.70) |
| Model 1* | 1.00 (ref.) | 1.53 (1.34–1.75) | 1.97 (1.71–2.27) | 2.00 (1.65–2.44) |
| Model 2† | 1.00 (ref.) | 1.43 (1.25–1.64) | 1.81 (1.57–2.09) | 1.81 (1.48–2.20) |
| Model 3‡ | 1.00 (ref.) | 1.44 (1.26–1.65) | 1.80 (1.56–2.09) | 1.83 (1.50–2.24) |
BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; MI, myocardial infarction; and ref., defined as reference.
Model 1 adjusted for age, sex.
Model 2 further adjusted for BMI, educational status, smoking and drinking status, physical activity, famine severity, healthy diet (yes/no).
Model 3 further adjusted for metabolic syndrome (yes/no).
In our main analysis we performed comparisons within the same age groups between individuals from the less severely affected famine area and the severely affected famine area. In all 3 famine‐exposed groups, compared with those who lived in the less severely affected famine area, individuals in the severely affected famine area had significantly increased risk of total CVD (Figure 1). Moreover, the association was much greater in the childhood‐exposed group for the risk of having MI and stroke, and in the fetally exposed group for the risk of having CHD (Figure 1). After adjustment for other socioeconomic factors, the results did not change significantly (Table S2). The sex stratification analysis is shown in Figure S2.
Figure 1. Comparison between less severely affected individuals and severely affected individuals for the risk of total cardiovascular disease, myocardial infarction, stroke, and coronary heart disease, respectively.
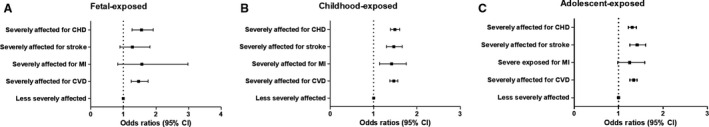
The classification was based on the famine exposure period and duration: fetal‐exposed (A), childhood‐exposed (B), and adolescent‐exposed (C). The model adjusted for age, sex, BMI, educational status, smoking and drinking status, physical activity, healthy diet (yes/no), and metabolic syndrome (yes/no). The black square represents the odds ratio of the risk estimation, and the bar represents its 95% CI. BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; and MI, myocardial infarction.
In the stratification analysis (Figure 2), the association was stronger in women, non–current smokers, non–current drinkers, those who were without moderate to vigorous physical activity, and those who were without hypertension, dyslipidemia, MetS, or diabetes mellitus among all 3 groups. A healthy diet, BMI, educational status, and location in China did not show obvious differences. There exists significant interaction of sex, BMI, physical activity, diabetes mellitus, hypertension, MetS, and location in China with famine exposure (all P for interaction<0.05).
Figure 2. Stratification analysis of famine exposure and risk of CVD according to basic factors including age, sex, smoking and drinking status, physical activity, diet status, educational status, diabetes mellitus (no/yes), hypertension (no/yes), dyslipidemia (no/yes), metabolic syndrome (no/yes), and north/south of China in fetal‐exposed (A), childhood‐exposed (B), and adolescent‐exposed (C) groups, respectively.
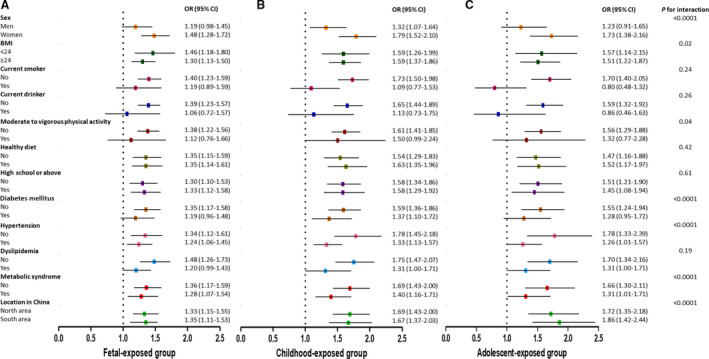
Different colors are used to distinguish the stratification factors. The model is adjusted for age, sex, BMI, educational status, smoking and drinking status, physical activity, famine severity, healthy diet (yes/no), and metabolic syndrome (yes/no). The square in the middle represents the odds ratio of the risk estimation, and the bar represents its 95% CI. BMI indicates body mass index; CVD, cardiovascular disease; OR, odds ratio.
When a finer classification of the exposure duration was made, the results remained similar (Figure 3). Individuals who were exposed to famine in late childhood (born in 1949‐1951) were associated with the highest risk of total CVD and MI (Figure 3). The sex stratification analysis is shown in Figure S3. An age‐balance analysis using the relatively older and younger age group combination as the reference showed that childhood‐exposed groups were significantly associated with an increased risk of total CVD, MI, stroke, and CHD (Table S3). A mediation analysis indicated that part of the impact of famine exposure on CVD is mediated via MetS (Figure S4).
Figure 3. Finer classification according to famine exposure duration in the estimation of risk for total cardiovascular disease (A), myocardial infarction (B), stroke (C), and coronary heart disease (D), respectively.
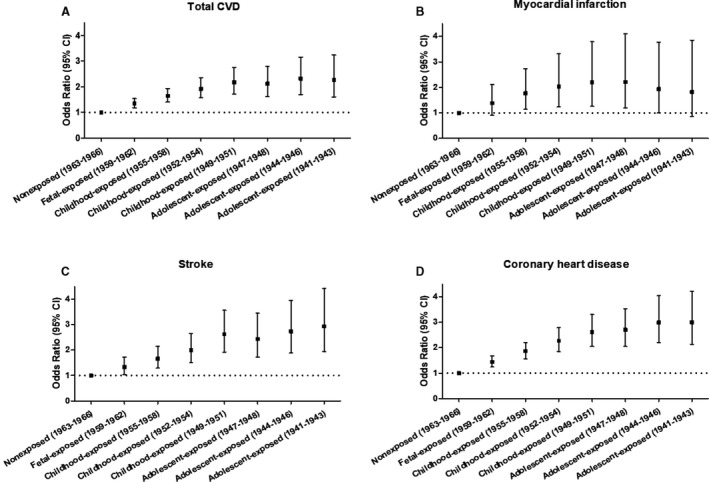
Finer classification of the exposure duration including 8 groups: (1) born between January 1, 1959 and December 31, 1962, fetal exposed (n=29 387); (2) born between January 1, 1955 and December 31, 1958, childhood exposed (n=41 929); (3) born between January 1, 1952 and December 31, 1954, childhood exposed (n=32 994); (4) born between January 1, 1949 and December 31, 1951, childhood exposed (n=27 447); (5) born between January 1, 1947 and December 31, 1948, adolescent exposed (n=15 198); (6) born between January 1, 1944 and December 31, 1946, adolescent exposed (n=18 040); (7) born between January 1, 1941 and December 31, 1943, adolescent exposed (n=14 699); (8) born between January 1, 1963 and December 31, 1966, nonexposed (n=29 738). The model adjusted for age, sex, BMI, educational status, smoking and drinking status, physical activity, famine severity, healthy diet (yes/no), and metabolic syndrome (yes/no). The black square represents the odds ratio of the risk estimation, and the bar represents its 95% CI. BMI indicates body mass index; CVD, cardiovascular disease.
Discussion
This large population‐based study found that early‐life exposure to undernutrition is associated with an increased risk of total CVD, MI, stroke, and CHD, and this association might be partly mediated via MetS. To our knowledge, this is the largest population‐based study ever done to investigate the association between early‐life famine exposures and CVD risks later in life among a Chinese population. These findings have important epidemiological and clinical implications.
The hypothesis that early‐life (preadult) factors may have a role in the pathophysiology of metabolic diseases in adulthood has attracted substantial research interest, particularly in the past 2 decades.7, 25, 28, 29, 30 However, data on the association between early‐life famine exposure with CVD risks remain limited and inconclusive.12, 13, 14, 15, 31, 32 A study on the Dutch famine with a total of 736 participants found that the prevalence of CHD was higher in those exposed in early gestation than in nonexposed people.31 Another study of 7845 women in the Dutch famine found that severe exposure to famine was significantly associated with increased risk of CHD but that the association was attenuated after adjustment for confounders.12 Two other studies on the Dutch famine did not find significant associations of famine exposure with coronary artery disease risk, Framingham risk,32 or subsequent mortality from CVD.15 A retrospective study conducted among the survivors of the Leningrad siege with a small sample size (n=356) found no significant differences in the prevalence of cardiovascular diseases between the survivors and their age‐ and sex‐matched controls.14 In a Chinese population, Shi et al previously reported that early‐life exposure to the Chinese famine exacerbated the association between hypertension and CVD.13 In the current nationwide epidemiology study we provided evidence supporting the association between early‐life famine exposure and CVD risk in later life. It is worth mentioning that the negative findings from the above studies might partly be explained by their relatively small sample sizes and the limited numbers of events.
More importantly, our findings suggest that the association between early‐life famine exposure and CVD risk in later life might be mediated via MetS. Both type 2 diabetes mellitus and MetS are important risk factors for CVD, and, in turn, CVD is the main macrovascular complication of diabetes mellitus.33, 34 Early‐life exposure to famine has been shown to be related to the risk of diabetes mellitus7, 8, 9, 19 and MetS6, 20, 25, 29 in epidemiological studies. As shown in previous studies, the severe malnutrition exposure in early life could permanently alter the structure and/or function of the heart, which could elevate the risks of MetS as well as CVD.35 In a Dutch study researchers found that early‐life malnutrition might cause less physical activity and more intake of fat, which could lead to elevated total cholesterol and triglyceride levels.36 The elevated atherogenic lipid profiles might increase the risk of CVD in later life.
The mechanism linking early‐life exposure and CVD risks is not well understood. Experimental studies have shown that extreme maternal dietary restriction results in a reduced life expectancy37 and a higher prevalence of hypertension,38 obesity39 and diabetes mellitus38 in the offspring. It has been proposed that early‐life malnutrition because of famine causes epigenetic changes that persist throughout life, which lead to the structural changes in the cardiovascular system.35 This hypothesis is supported by the report that the survivors of severe acute malnutrition in childhood had smaller left ventricular outflow tract diameter, stroke volume, and cardiac output.40 Moreover, findings from the Dutch famine study demonstrate that exposure to famine in early life increased the preference for fatty food and the prevalence of dyslipidemia,41 which in turn could promote the development of CVD.3 In the current study we observed that the influence of early‐life undernutrition is unlikely to be compensated by healthy lifestyle factors during adulthood. Among those following a healthy lifestyle, including healthy diet, no smoking or drinking, higher levels of education, and normal weight, early‐life famine exposure still imposes an increased risk of CVD. These findings are similar to those of earlier studies13, and they highlight the importance of early‐life nutrition in the prevention of adult chronic diseases. In other respects the clustering of multimorbidity may also be different across famine‐exposure groups. In the current study we found that the prevalence of obesity, hypertension, diabetes mellitus, and dyslipidemia were higher in the exposed group. Just as an early life exposed to the Chinese famine was associated with diabetes mellitus and obesity, and we also found that part of the famine effect on CVD is mediated via MetS, the increased risk of CVD among those exposed to famine may be because of the clustering of multimorbidity. However, in stratified analysis, early‐life exposure to famine is associated with increased risk of CVD both in individuals with or without metabolic syndrome. The relationship between famine and CVD might not be fully explained through the MetS pathway alone.
What needs to be mentioned is the sex difference in the current study. In the stratified analysis we observed sex differences in the association of famine exposure and risk of having CVD. The association was more prominent among women in all 3 exposure groups. As suggested by a previous study, a male‐sex–preference culture in China may have masked the true health impact of a famine on men42; thus, more significant long‐term effects of famines and other adversities on women than on men are often observed in such research. Moreover, the son‐preference culture may lead to a better educational level and health outcome for men.42 In our study the proportion of having high‐school level and above educational status was significantly higher in men compared with women (41.12% versus 33.91%, P<0.0001). On the other hand, women may be more adaptable to famine than men. It has been shown that famine exposure was related to a lower sex ratio leading to more female babies.43 Thus, the increased survival of women may predispose them to CVD risk in adulthood.
Aging has been proposed as an important risk factor for CVD.44 The exposure groups in the famine cohort were classified according to the birth date of the participants, which cannot avoid the difference in age and might add potential confounding in the evaluation.28 To control the confounding effect of age on the association between famine exposure and risk of CVD, we performed several analyses to address this issue. The results were encouraging, and after adjustment for age, the association was not attenuated. Furthermore, we calculated the influence of famine exposure severity on the risk of CVD, MI, stroke, and CHD within the same exposure (age) groups, respectively. The results indicated that severe exposure to famine in early life is associated with significantly increased risk of CVD in adulthood compared with those who were less exposed. Even the finer classification of the age groups showed similar results. The results indicated that the effect of famine exposure on total CVD risk is more prominent among childhood‐ and adolescent‐exposure groups compared with those fetally exposed. The effect for total CVD, stroke, and CHD in late‐childhood–exposed and adolescent‐exposed groups was not obviously different, but for MI, the highest effect was observed in the childhood‐exposed group. In a previous study focusing on the Chinese famine, the childhood and adolescent exposure to famine was also associated with a higher risk of diabetes mellitus compared with fetally exposed individuals, especially in women.19 Given the son‐preference culture in China, when children were exposed to famine, the average welfare of the surviving girls is much worse than that of boys during childhood. Thus, the consequence of preferring sons to daughters may worsen women's health outcomes including those involving glucose and lipid metabolism in later life,42 which lead to a higher risk of having CVD in adulthood. However, the exact mechanism behind these phenomena needs further investigation.
The major strength of the present study is the large, well‐characterized number of cohorts, which guaranteed a large number of cardiovascular disease cases and limited the sampling bias. Importantly, we performed a comparison between participants in the less severely affected famine area and the severely affected famine area within the same age group, which provided much more convincing results in disclosing the effect of famine exposure on CVD risk. In addition, we collected detailed lifestyle risk factors and biochemical tests for the confounding adjustment and stratified analysis. There are several limitations that should be acknowledged in the current study. First, using the birth date of participants to define exposure to famine might have led to a misclassification bias because the Chinese famine did not have a definitive beginning and ending time, which prevented us from precisely classifying the participants by their exposure period. Second, we used the excess death rate of the locations as an index to distinguish the severity of the famine exposure. With this method, other death‐related factors such as bad weather, infection, and natural causes that could also increase the rate of premature death were not fully considered. Third, there existed inevitable age differences among the exposure groups, which could have influenced the risk estimation for CVD. However, results from several sensitivity tests do not support the perception that the observed association of famine exposure with increased risk of CVD was due to age alone. Fourth, the cardiovascular diseases in this study were self‐reported, which leads to an inevitable recall bias. Fifth, we did not collect enough data on birth weight in the current study, and birth weight was reported to be associated with the relationship between famine exposure and risk of CVD later in life. Thus, we could not fully exclude the influence of birth weight on the association. Sixth, because of the cross‐sectional design of the study, we were not able to detect the association between famine exposure and incidence of CVD and its related mortality. Last but not the least, in the current study, we did not have data on the situation of lifestyle factors such as physical activity in the early life of the participants, which might have influenced the development of the heart. Further studies are needed to disclose the exact mechanisms behind the association.
Conclusions
In conclusion, early‐life exposure to famine significantly increased the risk of CVD in adulthood. This association might be partly mediated via MetS. The potential mechanisms behind these associations need to be elucidated in future studies.
Appendix
REACTION Study Group
Steering Committee: Guang Ning (Principal Investigator), National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Yiming Mu, Chinese People's Liberation Army General Hospital, Beijing, China; Jiajun Zhao, Shandong Provincial Hospital affiliated to Shandong University, Jinan, China; Weiqing Wang, National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Chao Liu, Jiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing, China; Yufang Bi, National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Donghui Li, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas; Shenghan Lai, The Johns Hopkins University School of Medicine, Baltimore, MD; Zachary T. Bloomgarden, Icahn School of Medicine at Mount Sinai, New York, NY. Working Group: Weiqing Wang, Yufang Bi, Jieli Lu, National Clinical Research Center for Metabolic Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Yiming Mu, Chinese People's Liberation Army General Hospital, Beijing, China; Jiajun Zhao, Shandong Provincial Hospital affiliated to Shandong University, Jinan, China; Chao Liu, Jiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing, China; Lulu Chen, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Lixin Shi, Affiliated Hospital of Guiyang Medical College, Guiyang, China; Qiang Li, The Second Affiliated Hospital of Harbin Medical University, Harbin, China; Tao Yang, The First Affiliated Hospital with Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China; Li Yan, Sun Yat‐sen Memorial Hospital, Sun Yat‐sen University, Guangzhou, China; Qin Wan, The Affiliated Hospital of Luzhou Medical College, Luzhou, China; Shengli Wu, Karamay Municipal People's Hospital, Xinjiang, China; Guixia Wang, The First Hospital of Jilin University, Changchun, China; Zuojie Luo, The First Affiliated Hospital of Guangxi Medical University, Nanning, China; Xulei Tang, The First Hospital of Lanzhou University, Lanzhou, China; Gang Chen, Fujian Provincial Hospital, Fujian Medical University, Fuzhou, China; Yanan Huo, Jiangxi Provincial People's Hospital Affiliated to Nanchang University, Nanchang, China; Zhengnan Gao, Dalian Municipal Central Hospital, Dalian, China; Qing Su, Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China; Zhen Ye, Zhejiang Provincial Center for Disease Control and Prevention, Zhejiang, China; Youmin Wang, The First Affiliated Hospital of Anhui Medical University, Hefei, China; Guijun Qin, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China; Huacong Deng, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China; Xuefeng Yu, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Feixia Shen, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China; Li Chen, Qilu Hospital of Shandong University, Jinan, China.
Sources of Funding
The research reported in this publication was supported by the Ministry of Science and Technology of the People’s Republic of China (award nos. 2016YFC1305600, 2016YFC1305202, 2016YFC1304904, 2017YFC1310700, and 2018YFC1311800); the National Natural Science Foundation of China (award nos. 81700764, 81670795, 81970691, 81970728, 81800683 and 81561128019), Shanghai municipal health commission (award nos. 201740040), and Shanghai Science and Technology Commission (award nos. 19411964200).
Disclosures
None.
Supporting information
Tables S1–S3 Figures S1–S4
Acknowledgments
The authors thank all study participants.
Author contributions: Du, Yu, Bi, Lu, Ning, and W. Wang conceived and designed the study. Du, Zheng, Zhu analyzed data. M. Xu, Y. Xu, T. Wang, M. Li, Z. Zhao, D. Zhang, Dai, Y. Chen, Tang, Hu, Ye, Shi, Su, Yu, Yan, G. Qin, Wan, G. Chen, Gao, G. Wang, Shen, Luo, Y. Qin, L. Chen, Huo, Q. Li, Y. Zhang, Liu, Y. Wang, Wu, Yang, Deng, L. Chen, J. Zhao, and Mu collected data. All authors were involved in writing and revising the paper and had final approval of the submitted and published versions. Ning and W. Wang are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2020;9:e014175 DOI: 10.1161/JAHA.119.014175.)
Contributor Information
Yufang Bi, Email: byf10784@rjh.com.cn.
Jieli Lu, Email: jielilu@hotmail.com.
the REACTION Study Group:
References
- 1. Qin X, Huo Y, Langman CB, Hou F, Chen Y, Matossian D, Xu X, Wang X. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: a meta‐analysis. Clin J Am Soc Nephrol. 2011;6:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Itani O, Kaneita Y, Tokiya M, Jike M, Murata A, Nakagome S, Otsuka Y, Ohida T. Short sleep duration, shift work, and actual days taken off work are predictive life‐style risk factors for new‐onset metabolic syndrome: a seven‐year cohort study of 40,000 male workers. Sleep Med. 2017;39:87–94. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Wang DD, Ley SH, Howard AG, He Y, Lu Y, Danaei G, Hu FB. Potential impact of time trend of life‐style factors on cardiovascular disease burden in China. J Am Coll Cardiol. 2016;68:818–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin‐angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. [DOI] [PubMed] [Google Scholar]
- 5. Smil V. China's great famine: 40 years later. BMJ. 1999;319:1619–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Jaddoe VW, Qi L, He Y, Wang D, Lai J, Zhang J, Fu P, Yang X, Hu FB. Exposure to the Chinese famine in early life and the risk of metabolic syndrome in adulthood. Diabetes Care. 2011;34:1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang N, Cheng J, Han B, Li Q, Chen Y, Xia F, Jiang B, Jensen MD, Lu Y. Exposure to severe famine in the prenatal or postnatal period and the development of diabetes in adulthood: an observational study. Diabetologia. 2017;60:262–269. [DOI] [PubMed] [Google Scholar]
- 8. Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:787–794. [DOI] [PubMed] [Google Scholar]
- 9. Grabowski E, Letelier RM, Laws EA, Karl DM. Coupling carbon and energy fluxes in the North Pacific subtropical gyre. Nat Commun. 2019;10:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Z, Ji L, Ma RCW, Zimmet P. Early life exposure to 1959–1961 Chinese famine exacerbates association between diabetes and cardiovascular disease. J Diabetes. 2020;12:134–141. [DOI] [PubMed] [Google Scholar]
- 11. Sun Y, Zhang L, Duan W, Meng X, Jia C. Association between famine exposure in early life and type 2 diabetes mellitus and hyperglycemia in adulthood: results from the China Health and Retirement Longitudinal Study (CHARLS). J Diabetes. 2018;10:724–733. [DOI] [PubMed] [Google Scholar]
- 12. van Abeelen AF, Elias SG, Bossuyt PM, Grobbee DE, van der Schouw YT, Roseboom TJ, Uiterwaal CS. Cardiovascular consequences of famine in the young. Eur Heart J. 2012;33:538–545. [DOI] [PubMed] [Google Scholar]
- 13. Shi Z, Nicholls SJ, Taylor AW, Magliano DJ, Appleton S, Zimmet P. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J Hypertens. 2018;36:54–60. [DOI] [PubMed] [Google Scholar]
- 14. Rotar O, Moguchaia E, Boyarinova M, Kolesova E, Khromova N, Freylikhman O, Smolina N, Solntsev V, Kostareva A, Konradi A, et al. Seventy years after the siege of Leningrad: does early life famine still affect cardiovascular risk and aging? J Hypertens. 2015;33:1772–1779; discussion 1779. [DOI] [PubMed] [Google Scholar]
- 15. Ekamper P, van Poppel F, Stein AD, Bijwaard GE, Lumey LH. Prenatal famine exposure and adult mortality from cancer, cardiovascular disease, and other causes through age 63 years. Am J Epidemiol. 2015;181:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bi Y, Lu J, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Wan Q, et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2014;6:147–157. [DOI] [PubMed] [Google Scholar]
- 17. Wu X, Du R, Hu C, Cheng D, Ma L, Li M, Xu Y, Xu M, Chen Y, Li D, et al. Resting heart rate is associated with metabolic syndrome and predicted 10‐year risk of cardiovascular disease: a cross‐sectional study. J Diabetes. 2019;11:884–894. [DOI] [PubMed] [Google Scholar]
- 18. Wang N, Chen Y, Ning Z, Li Q, Han B, Zhu C, Chen Y, Xia F, Jiang B, Wang B, et al. Exposure to famine in early life and nonalcoholic fatty liver disease in adulthood. J Clin Endocrinol Metab. 2016;101:2218–2225. [DOI] [PubMed] [Google Scholar]
- 19. Wang N, Wang X, Han B, Li Q, Chen Y, Zhu C, et al. Is exposure to famine in childhood and economic development in adulthood associated with diabetes? J Clin Endocrinol Metab. 2015;100:4514–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, Chen Y, Lin D, Wang B, Jensen MD, et al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017;36:253–259. [DOI] [PubMed] [Google Scholar]
- 21. Moriarty DG, Zack MM, Kobau R. The Centers for Disease Control and Prevention's Healthy Days Measures—population tracking of perceived physical and mental health over time. Health Qual Life Outcomes. 2003;1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu J, Bi Y, Wang T, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Wan Q, et al. The relationship between insulin‐sensitive obesity and cardiovascular diseases in a Chinese population: results of the REACTION study. Int J Cardiol. 2014;172:388–394. [DOI] [PubMed] [Google Scholar]
- 23. Lu J, Mu Y, Su Q, Shi L, Liu C, Zhao J, Chen L, Li Q, Yang T, Yan L, et al. Reduced kidney function is associated with cardiometabolic risk factors, prevalent and predicted risk of cardiovascular disease in Chinese adults: results from the REACTION study. J Am Heart Assoc. 2016;5:e003328 DOI: 10.1161/JAHA.116.003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo ZMR, Zhang X. Famine and overweight in China. Rev Agric Econ. 2006;28:296–304. [Google Scholar]
- 25. Wang Z, Zou Z, Wang S, Yang Z, Ma J. Chinese famine exposure in infancy and metabolic syndrome in adulthood: results from the China Health and Retirement Longitudinal Study. Eur J Clin Nutr. 2019;73:724–732. [DOI] [PubMed] [Google Scholar]
- 26. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association and National Heart, Lung and Blood Institute . Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, Ma G, Hu FB. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59:2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li C, Tobi EW, Heijmans BT, Lumey LH. The effect of the Chinese famine on type 2 diabetes mellitus epidemics. Nat Rev Endocrinol. 2019;15:313–314. [DOI] [PubMed] [Google Scholar]
- 29. Zheng X, Wang Y, Ren W, Luo R, Zhang S, Zhang JH, Zeng Q. Risk of metabolic syndrome in adults exposed to the great Chinese famine during the fetal life and early childhood. Eur J Clin Nutr. 2012;66:231–236. [DOI] [PubMed] [Google Scholar]
- 30. Hoet JJ. The role of fetal and infant growth and nutrition in the causality of diabetes and cardiovascular disease in later life. SCN News. 1997;14:10–13. [PubMed] [Google Scholar]
- 31. Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder‐Tanka JM, van Montfrans GA, Michels RP, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lumey LH, Martini LH, Myerson M, Stein AD, Prineas RJ. No relation between coronary artery disease or electrocardiographic markers of disease in middle age and prenatal exposure to the Dutch famine of 1944–5. Heart. 2012;98:1653–1659. [DOI] [PubMed] [Google Scholar]
- 33. International Hypoglycaemia Study Group . Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385–396. [DOI] [PubMed] [Google Scholar]
- 34. Guo Y, Musani SK, Sims M, Pearson TA, DeBoer MD, Gurka MJ. Assessing the added predictive ability of a metabolic syndrome severity score in predicting incident cardiovascular disease and type 2 diabetes: the Atherosclerosis Risk in Communities Study and Jackson Heart Study. Diabetol Metab Syndr. 2018;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lumey LH, Stein AD, Kahn HS, Romijn JA. Lipid profiles in middle‐aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. Am J Clin Nutr. 2009;89:1737–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ozanne SE, Hales CN. Lifespan: catch‐up growth and obesity in male mice. Nature. 2004;427:411–412. [DOI] [PubMed] [Google Scholar]
- 38. Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. [DOI] [PubMed] [Google Scholar]
- 39. Breier BH, Vickers MH, Ikenasio BA, Chan KY, Wong WP. Fetal programming of appetite and obesity. Mol Cell Endocrinol. 2001;185:73–79. [DOI] [PubMed] [Google Scholar]
- 40. Tennant IA, Barnett AT, Thompson DS, Kips J, Boyne MS, Chung EE, Chung AP, Osmond C, Hanson MA, Gluckman PD, et al. Impaired cardiovascular structure and function in adult survivors of severe acute malnutrition. Hypertension. 2014;64:664–671. [DOI] [PubMed] [Google Scholar]
- 41. Lussana F, Painter RC, Ocke MC, Buller HR, Bossuyt PM, Roseboom TJ. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am J Clin Nutr. 2008;88:1648–1652. [DOI] [PubMed] [Google Scholar]
- 42. Mu R, Zhang X. Why does the great Chinese famine affect the male and female survivors differently? Mortality selection versus son preference. Econ Hum Biol. 2011;9:92–105. [DOI] [PubMed] [Google Scholar]
- 43. Song S. Does famine influence sex ratio at birth? Evidence from the 1959–1961 Great Leap Forward Famine in China. Proc Biol Sci. 2012;279:2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarink D, Nedkoff L, Briffa T, Shaw JE, Magliano DJ, Stevenson C, Mannan H, Knuiman M, Hung J, Hankey GJ, et al. Trends in age‐ and sex‐specific prevalence and incidence of cardiovascular disease in Western Australia. Eur J Prev Cardiol. 2018;25:1280–1290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3 Figures S1–S4


