Abstract
Background
Aerobic exercise capacity is inversely associated with cardiovascular and all‐cause mortality in men and women without coronary artery disease (CAD); however, a higher amount of vigorous exercise is associated with a J‐shaped relationship in CAD patients. Therefore, the optimal type and amount of exercise for CAD patients is unclear. Coronary artery calcification (CAC) is associated with increased cardiovascular disease (CVD) events and mortality. Fatty plaque is more likely to rupture and cause coronary events than other types. We examined the association between exercise capacity, fatty plaque, CAC score and CVD events in CAD patients.
Methods and Results
A total of 270 subjects with stable CAD were divided into tertiles based on metabolic equivalents of task (METs) calculated from exercise treadmill testing. Self‐reported exercise was obtained. Coronary computed tomographic angiography measured coronary plaque volume and CAC score. After adjustment, fatty plaque volume was not different among the 3 MET groups. For each 1 MET increase, CAC was 66.2 units lower (P=0.017). Those with CAC >400 and ≥8.2 METs had fewer CVD events over 30 months compared to <8.2 METs (P=0.037). Of moderate intensity exercisers (median, 240 min/wk; 78% walking only), 62.4% achieved ≥8.2 METs and lower CAC scores (P=0.07). Intensity and duration of exercise had no adverse impact on coronary plaque or CVD events.
Conclusions
Achieving ≥8.2 METs with moderate exercise intensity and volume as walking resulted in lower CAC scores and fewer CVD events. Therefore, vigorous exercise intensity and volume may not be needed for CAD patients to derive benefit.
Registration
URL: https://www.clinicaltrials.gov; Unique Identifier: NCT01624727.
Keywords: coronary artery calcification, coronary artery disease, coronary computed tomographic angiography, coronary fatty plaque, exercise capacity
Subject Categories: Computerized Tomography (CT), Exercise, Cardiovascular Disease, Exercise Testing, Coronary Artery Disease
Clinical Perspective
What Is New?
Fatty plaque, the type which ruptures causing acute coronary syndromes, was not different among coronary artery disease subjects based on metabolic equivalent of task (MET) tertiles: 4.0 to 8.1, 8.2 to 10.5, and ≥10.6 METs.
For each 1 MET increase, coronary artery calcification (CAC) score was 66.2 units lower; those with CAC >400 and achieving ≥8.2 METs had fewer CVD events compared to <8.2 METs over 30 months;
Of moderate intensity exercisers (median 240 min/week; 78% walking only), 62.4% achieved ≥8.2 METs with a trend toward lower CAC scores (P=0.07).
What Are the Clinical Implications?
Since CAC scores predict CVD events and mortality, maintaining high cardiorespiratory fitness (≥8.2 METs) with moderate exercise intensity and volume (median 240 min/wk) as walking, resulted in lower CAC scores and fewer CVD events in coronary artery disease subjects.
Therefore, vigorous exercise intensity and volume may not be needed for coronary artery disease patients to benefit as long as high cardiorespiratory fitness is maintained which can be achieved with moderate intensity and volume of exercise as walking.
Nonstandard Abbreviations and Acronyms .
CAC coronary artery calcification
CAD coronary artery disease
CVD cardiovascular disease
IQR interquartile range
METs metabolic equivalents of task
Introduction
Aerobic exercise capacity measured by metabolic equivalents of task (METs) has shown a dose‐dependent and graded inverse relationship with cardiovascular disease (CVD) morbidity and mortality and all‐cause mortality in men and women.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 For every 1‐MET increase in exercise capacity, total mortality was 16% lower in men without CVD, 9% lower in men with CVD, and 17% lower in women without CVD.1, 2, 3
Computed tomographic–based coronary artery calcium (CAC) scores are a reliable measure of subclinical coronary artery disease (CAD). In the CARDIA (Coronary Artery Risk Development in Young Adults) study, CAC scores of 1 to 19, 20 to 99, and >100 were associated with 2.6‐, 5.8‐, and 9.8‐fold higher increases in incident coronary heart disease and death, respectively, at 12.5‐year follow‐up.12 Higher CAC scores predict an increased risk of cardiovascular events and mortality in other studies.12, 13, 14, 15 Therefore, higher CAC scores are of concern. Several reports suggest that higher exercise volume or vigorous exercise may accelerate CAC formation in subjects who do not have clinical CAD.13, 16, 17, 18, 19 For example, over a 25‐year period in the CARDIA Study, the CAC score was 1.86‐fold higher in those who exercised 450 minutes per week compared with those exercising <150 minutes per week.17 In the Cooper Center Longitudinal Clinic Study of 21,758 men without clinical CAD (mean age, 51.7±8.4 years) referred for preventive health screening between 1998 and 2013, those who exercised ≥3000 MET minutes per week had an 11% higher risk (95% CI, 1.03–1.20) of a CAC score of at least 100 (mean±SD, 800±1120) compared with those exercising <3000 MET minutes per week.13 In the >3000‐MET min/wk group, those with CAC ≥100 had a 4‐fold higher rate of all‐cause death (6% versus 1.2%, respectively) and 6‐ to 8‐fold higher risk of cardiovascular death (1.8% versus 0.2%, respectively) compared with those with CAC <100 at mean follow‐up of 10.4 years.13 These findings suggest that mortality is higher with CAC >100 compared with those with CAC <100 and similar amounts of exercise.
A J‐shaped association has been reported between exercise volume and cardiac events in subjects with stable CAD with the most highly active patients having a 2.36‐fold increased risk of cardiovascular mortality (95% CI, 1.05–5.34)20 and a 3.19‐fold increase (95% CI, 1.42–6.79) in all ischemic heart disease–related mortality.21 Therefore, it is unclear what the optimal exercise recommendation is for patients with CAD. The relationship between aerobic fitness, exercise intensity and volume, and degree of coronary atherosclerosis is unknown in subjects with CAD. In order to gain information on this relationship and beneficial exercise levels for patients with clinical CAD, we examined the effect of exercise capacity and exercise intensity and volume on coronary plaque composition, CAC scores, and CVD events in subjects with CAD. We were especially interested in fatty plaque given that it is lipid rich and thus more likely to rupture, leading to thrombosis and acute myocardial infarction, compared with fibrous or calcified plaque.22
Methods
Imaging methods, analytical methods, and study materials have been made available to other researchers for purposes of reproducing the results.23, 24 The exercise data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
The current report is a cross‐sectional analysis at baseline of the relationship between coronary plaque subtypes and exercise capacity and 30‐month follow‐up for cardiovascular events in the HEARTS (Slowing Heart Disease With Lifestyle and Omega‐3 Fatty Acids) trial conducted at Beth Israel Deaconess Medical Center (Boston, MA). The trial is a randomized, parallel, single‐center study of 3.36 g daily of the omega‐3 ethyl esters, eicosapentaenoic acid and docosahexaenoic acid, added to statin therapy compared with statin alone over 30 months. The primary end point of the trial is the effect of omega‐3 ethyl esters on progression of coronary arterial plaque at 30 months of follow‐up and has been reported.25 Several prespecified secondary end points have been reported.26, 27 The protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board, and all subjects signed informed consent.
Participants
Eligible participants were aged 37 to 80 years and had stable CAD defined as at least one of the following: ≥50% stenosis in at least 1 coronary artery at cardiac catheterization; previous myocardial infarction (≥6 months previous) or percutaneous coronary intervention (≥6 months previous); coronary bypass surgery (≥12 months previous); abnormal exercise treadmill test (defined as at least 1 mm of horizontal or downsloping ST depression in at least 2 contiguous ECG leads); or an area of reversible ischemia on nuclear imaging, pharmacological stress, or stress echocardiography with subsequent revascularization. All subjects underwent a maximal, graded exercise treadmill stress test at baseline using the Bruce protocol. The test was stopped according to the usual criteria for exercise testing: either exhaustion or reaching 100% of the maximum heart rate (220–age). Exercise capacity was measured by METs, which were calculated using the speed and grade of the treadmill as described.28
Clinical and Biochemical Parameters
A detailed history, physical examination, height, weight, waist measurement, blood pressure measurement, ECG, and ascertainment of medications were obtained at baseline. Blood samples were obtained after a 12‐hour fast, and plasma and serum were prepared. Glucose, hemoglobin A1c, chemical profile, total white blood cell count, absolute neutrophil, lymphocyte, monocyte and platelet counts, and lipid panel were measured at Quest Diagnostics (Cambridge, MA).
Exercise and Cardiac Events
Detailed physical activity was collected at baseline and included the type of exercise, number of sessions per week, and minutes per session. Exercise was then converted to total minutes per week. Vigorous activities (≥6 METs) included running or jogging, biking, running on a treadmill, elliptical, stepper, swimming, rowing, dancing, exercise or dance class, and strenuous sports (racquet sports, tennis, and soccer).29 Moderate‐intensity activities (3.0–5.9 METs) included nonstrenuous sports, walking and hiking, golfing and bowling, home exercises or calisthenics, and home maintenance or gardening.29 Subjects reported cardiac events at 6‐month follow‐up visits until final follow‐up at 30 months. Events were documented through review of medical records.
Image Acquisition, Reconstruction, and Coronary Plaque Analysis
Prospective ECG gated imaging was performed at Beth Israel Deaconess Medical Center using a 320‐row detector scanner (Aquilion ONE; Toshiba Medical Systems, Otawara, Japan). The protocol for performance of coronary computed tomographic angiography, plaque identification, and quantification has been published, with representative images shown in Supplement 2 of a previous work (Hauser et al (ref 24)).23, 24, 25, 27 Semiautomated software (SUREPlaque, version 6.3.2; Vital Images, Minnetonka, MN) was used to reconstruct 3‐dimensional coronary computed tomographic angiography images for coronary segment plaque volume analysis.30, 31, 32, 33 Segments with previous revascularization or significant calcification causing calcium‐bloom artifact were excluded. Plaque analysis was performed independently by 2 readers blinded to treatment allocation. Hounsfield unit densities defined plaques as fatty (−100 to 49 Hounsfield units), fibrous (50–150 Hounsfield units), and calcified plaque as >150 Hounsfield units. Noncalcified plaque was the sum of fatty and fibrous plaque. The intra‐ and interobserver agreement indices were 0.99 and 0.98, respectively, showing excellent correlation between readings.25 Plaque volumes were indexed to the length of the artery examined; indexed plaque volume was defined as plaque volume (mm3) divided by artery segment length (mm).
CAC Score Quantification
For CAC measurements, a noncontrast cardiac computed tomography scan was performed before the coronary computed tomographic angiography using prospective ECG triggering over a single heartbeat with a gantry rotation and x‐ray exposure time of 0.35 seconds and 0.5‐mm slice collimation. All images were reconstructed and interpreted in a blinded fashion by 2 independent observers. In the noncontrast scan, total coronary artery calcium volume score was measured by the Agatston technique on a commercially available computed tomography workstation (Vitrea, FX version 3.0 workstation; Vital Images, Minnetonka, MN).34 The volume score was computed as the sum of the total volume of calcium of the calcified regions in all vessels.
Statistical Analysis
Subjects were divided into tertiles based on METs achieved during exercise testing. Because of a large number of subjects in the 8.2‐MET category, group 1 has a slightly smaller number of subjects than groups 2 and 3. Categorical variables were expressed as counts and percentages and analyzed using chi‐square tests. Normality tests were conducted using the Shapiro–Wilk test. Continuous variables were reported as the mean and SD for normally distributed variables or median and interquartile range [IQR] for non‐normally distributed variables. Plaque volumes were not normally distributed and therefore are reported as median [IQR]. Continuous variables were compared using unpaired (between‐group comparisons) t tests or ANOVA for 3‐group comparison for normally distributed variables or the Mann–Whitney U test (between‐group comparisons) or Kruskal–Wallis for non‐normally distributed variables. Collinearity was assessed with the variance inflation factor. Residual plots were analyzed to assess for eligibility of a linear regression model. Homoscedasticity was assessed. Scatter plots of the dependent variables were assessed to evaluate any nonlinearity. A linear regression model was followed given that it was most suitable. The association between indexed plaque volume components, CAC score, and exercise MET groups was examined using multivariate linear regression adjusting for patient characteristics. A 2‐sided P≤0.05 was considered statistically significant. Data analyses were performed using SPSS software (version 20.0; IBM Corp., Armonk, NY). Drs Malik and Asbeutah had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Results
A total of 270 subjects underwent maximal exercise treadmill testing. All subjects achieved at least 4 METs. Baseline characteristics are shown in Table 1. The highest MET group reported a median exercise of 200 minutes per week compared with 85 minutes in the lowest MET group. Compared with those in the lowest MET group, those in the highest MET group were significantly younger, more likely to be male, had less hypertension or diabetes mellitus, and lower body mass index, waist circumference, systolic blood pressure, glucose, hemoglobin A1c, triglyceride, and neutrophil count and were less likely to be taking an angiotensin receptor blocker, thiazide diuretic, furosemide, or calcium‐channel blocker.
Table 1.
Characteristics Stratified by the 3 Exercise MET Groups
| Characteristics | MET Exercise Group | ||||
|---|---|---|---|---|---|
| Group 1 4.0 to 8.1 METs n=77 | Group 2 8.2 to 10.5 METs n=98 | Group 3 ≥10.6 METs n=95 | ANOVA P Valuea | P Valueb | |
| Min exercise/wk mean (SD) | 142 (201) | 198 (224) | 229 (180) | 0.018 | |
| Min exercise/wk median [IQR] | 85 [0, 210] | 150 [0, 280] | 200 [120, 313] | <0.001 | |
| Demographic characteristics | |||||
| Age, mean±SD, y | 65.5±6.8 | 63.8±6.6 | 59.9±8.5 | <0.001 | <0.001 |
| Male sex, n (%) | 54 (70.1) | 81 (82.7) | 89 (93.7) | 0.039 | <0.001 |
| Inclusion criteria (may have >1), n (%) | |||||
| History of myocardial infarction | 37 (48.1) | 43 (43.9) | 44 (46.3) | 0.90 | 0.77 |
| History of percutaneous coronary intervention | 45 (58.4) | 59 (60.2) | 64 (67.4) | 0.85 | 0.48 |
| History of coronary bypass grafting | 17 (22.1) | 31 (31.6) | 14 (14.7) | 0.074 | 0.25 |
| Cardiovascular risk factors, n (%) | |||||
| History of hypertension | 66 (85.7) | 87 (88.8) | 72 (75.8) | 0.10 | 0.043 |
| History of diabetes mellitus | 30 (39.0) | 32 (32.7) | 13 (13.7) | 0.002 | <0.001 |
| Anthropometric and blood pressure, mean±SD | |||||
| Weight, kg | 92.3±16.0 | 92.1±14.7 | 88.8±11.2 | 0.11 | 0.13 |
| Body mass index, kg/m2 | 31.5±4.1 | 30.8±3.5 | 29.2±2.8 | <0.001 | <0.001 |
| Waist circumference, cm | 110.5±11.7 | 106.7±10.4 | 102.5±7.6 | <0.001 | <0.001 |
| Systolic blood pressure, mm Hg | 124.7±13.4 | 126.0±15.7 | 120.9±12.3 | 0.094 | 0.036 |
| Diastolic blood pressure, mm Hg | 70.2±9.0 | 74.2±10.6 | 74.3±8.7 | 0.034 | 0.002 |
| Biochemical profile, mean±SD | |||||
| Glucose, mmol/L | 6.15±1.88 | 6.00±2.13 | 5.47±1.13 | 0.18 | 0.048 |
| Hemoglobin A1c, % | 6.5±1.1 | 6.1±0.8 | 5.9±0.8 | 0.008 | <0.001 |
| Albumin creatinine ratio, median [IQR] | 8.4 [4.3, 25.0] | 4.0 [2.3, 9.4] | 3.5 [2.3, 5.7] | <0.001 | 0.30 |
| Lipids, mean±SD, mg/dLc | |||||
| Total cholesterol | 151.1±33.8 | 150.2±31.6 | 141.0±29.5 | 0.10 | 0.11 |
| Triglyceride, median [IQR] | 135.0 [97.0, 205.0] | 116.0 [88.3, 172.8] | 88.0 [69.8, 122.3] | 0.001 | <0.001 |
| High‐density lipoprotein‐C | 43.8±14.4 | 46.2±15.0 | 46.7±13.6 | 0.77 | 0.46 |
| Low‐density lipoprotein‐C | 84.7±27.0 | 85.0±23.3 | 80.4±24.8 | 0.31 | 0.47 |
| Complete blood count, mean±SD | |||||
| WBC, 109 cells/L | 6.9±1.9 | 6.7±1.8 | 6.1±1.5 | 0.18 | 0.092 |
| Monocytes, cells/μL | 553.0±181.2 | 522.6±163.3 | 503.4±151.0 | 0.13 | 0.10 |
| Neutrophils, cells/μL | 4416.8±1678.9 | 4325.4±1675.3 | 3872.7±1234.0 | 0.013 | 0.025 |
| Lymphocytes, cells/μL | 1744.4±676.9 | 1703.9±568.9 | 1537.7±473.0 | 0.91 | 0.85 |
| Platelets, cells/μL | 199.1±57.2 | 197.7±53.1 | 178.8±43.9 | 0.17 | 0.061 |
| Medications, n (%) | |||||
| Statin | 76 (98.7) | 91 (92.9) | 91 (95.8) | 0.48 | 0.18 |
| Aspirin | 75 (97.4) | 92 (93.9) | 92 (96.8) | 0.20 | 0.43 |
| Angiotensin‐converting enzyme inhibitor | 38 (49.4) | 54 (55.1) | 55 (57.9) | 0.74 | 0.53 |
| Angiotensin receptor blocker | 22 (28.6) | 16 (16.3) | 11 (11.6) | 0.53 | 0.013 |
| Thiazide diuretic | 22 (28.6) | 14 (14.3) | 16 (16.8) | 0.077 | 0.045 |
| Furosemide | 12 (15.6) | 6 (6.1) | 2 (2.1) | 0.025 | 0.003 |
| Calcium‐channel blocker | 22 (28.6) | 28 (28.6) | 13 (13.7) | 0.011 | 0.022 |
| Beta‐blocker | 61 (79.2) | 67 (68.4) | 67 (70.5) | 0.59 | 0.25 |
C indicates cholesterol; IQR, interquartile range; MET, metabolic equivalent of task; and WBC, white blood cells.
P values derived using Pearson's chi square for categorical variables and Kruskal–Wallis test for nonparametric data.
P value compares group 1 with group 3.
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglyceride to millimoles per liter, multiply by 0.01129.
Exercise Capacity
Table 2 reports that those in the highest MET group (≥10.6) had significantly larger amounts of fatty, fibrous, and total plaque compared with the lowest MET group in the univariate analysis. Figure 1 shows box plots for the coronary plaque results in Table 2. After adjustment for age, sex, body mass index, diastolic blood pressure, diabetes mellitus status, level of low‐density lipoprotein cholesterol and triglyceride, and use of medications, no difference was observed in fatty plaque among the 3 exercise groups (P=0.23; Bonferroni adjusted, P=1.0). The CAC score was 241.3 Agatston units lower in the highest MET group compared with the lowest in the univariate analysis (P=0.011; Table 2). After multivariate adjustment, the difference became more significant (P=0.008). Figure 2 reports the relationship between CAC score and METs achieved as a continuous variable. For every 1‐MET increase in exercise capacity, the CAC score was 66.2 Agatston units lower (P=0.017).
Table 2.
Comparison of Indexed Volume for Coronary Plaque Subtypes and CAC Score Stratified by the Exercise MET Groups in Uni‐ and Multivariate Analyses
| MET Groups | Univariate | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| Group 1 n=77 4.0 to 8.1 METs | Group 2 n=98 8.2 to 10.5 METs | Group 3 n=95 ≥10.6 METs | ANOVA P Value | P Value Group 1 vs Group 3 | β | P Valuea | |
| Plaque subtypesb | |||||||
| Fatty plaque | 7.6 [4.0, 12.8] | 8.5 [4.1, 13.1] | 9.9 [6.8, 15.3] | 0.008 | 0.008 | 0.63 | 0.23 |
| Fibrous plaque | 12.3 [7.4, 21.5] | 13.4 [7.3, 22.9] | 20.6 [12.1, 27.3] | <0.001 | <0.001 | 1.96 | 0.028 |
| Noncalcified plaque | 22.5 [11.6, 33.8] | 21.6 [11.2, 35.6] | 31.3 [18.3, 42.6] | 0.001 | 0.002 | 2.60 | 0.064 |
| Total plaque | 23.9 [14.7, 41.0] | 25.5 [15.0, 41.4] | 39.5 [22.9, 47.6] | 0.001 | 0.002 | 3.50 | 0.038 |
| CACc | 542.9 [227.4, 1327.3] | 461.9 [84.0, 1216.1] | 297.8 [120.4, 769.8] | 0.050 | 0.011 | −241.3 | 0.008 |
Data presented as median [interquartile range]. CAC indicates coronary artery calcium score; LDL‐C, low‐density lipoprotein cholesterol; and MET, metabolic equivalent of task.
Adjusted for age, sex, body mass index, diastolic blood pressure, diabetes mellitus status, level of LDL‐C and triglyceride, and medications, which include statin, angiotensin receptor blocker, thiazide diuretic, furosemide, and calcium‐channel blocker. Bonferroni adjusted P values are P=1.0 for fatty plaque; P=0.14 for fibrous plaque; P=0.32 for noncalcified plaque; P=0.19 for total plaque; and P=0.04 for CAC score.
Plaque volume expressed as mm3 divided by artery segment length (mm).
CAC score using Agatston technique and expressed in Agatston units.
Figure 1. Box plots of coronary plaque results for fatty, fibrous, noncalcified, and total plaque shown in Table 2.
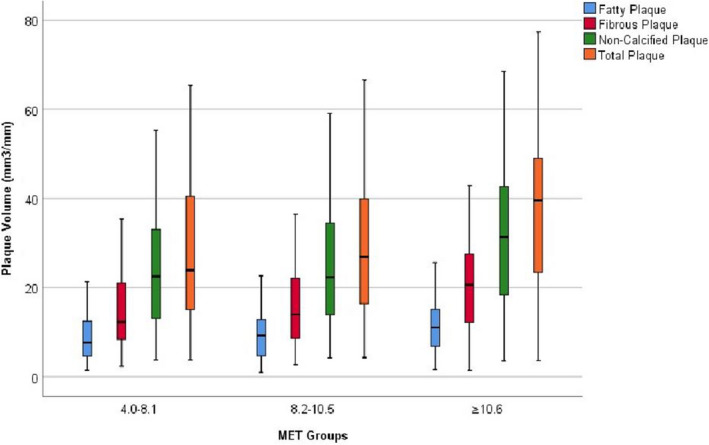
The horizontal bar is the median with the 25th percentile (first quartile; Q1) and 75th percentile (third quartile; Q3) represented by the bars below and above the median, respectively. The lower end of the whiskers is calculated as (Q1–1.5*IQR [interquartile range]) and the upper end of the whiskers is calculated as (Q3+1.5*IQR). Adjusted P values for comparison among the 3 groups as shown in Table 2 are: fatty plaque: 0.23; fibrous plaque: 0.028; non‐calcified plaque: 0.064 and total plaque: 0.038. IQR indicates interquartile range; MET, metabolic equivalents of task.
Figure 2. Relationship between CAC score and METs achieved as a continuous variable.
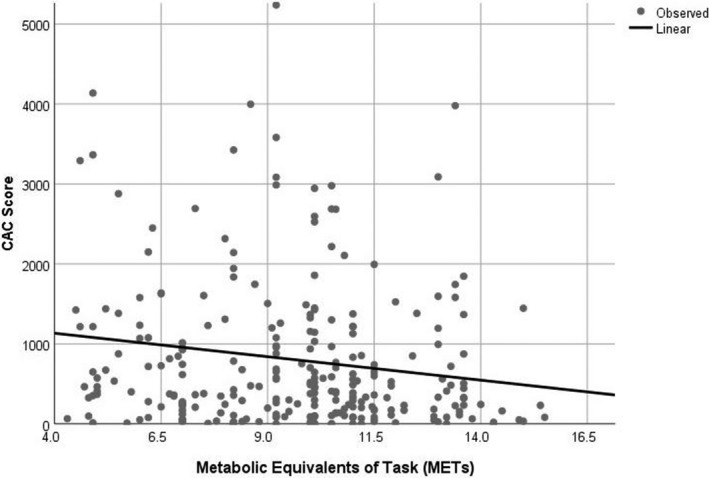
For every 1‐MET increase in exercise capacity, the CAC score was 66.2 Agatston units lower (P=0.017). CAC indicates coronary artery calcium score; MET, metabolic equivalents of task.
Exercise Intensity and Volume
Subjects were divided into 3 groups: no exercise, moderate‐intensity exercise, and high‐intensity exercise. Those subjects who exercised reported regular frequency on a weekly basis. Table 3 reports that hemoglobin A1c, albumin creatinine ratio, total cholesterol, triglyceride, low‐density lipoprotein cholesterol, neutrophil count, and statin were significantly different among the 3 groups. Table 4 reports no difference in fatty, fibrous, noncalcified, or total plaque or CAC score among the 3 exercise intensity groups. Figure 3 reports the relationship between CAC score and minutes per week of moderate and high exercise intensity as continuous variables. As minutes per week increased, the CAC score remained relatively constant for moderate‐intensity exercise (Figure 3; β=0.108, P=0.78), with a slight, but nonsignificant, decrease for high‐intensity exercise (Figure 3; (β=−0.72, P=0.42). These findings suggest no adverse effect of increasing min/wk of either moderate‐ or high‐intensity exercise on CAC score.
Table 3.
Characteristics Stratified by the 3 Exercise Intensity Groups
| Characteristics | No Exercise (n=75) | Moderate‐Intensity Exercise (n=89) | High‐Intensity Exercise (n=106) | P Valuea |
|---|---|---|---|---|
| Min exercise/wk, mean (SD) | 0 (0) | 285 (225) | 255 (115) | <0.001 |
| Min exercise/wk, median [IQR] | 0 [0] | 225 [124, 339] | 315 [210, 522] | <0.001 |
| Demographic characteristics | ||||
| Age, mean±SD, y | 63.5 (7.7) | 64.0 (6.5) | 61.8 (8.4) | 0.10 |
| Male sex, n (%) | 58 (77.3) | 75 (80.1) | 96 (89.7) | 0.062 |
| Inclusion criteria (may have >1), n (%) | ||||
| History of myocardial infarction | 27 (36.0) | 49 (52.7) | 51 (47.7) | 0.31 |
| History of percutaneous coronary intervention | 48 (64.0) | 58 (62.4) | 65 (60.7) | 0.99 |
| History of coronary bypass grafting | 15 (20.0) | 22 (23.7) | 28 (26.2) | 0.91 |
| Cardiovascular risk factors, n (%) | ||||
| History of hypertension | 68 (90.1) | 74 (79.6) | 88 (82.8) | 0.14 |
| History of diabetes mellitus | 25 (33.3) | 28 (30.1) | 25 (23.4) | 0.31 |
| Anthropometric and blood pressure, mean±SD | ||||
| Weight, kg | 92.6 (13.9) | 88.8 (15.5) | 92.8 (13.4) | 0.11 |
| Body mass index, kg/m2 | 31.4 (3.8) | 30.1 (3.9) | 30.6 (3.3) | 0.085 |
| Waist circumference, cm | 108.9 (10.6) | 105.9 (10.8) | 105.5 (10.0) | 0.076 |
| Systolic blood pressure, mm Hg | 127.4 (14.4) | 124.4 (16.7) | 123.1 (12.4) | 0.14 |
| Diastolic blood pressure, mm Hg | 74.0 (10.5) | 72.6 (9.8) | 74.0 (9.0) | 0.46 |
| Biochemical profile, mean±SD | ||||
| Glucose, mmol/L | 109.8 (32.5) | 111.7 (42.2) | 101.3 (25.1) | 0.09 |
| Hemoglobin A1c, % | 6.4 (1.0) | 6.3 (1.1) | 5.9 (0.6) | 0.001 |
| Albumin creatinine ratio, median [IQR] | 5.4 [3.2, 11.2] | 4.7 [2.9, 11.1] | 3.8 [2.1, 7.6] | 0.041 |
| Lipids, mean±SD, mg/dLb | ||||
| Total cholesterol | 164.5 (43.3) | 148.3 (28.5) | 147.0 (32.4) | 0.003 |
| Triglyceride, median [IQR] | 131.0 [109.0, 197.0] | 116.0 [77.0, 163.0] | 108.5 [72.3, 150.0] | 0.009 |
| High‐density lipoprotein‐C | 46.1 (13.3) | 47.5 (15.1) | 46.9 (13.9) | 0.81 |
| Low‐density lipoprotein‐C | 88.7 (36.7) | 74.2 (20.7) | 76.0 (24.3) | 0.002 |
| Complete blood count, mean±SD | ||||
| WBC, 109 cells/L | 6.9 (1.8) | 6.8 (1.9) | 6.4 (2.5) | 0.36 |
| Monocytes, cells/μL | 546.2 (193.7) | 522.7 (139.9) | 515.2 (161.6) | 0.46 |
| Neutrophils, cells/μL | 4278.0 (1497) | 4110.1 (1699.0) | 3921.3 (1386.2) | 0.041 |
| Lymphocytes, cells/μL | 1792.9 (621.7) | 1586.8 (638.7) | 1805.6 (1999.0) | 0.54 |
| Platelets, cells/μL | 202.9 (54.9) | 190.5 (50.9) | 185.3 (48.6) | 0.082 |
| Medications, n (%) | ||||
| Statin | 68 (90.1) | 91 (97.8) | 104 (97.2) | 0.046 |
| Aspirin | 71 (94.7) | 88 (94.6) | 105 (98.1) | 0.36 |
| Angiotensin‐converting enzyme inhibitor | 41 (54.7) | 53 (57.0) | 59 (55.1) | 0.95 |
| Angiotensin receptor blocker | 16 (21.3) | 14 (15.1) | 18 (16.8) | 0.55 |
| Thiazide diuretic | 16 (21.3) | 16 (17.2) | 20 (18.7) | 0.79 |
| Furosemide | 8 (10.7) | 7 (7.5) | 7 (6.5) | 0.59 |
| Calcium‐channel blocker | 21 (28.0) | 24 (25.8) | 21 (19.6) | 0.18 |
| Beta‐blocker | 60 (80.0) | 70 (75.3) | 71 (66.4) | 0.11 |
C indicates cholesterol; IQR, interquartile range; MET, metabolic equivalent of task; and WBC, white blood cells.
P values derived using Pearson's chi square for categorical variables, ANOVA for normally distributed data, and Kruskal–Wallis test for nonparametric data.
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglyceride to millimoles per liter, multiply by 0.01129.
Table 4.
Comparison of Indexed Volume for Coronary Plaque Subtypes and CAC Score Stratified by the Exercise Intensity Groups
| No Exercise (n=75) | Moderate Intensity (n=89) | High Intensity (n=106) | ANOVA P Value | P Value No Exercise vs High Intensity | |
|---|---|---|---|---|---|
| Plaque subtypesa | |||||
| Fatty plaque | 9.6 [5.5, 14.1] | 8.0 [4.3, 13.1] | 9.6 [5.6, 14.2] | 0.42 | 0.88 |
| Fibrous plaque | 16.0 [9.4, 23.9] | 15.0 [8.9, 22.4] | 17.6 [17.6, 25.2] | 0.29 | 0.59 |
| Noncalcified plaque | 25.3 [15.0, 37.2] | 22.4 [15.0, 35.3] | 27.4 [15.0, 47.5] | 0.36 | 0.69 |
| Total plaque | 30.2 [18.1, 43.8] | 27.3 [15.8, 41.8] | 33.3 [18.6, 46.2] | 0.32 | 0.75 |
| CACb | 356.9 [83.6, 978.0] | 455.2 [170.2, 984.6] | 516.6 [142.0, 1350.6] | 0.28 | 0.46 |
Data presented as median [interquartile range]. CAC indicates coronary artery calcium score.
Plaque volume expressed as mm3 divided by artery segment length (mm).
CAC score using Agatston technique and expressed in Agatston units.
Figure 3. Relationship between CAC score and minutes per week of moderate‐intensity exercise (A) and high exercise intensity (B) as continuous variables.
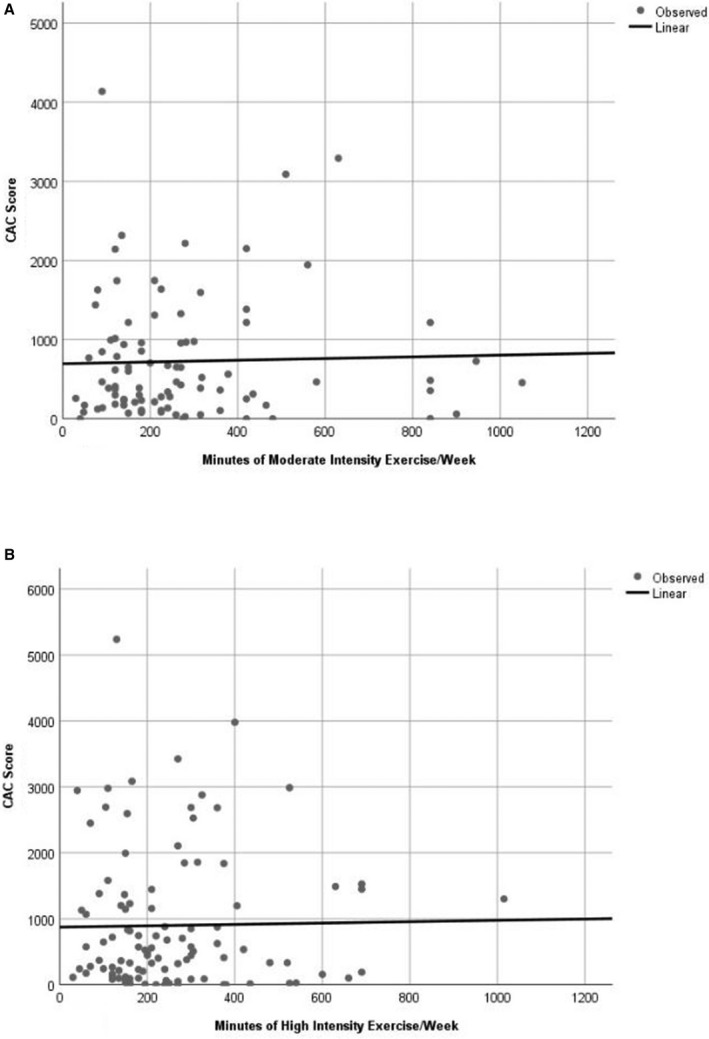
As minutes per week increased, the CAC score remained relatively constant for moderate‐intensity exercise (A) with a β=0.108 and P=0.78 with a slight, but not significant, decrease for high‐intensity exercise (B) with a β=−0.72 and P=0.42. CAC indicates coronary artery calcium score; MET, metabolic equivalents of task.
Of those doing moderate exercise, 62.4% achieved ≥8.2 METs, with 30.1% of these achieving ≥10.6 METs. They achieved this with a median [IQR] of 240 [140, 365] and 213 [143,315] minutes of exercise per week, respectively. Because CVD events were lower in the total group of those achieving ≥8.2 METs (see Cardiac Event Section below), we stratified results by 8.2 METs. Table 5 reports that those achieving ≥8.2 METs had a trend toward a lower CAC score compared with those achieving <8.2 METs (median [IQR], 370.7 [132.5, 826.9 versus 575.0 [222.8, 1408.3]; P=0.07); 78% of these were walking only. After adjustment for the differences in characteristics reported in Table 5—significantly lower waist circumference, total cholesterol and triglyceride level, and albumin creatinine ratio ratio compared with those achieving <8.2 METs—no material difference was noted in CAC score or plaque composition.
Table 5.
Characteristics, Plaque Composition, and CAC Score Stratified by MET Group for Those Exercising at Moderate Intensity
| MET Exercise Group | |||
|---|---|---|---|
| 4.0 to 8.1 METs (n=33) | ≥8.2 METs (n=56) | P Valuea | |
| Min exercise/wk, mean (SD) | 260 (236) | 294 (215) | 0.48 |
| Min exercise/wk, median [IQR] | 180 [90, 315] | 240 [140, 365] | 0.12 |
| Demographic characteristics | |||
| Age, mean±SD, y | 64.5 (6.6) | 63.7 (7.1) | 0.62 |
| Male sex, n (%) | 25 (71.4) | 50 (86.2) | 0.081 |
| Inclusion criteria (may have >1), n (%) | |||
| History of myocardial infarction | 17 (48.6) | 32 (65.3) | 0.57 |
| History of percutaneous coronary intervention | 22 (62.9) | 36 (62.1) | 0.74 |
| History of coronary bypass grafting | 9 (25.7) | 13 (22.4) | 0.70 |
| Cardiovascular risk factors, n (%) | |||
| History of hypertension | 26 (74.3) | 25 (82.8) | 0.33 |
| History of diabetes mellitus | 13 (37.1) | 15 (25.9) | 0.25 |
| Anthropometric and blood pressure, mean±SD | |||
| Weight, kg | 93.3 (18.6) | 86.3 (12.5) | 0.057 |
| Body mass index, kg/m2 | 31.9 (4.6) | 29.0 (2.7) | 0.001 |
| Waist circumference, cm | 110.3 (12.7) | 103.2 (8.1) | 0.004 |
| Systolic blood pressure, mm Hg | 125.6 (16.4) | 123.2 (17.4) | 0.51 |
| Diastolic blood pressure, mm Hg | 70.8 (8.8) | 73.3 (10.2) | 0.22 |
| Biochemical profile, mean±SD | |||
| Glucose | 111.3 (37.6) | 110.6 (44.2) | 0.94 |
| Hemoglobin A1c, % | 6.4 (0.92) | 6.2 (1.2) | 0.38 |
| Albumin creatinine ratio, median [IQR] | 6.3 [4.3, 26.5] | 3.9 [2.8, 7.6] | 0.016 |
| Lipids, mean±SD, mg/dLb | |||
| Total cholesterol | 159.2 (34.9) | 144.2 (27.4) | 0.024 |
| Triglyceride, median [IQR] | 141.0 [79.0187.0] | 109.0 [74.5147.0] | 0.010 |
| High‐density lipoprotein‐C | 48.9 (19.9) | 46.7 (11.9) | 0.51 |
| Low‐density lipoprotein‐C | 77.6 (27.7) | 73.4 (19.1) | 0.40 |
| Complete blood count, mean±SD | |||
| WBC, 109 cells/L | 7.0 (1.9) | 6.7 (1.9) | 0.36 |
| Monocytes, cells/μL | 518.4 (135.1) | 525.2 (140.4) | 0.82 |
| Neutrophils, cells/μL | 4528.4 (1627.5) | 4412.2 (1708.4) | 0.75 |
| Lymphocytes, cells/μL | 1766.3 (808.2) | 1513.1 (505.9) | 0.066 |
| Platelets, cells/μL | 201.5 (62.5) | 185.4 (41.4) | 0.19 |
| Plaque subtypes, median [IQR]c | |||
| Fatty plaque | 5.4 [3.2, 12.2] | 8.6 [5.5, 13.8] | 0.13 |
| Fibrous plaque | 11.7 [5.3, 19.5] | 16.8 [9.7, 23.7] | 0.065 |
| Noncalcified plaque | 15.5 [8.5, 32.0] | 26.1 [15.3, 36.9] | 0.087 |
| Total plaque | 19.9 [8.8, 39.7] | 29.5 [18.7, 44.9] | 0.093 |
| CACd | 575.0 [222.8, 1408.3] | 370.7 [132.5, 826.9] | 0.070 |
| Medications, n (%) | |||
| Statin | 35 (100.0) | 56 (96.6) | 0.45 |
| Aspirin | 35 (100.0) | 53 (91.4) | 0.074 |
| Angiotensin‐converting enzyme inhibitor | 18 (51.4) | 35 (60.3) | 0.45 |
| Angiotensin receptor blocker | 6 (17.1) | 8 (13.8) | 0.27 |
| Thiazide diuretic | 7 (20.0) | 9 (15.5) | 0.58 |
| Furosemide | 4 (11.4) | 3 (5.2) | 0.27 |
| Calcium‐channel blocker | 12 (34.3) | 12 (20.7) | 0.43 |
| Beta‐blocker | 25 (71.4) | 45 (77.6) | 0.89 |
CAC indicates coronary artery calcium score; IQR, interquartile range; MET, metabolic equivalent of task; and WBC, white blood cells.
P values derived using Pearson's chi square for categorical variables, Student t test for normally distributed data, and Mann–Whitney U test for nonparametric data.
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglyceride to millimoles per liter, multiply by 0.01129.
Plaque volume expressed as mm3 divided by artery segment length (mm).
CAC score using the Agatston technique and expressed in Agatston units.
Of those doing high‐intensity exercise, 87.9% achieved ≥8.2 METs, with 52.3% of these achieving ≥10.6 METs. They exercised a total median [IQR] of 315 [210, 522] minutes of exercise per week. Approximately half of this was moderate‐intensity exercise and half was high‐intensity exercise. Those achieving ≥8.2 METs had a trend toward a lower CAC score compared with those achieving <8.2 METs (median [IQR], 397.4 [101.5, 1261.7] versus 744.8 [412.4, 2123.3]; P=0.098).
Cardiac Events
Cardiac events included unstable angina, myocardial infarction, percutaneous coronary interventions, positive exercise treadmill test, and death. Subjects in the highest MET group had significantly fewer cardiac events compared with those in the lowest MET group at 30‐month follow‐up (4.25% versus 12.9%, respectively; P=0.038). Figure 4 reports the Kaplan–Meier plots for time to first cardiovascular event for the 3 MET groups for those with CAC score >400. The combination of MET groups 2 and 3 (which is ≥8.2 METs) had significantly fewer adverse cardiovascular events compared with <8.2 METs (P=0.037). When time to events by minutes of exercise per week was examined by intensity of exercise, there was no significant difference among the 3 MET groups (P=0.21; data not shown).
Figure 4. Kaplan–Meier plots for time to cardiovascular events for the 3 MET groups for those with CAC score >400.
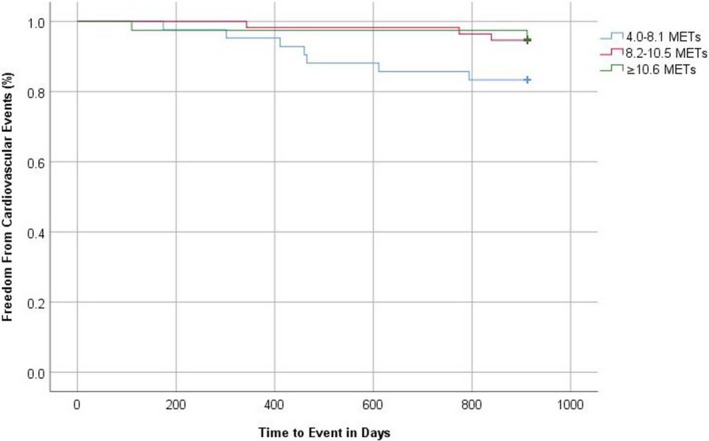
The combination of MET groups 2 and 3 had significantly fewer cardiovascular outcomes (P=0.037). CAC indicates coronary artery calcium score; MET, metabolic equivalents of task.
Discussion
Exercise Capacity and Coronary Fatty Plaque
In the current study, we examined the relationship between exercise capacity, coronary plaque composition, and CAC scores in subjects with stable CAD. Several new findings emerge. First, after adjustment, no difference in the amount of fatty plaque was observed among the 3 exercise MET groups. Those in the highest MET group exercised a median of 200 minutes per week compared with the lowest MET group who exercised a median of 85 minutes per week. Fatty plaque is lipid rich and more likely to rupture, leading to thrombosis and acute myocardial infarction, compared with fibrous or calcified plaque.22 This finding suggests that there is no adverse effect of exercise capacity or self‐reported exercise duration on fatty plaque.
Exercise Capacity and CAC Score
Second, those in the highest MET exercise category had a significantly lower CAC score in univariate analysis, which strengthened in significance after adjustment, compared with the lower MET categories. Similar findings were noted in a cross‐sectional study of 8565 healthy Korean men without clinical CAD. Those in the highest quartile of cardiorespiratory fitness, as measured by calorimetry to calculate VO2 max (maximum capacity to use oxygen during exercise, which reflects cardiopulmonary fitness), were 40% less likely (95% CI, 0.48–0.73) to have a CAC score >75th percentile.35
Exercise Capacity and Cardiovascular Events
Third, in the current study, those with exercise capacity ≥10.6 METs had a 68% lower rate of cardiac events over the course of 30‐month follow‐up compared with those in the lowest MET group. In those with CAC score >400, those achieving ≥8.2 METs had significantly better survival free of CVD events (P=0.037). Our findings in CAD subjects are in line with those of the Cooper Clinic study in subjects without CAD. When cardiorespiratory fitness was estimated based on METs achieved in 8425 asymptomatic men in the Cooper Clinic study, higher levels of exercise capacity were associated with decreased cardiovascular events across the spectrum of CAC scores at an average follow‐up of 8.4 years.36 Similarly, in 25,972 asymptomatic Korean subjects, the hazard ratio for all‐cause mortality in those with CAC >400 and exercise capacity <10 METs was 3.328‐fold higher (95% CI, 1.850–5.988) than the hazard ratio of 1.108 (95% CI, 0.512–2.400) in those achieving >10 METs (P=0.024) after adjustment.37 Thus, exercise capacity >10 METs was protective in the setting of CAC scores >400. These 2 studies suggest that in the setting of higher CAC scores, high cardiorespiratory fitness estimated from METs achieved lowers the risk for cardiovascular events and mortality. The current findings similarly support that achieving ≥8.2 METs protects against cardiovascular events in CAD subjects with CAC scores >400. To our knowledge, this is the first report on the relationship between fatty plaque, CAC score, exercise capacity, and cardiovascular events in subjects with CAD.
Exercise Intensity and Volume and Coronary Plaque
In the current report, no difference was found in CAC score or plaque subtypes among those who did no exercise and those who exercised at moderate‐ or high‐intensity levels. In fact, both the moderate‐intensity exercisers and high‐intensity exercisers achieving ≥8.2 METs had a trend toward lower CAC scores. The lack of adverse impact on CAC score and coronary plaque composition by exercise intensity is important because higher exercise volume and/or intensity have been reported to accelerate coronary plaque calcification in several studies in subjects without clinical CAD (reviewed in Table 6).13, 16, 17, 18, 19 In the Cooper Center Longitudinal Clinic Study of 21,758 men without clinical CAD (mean age, 51.7±8.4 years), those who exercised ≥3000 MET minutes per week had an 11% higher risk (95% CI, 1.03–1.20) of a CAC score of at least 100 (mean±SD, 800±1120) compared with those exercising <3000 MET minutes per week.13 In a cohort of 248 male athletes with self‐reported exercise data converted to METs, men in the >2000 MET minutes per week group had a significantly higher CAC score and a higher prevalence of calcified plaque (adjusted odds ratio, 3.57; 95% CI, 1.28–9.97) compared with the <1000 MET minutes per week group.16 In a cross‐sectional study of vigorous exercise, 152 masters athletes (70% male) aged >40 years who ran ≥10 miles or cycled ≥30 miles per week for ≥10 years and competed in ≥10 endurance events over a 10‐year period had a higher prevalence of calcified plaques (72.7% versus 30.8%; P=0.0002) and a higher prevalence of atherosclerotic plaques of any luminal irregularity (44.3% versus 22.2%; P=0.009) compared with 92 sedentary males.18 Only male athletes had a CAC score ≥300 Agatston units and a luminal stenosis ≥50%. Median CAC score in male athletes was 86 versus 3 in sedentary males (P=0.02). Of note, the number of years of training was the only independent variable associated with CAC >70th percentile for age or luminal stenosis ≥50% in male athletes (odds ratio, 1.08; 95% CI, 1.01–1.15; P=0.016), a finding suggesting that duration of vigorous exercise increases the risk of CAC.18 In a cross‐sectional study of vigorous exercise in 50 experienced male marathon runners who participated in 25 consecutive Twin Cities Marathon races, the runners had a significantly higher mean calcified plaque volume as compared with 23 sedentary controls (84 versus 44 mm3; P<0.0001) and higher noncalcified plaque volume (116 versus 82 mm3; P=0.04).19As noted earlier, CAC score was 1.86‐fold higher in those who exercised 450 minutes per week in the CARDIA study compared with those exercising <150 minutes per week.17 Although high exercise duration >450 minutes per week in CARDIA was associated with higher CAC scores, when examined by cardiorespiratory fitness, those in the highest fitness level (≥13.5 METs for males and ≥10 METs for females) had a 41% lower odds ratio of CAC 15 years later (odds ratio, 0.59; 95% CI, 0.36–0.97).38 These findings, along with the current report, suggest that high fitness has a beneficial impact on CAC score. In contrast to CARDIA, the current report suggests no adverse effect of exercise intensity and volume on coronary plaque or CAC.
Table 6.
Comparison of Coronary Plaque Composition and CAC Score in Exercise Studies
| Author Year | N | Male (%) | Amount of Exercise | Fatty Plaque | Fibrous Plaque | Noncalcified Plaque | Calcified Plaquea | Mixed Plaque (Calcified and Noncalcified) | Total Plaque | CAC Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Current study 2019 | 270 | 83% | MET group 3 (≥10.6) compared with MET group 1 (4.0–8.1) | ↔ | ↑ | ↔ | N/A | N/A | ↑ | ↓ |
| Merghan18 2017 | 152 | 70% | Athletes with mean endurance training of 7.7±3.5 h/wk for average of 31.0±12.6 years compared with controls who exercised for 1.9±0.5 h/wk | N/A | N/A | ↔ | ↑ | ↓ | ↑ | ↑ |
| Aengevaeren16 2017 | 284 | 100% | >2000 MET min/wk group compared with <1000 MET‐min/wk group | N/A | N/A | ↔ | ↑ | ↓ | NA | ↑ |
| Laddu17 2017 | 3175 | 43.5% | >450 min exercise/wk compared with <150 minutes exercise/wk | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| Schwartz19 2014 | 50 | 100% | 50 male marathon runners who participated in 25 consecutive Twin Cities Marathon races compared with 23 sedentary controls | N/A | N/A | ↑ | ↑ | N/A | ↑ | N/A |
| DeFina13 2019 | 21, 758 | 100% | Men exercising ≥3000 MET min/wk compared with <1500 MET min/wk | N/A | N/A | N/A | N/A | N/A | N/A | ↑ |
| Imran39 2016 | 2971 | 40% | Walking >15 to 22.5 MET h/wk compared with ≤3.75 MET h/wk | N/A | N/A | N/A | N/A | N/A | N/A | ↓ |
↓ indicates decrease; ↑, increase; ↔, no difference; CAC, coronary artery calcium score; MET, metabolic equivalent of task; and NA, not applicable.
Calcified plaque within individual arterial segments examined.
Exercise Intensity and Volume and CVD Events
Our Kaplan–Meier time to cardiovascular event curves showed no difference in cardiovascular events by volume of exercise for the 3 exercise intensity groups. These findings are important because studies of subjects with CAD have shown a J‐ or U‐shaped relationship between amount of physical activity and cardiovascular outcomes. The National Walkers’ and Runners’ Health study examined whether greater exercise volume reduces mortality in a study of 2377 post–myocardial infarction patients over 10.4 years of follow‐up.21 Compared with the lowest exercise group (<1.1 MET hours per day), CVD‐related mortality decreased by 21% for 1.07 to 1.8 MET hours per day of running or walking (P=0.11), 24% for 1.8 to 3.6 MET hours per day (P=0.04), 50% for 3.6 to 5.4 MET hours per day (P=0.001), and 63% for 5.4 to 7.2 MET hours per day (P<0.001), but decreased only 12% for 7.2 MET hours per day (P=0.68). Compared with the 12% risk reduction at 7.2 MET hours per day, those exercising >7.2 MET hours per day (the equivalent of running >7.1 km a day or walking >10.7 km per day) had a 3.19‐fold increased risk (95% CI, 1.42–6.79, P=0.006) for all ischemic heart disease–related mortalities and a 2.62‐fold increase (95% CI, 1.29–5.06; P=0.009) in cardiovascular mortality.21 In the German KAROLA (Langzeiterfolge der Kardiologischen Anschlussheilbehandlung) study of 1038 patients with stable CAD, those who performed daily strenuous physical activity and those who rarely exercised had a higher all‐cause and cardiovascular mortality rate compared with those performing moderate exercise daily or in 2 to 4 sessions per week over 8.1 years of follow‐up.20 The most highly active patients had a 2.36‐fold increased risk of cardiovascular mortality (95% CI, 1.05–5.34). Thus, a high volume of strenuous or vigorous physical activity may attenuate the benefit of physical activity and actually increase cardiovascular events and mortality in CAD subjects. In contrast, the findings in the current study support no difference in events with either moderate‐ or high‐intensity exercise. It is important that 62.4% of those who exercised at moderate intensity were able to achieve ≥8.2 METs; of these, 30.1% achieved ≥10.6 METs, and the combined group of those achieving ≥8.2 METs had a trend toward lower CAC scores. Of those doing moderate exercise, the majority (78%) were walking only. These results should reassure patients with stable CAD that they can reap similar benefits from moderate‐intensity as opposed to high‐intensity exercise on prevention of cardiovascular events and elevated CAC scores.
Moderate Intensity Walking and Coronary Artery Calcium
Based on our finding that those doing moderate intensity exercise and achieving ≥8.2 METs had a trend toward a lower CAC score and that 78% of these were walking only, it's worthy to note that moderate intensity walking has been associated with less CAC in healthy subjects. In an analysis of 2971 participants (60% female) of the National Heart, Lung, and Blood Institute Family Heart Study who had no history of myocardial infarction, coronary bypass graft surgery, or percutaneous coronary angioplasty, the group who walked >15 to 22.5 MET hours per week had a 46% lower prevalence of a CAC score of at least 100 as compared with those who walked ≤3.75 MET hours per week after adjusting for age, sex, race, smoking, alcohol use, total exercise metabolic activity index (excluding walking), and familial clustering.39 These findings, coupled with the findings of the current study, support the recommendation of moderate volume and intensity of exercise, such as walking, as being beneficial for subjects with and without CAD.
Major strengths of the current analysis include examining both fatty plaque and CAC score in relation to exercise capacity, exercise intensity, and duration and subsequent cardiac events over 30 months in subjects with CAD. Limitations include measurement of exercise capacity only at baseline, but subjects were encouraged to maintain levels of activity at that time during the 30‐month follow‐up for cardiovascular events. Another limitation is that exercise was self‐reported, and this could impact our results on exercise duration. However, the acquisition method included meeting with each subject individually using a chart to collect the type of exercise, minutes per session, and frequency per week, allowing for solid definition of total physical activity intensity and volume.
Conclusions
High exercise capacity was associated with lower coronary calcium scores and no difference in coronary fatty plaque volume in CAD subjects. Moreover, exercise capacity ≥8.2 METs was associated with fewer cardiovascular events over 30 months compared with <8.2 METS. In contrast, exercise intensity or duration had no impact on CAC score and plaque subtypes or cardiovascular events and therefore was not harmful. Moderate exercise intensity at a median [IQR] of 240 [140,365] minutes per week achieved ≥8.2 METs with a trend toward a lower CAC score. These data suggest that exercise, which increases cardiorespiratory fitness to ≥8.2 METs and can be achieved by moderate exercise in the form of walking, may provide the most benefit for coronary plaque burden and cardiovascular events in subjects with CAD and strengthen recommendations for moderate exercise as beneficial for CAD subjects. Therefore, vigorous exercise intensity and volume may not be needed for CAD patients to benefit, with the more important factor being maintenance of cardiorespiratory fitness at ≥8.2 METs, which can be achieved with moderate volume and intensity of exercise such as walking.
Sources of Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) Specialized Centers of Clinically Oriented Research (SCCOR) program grant to Dr Welty: P50 HL083813 and supported by the Harvard Clinical and Translational Science Center Award NIH UL1 TR001102.
Disclosures
None.
Acknowledgments
We thank the study subjects for participating.
J Am Heart Assoc. 2020;9:e014919 DOI: 10.1161/JAHA.119.014919
The data were presented at the American Heart Association Scientific Sessions, November 11, 2018, in Chicago, IL.
Abdulaziz Malik is currently located at the Division of Internal Medicine, Boston Medical Center, One Boston Medical Center, Boston, MA.
References
- 1. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 2. Blair SN, Kampert JB, Kohl HW III, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all‐cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 3. Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al‐Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554–1559. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed HM, Blaha MJ, Nasir K, Rivera JJ, Blumenthal RS. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109:288–295. [DOI] [PubMed] [Google Scholar]
- 5. Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. [DOI] [PubMed] [Google Scholar]
- 6. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Tosuka K, Shimano H, Ohashi Y, et al. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 7. Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A, Manolis A, Kokkinos JP, Karasik P, Greenberg M, et al. Exercise capacity and mortality in older men: a 20‐year follow‐up study. Circulation. 2010;122:790–797. [DOI] [PubMed] [Google Scholar]
- 8. Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all‐cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. [DOI] [PubMed] [Google Scholar]
- 9. Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CNB, Lauer MS, Marwick TH, Pandey DK, Wicklund RH, Thisted RA. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. [DOI] [PubMed] [Google Scholar]
- 10. Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all‐cause death in asymptomatic women: a 20‐year follow‐up of the Lipid Research Clinics Prevalence Study. JAMA. 2003;290:1600–1607. [DOI] [PubMed] [Google Scholar]
- 11. Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle‐aged Norwegian men. N Engl J Med. 1993;328:533–537. [DOI] [PubMed] [Google Scholar]
- 12. Carr J, Jacobs D, Terry J, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeFina LF, Radford NB, Barlow CE, Willis BL, Leonard D, Haskell WL, Farrell SW, Pavlovic A, Abel K, Berry JD, et al. Association of all‐cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Detrano R, Guerci A, Carr J, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 15. Miedema MD, Dardari ZA, Nasir K, Blankstein R, Knickelbine T, Oberembt S, Shaw L, Rumberger J, Michos ED, Rozanski A, et al. Association of coronary artery calcium with long‐term, cause‐specific mortality among young adults. JAMA Netw Open. 2019;2:e197440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aengevaeren V, Mosterd A, Braber T, Prakken NH, Doevendans PA, Grobbee DE, Thompson PD, Eijsvogels TM, Velthuis BK. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes: clinical perspective. Circulation. 2017;136:138–148. [DOI] [PubMed] [Google Scholar]
- 17. Laddu D, Rana J, Murillo R, Sorel ME, Quesenberry CP, Allen NB, Gabriel KP, Carnethon MR, Liu K, Reis JP, et al. 25‐year physical activity trajectories and development of subclinical coronary artery disease as measured by coronary artery calcium: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Mayo Clin Proc. 2017;92:1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, David S, Yeo TJ, Narain R, Malhotra A, et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation. 2017;136:126–137. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz RS, Kraus SM, Schwartz JG, Wickstrom KK, Peichel G, Garberich RF, Lesser JR, Oesterle SN, Knickelbine T, Harris KM, et al. Increased coronary artery plaque volume among male marathon runners. Mo Med. 2014;111:89–94. [PMC free article] [PubMed] [Google Scholar]
- 20. Mons U, Hahmann H, Brenner H. A reverse J‐shaped association of leisure time physical activity with prognosis in patients with stable coronary heart disease: evidence from a large cohort with repeated measurements. Heart. 2014;100:1043–1049. [DOI] [PubMed] [Google Scholar]
- 21. Williams PT, Thompson PD. Increased cardiovascular disease mortality associated with excessive exercise in heart attack survivors. Mayo Clin Proc. 2014;89:1187–1194. [DOI] [PubMed] [Google Scholar]
- 22. van der Wal AC, Becker AE. Atherosclerotic plaque rupture–pathologic basis of plaque stability and instability. Cardiovasc Res. 1999;41:334–344. [DOI] [PubMed] [Google Scholar]
- 23. Khosa F, Khan AN, Nasir K, Bedayat A, Malik Z, Jon AF, Cheema AR, Clouse ME, Welty FK. Comparison of coronary plaque subtypes in male and female patients using 320‐row MDCTA. Atherosclerosis. 2013;226:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hauser TH, Salastekar N, Schaefer EJ, Desai T, Goldfine HL, Fowler KM, Weber GM, Welty F, Clouse M, Shoelson SE, et al; Targeting Inflammation Using Salsalate in Cardiovascular Disease (TINSAL‐CVD) Study Team . Effect of targeting inflammation with salsalate: the TINSAL‐CVD randomized clinical trial on progression of coronary plaque in overweight and obese patients using statins. JAMA Cardiol. 2016;1:413–423. [DOI] [PubMed] [Google Scholar]
- 25. Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease. A randomized clinical trial. J Am Heart Assoc. 2017;6:e006981 DOI: 10.1161/JAHA.117.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elajami TK, Alfaddagh A, Lakshminarayan D, Soliman M, Chandnani M, Welty FK. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes mellitus and coronary artery disease. J Am Heart Assoc. 2017;6:e004740 DOI: 6. 10.1161/JAHA.116.004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saleh M, Alfaddagh A, Elajami TK, Ashfaque H, Haj‐Ibrahim H, Welty FK. Diastolic blood pressure predicts coronary plaque volume in patients with coronary artery disease. Atherosclerosis. 2018;277:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American College of Sports Medicine (ACSM) . Metabolic Calculation Handbook. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 29. U.S. Department of Health and Human Services . Physical Activity Guidelines for Americans, 2nd ed. Washington, DC: U.S. Department of Health and Human Services; 2018. Available at: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed February 14, 2020. [Google Scholar]
- 30. Rinehart S, Vazquez G, Qian Z, Murrieta L, Christian K, Voros S. Quantitative measurements of coronary arterial stenosis, plaque geometry, and composition are highly reproducible with a standardized coronary arterial computed tomographic approach in high‐quality CT datasets. J Cardiovasc Comput Tomogr. 2011;5:35–43. [DOI] [PubMed] [Google Scholar]
- 31. Brodoefel H, Burgstahler C, Sabir A, Yam CS, Khosa F, Claussen CD, Clouse ME. Coronary plaque quantification by voxel analysis: dual‐source MDCT angiography versus intravascular sonography. Am J Roentgenol. 2009;192:W84–W89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C, Belur P, Hulten E, Villines TC. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta‐analysis. JACC Cardiovasc Imaging. 2011;4:537–548. [DOI] [PubMed] [Google Scholar]
- 33. Brodoefel H, Burgstahler C, Heuschmid M, Reimann A, Khosa F, Kopp A, Schroeder S, Claussen CD, Clouse ME. Accuracy of dual‐source CT in the characterisation of non‐calcified plaque: use of a colour‐coded analysis compared with virtual histology intravascular ultrasound. Br J Radiol. 2009;82:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 35. Sung J, Cho SJ, Choe YH, Hong KP. Prevalence of coronary atherosclerosis in asymptomatic middle‐age men with high aerobic fitness. Am J Cardiol. 2012;109:839–843. [DOI] [PubMed] [Google Scholar]
- 36. Radford N, DeFina L, Leonard D, Barlow CE, Willis BL, Gibbons LW, Gilchrist SC, Khera A, Levine BD. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle‐age men. Circulation. 2018;137:1888–1895. [DOI] [PubMed] [Google Scholar]
- 37. Choi SY, Sung J, Park HE, Han D, Chang HJ. Combined effects of exercise capacity and coronary atherosclerotic burden on all‐cause mortality in asymptomatic Koreans. Atherosclerosis. 2016;251:396–403. [DOI] [PubMed] [Google Scholar]
- 38. Lee CD, Jacobs DR Jr, Hankinson A, Iribarren C, Sidney S. Cardiorespiratory fitness and coronary artery calcification in young adults: the CARDIA study. Atherosclerosis. 2009;203:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Imran TF, Patel Y, Ellison RC, Carr JJ, Arnett DK, Pankow JS, Heiss G, Hunt SC, Gaziano JM, Djoussé L. Walking and calcified atherosclerotic plaque in the coronary arteries: the national heart, lung, and blood institute family heart study. Arterioscler Thromb Vasc Biol. 2016;36:1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]


