Abstract
We present the AI-discovered aetiology of COVID-19, based on a precise disease model of COVID-19 built under five weeks that best matches the epidemiological characteristics, transmission dynamics, clinical features, and biological properties of COVID-19 and consistently explains the rapidly expanding COVID-19 literature. We present that SARS-CoV-2 implements a unique unbiased survival strategy of balancing viral replication with viral spread by increasing its dependence on (i) ACE2-expressing cells for viral entry and spread, (ii) PI3K signaling in ACE2-expressing cells for viral replication and egress, and (iii) viral- non-structural-and-accessory-protein-dependent immunomodulation to balance viral spread and viral replication. We further propose the combination of irinotecan (an in-market topoisomerase I inhibitor) and etoposide (an in-market topoisomerase II inhibitor) could potentially be an exceptionally effective treatment to protect critically ill patients from death caused by COVID-19-specific cytokine storms triggered by sepsis, ARDS, and other fatal comorbidities.
Keywords: Aetiology, Treatment, Cytokine storm, ICU, COVID-19, ACE2, Irinotecan, Etoposide, SARS-CoV-2
Introduction
The global outbreak of 2019 novel coronavirus disease (COVID-19) demands accurate and rapid discoveries of effective and safe treatments despite the limited availability of clinical data.
While the conventional drug discovery approach is too slow and costly to meet the demand in antiviral settings, AI is an emerging powerful approach to deeper disease understanding and faster drug discovery.
Demiurge Technologies AG is a Swiss research-based AI-biopharmaceutical company that transforms ultra-large-scale publicly available life science data into ultra-fine-detail precise disease models in areas with unmet medical needs. The company pioneers a self-correcting scientific approach that validates the quality of disease models with the accuracy of AI-based predictions of phase 3 clinical trial outcomes.
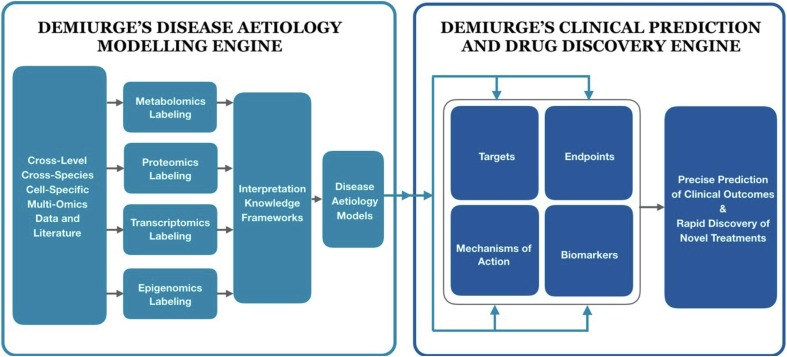
We started deep analyses of published studies, clinical data, and case reports on COVID-19 since early February 2020 with the Demiurge Unified AI platform for disease modeling, clinical prediction, and drug discovery (Fig. 1 ).
Fig. 1.
Demiurge unified AI platform for disease modeling, clinical prediction, and drug discovery.
By harnessing the complementary strengths of machine intelligence and human intelligence, we have brought missed data and mislabeled data to a minimum in our investigation of the causal aetiology of COVID-19. In addition, we also leverage our validated precise disease models for other indications in virology, oncology, neurology, immunology and cardiovascular metabolism in our investigation of the cross-level pathways commonly implicated in COVID-19 and those other indications. Finally, we built a precise disease model of COVID-19 under five weeks that best matches the epidemiological characteristics, transmission dynamics, clinical features, and biological properties of COVID-19 and consistently explains the rapidly expanding COVID-19 literature.
Aetiology
We present the following the causal aetiology of COVID-19 as the scientific rationale for the combination therapy of topoisomerase I inhibitors and topoisomerase II inhibitors, which could potentially be an effective and safe treatment to protect critically ill patients from death caused by COVID-19-specific cytokine storms and other fatal comorbidities:
Viral survival strategy
Severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2) adopts a unique unbiased survival strategy of balancing viral replication with viral spread, in stark contrast to other viruses that typically trade off one against the other. For example, MERS coronavirus adopts a survival strategy that prioritizes viral replication over viral spread, whereas influenza viruses adopt a survival strategy that prioritizes viral spread over viral replication.
SARS-CoV-2 implements this unique unbiased survival strategy by increasing its dependence on (i) ACE2-expressing cells for viral entry and spread, (ii) PI3K signaling in ACE2-expressing cells for viral replication and egress, and (iii) viral-non-structural-and-accessory-protein-dependent immunomodulation to balance viral spread and viral replication.
COVID-19’s course of infection bifurcates between (i) a self-limiting stage for mildly-to-moderately symptomatic patients when the unbiased survival strategy of SARS-CoV-2 is disadvantageous against the host immunity, and (ii) a self-amplifying stage for severely-to-critically ill patients when the unbiased survival strategy of SARS-CoV-2 is advantageous over the host immunity.
Viral entry
SARS-CoV-2 directly enhances its affinity with the functional host-cell receptor angiotensin-converting enzyme 2 (ACE2), and thus targets only stem-like cell types whose ACE2-dependent baseline activities regulate a myriad of homeostatic cellular processes that maintain genome, proteome, ion, energy, and immune system stability in a tissue-specific fashion.
ACE2 is expressed in (i) type II alveolar cells in the lung; (ii) hepatocytes and cholangiocytes in the liver; (iii) erythrocytes, Paneth cells, and enteroendocrine cells in the intestine; (iv) oligodendrocyte progenitor cells and astrocytes in the brain; (v) proximal tubule cells, podocytes, and intercalated cells in the kidney [1]. ACE2 is also expressed in endothelial stem cells and endothelial progenitor cells [2], insulin-secreting beta pancreatic cells [3], and bone marrow-derived mesenchymal stem cells [4].
Therefore, the entry of SARS-CoV-2 has a broad range of target host cell types widely distributed in the human body.
Viral replication
After entry, SARS-CoV-2 hijacks the PI3K signaling of ACE2-expressing host cells to drive viral replication. Furthermore, SARS-CoV-2 enhances the activation of the distal promoters of the ACE2 gene in the host cells, which further suppresses the ACE2 expression in the host cells and disrupts the normal homeostatic functions of the host cells.
The ACE2 distal promoter transcript (DPT) is an evolutionarily conserved binding motif of ACE2 inhibitors, whereas the ACE2 proximal promoter transcript (PPT) is an evolutionarily conserved binding motif of ACE2 enhancers [5]. The DPT/PPT ratio is tissue-specific such that the DPT is significantly more than the PPT in the lung (>49-fold), whereas the PPT is significantly more than the DPT in the kidney, brain, and heart (>20-fold) [6]. It is unusual that the ACE2 DPT/PPT ratio, rather than the ACE2 genotype, determines ACE2 transcript levels.
Lung vulnerability
Given that type II alveolar cells exclusively express ACE2 in the lung and features a 49-fold DPT/PPT ratio [6], COVID-19 infection would suppress ACE2 expression in type II alveolar cells with orders-of-magnitude higher potency than in other ACE2-expressing cell types that feature much lower DPT/PPT ratios.
The sharply reduced ACE2 expression in type II alveolar cells will enhance the binding of angiotensin II to AT2 receptors, resulting in (i) reduced pulmonary surfactant secretion, (ii) enhanced lung elastance, (iii) reduced blood oxygenation, (iv) enhanced vascular permeability, (v) enhanced pulmonary edema, (vi) increased alveolar wall thickness, (vii) enhanced inflammatory cell infiltrates, (viii) formation of hyaline membranes, (ix) enhanced pulmonary fibrosis, (x) reduced lung regeneration, (xi) air space collapse, and (xii) eventually lung failure [7].
Therefore, human lungs are selectively vulnerable to ACE2 deficiencies induced by COVID-19, and lung injuries are the predominant clinical features of COVID-19.
Immunomodulation
There are 380 amino acid substitutions of the SARS-CoV-2 genome compared with the human SARS-CoV genome [8]. Consequentially, the non-structural proteins and accessory proteins [9] of SARS-CoV-2 leverage the disrupted homeostatic functions of the host cells to create a favorable microenvironment. In particular, SARS-CoV-2 activates the innate immune response to increase dendritic cell contact to the host cells so as to enhance the PI3K pathway-mediated viral replication [10] and (ii) suppresses the adaptive immune response to further reduce the apoptosis of host cells so as to sustain viral replication and egress.
Therefore, COVID-19 has exhibited an immunomodulatory profile that over-stimulates the innate immune response (enhanced IL2, IL7, IL10, TNF-alpha, monocyte, and GM-CSF levels) [11] and over-suppresses the adaptive immune response (reduced lymphocyte count, without significant changes in B cells and NK cells) [12]. The over-suppression of the adaptive immune response is further corroborated by the increased PD-1 and TIM-3 expression on CD4 + and CD8 + T lymphocytes in COVID-19 patients [11], as well as the decreased IL-6 levels (<0.5 pg/mL) in 91% of hospitalized patients [12].
Therefore, the COVID-19 immunomodulatory profile is the causal driver of lymphocytopenia, the most common clinical hallmark for COVID19 patients on admission (83.2%), when only half of them have fever on admission (43.8%) [13].
Clinical outcomes
The clinical outcomes of COVID-19 are driven by the tissue-specific and patient-specific co-development of ACE2-deficiency-induced injury and innate-adaptive-imbalance-induced lymphocytopenia.
First, the synergistic co-development of ACE2-deficiency-induced injury in the lung and innate-adaptive-imbalance-induced lymphocytopenia will eventually result in the primary respiratory clinical outcomes of critically ill patients, such as sepsis (100%), respiratory failure (98%) and ARDS (93%), which are the primary mortality factors of COVID-19 patients [12].
Second, the synergistic co-development of ACE2-deficiency-induced injury in other organs featuring lower DPT/PPT ratio (such as kidney, heart, and brain) [6] and innate-adaptive-imbalance-induced lymphocytopenia will result in secondary clinical outcomes, such as heart failure (52%), secondary infection (50%), acute cardiac injury (59%) and acute kidney injury (50%), which are the secondary mortality factors of COVID-19 patients [12].
The synergistic co-development of ACE2-deficiency-induced injury and innate-adaptive- imbalance-induced lymphocytopenia is consistent with the clinical course of hospitalized COVID-19 patients: sepsis (Day10) -> ARDS (Day 12) -> acute kidney/cardiac injury (Day 15) -> secondary infection (Day 17) -> death (Day 19) [12].
As an escape gene of X chromosome inactivation, ACE2 shows a heterogeneous sex bias such that males have higher ACE2 expression in the lungs than females [14]. As a result, male COVID-19 patients are more likely to develop lung injuries and to become severely and critically ill patients than female COVID-19 patients. Elderly people are also more vulnerable than young people to COVID-19 infection due to age-dependent decline of ACE2 expression [15].
Inflammaging is the long-term chronic physiological stimulation of the innate immune system and inflammaging is more common in the elderly than in the young [16]. Hence, elderly people are more likely to develop severe innate-adaptive-imbalance-induced lymphocytopenia and become severely and critically ill patients than are young people. Nevertheless, the causal driver of exacerbated lymphocytopenia is inflammaging rather than age, so young people with inflammaging are more vulnerable to COVID-19 infection than elderly people without inflammaging.
The neurological symptoms of COVID-19 (such as hear loss, taste loss, impaired consciousness, cerebrovascular diseases, etc.) [17] may be caused predominantly by ACE2 deficiency in the brain rather than immune homeostasis disruption, because the neurological symptoms associated with COVID-19 have an early onset and there is no lymphocyte count difference between non-severe patients with and without CNS symptom [17].
Treatment
Unbiased treatment strategy
COVID-19 is fundamentally different from all the other viruses that the world has hitherto known. As an RNA virus, COVID-19 has inherently strong adaptive mutability, which is further enhanced by the favorable intercellular microenvironment due to the disrupted homeostatic functions of the ACE2-expressing host cells.
Consequentially, COVID-19 could develop robust resistance to effective but biased treatments that predominantly inhibit either viral replication or viral immunomodulation, and vaccines targeting the S protein can only provide temporary protection at the expense of accelerating adaptive mutation and potentially inducing immune crossreactivity.
While effective but biased treatments could switch SARS-CoV-2 to the asymptomatic latent infection mode, SARS-CoV-2 can still chronically interfere with systemic genome, proteome, ion, energy, and immune system homeostases by affecting the baseline activities of ACE2-expressing host cells in multiple organs over the years. When the cumulative disruption of multi-level homeostases is sufficient to switch SARS-CoV-2 back to the symptomatic productive infection mode, asymptomatic SARS-CoV-2 carriers will initiate an accelerated disease progression and trigger severe cytokine storms and fatal comorbidities.
Therefore, COVID-19 treatments must be rationally designed to avoid fueling the adaptive mutation and the latent infection of SARS-CoV-2. In accordance with the unbiased survival strategy of SARS-CoV-2 that balances viral replication and viral spread, the effective treatment for COVID-19 must also be unbiased such that both viral replication and host immunomodulation are equally targeted.
Treatment for critically ill patients
It is paramount to develop effective treatments for critically ill patients because the critically ill COVID-19 patient population has a 61.5% mortality rate in a Wuhan critically ill patient cohort [18]. It is equally essential to discover scalable treatments that are cost-effective and globally available to reduce the mortality rate of COVID-19 in various countries of different infection control capabilities.
To that end, we discovered the combination of irinotecan (an in-market topoisomerase I inhibitor approved for cancer chemotherapy) and etoposide (an in-market topoisomerase II inhibitor approved for cancer chemotherapy) could potentially be an exceptionally effective treatment to protect critically ill patients from death caused by COVID-19-specific cytokine storms triggered by sepsis, ARDS, and other fatal comorbidities.
TOP I inhibitors can potently suppress the overstimulated innate immune response triggered by the COVID-19-mediated immunomodulation that depends on SARS-CoV-2’s non-structural proteins and accessory proteins.
In particular, TOP I inhibitors selectively suppress the innate immune response by reducing the expression of 84 genes that encode pro-inflammatory cytokines in infected cells, while maintaining the viability of host cells without affecting viral replication.
On the other hand, TOP II inhibitors can potently suppress viral replication triggered by the ACE2-dependent viral entry mechanism that depends on SARS-CoV-2’s structural proteins.
In particular, TOP II inhibitors selectively suppress both viral replication and cellular proliferation by suppressing the downstream pathways of PI3K signaling, yet at the risk of exacerbating the discrepancy between innate immune cells and adaptive immune cells by suppressing the proliferation of all immune cells.
There are direct in vivo and in vitro data that support the efficacy and safety of TOP I inhibitors and TOP II inhibitors in reducing host deaths caused by infection-induced hyperinflammation:
Topoisomerase I inhibitors have been shown in vitro and in vivo to prevent the host deaths caused by virus-infection-induced innate immune response [19]. In addition to the in vitro data in several virus cell lines showing the broad-spectrum anti-hyperinflammation efficacy of TOP I inhibitors [19], the in vivo data on mice with viral-bacterial co-infections (influenza virus infection followed by staphylococcus aureus infection) [19] are highly clinically translatable because the combined immunomodulatory profile of influenza virus + Staphylococcus aureus could approximately replicate the immunostimulatory profile of COVID-19.
Topoisomerase II inhibitors, especially etoposide, have been effective treatments for the cytokine storm caused by hemophagocytic lymphohistiocytosis (HLH) [20]. They have been shown in vitro to suppress RNA virus replication, and they work synergistically with TOP I inhibitors in a combination therapy [19].
The toxicity profile of the immunosuppressive TOP I inhibitors in cancer patients would not translate to COVID-19 critically ill patients, because cancerous cells are endogenous agents that chronically induce the over-suppression of the immune system, whereas SARS-CoV-2 is an exogenous pathogen that acutely induces the over-stimulation of the immune system. Therefore, immunosuppressive TOP I inhibitors (e.g., irinotecan) will be both effective and safe for COVID-19 critically ill patients who have developed hyperinflammation-driven clinical outcomes.
The toxicity profile of the immunosuppressive TOP II inhibitors in HLH patients would not translate to COVID-19 critically ill patients either, because COVID-19 and HLH have fundamentally different causal drivers of their respective cytokine storms: overstimulated innate immune response is the causal driver of cytokines storms in COVID19 infection, whereas overstimulated adaptive immune response is the causal driver of cytokine storms in HLH. The aetiology of COVID-19 further differs from the aetiology of HLH in the following three respects: (i) COVID-19 features lymphocyte reduction, whereas HLH features lymphocyte proliferation [12], [21]; (ii) NK cells are not affected in COVID-19, but NK-cells are impaired in HLH [11], [22]; (iii) COVID19 features reduced IL2, reduced IL6 and increased IL10, whereas HLH features increased IL2, increased Il6 and reduced IL10 [12], [13].
Given that lymphocytopenia is the most common clinical hallmark for COVID19 patients on admission (83.2%) [13], etoposide monotherapy may have a novel toxicity profile in critically ill COVID-19 patients by exacerbating the already lethal level of lymphocytopenia.
Therefore, to the end of ensuring maximal clinical efficacy and safety for critically ill COVID-19 patients, we further recommend that the optimal treatment sequence is irinotecan followed by etoposide. The administration of irinotecan will first attenuate innate-adaptive-imbalance- induced lymphocytopenia and thus create a safe therapeutic window for etoposide to safely curb viral replication and for irinotecan + etoposide to deliver synergistic efficacy on attenuating cytokine storms while maintaining cell viability and minimizing host toxicity for critically ill COVID-19 patients.
Clinical use and study
Given that more than 90% of critically ill COVID-19 patients are ineligible for placebo-controlled open-label clinical trials due to legitimate ethical concerns and severe medical conditions, and only 7% of critically ill patients in ICUs were eligible to be admitted into a retrospective clinical study [18], we thus recommend the off-label use of irinotecan + etoposide out of compassion in parallel with a pilot clinical study for critically ill COVID-19 patients.
For the global control of a COVID-19 pandemic, it is paramount to develop effective treatments for critically ill patients because COVID-19 has a higher mortality rate of critically ill patients than SARS-CoV and MERS-CoV. It is equally essential to discover scalable treatments that are cost-effective and globally available to reduce the mortality rate of COVID-19 in various countries of different infection control capabilities.
Irinotecan (an in-market topoisomerase I inhibitor) and etoposide (an in-market topoisomerase II inhibitor) are available in more than 40 countries, and they are on the World Health Organization’s List of Essential Medicines that are the safest and most effective medicines needed in a health system. Therefore, the combination of irinotecan and etoposide could be a globally scalable effective treatment for critically ill COVID-19 patients as well as for moderately ill COVID-19 patients with different dosage regimens.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu, Y., Jiang, M., Gao, L., & Huang, X., Single cell analysis of ACE2 expression reveals the potential targets for 2019-nCoV. (2020). doi:10.20944/PREPRINTS202002.0221.V1.
- 2.Jarajapu Y.P.R. Activation of the ACE2/angiotensin-(1–7)/mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes. 2013;62:1258–1269. doi: 10.2337/db12-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Liang J., Leung P.S. The ACE2/Ang-(1–7)/Mas axis regulates the develop- ment of pancreatic endocrine cells in mouse embryos. PLoS ONE. 2015;10:1–17. doi: 10.1371/journal.pone.0128216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He H. Mesenchymal stem cells overexpressing angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced lung injury. Cell Transplant. 2015;24:1699–1715. doi: 10.3727/096368914X685087. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen K.B., Chhabra K.H., Nguyen V.K., Xia H., Lazartigues E. The transcrip- tion factor HNF1α induces expression of angiotensin-converting enzyme 2 (ACE2) in pancreatic islets from evolutionarily conserved promoter motifs. Biochim Biophys Acta. 2013;1829:1225–1235. doi: 10.1016/j.bbagrm.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen V.K., Pedersen K.B., Xia H., Lazartigues E. Tissue-specific expression of angiotensin-converting enzyme 2 (ACE2) from two promoter regions is unaffected by elevated levels of renin and angiotensinogen. FASEB J. 2012 [Google Scholar]
- 7.Imai Y. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu A. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D.X., Fung T.S., Chong K.K.L., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Montfort T. Dendritic cells potently purge latent HIV-1 beyond TCR-stimulation, activating the PI3K-Akt-mTOR pathway. EBioMedicine. 2019;42:97–108. doi: 10.1016/j.ebiom.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou, Y. et al. PERSPECTIVE Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. [DOI] [PMC free article] [PubMed]
- 12.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China : a retrospective cohort study. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.-J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;1–13 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tukiainen T. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peña-Silva R.A. Impact of ACE2 deficiency and oxidative stress on cerebrovas-cular function with aging. Stroke. 2012;43:3358–3363. doi: 10.1161/STROKEAHA.112.667063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanada F. Source of chronic inflammation in aging. Front Cardiovasc Med. 2018;5:1–5. doi: 10.3389/fcvm.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao, L. et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv 2020.02.22.20026500 (2020). doi:10.1101/2020.02.22.20026500.
- 18.Yang X. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;2600:1–7. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rialdi A. Topoisomerase 1 inhibition suppresses inflammatory genes and protects from death by inflammation. Science (80-) 2016;352 doi: 10.1126/science.aad7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmberg K. Consensus recommendations for the diagnosis and management of hemophagocytic lymphohistiocytosis associated with malignancies. Haematologica. 2015;100:997–1004. doi: 10.3324/haematol.2015.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vick E.J., Patel K., Prouet P., Martin M.G. Proliferation through activation: Hemophagocytic lymphohistiocytosis in hematologic malignancy. Blood Adv. 2017;1:779–791. doi: 10.1182/bloodadvances.2017005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otrock Z.K., Eby C.S. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90:220–224. doi: 10.1002/ajh.23911. [DOI] [PubMed] [Google Scholar]