Abstract
Background
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited condition associated with ventricular arrhythmias and myocardial dysfunction; however, limited data exist on identifying patients at highest risk. The purpose of the study was to determine whether measures of right ventricular (RV) dysfunction on echocardiogram including RV strain were predictive of structural disease progression in ARVC.
Methods and Results
A retrospective analysis of serial echocardiograms from 40 patients fulfilling 2010 task force criteria for ARVC was performed to assess structural progression defined by an increase in proximal RV outflow tract dimensions (parasternal short or long axis) or decrease in RV fractional area change. Echocardiograms were analyzed for RV free‐wall peak longitudinal systolic strain using 2‐dimensional speckle tracking. Risk of structural progression and 5‐year change in RV outflow tract measurements were compared with baseline RV strain. Of the 40 ARVC patients, 61% had structural progression with an increase in the mean parasternal short‐axis RV outflow tract dimension from 36.2 to 38.5 mm (P=0.022) and 68% by increase in parasternal long‐axis RV outflow tract dimension from 36.1 to 39.2 mm (P=0.001). RV fractional area change remained stable over time. Baseline RV strain was significantly associated with the risk of structural progression and 5‐year rate of change. Patients with an RV strain more positive than −20% had a higher risk (odds ratio: 18.4; 95% CI, 2.7–125.8; P=0.003) of structural progression.
Conclusions
RV free wall strain is associated with the rate of structural progression in patients with ARVC. It may be a useful marker in determining which patients require closer follow‐up and treatment.
Keywords: arrhythmogenic right ventricular cardiomyopathy, echocardiography, strain imaging
Subject Categories: Echocardiography, Imaging
Nonstandard Abbreviations and Acronyms
- RV
right ventricular
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- ARVD
arrhythmogenic right ventricular dysplasia
- PSAX
parasternal short axis
- PLAX
parasternal long axis
- RVOT
right ventricular outflow tract
- RV‐FAC
right ventricular fractional area change
- LV
left ventricular
- TFC
task force criteria
- LVEF
left ventricular ejection fraction
- PVC
premature ventricular complexes
- PKP2
plakophillin‐2
- DSG2
desmoglein‐2
- DSC2
desmocollin‐2
- DSP
desmoplakin
- JUP
plakoglobin
- TMEM43
transmembrane protein 43
- PLN
phospholamban
- DICOM
Digital Imaging and Communications in Medicine
- RBBB
right bundle‐branch block
- SCD
sudden cardiac death
- VT
ventricular tachycardia
Clinical Perspective
What Is New?
Echocardiographic right ventricular strain and strain rate at baseline are associated with the rate of future progression of structural abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy.
What Are the Clinical Implications?
Patients with suspected arrhythmogenic right ventricular cardiomyopathy and abnormal baseline right ventricular strain or strain rate could be at higher risk of disease progression and may warrant closer observation.
Introduction
Arrhythmogenic right ventricular (RV) cardiomyopathy (ARVC) is a genetic disease characterized pathologically by fibrofatty replacement of the myocardium and clinically by ventricular dysfunction and arrhythmias.1, 2 Since the first major description of the disease,3 attention has been devoted to establishing diagnostic criteria,4 identifying genotypes,5 predicting prognosis,6, 7, 8 and developing guidelines for management.7, 8 Prognostication of ARVC is challenging because clinical manifestations typically become apparent between the second and fourth decades of life.8, 9, 10 In addition, substantial interpatient variability in structural progression of disease has been noted.11 Therefore, accurate and reliable prediction of structural progression is an important area of clinical interest, as presenting events can be fatal. Noninvasive measures determined by the 2010 task force criteria (TFC) include structural and functional parameters for diagnosis of ARVC by echocardiography including RV outflow tract (RVOT) dimension and RV fractional area change (RV‐FAC).4 Changes to these imaging parameters have been used to identify and follow disease progression.11 Echocardiography has emerged as an ideal modality because it is noninvasive, widely available,12 and safe for use in patients defibrillators, as is common in ARVC.
Echocardiographic myocardial strain has emerged as a principal method for the quantification of subclinical left ventricular (LV) dysfunction in a variety of clinical settings,13 including chemotoxicity14 and ischemic heart disease.15 The role of echocardiographic strain in evaluating RV dysfunction in patients with ARVC is not fully elucidated, although several studies have indicated a possible role for this technique. Prior studies report reduced RV free wall strain and strain rate in patients with ARVC compared with measurements in healthy controls.16, 17, 18, 19, 20 Limited data have evaluated the utility of various echocardiographic parameters in ARVC for predicting disease progression over time. In one study, variability in RV and LV contraction duration was associated with greater arrhythmia risk.21 A recent study in first‐degree relatives of patients with ARVC found abnormal RV deformation in the subtricuspid region to be associated with disease progression.22 However, no studies have systematically characterized the role of traditional and novel measures of RV dysfunction as they relate to ARVC disease progression. Moreover, further characterization of the relationship between RV strain and structural progression over time in ARVC would be beneficial for risk stratification.
The purpose of this study was to evaluate echocardiographic free‐wall longitudinal strain of the RV as a predictor of progression of structural disease in ARVC using data from patients who underwent serial echocardiograms enrolled in the Johns Hopkins ARVC registry. We hypothesized that worse peak RV strain and strain rate would be significantly associated with progression of disease, as defined by increasing RVOT dimension and decreased RV‐FAC.4
Methods
Study Population
The study population consisted of 40 patients with ARVC from the Johns Hopkins arrhythmogenic right ventricular dysplasia/cardiomyopathy registry (http://ARVD.com). All patients were diagnosed with ARVC and fulfilled the 2010 task force criteria.4 To be included in this study, patients must have undergone at least 2 separate echocardiograms >6 months apart that were digitally available and of sufficient quality for analysis. The first and last available examinations were used for analysis, and the first exam was considered the baseline echocardiogram. The study was approved by the Johns Hopkins Medicine institutional review boards, and patients in the Johns Hopkins University arrhythmogenic right ventricular dysplasia/cardiomyopathy registry provided written informed consent. Participants did not receive compensation for this study. The data that support the findings of this study are available from the corresponding author on reasonable request.
Clinical Characterization
Fulfillment of TFC4 was assessed for each patient, as similarly performed by Mast et al.11 All patients had complete transthoracic echocardiograms including dedicated RV apical views. LV ejection fraction was calculated on 2‐dimensional parasternal long‐axis (PLAX) images using the modified Quinones formula.23 Cardiac magnetic resonance was performed per standard ARVC protocols described elsewhere and was used only to assess fulfillment of TFC structural criteria.24
All participants had undergone 12‐lead ECG recordings as part of assessment of TFC. ECG recordings were analyzed for fulfillment of TFC,4 including depolarization criteria (ε waves and terminal activation duration ≥55 ms) and repolarization criteria (precordial T‐wave inversion, V1–V6). In addition, signal‐averaged ECG was evaluated for the presence of late potentials. History of arrhythmias (nonsustained or sustained ventricular tachycardia of criteria‐defined axis and morphology) was also noted. Holter monitors were evaluated for premature ventricular complex count and were considered abnormal if >500 was observed in 24 hours, per the TFC.
All index patients also underwent genetic testing by molecular genetic screening of 5 ARVC‐associated desmosomal genes (PKP2 [plakophillin‐2], DSG2 [desmoglein‐2], DSC2 [desmocollin‐2], DSP [desmoplakin], and JUP [plakoglobin]), as well as nondesmosomal genes (TMEM43 [transmembrane protein 43] and PLN [phospholamban]).
Assessment of Structural Progression
Echocardiograms performed at baseline and follow‐up were exported from Synapse Cardiovascular (FUJIFILM Medical Systems) in DICOM (Digital Imaging and Communications in Medicine) format and analyzed using Image‐Arena v4 (TomTec Imaging Systems), a vendor‐neutral imaging platform.
Measurements were obtained for proximal RVOT dimensions in both PLAX and parasternal short‐axis (PSAX) views during end‐diastole, as shown in Figure 1. RV‐FAC was assessed as described in the TFC4 as the percentage of RV area decrease between diastole and systole obtained on apical imaging. All echocardiograms were analyzed by a single operator (S.W.) to exclude interobserver variability, and measurements were performed blinded to clinical data.
Figure 1. Measurement of right ventricular outflow tract (RVOT) dimensions.
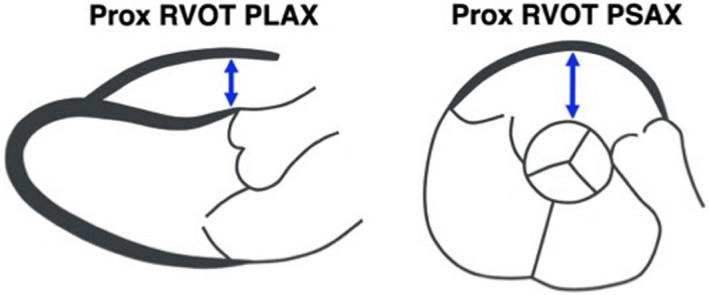
Measurement of proximal (Prox) RVOT dimensions on the parasternal long‐axis (PLAX) and short‐axis (PSAX) views.
Structural progression was assessed by calculating the change in these echocardiographic parameters between the baseline and follow‐up studies. These changes were normalized as yearly and 5‐year rates of change, and patients were subsequently grouped into quartiles by rate of change. Patients were characterized as progressors or nonprogressors for each variable; progression was defined as an increase in PSAX RVOT size, increase in PLAX RVOT size, and decrease in RV‐FAC.
Assessment of Strain and Strain Rate
RV free‐wall peak longitudinal systolic strain and strain rate were measured on DICOM images of an RV‐focused apical 4‐chamber view by endocardial speckle‐tracking using the 2D Cardiac Performance Analysis module of Image‐Arena. All available cardiac cycles were examined, and the best‐quality clip with optimal tracking was chosen. The peak values of free‐wall strain and strain rate were defined as the deepest points of the respective curves (Figure 2). The exact time of peak may vary but is generally around end systole for strain and early systole for strain rate. Furthermore, the peaks may not occur at the same time for the free wall and septum; however, only free‐wall values were used in our analysis, and septal strain values were excluded. LV endocardial peak longitudinal systolic strain was measured using the AutoStrain package on similarly selected cycles with optimal tracking, where the endocardium was automatically recognized and fine‐tuned manually at end diastole (first frame after mitral valve closure) and end systole (first frame after aortic valve closure). A sample image of RV strain on a patient with ARVC is shown in Figure 2. A similar technique is described by Hamada‐Harimura et al.25
Figure 2. Representative image of right ventricular strain measurement.
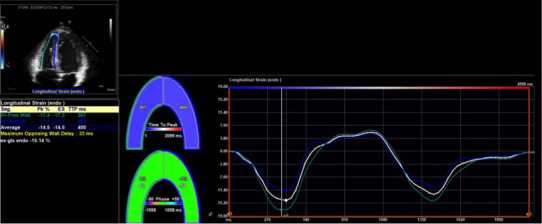
A sample image from TomTec Image‐Arena is shown. Software detected the myocardial–endocardial interface and was manually adjusted by an operator to ensure accuracy. The software then calculated strain and strain rate.
Statistical Analysis
Continuous data are presented as mean±SD or median with interquartile range. Categorical variables are presented as counts and percentages. A 2‐sided P<0.05 was considered significant. Differences between baseline and follow‐up echocardiographic measurements were compared using paired t tests for continuous variables and χ2 tests for categorical variables. Multivariable logistic regression (for risk of progression) and linear regression (for quartile of progression rate) adjusting for age and sex were used to assess the relationship between baseline RV strain and strain rate and structural progression. This was done separately for RVOT PSAX, RVOT PLAX, and RV‐FAC. Statistical analysis was performed using Stata v14.2 (StataCorp).
Results
Clinical characteristics for the patient population are shown in Table 1. All 40 participants fulfilled the TFC for definite ARVC. There were 29 probands and 11 affected family members; 21 patients (53%) were women. Mean age was 35.2±12.6 years at baseline, and median time to follow‐up echocardiogram was 3.6 years (interquartile range: 1.3–6.8 years). Minimum age at baseline was 19 years, and maximum age was 73 years. A pathogenic or likely pathogenic variant was identified in 65% of the study population. Variants in PKP2 were most common (n=21, 53%). From echocardiography, 23 patients (58%) met major criteria at baseline, whereas 29 patients (73%) met major criteria at follow‐up. LV ejection fraction was 58±7.3% at baseline and 56±10.9% at follow‐up (P=0.035). LV global longitudinal peak systolic strain was relatively stable during follow‐up (−20.1% to −19.3%; P=0.253).
Table 1.
Clinical Characteristics of Study Population
| Characteristic | Baseline Exam, n (%) | Follow‐Up Exam* | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y, mean (SD) | 35.2 (±12.6) | 39.7 (±12.3) | … |
| Male | 19 (48) | … | … |
| White | 40 (100) | … | … |
| Probands | 29 (73) | … | … |
| ICD | 34 (85) | 37 (93) | 0.288 |
| Genetics | |||
| Pathogenic mutation | 26 (65) | … | … |
| PKP2 | 21 (53) | … | … |
| DSG2 | 2 (5) | … | … |
| DSP | 0 (0) | … | … |
| DSC2 | 1 (3) | … | … |
| PLN | 1 (3) | … | … |
| SCN5A (non‐TFC mutation) | 1 (3) | … | … |
| No pathogenic mutation | 14 (35) | … | … |
| TFC | |||
| Structural (by echocardiography) | |||
| Major | 23 (58) | 29 (73) | 0.160 |
| Minor | 3 (8) | 1 (2) | 0.305 |
| Depolarization | |||
| Epsilon waves (major) | 5 (13) | 8 (20) | 0.363 |
| Depolarization minor TFC† | 23 (58) | 23 (58) | 1.000 |
| Terminal activation duration ≥55 ms | 15 (38) | 20 (50) | 0.294 |
| Repolarization | |||
| TWI in V1–V3 (major) | 31 (78) | 33 (83) | 0.420 |
| TWI V1–V2 (minor) | 35 (88) | 35 (88) | 0.754 |
| TWI V4–V6 (minor) | 6 (15) | 9 (23) | 0.390 |
| TWI V1–V4 in presence of RBBB (minor) | 5 (13) | 6 (15) | 0.745 |
| Arrhythmias | |||
| VT, superior major axis (major) | 8 (20) | 9 (23) | 0.834 |
| VT, inferior or unknown axis (minor) | 22 (55) | 25 (63) | 0.479 |
| PVC >500 in 24 h (minor)‡ | 29 (73) | 34 (85) | 0.815 |
| Family history | |||
| Pathogenic ARVC mutation carrier | 26 (65) | … | … |
| ARVC confirmed in first‐degree relative | 13 (33) | … | … |
| ARVC confirmed, second‐degree relative | 2 (5) | … | … |
| SCD <35 in first‐degree relative owed to ARVC | 4 (10) | … | … |
P value was obtained after performing χ2 analysis. ARVC indicates arrhythmogenic right ventricular cardiomyopathy; DSC2, desmocollin‐2; DSG2, desmoglein‐2; DSP, desmoplakin; ICD, implantable cardioverter‐defibrillator; PKP2, plakophillin‐2; PLN, phospholamban; PVC, premature ventricular complexes; RBBB, right bundle‐branch block; SCN5A, sodium channel gene alpha subunit; TWI, T wave inversions; SCD, sudden cardiac death; TFC, task force criteria; and VT, ventricular tachycardia.
Empty cells indicate no change from baseline.
Depolarization minor criteria were scored if terminal activation duration was >55 ms on ECG or late potentials by signal‐averaged ECG.
Holter monitoring had been performed on only 33 patients at baseline and 38 patients at follow‐up.
Between baseline and follow‐up studies, mean RVOT PSAX increased from 36.2 to 38.5 mm (P=0.022) with structural progression (increase in size) in 23 patients (60%); mean RVOT PLAX increased from 36.1 to 39.2 mm (P=0.001) with progression in 25 patients (67%); and mean RV‐FAC was relatively unchanged, decreasing from 31.6% to 30.6% (P=0.331) with 21 patients (52%) classified as progressed. Baseline age, sex, LV ejection fraction, RV‐FAC, RV basal diameter, and fulfillment of major structural TFC by echocardiography were compared according to progression status, and results are summarized in Table 2. Patients who exhibited progression by RVOT PSAX overall had lower RV‐FAC (28.5±9.6% versus 38.1±6.6%, P=0.002), larger RV basal diameters (4.6±0.88 versus 3.9±0.42 cm, P=0.005), and a greater percentage of patients meeting major structural criteria by echocardiography (74% versus 27%, P=0.004) at baseline compared with patients who did not progress by RVOT PSAX. In addition, progressors by RVOT PSAX tended to be men, compared with nonprogressors (61% versus 27%, P=0.039). Patients who exhibited progression (decrease) in RV‐FAC had significantly greater RV‐FAC measurements at baseline (35.2±9.7 versus 27.7±8.6, P=0.014) compared with patients who did not progress by RV‐FAC.
Table 2.
Comparison of Demographics and LVEF by Progression Status
| RVOT PSAX Progression | RVOT PLAX Progression | RV‐FAC Progression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n=23) | No (n=15) | P Value | Yes (n=25) | No (n=12) | P Value | Yes (n=21) | No (n=19) | P Value | |
| Age, y, mean±SD | 34.5±12.5 | 33.6±10.7 | 0.825 | 36.2±12.9 | 31.0±7.7 | 0.212 | 34.7±11.7 | 35.8±13.8 | 0.791 |
| Male (%) | 14 (61) | 4 (27) | 0.039 | 11 (44) | 6 (50) | 0.732 | 8 (38) | 11 (58) | 0.210 |
| LVEF, %, mean±SD | 57.6±8.2 | 60.8±5.0 | 0.187 | 58.2±6.9 | 60.0±8.3 | 0.504 | 57.0±7.3 | 60.1±7.2 | 0.184 |
| Baseline RV‐FAC, %, mean±SD | 28.5±9.6 | 38.1±6.6 | 0.002 | 32.1±9.3 | 33.4±10.6 | 0.708 | 35.2±9.7 | 27.7±8.6 | 0.014 |
| Baseline RV basal diameter, cm, mean±SD | 4.6±0.88 | 3.9±0.42 | 0.005 | 4.3±0.79 | 4.2±0.90 | 0.703 | 4.4±0.66 | 4.4±1.0 | 0.969 |
| Major structural TFC satisfied by echocardiography at baseline, n (%) | 17 (74) | 4 (27) | 0.004 | 16 (64) | 4 (33) | 0.080 | 12 (57) | 11 (58) | 0.962 |
Baseline age, sex, LVEF, RV‐FAC, RV basal diameter, and fulfillment of major structural TFC by echocardiography were compared across progression status. P values were calculated using t tests (age, LVEF, RV‐FAC, RV basal diameter) and χ2 analysis (sex, major structural TFC by echocardiography). LVEF indicates left ventricular ejection fraction; PLAX, parasternal long axis; PSAX, parasternal short axis; RV, right ventricular; RV‐FAC, right ventricular fractional area change; RVOT, right ventricular outflow tract; and TFC, Task Force Criteria.
Table 3 shows the association of baseline RV strain and strain rate with the risk of structural progression and across quartiles of 5‐year rate of change for each of the 3 parameters (adjusted for age and sex). Overall, both RV strain and strain rate were significantly associated with the likelihood of future structural progression and the rate of change of PSAX RVOT and PLAX RVOT dimensions. Patients with more negative strain (eg, −22.7% for PSAX quartile 1) or strain rate were less likely to progress, whereas less negative numbers (eg, −14.5% for PSAX quartile 4) were associated with a faster rate of structural progression. No significant differences in strain or strain rate were seen between structural progressors and nonprogressors or by quartile of rate of change according to RV‐FAC. A comparison of distribution of baseline RV strain across each quartile is shown in Figure 3.
Table 3.
Association of Baseline RV Strain and Strain Rate With Risk and Rate of Structural Progression by PSAX‐RVOT, PLAX‐RVOT, and RV‐FAC
| Structural Progression | Quartile of 5‐y Rate of Change (1=Best, 4=Worst) | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | P Value* | 1 | 2 | 3 | 4 | P Value† | |
| PSAX RVOT | ||||||||
| RV strain, %, mean±SD | −15.4±4.5 | −22.7±3.9 | 0.007 | −22.7±3.6 | −20.4±5.4 | −15.5±4.5 | −14.5±4.8 | 0.001 |
| RV strain rate, 1/s, mean±SD | −0.64±0.21 | −0.97±0.23 | 0.003 | −0.91±0.18 | −0.90±0.31 | −0.68±0.28 | −0.59±0.17 | 0.011 |
| PLAX RVOT | ||||||||
| RV strain, %, mean±SD | −17.3±4.9 | −20.6±6.6 | 0.160 | −20.9±7.0 | −19.5±4.3 | −18.3±3.7 | −14.6±5.5 | 0.011 |
| RV strain rate, 1/s, mean±SD | −0.71±0.25 | −0.91±0.27 | 0.043 | −0.87±0.16 | −0.90±0.34 | −0.76±0.26 | −0.57±0.22 | 0.008 |
| RV‐FAC | ||||||||
| RV strain, %, mean±SD | −18.6±5.7 | −16.8±6.4 | 0.505 | −15.9±7.0 | −17.0±6.1 | −18.9±6.7 | −19.2±4.2 | 0.219 |
| RV strain rate, 1/s, mean±SD | −0.79±0.32 | −0.71±0.24 | 0.497 | −0.63±0.28 | −0.74±0.22 | −0.84±0.40 | −0.78±0.20 | 0.254 |
RV strain and strain rate are compared between progressors and nonprogressors and across quartiles of 5‐y rate of change. PLAX indicates parasternal long axis; PSAX, parasternal short axis; RV, right ventricular; RV‐FAC, right ventricular fractional area change; and RVOT, right ventricular outflow tract.
P values from multivariable logistic regression (adjusting for age and sex).
P values from ordinal logistic regression (adjusting for age and sex).
Figure 3. Comparison of RV peak longitudinal systolic strain and strain rate across quartiles of 5‐year rate of change in PSAX RVOT, PLAX RVOT, and RV‐FAC.
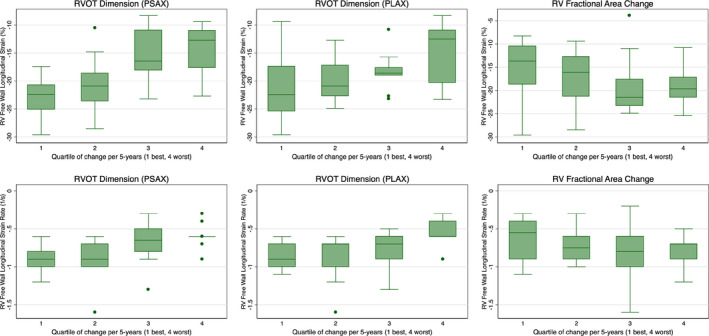
The distribution of RV peak longitudinal systolic strain on baseline echocardiography is compared across patients, arranged by 5‐year quartile rate of change of each structural marker. PLAX indicates parasternal long axis; PSAX, parasternal short axis; RV, right ventricular; RV‐FAC, right ventricular fractional area change; and RVOT, right ventricular outflow tract.
We further defined an abnormal RV peak longitudinal systolic strain as being more positive (greater) than −20%. There were 24 patients (60%) with abnormal RV strain at baseline. These patients were at substantially greater risk of structural progression than those with a normal baseline RV strain: 18 times higher risk of RVOT PSAX progression (odds ratio: 18.4; 95% CI, 2.7–125.8; P=0.003) adjusting for age and sex and 3.7 times higher risk of RVOT PLAX progression (odds ratio: 3.65; 95% CI, 0.60–22.4; P=0.161).
Discussion
In this study, we evaluated 2‐dimensional echocardiographic RV strain as a predictor of structural disease progression in ARVC. This study has several notable findings. First, our results indicate that many patients exhibited progression in RVOT dimensions (60% for PSAX and 67% for PLAX) and less so for RV‐FAC during the follow‐up period. This finding is expected given that ARVC is a progressive disease and because similar findings have been seen in prior studies that have evaluated for progression on serial echocardiograms.11, 26, 27 Mast et al11 reported progression of all 3 measures (PSAX RVOT, PLAX RVOT, and RV‐FAC) in two‐thirds of their study population over a mean follow‐up time of 6.4 years, and this result was reproduced in our study.
Our results also indicate that worse RV free‐wall peak longitudinal systolic strain and strain rate are associated with increases in RVOT size, as obtained from PSAX and PLAX views. Specifically, when baseline strain and strain rate were compared for patients who demonstrated structural progression and those who did not, worse strain and strain rate were observed among the progressors. Higher quartiles of 5‐year RV structural progression (by both PSAX RVOT and PLAX RVOT) were associated with diminished RV free‐wall strain and strain rate at baseline (Table 3). Furthermore, patients with more abnormal RV strain (greater than −20%) had an 18‐fold risk (P=0.002) of progression in PSAX RVOT. Thus, RV free‐wall strain and strain rate appear to be predictive of structural progression of disease in patients with ARVC, and patients with relatively normal baseline values progressed slowly, whereas those with abnormal values progressed more rapidly. These measures may have important clinical utility because structural changes and progression are associated with arrhythmic events and development of heart failure.21, 28, 29
Regarding measurement of RVOT, prior studies have shown that RVOT measures by echo are reproducible and correlate with cardiac magnetic resonance imaging measures. One study reported high reproducibility, with interclass correlation values >0.91 for inter‐ and intraobserver correlation of 2‐dimensional RVOT measurements on echo.30 In addition, RVOT dimension on transthoracic echocardiogram exhibited good correlation with the same measure on cardiac magnetic resonance (r=0.87; 95% CI, 0.75–0.93; P<0.0001) and good absolute agreement with a mean difference of 0.2 mm between the 2 imaging modalities.30 This metric was used in our study and was utilized by the Heart Rhythm Society 2010 TFC in the diagnosis of ARVC. Another study11 reported a 2‐mm increase in RVOT dimension over time in a population of ARVC patients and was found to be significant, similar to our current findings. Based on the reproducibility of RVOT measurements and findings in prior studies, changes in RVOT size in this study of 2.3 and 3.2 mm on PSAX and PLAX views, respectively, are appropriately significant, and they correspond to relative changes of ≈6.3% and 8.6% from baseline to follow‐up.
It should be noted that patients who progressed by RVOT PSAX had lower baseline RV‐FAC, larger RV basal diameters, and greater fulfillment of major structural criteria by echocardiography at baseline compared with patients who did not progress by RVOT PSAX (Table 2). These findings are consistent with the notion that ARVC is a progressive disease; patients who exhibited progression tended to have greater evidence of disease at baseline. In addition, these findings were not observed among progressors by RVOT PLAX. It is possible that variability in imaging technique is responsible for this discrepancy. However, it also suggests that RV strain and strain rate, in addition to other structural markers of disease, can be used to predict structural progression. Patients who progressed by RVOT PSAX tended to be male; however, the implications of this finding are unclear and perhaps incidental, as this association was not seen for RVOT PLAX and RV‐FAC. Patients who progressed by RV‐FAC tended to have larger RV‐FAC at baseline, perhaps reflecting a larger “window” for decrease in RV function with higher RV‐FAC at baseline.
In a prior study by Mast et al,22 subtricuspid RV deformation was evaluated in first‐degree relatives of patients with ARVC, using criteria that incorporated strain. The authors concluded that abnormal RV deformation seemed to precede established signs of ARVC. However, the study performed by Mast et al22 utilized prespecified deformation patterns to predict disease progression, whereas our study evaluated RV free‐wall peak longitudinal systolic strain and strain rate. Our structural predictors for disease progression are related to the described deformation patterns but are arguably simpler to implement. Furthermore, the patient population in the study by Mast et al22 consisted of first‐degree relatives of patients with ARVC and excluded patients who fulfilled TFC for structural abnormalities. In contrast, our study evaluated patients who carried diagnoses of definite ARVC regardless of criteria fulfilled. Thus, our study expands the patient population in which strain imaging may be useful.
Speckle‐tracking strain can also assess ventricular contraction homogeneity using mechanical dispersion, which is defined as the standard deviation of the time from the Q/R on ECG to peak negative longitudinal strain for all RV or LV segments. Prior studies have evaluated RV and LV mechanical dispersion, in addition to RV strain, to predict arrhythmias in ARVC and differentiate early ARVC from RVOT–ventricular tachycardia patients.31, 32, 33 Leren et al32 found RV mechanical dispersion to be a marker of prior arrhythmic events in patients with early ARVC, but the authors also identified an association between diminished RV global longitudinal strain and arrhythmia events in a broader population that included patients with definite ARVC. Lie et al33 also found an association between RV longitudinal strain and life‐threatening ventricular arrhythmias but identified a stronger relationship using LV mechanical dispersion. In contrast to these studies, our study validates the utility of strain techniques in the definite ARVC population and demonstrates the ability of strain to predict other markers of ARVC severity, namely, structural progression. In addition, compared with mechanical dispersion, RV free‐wall strain is more widely available and less technically demanding to assess, making it more suitable for serial measurements over time to assess structural progression.
Results for RV‐FAC disagree with our hypothesis; however, in our study population, the RV‐FAC remained relatively stable during the follow‐up period, with very few patients progressing. Saguner et al26 found that reduced FAC was highly predictive of major adverse cardiovascular events in patients with ARVC. However, they also reported no association between an impaired tricuspid annular plane systolic excursion and subtricuspid regional wall motion abnormalities. Consequently, it is possible that strain and strain rate are more predictive of changes in the RVOT dimensions for patients with ARVC than for overall RV systolic function. Findings for RV‐FAC may also have been limited by a relatively small patient population. In addition, the technical challenges of accurately determining RV‐FAC on 2‐dimensional echocardiographic imaging may have led to variability in assessment of RV function.
Limitations
This study has several limitations. First, it was a retrospective study from a single referral center with variable follow‐up periods and relatively small sample size; however, because of the rare nature of ARVC, the study is still one of the largest of its kind. The measurement of strain and strain rate can be inconsistent depending on the user, image quality, software, or vendor of the echocardiography system. This is particularly true for the right ventricle, which remains challenging to assess quantitatively by echocardiography. However, we mitigated this by having only a single operator evaluate RV strain under the same software/hardware conditions. Furthermore, the operator was blinded to clinical data during analysis. It should also be noted that strain and wall dimension are related. However, apart from comparing against changes in RV‐FAC, RV free‐wall strain was used to identify changes in the RVOT, an anatomically separated location from where strain was measured. Last, echocardiography may be less sensitive than magnetic resonance imaging for determining structural progression of disease, given the ability of magnetic resonance imaging to identify segmental RV dilatation or hypokinesis with higher resolution.34 Nevertheless, echocardiography is more widely available clinically, particularly for ARVC patients, the majority of whom have defibrillators requiring special magnetic resonance imaging protocols.
Conclusions
Our study recognizes echocardiographic strain as a useful technique to predict structural progression of disease in ARVC. These findings suggest that echocardiographic strain and strain rate could be useful in identification of patients at risk of disease progression who may require closer follow‐up and treatment.
Sources of Funding
The Johns Hopkins arrhythmogenic right ventricular dysplasia (ARVD)/cardiomyopathy program is supported by the Leonie‐Wild Foundation, the Dr. Francis P. Chiaramonte Private Foundation, the Leyla Erkan Family Fund for ARVD Research, the Dr. Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella Family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments. This work is also supported by a grant from the Fondation Leducq’ (Calkins).
Disclosures
Dr Calkins is a consultant for Medtronic Inc. and St. Jude Medical/Abbott. Dr Calkins receives research support from Boston Scientific Corp. Crystal Tichnell and Cynthia James receive salary support from this grant. Dr James has received a lecture fee from Abbott. Dr Tandri receives research support from Abbott. The remaining authors have no disclosures to report.
Acknowledgments
We are grateful to the arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and families who have made this work possible.
J Am Heart Assoc. 2020;9:e015016 DOI: 10.1161/JAHA.119.015016
References
- 1. Sen‐Chowdhry S, Lowe MD, Sporton SC, McKenna WJ. Arrhythmogenic right ventricular cardiomyopathy: clinical presentation, diagnosis, and management. Am J Med. 2004;117:685–695. [DOI] [PubMed] [Google Scholar]
- 2. Gemayel C, Pelliccia A, Thompson PD. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2001;38:1773–1781. [DOI] [PubMed] [Google Scholar]
- 3. Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. [DOI] [PubMed] [Google Scholar]
- 4. Marcus FI, McKenna WJ, Duane S, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace. 2011;13:1077–1109. [DOI] [PubMed] [Google Scholar]
- 6. Bosman LP, Sammani A, James CA, Cadrin‐Tourigny J, Calkins H, van Tintelen JP, Hauer RNW, Asselbergs FW, te Riele ASJM. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta‐analysis. Heart Rhythm. 2018;15:1097–1107. [DOI] [PubMed] [Google Scholar]
- 7. Corrado D, Wichter T, Link MS, Hauer RNW, Marchlinski FE, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2015;132:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 9. Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld ACP, Sawant AC, Kassamali B, et al. Clinical presentation, long‐term follow‐up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015;8:437–446. [DOI] [PubMed] [Google Scholar]
- 10. Nava A, Bauce B, Basso C, Muriago M, Rampazzo A, Villanova C, Daliento L, Buja G, Corrado D, Danieli GA, et al. Clinical profile and long‐term follow‐up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2000;36:2226–2233. [DOI] [PubMed] [Google Scholar]
- 11. Mast TP, James CA, Calkins H, Teske AJ, Tichnell C, Murray B, Loh P, Russell SD, Velthuis BK, Judge DP, et al. Evaluation of structural progression in arrhythmogenic right ventricular dysplasia/cardiomyopathy. JAMA Cardiol. 2017;2:293–302. [DOI] [PubMed] [Google Scholar]
- 12. Mast TP, Teske AJ, Doevendans PA, Cramer MJ. Current and future role of echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Cardiol J. 2015;22:362–374. [DOI] [PubMed] [Google Scholar]
- 13. Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle‐tracking echocardiography. J Am Coll Cardiol. 2017;69:1043–1056. [DOI] [PubMed] [Google Scholar]
- 14. Thavendiranathan P, Poulin F, Lim K‐D, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy. J Am Coll Cardiol. 2014;63:2751. [DOI] [PubMed] [Google Scholar]
- 15. Grenne B, Eek C, Sjøli B, Dahlslett T, Uchto M, Hol PK, Skulstad H, Smiseth OA, Edvardsen T, Brunvand H. Acute coronary occlusion in non‐ST‐elevation acute coronary syndrome: outcome and early identification by strain echocardiography. Heart. 2010;96:1550. [DOI] [PubMed] [Google Scholar]
- 16. Prakasa KR, Wang J, Tandri H, Dalal D, Bomma C, Chojnowski R, James C, Tichnell C, Russell S, Judge D, et al. Utility of tissue Doppler and strain echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2007;100:507–512. [DOI] [PubMed] [Google Scholar]
- 17. Teske AJ, Cox MG, De Boeck BW, Doevendans PA, Hauer RN, Cramer MJ. Echocardiographic tissue deformation imaging quantifies abnormal regional right ventricular function in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Soc Echocardiogr. 2009;22:920–927. [DOI] [PubMed] [Google Scholar]
- 18. Vitarelli A, Cortes Morichetti M, Capotosto L, De Cicco V, Ricci S, Caranci F, Vitarelli M. Utility of strain echocardiography at rest and after stress testing in arrhythmogenic right ventricular dysplasia. Am J Cardiol. 2013;111:1344–1350. [DOI] [PubMed] [Google Scholar]
- 19. Pieles GE, Grosse‐Wortmann L, Hader M, Fatah M, Chungsomprasong P, Slorach C, Hui W, Fan C‐PS, Manlhiot C, Mertens L, et al. Association of echocardiographic parameters of right ventricular remodeling and myocardial performance with modified task force criteria in adolescents with arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 2019;12:e007693. [DOI] [PubMed] [Google Scholar]
- 20. Prati G, Vitrella G, Allocca G, Muser D, Buttignoni SC, Piccoli G, Morocutti G, Delise P, Pinamonti B, Proclemer A, et al. Right ventricular strain and dyssynchrony assessment in arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 2015;8:e003647. [DOI] [PubMed] [Google Scholar]
- 21. Sarvari SI, Haugaa KH, Anfinsen O‐G, Leren TP, Smiseth OA, Kongsgaard E, Amlie JP, Edvardsen T. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J. 2011;32:1089–1096. [DOI] [PubMed] [Google Scholar]
- 22. Mast TP, Taha K, Cramer MJ, Lumens J, van der Heijden JF, Bouma BJ, van den Berg MP, Asselbergs FW, Doevendans PA, Teske AJ. The prognostic value of right ventricular deformation imaging in early arrhythmogenic right ventricular cardiomyopathy. JACC Cardiovasc Imaging. 2019;12:446–455. [DOI] [PubMed] [Google Scholar]
- 23. Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WLJ, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two‐dimensional echocardiography. Circulation. 1981;64:744–753. [DOI] [PubMed] [Google Scholar]
- 24. Tandri H, Calkins H, Nasir K, Bomma C, Castillo E, Rutberg J, Tichnell C, Lima JAC, Bluemke DA. Magnetic resonance imaging findings in patients meeting task force criteria for arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2003;14:476–482. [DOI] [PubMed] [Google Scholar]
- 25. Hamada‐Harimura Y, Seo Y, Ishizu T, Nishi I, Machino‐Ohtsuka T, Yamamoto M, Sugano A, Sato K, Sai S, Obara K, et al. Incremental prognostic value of right ventricular strain in patients with acute decompensated heart failure. Circ Cardiovasc Imaging. 2018;11:e007249. [DOI] [PubMed] [Google Scholar]
- 26. Saguner AM, Vecchiati A, Baldinger SH, Rüeger S, Medeiros‐Domingo A, Mueller‐Burri AS, Haegeli LM, Biaggi P, Manka R, Lüscher TF, et al. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Cardiovasc Imaging. 2014;7:230–239. [DOI] [PubMed] [Google Scholar]
- 27. Aneq MÅ, Lindström L, Fluur C, Nylander E. Long‐term follow‐up in arrhythmogenic right ventricular cardiomyopathy using tissue Doppler imaging. Scand Cardiovasc J. 2008;42:368–374. [DOI] [PubMed] [Google Scholar]
- 28. Gilotra NA, Bhonsale A, James CA, te Riele ASJ, Murray B, Tichnell C, Sawant A, Ong CS, Judge DP, Russell SD, et al. Heart failure is common and under‐recognized in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Heart Fail. 2017;10:e003819. [DOI] [PubMed] [Google Scholar]
- 29. Calkins H, Corrado D, Marcus F. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2017;136:2068–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gotschy A, Saguner AM, Niemann M, Hamada S, Akdis D, Yoon J‐N, Parmon EV, Delgado V, Bax JJ, Kozerke S, et al. Right ventricular outflow tract dimensions in arrhythmogenic right ventricular cardiomyopathy/dysplasia—a multicentre study comparing echocardiography and cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2017;19:516–523. [DOI] [PubMed] [Google Scholar]
- 31. Saberniak J, Leren IS, Haland TF, Beitnes JO, Hopp E, Borgquist R, Edvardsen T, Haugaa KH. Comparison of patients with early‐phase arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract ventricular tachycardia. Eur Heart J Cardiovasc Imaging. 2017;18:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leren IS, Saberniak J, Haland TF, Edvardsen T, Haugaa KH. Combination of ECG and echocardiography for identification of arrhythmic events in early ARVC. JACC Cardiovasc Imaging. 2017;10:503–513. [DOI] [PubMed] [Google Scholar]
- 33. Lie OH, Rootwelt‐Norberg C, Dejgaard LA, Leren IS, Stokke MK, Edvardsen T, Haugaa KH. Prediction of life‐threatening ventricular arrhythmia in patients with arrhythmogenic cardiomyopathy: a primary prevention cohort study. JACC Cardiovasc Imaging. 2018;11:1377–1386. [DOI] [PubMed] [Google Scholar]
- 34. Li KHC, Bazoukis G, Liu T, Li G, Wu WKK, Wong SH, Wong WT, Chan YS, Wong MCS, Wassilew K, et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) in clinical practice. J Arrhythm. 2018;34:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


