Graphical abstract
Abbreviation: AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; ATF4, activating transcription factor 4; Caprin 1, cell cycle associated protein 1; CK2, Casein kinase 2; DDX3X, DEAD box RNA helicase 3 X-linked; DOX, doxorubicin; eIF2, eukaryotic initiation factor 2; eIF4F, eukaryotic initiation factor 4F; ERK, extracellular signal-regulated kinase; FTD, frontotemporal dementia; FUS/TLS, fused in sarcoma/translocated in liposarcoma; GCN2, general control non-derepressible 2; GCN4, general control non-derepressible 4; G3BP1/2, Ras GTPase-activating protein-binding protein 1 and 2; HDAC, histone deacetylases; HRI, heme-regulated inhibitor; hsp, heat shock protein; JNK, c-Jun N-terminal kinase; LLPS, liquid-liquid phase separation; mRNP, messenger ribonucleoprotein; mTOR, mammalian target of rapamycin; NLRP3, NLR Family, Pyrin Domain Containing Protein 3; N protein, nucleocapsid protein; PAI-1, plasminogen activator inhibitor-1; P-body, processing body; PERK, PKR-like endoplasmatic reticulum kinase; PKR, protein kinase RNA-activated; PTM, post-translational modifications; RBP, RNA-binding protein; ROS, reactive oxygen species; SA, sodium arsenite; SG, stress granule; TDP-43, TAR DNA binding protein 43; TIA-1, T cell restricted intracellular antigen-1; TIAR-2, homolog of human TIA-1 inC. elegans pharyngeal muscles; VCP, valosin-containing protein; 4E-BP1, eIF4E-binding protein 1; 5-FU, 5-fluorouracil
Chemical compounds studied in this article: Bortezomib (PubChem CID: 387447); E)-3-(2,3,4,5-tetrabromophenyl)prop-2-enoic acid (PubChem CID: 16760346); ISRIB (PubChem CID: 1011240); Olaparib (PubChem CID: 23725625); Paclitaxel (PubChem CID: 36314); PP242 (PubChem CID:135565635); Psammaplysin F (PubChem CID: 46888580); Puromycin (PubChem CID: 439530); Silvestrol (PubChem CID: 11787114); Sorafenib (PubChem CID: 216239); Trehalose (PubChem CID: 7427); 5-Fluorouracil (PubChem CID: 3385)
Keywords: eIF2α, G3BP, Microtubule, Post-translational modification, Neurodegenerative disease, Virus infection
Abstract
Stress granules (SGs) are assemblies of mRNA and proteins that form from mRNAs stalled in translation initiation in response to stress. Chronic stress might even induce formation of cytotoxic pathological SGs. SGs participate in various biological functions including response to apoptosis, inflammation, immune modulation, and signalling pathways; moreover, SGs are involved in pathogenesis of neurodegenerative diseases, viral infection, aging, cancers and many other diseases. Emerging evidence has shown that small molecules can affect SG dynamics, including assembly, disassembly, maintenance and clearance. Thus, targeting SGs is a potential therapeutic strategy for the treatment of human diseases and the promotion of health. The established methods for detecting SGs provided ready tools for large-scale screening of agents that alter the dynamics of SGs. Here, we describe the effects of small molecules on SG assembly, disassembly, and their roles in the disease. Moreover, we provide perspective for the possible application of small molecules targeting SGs in the treatment of human diseases.
1. Stress granules
A large portion of mRNA in mammalian eukaryotic cells completes transcription in the nucleus and is then transported to the cytoplasm for translation and expression. When eukaryotic cells are stimulated or disturbed, the mature mRNA in cells cannot be translated into proteins immediately. These temporarily untranslated mRNA or translation-stalled mRNA then polymerize with RNA-binding proteins (RBPs) to form messenger ribonucleoprotein (mRNP) granules without a membrane structure, known as Cajal bodies, stress granules (SGs), processing bodies (P-bodies), RNA transport granules, or germ granules [1,2]. While mRNP granule types are complex and diverse, there are three commonalities between mRNP granules [3]: first, mRNP granules usually contain non-translated or poorly translated mRNA, and these mRNA can re-enter polysome for translation after cellular adaption or environmental recovery. Second, different mRNP granules may contain the same mRNA or RBP and these components can be relocated from one mRNP granule to another granule. Third, different mRNP granules can interact dynamically, involving docking, fusion, and becoming another mRNP granule after maturation. mRNP granules have a very important effect on mRNA function and cell signalling, and are also closely related to diseases [4]. One of the most studied mRNP granules is SGs. SGs are a type of dynamic granular substance formed of mRNA of stagnant translation and RBPs in the cytoplasm of eukaryotic cells, the formation of which is stimulated by various stresses including oxidative stress, heat shock, hypoxia, or viral infection (Fig. 1 ). It is an adaptive regulatory mechanism that protects cells from apoptosis under adverse conditions [5,6]. Besides, SG formation and dynamic can affect mRNA localization, translation, and degradation as well as signalling pathways and antiviral responses [7].
Fig. 1.
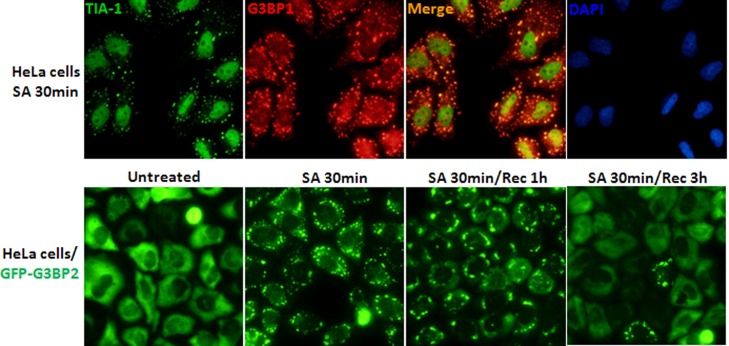
SG dynamics. SG dynamics is observed by fluorescence microscopy with immunostaining against SG-nucleating proteins or cells expressing GFP-tagged SG-nucleating proteins including G3BP1, G3BP2 and TIA-1, which are commonly used as SG markers. (A). Immunostaining results show G3BP1 and TIA-1 are extensively overlapped in SA (sodium arsenite)-induced SGs. (B). HeLa cells stably expressing GFP-tagged G3BP2 protein show SGs are efficiently assembled after 30 min treatment of SA, partially disassembled after 1 h recovery (Rec) from SA treatment and completely disassembled after 3 h Rec from SA treatment.
2. The biological functions of SGs
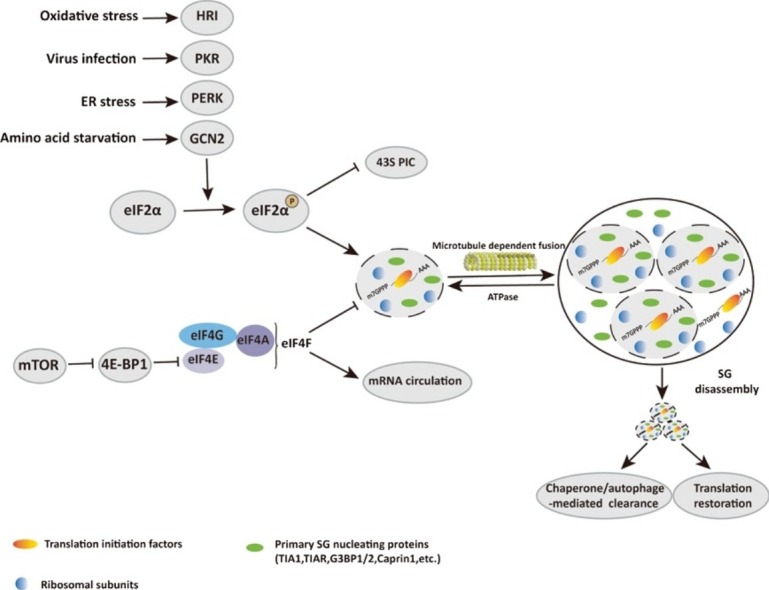
The assembly of SGs is closely related to the translation initiation of eukaryotes. The canonical translation initiation in eukaryotes begins with circularization of mRNA by eukaryotic initiation factor 4F (eIF4F) complexes and assembly of 43S preinitiation complexes (43S PIC) [8,9]. The 43S complexes start searching for the initiation codon along mRNA, and once the initiation codon is identified, eukaryotic initiation factor 2 (eIF2) hydrolyses GTP to GDP, which triggers dissociation of eIFs from ribosome and allow the join of 60S ribosomal subunit to form a complete 80S initiation complex. A key step in this process is the formation of ternary complex comprising eIF2, GTP and Met-tRNAi Met. eIF2 plays a central role in translation initiation and is tightly regulated. When eukaryotic cells encounter harmful external stimuli, such as heat shock, viral infection, oxidative stress, hypoxia, or other stresses, the α subunit of eIF2 (eIF2α) is phosphorylated at serine 51 by stress-induced kinases including PKR (protein kinase RNA-activated), PERK (PKR-like endoplasmic reticulum kinase), HRI (heme-regulated inhibitor) and GCN2 (general control non-derepressible 2). Phosphorylation of eIF2α blocks the formation of eIF2-GTP-Met-tRNAi Met ternary complex, resulting in inhibition of global translation and activation of gene-specific translation such as yeast general control non-derepressible 4 (GCN4) and mammalian activating transcription factor 4 (ATF4). The translation-stalled mRNAs are then recruited by RBPs to promote the formation of SGs [[10], [11], [12], [13], [14], [15]]. However, eIF2α phosphorylation cannot certainly cause SG formation. For example, doxorubicin (DOX) efficiently triggers phosphorylation of eIF2α while fails to promote SG formation [16]. The inactivation of eIF4F complexes through the disruption of eIF4E and eIF4G binding or inhibition of eIF4A activity by hippuristanol [17], H2O2 [18], pateamine A [19] can also lead to translation inhibition and SG formation [20].
In addition to mRNA, SG components include various RBPs, translation initiation factor and non-RBPs such as signalling proteins. SG composition is different in the same type of cells subjected to different types of stress or in different cells subjected to the same type of stress. SGs also share some ubiquitous RBPs to facilitate SG aggregation and nucleation, such as Ras GTPase-activating protein-binding protein 1 and 2 (G3BP1/2), cell cycle associated protein 1 (Caprin1) and T cell restricted intracellular antigen-1 (TIA-1) [21,22]. These proteins are often used as protein markers of SGs to facilitate SG research (Fig. 1). With the deepening of research, SG proteins and proteins that regulate SG assembly/disassembly have been discovered.
SGs are not of a uniform structure; rather, there are two distinct layers: the core and the shell [23]. These two layers of SGs may have different components, functions and dynamics. Two models for discrete phases of SGs assembly have been proposed, the cores first model and the liquid-liquid phase separation (LLPS) first model. In the cores first model, the core is surrounded by a less concentrated and more dynamic shell, and the core size and dynamics of SGs do not change over time [9,24]. In the LLPS first model, the condensation of the liquid inside the cell can drive the assembly of SGs. In some special circumstances, liquid aggregates of SGs are transformed into solid-like states, which may causes fibrosis or devastating protein aggregation diseases [25].
As the stress disappears, the clearance of SGs from the cytoplasm of cells occurs rapidly, i.e., within minutes (Fig. 1). The SG depolymerisation process is opposite to the aggregation process. First, the less stable shell is dissolved and then the core is removed [26]. SGs are generally believed to be sites of temporary mRNA storage and triage. The mRNAs in SG will have at least two directions after depolymerisation. The first is that, after chaperone or autophagy-mediated clearance of SGs upon recovery from stress or stress adaptation, specific mRNAs reversely mobilise back to the polysome and translation is resumed. The second is that specific mRNAs are translocated from SGs to P-bodies for storage or degradation [[26], [27], [28], [29], [30]]. The disassembly of SGs depends on the activation of heat hock proteins and the dephosphorylation of eIF2α, as well as the regulation of the microtubule system [31,32].
3. SGs and human diseases
Increasing evidence suggests that SGs are involved in many human diseases (Fig. 2 ), including cancers, neurodegenerative diseases, viral infections and autoimmune disease, well-documented by elegant reviews [[33], [34], [35], [36], [37]]. SGs affect multiple pathways by intercepting and sequestering signalling components, such as RACK1 (p38/JNK signalling), TRAF2 (NF-κB signalling), Raptor (mTOR signalling) and RhoA/ROCK1 (Wnt signalling) [[33], [34], [35], [36], [37]]. When exposed to stress environments, cells may arrest the cell cycle and repair stress-induced damage, or proceed to apoptosis. Acute stress-induced SGs selectively recruit and exclude mRNAs, thereby promoting the translation of stress-related response genes and enabling cells to quickly return to a normal state after stress. Conversely, if SG formation is blocked during stress, the cell survival rate is significantly reduced. However, SGs induced by pathological chronic stress (neurodegeneration, nutrient starvation) lack several classical SG components that contribute to the pro-survival functions of canonical SGs (RACK1, small ribosomal proteins), and conversely have a pro-death function [38,39].
Fig. 2.
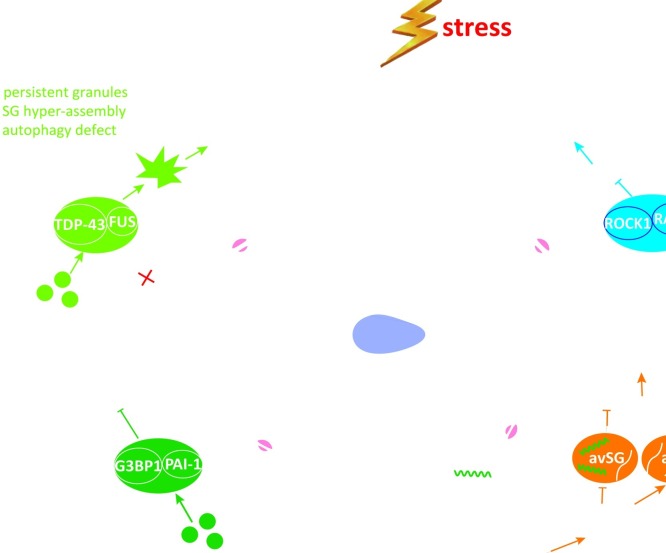
The physiological and pathological roles of SGs. By recruitment of a variety of RBPs and signaling proteins into SGs to regulate cellular processes including stress response and adaption, SGs are important to maintain homeostasis and human health. Disruption of SG dynamics is involved in the progression of aging and many types of human diseases, including cancer, chemotherapy resistance, neurodegenerative diseases and viral infection. (A). Mutation in disease-associated RBPs (e.g., FUS, TDP-43, hnRNPA1) promotes the formation of abnormal and persistent SGs, which lead to neurotoxic protein aggregates and progress towards neurodegenerative diseases. (B). Tumor microenvironment and many chemotherapeutic drugs act as stressful condition and might promote SG formation. Tumor cells hijack SG property to escape from apoptosis, resulting in tumorigenesis and drug resistance. (C). Aging-associated changes in cellular environment might lead to disruption of SG dynamics. During aging, downregulation of G3BP1 expression causes defect in SG formation. Thus, aging-promoting protein PAI-1 fails to localize to SG, which further accelerates aging. (D). In response to viral infection, SGs are induced in host cells to inhibit viral replication, via repression on viral mRNA translation and stimulation on host innate immune response. However, viral proteins (e.g., protease and nucleocapsid protein) antagonize SG formation to favor viral mRNA translation. Alternatively, viral proteins could also induce aSG (atypical SG, viral mRNAs are excluded) to inhibit translation of cellular mRNA while allow translation of viral mRNA.
3.1. Cancer
The tumour microenvironment is full of stresses, such as a high concentration of ROS, hypoxia and hypoglycaemia, which can strongly trigger SG formation. SGs are observed in many human cancers, such as pancreatic cancer, hepatocellular carcinoma, glioma/glioblastoma, sarcoma, mantle cell myeloma, colorectal cancer, head and neck cancers and prostate cancer [36]. SGs regulate the expression of oncogenes, tumour metabolism, and adapt tumour cells to the microenvironment, which are directly related to the development of tumours and the efficiency of anticancer drugs. Several anti-tumour drugs have been shown to induce the formation of SGs. On the one hand, SGs are partially involved in apoptosis regulation. For example, eIF2α phosphorylation-dependent SGs (Type I) induced by sodium arsenite (SA) and bortezomib [40] may protect cells in the stress response, inhibit apoptosis and promote cell survival by the sequestration of signalling molecules, such as RACK1 [41], ROCK1 [42] and Raptor [43]. Disturbing the formation of anti-apoptotic type I SGs not only influences tumour progression but also sensitises cancer cells to chemotherapeutic agents. On the other hand, eIF2α phosphorylation-independent SGs (Type II), induced by sodium selenite and silvestrol, may impair cell resistance to stress, reduce cell survival and promote apoptosis [39,44,45]. Induction of pro-apoptotic type II SGs might be one mechanism underlying their anti-tumour effect.
3.2. Virus infection
Viruses are another type of adverse stress to host cells. Viruses interfere with the gene expression of infected cells, and antiviral responses drive the formation of SGs to control viral RNA translation and viral replication. Virus-induced SGs have a unique composition (such as Trim25) and function, and have been named anti-viral SGs (avSGs) to distinguish them from typical SGs [46]. Based on the ways of regulating SG formation, viruses are divided into different types. The first type of virus temporarily triggers SG formation early in the replication cycle, but later limits SG formation. These viruses include poliovirus, genovirus and orthovirus [47]. Mammalian orthoreoviruses (MRV) induce SG formation via eIF2α phosphorylation in a strain- and cell type-dependent manner. As the infection progresses, viral infections leads to the inhibition of SGs. In addition, several strains of MRV inhibit SG formation induced by arsenite to facilitate infection [48]. The second type of virus can inhibit SG formation during the entire infection process, such as influenza A virus, which inhibits SG formation by blocking eIF2α phosphorylation to avoid the repression of virus replication [49]. The third type of virus induces SG formation to help virus RNA replication. Respiratory syncytial virus (RSV), picornavirus and coronavirus may benefit from inducing SG formation as part of the mechanism by which they inhibit host cell protein synthesis and innate immunity [[50], [51], [52]]. The eIF2α kinase PKR is the primary sensor responsible for the rapid inhibition of translation initiation. PKR-mediated SGs can block the translation of viral RNA and interfere with virus replication in response to virus infection. Some viruses, however, encode for PKR inhibitors, thus avoiding PKR-mediated eIF2α phosphorylation and SG formation [53]. The drugs that induce SGs by bypassing PKR and/or eIF2α phosphorylation may have therapeutic potential to control viral infection.
Recently, SARS-CoV-2 nucleocapsid (N) protein has been identified to inhibit SG formation by LLPS of N protein from the SG essential protein G3BP1/2, which enhances viral RNA replication and translation [33,54,55]. SARS-CoV-2 suppression of SG formation may be mediated by the interaction of N protein with G3BP1/2 and/or CSNK2B/CSNK2A2, the subunits of casein kinase 2 (CK2) [56]. CK2 activity is enhanced following SARS-CoV-2 infection in cells which increase SG disassembly through the promotion of G3BP1 phosphorylation. Several small molecules, including CK2 inhibitors, have been suggested to inhibit the process of viral RNA replication via SG disassembly [56,57].
3.3. Neurodegenerative diseases
SGs have been linked to several neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD) and Alzheimer’s disease (AD). A number of proteins in SG components, such as TDP-43, FUS, hnRNPA1, hnRNPA2, TIA1, MATR3, EWSR1, TAF15, ATX1 and ATX2 [58,59], have been shown to be associated with neurodegenerative diseases. Misfolded proteins or mutations in pathogenic RBPs cause abnormal protein aggregation and SG formation. When stress becomes chronic, persistent and more severe, original reversible SGs change into irreversible stable amyloid-like assemblies and insoluble fibre aggregates, leading to the loss of normal RBP function in the nucleus and the induction of neurotoxicity in the cytoplasm [58,59]. Moreover, partially mutated proteins (VCP, optineurin, p62, ubiquilin-2) may result in the dysfunction of autophagy and cause defects in the clearance of abnormal SGs, subsequently exacerbating the disease process [62]. At present, research on SGs and neurodegenerative lesions are focused on RBP biological models. RBPs guide the transport, translation and degradation of intracellular RNA, thereby regulating and controlling protein expression. Irreversible SGs impair the function of RBPs and interfere with mRNA transport and transcription, and worsen neurodegenerative diseases. Thus, reversing the irreversible solid-state of SGs may enables SGs to maintain reversible liquid properties, or promote SG disassembly and autophagy clearance, which is beneficial to the treatment of neurodegenerative diseases.
3.4. Aging
Aging is accompanied by many intracellular processes such as cellular metabolic change, mitochondrial dysfunction and abnormal protein quality control, which could result in disrupted protein homeostasis and the abnormal formation, maintenance, disassembly and clearance of SGs, consequently leading to chronic or pathological SGs [35]. These aberrant SGs may accelerate the aging process and aging-associated diseases. In aged C. elegans, the SG proteins PAB-1 and TIAR-2 can form aggregates, associated with shorter lifespan [63], suggesting a connection between SG protein aggregation and shorter lifespan. An abnormal protein-RNA interaction may lead these proteins to be aggregated and form irreversible aggregations [64], leading to persistent SGs, which would cause the pathogenesis of aging-related diseases [[65], [66], [67]]. In addition, the SG components can also disturb SG dynamics, i.e., TDP-43 can increase amyloid-β oligomeric aggregates and accelerate aging and the onset of neurodegenerative diseases [68,69]. However, studies have also shown that cellular senescence can cause eIF2α hyperphosphorylation and impair SG formation in the stress response in a G3BP1-dependent manner [70]. Omer and colleagues found that SG assembly is defective in aged cells and SG assembly has an anti-aging effect by recruitment of the pro-aging protein, plasminogen activator inhibitor-1 (PAI-1) to SGs [71]. Whether preventing chronic SG formation or inducing the formation of canonical SGs could retard aging process is worth investigating.
3.5. Inflammatory diseases
In inflammatory diseases, the inflammasome is an important component that activates proinflammatory cytokines and induces inflammatory cell death. However, SGs and the NLRP3 inflammasome compete for DDX3X molecules to coordinate the activation of innate responses and subsequent cell fate decisions under stress conditions. SG formation leads to the sequestration of DDX3X and thereby inhibits NLRP3 inflammasome activation [72]. In the immune response, the eIF2α of T cells undergoing antigen presentation for the first time is phosphorylated to form SGs that wrap the mRNA of cytokines inside. When antigen presentation occurs again, some mechanism is initiated to dissolve SGs and release the mRNA, thus translating and secreting cytokines [73]. SGs suppress the stress-induced inflammatory response by recruiting inflammatory factors TRAF2 and inhibiting TNF-α-mediated NF-κB proinflammatory signalling [74].
SGs are also associated with several other diseases, such as atrial fibrillation, organ fibrosis, autoimmune disease and brain ischemia [75]. It is expected that more diseases will be linked with SGs soon.
4. Small molecules targeting SGs
At present, many compounds have been identified to affect the process of SG assembly, maintenance and disassembly via different mechanisms. SGs transiently exist in specific stress states and are regulated by a large number of post-translational modifications, protein remodelling complexes and microtubule networks. Regulating SG assembly or disassembly may be a key method of controlling cell fate or treating disease. Hence, it is a promising field to develop potential therapeutic strategies by targeting SG proteins in related diseases. In this part, we will focus on the reported small molecules that affect SG aggregation or depolymerisation (Table 1, Table 2, Table 3, Table 4 ), summarise them according to their functions, and discuss the potential for drug development targeting SGs.
Table 1.
Compounds increasing SG assembly.
| Category | Compound | Chemical formula | Mechanism | References |
|---|---|---|---|---|
| Oxidative stressor | Sodium arsenite | NaAsO2 | Induce eIF2α phosphorylation | [154] |
| Sodium selenite | Na2SeO3 | Induce 4EBP1 dephosphorylation | [39] | |
| Hydrogen peroxide* | H2O2 | Induce 4EBP1 hypophosphorylation | [18] | |
| Diethyl maleate (DEM) | 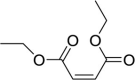 |
Induce eIF2α phosphorylation | [18] | |
| Menadione | 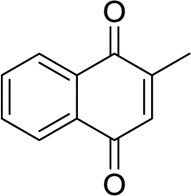 |
Unknown | [156] | |
| Osmotic and oxidative stressor | Sorbitol | 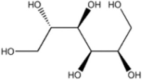 |
Induce eIF2α phosphorylation | [124,157] |
| ER stressor | Thapsigargin | 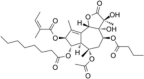 |
Induce eIF2α phosphorylation | [89] |
| Dithiothreitol (DTT) | 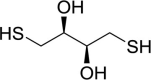 |
Induce eIF2α phosphorylation | [158] | |
| Proteasome inhibitor | Bortezomib | 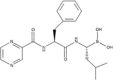 |
Induce eIF2α phosphorylation | [40] |
| Sorafenib | 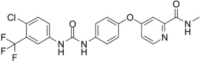 |
Induce eIF2α phosphorylation | [78] | |
| Lactacystin |  |
Induce eIF2α phosphorylation | [151] | |
| Mitochondrial inhibitor | Malonate | 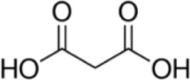 |
Induce 4EBP1 hypophosphorylation | [159] |
| Paraquat | Induce eIF2α phosphorylation | [160] | ||
| Carbonyl cyanide (trifluoromethoxy)phenylhydrazone (FCCP) | 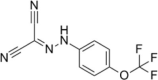 |
Induce enery deprivation | [161] | |
| Sodium azide | NaN3 | Decrease polysomes | [162] | |
| Clotrimazole | 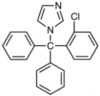 |
Unknown | [162] | |
| Nitric oxide-generating compound | Nitroso-N-acetylpenicillamine (SNAP) | 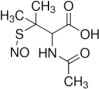 |
Induce eIF2α phosphorylation and 4EBP1 dephosphorylation | [90] |
| 3-Morpholinosydnonimine (SIN-1) |  |
Induce eIF2α phosphorylation and 4EBP1 dephosphorylation | [90] | |
| Microtubule stabilizer | Paclitaxel |  |
Promote microtubule as-sembly and stabilization | [16,106] |
| Microtubule disruption drug | Vinorelbine | 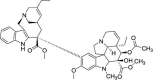 |
Induce eIF2α phosphorylation and 4EBP1 dephosphorylation | [16,109] |
| Vinblastine* |  |
Induce eIF2α phosphorylation and 4EBP1 dephosphorylation | [16,109] | |
| Vincristine | 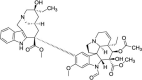 |
Induce eIF2α phosphorylation and 4EBP1 dephosphorylation | [16,109] | |
| Darinaparsin | 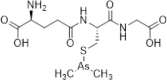 |
Inhibits microtubule polymerization | [164] | |
| Actin polymerization inhibitor | Latrunculin B | 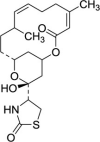 |
Disrupt actin fiber and facilitate the motility of SG components | [109] |
| DNA damage drug | Oxaliplatin | 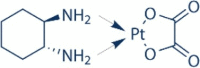 |
Induce eIF2α phosphorylation | [165] |
| Cisplatin |  |
Induce eIF2α phosphorylation | [166] | |
| eIF4A inhibitor | Hippuristanol | 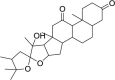 |
Inhibit eIF4A activity | [17,98] |
| Silvestrol | 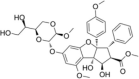 |
Stimulate eIF4A RNA-binding activity | [45,167] | |
| Tyrosine kinase inhibitor | Imatinib |  |
Induce eIF2α phosphorylation | [45,167] |
| Topoisomerase II inhibitor | Etoposide | 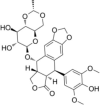 |
Induce eIF2α phosphorylation | [166] |
| RNA incorporating agents | Fluorouracil (5-FU) | 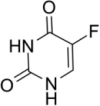 |
Induce eIF2α phosphorylation | [44,80] |
| 6-Thioguanine | 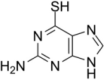 |
Incorporate into RNA | [80] | |
| 5-Azacytidine |  |
Incorporate into RNA | [80] | |
| Others | Boric acid | B(OH)3 | Induce eIF2α phosphorylation | [116] |
| Vanillin | 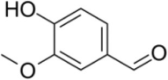 |
Unknown | [168] | |
| Pateamine A and its analogs | 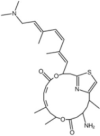 |
Stimulate eIF4A RNA-binding activity | [19,97] | |
| Furfural | 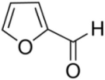 |
Unknown | [169] | |
| Deoxy-delta12,14-prostaglandin J2 (15d-PGJ2) |  |
Promote eIF4A inactivation | [170] | |
| Edeine | 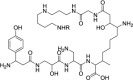 |
Prevent 60S binding to the 48S complex | [132] |
“*” represents that this drug has been reported that can both induce SGs assembly and inhibit SGs assembly, depending on cell type and dosage.
Table 2.
Compounds enhancing SG assembly.
| Category | Compound | Chemical formula | Mechanism | References |
|---|---|---|---|---|
| Protein synthesis inhibitor | Puromycin | 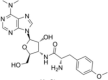 |
Destabilize polysomes | [153] |
| eIF2α dephosphorylation inhibitor | Salubrinal | 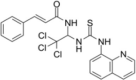 |
Inhibit eIF2α dephosphorylation | [171,172] |
| Casein Kinase 2 inhibitor | Tetrabromocinnamic acid | 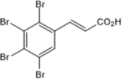 |
Prevent G3BP1 phosphorylation | [90] |
| IQA | 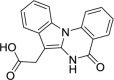 |
Prevent G3BP1 phosphorylation | [103] | |
| TMCB |  |
Prevent G3BP1 phosphorylation | [56] | |
| Silmitasertib |  |
Prevent G3BP1 phosphorylation | [56] | |
| PKC inhibitor | GF109203X |  |
Inhibit G3BP2 phosphorylation and delay eIF2α phosphorylation | [173] |
| Others | Troxerutin | 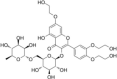 |
Unknown | [117] |
These compounds can not induce SGs assembly in large quantities when used alone. However, they enhance the role of other induced SGs production compounds, such as increase the size of sodium arsenite-induced SGs or block the disassembly of arsenite-induced SGs.
Table 3.
Compounds inhibiting SG assembly.
| Category | Compound | Chemical formula | Mechanism | References |
|---|---|---|---|---|
| Transcription inhibitor | 8-Hydroxyquinoline | 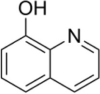 |
Unknown | [123] |
| Mitochondrial fission inducer | Tyrphostin A9 | 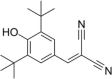 |
Unknown | [123] |
| β cell proliferation agonist | WS3 |  |
Unknown | [123] |
| Protein synthesis inhibitor | Cycloheximide |  |
Stabilize polysomes | [153] |
| Emetine | 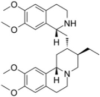 |
Stabilize polysomes | [153] | |
| Neomycin |  |
Unknown | [123] | |
| Anisomycin | 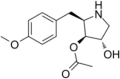 |
Unknown | [123] | |
| ROS scavenger | N-Acetyl-l-cysteine |  |
Reduce ROS production | [18] |
| PARP inhibitor | Olaparib | 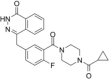 |
Inhibit PARP | [18] |
| Phosphatase inhibitor | Okadaic acid | Promote G3BP phosphorylation | [131] | |
| Vanadate | Na3VO4 | Promote G3BP phosphorylation | [131] | |
| HDAC inhibitor | Trichostatin A(TSA) | 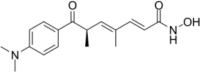 |
Inhibit HDAC6 | [131] |
| PERK inhibitor | ISRIB | 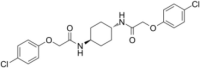 |
Target eIF2B and block signaling downstream of all eIF2α kinases | [89] |
| ATP-competitive mTOR inhibitor | Torkinib(PP242) | 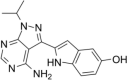 |
Inhibit mTORC1 activity and reduce accumulation of eIF4E-eIF4GI complexes | [91] |
| Torin1 | 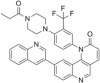 |
Inhibit mTORC1 activity and reduce accumulation of eIF4E-eIF4GI complexes | [91] | |
| Na+/K+-ATPase inhibitor | Proscillaridin A | 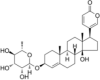 |
Unknown | [91] |
| Digitoxin |  |
Unknown | [123] | |
| Ouabain |  |
Unknown | [123] | |
| Topoisomerase II inhibitor | Mitoxantrone |  |
Inhibit TDP-43 accumulation | [123] |
| Bis(thiosemicarbazonato)-Copper Complexes | Diacetylbis(methylthiosemicarbazonato)-copperII (CuII(atsm)) |  |
Inhibit TDP-43 accumulation | [174] |
| Glyoxalbis(methylthiosemicarbazonato)-copperII (CuII(gtsm)) | 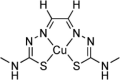 |
Inhibit TDP-43 accumulation | [174] | |
| Others | Lipoamide | 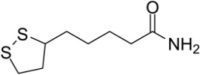 |
Inhibit FUS accumulation and disrupt SG phase separation | [124] |
| Lipolic acid | 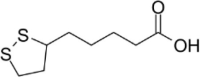 |
Inhibit FUS accumulation and disrupt SG phase separation | [124] | |
| Hydroxy-N′-[1-(2-hydroxyphenyl)ethylidene]benzohydrazide | 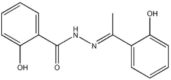 |
Target G3BP2 and influence G3BP2 function | [132] | |
| Psammaplysin F | 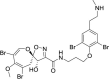 |
Decrease eIF2α phosphorylation | [83] | |
| Trehalose | 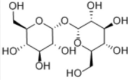 |
Promote eIF2α dephosphorylation | [85] | |
| Syringic acid | 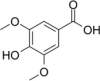 |
Unknown | [117] | |
| Penfluridol | 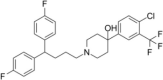 |
Unknown | [123] | |
| Gitoxigenin | 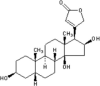 |
Unknown | [123] | |
| Benzethonium | 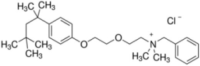 |
Unknown | [123] | |
| Pyrvinium | 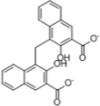 |
Unknown | [123] | |
| Quinacrine |  |
Unknown | [123] |
Table 4.
Compounds disturbing SG assembly.
| Category | Compound | Chemical formula | Mechanism | References |
|---|---|---|---|---|
| Microtubule disruption durgs | Nocodazole | 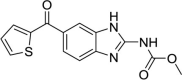 |
Promote microtubule depolymerization | [109] |
| Vinblastine* | 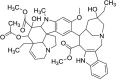 |
Promote microtubule depolymerization | [109] | |
| Vincristine* |  |
Promote microtubule depolymerization | [16,123] | |
| Actin filament stabilizer | Prieurianin | 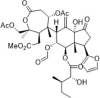 |
Unknown | [123] |
| ATP-competent kinase inhibitor | Staurosporine and its analog (RO-31−8220) | 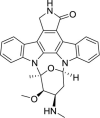 |
Disrupt the ATP-driven chemical reactions | [138] |
| 5′-Iodotubercidin |  |
Disrupt the ATP-driven chemical reactions | [138] |
These compounds interfere with the fusion and growth of SG, rather than the initial assembly of SG. When SA stimulates cells that pretreated with these compounds, the proportion of cells with SGs decreases, the number of SGs in each cell increases, and the proportion of large SGs decreases. "*" represents that this drug has been reported that can both induce SGs assembly and disturb SGs assembly, depending on cell type and dosage.
4.1. Targeting translation initiation
The accumulation of SGs and related pathogenesis is fundamentally regulated at three levels: formation, maintenance and clearance. The major SG formation-regulating signalling pathways include the eIF4F and eIF2α pathways, and the mammalian target of rapamycin (mTOR) also involved in signalling. The eIF2, GTP and Met-tRNAi Met ternary complex and eIF4F complex are two major points for the regulation of translation initiation. Under stress conditions, eIF2α kinases are activated to induce the phosphorylation of eIF2α at position 51 serine. The phosphorylated eIF2α (p-eIF2α) reduces the actions of eIF2B and inhibits translation initiation, leading to the disassembly of polysomes; consequently, untranslated mRNAs and translationally arrested PICs initiate SG assembly. These eIF2α kinases are key components in the integration of intracellular and extracellular signals regulating cellular translation in the stress response. Four eIF2α kinases are responsible for this process with different stresses: GCN2, PERK, PKR and HRI, which provide effective drug targets for therapeutic intervention.
4.1.1. Phosphorylation of eIF2α
Several small molecules have been reported to induce eIF2α phosphorylation and SG assembly, although different eIF2α kinases are employed. The Raf1/Mek/Erk kinase inhibitor sorafenib is approved for advanced hepatocarcinoma [76], and has previously been reported to induce ER stress by the activation of PERK phosphorylation of eIF2α [77,78]. Recently, it has been found that sorafenib induces SG formation in various cancer cells via PERK-mediated eIF2α phosphorylation [78]. SG formation reduces the sensitivity of cells to sorafenib, reduces cell death and improves cell survival. Thus, the disruption of SG formation can restore cell sensitivity to sorafenib. The anti-tumour drug 5-fluorouracil (5-FU) [79] has been found to induce SG assembly, inhibit cell proliferation and promote apoptosis via PKR-mediated eIF2α phosphorylation. SG assembly is associated with the 5-FU metabolite FUrd that is further converted into FUTP and incorporated into RNA to promote SG assembly [80].
Bortezomib is a peptide boronate inhibitor of the 26S proteasome approved by the FDA for the treatment of myelomas and other haematological tumours [81,82]. Bortezomib efficiently induces SGs in many cancer cells, but fails to form SGs in Hs578 T breast cancer cells. The effects of bortezomib-induced eIF2α phosphorylation is mediated through the activation of HRI and GCN2, leading to SG formation, subsequently resulting in resistance to bortezomib in cancer cells [40].
In contrast, several small molecules have also been reported to inhibit eIF2α phosphorylation and impair SG assembly. Psammaplysin F, a marine sponge-derived metabolite, has effective activity against the malaria parasite [83,84]. Psammaplysin F treatment may lead to resistance to SG inducers in many cell lines (e.g., the Vero, MCF7, T47D, HeLa and MCF7MDR cell lines). One possible mechanism is that psammaplysin F decreases the phosphorylation of eIF2α under stress. Trehalose is a natural disaccharide sugar, widely occurring in various organisms including bacteria, plants, insects, yeast, fungi and invertebrates. Trehalose protects cells from protein denaturation and exhibits neuro-protective effects in several neurodegenerative diseases under heat, freezing, oxidation, drying and dehydration conditions. This effect is accompanied by p-eIF2α dephosphorylation, which accelerates the restoration of translation. Trehalose pretreatment also decreases p-eIF2α induced by ER stress or heat shock, sensitizes cells to stress and impairs cell survival [85]. Inhibition of chemotherapy drug-induced SGs by this type of small molecule may be beneficial to relieving drug resistance in cancer patients.
The integrated stress response, mediated by eIF2α, regulates protein synthesis under stress. ISRIB targets eIF2B and is the first reported antagonist of the ISR that blocks signalling downstream of all eIF2α kinases [86]. In non-stress situations, 43S preinitiation complexes containing eIF2-GTP-Met-tRNAi Met begin translation. To engage in a new round of initiation, eIF2-GDP released during this process needs to reload GTP to form new eIF2-GTP, in a reaction catalysed by the heteropentameric eIF2B [87]. ISRIB can reverse cognitive deficits following traumatic brain injury, protect against prion-induced neurodegeneration and prevent metastasis in a subset of cancers [86]. It has been reported that ISRIB reverses the effect of eIF2α phosphorylation and restores translation by targeting eIF2B. ISRIB prevents the formation of SGs induced by thapsigargin (a potent ER stressor that inhibits the ER calcium pump [88]) and arsenite through eIF2α phosphorylation, without reducing Pat-A or hippuristanol-induced SGs through eIF4A inhibition [89]. In another study, ISRIB also inhibited SIN-1-induced SG assembly in U2OS cells [90]. ISRIB also leads to the disassembly of pre-formed SGs by loading dissociating mRNAs with actively translating ribosomes [89]. ISRIB, with its ability to quickly disassemble SGs even in the presence of stress, is expected to develop into new therapies for SG-related diseases.
4.1.2. Disruption of the eIF4F complex
Assembly of the eIF4F complex requires eIF4A, eIF4E and eIF4G as a key step in mRNA circulation and translation initiation. It is tightly regulated by cell signalling, particularly through mTOR signalling. In normal conditions, mTOR phosphorylates eIF4E-binding protein 1 (4E-BP1), which blocks 4E-BP1 binding to eIF4E and forms the eIF4F complex. The eIF4F complex is a major target of environmental stress to induce eIF2α-independent SGs. In response to stresses or chemical compounds, mTOR is inactivated, which results in the accumulation of hypophosphorylated 4E-BP1 and enables its binding to eIF4E, causing displacement of the scaffolding protein eIF4G and the RNA helicase eIF4A from mRNA cap structures. Consequently, translation initiation and polysome formation are blocked, concomitantly with SG assembly. Disruption of the eIF4F complex may induce non-canonical SGs, which lack some of the core components of eIF2α phosphorylation-induced canonical SGs [91]. Thus, targeting the eIF4F complex might be a promising approach to regulating SG dynamics.
eIF4A inhibition and the disruption of eIF4E binding to eIF4G are the common mechanisms underlying the formation of eIF2α-independent SGs induced by several small molecules. Malonate, a classic competitive inhibitor of the respiratory electron transport chain, induces mitochondrial stress and inhibits mitochondrial respiration [92,93]. It has been reported that malonate induces SG aggregation through 4E-BP1-mediated inactivation of the eIF4E pathway, which may inhibit apoptosis in HeLa cells [94]. Like malonate, selenium, an essential micronutrient that promotes the production of reactive oxygen species (ROS) for targeting tumour cells [31], induces SG assembly by destroying the eIF4F complex via the dephosphorylation of 4E-BP1 [39]. Interestingly, selenite-induced SGs prompts U2OS human osteosarcoma cells to undergo apoptosis, probably because of the unique SG composition [39].
Of note, perturbing eIF4A activity induces SG assembly independent of eIF2α phosphorylation, which may exert anti-cancer activity [20,95]. 15-deoxy-delta12,14-prostaglandin J2 (15d-PGJ2) possesses multiple biological activities [96], which blocks translation by targeting cysteine 264 and inactivating eIF4A, without the involvement of eIF2α phosphorylation, suggesting that the translation inhibitory action of 15d-PGJ2 is likely to contribute to the antineoplastic activity of 15d-PGJ2 [96]. Pateamine A and its analogues, DMDA-PatA and B-PatA, induce SG formation through the prevention of eIF4A heterodimerisation with eIF4G and stabilising the eIF4A:mRNA complex, consequently limiting the availability of eIF4A incorporation into eIF4F complexes [19,97]. Hippuristanol and silvestrol have also been found to induce SG assembly by perturbing eIF4F ATPase, helicase and RNA-binding activities, explaining their anti-tumour and antiviral replication activity [17,98].
Some small molecules have been reported to have combined activities of eIF2α phosphorylation and eIF4F disruption. For example, the vinca alkaloids vinorelbine, vinblastine and vincristine promote polysome disassembly and SG assembly through simultaneous activation of eIF4E-BP1 and phosphorylation of eIF2α [16]. Blocking vinca alkaloid-induced SG assembly by inactivating 4EBP1 or inhibiting eIF2α phosphorylation decreases cancer cell viability and promotes apoptosis [16]. Nitric oxide-generating compounds 3-morpholinosydnonimine (SIN-1) and S-nitroso-N-acetylpenicillamine (SNAP) are protein synthesis inhibitors that trigger SG formation through NO-induced eIF2α phosphorylation and 4E-BP1 dephosphorylation [90]. NO plays a major role in this process. Interestingly, SIN-1-induced pro-apoptotic SGs are less dynamic and less mobile compared to typical arsenite-induced anti-apoptotic SGs because of the loss of eIF3B over time [90].
4.1.3. Targeting mTOR signalling
While the dissociation of eIF4E-eIF4G induces eIF2α-independent SGs, association of eIF4E-eIF4G is required for the assembly of eIF2α-dependent SGs. Therefore, disruption of eIF4E-eIF4G could inhibit the assembly of eIF2α-dependent SGs, or promote the disassembly of pre-existing eIF2α-dependent SGs. This was proposed by Fournier and colleagues, who found that mTOR inhibitor-mediated disruption of eIF4E-eIF4GI impairs SG formation under mild stress [91]. When the cell is treated with a mild stressor such as arsenite or bortezomib (both induce eIF2α phosphorylation), eIF4E binds to eIF4G, but the eIF4E-4GI complex is in an inactive state, accumulates gradually and eventually leads to the production of SGs. In this case, depletion or inactivation of mTOR leads to hypophosphorylation of 4E-BP1, which then blocks the interactions between eIF4E and eIF4GI to promote the disassembly of formed SG [91].
PP242 is an ATP-competitive inhibitor of mTOR that blocks the phosphorylation of Akt at S473 and suppresses mTOR activity. PP242 directly binds to the ATP-binding site of mTOR and inhibits the catalytic activity of both mTORC1 and mTORC2 [99]. It also decreases the phosphorylation of 4E-BP1 to prevent the binding of eIF4E to eIF4GI, thus inhibiting SG formation [91]. The depletion of mTOR significantly inhibits SG formation in cells treated with PP242 [91]. Similar to PP242, the kinase inhibitor torin1 is also a selective ATP-competitive inhibitor of mTOR [100], which inhibits mTORC1-dependent phosphorylation of 4E-BP1 and impairs SG formation [91]. These data suggest that the suppression of mTOR signalling may inhibit the assembly of mild stress-induced eIF2α-dependent SGs and sensitize tumour cells to apoptosis.
4.1.4. Inhibition of CK2
CK2 is a stress-activated protein kinase linked to the phosphorylation of several translation initiation factors, including eIF3 and eIF5 [101,102]. CK2 accelerates SG disassembly through the promotion of G3BP1 phosphorylation to inhibit SG assembly [103]. Thus, CK2 inhibitors may block SG disassembly and recover from the stress. Tetrabromocinnamic acid (TBCA) is a specific CK2 inhibitor [104] and increases residual SGs in cells during recovery from stress [103]. The another CK2 inhibitor, 5-oxo-5,6-dihydroindolo-(1,2-a)quinazolin-7-yl acetic acid (IQA), also inhibits the disassembly of virus-induced SGs [103]. A recent study has suggested that the CK2 inhibitors TMCB and silmitasertib could interfere with SG disassembly by SARS-CoV-2 N protein, but this needs to be confirmed [56,57].
4.2. Targeting the cytoskeleton
The recruitment of mRNPs in the cytoplasm to fuse into mature SGs requires transportation along microtubules by motor proteins; thus, microtubule integrity is essential for SG assembly. Microtubules are intracellular structures assembled with heterodimers of α and β tubulin, and are responsible for various kinds of movements in cells. Microtubules are involved in cell division, the organisation of intracellular structure and intracellular transportation. Microtubule disruption delays SG formation. SGs are still formed, but they are smaller in size, greater in number and variable in distribution [105].
Tubulin inhibitors are used in the treatment of cancer because they induce cell cycle arrest. Paclitaxel, a first-line anti-cancer drug, is a β-tubulin inhibitor and promotes microtubule assembly and stabilisation [106,107]. Paclitaxel induces SG formation in many cells [16,105]. Similarly, latrunculin B, an actin filament inhibitor [108], has been reported to accelerate microtubule-based motility via actin disruption, thus facilitating the motility of SGs and promoting the formation of large SGs [109].
In contrast, tubulin depolymerising agents could trigger SG disappearance. As a reversible microtubule inhibitor, nocodazole binds to β-tubulin and disrupts microtubule assembly and disassembly kinetics, thereby preventing mitosis and inducing apoptosis in tumour cells [110]. Nocodazole inhibits SG formation but does not prevent SA-induced eIF2α phosphorylation, suggesting that nocodazole acts downstream of eIF2α phosphorylation [109].
Vinblastine is a therapeutic drug for Hodgkin’s disease, lymphocytic lymphoma, histiocytic lymphoma, advanced testicular cancer, advanced breast cancer, Kaposi’s sarcoma and Letterer-Siwe disease [111,112]. Vinblastine has higher affinity for tubulin and causes microtubule depolymerisation [113]. Similarly to nocodazole, vinblastine disrupts the formation of SGs and the disassembly of the formed SGs [109]. However, a higher concentration or prolonged treatment with vinblastine potently promotes translation repression and induces microscopically visible SGs [16,109]. Since the cytoskeleton also serves as scaffold and is closely associated with the polysome for the active translation of mRNA [114], vinblastine at a high dose may induce robust polysome disassembly through the collapse of microtubules and the secondary aggregation of SGs [16].
These studies have provided helpful strategies for targeting microtubules to regulate SG formation, as well as strategies for overcoming resistance to tubulin inhibitors in cancer patients.
4.3. Targeting SG components
The core components of SGs includes G3BP1/2, TIA-1, TIAR, Caprin1, TDP-43, a number of energy-driven protein/nucleic acid chaperones or remodelling complexes and untranslated mRNA. Targeting the components may influence SG dynamics.
4.3.1. TIA-1
TIA-1 has been reported to prevent cell proliferation, tumour growth and invasion [115]. Under physiological conditions, TIA-1 binds to an adenine/uridine-rich element in the 3’-UTR of TNFα mRNA and represses TNFα mRNA translation. In contrast, TIA-1 increases TNFα mRNA translation in response to stress. Boric acid exhibits anticarcinogenic and bone strengthening effects by balancing the anti-apoptotic eIF2α-SG pathway and pro-apoptotic eIF2α-ATF4 pathway via TIA-1 translocation from the nucleus to SGs [116]. Similarly, troxerutin, known as vitamin P4 with multiple nutritional and pharmacological properties (antioxidant, anti-inflammatory, radioprotective and hepatoprotective activities), has also been shown to promote SG formation in a TIA1-dependent manner. This effect is diminished in TIA-1 suppressed cells, suggesting that enhanced SG formation by troxerutin might recruit essential stress-fighting mRNAs/proteins into SGs and thereby impair the cellular machines required for survival and recovery [117].
4.3.2. TDP-43
TAR DNA binding protein 43 (TDP-43) is an RBP pathologically and genetically linked to ALS, FTD, Alzheimer’s disease, Parkinson’s disease and Huntington’s disease [118,119]. Several studies have shown that the accumulation of TDP-43 in SGs is mediated through activation of the stress kinase c-Jun N-terminal kinase (JNK) [120]. Inhibiting extracellular signal-regulated kinase (ERK) could prevent the accumulation of TDP-43 and human antigen R-(HuR)-positive SG formation. Mutations in the TDP-43 coding gene TARDBP as well as changes in the expression level of TDP-43 proteins may disrupt the dynamics of SGs [120].
Mitoxantrone is a type II topoisomerase inhibitor approved for the treatment of acute myeloid leukaemia and multiple sclerosis [122]. Mitoxantrone reduces TDP-43 in SGs and decreases SG formation in disease-relevant cell models harbouring mislocalised TDP-43 protein [121,123]. Mitoxantrone prevents SG localisation of mutant TDP-43 and reduces the accumulation of mutant TDP-43 into persistent cytoplasmic puncta in induced pluripotent stem cell-derived spinal motor neurons (iPS-MNs). Mitoxantrone also significantly reduces the cumulative death rate in mouse primary neurons expressing TDP-43 (M337 V)-EGFP [123], suppresses the recruitment of FUS to SGs, and reduces the number and size of liquid FUS droplets formed in vitro [124].
Emerging evidence suggests that LLPS-driven SG formation is associated with the pathogenesis of neurodegenerative diseases, aging, cancer and virus infections [125] because of increased irreversible hydrogelation that interferes with normal cellular functions. Hyperphosphorylated TDP-43 undergoes LLPS to colocalise with other stress granule components and promote aberrant protein aggregation, forming chronic SGs. The small molecule inhibitors of tankyrase-1/2, XAV939, G007-LK and IWR-1-endo, suppress the formation of cytoplasmic TDP-43 and increase LLPS of TDP-43 from other SG components [126]; this prevents the pathogenesis of neurodegenerative diseases and may be used to treat ALS and FTD. LLPS can be enhanced by poly(ADP-ribosyl)ation (PARylation), an important post-translational modification (PTM) involved in DNA damage repair and apoptosis. Additionally, a PARP inhibitor was found to attenuate TDP-43-mediated neurotoxicity in an ALS model [127]. It has been reported that the PARP inhibitor olaparib delays the recruitment of TDP-43 and hnRNP A1 to SGs and delays SG assembly, which is beneficial in ALS and FTD [127]. In pathologic fibrillation, SGs are unlinked from the chemical chaperone trimethylamine N-oxide (TMAO), which enhances RNP liquid condensation and inhibits protein fibrillation, suggesting a therapeutic strategy for TDP-43 aggregation-associated degenerative disorders [128].
4.3.3. G3BP
G3BP1 and G3BP2 contain RNA-binding domains, and are associated with RNA binding [129]. G3BP1/2 are core component of SGs and essential for SG formation. Arsenite induces SG formation, probably via the induction of the dephosphorylation of G3BP at Ser149 [22,130], and increases the interaction of G3BP with histone deacetylase 6 (HDAC6) [131]. Phosphatase inhibitors such as okadaic acid and vanadate inhibit the interaction of G3BP and HDAC6 and increase G3BP phosphorylation level and regulate SG formation [22,131].
Since G3BP1/2 are essential for the initiation of SG formation, small molecules targeting G3BP1/2 could be expected as a new approach for cancer therapy. Compound C108, 2-hydroxy-N-[1-(2-hydroxyphenyl)ethylidene]benzohydrazide, could alleviate the function of G3BP2 and inhibit the activity of tumour-initiating cells [132], showing anti-tumour activity. A recent study showed that SARS-CoV-2 N protein interacts with G3BP1/2 to inhibit SG formation; this inhibits virus RNA replication, suggesting a potential therapeutic target for COVID-19 [54]. However, the small molecules targeting this complex need to be tested.
4.3.4. ATPase
The assembly, fusion, depolymerisation and transportation of SG components require ATP. Consequently, intracellular ATP levels can affect SG dynamics [90]. The formation of SGs and maintenance of SG core proteins in the dynamic pool of SGs require ATP driven chemical reactions rather than specific kinase activity [133]. It seems that the different ATPase complexes have different effects on SG assembly and disassembly [133]. The movement of SGs along the microtubule also requires ATP energy [134,135]. Thus, ATPase inhibitors may affect both the assembly and disassembly of SGs.
Soil-dwelling microbes produce the chemical staurosporine and its analogue RO-31-8220, which are both ATP-competent kinase inhibitors [134,135] that may inhibit SA-induced SGs [138]. 5′-Iodotubercidin (5′-ITU), a kinase inhibitor [139], interferes with the fusion and growth of SGs by inhibiting ATPase activity, but does not affect the disassembly of SGs during recovery from SA stress [138].
4.3.5. FUS
The RNA/DNA binding protein fused in sarcoma/translocated in liposarcoma (FUS/TLS) is linked to ALS [140]. Like TDP-43, FUS/TLS is sequestered in the cytosol of ALS-affected motor neurons. Mutations in FUS cause severe loss of motor neurons in the spinal cord associated with juvenile ALS. The unique feature of FUS pathogenesis in ALS is their nuclear clearance and simultaneous cytoplasmic aggregation in affected motor neurons [118]. FUS accumulation in SGs is a reversible process, which may lead to pathological aggregation of FUS in SGs under chronic stress [60,61]. FUS droplets form by LLPS, and become gel-like forms containing amyloid fibrils and are thus less dynamic. Additionally, some disease-related FUS mutations can exacerbate this phenomenon and aggravate the condition [80]. Lipoamide and lipolic acid have been reported to act on FUS to reduce SG formation and promote the dissolution of pre-existing SGs. Lipoamide and lipoic acid have been shown to improve FUS condensation behaviour, prevent SG component aggregation in a C. elegans model, and recover neuron and organism FUS-associated defects in a Drosophila model of ALS [80].
4.4. Post-translational modifications
Post-translational modifications (PTMs) including methylation, acetylation, ubiquitination, sumoylation, glycosylation, phosphorylation and neddylation play crucial roles in regulating the functions of proteins by inducing their covalent linkage to new functional chemical groups. PTMs are involved in various signalling pathways that regulate distinct cellular processes. Dysregulated PTMs have been shown to influence RBPs, the key regulators in RNA dynamics, and cause various diseases including cancers, neurodegenerative disorders, virus infection and cardiovascular disease [141,142], suggesting that targeting PTMs is a therapeutic option for these diseases.
Ubiquitination and the ubiquitin-like proteins NEDD8 and SUMO, which mediate neddylation and sumoylation, regulate various cellular processes [[143], [144], [145]], and involved in the disassembly of SGs. Thus, the pharmacological inhibition of ubiquitination may improve the efficiency of cancer therapies. Neddylation induces SG assembly under a stress state via enhanced polysomic disassembly following translation arrest. However, so far, no pharmacological agent has been reported to act on neddylation.
Acetylation and deacetylation, another major form of protein modification, are also involved in SG formation and disassembly. Histone acetylases and histone deacetylases (HDACs) regulate the acetylation of proteins. It has been shown that HDAC6, but not other HDACs, may directly interact with the SG core component G3BP1 to enhance SG formation. Pharmacological inhibition or genetic ablation of HDAC6 blocks SG assembly and sensitises cells to apoptosis under stress [131]. This effect of HDAC6 might be linked to the deacetylation of tubulin [146,147], which is important for SG dynamics, since the pan-HDAC inhibitor TSA results in microtubule network hyperacetylation and decreases SG formation [131]. Thus, targeting HDAC6 deacetylase activity or its interaction with G3BP are worth considering for SG regulation and cancer therapy. Interestingly, the NAD+-dependent deacetylase SIRT6 interacts with G3BP to induce SG assembly in response to stress [148], and inhibiting the activity of SIRT6 impairs SG formation. Thus, SIRT6 antagonists may be a strategy to inhibit the formation of anti-apoptotic SGs and induce apoptosis in tumour cells.
4.5. Targeting SG clearance
SGs are disassembled when the stress is relieved, which is accompanied by translation restoration. Similar to SG formation, the disassembly of SGs is also a multi-step process and is mediated by chaperones and autophagy [26]. The roles of the hsp70 family in chaperone-mediated SG dissolution have been studied by several groups. For example, hsp70, hsp40, hsp104 and HspB8 (the small heat shock protein hsp22) participate in SG removal and relieve the accumulation of untranslated ribosomal products in SGs. Therefore, it is worth investigating the effect of small molecules targeting these chaperone proteins on SG dynamics.
Autophagy removes SGs, in a process called granulophagy. Autophagic vesicles may engulf SGs and clear the particles. The process requires valosin-containing protein (VCP), an ATPase required for the autophagy disaggregation of SG components [29]. Trehalose, an autophagy enhancer [149], has been found to delay SG assembly and facilitate SG premature post-stress disassembly in neuroblastoma SH-SY5Y cells, implicating that trehalose is a possible therapeutic approach in neurodegenerative diseases. However, the action of trehalose is mediated via an increase in p-eIF2α rather than the autophagosome pathway. One study showed that only a minor fraction of aberrant SGs is cleared by autophagy, whereas the majority of SG disassembly requires the HSPB8-BAG3-HSP70 chaperone [149]. Whether autophagy enhancers could accelerate SG disassembly, and thereby be developed as therapeutic agent against cancers and neurodegenerative diseases, still needs further investigation.
The proteasome is also responsible for the degradation of mRNAs in SGs, which was confirmed by the induction of SG assembly by pharmacological and clinically used proteasome inhibitors [150]. The proteasome inhibitor bortezomib induces SG assembly via the stimulation of eIF2α phosphorylation by the kinases HRI, PKR, GCN2 and PERK [40,151]. This disrupts multiple pathways in the cell and bone marrow microenvironment, leading to apoptosis and inhibiting cell cycle progression, angiogenesis and proliferation [82]; this is a common approach in the treatment of cancers [81,151]. In contrast, prolonged inhibition of the proteasome instead leads to partial recovery of translation and SG disassembly due to stress adaptation [40,151], suggesting that the assembly of SGs in response to proteasome inhibition is time-dependent and reversible [40,151]. Thus, the application of proteasome inhibitors has to consider the involvement of SGs.
4.6. Targeting polysome dissociation
The polysome is a complex formed by cytoplasmic RNAs and ribosomes undergoing active translation. During the stress process, the polysomes originally in the translationally active state begin to dissociate, and the excess free mRNA from ribosomes associate with aggregation-prone RBPs or misfolded proteins, thereby promoting SG protein recruitment and SG assembly [152].
Emetine and cycloheximide are protein synthesis inhibitors that stabilise polysomes by freezing ribosomes to keep ribosomes loaded with mRNA; this prevents the ribosome from reaching the stop codon. mRNA can shuttle between SGs and polysomes under stress. Emetine and cycloheximide prevent the assembly of SA-induced SGs by trapping mRNA in polysomes and maintaining polysomes, preventing free mRNA from accumulating in the cytoplasm [150,153]. On the contrary, puromycin, an inhibitor of protein synthesis that destabilises polysomes by promoting translational premature termination, enhances SA-induced SGs rather than inhibiting SG aggregation, suggesting that the drugs promoting polysome stabilization or dissociation can affect SG formation.
5. Conclusion and perspective
Here, we have summarised recent advances in small molecules targeting SGs (Fig. 3 ). SGs have been identified in many biological processes and diseases. The assembly and disassembly of SGs determine further storage, translation remodelling or degradation of untranslated mRNA, which affect cell death or survival under specific conditions. In cancer treatment, on the one hand, the formation of SGs can lead to cell survival and increase cell resistance to chemotherapeutic drugs. The combined use of drugs that inhibit SG formation or promote SG disassembly with chemotherapeutic drugs may alleviate drug resistance. On the other hand, some drugs may enhance the effects of chemotherapy by inducing SG-mediated cell apoptosis. Furthermore, the persistence of stress particles leads to chronic SG formation and irreversible pathogenesis, for example in neurodegeneration and aging, It is therefore possible that targeting chronic SGs that inhibit the abnormal aggregation of related SGs or promote SG clearance may be a novel therapeutic strategy in neurodegenerative diseases and other chronic diseases. The formation and the biological functions of SGs are complex. Many questions need to be answered by research and the development of SG-targeting drugs. (1) Studies on SGs are largely confined to cell culture and C. elegans because of the absence of a suitable in vivo mammalian model. Ideally, a mouse model will be developed that can directly assess stress particles in living animals and more intuitively present direct associations between SGs, drugs and diseases. (2) The side effects of the long-term administration of SG-interfering agents remain unclear. (3) Most SG contents are not the direct target of small molecules. So, the identification of druggable targets in SGs will reveal new biological functions and mechanisms of SG biology. Thus, SGs deserve more in-depth research in order to find novel therapeutic strategies for human diseases.
Fig. 3.
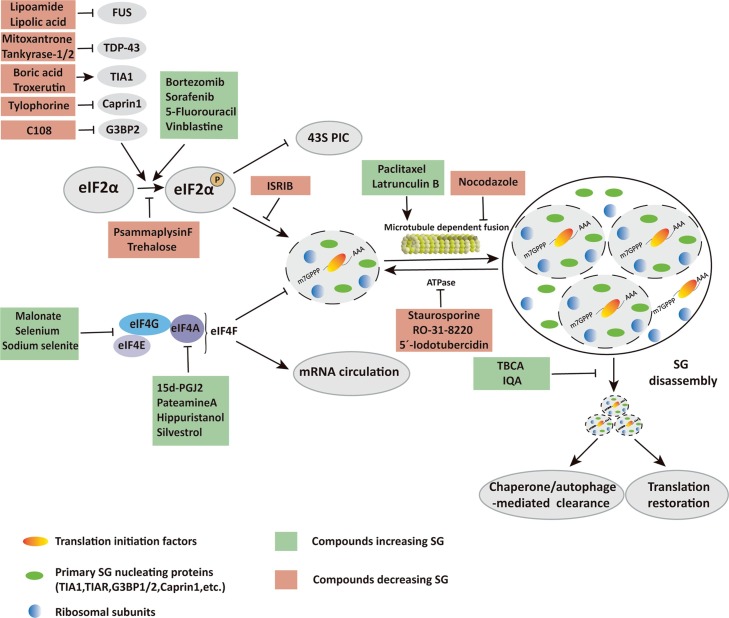
Compounds targeting SG. Compounds targeting SGs at diverse steps of SG assembly and SG disassembly are summarized and schematically represented.
Declaration of Copeting Interest
The authors have no competing interests to declare.
Acknowledgement
This work was supported by grant from National Natural Science Foundation of China (No. 31970755).
References
- 1.Banani S.F., Lee H.O., Hyman A.A., Rosen M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Bio. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas M.G., Loschi M., Desbats M.A., Boccaccio G.L. RNA granules: the good, the bad and the ugly. Cell. Signal. 2011;23:324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchan J.R. mRNP granules. RNA Biol. 2014;11:1019–1030. doi: 10.4161/15476286.2014.972208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson P., Kedersha N. RNA granules. J. Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson P., Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- 6.Arimoto-Matsuzaki K., Saito H., Takekawa M. TIA1 oxidation inhibits stress granule assembly and sensitizes cells to stress-induced apoptosis. Nat. Commun. 2016:7. doi: 10.1038/ncomms10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kedersha N., Ivanov P., Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci. 2013;38:494–506. doi: 10.1016/j.tibs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haimov O., Sinvani H., Dikstein R. Cap-dependent, scanning-free translation initiation mechanisms. Biochim. Biophys. Acta. 2015;1849:1313–1318. doi: 10.1016/j.bbagrm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Sonenberg N., Hinnebusch A.G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava S.P., Kumar K.U., Kaufman R.J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 11.Anderson P., Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 13.McEwen E., Kedersha N., Song B., Scheuner D., Gilks N., Han A., et al. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 2005;280:16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- 14.Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 15.Anderson P., Kedersha N. Stressful initiations. J. Cell. Sci. 2002;115:3227–3234. doi: 10.1242/jcs.115.16.3227. [DOI] [PubMed] [Google Scholar]
- 16.Szaflarski W., Fay M.M., Kedersha N., Zabel M., Anderson P., Ivanov P. Vinca alkaloid drugs promote stress-induced translational repression and stress granule formation. Oncotarget. 2016;7:30307–30322. doi: 10.18632/oncotarget.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cencic R., Pelletier J. Hippuristanol-A potent steroid inhibitor of eukaryotic initiation factor 4A. Translation. 2016;4 doi: 10.1080/21690731.2015.1137381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emara M.M., Fujimura K., Sciaranghella D., Ivanova V., Ivanov P., Anderson P. Hydrogen peroxide induces stress granule formation independent of eIF2α phosphorylation. Biochem. Biophys. Res. Co. 2012;423:763–769. doi: 10.1016/j.bbrc.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang Y., Kedersha N., Low W., Romo D., Gorospe M., Kaufman R., et al. Eukaryotic initiation factor 2α-independent pathway of stress granule induction by the natural product pateamine a. J. Biol. Chem. 2006;281:32870–32878. doi: 10.1074/jbc.M606149200. [DOI] [PubMed] [Google Scholar]
- 20.Mazroui R., Sukarieh R., Bordeleau M.E., Kaufman R.J., Northcote P., Tanaka J., et al. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2α phosphorylation. Mol. Biol. Cell. 2006;17:4212–4219. doi: 10.1091/mbc.E06-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., Dember L.M., et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tourrière H., Chebli K., Zekri L., Courselaud B., Blanchard J.M., Bertrand E., et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Souquere S., Mollet S., Kress M., Dautry F., Pierron G., Weil D. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. J. Cell. Sci. 2009;122:3619–3626. doi: 10.1242/jcs.054437. [DOI] [PubMed] [Google Scholar]
- 24.Protter D.S.W., Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26:668–679. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin Y., Brangwynne C.P. Liquid phase condensation in cell physiology and disease. Science. 2017;357:f4382. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler J.R., Matheny T., Jain S., Abrisch R., Parker R. Distinct stages in stress granule assembly and disassembly. Elife. 2016;5 doi: 10.7554/eLife.18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchan J.R., Muhlrad D., Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol. 2008;183:441–455. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decker C.J., Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. CSH Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchan J.R., Kolaitis R., Taylor J.P., Parker R. Eukaryotic stress granules are cleared by granulophagy and Cdc48/VCP function. Cell. 2013;153:1461–1474. doi: 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganassi M., Mateju D., Bigi I., Mediani L., Poser I., Lee H.O., et al. A surveillance function of the HSPB8-BAG3-HSP70 chaperone complex ensures stress granule integrity and dynamism. Mol. Cell. 2016;63:796–810. doi: 10.1016/j.molcel.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Kedersha N., Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. T. 2002;30:963. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 32.Loschi M., Leishman C.C., Berardone N., Boccaccio G.L. Dynein and kinesin regulate stress-granule and P-body dynamics. J. Cell. Sci. 2009;122:3973–3982. doi: 10.1242/jcs.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick C., Khaperskyy D.A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 2017;17:647–660. doi: 10.1038/nri.2017.63. [DOI] [PubMed] [Google Scholar]
- 34.Wolozin B., Ivanov P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019;20:649–666. doi: 10.1038/s41583-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X., Jin X., Liu B. The involvement of stress granules in aging and aging-associated diseases. Aging Cell. 2020;19 doi: 10.1111/acel.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao X., Jiang L., Gong Y., Chen X., Ying M., Zhu H., et al. Stress granule: a promising target for cancer treatment. Br. J. Pharmacol. 2019;176:4421–4433. doi: 10.1111/bph.14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahboubi H., Stochaj U. Cytoplasmic stress granules: dynamic modulators of cell signaling and disease. Biochim. Biophys. Acta. 2017;1863:884–895. doi: 10.1016/j.bbadis.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Reineke L.C., Neilson J.R. Differences between acute and chronic stress granules, and how these differences may impact function in human disease. Biochem. Pharmacol. 2019;162:123–131. doi: 10.1016/j.bcp.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimura K., Sasaki A.T., Anderson P. Selenite targets eIF4E-binding protein-1 to inhibit translation initiation and induce the assembly of non-canonical stress granules. Nucleic Acids Res. 2012;40:8099–8110. doi: 10.1093/nar/gks566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marie-Josée Fournier CGAR . 2010. The Chemotherapeutic Agent Bortezomib Induces the Formation of Stress Granules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arimoto K., Fukuda H., Imajoh-Ohmi S., Saito H., Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 2008;10:1324–1332. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- 42.Tsai N., Wei L. RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cell. Signal. 2010;22:668–675. doi: 10.1016/j.cellsig.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thedieck K., Holzwarth B., Prentzell M.T., Boehlke C., Klasener K., Ruf S., et al. Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells. Cell. 2013;154:859–874. doi: 10.1016/j.cell.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 44.García M.A., Carrasco E., Aguilera M., Alvarez P., Rivas C., Campos J.M., et al. The chemotherapeutic drug 5-fluorouracil promotes PKR-mediated apoptosis in a p53-independent manner in colon and breast cancer cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slaine P., Kleer M., Smith N., Khaperskyy D., McCormick C. Stress granule-inducing eukaryotic translation initiation factor 4A inhibitors block influenza a virus replication. Viruses. 2017;9:388. doi: 10.3390/v9120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo J.S., Takahasi K., Ng C.S., Ouda R., Onomoto K., Yoneyama M., et al. DHX36 enhances RIG-I signaling by facilitating PKR-mediated antiviral stress granule formation. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White J.P., Lloyd R.E. Regulation of stress granules in virus systems. Trends Microbiol. 2012;20:175–183. doi: 10.1016/j.tim.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin Q., Hastings C., Miller C.L. Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection. J. Virol. 2009;83:11090–11101. doi: 10.1128/JVI.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khaperskyy D.A., Hatchette T.F., McCormick C. Influenza A virus inhibits cytoplasmic stress granule formation. Faseb J. 2011;26:1629–1639. doi: 10.1096/fj.11-196915. [DOI] [PubMed] [Google Scholar]
- 50.Lindquist M.E., Lifland A.W., Utley T.J., Santangelo P.J., Crowe J.E. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. J. Virol. 2010;84:12274–12284. doi: 10.1128/JVI.00260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raaben M., Groot Koerkamp M.J.A., Rottier P.J.M., de Haan C.A.M. Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies. Cell. Microbiol. 2007;9:2218–2229. doi: 10.1111/j.1462-5822.2007.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X., Hu Z., Fan S., Zhang Q., Zhong Y., Guo D., et al. Picornavirus 2A protease regulates stress granule formation to facilitate viral translation. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malinowska M., Niedźwiedzka-Rystwej P., Tokarz-Deptuła B., Deptuła W. Stress granules (SG) and processing bodies (PB) in viral infections. Acta Biochim. Pol. 2016:63. doi: 10.18388/abp.2015_1060. [DOI] [PubMed] [Google Scholar]
- 54.Luo L., Li Z., Ma P., Zou Y., Li P., Liang A., et al. 2020. SARS-CoV-2 Nucleocapsid Protein Impairs SG Assembly by Partitioning Into G3BP Condensate. Available at SSRN: https://ssrn.com/abstract=3646571. [Google Scholar]
- 55.Savastano A., de Opakua A.I., Rankovic M., Zweckstetter M. BioRxiv; 2020. Nucleocapsid Protein of SARS-CoV-2 Phase Separates Into RNArich Polymerase-containing Condensates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehdi Bouhaddou D.M.B.M., Rezelj M.C.M.B., Robyn M., Kaake J.B.A.L., Ajda Rojc K.O.J.M., Miorin E.M.C.K., Robinot T.V.B.E., et al. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020 doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobra I., Pankivskyi S., Samsonova A., Pastre D., Hamon L. Relation between stress granules and cytoplasmic protein aggregates linked to neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2018:18. doi: 10.1007/s11910-018-0914-7. [DOI] [PubMed] [Google Scholar]
- 59.Brown D.G., Shorter J., Wobst H.J. Emerging small-molecule therapeutic approaches for amyotrophic lateral sclerosis and frontotemporal dementia. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2019.126942. [DOI] [PubMed] [Google Scholar]
- 60.Ling S., Polymenidou M., Cleveland D.W. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baron D.M., Kaushansky L.J., Ward C.L., Sama R.R.K., Chian R., Boggio K.J., et al. Amyotrophic lateral sclerosis-linked FUS/TLS alters stress granule assembly and dynamics. Mol. Neurodegener. 2013;8:30. doi: 10.1186/1750-1326-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L., Liu B. Relationships between stress granules, oxidative stress, and neurodegenerative diseases. Oxid. Med. Cell. Longev. 2017;2017:1–10. doi: 10.1155/2017/1809592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lechler M.C., David D.C. More stressed out with age? Check your RNA granule aggregation. Prion. 2017;11:313–322. doi: 10.1080/19336896.2017.1356559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alberti S., Halfmann R., King O., Kapila A., Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neumann M., Rademakers R., Roeber S., Baker M., Kretzschmar H.A., Mackenzie I.R.A. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Co. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 67.Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 68.Gasset-Rosa F., Chillon-Marinas C., Goginashvili A., Atwal R.S., Artates J.W., Tabet R., et al. Polyglutamine-expanded huntingtin exacerbates age-related disruption of nuclear integrity and nucleocytoplasmic transport. Neuron. 2017;94:48–57. doi: 10.1016/j.neuron.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woerner A.C., Frottin F., Hornburg D., Feng L.R., Meissner F., Patra M., et al. Cytoplasmic protein aggregates interfere with nucleocytoplasmic transport of protein and RNA. Science. 2016;351:173–176. doi: 10.1126/science.aad2033. [DOI] [PubMed] [Google Scholar]
- 70.Moujaber O., Mahboubi H., Kodiha M., Bouttier M., Bednarz K., Bakshi R., et al. Dissecting the molecular mechanisms that impair stress granule formation in aging cells. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:475–486. doi: 10.1016/j.bbamcr.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Omer A., Patel D., Lian X.J., Sadek J., Di Marco S., Pause A., et al. Stress granules counteract senescence by sequestration of PAI-1. EMBO Rep. 2018:19. doi: 10.15252/embr.201744722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samir P., Kesavardhana S., Patmore D.M., Gingras S., Malireddi R.K.S., Karki R., et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573:590–594. doi: 10.1038/s41586-019-1551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheu S., Stetson D.B., Reinhardt R.L., Leber J.H., Mohrs M., Locksley R.M. Activation of the integrated stress response during T helper cell differentiation. Nat. Immunol. 2006;7:644–651. doi: 10.1038/ni1338. [DOI] [PubMed] [Google Scholar]
- 74.Kim W.J., Back S.H., Kim V., Ryu I., Jang S.K. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol. Cell. Biol. 2005;25:2450–2462. doi: 10.1128/MCB.25.6.2450-2462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong G., Liang F., Sun B., Wang C., Liu Y., Guan X., et al. Presence and function of stress granules in atrial fibrillation. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelgalil A.A., Alkahtani H.M., Al-Jenoobi F.I. Sorafenib. Profiles Drug Subst. Excip. Relat. Methodol. 2019;44:239–266. doi: 10.1016/bs.podrm.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Rahmani M., Davis E.M., Crabtree T.R., Habibi J.R., Nguyen T.K., Dent P., et al. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol. Cell. Biol. 2007;27:5499–5513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adjibade P., St-Sauveur V.G., Quevillon H.M., Fournier M.J., Savard A., Coudert L., et al. Sorafenib, a multikinase inhibitor, induces formation of stress granules in hepatocarcinoma cells. Oncotarget. 2015;6:43927–43943. doi: 10.18632/oncotarget.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harris R.B.D.A. Clinical pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989 doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 80.Kaehler C., Isensee J., Hucho T., Lehrach H., Krobitsch S. 5-Fluorouracil affects assembly of stress granules based on RNA incorporation. Nucleic Acids Res. 2014;42:6436–6447. doi: 10.1093/nar/gku264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manasanch E.E., Orlowski R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017;14:417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan C.R.C., Abdul-Majeed S., Cael B., Barta S.K. Clinical pharmacokinetics and pharmacodynamics of Bortezomib. Clin. Pharmacokinet. 2019;58:157–168. doi: 10.1007/s40262-018-0679-9. [DOI] [PubMed] [Google Scholar]
- 83.Christen K.E., Davis R.A., Kennedy D. Psammaplysin F increases the efficacy of bortezomib and sorafenib through regulation of stress granule formation. Int. J. Biochem. Cell Biol. 2019;112:24–38. doi: 10.1016/j.biocel.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 84.Liu S., Fu X., Schmitz F.J., Kelly-Borges M. Psammaplysin F, a new bromotyrosine derivative from a sponge, Aplysinella sp. J. Nat. Prod. 1997;60:614–615. doi: 10.1021/np970070s. [DOI] [PubMed] [Google Scholar]
- 85.Dimasi P., Quintiero A., Shelkovnikova T.A., Buchman V.L. Modulation of p-eIF2α cellular levels and stress granule assembly/disassembly by trehalose. Sci. Rep.-UK. 2017;7:44088. doi: 10.1038/srep44088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anand A.A., Walter P. Structural insights into ISRIB, a memory-enhancing inhibitor of the integrated stress response. FEBS J. 2020;287:239–245. doi: 10.1111/febs.15073. [DOI] [PubMed] [Google Scholar]
- 87.Lopez-Lastra M., Rivas A., Barria M.I. Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation. Biol. Res. 2005;38:121–146. doi: 10.4067/s0716-97602005000200003. [DOI] [PubMed] [Google Scholar]
- 88.Reid D.W., Chen Q., Tay A.S.L., Shenolikar S., Nicchitta C.V. The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell. 2014;158:1362–1374. doi: 10.1016/j.cell.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sidrauski C., McGeachy A.M., Ingolia N.T., Walter P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife. 2015;4 doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aulas A., Lyons S.M., Fay M.M., Anderson P., Ivanov P. Nitric oxide triggers the assembly of “type II” stress granules linked to decreased cell viability. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-1173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fournier M.J., Coudert L., Mellaoui S., Adjibade P., Gareau C., Cote M.F., et al. Inactivation of the mTORC1-Eukaryotic translation initiation factor 4E pathway alters stress granule formation. Mol. Cell. Biol. 2013;33:2285–2301. doi: 10.1128/MCB.01517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bowman C.E., Wolfgang M.J. Role of the malonyl-CoA synthetase ACSF3 in mitochondrial metabolism. Adv. Biol. Regul. 2019;71:34–40. doi: 10.1016/j.jbior.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quastel J.H., Wooldridge W.R. Some properties of the de-hydrogenating enzymes of bacteria. Biochem. J. 1928;22:689–702. doi: 10.1042/bj0220689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu X., Gao X., Ge L., Cui X., Su C., Yang W., et al. Malonate induces the assembly of cytoplasmic stress granules. FEBS Lett. 2016;590:22–33. doi: 10.1002/1873-3468.12049. [DOI] [PubMed] [Google Scholar]
- 95.Malka-Mahieu H., Newman M., Desaubry L., Robert C., Vagner S. Molecular pathways: the eIF4F translation initiation complex-new opportunities for cancer treatment. Clin. Cancer Res. 2017;23:21–25. doi: 10.1158/1078-0432.CCR-14-2362. [DOI] [PubMed] [Google Scholar]
- 96.Li J., Guo C., Wu J. 15-deoxy-Δ-12,14-Prostaglandin J2 (15d-PGJ2), an endogenous ligand of PPAR-γ: function and mechanism. PPAR Res. 2019;2019:1–10. doi: 10.1155/2019/7242030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korneeva N.L. Translational dysregulation by pateamine A. Chem. Biol. 2007;14:5–7. doi: 10.1016/j.chem-biol.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naineni S.K., Itoua M.R., Cencic R., Putnam A.A., Amador L.A., Rodriguez A.D., et al. A comparative study of small molecules targeting eIF4A. RNA. 2020;26:541–549. doi: 10.1261/rna.072884.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feldman M.E., Apsel B., Uotila A., Loewith R., Knight Z.A., Ruggero D., et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thoreen C.C., Kang S.A., Chang J.W., Liu Q., Zhang J., Gao Y., et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arrigoni G., Pagano M.A., Sarno S., Cesaro L., James P., Pinna L.A. Mass spectrometry analysis of a protein kinase CK2β subunit interactome isolated from mouse brain by affinity chromatography. J. Proteome Res. 2008;7:990–1000. doi: 10.1021/pr070500s. [DOI] [PubMed] [Google Scholar]
- 102.Meggio F., Pinna L.A. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 103.Reineke L.C., Tsai W.C., Jain A., Kaelber J.T., Jung S.Y., Lloyd R.E., et al. Casein kinase 2 is linked to stress granule dynamics through phosphorylation of the stress granule nucleating protein G3BP1. Mol. Cell. Biol. 2017;37 doi: 10.1128/MCB.00596-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pagano M.A., Poletto G., Di Maira G., Cozza G., Ruzzene M., Sarno S., et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 105.Nadezhdina E.S., Lomakin A.J., Shpilman A.A., Chudinova E.M., Ivanov P.A. Microtubules govern stress granule mobility and dynamics. Biochim. Biophys. Acta (BBA) 2010;1803:361–371. doi: 10.1016/j.bbamcr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 106.Schiff P.B., Horwitz S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. U. S. A. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blagosklonny M.V., Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int. J. Cancer. 1999;83:151–156. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 108.Spector I., Shochet N.R., Blasberger D., Kashman Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 109.Ivanov P.A., Chudinova E.M., Nadezhdina E.S. Disruption of microtubules inhibits cytoplasmic ribonucleoprotein stress granule formation. Exp. Cell Res. 2003;290:227–233. doi: 10.1016/s0014-4827(03)00290-8. [DOI] [PubMed] [Google Scholar]
- 110.Signoretto E., Honisch S., Briglia M., Faggio C., Castagna M., Lang F. Nocodazole induced suicidal death of human erythrocytes. Cell. Physiol. Biochem. 2016;38:379–392. doi: 10.1159/000438638. [DOI] [PubMed] [Google Scholar]
- 111.Sears J.E., Boger D.L. Total synthesis of vinblastine, related natural products, and key analogues and development of inspired methodology suitable for the systematic study of their structure-function properties. Accounts Chem. Res. 2015;48:653–662. doi: 10.1021/ar500400w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pandya P., Agarwal L.K., Gupta N., Pal S. Molecular recognition pattern of cytotoxic alkaloid vinblastine with multiple targets. J. Mol. Graph. Model. 2014;54:1–9. doi: 10.1016/j.jmgm.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 113.Nogales E. Structural insights into microtubule function. Annu. Rev. Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- 114.Kim S., Coulombe P.A. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat. Rev. Mol. Cell Bio. 2010;11:75–81. doi: 10.1038/nrm2818. [DOI] [PubMed] [Google Scholar]
- 115.Izquierdo J.M., Alcalde J., Carrascoso I., Reyes R., Ludeña M.D. Knockdown of T-cell intracellular antigens triggers cell proliferation, invasion and tumour growth. Biochem. J. 2011;435:337–344. doi: 10.1042/BJ20101030. [DOI] [PubMed] [Google Scholar]
- 116.Henderson K.A., Kobylewski S.E., Yamada K.E., Eckhert C.D. Boric acid induces cytoplasmic stress granule formation, eIF2α phosphorylation, and ATF4 in prostate DU-145 cells. Biometals. 2015;28:133–141. doi: 10.1007/s10534-014-9809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu L., Chen X., Liao X., Yan Y. Screening novel stress granule regulators from a natural compound library. Protein Cell. 2017;8:618–622. doi: 10.1007/s13238-017-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guerrero E.N., Wang H., Mitra J., Hegde P.M., Stowell S.E., Liachko N.F., et al. TDP-43/FUS in motor neuron disease: complexity and challenges. Prog. Neurobiol. 2016;145–146:78–97. doi: 10.1016/j.pneurobio.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dewey C.M., Cenik B., Sephton C.F., Johnson B.A., Herz J., Yu G. TDP-43 aggregation in neurodegeneration: Are stress granules the key? Brain Res. 2012;1462:16–25. doi: 10.1016/j.brainres.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meyerowitz J., Parker S.J., Vella L.J., Ng D., Price K.A., Liddell J.R., et al. C-Jun N-terminal kinase controls TDP-43 accumulation in stress granules induced by oxidative stress. Mol. Neurodegener. 2011;6:57. doi: 10.1186/1750-1326-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Orru S., Coni P., Floris A., Littera R., Carcassi C., Sogos V., et al. Reduced stress granule formation and cell death in fibroblasts with the A382T mutation of TARDBP gene: evidence for loss of TDP-43 nuclear function. Hum. Mol. Genet. 2016;25:4473–4483. doi: 10.1093/hmg/ddw276. [DOI] [PubMed] [Google Scholar]
- 122.Bellosillo B., Colomer D., Pons G., Gil J. Mitoxantrone, a topoisomerase II inhibitor, induces apoptosis of B-chronic lymphocytic leukaemia cells. Br. J. Haematol. 1998;100:142–146. doi: 10.1046/j.1365-2141.1998.00520.x. [DOI] [PubMed] [Google Scholar]
- 123.Fang M.Y., Markmiller S., Vu A.Q., Javaherian A., Dowdle W.E., Jolivet P., et al. Small-molecule modulation of TDP-43 recruitment to stress granules prevents persistent TDP-43 accumulation in ALS/FTD. Neuron. 2019;103:802–819. doi: 10.1016/j.neuron.2019.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wheeler R.J., Hyun O.L., Ina P., Arun P., Thom D., Satoshi K., et al. bioRxiv; 2019. Small Molecules for Modulating Protein Driven liquid-liquid Phase Separation in Treating Neurodegenerative Disease. [Google Scholar]
- 125.Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A.P., Kim H.J., et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McGurk L., Gomes E., Guo L., Mojsilovic-Petrovic J., Tran V., Kalb R.G., et al. Poly(ADP-Ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol. Cell. 2018;71:703–717. doi: 10.1016/j.molcel.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duan Y., Du A., Gu J., Duan G., Wang C., Gui X., et al. PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins. Cell Res. 2019;29:233–247. doi: 10.1038/s41422-019-0141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choi K., Tsoi P.S., Moosa M.M., Paulucci-Holthauzen A., Liao S.J., Ferreon J.C., et al. A chemical chaperone decouples TDP-43 disordered domain phase separation from fibrillation. Biochemistry-US. 2018;57:6822–6826. doi: 10.1021/acs.biochem.8b01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Irvine K., Stirling R., Hume D., Kennedy D. Rasputin, more promiscuous than ever: a review of G3BP. Int. J. Dev. Biol. 2004;48:1065–1077. doi: 10.1387/ijdb.041893ki. [DOI] [PubMed] [Google Scholar]
- 130.Gallouzi I., Parker F., Chebli K., Maurier F., Labourier E., Barlat I., et al. A novel phosphorylation-dependent RNase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol. Cell. Biol. 1998;18:3956–3965. doi: 10.1128/mcb.18.7.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kwon S., Zhang Y., Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Gene Dev. 2007;21:3381–3394. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gupta N., Badeaux M., Liu Y., Naxerova K., Sgroi D., Munn L.L., et al. Stress granule-associated protein G3BP2 regulates breast tumor initiation. Proc. Natl. Acad. Sci. 2017;114:1033–1038. doi: 10.1073/pnas.1525387114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jain S., Wheeler J.R., Walters R.W., Agrawal A., Barsic A., Parker R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–498. doi: 10.1016/j.cell.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kang J., Lim L., Song J. ATP enhances at low concentrations but dissolves at high concentrations liquid-liquid phase separation (LLPS) of ALS/FTD-causing FUS. Biochem. Biophys. Res. Co. 2018;504:545–551. doi: 10.1016/j.bbrc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 135.Perez-Pepe M., Fernández-Alvarez A.J., Boccaccio G.L. Life and work of stress granules and processing bodies: new insights into their formation and function. Biochemistry-US. 2018;57:2488–2498. doi: 10.1021/acs.biochem.8b00025. [DOI] [PubMed] [Google Scholar]
- 138.Eum H., Shin Y., Song Y., Kim Y., Kang S. ATP-driven reactions are required for the assembly of large stress granules. Biochem. Biophys. Res. Co. 2020;521:238–244. doi: 10.1016/j.bbrc.2019.10.116. [DOI] [PubMed] [Google Scholar]
- 139.Massillon D., Stalmans W., van de Werve G., Bollen M. Identification of the glycogenic compound 5-iodotubercidin as a general protein kinase inhibitor. Biochem. J. 1994;299(Pt 1):123–128. doi: 10.1042/bj2990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vance C., Rogelj B., Hortobagyi T., De Vos K.J., Nishimura A.L., Sreedharan J., et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grammatikakis I., Abdelmohsen K., Gorospe M. Posttranslational control of HuR function. Wiley Interdiscip. Rev. RNA. 2017;8:e1372. doi: 10.1002/wrna.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee E.K. Post-translational modifications of RNA-binding proteins and their roles in RNA granules. Curr. Protein Pept. SCI. 2012;13:331. doi: 10.2174/138920312801619411. [DOI] [PubMed] [Google Scholar]
- 143.Komander D., Rape M. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 144.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 145.van der Veen A.G., Ploegh H.L. Ubiquitin-like proteins. Annu. Rev. Biochem. 2012;81:323–357. doi: 10.1146/annurev-biochem-093010-153308. [DOI] [PubMed] [Google Scholar]
- 146.Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 147.Boyault C., Sadoul K., Pabion M., Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 148.Jedrusik-Bode M., Studencka M., Smolka C., Baumann T., Schmidt H., Kampf J., et al. The sirtuin SIRT6 regulates stress granule formation in C. elegans and mammals. J. Cell. Sci. 2013;126:5166–5177. doi: 10.1242/jcs.130708. [DOI] [PubMed] [Google Scholar]
- 149.Hosseinpour-Moghaddam K., Caraglia M., Sahebkar A. Autophagy induction by trehalose: molecular mechanisms and therapeutic impacts. J. Cell. Physiol. 2018;233:6524–6543. doi: 10.1002/jcp.26583. [DOI] [PubMed] [Google Scholar]
- 150.Novac O. Inhibitors of protein synthesis identified by a high throughput multiplexed translation screen. Nucleic Acids Res. 2004;32:902–915. doi: 10.1093/nar/gkh235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mazroui R., Di Marco S., Kaufman R.J., Gallouzi I.E. Inhibition of the ubiquitin-proteasome system induces stress granule formation. Mol. Biol. Cell. 2007;18:2603–2618. doi: 10.1091/mbc.E06-12-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bounedjah O., Desforges B., Wu T., Pioche-Durieu C., Marco S., Hamon L., et al. Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res. 2014;42:8678–8691. doi: 10.1093/nar/gku582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kedersha N., Cho M.R., Li W., Yacono P.W., Chen S., Gilks N., et al. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 2000;151:1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kedersha N.L., Gupta M., Li W., Miller I., Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J. Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Byrd A.K., Zybailov B.L., Maddukuri L., Gao J., Marecki J.C., Jaiswal M., et al. Evidence that G-quadruplex DNA accumulates in the cytoplasm and participates in stress granule assembly in response to oxidative stress. J. Biol. Chem. 2016;291:18041–18057. doi: 10.1074/jbc.M116.718478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dewey C.M., Cenik B., Sephton C.F., Dries D.R., Mayer P.R., Good S.K., et al. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol. Cell. Biol. 2011;31:1098–1108. doi: 10.1128/MCB.01279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fujimura K., Kano F., Murata M. Identification of PCBP2, a facilitator of IRES-mediated translation, as a novel constituent of stress granules and processing bodies. RNA. 2008;14:425–431. doi: 10.1261/rna.780708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fu X., Gao X., Ge L., Cui X., Su C., Yang W., et al. Malonate induces the assembly of cytoplasmic stress granules. FEBS Lett. 2016;590:22–33. doi: 10.1002/1873-3468.12049. [DOI] [PubMed] [Google Scholar]
- 160.Aulas A., Vande Velde C. Alterations in stress granule dynamics driven by TDP-43 and FUS: a link to pathological inclusions in ALS? Front. Cell. Neurosci. 2015;9:423. doi: 10.3389/fncel.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rigby W.F., Stubbs T., Blackwell T.K., Stoecklin G., Anderson P., Kedersha N., et al. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23:1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Buchan J.R., Yoon J., Parker R. Stress-specific composition, assembly and kinetics of stress granules in Saccharomyces cerevisiae. J. Cell. Sci. 2011;124:228–239. doi: 10.1242/jcs.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mason T.A., Kolobova E., Liu J., Roland J.T., Chiang C., Goldenring J.R. Darinaparsin is a multivalent chemotherapeutic which induces incomplete stress response with disruption of microtubules and Shh signaling. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martins I., Kepp O., Schlemmer F., Adjemian S., Tailler M., Shen S., et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 166.Vilas-Boas F.D.A.S., Da Silva A.M., de Sousa L.P., Lima K.M., Vago J.P., Bittencourt L.F.F., et al. Impairment of stress granule assembly via inhibition of the eIF2alpha phosphorylation sensitizes glioma cells to chemotherapeutic agents. J. Neurooncol. 2016;127:253–260. doi: 10.1007/s11060-015-2043-3. [DOI] [PubMed] [Google Scholar]
- 167.Bordeleau M., Robert F., Gerard B., Lindqvist L., Chen S.M.H., Wendel H., et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J. Clin. Invest. 2008 doi: 10.1172/JCI34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iwaki A., Ohnuki S., Suga Y., Izawa S., Ohya Y. Vanillin inhibits translation and induces messenger ribonucleoprotein (mRNP) granule formation in saccharomyces cerevisiae: application and validation of high-content, image-based profiling. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Iwaki A., Kawai T., Yamamoto Y., Izawa S. Biomass conversion inhibitors furfural and 5-Hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae. Appl. Environ. Microb. 2013;79:1661–1667. doi: 10.1128/AEM.02797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim W.J., Kim J.H., Jang S.K. Anti-inflammatory lipid mediator 15d-PGJ2 inhibits translation through inactivation of eIF4A. EMBO J. 2007;26:5020–5032. doi: 10.1038/sj.emboj.7601920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boyce M. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 172.Walker A.K., Soo K.Y., Sundaramoorthy V., Parakh S., Ma Y., Farg M.A., et al. ALS-associated TDP-43 induces endoplasmic reticulum stress, which drives cytoplasmic TDP-43 accumulation and stress granule formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kobayashi T., Winslow S., Sunesson L., Hellman U., Larsson C. PKCalpha binds G3BP2 and regulates stress granule formation following cellular stress. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Parker S.J., Meyerowitz J., James J.L., Liddell J.R., Nonaka T., Hasegawa M., et al. Inhibition of TDP-43 accumulation by bis(thiosemicarbazonato)-copper complexes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042277. [DOI] [PMC free article] [PubMed] [Google Scholar]