Abstract
Background
Recent studies suggest that lymphatic congestion plays a role in development of late Fontan complications, such as protein‐losing enteropathy. However, the role of the lymphatic circulation in early post‐Fontan outcomes is not well defined.
Methods and Results
This was a retrospective, single‐center study of patients undergoing first‐time Fontan completion from 2012 to 2017. The primary outcome was early Fontan complication ≤6 months after surgery, a composite of death, Fontan takedown, extracorporeal membrane oxygenation, chest tube drainage >14 days, cardiac catheterization, readmission, or transplant. Complication causes were assigned to 1 of 4 groups: (1) Fontan circuit obstruction, (2) ventricular dysfunction or atrioventricular valve regurgitation, (3) persistent pleural effusions in the absence of Fontan obstruction or ventricular dysfunction, and (4) chylothorax or plastic bronchitis. T2‐weighted magnetic resonance imaging sequences were used to assess for lymphatic perfusion abnormality. The cohort consisted of 238 patients. Fifty‐eight (24%) developed early complications: 20 of 58 (34.5%) in group 1, 8 of 58 (14%) in group 2, 18 of 58 (31%) in group 3, and 12 of 58 (20%) in group 4. Preoperative T2 imaging was available for 126 (53%) patients. Patients with high‐grade lymphatic abnormalities had 6 times greater odds of developing early complications (P=0.001).
Conclusions
There is substantial morbidity in the early post‐Fontan period. Half of those who developed early complications had lymphatic failure or persistent effusions unrelated to structural or functional abnormalities. Preoperative T2 imaging demonstrated that patients with higher‐grade lymphatic perfusion abnormalities were significantly more likely to develop early complications. This has implications for risk stratification and optimization of patients before Fontan palliation.
Keywords: cardiovascular magnetic resonance imaging, congenital heart disease, Fontan procedure, lymph, morbidity/mortality
Subject Categories: Magnetic Resonance Imaging (MRI), Congenital Heart Disease, Cardiovascular Surgery, Clinical Studies, Complications
Nonstandard Abbreviations and Acronyms
- AWR
Atrioventricular valve regurgitation
- ECFF
Extracardiac fenestrated fontan
- ECMO
Extracorporeal membrane oxygenation
- HLHS
Hypoplastic left heart syndrome
- SPC
Systemic‐pulmonary arterial collateral
Clinical Perspective
What Is New?
This study assessed the extent of early postoperative morbidity in patients undergoing total cavopulmonary anastomosis at a single center, and found that nearly one third of patients experienced complications within the first 6 months of surgery.
Furthermore, it showed that the presence of mediastinal and pulmonary lymphatic perfusion conferred 6‐fold higher odds of developing early complications.
What Are the Clinical Implications?
Despite significant improvements in mortality associated with the Fontan operation, this patient population continues to experience a high burden of early postoperative morbidity.
Commonly investigated causes of early morbidity, such as obstruction within the Fontan circulation, decreased ventricular function, and increased atrioventricular valve regurgitation, are only found in half of the patients who develop early postoperative complications.
Preoperative assessment of lymphatic perfusion patterns may improve preoperative risk stratification and identify patients in whom lymphatic intervention might reduce the risk of early postoperative morbidity and mortality.
The interplay between the lymphatic and cardiovascular circulations in congenital heart disease is a burgeoning area of research.1, 2, 3 Recent studies suggest that lymphatic congestion attributable to elevated central venous pressure plays a role in the development of protein‐losing enteropathy and plastic bronchitis in patients with Fontan circulation.4, 5, 6 These conditions, which are known to have a basis in abnormal lymphatic perfusion, are both significant contributors to late Fontan failure.7, 8, 9 However, the role of the lymphatic circulation in the development of early Fontan complications is not as well described. Delineation of pre‐Fontan lymphatic anatomical characteristics by T2‐weighted magnetic resonance imaging (MRI) sequences and dynamic contrast magnetic resonance lymphangiography, as well as its association with Fontan failure, has been previously described by our group.4, 5 The primary objective of the current study is to describe the prevalence and cause of all early post‐Fontan morbidity. The secondary objective of this study is to identify associations between pre‐Fontan lymphatic anatomical characteristics and early post‐Fontan complications, in those patients in whom lymphatic circulation imaging was available. Establishing this framework for early Fontan morbidity may allow us to more accurately identify and target therapies for patients who develop complications related to their short‐term transition from a superior cavopulmonary connection to a total cavopulmonary circulation.
Methods
The data that support the findings of this study are available from the corresponding author on request.
Subject Ascertainment
The institutional surgical database was queried for all patients who had a Fontan operation from June 1, 2012, to July 1, 2017. Patients presenting for a Fontan revision (regardless of whether the primary Fontan surgery was performed at our institution or elsewhere), those presenting for second attempt at Fontan completion after previous takedown, or those who had a previous hepatic vein exclusion procedure (Kawashima operation) were excluded. Patients with previous Kawashima were excluded because most of their venous return entered the cavopulmonary circuit before Fontan completion and, as such, the hemodynamic change expected post‐Fontan would not be as pronounced as it would be in the rest of the patient population. This study was approved by the institutional review board, with waiver of the need for informed consent.
Data Collection
A retrospective medical record review was performed for all subjects. Demographic data included date of birth, sex, age, and weight at Fontan completion. Clinical variables included cardiac anatomical characteristics, surgical history, and catheterization history. Imaging variables included pre‐Fontan MRI data (eg, cardiac index, degree of atrioventricular valve regurgitant fraction, and systemic‐pulmonary arterial collateral burden) and preoperative transthoracic echocardiographic data (ventricular function and atrioventricular valve regurgitation). Surgical variables included type of Fontan performed, use of fenestration, concurrent atrioventricular valvuloplasty or pulmonary arterioplasty, cardiopulmonary bypass time, and aortic cross‐clamp time. Postoperative clinical variables included number of days with chest tube, duration of mechanical ventilation and ionotropic support, cardiac catheterization, reoperation, extracorporeal membrane oxygenation (ECMO), and death.
Pre‐Fontan lymphatic anatomical characteristics, as defined by T2 imaging, are a relatively novel exposure variable. Over the 5‐year span that these data were collected, new MRI techniques were developed that allowed efficient imaging of the lymphatic circulation. These techniques included T2‐weighted MRI sequences of the thoracic cavity and dynamic contrast magnetic resonance lymphangiography. As these were new techniques, they were applied variably during the early portion of the cohort and more consistently toward the end. Pre‐Fontan MRI assessment was performed at the discretion of the primary cardiologist. In this study, T2‐weighted imaging was used for the assessment of lymphatic anatomical characteristics.
MRI Protocol
All studies were performed on a 1.5‐T scanner (Magnetom Avanto; Siemens Healthcare). A T2‐weighted MRI lymphatic study was performed using a respiratory‐navigated and cardiac‐gated 3‐dimensional turbo spin‐echo sequence with the following parameters: 256×256; field of view, 300 to 450; repetition time/echo time, 2500/650; flip angle, 140°; and voxel size, 1.1×1.1×1.1 mm. The range of scan time was between 2 and 5 minutes, depending on the size of the patient. The sequence produces a 3‐dimensional volume, which can be formatted into coronal and custom plane maximal‐intensity projection reconstructions of the source images.5
Lymphatic Perfusion Pattern
Patterns of lymphatic perfusion of the thoracic cavity were characterized using the T2‐weighted images. Lymphatic abnormalities were graded on the basis of previously published categorizations used at our institution.10 Patients with little or no abnormalities of the thoracic duct were labeled “type 1.” Patients with enhancement of the supraclavicular region and a dilated or tortuous thoracic duct were labeled “type 2.” If there was T2 enhancement of the mediastinum and supraclavicular region, the pattern was labeled “type 3,” and T2‐weighted enhancement that extended from the mediastinum into the lung parenchyma was labeled “type 4.” Types 3 and 4 were grouped together and additionally analyzed as a composite category of “high‐grade” lymphatic abnormalities (Figure 1A and 1B). Two reviewers (R.M.G., Y.D.) viewed all the MRI images and separately graded the lymphatic perfusion pattern. Any discrepancy in grading between the 2 readers was noted and subsequently resolved by the readers viewing the scan in question together, and coming to an agreement about the grade of perfusion pattern.
Figure 1. Lymphatic perfusion patterns.
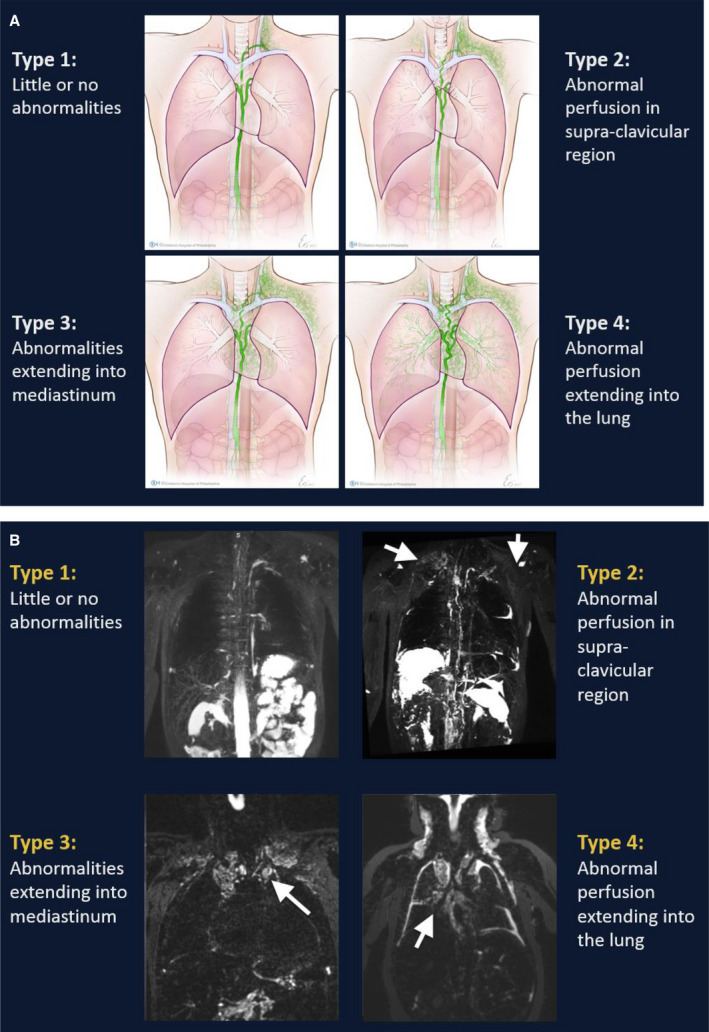
A, Schematic representation. B, T2 imaging. Reprinted from Biko et al10 with permission. Copyright ©2019, the Radiological Society of North America.
The primary outcome was early Fontan complication, a composite defined as the occurrence of at least one of the following events within 6 months of surgery: death, Fontan takedown, ECMO, chest tube drainage >14 days, cardiac catheterization, readmission, or heart transplant listing. Prior studies have demonstrated an independent relationship between prolonged chest tube drainage >14 days and increased risk of post‐Fontan morbidity and mortality.9, 11, 12 In addition, there may be institutional practice variations on routine post‐Fontan catheterizations and accordingly the use of postoperative catheterization incidence as a marker of early Fontan complications is likely center specific. However, at our institution, cardiac catheterizations within the first 6 months were only performed if there was a serious clinical concern. Accordingly, both chest tube drainage >14 days and cardiac catheterization were included as part of the composite outcome of early Fontan complication.
For the purpose of this study, the cause of early complications was categorized into 4 groups, a modification of the framework proposed by Book et al for the categorization of late Fontan failure.13 Group 1 includes patients with “structural failure” attributable to obstruction at any location in the Fontan circuit (attributable to stenosis or thrombus). Group 2 includes patients with “pump failure,” who have evidence of at least moderate systolic or diastolic dysfunction and/or at least moderate atrioventricular valve regurgitation. Group 3 includes patients with persistent pleural effusions in the absence of “structural failure” or “pump failure.” Group 4 describes patients with “lymphatic failure” manifesting as chylothorax or plastic bronchitis.
Statistical Analysis
Demographic, clinical, operative, and outcome variables were reported using descriptive statistics. Means/SDs are reported for normal continuous variables, medians with interquartile ranges are reported for nonnormal continuous variables, and counts with percentages are reported for categorical variables.
Mann‐Whitney U, χ2, and Fisher's exact tests were used for bivariate associations, with a 2‐tailed P<0.05. Odds ratios are reported for the association between lymphatic abnormality type and outcomes.
Sensitivity analyses were performed with the 12 patients who met criteria for the primary outcome because of non–Fontan‐related reasons. After removing them from the “early complications” group, the results remained the same. Consequently, the results are presented with those 12 patients removed from the cohort. To address potential bias from T2 imaging that was obtained inconsistently during the first 2 years of the study, additional associations between availability of lymphatic imaging and development of early complications were performed.
Results
Demographic and Surgical Data
There were 249 patients who underwent Fontan completion during the established time frame. We excluded 8 patients who had previous Kawashima operation and 3 who had presented for Fontan revision. The initial analytic cohort was composed of 238 patients (Figure 2), and no patients were lost to follow‐up during the 6‐month postoperative period. However, 12 patients had early postoperative complications attributable to noncardiac causes and were ultimately excluded from the final data analysis. Thus, the final analytic study cohort was composed of 226 patients; 56% (126) had hypoplastic left heart syndrome and 71% (160) had a systemic right ventricle (Table 1). The mean age and weight at surgery were 3.4±1.7 years and 14.3±4.3 kg, respectively; and 81% of patients underwent an extracardiac fenestrated Fontan.
Figure 2. Flow diagram of patients presenting for Fontan completion.
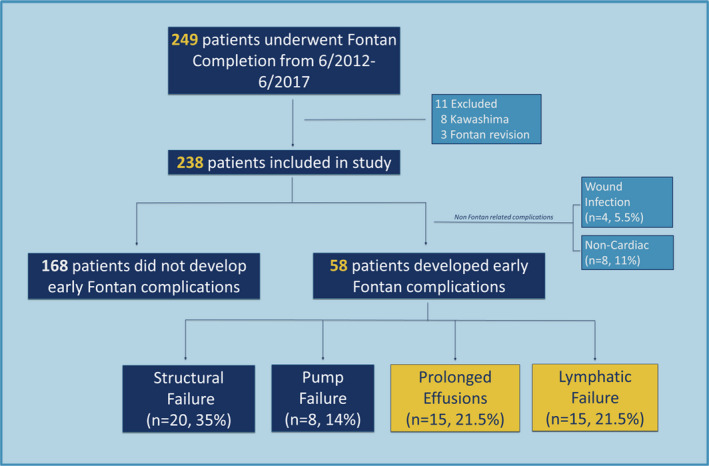
Table 1.
Baseline Characteristics of Cohort
| Characteristic | All Patients (n=226) | No Complications (n=168) | Early Complications (n=58) | P Value | |||
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Men | 138 | (61) | 99 | (59) | 39 | (67) | 0.263 |
| Women | 88 | (39) | 69 | (41) | 19 | (33) | |
| Anatomical feature | |||||||
| HLHS | 126 | (56) | 98 | (58) | 28 | (48) | 0.184 |
| Other | 100 | (44) | 70 | (42) | 30 | (52) | |
| Systemic ventricle | |||||||
| RV | 160 | (71) | 113 | (67) | 47 | (81) | 0.047b |
| LV | 66 | (29) | 55 | (33) | 11 | (19) | |
| Systemic venous anomaly | |||||||
| No | 186 | (82) | 137 | (82) | 49 | (84) | 0.614 |
| Yes | 40 | (18) | 31 | (18) | 9 | (16) | |
| Pulmonary venous anomaly | |||||||
| No | 214 | (95) | 160 | (95) | 54 | (93) | 0.51 |
| Yes | 12 | (5) | 8 | (5) | 4 | (7) | |
| Heterotaxy | |||||||
| No | 209 | (92) | 156 | (93) | 53 | (91) | 0.774 |
| Yes | 17 | (8) | 12 | (7) | 5 | (9) | |
| Stage 3 | |||||||
| ECFF | 184 | (81) | 133 | (79) | 51 | (88) | 0.491 |
| Othera | 42 | (18) | 35 | (21) | 7 | (12) | |
| Genetic anomaly | |||||||
| No | 197 | (87) | 149 | (89) | 48 | (83) | 0.244 |
| Yes | 29 | (13) | 19 | (11) | 10 | (17) | |
| Median | IQR | Median | IQR | Median | IQR | ||
|---|---|---|---|---|---|---|---|
| CPB time, min | 66.5 | (60–77) | 64 | (59–76) | 69.5 | (61–82) | 0.025b |
| XC, min | 26 | (22–33) | 25 | (21–31) | 29 | (23–37) | 0.002b |
| % AVVR by MRIc | 6 | (1–10) | 5 | (1–9) | 8 | (1–19) | 0.091 |
| % SPC by MRId | |||||||
| <35% | 143 | (73) | 101 | (74) | 42 | (72) | 0.394 |
| 35%–50% | 42 | (21) | 28 | (21) | 14 | (24) | |
| >50% | 9 | (5) | 7 | (5) | 2 | (3) | |
| T2 imaging | |||||||
| Obtained | 122 | (54) | 86 | (51) | 36 | (62) | 0.152 |
| Not obtained | 104 | (46) | 82 | (49) | 22 | (38) | |
Data presented as number (percentage) or median (interquartile range), with P value. AVVR indicates atrioventricular valve regurgitation; CPB, cardiopulmonary bypass; ECFF, extracardiac fenestrated Fontan; HLHS, hypoplastic left heart syndrome; LV, left ventricle; MRI, magnetic resonance imaging; RV, right ventricle; SPC, systemic‐pulmonary arterial collateral; and XC, cross‐clamp time.
Significant P value.
Includes lateral tunnel fenestrated Fontans and all nonfenestrated Fontans.
AVVR quantification by MRI was only available for 173 patients (118 had no complications, 55 had early complications).
SPC quantification by MRI was only available for 194 patients (136 had no complications, 58 had early complications).
Clinical Outcomes
Fifty‐eight patients (25.7%) met criteria for the definition of early complications. There were 142 unique events in these 58 patients, including 40 patients with ≥1 catheterization and 33 patients with ≥1 hospital readmission, within 6 months of the Fontan operation (Table 2). Prevalence of the most severe morbidity was low (4.0%), with ECMO, Fontan takedown, or transplant listing occurring in 9 individual patients. There were 2 deaths (0.9%) within 6 months of primary Fontan completion.
Table 2.
Early Complications
| Type of Complication | No. (%) of Patients (n=58)a |
|---|---|
| >14 d with chest tube | 28 (48) |
| Readmissions | 33 (57) |
| Cardiac catheterizations | 40 (70) |
| Catheter‐based intervention | 27 (46) |
| ECMO | 6 (10) |
| Listed for transplant | 3 (5) |
| Fontan takedown | 3 (5)b |
| Death | 2 (3)c |
ECMO indicates extracorporeal membrane oxygenation.
Each complication type is counted only once.
All 3 patients were on ECMO before their Fontan takedown.
Both patients were on ECMO.
There were no significant differences in demographic and procedural variables between the groups who did and did not develop early complications, except for a higher representation of patients with systemic right ventricles (81% versus 67%; P=0.047) and longer cardiopulmonary bypass (median time, 69.5 versus 64 minutes; P=0.025) and cross‐clamp times (median time, 29 versus 25 minutes; P=0.002) in those patients who developed early complications (Table 1).
Complication Cause
Of the 58 patients who developed early complications, 20 (34.5%) met criteria for “structural failure” (group 1), with angiographic evidence of obstruction in the Fontan circuit or direct evidence of structural abnormality at the time of reoperation. There were 8 patients (14%) who met criteria for “pump failure” (group 2) with echocardiographic and/or directly measured hemodynamics demonstrating decreased function or significant atrioventricular valve regurgitation. Three of these patients were listed for heart transplantation. There were 15 patients (21.5%) who met criteria for “persistent effusions” without structural abnormalities or pump failure (group 3), and 15 patients in group 4 (21.5%) who had direct evidence of lymphatic failure (plastic bronchitis or chylous effusions) (Figure 2).
Imaging Categorization
There were 195 patients (82%) with pre‐Fontan MRIs. Quantification of atrioventricular valve regurgitant fraction was available in 173 patients (73% of the total cohort and 89% of those with MRIs), with median regurgitation of 6%. Quantification of systemic‐pulmonary arterial collateral burden was available for 194 patients (99% of those with MRIs) and expressed as percentage of aortic flow. Most patients (134) had systemic‐pulmonary arterial collateral burden <35% of aortic flow, 42 patients had a collateral burden between 35% and 50%, and 9 patients had >50% APC flow (Table 1). Of the 51 patients with >35% aortopulmonary collateral flow, all but 8 had collateral embolization before Fontan completion.
There were 126 patients who had T2‐weighted sequences with which to grade lymphatic perfusion patterns (53% of total cohort, 65% of those who had MRIs). Of the 126 with T2 imaging, there were 39 patients with type 1, 41 patients with type 2, and 35 patients with type 3 patterns. Type 4 was rare, seen in only 7 patients. There was no difference between the prevalence of a known genetic syndrome between the types of lymphatic perfusion pattern (P=0.46).
There was no statistically significant difference between the groups with and without available T2 imaging for development of early complications (P=0.152). T2 imaging was performed in 62% of patients with early complications versus 51% of patients with no complications. However, there were anatomical differences between the patients who had clinical T2 imaging obtained and those who did not. Patients with a systemic right ventricle were more likely to have had T2 imaging (P=0.011) (Table 3).
Table 3.
Analysis of Patients With Clinical T2 Imaging Performed
| Variable | No T2 Imaging (n=104) | T2 Imaging (n=122) | P Value | ||
|---|---|---|---|---|---|
| Sex | |||||
| Men | 68 | (65) | 70 | (57) | 0.219 |
| Women | 36 | (35) | 52 | (43) | |
| Systemic ventricle | |||||
| RV | 65 | (63) | 95 | (78) | 0.011a |
| LV | 39 | (38) | 27 | (22) | |
| Systemic venous anomaly | |||||
| No | 86 | (83) | 100 | (82) | 0.887 |
| Yes | 18 | (17) | 22 | (18) | |
| Pulmonary venous anomaly | |||||
| No | 99 | (95) | 115 | (94) | 1 |
| Yes | 5 | (5) | 7 | (6) | |
| Heterotaxy | |||||
| No | 98 | (94) | 111 | (91) | 0.451 |
| Yes | 6 | (6) | 11 | (9) | |
| Heterotaxy type | |||||
| Polysplenia | 2 | (33) | 1 | (9) | 0.515 |
| Asplenia | 4 | (67) | 10 | (91) | |
| Stage 3 | |||||
| ECFF | 83 | (80) | 101 | (83) | 0.609 |
| Otherb | 21 | (20) | 21 | (17) | |
| Genetic anomaly | |||||
| No | 91 | (88) | 106 | (87) | 0.89 |
| Yes | 13 | (13) | 16 | (13) | |
| CPB time, min | 67 | (59–79) | 65.6 | (60–77) | 0.521 |
| XC, min | 26 | (21–34) | 26.5 | (22–32) | 0.910 |
| % AVVR by MRIc | 5 | (1–8.5) | 7 | (1–11) | 0.075 |
| % SPC by MRId | |||||
| <35% | 52 | (76) | 82 | (69) | 0.5 |
| 35%–50% | 14 | (21) | 29 | (25) | |
| >50% | 2 | (3) | 7 | (6) | |
Data presented as number (percentage) or median (interquartile range), with P value. AVVR indicates atrioventricular valve regurgitation; CPB, cardiopulmonary bypass; ECFF, extracardiac fenestrated Fontan; LV, left ventricle; MRI, magnetic resonance imaging; RV, right ventricle; SPC, systemic‐pulmonary arterial collateral; and XC, cross‐clamp time.
Significant P value.
Includes lateral tunnel fenestrated Fontans and all nonfenestrated Fontans.
AVVR quantification by MRI was only available for 173 patients (60 had no T2 imaging, 113 had T2 imaging).
SPC quantification by MRI was only available for 194 patients (72 had no T2 imaging, 122 had T2 imaging).
Lymphatic Perfusion Pattern and Early Complications
There were 39 patients who had type 1 lymphatic perfusion pattern, indicating little to no abnormalities of thoracic lymphatic drainage. Of these, only 6 (15%) had early complications (Table 4). Of patients with type 2 perfusion pattern, 9 of 41 (22%) developed early complications. On the other hand, 15 of 35 (43%) of patients with a type 3 pattern developed early complications, and all 7 patients with type 4 pattern developed early complications requiring intervention, including 2 patients who developed plastic bronchitis and 2 other patients who underwent Fontan takedown. Patients who had type 3 perfusion pattern had 5.5 greater odds of developing complications compared with patients with a type 1 pattern. All patients with type 4 perfusion pattern developed early complications. Combining groups 3 and 4 as patients with high‐grade lymphatic abnormalities, they had 6.05 greater odds of developing complications compared with those with a type 1 pattern. Controlling for ventricular morphological characteristics and cardiopulmonary bypass time, these patients with high‐grade lymphatic abnormalities had an adjusted odds ratio of 6.28 (95% CI, 2.13–18.5) for developing early complications than the group who had a type 1 pattern (P=0.001) (Table 5).
Table 4.
Lymphatic Perfusion Outcomes and Early Complications
| Perfusion Pattern | Total (n=122) | Early Complications (n=37) | No Complications (n=85) | P Value |
|---|---|---|---|---|
| Type 1 | 39 | 6 | 33 | 0.001 |
| Type 2 | 41 | 9 | 32 | |
| Type 3 | 35 | 15 | 20 | |
| Type 4 | 7 | 7 | 0 |
Table 5.
Odds of Developing Early Complications Based on Lymphatic Perfusion Pattern
| Lymphatic Perfusion Pattern | No. (%) of Patients (N=122)a | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)b | P Value |
|---|---|---|---|---|---|
| Type 1 | 39 (32) | Reference | ··· | ··· | ··· |
| Type 2 | 41 (34) | 1.55 (0.49–4.85) | 0.454 | 1.56 (0.48–5.00) | 0.458 |
| Type 3+4c | 42 (35)d | 6.05 (2.10–17.46)d | 0.001d | 6.28 (2.13–18.5)d | 0.001d |
OR indicates odds ratio.
Total number of patients with T2 imaging available for an analysis of lymphatic perfusion pattern.
Results hold after adjusting for cardiopulmonary bypass (CPB) time and ventricular morphological characteristics (left ventricle vs right ventricle [RV], with RV as reference group: OR, 0.46 [95% CI, 0.15–1.39] [P=0.168]; CBP time: OR, 1.02 [95% CI, 0.99–1.06] [P=0.213]).
Results for type 3 and type 4 hold separately.
Significant P value.
Discussion
Nearly one third of patients in our cohort developed complications within the first 6 months after Fontan completion surgery. On the surface, this proportion is jarringly higher than the incidence of short‐term Fontan complications that is reported in the literature. However, the assessment of early Fontan outcomes to date has largely focused on short‐term Fontan failure. In concordance with existing reports, the prevalence of the most severe complications was low, with ECMO, Fontan takedown, transplant listing, or death occurring in only 4% of our study cohort. However, by expanding our focus beyond short‐term Fontan failure, we were able to capture the extent of the morbidity experienced by this patient population as their cardiovascular system adapts to Fontan physiological characteristics. In the 6 months after Fontan completion, we found that 30% of patients experienced some combination of prolonged pleural effusions, unplanned catheterization, and/or readmission. This represents a significant emotional, physical, and financial cost to patients, their families, and the healthcare system. It challenges us to decrease the rates of early post‐Fontan morbidity, just as we have decreased the prevalence of perioperative mortality.
Risk factors identified in this study for the development of early post‐Fontan complications included prolonged bypass and cross‐clamp times. This has been well documented in previous studies.9, 11, 12 It is not surprising as both induce systemic inflammation and can lead to myocardial dysfunction.14 Previous studies have demonstrated that the right ventricular dysfunction that results from serving as the systemic ventricle for many years has been shown to affect long‐term outcomes.7, 8, 11 Not surprisingly, there were significantly more patients with a systemic right ventricle in the group who developed early complications. With regard to the presence and degree of preoperative atrioventricular valve regurgitation, there was no difference in the development of early complications, as other studies have also demonstrated.11, 15, 16 In our patient population, this finding may be partially attributed to the concomitant valvuloplasties performed in two thirds of the patients with moderate or severe atrioventricular valve regurgitation at the time of Fontan completion.
Unique to this study, we grouped the patients who developed complications according to the mostly likely cause of their morbidity. Currently, the most commonly available diagnostic techniques in pediatric cardiology are transthoracic echocardiography and cardiac catheterization. These modalities are best at evaluating ventricular function, valve regurgitation, and Fontan hemodynamics. Not surprisingly, common practice is to primarily consider ventricular dysfunction or structural issues with the Fontan circuit when a patient is clinically unwell in the postoperative period. In this cohort, slightly more than one fourth of patients with early complications had evidence of obstruction within the Fontan circuit, attributable to thrombus, vessel stenosis, or other causes. Another 8 patients had evidence of decreased ventricular function and/or atrioventricular valve regurgitation. However, detailed review of the procedural, imaging, and clinical data revealed that “structural failure” and “pump failure” accounted for only half of early Fontan morbidity. This begs the question, “what else is causing complications post‐Fontan?”
The other half of the patients who experienced postoperative morbidity developed complications related to plastic bronchitis, chylous effusions, or persistent pleural effusions in the absence of abnormal Fontan structure or function. The former 2 causes are directly related to abnormal lymphatic perfusion. The presence of persistent nonchylous effusions in the absence of structural or functional reasons for elevated Fontan pressure has not yet been well explained. However, we theorize that it could be explained by an imbalance in the normal physiologic relationship1, 10 between the lymphatic and cardiovascular circulation. More specifically, we speculate that the lymphatic vessels are unable to adequately resorb fluid from the extracellular space. In patients with normal central venous pressures or slightly elevated central venous pressure (as in the Glenn circulation), this abnormality may not manifest clinically. However, the persistently elevated central venous pressure inherent to Fontan physiological characteristics may overwhelm the abnormal lymphatic circulation and thus result in pleural effusions.1, 10, 17
Although the exact mechanism by which abnormalities in lymphatic function lead to post‐Fontan complications is not yet elucidated, our data do highlight the significance of abnormal lymphatic perfusion in the prediction of early post‐Fontan outcomes. The adoption of new imaging techniques in this patient population allowed us to identify preoperative high‐grade abnormal thoracic lymphatic perfusion as a risk factor for the development of early post‐Fontan complications. Of those patients who had T2 MRI sequences performed, the presence of mediastinal and pulmonary lymphatic perfusion (types 3 and 4) conferred a 6‐fold higher risk of developing early postoperative complications. Furthermore, the 7 patients with pulmonary lymphatic perfusion (type 4) experienced some of the most severe morbidity seen in this cohort. Three of those patients required ECMO support and subsequent Fontan takedown, one because of the short‐term development of plastic bronchitis. Of the remaining 4 patients, 1 developed plastic bronchitis, 1 had prolonged chylous effusions requiring multiple readmissions and chest tube placement, and 2 required immediate postoperative cardiac catheterizations. Although these findings do not allow us to comment on the causal nature of the relationship, it is certainly suggestive of an association between high‐grade lymphatic abnormalities and worse early Fontan outcomes.
Limitations
The most notable limitation is that the entire cohort did not have T2 imaging to determine lymphatic perfusion pattern. Because of the initial variability in clinical use of T2 imaging at our institution, this was not performed routinely for all pre‐Fontan assessments. Data analysis demonstrated that there was no statistically significant difference between the groups who did and did not have T2 imaging available, for development of early complications. However, the raw data reveal that there is a higher proportion of patients who developed complications in the group who had T2 imaging (62%) compared with those who did not (51%). When the sequence was first introduced, patients referred for T2 imaging may have had a worse clinical status compared with the rest of the cohort. Given the moderate sample size (122 and 104 patients with and without T2 imaging, respectively), it is possible that there is a true difference between the groups, but the study was not powered to detect that difference. All MRIs performed on patients undergoing single‐ventricle palliation now include T2 imaging, and this limitation will no longer exist for future studies.
Another limitation was the small percentage of patients with pleural effusions who had cellular analysis of the fluid performed. Routine fluid analysis on all patients with chest tube drainage for more than several days would have provided critical information in identifying concrete evidence of lymphatic dysfunction in patients who had prolonged pleural effusions.
Conclusions
These results have significant implications for the care of patients undergoing Fontan completion and highlight several important points. (1) As seen in our cohort, when we look beyond acute Fontan failure, we find that there is still substantial morbidity and complications in the early postoperative period. (2) Most of our current therapies and investigative tools are targeted to patients with structural or cardiac function abnormalities, which account for only half of early Fontan complications in this patient population. (3) Patients with preoperative evidence of high‐grade lymphatic perfusion abnormalities account for a higher proportion of patients who develop early complications, compared with those with low‐grade lymphatic anomalies. These findings force us to consider new techniques and therapies for targeting prolonged effusions and abnormal lymphatic perfusion as a means of improving early postoperative outcomes. In addition, this work lays the foundation for prospective research on the relationship between preoperative lymphatic perfusion patterns and early post‐Fontan outcomes. Such work has the potential to improve preoperative risk stratification and enable identification of patients with high‐grade lymphatic abnormalities, in whom intervention could reduce the risk of early postoperative morbidity and mortality.
Sources of Funding
Dr O'Byrne receives support from the National Institutes of Health/National Heart, Lung, and Blood Institute (HL130420‐01). Dr Ghosh is supported by the National Institutes of Health Institutional Training Grant (T32GM008562). The funding agencies had no role in the planning or execution of the study, nor did they edit the article. The article represents the opinions of the authors alone.
Disclosures
None.
Acknowledgments
The authors would like to acknowledge the Cardiac Center Clinical Research Core at the Children's Hospital of Philadelphia for providing statistical support for this article.
(J Am Heart Assoc. 2020;9:e015318 DOI: 10.1161/JAHA.119.015318.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic system in cardiovascular medicine. Circ Res. 2016;118:515–530. [DOI] [PubMed] [Google Scholar]
- 2. Rito Lo M, Al‐Radi O, Saedi A, Kotani Y, Sivarajan VB, Russell JL, Caldarone CA, Van Arsdell GS, Honjo O. Chylothorax and pleural effusion in contemporary extracardiac fenestrated Fontan completion. J Thorac Cardiovasc Surg. 2018;155:2069–2077. [DOI] [PubMed] [Google Scholar]
- 3. Kreutzer C, Kreutzer G. The lymphatic system: the Achilles heel of the Fontan‐Kreutzer circulation. World J Pediatr Congenit Heart Surg. 2017;8:613–623. [DOI] [PubMed] [Google Scholar]
- 4. Dori Y, Keller MS, Rome JJ, Gillespie MJ, Glatz AC, Dodds K, Goldberg DJ, Goldfarb S, Rychik J, Itkin M. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133:1160–1170. [DOI] [PubMed] [Google Scholar]
- 5. Dori Y, Keller MS, Fogel MA, Rome JJ, Whitehead KK, Harris MA, Itkin M. MRI of lymphatic abnormalities after functional single‐ventricle palliation surgery. AJR Am J Roentgenol. 2014;203:426–431. [DOI] [PubMed] [Google Scholar]
- 6. d'Udekem Y, de Leval M. The elusive and ungrateful lymphatic circulation may be a key determinant of Fontan failure. J Thorac Cardiovasc Surg. 2018;155:2067–2068. [DOI] [PubMed] [Google Scholar]
- 7. Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, Justo RN, Radford DJ, Weintraub RG, Bullock A, Celermajer DS, d'Udekem Y; Australia and New Zealand Fontan Registry. The extracardiac conduit Fontan procedure in Australia and New Zealand: hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardiothorac Surg. 2014;46:465–473. [DOI] [PubMed] [Google Scholar]
- 8. d'Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, Bullock A, Justo RN, Grigg LE, Sholler GF, Hope S, Radford DJ, Gentles TL, Celermajer DS, Winlaw DS. Redefining expectations of long‐term survival after the Fontan procedure: twenty‐five years of follow‐up from the entire population of Australia and New Zealand. Circulation. 2014;130:S32–S38. [DOI] [PubMed] [Google Scholar]
- 9. Allen KYA, Downing TE, Glatz ACGM, Rogers LSR, Ravishankar CR, Rychik JR, Fuller S, Montenegro LMM, Steven JMS, Spray TLS, Nicolson SC, Gaynor JW, Goldberg DJ. Effect of Fontan‐associated morbidities on survival with intact Fontan circulation. Am J Cardiol. 2017;119:1866–1871. [DOI] [PubMed] [Google Scholar]
- 10. Biko DM, DeWitt AG, Pinto EM, Morrison RE, Johnstone JA, Griffis H, OByrne ML, Fogel MA, Harris MA, Partington SL, Whitehead KK, Saul D, Goldberg DJ, Rychik J, Glatz AC, Gillespie MJ, Rome JJ, Dori Y. MRI evaluation of lymphatic abnormalities in the neck and thorax after Fontan surgery: relationship with outcome. Radiology. 2019;291:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, Rush CH, Gaynor JW, Goldberg DJ. 18 Years of the Fontan operation at a single institution. J Am Coll Cardiol. 2012;60:1018–1025. [DOI] [PubMed] [Google Scholar]
- 12. Downing TE, Allen KY, Goldberg DJ, Rogers LS, Ravishankar C, Rychik J, Fuller S, Montenegro LM, Steven JM, Gillespie MJ, Rome JJ, Spray TL, Nicolson SC, Gaynor JW, Glatz AC. Surgical and catheter‐based reinterventions are common in long‐term survivors of the Fontan operation. Circ Cardiovasc Interv. 2017;10:e004924. [DOI] [PubMed] [Google Scholar]
- 13. Book WM, Gerardin J, Saraf A, Marie Valente A, Rodriguez F III. Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis. 2016;11:296–308. [DOI] [PubMed] [Google Scholar]
- 14. Wessel DL. Managing low cardiac output syndrome after congenital heart surgery. Crit Care Med. 2001;29:S220–S230. [DOI] [PubMed] [Google Scholar]
- 15. Murphy MO, Glatz AC, Goldberg DJ, Rogers LS, Ravishankar C, Nicolson SC, Steven JM, Fuller S, Spray TL, Gaynor JW. Management of early Fontan failure: a single‐institution experience. Eur J Cardiothorac Surg. 2014;46:458–464. [DOI] [PubMed] [Google Scholar]
- 16. Ovroutski S, Sohn C, Barikbin P, Miera O, Alexi‐Meskishvili V, Hübler M, Ewert P, Hetzer R, Berger F. Analysis of the risk factors for early failure after extracardiac Fontan operation. Ann Thorac Surg. 2013;95:1409–1416. [DOI] [PubMed] [Google Scholar]
- 17. Simons M, Eichmann A. Physiology: lymphatics are in my veins. Science. 2013;341:622–624. [DOI] [PMC free article] [PubMed] [Google Scholar]


