Abstract
Background
Concurrent presence of aortic stenosis and aortic regurgitation is termed mixed aortic valve disease (MAVD). Although multiple articles have addressed patients with “isolated” aortic stenosis or aortic regurgitation, the natural history, impact, and outcomes of MAVD are not well defined. Here, we evaluate long‐term outcomes in patients with MAVD and cardiovascular adaptations to chronic MAVD.
Methods and Results
This observational cohort study evaluated 862 adult patients (56.8% male) with preserved left ventricular ejection fraction and at least moderate aortic regurgitation and moderate aortic stenosis. Primary outcome was all‐cause mortality. Subgroup analysis was based on treatment modality (aortic valve replacement [AVR] versus medical management). A regression analysis of longitudinal echocardiographic parameters was performed to assess the natural history of MAVD. Mean age was 68±15 years, and mean left ventricular ejection fraction was 58±5%. At 4.6 years (25th–75th percentile range, 1.0–8.7), 58.6% of patients underwent an AVR and 48.8% patients died. In both unadjusted and adjusted Cox survival analysis, AVR was associated with improved survival (hazard ratio, 0.41; 95% CI, 0.34–0.51, P<0.001). Impact of AVR persisted when stratifying the cohort by symptom status and baseline aortic valve area (log rank, P<0.001 for both) and after propensity‐score matching (hazard ratio, 0.40; 95% CI, 0.32–0.50; P<0.001). In the longitudinal analysis, there were statistically significant changes over time in aortic valve peak gradient (P<0.001) and aortic valve area (P<0.001) and only mild increases in left ventricular end‐diastolic (P<0.007) and ‐systolic (P<0.001) volumes.
Conclusions
MAVD confers a high risk of all‐cause mortality. However, AVR significantly reduces this risk independent of aortic valve area, symptom status, and after controlling for confounding variables.
Keywords: aortic regurgitation, aortic stenosis, mixed aortic valve disease, survival
Subject Categories: Valvular Heart Disease, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Echocardiography
Clinical Perspective
What Is New?
Untreated mixed aortic valve disease (defined as concomitant presence of at least moderate aortic stenosis and regurgitation) is associated with poor prognosis even in the presence of normal ejection fraction.
What Are the Clinical Implications?
Given that aortic valve replacement dramatically improves survival independent of symptoms, early intervention in patients with mixed aortic valve disease may be warranted.
Introduction
Concurrent presence of aortic stenosis (AS) and aortic regurgitation (AR) is termed mixed aortic valve disease (MAVD). Although multiple articles addressed patients with “isolated” AS or AR, the natural history, impact, and outcome of MAVD are not well defined. Decision making regarding treatment with either surgical or transcatheter interventions is extrapolated from evidence from either isolated AS or AR and is usually guided by the dominant lesion.1 Therefore, intervention is usually recommended in the presence of symptoms and specific structural or hemodynamic factors such as left ventricular (LV) ejection fraction (LVEF), aortic valve area (AVA), mean aortic gradient, maximum aortic velocity, and LV volumes.2, 3 However, in MAVD, the development of symptoms and progression of structural and hemodynamic parameters may have a different pattern than in isolated AS or AR. For instance, as previously proposed by our group, if the AS component causes concentric LV hypertrophy, the increase in LV end‐diastolic volume from significant AR leads the left ventricle to fill on a steeper portion of the pressure‐volume curve, potentially causing earlier onset of symptoms than if concomitant AR were not present.4 Concomitantly, AR may also augment the aortic gradient (through increased forward stroke‐volume component) and wall tension. These features may explain why the combination of both lesions may produce hemodynamic compromise in patients in whom neither lesion alone is severe enough to warrant surgery. Consequently, the analysis of long‐term outcomes in this population as well as the recognition of factors that are associated with favorable outcomes could help in a better characterization and understanding of MAVD.
The aims of the present study are to: (1) evaluate the long‐term outcomes and factors associated with outcomes in a contemporary cohort of patients with MAVD with extended follow‐up and (2) evaluate the cardiovascular adaptations to chronic MAVD.
Methods
The authors declare that all supporting data are available within the article.
This was observational retrospective cohort study of patients who satisfied the following criteria: age ≥18 years; LVEF ≥50%; at least moderate AS defined as AVA ≤1.5 cm2 according to 2017 European Association of Cardiovascular Imaging/American Society of Echocardiography Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis5; at least moderate AR according to 2014 American Heart Association/American College of Cardiology Guidelines for the Management of Patients With Valvular Heart Disease6; and clinical evaluation and echocardiographic study at our tertiary care center performed between January 2003 and December 2013 to allow for at least 5 years of follow‐up. A total of 862 patients satisfied these criteria.
The protocol for the present study was approved by the institutional review board of the Cleveland Clinic (Cleveland, OH). Given retrospective nature of the study, the requirement for informed consent was waived.
Baseline characteristics for the current study were manually extracted from electronic medical records at the time of the medical encounter in which the patient was identified to meet the inclusion criteria. Variables included previous diagnoses of hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, obstructive coronary artery disease (CAD), congestive heart failure, smoking, stroke, peripheral vascular disease, chronic renal failure, connective tissue disorder, aortic dissection, radiation heart disease, and previous cardiac surgery. Individual variables, including height, weight, serum hemoglobin, serum creatinine, serum low‐density lipoprotein cholesterol, serum high‐density lipoprotein cholesterol, and serum triglycerides, current medication use, including aspirin, angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), b‐adrenergic blocker, statin, hydralazine, and oral anticoagulation use, and immunosuppressive treatment were recorded.
Echocardiography
Echocardiographic data were obtained using commercially available ultrasound systems. All patients underwent comprehensive examinations, including M‐mode and 2‐dimensional echocardiography and spectral and color Doppler, conducted by an experienced sonographer and interpreted by an echocardiographer using standard criteria.7 We used data from original echocardiographic reports in our analysis, except for the ratio between early mitral flow velocity and the average of the lateral and septal mitral annulus velocities. All ratio between early mitral flow velocity and the average of the lateral and septal mitral annulus velocities measurements were done by a single observer (Y.S.).
Continuous‐wave Doppler examinations were performed to obtain maximum jet velocity. Maximal instantaneous and mean pressure gradients across the aortic valve were calculated using a modified Bernoulli equation. Aortic valve area was calculated using the continuity equation. Moderate AS was defined as AVA >1 cm2 and a mean gradient of 20 to 40 mm Hg. Severe AS was defined as AVA <1 cm2.5
Severity of AR was derived using a multiparametric approach that included jet width in LV outflow tract with color Doppler, jet deceleration rate with continuous‐wave Doppler, presence of diastolic flow reversal in the descending aorta, vena contracta width, jet width/LV outflow tract width percent, and regurgitant volume and fraction.8 We recently published the interobserver variability of mulitparametric approach for assessment of AR severity, as well as its agreement with magnetic resonance imaging.9 The final overall value of kappa for multireader concordance was 0.7, with the correlation between magnetic resonance imaging and consensus AR grading having a correlation coefficient r=0.91.
Other echocardiographic parameters recorded according to the guidelines included LVEF, LV dimensions and volumes, left atrial dimensions, and right ventricular systolic pressure.7
Outcomes
The date of the patient's baseline echocardiogram at our institution was defined as the beginning of the observational period. Patients were followed by chart review with date of last follow‐up or death recorded. Mortality data were obtained from medical records or available obituary databases. Primary outcome was all‐cause mortality. The survival analysis was performed by dividing the sample in 2 predefined subgroups: (1) patients with MAVD who underwent either surgical (AVR) or transcatheter (TAVR) aortic valve replacement (AVR) during the follow‐up period and (2) patients with MAVD who during the entirety of the follow‐up period were medically managed.
Statistical Analysis
Continuous variables were expressed as mean±SD or median and 25th to 75th percentile ranges for skewed distributions and were compared using Student t test or ANOVA (for normally distributed variables) or Mann–Whitney U test (for non‐normally distributed variables). Categorical data are expressed as percentages and were compared using chi‐square or Fisher's exact test, as appropriate. Survival curves were constructed using the Kaplan–Meier method and compared using the log‐rank statistic. Step‐wise multivariable Cox proportional hazards analyses were performed to assess associations between outcomes and clinical and echocardiographic variables. Impact of AVR was modeled as both a standard and time‐dependent covariate. We also tested for possible interactions between AVR and other significant and/or clinically relevant variables, including sex, age, symptoms, and aortic valve area. Table 1 lists all initial parameters entered into the multivariable step‐wise model. To assess for potential impact of AVR after controlling for potential confounding factors that could drive selection for AVR, we calculated a propensity score (PS). The PS was estimated with the use of a nonparsimonious multivariable logistic‐regression model, with AVR as the dependent variable and the following characteristics as covariates: age, sex, hypertension, heart failure, atrial fibrillation, hyperlipidemia, stroke, obstructive CAD, LVEF, diabetes mellitus, Society of Thoracic Surgeons (STS) score, bicuspid aortic valve, peak aortic valve gradient, AVA, LV mass, and AR severity as predictors. Missing data in continuous variables were imputed using simple imputation by robust linear models. There were no missing data in binary variables.
Table 1.
Initial Parameters Entered Into the Multivariable Forward Step‐wise Cox Proportional Hazards Regression Model
| Clinical variables |
| Age |
| Sex |
| Diabetes mellitus |
| Chronic kidney disease |
| Hyperlipidemia |
| Obstructive coronary artery disease |
| Peripheral arterial disease |
| Stroke |
| Atrial fibrillation |
| Hypertension |
| Smoking |
| Connective tissue disease |
| STS score |
| NYHA class |
| Radiation heart disease |
| Treatment variables |
| Aortic valve replacement |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker |
| Statin |
| Beta‐blocker |
| Echocardiographic variables |
| Aortic valve area |
| Aortic valve peak gradient |
| Aortic valve regurgitation grade |
| Left ventricular end‐diastolic volume index |
| Left ventricular end‐systolic volume index |
| Right ventricular systolic pressure |
| Left atrial diameter |
| Left ventricular mass index |
| Bicuspid aortic valve |
NYHA indicates New York Heart Association; STS, Society of Thoracic Surgeons.
We then used the PS in 2 ways. We first performed PS matching to obtain 2 patient groups (AVR and controls) that were of identical size and well balanced in their baseline characteristics. Matching was performed with the use of a 1:1 greedy matching protocol without replacement, with a caliper width equal to 0.5 of the SD of the logit of the PPS. We used conditions 1 and 2, as proposed by Rubin, to define appropriate PS matching.10 For condition 1, that states that the difference in the means of the PSs in the 2 groups being compared must be small, we used the rule that the absolute value of the standardized difference of the linear PS, comparing the treated group to the control group, should be below 10%. For condition 2, PS matching was assumed to be appropriate if the ratio of the variances of the PS in the 2 groups was close to 1 (ie, >1/2 and <2). Survival curves (AVR versus medical management) for cumulative events as a function over time in the propensity‐matched cohort were obtained using the Kaplan–Meier method and compared using the log‐rank statistic. Second, we calculated inverse probability of treatment weights (IPTW) derived from PSs to reduce potential imbalance in baseline variables between patients with and without AVR. A Cox proportional hazards model, that included IPTW, was then used to obtain adjusted estimates of hazard ratios.
We also assessed whether degree of MAVD severity affects survival in a subset of patients who did not undergo any aortic valve intervention. Previous studies have shown that peak aortic valve gradient (or its mathematical equivalent, peak aortic valve velocity) acts as a measure of global burden of MAVD disease.11, 12 We modeled the nonlinear impact of peak aortic valve gradient using restricted cubic spline with 4 knots in a Cox proportional hazards model that also included age, sex, STS score, New York Heart Association (NYHA) class, radiation heat disease, obstructive CAD, and use of ACE inhibitors or ARBs.
To investigate the natural time course of changes in echocardiographic parameters of aortic valve disease severity and LV size in MAVD, we performed longitudinal data analysis.13 We separated patients into 2 groups based on whether or not patients eventually underwent AVR. Subjects had to have a baseline and at least 1 follow‐up echocardiogram performed ≥720 days after the initial study to be included. Studies performed after >3 years of follow‐up were excluded. In patients who eventually underwent AVR, only echocardiographic data preceding AVR were accepted for analysis. Longitudinal data analysis was performed using a generalized linear mixed‐effects model with unstructured covariance for random effects using SPSS. We tested the effects of groups as a factor, time as a covariate, as well as time×group interactions.
Statistical analysis was performed using IBM SPSS Statistics (version 25; SPSS, Inc., Chicago, IL) and R software (version 3.3; R Foundation for Statistical Computing, Vienna, Austria). A P value of <0.05 was considered significant.
Results
Demographic and baseline clinical and echocardiographic characteristics for the entire cohort as well as the predefined subgroups are shown in Tables 2 and 3. In summary, patients who underwent intervention were younger and had fewer rates of comorbidities than those who were medically managed. The majority of patients in both groups were asymptomatic or minimally symptomatic. In terms of baseline echocardiographic findings, patients who underwent AVR had higher rates of bicuspid valve, higher aortic valve gradients, smaller aortic valve areas, and more‐dilated ventricles. Compared with patients with tricuspid aortic valve, bicuspid aortic valve patients were younger (72±13 versus 53±16 years; P<0.001), more frequently male (53.3% versus 72.9%; P<0.001), had lower rates of radiation heart disease (7.4% versus 0.7%; P=0.005), lower rates of chronic kidney disease (12.7% versus 4.5%; P=0.004), were more asymptomatic or minimally symptomatic (82.3% versus 93.5%; P=0.001), had more‐dilated ventricles (left ventricular end‐diastolic volume, 107±34 versus 129±45 mL, P<0.001; left ventricular end‐systolic volume, 45±16 versus 51±20 mL, P<0.001) and higher LV mass (121±41 versus 131±48 g; P=0.009), and had more‐severe grades of AR (2.3±0.5 versus 2.5±0.6; P<0.001).
Table 2.
Characteristics of the General Cohort and Subgroups (Aortic Valve Procedure and Medical Management) of Patients With MAVD at Baseline
| Characteristics | Entire Cohort (N=862) | Medical Management (N=357) | AVR (N=505) | P Value |
|---|---|---|---|---|
| Age at diagnosis, y | 68±15 | 73±14 | 65±15 | <0.001 |
| Male sex, n (%) | 490 (56.8) | 161 (45.1) | 329 (65.1) | <0.001 |
| Race | ||||
| White | 709 (82.3) | 282 (79.0) | 427 (84.6) | 0.035 |
| Black | 45 (5.2) | 26 (7.3) | 19 (3.8) | 0.022 |
| Asian | 11 (1.3) | 2 (0.6) | 9 (1.8) | 0.115 |
| Hispanic | 3 (0.3) | 2 (0.6) | 1 (0.2) | 0.374 |
| Other/declined | 94 (10.9) | 45 (12.6) | 49 (9.7) | 0.178 |
| Height, cm | 169±14 | 165±15 | 170±13 | <0.001 |
| Weight, kg | 79±22 | 75±23 | 82±21 | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 575 (66.7) | 259 (72.5) | 316 (62.6) | 0.001 |
| Hyperlipidemia | 516 (59.9) | 201 (56.3) | 315 (62.4) | 0.09 |
| Diabetes mellitus | 154 (18.2) | 64 (17.9) | 90 (17.8) | 0.938 |
| Atrial fibrillation | 175 (20.3) | 86 (24.1) | 89 (17.6) | 0.018 |
| Obstructive coronary artery disease | 306 (35.5) | 122 (34.2) | 184 (36.4) | 0.533 |
| Congestive heart failure | 136 (15.8) | 73 (20.4) | 63 (12.5) | 0.001 |
| Smoker | 209 (24.2) | 75 (21.0) | 134 (26.5) | 0.069 |
| Stroke | 74 (8.6) | 39 (10.9) | 35 (6.9) | 0.037 |
| Peripheral artery disease | 122 (14.2) | 50 (14.0) | 72 (14.3) | 0.943 |
| Chronic kidney disease | 97 (11.3) | 54 (15.1) | 43 (8.5) | 0.002 |
| Connective tissue disorder | 14 (1.6) | 9 (2.5) | 5 (1.0) | 0.078 |
| Aortic dissection | 7 (0.8) | 3 (0.8) | 4 (0.8) | 0.932 |
| Radiation heart disease | 54 (6.3) | 20 (5.6) | 34 (6.7) | 0.513 |
| Previous cardiac surgery | 185 (21.5) | 85 (23.8) | 100 (19.8) | 0.146 |
| Society of Thoracic Surgeons % score | 2.7±2.9 | 3.6±3.6 | 2.0±2.0 | <0.001 |
| Symptom status, n (%) | ||||
| NYHA class 1 to 2 | 727 (84.3) | 298 (83.5) | 427 (84.6) | 0.808 |
| NYHA class 3 to 4 | 135 (15.7) | 57 (16.0) | 78 (15.4) | 0.808 |
| Medications, n (%) | ||||
| Aspirin | 481 (55.8) | 197 (55.2) | 284 (56.2) | 0.829 |
| ACE inhibitors/ARBs | 378 (43.9) | 155 (43.4) | 223 (44.2) | 0.885 |
| Beta‐blockers | 444 (51.5) | 181 (50.7) | 263 (52.1) | 0.752 |
| Hydralazine | 16 (1.9) | 8 (2.2) | 8 (1.6) | 0.474 |
| Statin | 476 (55.2) | 178 (49.9) | 298 (59.0) | 0.01 |
| Oral anticoagulants | 145 (16.8) | 71 (19.9) | 74 (14.7) | 0.039 |
| Immunosuppressive treatment | 12 (1.4) | 7 (2.0) | 5 (1.0) | 0.227 |
| Serum hemoglobin, mg/dL | 13.1±1.9 | 12.4±1.9 | 15.5±1.7 | <0.001 |
| Serum creatinine, mg/dL | 1.2±1.0 | 1.3±1.2 | 1.1±0.9 | 0.014 |
| Low‐density lipoprotein cholesterol, mg/dL | 95±51 | 94±36 | 96±58 | 0.597 |
| High‐density lipoprotein cholesterol, mg/dL | 53±17 | 55±18 | 52±17 | 0.06 |
| Triglycerides, mg/dL | 124±82 | 117±71 | 128±87 | 0.085 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; AVR, aortic valve replacement; MAVD, mixed aortic valve disease; NYHA, New York Heart Association.
Table 3.
Echocardiographic Characteristics of the General Cohort and Subgroups (Aortic Valve Procedure and Medical Management) of Patients With MAVD at Baseline
| Characteristics | General Cohort | Medical Management | AVR | P Value |
|---|---|---|---|---|
| N=862 | N=357 | N=505 | ||
| LV ejection fraction, % | 58±5 | 58±5 | 58±5 | 0.988 |
| LV end‐diastolic volume, mL | 112±48 | 102±41 | 118±52 | <0.001 |
| LV end‐systolic volume, mL | 46±23 | 42±21 | 49±25 | 0.001 |
| LV end‐diastolic diameter, cm | 4.5±0.7 | 4.4±0.7 | 4.6±0.7 | <0.001 |
| LV end‐systolic diameter, cm | 2.90±0.64 | 2.8±0.6 | 3.0±0.7 | <0.001 |
| LV mass index, g/m2 | 124±39 | 120±38 | 127±40 | 0.07 |
| LV hypertrophy | 563 (65) | 222 (62) | 341 (68) | 0.1 |
| Left atrial area, cm2 | 23±6 | 23±6 | 23±6 | 0.908 |
| Aortic valve anatomy | ||||
| Tricuspid valve | 699 (81.1) | 315 (88.2) | 384 (76.0) | <0.001 |
| Bicuspid valve | 155 (18.0) | 41 (11.5) | 114 (22.6) | <0.001 |
| Other | 8 (0.9) | 1 (0.3) | 7 (1.4) | 0.095 |
| Aortic valve gradients | ||||
| Mean gradient, mm Hg | 36±18 | 29±17 | 40±17 | <0.001 |
| Peak gradient, mm Hg | 64±29 | 53±27 | 72±28 | <0.001 |
| Aortic valve area, cm2 | 0.93±0.30 | 1.02±0.30 | 0.86±0.20 | <0.001 |
| Aortic regurgitation severity | ||||
| Moderate | 676 (78.4) | 291 (81.5) | 385 (76.2) | 0.064 |
| Severe | 186 (21.6) | 66 (18.5) | 120 (23.8) | 0.064 |
| Diastolic functiona | ||||
| Normal | 59 (10.6) | 16 (6.8) | 43 (13.3) | 0.015 |
| Abnormal relaxation | 385 (69.0) | 171 (73.1) | 214 (66.0) | 0.077 |
| Pseudo‐normal | 108 (19.4) | 43 (18.4) | 65 (20.1) | 0.619 |
| Restrictive | 6 (1.1) | 4 (1.7) | 2 (0.6) | 0.217 |
| E/e’b | 17±10 | 17±10 | 16±9 | 0.13 |
| RV systolic pressure, mm Hg | 38±13 | 39±13 | 37±12 | 0.063 |
AVR indicates aortic valve replacement; E/e’, ratio between mitral valve and average mitral annulus early wave velocities; LV, left ventricular; MAVD, mixed aortic valve disease; RV, right ventricular.
Diastolic function assessment available in 558 patients.
E/e’ ratio available in 498 patients.
AVR was performed in 505 of the 862 patients (58.6%) at a median time of 50 days after initial echocardiogram (25th–75th percentile range, 6–560). Most of the procedures were surgical AVR (93.3%), and 31.2% had a concomitant aortic surgery. A minority of intervened patients underwent a TAVR (6.7%). Compared with patients with tricuspid aortic valve, a higher proportion of patients with bicuspid aortic valve underwent AVR (55.3% versus 73.5%; P<0.001), and a higher proportion of patients had a concomitant aortic intervention of any type (12.6% versus 37.4%; P<0.001). Of 184 patients with a history of obstructive CAD who underwent AVR, 23 underwent TAVR and 161 underwent SAVR. In patients who underwent SAVR, 102 underwent concomitant coronary artery bypass graft.
An aortic valve procedure was performed in 237 of 443 of initially asymptomatic patients, with 137 having surgery after >90 days, most frequently driven by symptom development or increasing severity of valve disease. In the remaining 100 asymptomatic patients who underwent an aortic valve procedure within the first 90 days, indications for surgery were findings consistent with critical (in 7 patients) or severe rapidly progressive (in 33 patients) AS, aortic aneurysm in 31 patients, CAD in 13 patients, severe AR in 12 patients, positive stress test in 3 patients, and other cardiac surgery in a remaining patient.
Outcomes
Median follow‐up was 5.6 years (25th–75th percentile, 1.8–9.4). Median follow‐up was 8.4 years (25th–75th percentile, 2.8–10.7) in patients who were alive at the last contact and 4.0 years (25th–75th percentile, 1.5–7.2) in patients who died during the study.
During follow‐up, there were 421 (48.8%) recorded deaths for the entire cohort. There were 243 (68.1%) deaths in the medical management group and 178 (35.2%) deaths in the AVR group. Only 9 (1.8%) patients in the AVR group died within 30 days of the AVR date. Table 4 shows proportional hazards analysis data. As can be seen, independent clinical factors associated with survival were age, STS score, chronic kidney disease, radiation heart disease, NYHA class, if they were on either an ACE inhibitor or ARB, and whether or not they underwent AVR. Independent echocardiographic factors associated with were bicuspid aortic valve anatomy, AR severity, and right ventricular systolic pressure. There were no significant interactions between these clinically relevant factors.
Table 4.
Time‐Independent Proportional Hazards Analysis Showing Factors Associated With All‐Cause Mortality Without Adjustment and After Adjustment With Inverse Proportional Weights
| Time‐Independent Factors Associated With Long‐Term Mortality | No Adjustment | With Inverse Probability Weighting | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age | 1.04 (1.03–1.05) | <0.001 | 1.05 (1.04–1.06) | <0.001 |
| Society of Thoracic Surgeons % score | 1.08 (1.05–1.13) | <0.001 | 1.09 (1.05–1.13) | <0.001 |
| Chronic kidney disease | 1.90 (1.44–2.52) | <0.001 | 1.77 (1.33–2.35) | <0.001 |
| Radiation heart disease | 2.88 (1.95–4.24) | <0.001 | 2.70 (1.80–4.04) | <0.001 |
| ACE inhibitors/ARBs | 0.75 (0.61–0.91) | 0.004 | 0.76 (0.62–0.93) | 0.006 |
| NYHA class | 1.66 (1.28–2.14) | <0.001 | 1.51 (1.16–1.96) | 0.002 |
| Bicuspid aortic valve | 0.45 (0.28–0.73) | 0.001 | 0.48 (0.30–0.76) | 0.005 |
| Aortic valve intervention | 0.41 (0.34–0.51) | <0.001 | 0.40 (0.32–0.50) | <0.001 |
| Aortic regurgitation severity grade | 1.37 (1.10–1.70) | 0.005 | 1.41 (1.13–1.75) | 0.002 |
| Right ventricular systolic pressure | 1.01 (1.00–1.02) | 0.019 | 1.01 (1.00–1.02) | 0.003 |
| Left ventricular mass index | 1.004 (1.001–1.007) | 0.006 | 1.004 (1.001–1.007) | 0.01 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; NYHA, New York Heart Association. (dichotomized into I or II and III or IV).
Table 5 shows proportional hazards analysis after adjusting for the time‐dependent nature of the AVR. The independent clinical factors associated with survival were age, STS score, chronic kidney disease, radiation heart disease, NYHA class, if they were on either an ACE inhibitor or ARB, and whether or not they underwent an aortic valve procedure. Independent echocardiographic factors associated with survival were bicuspid aortic valve anatomy, AR severity, right ventricular systolic pressure, and LV mass index.
Table 5.
Time‐Dependent Proportional Hazards Analysis Showing Factors Associated With All‐Cause Mortality
| Time‐Dependent Factors Associated With Long‐Term Mortality | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Aortic valve intervention as time‐dependent covariate | 0.59 (0.47–0.75) | <0.001 |
| Age | 1.05 (1.04–1.06) | <0.001 |
| Society of Thoracic Surgeons % score | 1.10 (1.07–1.14) | <0.001 |
| Chronic kidney disease | 1.85 (1.40–2.45) | <0.001 |
| Radiation heart disease | 2.74 (1.86–4.03) | <0.001 |
| ACE inhibitors/ARBs | 0.76 (0.62–0.92) | 0.006 |
| NYHA class | 1.56 (1.21–2.01) | 0.001 |
| Bicuspid aortic valve | 0.43 (0.27–0.68) | <0.001 |
| Aortic regurgitation severity grade | 1.33 (1.07–1.65) | 0.01 |
| Right ventricular systolic pressure | 1.01 (1.002–1.02) | 0.012 |
| Left ventricular mass index | 1.005 (1.001–1.008) | 0.004 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; NYHA, New York Heart Association.
Figure 1 shows adjusted and unadjusted survival curves of patients with MAVD divided according to undergoing AVR or not. Patients with AVR had an improved survival compared with those that were medically managed (log rank, P<0.001). This difference persists after adjusting for the variables identified as factors associated with survival in the proportional hazards analysis (log rank, P<0.001).
Figure 1.
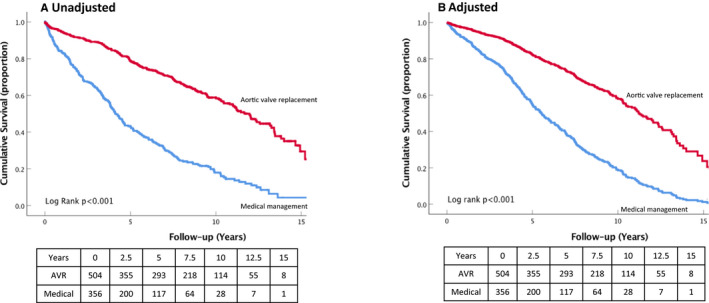
Unadjusted (A) and adjusted (B) Kaplan–Meier curves for all‐cause mortality in the AVR and medical management subgroups of patients with MAVD. AVR indicates aortic valve replacement; MAVD, mixed aortic valve disease.
Figures 2 and 3 show the survival curves for the 2 subgroups when stratified by median AVA (0.9 cm2) and symptom status (NYHA class 1 versus NYHA classes 2–4). In both cases, impact of AVR was present in the 2 strata (log rank, P<0.001 for both). Patients with a bicuspid aortic valve had improved survival compared with those with tricuspid valve in both unadjusted and adjusted models (P<0.001).
Figure 2.
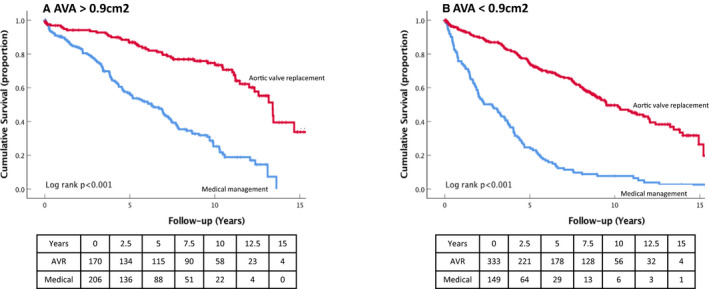
Kaplan–Meier curves for all‐cause mortality in the AVR and medical management subgroups of patients with MAVD stratified by AVA >1.0 cm2 (A) or ≤1.0 cm2 (B). AVA indicates aortic valve area; AVR, aortic valve replacement; MAVD, mixed aortic valve disease.
Figure 3.
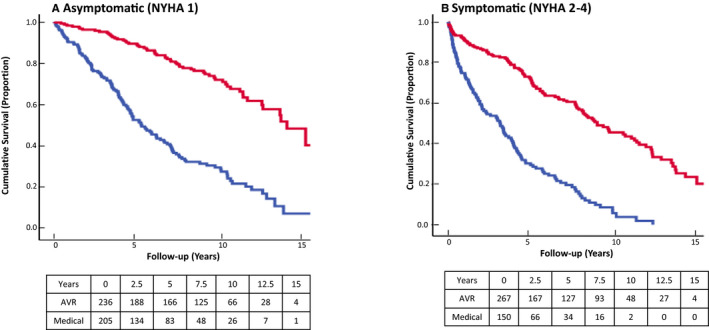
Kaplan–Meier curves for all‐cause mortality in the AVR and medical management subgroups of patients with MAVD stratified by NYHA class I (A) or NYHA class II to IV (B). AVR indicates aortic valve replacement; MAVD, mixed aortic valve disease; NYHA, New York Heart Association.
PS Matching and IPTW
Given the differences in baseline characteristics between patients in the 2 prespecified subgroups, we performed PS matching. Propensity‐matched populations’ baseline clinical and echocardiographic characteristics are shown in Table 6. Application of IPTW resulted in balanced covariates between 2 groups (Figure 4). Standardized differences for all covariates were below the 0.1 threshold (with the exception of the standardized differences for statin use, hemoglobin, AVA, and LVEF that were below the 0.25 threshold), suggesting minimal differences in the weighted distributions between those who underwent AVR and those treated conservatively following application of IPTW. The very significant impact of AVR on survival was still present (Figure 5). Finally, Table 4 (last column) shows results from the Cox proportional hazards model using IPTW. The model adjusted by IPTW showed that AVR was again independently associated with better survival (hazard ratio, 0.40; 95% CI, 0.32–0.50; P<0.001). Finally, in a subset of 498 subjects in whom we were able to measure ratio between early mitral flow velocity and the average of the lateral and septal mitral annulus velocities, we performed additional survival analyses. Ratio between early mitral flow velocity and the average of the lateral and septal mitral annulus velocities was a significant survival predictor on univariable analysis (hazard ratio, 1.04; 95% CI, 1.02–1.05); however, it lost its significance on multivariable analysis.
Table 6.
Characteristics of the Propensity Matched Cohort and Subgroups (Aortic Valve Procedure and Medical Management) of Patients With MAVD at Baseline
| Characteristics | Medical Management (N=243) | AVR (N=243) | P Value |
|---|---|---|---|
| Age at diagnosis, y | 71±15 | 70±13 | 0.632 |
| Male sex, n (%) | 134 (55.1) | 134 (55.1) | 1 |
| Race | |||
| White | 191 (78.6) | 204 (84.0) | 0.131 |
| Black | 19 (7.8) | 16 (6.6) | 0.599 |
| Asian | 1 (0.4) | 1 (0.4) | 1 |
| Hispanic | 1 (0.4) | 0 (0.0) | 0.317 |
| Other/declined | 31 (12.8) | 22 (9.1) | 0.145 |
| Height, cm | 168±11 | 169±12 | 0.421 |
| Weight, kg | 78±25 | 80±19 | 0.34 |
| Comorbidities, n (%) | |||
| Hypertension | 169 (69.5) | 165 (67.9) | 0.696 |
| Hyperlipidemia | 137 (56.4) | 152 (62.6) | 0.166 |
| Diabetes mellitus | 46 (18.9) | 46 (18.9) | 1 |
| Atrial fibrillation | 52 (21.4) | 52 (21.4) | 1 |
| Obstructive coronary artery disease | 91 (37.4) | 86 (35.4) | 0.637 |
| Congestive heart failure | 40 (16.5) | 41 (16.9) | 0.903 |
| Smoker | 57 (23.5) | 65 (26.7) | 0.403 |
| Stroke | 25 (10.3) | 21 (8.6) | 0.535 |
| Peripheral artery disease | 28 (11.5) | 45 (18.5) | 0.031 |
| Chronic kidney disease | 34 (14.0) | 25 (10.3) | 0.211 |
| Connective tissue disorder | 5 (2.1) | 3 (1.2) | 0.476 |
| Aortic dissection | 2 (0.8) | 4 (1.6) | 0.411 |
| Radiation heart disease | 17 (7.0) | 16 (6.6) | 0.857 |
| Previous cardiac surgery | 56 (23.0) | 61 (25.1) | 0.596 |
| Society of Thoracic Surgeons % score | 2.7±2.3 | 2.6±2.4 | 0.583 |
| Symptom status, n (%) | |||
| NYHA class 1 to 2 | 211 (86.8) | 198 (81.5) | 0.106 |
| NYHA class 3 to 4 | 32 (13.2) | 45 (18.5) | 0.106 |
| Medications, n (%) | |||
| Aspirin | 137 (56.4) | 143 (58.8) | 0.582 |
| ACE inhibitors/ARBs | 107 (44.0) | 119 (49.0) | 0.275 |
| Beta‐blockers | 115 (47.3) | 127 (52.3) | 0.276 |
| Hydralazine | 5 (2.1) | 8 (3.3) | 0.399 |
| Statin | 123 (50.6) | 150 (61.7) | 0.014 |
| Oral anticoagulants | 46 (18.9) | 51 (21.0) | 0.57 |
| Immunosuppressive treatment | 3 (1.2) | 4 (1.6) | 0.703 |
| Serum hemoglobin, mg/dL | 12.6±1.9 | 13.2±1.7 | <0.001 |
| Serum creatinine, mg/dL | 1.2±1.2 | 1.2±1.2 | 0.896 |
| Low‐density lipoprotein cholesterol, mg/dL | 94±35 | 100±77 | 0.352 |
| High‐density lipoprotein cholesterol, mg/dL | 53±16 | 53±16 | 0.978 |
| Triglycerides, mg/dL | 119±78 | 128±88 | 0.249 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; AVR, aortic valve replacement; MAVD, mixed aortic valve disease; NYHA, New York Heart Association.
Figure 4.
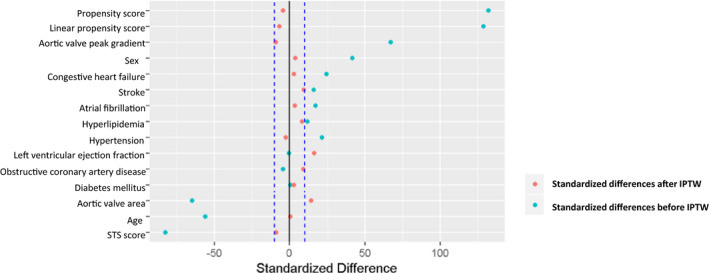
Standardized differences for all covariates suggesting minimal differences in the weighted distributions between those who underwent AVR and those treated conservatively following application of IPTW. AVR indicates aortic valve replacement; IPTW, inverse probability of treatment weights; STS, Society of Thoracic Surgeons.
Figure 5.
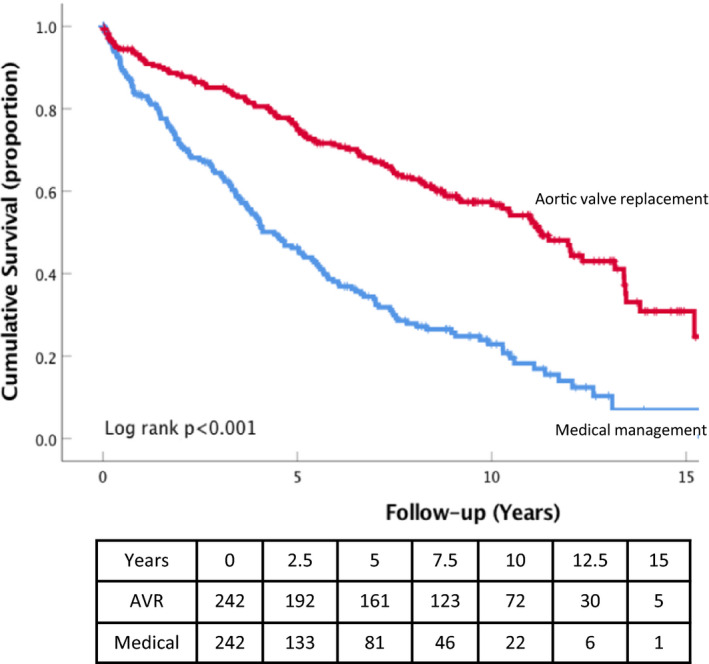
Kaplan–Meier curves for all‐cause mortality in the AVR and medical management subgroups of patients with MAVD for the propensity‐matched cohort. AVR indicates aortic valve replacement; MAVD, mixed aortic valve disease.
Our next aim was to assess the relationship between the outcomes and degree of MAVD severity (quantified as peak aortic valve gradient11, 12) in a subset of patients who did not undergo any aortic valve intervention. Figure 6 shows that, after controlling for other potential factors in a proportional hazards model, risk of all‐cause mortality increases steeply with increase of peak aortic valve gradients until it reaches 45 mm Hg, after which it continues to increase at a slower rate.
Figure 6.
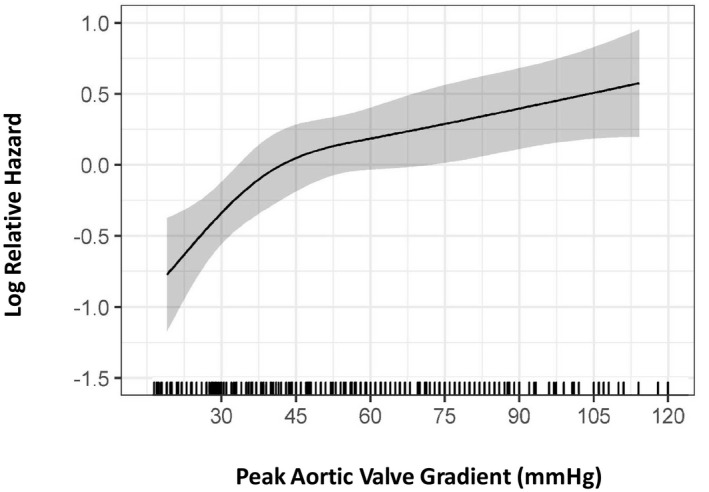
Relationship between relative hazard (y axis) and peak aortic valve gradient (x axis) in a subset of 357 mixed aortic valve disease patients who did not undergo aortic valve intervention during follow‐up. Visual analysis of the curves shows a steep increase in relative hazard as the peak aortic valve gradient reaches 45 mm Hg, after which the hazard plateaus. This nonlinear impact of peak aortic valve gradient was modeled using restricted cubic splines in a multivariable Cox proportional hazards model (see Methods for details). Grayscale area represents 95% CIs.
Longitudinal Data Analysis
A total of 234 patients (118 medically managed throughout the study period; 116 with AVR >2 years after the initial echocardiogram) satisfied the entry criteria. A total of 780 echocardiograms, with a median of 4 echocardiograms (25th–75th percentile range, 2–8) per patient, were analyzed. Again, we analyzed the data over a period of 3 years, with all postoperative echocardiograms excluded (Figure 7).
Figure 7.
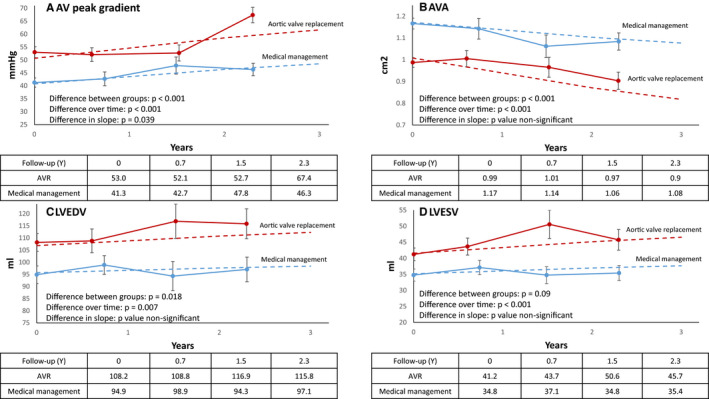
Longitudinal data analysis of echocardiographic parameters. Dotted lines represent regression lines obtained from mixed‐model results. (A) AV peak gradient in the AVR and medical management subgroups of patients with MAVD. (B) AV area in the AVR and medical management subgroups of patients with MAVD. (C) LVEDV in the AVR and medical management subgroups of patients with MAVD. (D) LVESV in the AVR and medical management subgroups of patients with MAVD. AV indicates aortic valve; AVA, aortic valve area; AVR, aortic valve replacement; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; MAVD, mixed aortic valve disease.
Patients who eventually underwent AVR had higher initial aortic valve peak gradients (P<0.001), and aortic valve peak gradients increased in both groups over time (P<0.0001); however, more so in patients who eventually underwent AVR (P=0.01; Figure 7A). As expected, patients who eventually underwent AVR also had smaller AVA (P<0.0001), with AVA decreasing in both groups over time (P<0.001), with no between‐group difference in the amount of decrease (Figure 7B). Patients who eventually underwent AVR also had larger initial left ventricular end‐diastolic volume and left ventricular end‐systolic volume (P=0.018 and 0.009, respectively); over time, both left ventricular end‐diastolic volume and left ventricular end‐systolic volume showed a very small, but statistically significant, increase (P=0.007 and P<0.001, respectively), with no difference in rate of dilation between the 2 groups (Figure 7C and 7D). Finally, LVEF and AR severity were similar in both groups, with no change over time.
Discussion
This study evaluated the factors affecting long‐term outcomes in a contemporary cohort of patients with MAVD defined as a concomitant presence of at least moderate AR and AS. Our main finding was that survival was dramatically improved in patients who underwent either SAVR or TAVR. Apart from lack of AVR, the factors most strongly associated with poor outcome were STS score, age, chronic kidney disease, and radiation heart disease, with tricuspid (ie degenerative) disease of the aortic valve, presence of symptoms, AR severity, LV hypertrophy, and use of ACE inhibtors/ARBs showing less‐significant associations. AVR improved survival regardless of symptoms or potentially modifying factors, such as smaller AVA. This was further confirmed by IPTW‐adjusted and propensity‐matched analyses.
Given this strong association between aortic valve intervention and better survival, we investigated which factors could be responsible for all‐cause mortality in a subgroup of patients who did not undergo aortic valve intervention. We found that peak aortic valve gradient (a measure of overall hemodynamic impact of MAVD) has an independent nonlinear association with survival, with a risk of death rising steeply with the peak aortic valve gradient increasing to 45 mm Hg and becoming flatter afterward, Finally, we show that the dominant echocardiographic change during follow‐up of patients was an increase in aortic valve gradients and decrease in valve area. Therefore, it can be considered that AS worsening is the main contributor to progression of MAVD. In contrast, increase in LV volumes, while statistically significant, was small and both LVEF and AR severity grade did not change over time. Therefore, AR severity, while a significant survival predictor at baseline, appears to remain stable during follow‐up.
To the best of our knowledge, this is the largest study reporting outcomes in patients with MAVD. In contrast to previous studies that combined hard (death) and soft (valve surgery or hospitalization) end points, this study solely relied on all‐cause mortality. Previous studies in the MAVD population have also differed in the patients included. Studies differed by including patients using different criteria (eg, with at least mild MAVD, only moderate MAVD, or at least moderate MAVD).4, 11, 12 Still, results from these studies are concordant with our study by showing high event rates. In a recent study, the researchers report an 84% rate of adverse effects (ie, development of severe symptoms, need for aortic valve replacement, and cardiac death) after 7 years of follow‐up.11 Similarly, after 6 years of follow‐up, Zilberszac et al found a prevalence of 81% of the same outcome.12 Finally, in the previous study by our group, rate of aortic valve replacement or death after 5 years of follow‐up was 70%.4 Similarly, in a current study, 5‐year death rate in the entire cohort was 50%, indicating that presence of MAVD, as defined by our criteria, should be taken seriously.
Better clinical outcome in patients with bicuspid MAVD is intriguing. Although bicuspid aortic valve patients were both younger and healthier, they had better survival than patients with tricuspid MAVD even after adjusting for age and clinical and echocardiographic parameters. One possibility is that tricuspid MAVD, in itself, is a marker of degenerative processes.
Clinical Implications
Despite studies documenting poor outcome of MAVD patients, there are no guideline recommendations for the timing of intervention. The usual approach involves extrapolation from the recommendations for the predominant lesion, either AS or AR.1 Based on earlier studies,4, 11, 12 one can suggest that early AVR for MAVD patients without comorbidities in centers with low risk for such procedures is a feasible alternative. Still, one could argue that inclusion of AVR as one of the outcomes weakens the composite end point.14 We show here that in patients who did not undergo aortic valve intervention, risk of death increases steeply with increase of peak aortic valve gradient, with risk plateauing once peak aortic valve gradient reaches 45 mm Hg. In other words, peak aortic valve gradient of 45 mm Hg could be used as a cut‐off value for aortic valve intervention.
Natural History of MAVD
AS and AR lead to different cardiac remodeling phenotypes. Pressure overload of AS leads to concentric LV hypertrophy, whereas volume overload AR leads to LV dilatation and eccentric hypertrophy. But when MAVD leads to concomitant pressure and volume overload, development of AS‐induced concentric hypertrophy and associated increased chamber stiffness will reduce the ability of the LV to remodel to accommodate the increased stroke volume of AR. This leads to left ventricular end‐diastolic dimensions that are less than one observed in pure AR.15 This would lead to the left ventricle filling on a steeper portion of the pressure‐volume curve, potentially causing the earlier onset of symptoms than is observed in isolated AR or AS.4
Based on our assumption that AV area measures the contribution of AS, AR severity grade and LV volumes measure the contribution of AR, and AV gradients measure the contribution of both AS and AR to the severity of MAVD; we found that the AS was the main contributor to progression of MAVD. In our cohort, annual decrease in AVA was approximately 0.07 cm2/yr, a value similar to that previously reported in the literature.16, 17, 18, 19 On the contrary, severity grading of AR did not change over time, and, more important, an increase in ventricular volumes over time was small and unlikely to be clinically relevant despite statistical significance. In our study, the annual increase in left ventricular end‐diastolic volume and left ventricular end‐systolic volume was ≈1 mL/yr, significantly less than previously reported grades of progression of LV volumes in isolated AR.20, 21, 22 This observation, in which MAVD limits the degree of LV dilation in response to the volume load of regurgitation, had also been observed in the study by Egbe et al.11 These findings diverge from recommendations in isolated AR, in which LV dilation can be used as an indicator to time intervention in order to improve outcomes.23 This is a crucial difference to consider when comparing MAVD to isolated AR, given that common markers of progression in AR should not be part of the decision‐making process in MAVD, and also raises questions regarding the sensitivity of available echocardiographic parameters to quantify AR severity and its progression in the concomitant presence of AS. Finally, rate of AV gradients increase was slower than previously reported in both MAVD and isolated AS.11, 16, 17, 18, 19
Limitations
This was a retrospective, observational study from a tertiary referral center with its inherent selection biases. NYHA class was attributed to cardiac causes, although it may have been attributable to other etiologies in some patients. Symptom status elicited by the physician during the history and physical examination is subjective, given that some patients do not report symptoms promptly, some deny symptoms, and some might attribute their symptoms to other causes. Also, our data may not be generalized across all other centers. Except for ratio between early mitral flow velocity and the average of the lateral and septal mitral annulus velocities, we used data from original echocardiographic reports in our analysis. This approach likely increased measurement error attributed to interobserver variability. However, the strength of this approach is that, given that the data were obtained at the beginning of the study period, this makes any potential measurement error independent of final outcome.
A small number of subjects underwent a TAVR procedure. Although this may have introduced heterogeneity into the AVR treatment effects, TAVR has largely been shown to lead to outcomes similar to SAVR.24, 25 We assumed that there was a linear progression of changes in echocardiographic parameters, which may not always hold true, especially in advanced stages of the MAVD.26 This may have led to underestimating rates of progression. Also, retrospective studies like ours can demonstrate only associations and not causality. Finally, we report all‐cause, not cardiac, mortality as the primary end point. However, it has been demonstrated previously that all‐cause mortality is less biased than cardiac mortality.27
Finally, while we succeeded to obtain overall well‐balanced propensity‐matched samples that satisfied Rubin conditions, some parameters were less well balanced, which may have introduced a bias in our data.
Conclusions
MAVD confers a high risk of all‐cause mortality. Although AVR significantly reduced this risk, independent of valve area and symptom status, a substantial risk of death remained even after AVR. Peak aortic valve gradients >45 mm Hg are associated with poor prognosis in MAVD for those who did not undergo aortic valve intervention. These results build on previous knowledge and add to the notion that in the MAVD population, there may be a role for closer follow‐up and earlier invasive treatment.
Additionally, the phenotypic characteristics of MAVD differ from isolated AS or AR given that these patients display only mild LV dilation. These features may have an important role in accelerating the development of symptoms and worsening the manifestations of MAVD.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e014591 DOI: 10.1161/JAHA.119.014591.)
References
- 1. Unger P, Rosenhek R, Dedobbeleer C, Berrebi A, Lancellotti P. Management of multiple valve disease. Heart. 2011;97:272–277. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM III, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 4. Rashedi N, Popovic ZB, Stewart WJ, Marwick T. Outcomes of asymptomatic adults with combined aortic stenosis and regurgitation. J Am Soc Echocardiogr. 2014;27:829–837. [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. [DOI] [PubMed] [Google Scholar]
- 6. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 8. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 9. Dahiya A, Bolen M, Grimm RA, Rodriguez LL, Thomas JD, Marwick TH. Development of a consensus document to improve multireader concordance and accuracy of aortic regurgitation severity grading by echocardiography versus cardiac magnetic resonance imaging. Am J Cardiol. 2012;110:709–714. [DOI] [PubMed] [Google Scholar]
- 10. Rubin DB. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169‐188. [Google Scholar]
- 11. Egbe AC, Luis SA, Padang R, Warnes CA. Outcomes in moderate mixed aortic valve disease: is it time for a paradigm shift? J Am Coll Cardiol. 2016;67:2321–2329. [DOI] [PubMed] [Google Scholar]
- 12. Zilberszac R, Gabriel H, Schemper M, Zahler D, Czerny M, Maurer G, Rosenhek R. Outcome of combined stenotic and regurgitant aortic valve disease. J Am Coll Cardiol. 2013;61:1489–1495. [DOI] [PubMed] [Google Scholar]
- 13. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley‐Interscience; 2004. [Google Scholar]
- 14. Cremer PC, Rodriguez LL, Jaber WA. Early surgery for mixed aortic valve disease: a precocious or premature proposition? J Am Coll Cardiol. 2016;68:1925–1926. [DOI] [PubMed] [Google Scholar]
- 15. Parker MW, Aurigemma GP. The simple arithmetic of mixed aortic valve disease: LVH + volume load = trouble. J Am Coll Cardiol. 2016;67:2330–2333. [DOI] [PubMed] [Google Scholar]
- 16. Roger VL, Tajik AJ, Bailey KR, Oh JK, Taylor CL, Seward JB. Progression of aortic stenosis in adults: new appraisal using Doppler echocardiography. Am Heart J. 1990;119:331–338. [DOI] [PubMed] [Google Scholar]
- 17. Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. 2000;101:2497–2502. [DOI] [PubMed] [Google Scholar]
- 18. Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol. 1989;13:545–550. [DOI] [PubMed] [Google Scholar]
- 19. Brener SJ, Duffy CI, Thomas JD, Stewart WJ. Progression of aortic stenosis in 394 patients: relation to changes in myocardial and mitral valve dysfunction. J Am Coll Cardiol. 1995;25:305–310. [DOI] [PubMed] [Google Scholar]
- 20. Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popovic ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines: incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging. 2014;7:352–362. [DOI] [PubMed] [Google Scholar]
- 21. Goldbarg SH, Halperin JL. Aortic regurgitation: disease progression and management. Nat Clin Pract Cardiovasc Med. 2008;5:269–279. [DOI] [PubMed] [Google Scholar]
- 22. Kusunose K, Cremer PC, Tsutsui RS, Grimm RA, Thomas JD, Griffin BP, Popovic ZB. Regurgitant volume informs rate of progressive cardiac dysfunction in asymptomatic patients with chronic aortic or mitral regurgitation. JACC Cardiovasc Imaging. 2015;8:14–23. [DOI] [PubMed] [Google Scholar]
- 23. Mentias A, Feng K, Alashi A, Rodriguez LL, Gillinov AM, Johnston DR, Sabik JF, Svensson LG, Grimm RA, Griffin BP, Desai MY. Long‐term outcomes in patients with aortic regurgitation and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2016;68:2144–2153. [DOI] [PubMed] [Google Scholar]
- 24. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 25. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 26. Popescu AC, Antonini‐Canterin F, Enache R, Nicolosi GL, Piazza R, Faggiano P, Cassin M, Dimulescu D, Ginghină C, Popescu BA. Impact of associated significant aortic regurgitation on left ventricular remodeling and hemodynamic impairment in severe aortic valve stenosis. Cardiology. 2013;124:174–181. [DOI] [PubMed] [Google Scholar]
- 27. Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–620. [DOI] [PubMed] [Google Scholar]


