Abstract
Background
Multiplexed molecular rapid diagnostic tests (RDTs) may allow for rapid and accurate diagnosis of the microbial etiology of pneumonia. However, little data are available on multiplexed RDTs in pneumonia and their impact on clinical practice.
Methods
This retrospective study analyzed 659 hospitalized patients for microbiological diagnosis of suspected pneumonia.
Results
The overall sensitivity of the Unyvero LRT Panel was 85.7% (95% CI 82.3–88.7) and the overall specificity was 98.4% (95% CI 98.2–98.7) with a negative predictive value of 97.9% (95% CI 97.6–98.1). The LRT Panel result predicted no change in antibiotics in 12.4% of cases but antibiotic de-escalation in 65.9% (405/615) of patients, of whom 278/405 (69%) had unnecessary MRSA coverage and 259/405 (64%) had unnecessary P. aeruginosa coverage.
Interpretation
In hospitalized adults with suspected pneumonia, use of an RDT on respiratory samples can allow for early adjustment of initial antibiotics, most commonly de-escalation.
Abbreviations: BAL, bronchoalveolar lavage (BAL); CAP, community-acquired pneumonia; ETA, endotracheal aspirate; FN, false negative; FP, false positive; HAP, hospital-acquired pneumonia; LRT(I), lower respiratory tract infection; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; NPV, negative predictive value; PPV, positive predictive value; RDT, rapid diagnostic test; TN, true negative; TP, true positive; VAP, ventilator-associated pneumonia
Keywords: Antimicrobial stewardship, Bacteria, Multiplexed PCR, Pneumonia, Rapid diagnostic test
1. Background
Pneumonia remains a leading cause of hospital admissions and is associated with substantial morbidity, mortality, healthcare costs, and days of work lost. (Yu et al., 2012) Community-acquired pneumonia (CAP) is estimated to cause ~1.5 million hospitalizations and ~100,000 deaths in the US each year, (Ramirez et al., 2017) equating to an aggregate cost of nearly $9.5 billion for 960,000 hospital stays. (Thomas et al., 2012; Tong et al., 2018) Hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), is the most common healthcare-associated infection. (Magill et al., 2018) Despite clinical advances, the burden of pneumonia on the healthcare system continues to increase, with hospitalization rates for pneumonia increasing by 35% due to Klebsiella spp., 23% due to Pseudomonas spp., and 23% due to Staphylococcus aureus between 2001 and 2011. (Wuerth et al., 2016) An aging population, (Kline and Bowdish, 2016) poor sensitivity diagnostic tools, (Ewig et al., 1996; Bandyopadhyay et al., 2000; Jain et al., 2015) prolonged courses of empirical antibiotics, (Foolad et al., 2018) and the emergence of antimicrobial resistance(Barrasa-Villar et al., 2017) are all contributing to the persistent and increasing burden of pneumonia.
A hospitalized patient with suspected pneumonia is generally treated empirically. Empirical treatment recommendations in CAP, HAP, and VAP guidelines are based on risk factors for the specific pathogens seen in each form of pneumonia and are consistently associated with overtreatment. (Attridge et al., 2011; Kett et al., 2011; Ekren et al., 2018) Empirical treatment of CAP includes coverage of atypical but uncommon organisms, (Arnold et al., 2007) and empirical antibiotic decisions are even more difficult for HAP/VAP due to other common antibiotic-resistant pathogens. Moreover, empirical treatment of both CAP and HAP/VAP focuses on MRSA and Pseudomonas coverage. (Kalil et al., 2016) Subsequent tailoring of antibiotics is recommended to treat the specific etiology depending on the microbiological diagnosis; (Pickens and Wunderink, 2019) however, the turnaround time for a final respiratory culture result can be upwards of 72 hours, during which time patients continue to receive empirical antibiotics. In addition, respiratory cultures are often negative due to their relatively poor sensitivity, particularly when obtained after initiation of antibiotic therapy, and can miss important pathogens when concealed by the growth of multiple organisms in culture. (Ewig et al., 1996; Bandyopadhyay et al., 2000; Jain et al., 2015)
These limitations of respiratory microbiological culture make effective antibiotic stewardship, defined as “coordinated interventions designed to improve and measure the appropriate use of antimicrobials by promoting the selection of optimal antimicrobial drug regimen, dose, duration of therapy, and route of administration”, difficult. (Barlam et al., 2016) As of September 2019, the Centers for Medicare and Medicaid Services now require US hospitals to implement antibiotic stewardship programs, highlighting the need for more appropriate antibiotic use. Antibiotic stewardship aims to minimize broad antibiotic use, which is associated with longer lengths of hospital stay, higher morbidity and mortality, higher hospital costs, nephrotoxicity, and nosocomial infections. (Falcone et al., 2012; Jensen et al., 2012; Vincent and Manges, 2015) Guideline-driven empirical therapy for pneumonia with broad-spectrum antibiotics and the lag time to definitive microbiological diagnosis mean that (i) de-escalation cannot be performed quickly, making the use of inappropriate antibiotics more likely; and (ii) the prolonged use of broad-spectrum antibiotics promotes the development of antimicrobial resistance. (Fowler et al., 2007; Fair and Tor, 2014; Karam et al., 2016) Therefore, an urgent need exists for the rapid detection of the causative pathogen in pneumonia in order to tailor antibiotics and encourage appropriate antibiotic stewardship.
Rapid diagnostic tests (RDTs) that reduce diagnostic turnaround times from days to hours can address some of the aforementioned clinical challenges. The 2019 Infectious Diseases Society of America CAP guidelines acknowledged the need for rapid, cost-effective, accurate tests to improve directed antibiotic therapy. (Metlay et al., 2019) This crisis has led to a recommendation that antimicrobial prescriptions in high-income countries should be made only when supported by rapid diagnostic evidence, where such tests are available. (O’Neill, 2018) However, the majority of current RDTs for pneumonia only detect a single organism, (Torres et al., 2016) and experiences with high-sensitivity RDTs developed for malaria and tuberculosis in resource-poor settings have given rise to concerns that false positive results may result in overtreatment. (Ranadive et al., 2017; Houben et al., 2018; Weinrib and Capraro, 2019) However, data on the clinical application and impact of a new generation of highly multiplexed RDTs, which detect multiple organisms in a single respiratory sample by molecular testing, are lacking.
The Unyvero Lower Respiratory Tract (LRT) Panel is an RDT that uses multiplex polymerase chain reaction (PCR) to identify 20 causative agents of severe lower respiratory tract infections (LRTIs) and ten antibiotic resistance determinants in clinical specimens. Targets comprise two Gram-positive bacteria (Staphylococcus aureus and Streptococcus pneumoniae), 14 Gram-negative bacteria (including Pseudomonas aeruginosa), three atypical pneumonia species (Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae), and one fungus (Pneumocystis jirovecii). The test uses an automated sample-to-answer platform with minimal hands-on time and takes approximately five hours, so it has the potential for use as a point-of-care test.
Using patients enrolled at two of the clinical sites for NCT01922024, a large non-interventional multicenter study that determined the operating characteristics of the Unyvero LRT Panel, we predicted the impact of Unyvero LRT Panel RDT results on adjustment of empiric antibiotic regimens in hospitalized patients with suspected pneumonia.
2. Methods
2.1. Study population, inclusion criteria, and ethical approval
This retrospective study analyzed subjects enrolled in NCT01922024 at Northwestern Memorial Hospital (NMH) in Chicago, IL and Beaumont Health (BH; comprising three hospitals in Royal Oak, Troy, and Grosse Pointe) in Michigan. NCT01922024 was a large non-interventional multicenter study conducted in 2015–2016 to determine the operating characteristics of the Unyvero LRT Panel. Any study subject enrolled in NCT01922024 with an available study ID linked to the electronic medical records (EMR) of NMH or BH was considered for inclusion. Inclusion criteria were hospitalized patients ≥18 years old; with a suspicion of a LRTI; and with an available surplus of >1 ml endotracheal aspirate (ETA) or bronchoalveolar lavage (BAL) fluid. Exclusion criteria were known infection with HIV, HBV, or tuberculosis. The Northwestern University and Beaumont Health Institutional Review Boards approved retrospective chart review of clinical parameters and outcomes prior to local study site closure (IRB reference number(s) STU00206068 and IORG00000367, FWA 00002516. The date of hospital admission to discharge; date and time of antibiotic administration; culture results from blood, urine, and sites other than respiratory; other microbiologic tests; and discharge disposition were retrieved from the EMR.
2.2. Unyvero LRT panel
The Unyvero LRT Panel is an RDT specifically designed for the detection of LRTIs. Specimens were processed with the Unyvero LRT Panel assay as per the manufacturer's instructions and as described previously. (Ozongwu et al., 2017; Gadsby et al., 2019) Organisms detected in the assay are shown in Table 1 .
Table 1.
The microorganisms and resistance markers detected by the Unyvero LRT Panel.
| Organism | Resistance | Gene |
|---|---|---|
| Acinetobacter spp.a | Carbapenem |
kpc ndm oxa-23 oxa-24 oxa-48 oxa-58 vim |
| Chlamydia pneumoniae | ||
| Citrobacter freundii | ||
| Enterobacter cloacae complexb | ||
| Escherichia coli | ||
| Haemophilus influenzae | ||
| Klebsiella oxytoca | ||
| Klebsiella pneumoniaec | ||
| Klebsiella variicola | 3rd generation cephalosporins | ctx-M |
| Legionella pneumophila | ||
| Moraxella catarrhalis | ||
| Morganella morganii | ||
| Mycoplasma pneumoniae | ||
| Pneumocystis jirovecii | Oxacillin/cefoxitin | mecA |
| Proteus spp.d | ||
| Pseudomonas aeruginosa | ||
| Serratia marcescens | ||
| Staphylococcus aureus | Penicillin | tem |
| Stenotrophomonas maltophilia | ||
| Streptococcus pneumoniae |
Acinetobacter spp. detected by LRT panel: A. baumannii, A. calcoaceticus, A. haemolyticus, A. junii, A. lwoffii, A. nosocomialis, A. parvus, A. pittii. bEnterobacter cloacae complex includes: E. asburiae, E. chengduensis, E. chuandaensis, E. cloacae, E. hormaechei (incl. Ssp. xiangfangensis), E. kobei, E. ludwigii, E. roggenkampii, E. sichuanensis as well as E. bugandensis (not yet recognized as member of the E. cloacae complex). cKlebsiella pneumoniae includes two variants: K. pneumoniae (variant 1), and K. quasipneumoniae (variant 2). dProteus spp. includes P. cibarius, P. hauseri, P. mirabilis, P. penneri, and P. vulgaris.
2.3. Definition of concordance and discordance of RDT and culture results
Results of the LRT Panel were compared to the final culture results in order to more accurately mimic clinical practice. A sample was defined as concordant if it was: (i) LRT Panel negative, culture negative; (ii) LRT Panel positive, culture positive for the same organism; (iii) LRT Panel positive, culture positive for the same organism and a non-panel organism; or (iv) LRT panel negative, culture positive for a non-panel organism. A sample was defined as discordant if it was: (i) LRT Panel positive, culture negative; (ii) LRT Panel positive, culture positive for a different panel organism; or (iii) LRT Panel negative, culture positive for a panel organism. A sample was defined as both concordant and discordant if the LRT Panel and culture results had the same organism plus an additional panel organism reported by either assay.
2.4. Assessment of predicted changes to empirical antibiotic administration
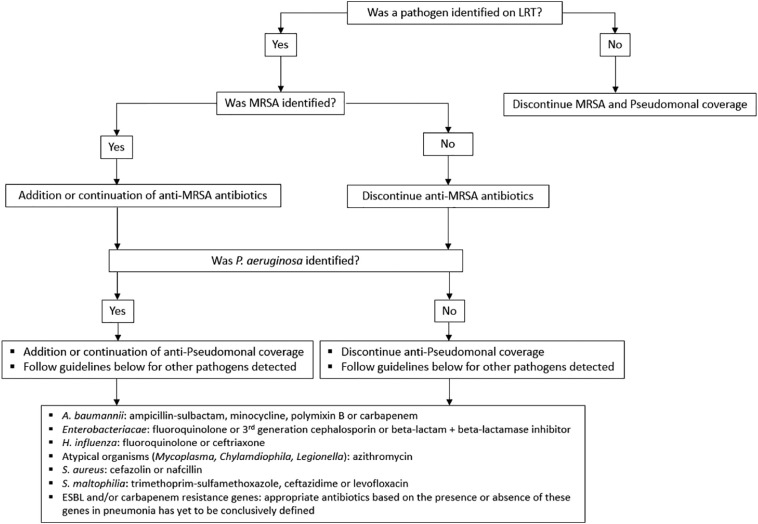
Our assessment of the potential changes to empirical antibiotics based on LRT Panel results assumed that pneumonia was the most likely source of infection. Appropriate versus inappropriate antibiotic regimens were based on the published guidelines for definitive treatment of CAP and HAP (Fig. 1 ) and the organisms detected by the LRT Panel. (Kalil et al., 2016; Metlay et al., 2019) In this study, we did not use resistance markers to guide de-escalation of antibiotic therapy with the exception of mecA for S. aureus, since mecA is the central determinant of MRSA. (Spratt, 1994) However, we did use detection of a carbapenemase gene to suggest the need to escalate antibiotic therapy. If MRSA or P. aeruginosa were not detected by the panel, then anti-MRSA and/or anti-pseudomonal therapy were deemed unnecessary.
Fig. 1.
Selection of appropriate antibiotic regimen based on published guidelines.
Predicted changes in therapy based on the LRT panel results were defined as: (1) No antibiotic change indicated, where an appropriate antibiotic regimen was used to treat the organism identified by the LRT Panel OR no pathogen was identified by LRT Panel and no anti-MRSA or anti-pseudomonal therapy was administered; (2) Favors de-escalation, where antibiotics could have been narrowed earlier based on the LRT Panel result; this category included cases where the LRT Panel result was negative but patients were on anti-MRSA and/or anti-pseudomonal therapy; (3) Favors expansion, where the antibiotic regimen used would not typically have adequately treated the pathogen identified by the LRT Panel; (4) De-escalation and expansion favored, where multiple antibiotics were used and one drug was too broad while the other was too narrow, e.g., LRT Panel reported P. aeruginosa but the empirical antibiotic regimen was vancomycin (too broad) and ceftriaxone (too narrow); (5) Initiate antibiotics, where antibiotics were not initiated at the time of testing but an organism was identified by the LRT Panel. Results from the LRT Panel were not available to the treating clinician during the study.
2.5. Statistical analysis
Results were analyzed using descriptive statistics. For sensitivity and specificity calculations, routine culture was considered the gold standard. All statistical analyses were performed in SPSS v21 (IBM Statistics Inc., Chicago, IL).
3. Results
3.1. Sample characteristics
Six-hundred and fifty-nine unique samples were enrolled in NCT01922024 at the two hospital systems. Study IDs were unavailable for 39 samples, and 5 samples had culture data but no antibiotic use data. Thus, 659 samples were available for determining the assay operating characteristics, 620 samples were available for concordance analysis, and 615 samples for antibiotic change analysis. Three-hundred and ninety-five samples were non-bronchoscopic or bronchoscopic BALs and 225 samples were ETAs obtained from patients in intensive care units and chronic ventilator units of the included hospitals.
3.2. Operating characteristics of the LRT panel and concordance between RDT and culture results
Compared to the bacterial culture standard, the sensitivity, specificity, positive predictive value, and negative predictive value of the Unyvero LRT Panel in this patient population are shown in Table 2 . While the sensitivity varied for different organisms, the specificity was uniformly high (96.5–99.5%). The overall sensitivity of the Unyvero LRT Panel was 85.7% (95% CI 82.3–88.7) and the overall specificity was 98.4% (95% CI 98.2–98.7). Accordingly, the assay had a very high negative predictive value of 97.9% (95% CI 97.6–98.1). This compared favorably to a 91.4% sensitivity and 99.5% specificity for pathogen detection in the original nine-center study (ClinicalTrials.gov: NCT01922024), which determined operating characteristics using culture plus an independent PCR test as the gold standard for common pathogens, rather than culture alone.
Table 2.
The operating characteristics of the Unyvero LRT panel.
| Organism | TP | FN | FP | TN | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Acinetobacter spp. | 23 | 2 | 14 | 620 | 92.0 (74.0–00.0) | 97.8 (96.3–98.8) | 62.2 (49.1–73.6) | 97.6 (96.0–98.6) |
| Citrobacter freundii | 0 | 1 | 6 | 652 | 0 (0–97.5) | 99.0 (98.0–99.7) | N/A | 99.9 (99.9–99.9) |
| Enterobacter cloacae complex | 29 | 6 | 3 | 621 | 82.9 (66.4–93.4) | 99.5 (98.6–99.9) | 90.6 (75.6–96.8) | 98.6 (97.4–99.4) |
| Escherichia coli | 38 | 3 | 15 | 603 | 92.7 (80.0–98.5) | 97.6 (96.0–98.6) | 71.7 (60.4–80.8) | 97.3 (95.7–98.4) |
| Haemophilus influenzae | 10 | 4 | 10 | 635 | 71.4 (41.9–91.6) | 98.5 (97.2–99.3) | 50.0 (33.2–66.8) | 97.9 (96.5–98.8) |
| Klebsiella oxytoca | 16 | 5 | 11 | 627 | 76.2 (52.8–91.8) | 98.3 (96.9–99.1) | 59.3 (43.6–73.3) | 97.6 (96.1–98.6) |
| Klebsiella pneumoniae | 35 | 12 | 10 | 602 | 74.5 (59.7–86.0) | 98.4 (97.0–99.2) | 77.8 (64.9–86.9) | 98.1 (96.9–98.8) |
| Klebsiella variicola | 1 | 0 | 4 | 654 | 100 (2.5–100) | 99.4 (98.5–99.8) | 20.0 (8.6–39.9) | 100 (100–100) |
| Moraxella catarrhalis | 6 | 0 | 7 | 646 | 100 (54.1–100) | 98.9 (97.8–99.6) | 46.2 (29.1–64.2) | 100 (100–100) |
| Morganella morganii | 1 | 0 | 4 | 654 | 100 (2.5–100) | 99.4 (98.5–99.8) | 20.0 (8.6–39.9) | 100 (100–100) |
| Proteus spp. | 19 | 3 | 12 | 625 | 86.4 (65.1–97.1) | 98.1 (96.7–99.0) | 61.3 (46.9–74.0) | 97.7 (96.3–98.7) |
| Pseudomonas aeruginosa | 72 | 9 | 16 | 562 | 88.9 (80.0–94.8) | 97.2 (95.5–98.4) | 81.8 (73.4–88.0) | 98.4 (97.1–99.1) |
| Serratia marcescens | 14 | 4 | 4 | 637 | 77.8 (52.4–93.6) | 99.4 (98.4–99.8) | 77.8 (56.1–90.6) | 99.4 (98.5–99.7) |
| Staphylococcus aureus | 109 | 17 | 17 | 516 | 86.5 (79.3–91.9) | 96.8 (94.9–98.1) | 86.5 (80.0–91.1) | 96.8 (95.1–97.9) |
| Stenotrophomonas maltophilia | 34 | 1 | 22 | 602 | 97.1 (85.1–99.9) | 96.5 (94.7–97.8) | 60.7 (50.5–70.1) | 99.8 (98.9–100.0) |
| Streptococcus pneumoniae | 7 | 2 | 3 | 647 | 77.8 (40.0–97.2) | 99.5 (98.7–99.9) | 70.0 (41.7–88.4) | 99.7 (99.0–99.9) |
| Overall | 414 | 69 | 158 | 9903 | 85.7 (82.3–88.7) | 98.4 (98.2–98.7) | 72.4 (69.1–99.4) | 97.9 (97.6–98.1) |
Abbreviations: TP, true positive; FN, false negative; FP, false positive; TN, true negative; PPV, positive predictive value; NPV, negative predictive value.
The concordance between the Unyvero LRT Panel and culture results is reported in Table 3 . According to our criteria, 75.0% of results were concordant, 9.2% were discordant, and 15.8% were both concordant and discordant. Of the discordant results, ~4% each were due to either LRT Panel negative/culture positive discordance or vice versa, suggesting that the LRT panel and culture misclassified similar numbers of cases for the major organisms responsible for LRTIs.
Table 3.
Concordance between LRT Panel and culture results.
| LRT panel and culture agreement | Total | % |
|---|---|---|
| CONCORDANT | 465 | 75.0 |
| LRT negative, Culture negative | 203 | 32.7 |
| LRT positive, Culture positive | 211 | 34.0 |
| LRT positive, Culture positive + Culture positive for non-panel organism | 34 | 5.5 |
| LRT negative, Culture positive for non-panel organism | 17 | 2.7 |
| DISCORDANT | 57 | 9.2 |
| LRT negative, Culture positive for panel organism | 23 | 3.7 |
| LRT positive, Culture negative | 25 | 4.0 |
| LRT positive, Culture negative for panel organism, Culture positive for non-panel organism | 3 | 0.5 |
| LRT and culture identifying different panel organisms | 6 | 1.0 |
| BOTH CONCORDANT and DISCORDANT | 98 | 15.8 |
| Due to additional LRT organism | 63 | 10.2 |
| Due to additional LRT organism, Culture positive for non-panel organism | 7 | 1.1 |
| Due to additional culture organism on panel | 19 | 3.0 |
| LRT and culture identifying same and different organisms | 9 | 1.5 |
3.3. Predicted changes to antibiotic therapy based on RDT testing
Having determined the organism and mecA status by the LRT Panel, we next predicted changes to antibiotic regimens based on the published guidelines for treatment of CAP and HAP (Fig. 1). (Kalil et al., 2016; Metlay et al., 2019) Reassured by the excellent negative predictive value of the LRT panel, we determined that if MRSA or P. aeruginosa were not detected by the panel, then anti-MRSA and/or anti-pseudomonal therapies were not indicated.
The predicted changes to antibiotic therapy based on the LRT Panel are shown in Table 4 . The LRT Panel result predicted no change in antibiotics in only 12.4% of cases, while in 65.9% (405/615) of patients the LRT Panel results favored de-escalation. Of these, 278/405 (69%) had unnecessary MRSA coverage and 259/405 (64%) had unnecessary P. aeruginosa coverage. In the favors de-escalation group, 25.2% (102/405) had a prior positive culture requiring either vancomycin or an anti-pseudomonal antibiotic or a simultaneous extra-pulmonary infection that would have been reasonable to treat with broad empirical therapy. In the favors expansion group, the most common organisms not initially covered by the empirical antibiotic regimen were Acinetobacter (n = 17), Pneumocystis jirovecii (n = 15), S. maltophilia (n = 47), P. aeruginosa (n = 18), and MRSA (n = 15).
Table 4.
Predicted changes to antibiotic therapy based on the LRT Panel results.
| Potential impact on therapy based on Unyvero LRT results alone | Total |
|---|---|
| No antibiotic change indicated | 76 (12.4%) |
| Favors de-escalation (antibiotics could have been narrowed) | 405 (65.9%) |
| Favors expansion (antibiotics could have been broadened) | 67 (10.0%) |
| Favors both de-escalation and expansion of antibiotics | 48 (7.8%) |
| Start antibiotics | 19 (3.1%) |
| Total Samples Available for Analysis | 615 (100%) |
When considering which RDT-based antibiotic decisions would have resulted in inappropriately narrow therapy, the LRT Panel did not detect a panel (n = 67) or non-panel organism (n = 79) detected by culture in 120 cases (Table 5 ). Of the panel organisms, a total of 9 missed P. aeruginosa and 18 missed S. aureus (13 MSSA, 5 MRSA) may have led to inappropriate narrowing of empirical therapy in 4.3% (27/620) of patients. Conversely, the LRT Panel detected 148 organisms not detected by culture, including 15 S. aureus and 12 P. aeruginosa, which may have prevented a similar number of inappropriate antibiotic de-escalations based on culture results. The LRT also detected a large number of other pathogens missed by culture that would have required specific treatments, including Acinetobacter (11 cases), S. maltophilia (19 cases), and P. jirovecii (13 cases). Escalation of therapy based on LRT-detected Gram-negative resistance patterns would be suggested for three carbapenem-resistant P. aeruginosa, one carbapenem-resistant E. coli, nine carbapenem-resistant Acinetobacter, and two carbapenem-resistant K. pneumoniae cases.
Table 5.
Concordance between Unyvero LRT Panel and culture results by organism.
| Panel organism | Concordant | Missed by culture | Missed by LRT panel |
|---|---|---|---|
| Acinetobacter spp. | 22 | 11 | 1 |
| Chlamydia pneumoniae | 0 | 0 | 0 |
| Citrobacter freundii | 0 | 6 | 1 |
| Enterobacter cloacae complex | 28 | 2 | 5 |
| Escherichia coli | 34 | 12 | 3 |
| Haemophilus influenzae | 5 | 9 | 3 |
| Klebsiella oxytoca | 14 | 8 | 5 |
| Klebsiella pneumoniae | 30 | 10 | 10 |
| Klebsiella variicola | 3 | 2 | 0 |
| Legionella pneumophila | 2 | 1 | 1 |
| Moraxella catarrhalis | 6 | 6 | 0 |
| Morganella morganii | 1 | 4 | 0 |
| Mycoplasma pneumoniae | 2 | 2 | 0 |
| Pneumocystis jirovecii | 3 | 13 | 1 |
| Proteus spp. | 19 | 9 | 3 |
| Pseudomonas aeruginosa | 63 | 12 | 9 |
| Serratia marcescens | 8 | 4 | 4 |
| Staphylococcus aureus | 98 | 15 | 18 |
| Stenotrophomonas maltophilia | 35 | 19 | 1 |
| Streptococcus pneumoniae | 6 | 3 | 2 |
| Total | 379 | 148 | 67 |
Of the non-panel organisms found on culture (Table 6 ), 56% (46/79) were common oropharyngeal colonizing organisms that generally do not necessitate antibiotic escalation, and a further 28% (22/79) were organisms that would have been covered even with empirical treatment that removed MRSA and Pseudomonas coverage.
Table 6.
Non-panel organisms detected by culture. Organisms with an * are considered oropharyngeal flora and are not routinely treated with antibiotics when identified on culture.
| Organism | Number (%) |
|---|---|
| Viridans Streptococcus* | 15 (19.0%) |
| Corynebacterium* | 13 (16.5%) |
| Enterobacter aerogenes | 10 (12.6%) |
| Coagulase negative Staphylococcus* | 7 (8.9%) |
| Citrobacter koseri | 5 (6.3%) |
| Alcaligenes | 4 (5.1%) |
| Enterococcus spp.* | 2 (2.5%) |
| Prevotella* | 1 (1.3%) |
| Pantoea | 1 (1.3%) |
| Enterobacter sakazakii | 1 (1.3%) |
| Group F Streptococcus* | 1 (1.3%) |
| Group C Streptococcus* | 1 (1.3%) |
| Moraxella/Psychrobacter | 1 (1.3%) |
| Pseudomonas putida | 1 (1.3%) |
| Pseudomonas fluorescens | 1 (1.3%) |
| Enterobacter gergoviae | 1 (1.3%) |
| Aeromonas | 1 (1.3%) |
| Eikenella corrodens | 1 (1.3%) |
| Providencia | 1 (1.3%) |
| Enterobacter amnigenus | 1 (1.3%) |
| Streptococcus mitis* | 1 (1.3%) |
| Beta hemolytic Streptococcus* | 1 (1.3%) |
| Raoultella spp. | 1 (1.3%) |
| Aspergillus | 1 (1.3%) |
| Actinomyces* | 1 (1.3%) |
| Streptococcus agalactiae | 1 (1.3%) |
| Burkholderia cepacia | 1 (1.3%) |
| Lactobacillus spp.* | 1 (1.3%) |
| Citrobacter youngae | 1 (1.3%) |
| Enterococcus faecium | 1 (1.3%) |
| Total non-panel organisms | 79 |
4. Discussion
The laboratory diagnosis of LRTIs is predominantly based on microbiological cultures, introducing delays and the prolonged use of empirical, broad-spectrum antibiotic therapy in large numbers of patients. Antibiotic resistance is a severe and increasing problem worldwide, mandating the increased use of improved RDTs to reduce antibiotic administration and increase the use of specific antimicrobial therapies. (Aliberti et al., 2013; Cilloniz et al., 2016) Also, in consideration of the current global COVID-19 pandemic, concerns about secondary bacterial infections in hospitalized COVID-19 patients have given rise to the need for timely and appropriate diagnosis of pneumonia to address the over and under-treatment of patients, enabling healthcare providers to practice better antibiotic stewardship, and helping to limit resistance and the development of super-bugs. (Gerberding, 2020) Rapid multiplex RDTs may play a critical role in this crisis setting. Rapid molecular techniques such as the Unyvero LRT Panel are a promising tool to help guide appropriate therapy and de-escalation from broad-spectrum antibiotic therapy in patients with suspected pneumonia. (Torres et al., 2016; Sullivan and Dien Bard, 2019) However, like any new medical technology, their potential clinical impact requires testing in clinical practice, and data on multiplex assays in particular are lacking. Here we analyzed the potential impact on antibiotic use in patients with suspected pneumonia with implementation of the Unyvero LRT Panel using data from a non-interventional study that originally evaluated the operating characteristics of the assay.
The overall sensitivity (85.7%) and specificity (98.4%) of the assay for organism detection in our mixed population of BALF and ETA samples were consistent with previous reports for this (88.8% and 94.9%33) and another (90% and 97.4%33) FDA-approved multiplex LRTI assay and comparable to the sensitivities and specificities reported for traditional ETA and BAL culture. (Baselski and Wunderink, 1994; Shin et al., 2011) A recent evaluation of the Unyvero LRT Panel in 175 BALF specimens reported a positive percentage agreement of 96.5% and negative percentage agreement of 99.6% with quantitative bacterial culture, (Collins et al., 2020) and the original prospective nine-center study, which compared the LRT Panel against a composite comparator of culture plus an independent PCR test, reported 91.4% sensitivity and 99.5% specificity for pathogen detection (ClinicalTrials.gov: NCT01922024). These discrepancies, particularly in sensitivity, are likely to be dominated by pre-analytical factors and the choice of comparator. For instance, despite ETA samples being recommended for use in the diagnosis of VAP in current guidelines, (Kalil et al., 2016) concordance between cultures from ETA samples and BALF are suboptimal. (Brun-Buisson et al., 2005; Scholte et al., 2014) Our population represented a mixed cohort of BALF and ETA samples compared against culture alone without supplementary molecular testing. Nevertheless, and importantly from the perspective of clinical implementation, the assay had a consistently very high negative predictive value of 97.9%. Therefore, the significant number of de-escalations based on a lack of MRSA (278/405, 69%) or P. aeruginosa (259/405, 64%) detection by the panel can be made with high degree of clinical confidence. This de-escalation strategy would be particularly advantageous when empirical antibiotics are used to cover organisms on the LRT panel that are difficult to culture including Legionella, Pneumocystis, Mycoplasma and Chlamydia.
The LRT Panel results could have changed the choice of antibiotic used in the majority of cases, potentially leading to more effective antibiotic stewardship both in terms of regimen and duration. In 66% of cases, the initial antibiotic regimen was too broad for the pathogen identified by the LRT Panel and subsequent culture, consistent with a recent report in the critical care setting that multiplex PCR for respiratory pathogens would alter the antibiotic choice earlier in >50% of cases. (Gadsby et al., 2019) Contrary to previous concerns that RDTs may lead to over-treatment, (Ranadive et al., 2017; Houben et al., 2018; Weinrib and Capraro, 2019) our data suggest that implementation of this RDT would de-escalate antibiotic use in the majority of cases. Even in cases where the LRT Panel result was negative for any organism, initial empirical antibiotic regimens usually still included anti-MRSA and anti-pseudomonal drugs. Conversely, in cases where the organism identified by the LRT panel was missed by culture, the patient was still usually covered by the empirical antibiotic regimen. In this scenario, LRT results may potentially prevent inappropriate de-escalation.
A recent study of the BioFire FilmArray Pneumonia panel concluded that the panel could also allow antibiotic adjustment in 71% of cases including discontinuation or de-escalation in 48% of patients. (Buchan et al., 2020) These findings, particularly the utility of a rapid diagnostic test for antibiotic de-escalation, were similar to the findings in our study of the LRT Panel. The two panels have important differences. The LRT Panel includes important etiologic agents of pneumonia like Stenotrophomonas maltophilia, Pneumocystis jirovecii, Klebsiella variicola, Morganella morganii and Citrobacter freundii; these organisms are not on the FilmArray Pneumonia panel. However, the FilmArray Pneumonia panel includes Klebsiella aerogenes, Streptococcus agalactiae and Streptococcus pyogenes, and common respiratory viruses not on the LRT panel. Another difference between these panels is that the LRT Panel includes ten resistance genes while the FilmArray Pneumonia Panel includes only six. The FilmArray Pneumonia Panel provides semi-quantitative results using four different bin categories corresponding to 104, 105, 106, or ≥107 copies/mL while the LRT Panel does not. Correlations to quantitative culture results reported as CFU/mL are however difficult, and agreement rates may be variable between organisms and in different specimen types; and concordance may be low, especially at lower CFU/mL densities. (Poole and Clark, 2020) The role of reporting semi-quantitative PCR results has yet to be defined in clinical practice.
Our evaluations regarding antibiotic escalation and de-escalation were based on the assumption that pneumonia was the most likely source of infection, as the study had enrolled only patients with a suspected LRTIs. In practice, in cases where another positive culture or extra-pulmonary infection is documented, the LRT Panel result should be used in conjunction with the other culture results to make decisions on therapy. If pneumonia is the only suspected site of infection, treatment could be narrowed and/or combination therapy discontinued, with important antibiotic stewardship implications. However, in patients presenting with sepsis or septic shock of unclear etiology, antibiotic decisions should not be based solely on an RDT.
This study has a number of limitations. We constructed a hypothetical estimate of the utility of the test, assuming 100% compliance with the assay and its results, which is unlikely to be achievable in practice. The decision to alter antimicrobial therapy was based on clinical guidelines, so mainly exploited results from two of the 20 organism targets and only two resistance markers, mecA and carbapenemase enzymes; it is possible that the negative predictive value for the detection of phenotypic resistance could be lower depending on prevalence of non-panel resistance mechanisms. (Spafford et al., 2019) Whether these potential false-negative and false-positive molecular results will degrade the utility of the assay awaits a prospective interventional study performed at multiple sites. The exclusion of expectorated sputum from the analysis may also have increased the specificity of the test for pathogens causing true disease.
4.1. Interpretation
Application of this multiplexed LRTI rapid diagnostic test would most commonly predict adjustment to antibiotic prescribing for patients in whom MRSA or P. aeruginosa were unlikely to be the causative organism. Given the consistently high negative predictive value of the RDT and that mecA is the central determinant of MRSA, (Spratt, 1994) even with the caveats of this study, our findings suggest that implementation of a multiplexed LRTI RDT is likely to alter the antibiotic management of significant numbers patients presenting with suspected pneumonia. When coupled with antimicrobial stewardship programs, rapid tests that identify organisms in respiratory samples can optimize antimicrobial utilization and patient outcomes.
Acknowledgments
We would like to thank Nextgenediting for their assistance with review and revision of the manuscript.
Footnotes
Conflicts of interest: RGW has been a consultant for Curetis and his institution has received investigator-initiated research funding. MDS is a study investigator for Curetis and has served as a consultant for Curetis. All other authors have no conflicts of interest to disclose.
Funding statement: This work is supported by the National Institute of Health Institutional Training Grant (3T32HL76139-13S2) and by NIH/NIAID grant U19AI135964.
Prior presentations: Preliminary data from this manuscript was presented in an abstract at the 2018 ASM Microbe and the 2019 American Thoracic Society International Conference.
References
- Aliberti S., Cilloniz C., Chalmers J.D. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax. 2013;68(11):997–999. doi: 10.1136/thoraxjnl-2013-203384. [DOI] [PubMed] [Google Scholar]
- Arnold F.W., Summersgill J.T., Lajoie A.S. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 2007;175(10):1086–1093. doi: 10.1164/rccm.200603-350OC. [DOI] [PubMed] [Google Scholar]
- Attridge R.T., Frei C.R., Restrepo M.I. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878–887. doi: 10.1183/09031936.00141110. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay T., Gerardi D., Metersky M. A comparison of induced and expectorated sputum for the microbiological diagnosis of community acquired pneumonia. Respiration. 2000;67(2):173–176. doi: 10.1159/000029482. [DOI] [PubMed] [Google Scholar]
- Barlam T.F., Cosgrove S.E., Abbo L.M. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasa-Villar J.I., Aibar-Remon C., Prieto-Andres P. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin. Infect. Dis. 2017;65(4):644–652. doi: 10.1093/cid/cix411. [DOI] [PubMed] [Google Scholar]
- Baselski V.S., Wunderink R.G. Bronchoscopic diagnosis of pneumonia. Clin. Microbiol. Rev. 1994;7(4):533–558. doi: 10.1128/cmr.7.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun-Buisson C., Fartoukh M., Lechapt E. Contribution of blinded, protected quantitative specimens to the diagnostic and therapeutic management of ventilator-associated pneumonia. Chest. 2005;128(2):533–544. doi: 10.1378/chest.128.2.533. [DOI] [PubMed] [Google Scholar]
- Buchan B.W., Windham S., Balada-Llasat J.M. Practical comparison of the biofire filmarray pneumonia panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. Journal of Clinical Microbiology. 2020;58(7):5. doi: 10.1128/JCM.00135-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilloniz C., Gabarrus A., Ferrer M. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415–425. doi: 10.1016/j.chest.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Collins M.E., Popowitch E.B., Miller M.B. Evaluation of a novel multiplex PCR panel compared to quantitative bacterial culture for the diagnosis of lower respiratory tract infections. J. Clin. Microbiol. 2020;58(5):2013–2019. doi: 10.1128/JCM.02013-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekren P.K., Ranzani O.T., Ceccato A. Evaluation of the 2016 Infectious Diseases Society of America/American Thoracic Society guideline criteria for risk of multidrug-resistant pathogens in patients with hospital-acquired and ventilator-associated pneumonia in the ICU. Am J Respir Crit Care Med. 2018;197(6):826–830. doi: 10.1164/rccm.201708-1717LE. [DOI] [PubMed] [Google Scholar]
- Ewig S., Bauer T., Hasper E. Value of routine microbial investigation in community-acquired pneumonia treated in a tertiary care center. Respiration. 1996;63(3):164–169. doi: 10.1159/000196538. [DOI] [PubMed] [Google Scholar]
- Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014;6:S14459. doi: 10.4137/PMC.S14459. PMC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M., Corrao S., Licata G. Clinical impact of broad-spectrum empirical antibiotic therapy in patients with healthcare-associated pneumonia: a multicenter interventional study. Intern. Emerg. Med. 2012;7(6):523–531. doi: 10.1007/s11739-012-0795-8. [DOI] [PubMed] [Google Scholar]
- Foolad F., Huang A.M., Nguyen C.T. A multicentre stewardship initiative to decrease excessive duration of antibiotic therapy for the treatment of community-acquired pneumonia. J. Antimicrob. Chemother. 2018;73(5):1402–1407. doi: 10.1093/jac/dky021. [DOI] [PubMed] [Google Scholar]
- Fowler S., Webber A., Cooper B. Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J. Antimicrob. Chemother. 2007;59(5):990–995. doi: 10.1093/jac/dkm014. [DOI] [PubMed] [Google Scholar]
- Gadsby N.J., McHugh M.P., Forbes C. Comparison of Unyvero P55 Pneumonia Cartridge, in-house PCR and culture for the identification of respiratory pathogens and antibiotic resistance in bronchoalveolar lavage fluids in the critical care setting. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38(6):1171–1178. doi: 10.1007/s10096-019-03526-x. [DOI] [PubMed] [Google Scholar]
- Gerberding J.L. Antibiotic resistance: the hidden threat lurking behind Covid-19. STAT NEWS. 2020;3 https://www.statnews.com/2020/03/23/antibiotic-resistance-hidden-threat-lurking-behind-covid-19/ [Google Scholar]
- Houben R.M., Lalli M., Kranzer K. What if they don’t have tuberculosis? The consequences and trade-offs involved in false-positive diagnoses of tuberculosis. Clin. Infect. Dis. 2018;68(1):150–156. doi: 10.1093/cid/ciy544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Self W.H., Wunderink R.G. Community-acquired pneumonia requiring hospitalization among US adults. N. Engl. J. Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.-U.S., Hein L., Lundgren B. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ open. 2012;2(2) doi: 10.1136/bmjopen-2011-000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A.C., Metersky M.L., Klompas M. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam G., Chastre J., Wilcox M.H. Antibiotic strategies in the era of multidrug resistance. Critical Care. 2016;20(1) doi: 10.1186/s13054-016-1320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kett D., Cano E., Quartin A. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11(3):181–189. doi: 10.1016/S1473-3099(10)70314-5. [DOI] [PubMed] [Google Scholar]
- Kline K.A., Bowdish D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016;29:63–67. doi: 10.1016/j.mib.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Magill S.S., O'Leary E., Janelle S.J. Changes in prevalence of health care-associated infections in U.S. Hospitals. N Engl J Med. 2018;379(18):1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J. HM Government and Welcome Trust; UK: 2018. Tackling drug-resistant infections globally: Final report and recommendations. 2016. [Google Scholar]
- Ozongwu C., Personne Y., Platt G. The Unyvero P55 'sample-in, answer-out' pneumonia assay: A performance evaluation. Biomol Detect Quantif. 2017;13:1–6. doi: 10.1016/j.bdq.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens C.I., Wunderink R.G. Principles and practice of antibiotic stewardship in the ICU. Chest. 2019;156:163–171. doi: 10.1016/j.chest.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole S., Clark T.W. Rapid syndromic molecular testing in pneumonia: The current landscape and future potential. J Infect. 2020;80(1):1–7. doi: 10.1016/j.jinf.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J.A., Wiemken T.L., Peyrani P. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin. Infect. Dis. 2017;65(11):1806–1812. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- Ranadive N., Kunene S., Darteh S. Limitations of rapid diagnostic testing in patients with suspected malaria: a diagnostic accuracy evaluation from Swaziland, a low-endemicity country aiming for malaria elimination. Clin. Infect. Dis. 2017;64(9):1221–1227. doi: 10.1093/cid/cix131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte J.B., van Dessel H.A., Linssen C.F. Endotracheal aspirate and bronchoalveolar lavage fluid analysis: interchangeable diagnostic modalities in suspected ventilator-associated pneumonia? J. Clin. Microbiol. 2014;52(10):3597–3604. doi: 10.1128/JCM.01494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.M., Oh Y.M., Kim M.N. Usefulness of quantitative endotracheal aspirate cultures in intensive care unit patients with suspected pneumonia. J. Korean Med. Sci. 2011;26(7):865–869. doi: 10.3346/jkms.2011.26.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spafford K., MacVane S., Humphries R. Evaluation of empiric beta-lactam susceptibility prediction among enterobacteriaceae by molecular beta-lactamase gene testing. J. Clin. Microbiol. 2019;57(10) doi: 10.1128/JCM.00674-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B.G. Resistance to antibiotics mediated by target alterations. Science. 1994;264(5157):388–393. doi: 10.1126/science.8153626. [DOI] [PubMed] [Google Scholar]
- Sullivan K.V., Dien Bard J. New and novel rapid diagnostics that are impacting infection prevention and antimicrobial stewardship. Curr. Opin. Infect. Dis. 2019;32(4):356–364. doi: 10.1097/QCO.0000000000000565. [DOI] [PubMed] [Google Scholar]
- Thomas C.P., Ryan M., Chapman J.D. Incidence and cost of pneumonia in Medicare beneficiaries. Chest. 2012;142(4):973–981. doi: 10.1378/chest.11-1160. [DOI] [PubMed] [Google Scholar]
- Tong S., Amand C., Kieffer A. Trends in healthcare utilization and costs associated with pneumonia in the United States during 2008–2014. BMC Health Serv. Res. 2018;18(1) doi: 10.1186/s12913-018-3529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A., Lee N., Cilloniz C. Laboratory diagnosis of pneumonia in the molecular age. Eur. Respir. J. 2016;48(6):1764–1778. doi: 10.1183/13993003.01144-2016. [DOI] [PubMed] [Google Scholar]
- Vincent C., Manges A. Antimicrobial use, human gut microbiota and Clostridium difficile colonization and infection. Antibiotics. 2015;4(3):230–253. doi: 10.3390/antibiotics4030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrib D.A., Capraro G.A. The uncertain clinical benefit of the T2Bacteria panel. Ann. Intern. Med. 2019;170(12):888–889. doi: 10.7326/M19-0971. [DOI] [PubMed] [Google Scholar]
- Wuerth B.A., Bonnewell J.P., Wiemken T.L. Trends in pneumonia mortality rates and hospitalizations by organism, United States, 2002–2011. Emerg. Infect. Dis. 2016;22(9):1624. doi: 10.3201/eid2209.150680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Rubin J., Dunning S. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J. Am. Geriatr. Soc. 2012;60(11):2137–2143. doi: 10.1111/j.1532-5415.2012.04208.x. [DOI] [PubMed] [Google Scholar]