Abstract
Pathogenic contamination has been considered as a significant worldwide water quality concern. Due to providing promising opportunities for the production of nanocomposite membranes with tailored porosity, adjustable pore size, and scaled-up ability of biomolecules incorporation, electrospinning has become the center of attention. This review intends to provide a detailed summary of the recent advances in the fabrication of antibacterial and antiviral electrospun nanofibers and discuss their application efficiency as a water filtration membrane. The current review attempts to give a functionalist perspective of the fundamental progress in construction strategies of antibacterial and antiviral electrospun nanofibers. The review provides a list of antibacterial and antiviral agents commonly used as water membrane filters and discusses the challenges in the incorporation process. We have thoroughly studied the recent application of functionalized electrospun nanofibers in the water disinfection process, with an emphasis on their efficiency. Moreover, different antibacterial and antiviral assay techniques for membranes are discussed, the gaps and limitations are highlighted and promising strategies to overcome barriers are studies.
Keywords: Antibacterial, Antiviral, Electrospun nanofiber, Water filtration
Graphical abstract
1. Introduction
Based on the exponential growth of the population, it has been estimated that the human population will reach about 9 billion by 2050 (Ray et al., 2016). Thus, the availability of clean drinking water sources to this enormous population will turn to one of the most critical problems mankind has to address (Parekh et al., 2018). The World Health Organization has stated that safe drinking water accessibility is the concern of about 785 million people and 2 million people use a contaminated water source for drinking (WHO, 2020). Aiming to obtain purified water, the unsought biological contaminants including bacteria or viruses and chemical pollutants such as toxic heavy metal ions have to be eliminated (Bridge et al., 2010; Aghalari et al., 2020).
Contaminated water is deemed to be responsible for significant kinds of waterborne diseases and considered as the main reason of about 5, 02,000 deaths throughout the world each year (WHO, 2019). The bacterial pathogens in drinking water supplies cause one of the biggest threats to public health which entail the outbreak of diseases such as gastroenteritis, cholera, giardiasis, cryptosporidiosis, etc. Shigella dysenteriae, Vibrio cholera, bacteria belonging to genus Legionella, Escherichia coli O157:H7 and Campylobacter jejuni are the most prevalent bacteria implicated in such outbreaks (Bruins et al., 2005; Li et al., 2008; Liu et al., 2014). Antimicrobial resistance is an emerging global health problem which is referred to the capacity of bacteria and viruses to tolerate antimicrobials agents. This event reduces the effectiveness of biocides designed to prevent infections caused by such pathogens. To overcome the problem, higher concentration of existing antimicrobial agents have to be utilized or novel antimicrobial agents or new strategies need to be developed (Gwenzi et al., 2020). The problem of occurrence of pathogens in drinking water gets more complicated by concerns of distribution of antibiotic-resistant bacteria which may greatly affect public (Xi et al., 2009). On the other hand, bacterial resistance to antibiotics, disinfectants in water threats human health, and consequently leads to serious economic concerns (Shomar et al., 2020).
However, the impact of waterborne viruses on human health is being less appreciated, as they are often associated with waterborne disease prevalence. Under a similar level of exposure, viruses have a less infectious dose and a higher illness risk of 10–10,000 times in comparison to bacterial pathogens (Zhang et al., 2019). Moreover, the survival rate of viruses in waters is influenced by different conditions like temperature and pH. The large numbers of viruses can survive for long times in drinking water, as hepatitis A virus, for which 99% inactivation happens about after 56 days (Wang et al., 2020). Viral pathogens that can be transmitted by water and illustrate a moderate to high negative effects on health are classified by the World Health Organization (WHO). The classification includes astrovirus, hepatitis A and E viruses, adenovirus, rotavirus, norovirus, caliciviruses, and enteroviruses. Also, some viruses like cytomegalovirus and polyomaviruses are excreted through urine and outspread through the water. Coronaviruses and influenza have been suggested to be able to transmit by drinking water, but the evidence is inconclusive (WHO, 2011; Cannon et al., 2011; Gall et al., 2015; Shamsollahi et al., 2019). Thus, due to potential infectious disease risks from excreta, well-managed centralized measures should be taken as water treatment works (WHO, 2020).
Water contaminants eliminating treatment can be categorized into three main approaches: Physical processes including filtration and distillation; biological processes, such as employing microorganisms as detoxifying agents; and chemical processes, such as aqueous phase oxidation methods (Chiam and Sarbatly, 2011; Ahmed et al., 2017).
Although about 600 disinfectants have been identified by now, the most currently used disinfectants for inactivating of pathogens in the water include chloramines, chlorine, chlorine dioxide, ozone, and chlorine gas due to their effectiveness (Hossain et al., 2014). Nevertheless, there are some known drawbacks for these traditional water disinfection methods including: (1) The long required reaction time to inactivate pathogens; (2) high disinfectant concentrations are required; (3) difficulty to prepare and apply due of their detrimental and fast degradation characteristics; and (4) reactivity with various components in natural water to form disinfection byproducts which commonly are carcinogens (Krasner et al., 2006; Richardson et al., 2007; Matilainen et al., 2011; Liu et al., 2014; Ncibi et al., 2017; Levchuk et al., 2018a; Sillanpää et al., 2018).
The perfect disinfectant should include the following properties: (1) extended range of antimicrobial abilities during the short time; (2) be safe for human health and do not generate toxic byproduct during and after their application; (3) be cost-effective and easily applicable; (4) exhibit high water solubility and do not be corrosive for any equipment; (5) manageable to safe disposal (Rutala and Weber, 2008).
A membrane is an obstacle between two phases which let materials to be selectively forward from one side to the other. There are two main kinds of porous or dense membranes based on the membrane's structure. The pore structure of a membrane determines the selectivity and transport characteristics of a membrane (Ahmed et al., 2015). The importance of membrane filtration approach (including reverse osmosis (RO), microfiltration (MF), ultrafiltration (UF) and nanofiltration (NF)) are growing compared to other technologies because their simple operation, low cost and high efficiency (Geise et al., 2010; Dervin et al., 2016). Microfiltration (MF) is a kind of membrane with a pore size ranging from 0.1–10 μm and potent to remove bacteria, particles and contaminants from a liquid solution (Warsinger et al., 2018). Ultrafiltration (UF) membranes with pore size in the range of 0.01–0.1 μm can reject contaminants including viruses, colloids, emulsified oils, and protein. The Nanofiltration (NF) membrane removes particles ranging from 0.001–0.01 μm in size and reverses osmosis (RO) membranes removes 0.001–0.0001 μm size particles (Suja et al., 2017).
Additionally, the broad utilization of chemical disinfectants has caused an expanded growing resistance to multiple biocidal agents and pathogenic resistance (Fahimirad et al., 2017; Fahimirad et al., 2018; Nadi et al., 2020). Thus, there is an urgent need for novel methods in removing bacteria from water supplies which meet the requirement of effective antimicrobial activity, superior filtration flux with acceptable retention potentials (Zodrow et al., 2014).
Nanofiber membranes, because of their high surface area to volume ratio, nano-sized pores, and high porosity, have been illustrated to improve the efficiency of conventional materials employed for the filtration and separation of particulate materials (Aussawasathien et al., 2008). A number of processing techniques including melt-blown, self-assembly, phase separation, template synthesis, and electrospinning have been employed to prepare nanofibers in recent years. Among them, electrospinning is the most promising, efficient method to produce web-like non-woven ultrafine fibers including microfibers (>1 μm) or nanofibers (<1000 nm) from different kinds of polymers. Moreover, incorporation of bioactive, antimicrobial and antiviral agents into nanofiber structure is easily possible through the electrospinning process (Fahimirad and Ajalloueian, 2019; Fahimirad and Hatami, 2019; Faccini et al., 2015).
The present work reviews previous studies on the production and application of electrospun nanofibers as antimicrobial water filtration membranes. The merits and demerits of these novel water microfiltration tools are discussed. Moreover, their antibacterial efficiency and disinfection activities are compared with commercial water membrane filters comprehensively. Finally, some points are recommended to be noticed as the subsequent future research plans.
The objectives of this review were to: (i) introduce the different procedures, which have been applied for incorporation of the various antimicrobial agents into electrospun nanofibers (ii) discuss the different antimicrobial tests used for proving antimicrobial activity of the fabricated electrospun water filters (iii) study the efficiency of the produced antimicrobial electrospun application in the water treatment industry.
2. Electrospinning process
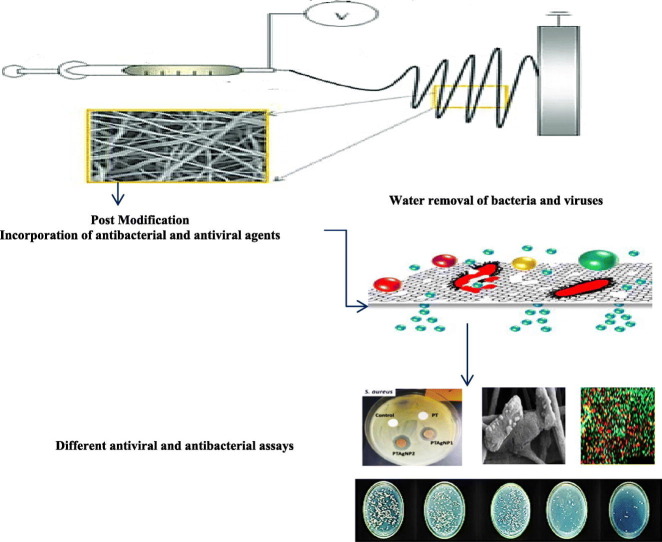
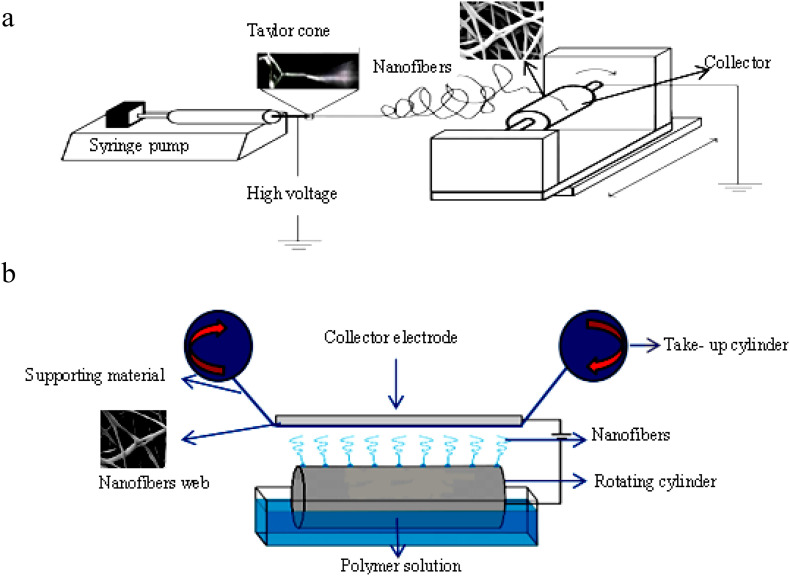
The electrospinning approach was invented by Cooley in 1900 (Cooley, 1900). This method is easy, cost-effective, uncomplicated, and has the potential for scale- up production. The flexibility in material selection and additive incorporation to obtain appropriate functionality, as well as its considerable capability to produce fibers in the sub-micron range with the high surface-area (up to 40 m2 g −1 based on the fiber diameter), are prominent privileges of electrospinning process for fabrication of nanofibers. In addition, effective porosity of electrospun nanofibers (almost about 80% with no upper limit) with many small pores, interconnected pore structure directly promote both infiltration rate and contaminant rejection ratio in comparison with conventional materials being used for MF applications (Nasreen et al., 2013; Wang et al., 2013). In this process, a prepared solution of polymer is loaded into a syringe and fed at a set flow rate to the spinneret. Due to the needle connected to a high voltage power supply under the electric field with a specific voltage, a Taylor cone is constructed by elongation of the polymer droplet at the end of the syringe into a characteristic conical shape. Enhancing the electrical field causes the formation of a steady jet elongated and whipped consecutively by electrostatic repulsion. The solvent evaporates when electrostatic forces prevail over surface tension and the jet gets finer, so nanofibers successively deposit on the collector (Ditaranto et al., 2018; Fig.1 a).
Fig. 1.
The schematic diagram of electrospinning a: The laboratory electrospinning (Needle electrospinning), b: The industrial Nanospider technique (Needle- less electrospinning).
The surface topography, the texture of the scaffold, fiber orientation and morphology are largely influenced by several parameters. These parameters include solution characteristics such as polymer molecular weight, molecular weight, and surface tension, the conformation of polymer chains, viscosity, solvent vapor pressure, pH value and electrical conductivity. Fiber properties are also directly influenced by operating situations such as temperature and humidity of the electrospinning chamber, feeding rate of the polymer solution, the collector rotating speed and distance between the spinneret and collector (Ahmed et al., 2015; Wang and Hsiao, 2016).
2.1. Scale-up production of electrospun nanofibers
Due to a very low production rate and deficiency of an economic and scale-up productive potential, despite of the various application capacities, needle electrospun nanofibers show difficulties in industrial applications. Using a single spinneret, 1 g weighted electrospun nanofibrous mat with takes several hours to fabricate. Multi-jet electrospinning methods, multi-needle electrospinning methods and needleless electrospinning methods are three different electrospinning classifications used for scaled- up nanofiber production. Due to repletion effect between jets, uniform web of nanofiber is not produced in multi-jet electrospinning methods. Employing an array of syringes as spinnerets with the appearance of the multiple jet electrospinning process improves the nanofiber production rate with ability of mixing different polymers at appropriate ratio (Munir and Ali, 2020). However, the highest mass production rate of nanofiber by electrospinning is achieved by needleless electrospinning. This technique is considered as a new electrospinning mode with electrospinning of nanofibers straightly from an open liquid surface. In this process, numerous jets are formed from the surface of polymer solution through the utility of a metal electrode with no influence of capillary effect unlike what happens normally in needle-like nozzles. Employing this method will improve the spinning unit production rate to 20,000 m2 d−1 (Wang and Hsiao, 2016; Lin, 2012; Fig. 1 b). Formation of multiple jets by employing rotating disks, rollers, conical wire coil, balls, rod, twisted wire spinnerets, bubbles and cones have been reported for effective needleless electrospinning used for commercial scale manufacture of nanofibers (Prabu and Dhurai, 2020). Application of this different spinneret shape leads to a variation of nanofiber production rate and fiber morphology (Munir and Ali, 2020).
3. Electrospun nanofiber in water treatment
Electrospun nanofibers (ENs) have been employed as water filtration for desalination, metal removal, filtration of organic materials/microparticles and microbial removal (Wang et al., 2013). Inter-connective porous morphology and uniform nano-pores of electrospun membranes are perfect characteristics for pressure- operated liquid filtration processes such as microfiltration, ultrafiltration, nanofiltration and reverse osmosis (Suja et al., 2017).
In addition, because of the adjustable nature of the electrospinning technique, the restrictions of hydrophobic polymeric membranes can be simply prevailed by various methods including mixing with inorganic nanoparticles, blending with hydrophilic polymers, and surface modification with hydrophilic agents. Also, the membrane post-heat treatment and using a hybrid system by spinning nanofibers directly over strong support are frequently employed strategies for improving the strength and qualities of the electrospun nanofibrous membrane (Gopal et al., 2006; Suja et al., 2017).
4. Common approaches for application of electrospun nanofiber in water disinfection
MF is a pressure-driven filtration process to remove contaminants, particles and bacteria (ranging from 0.1–10 μm) from a fluid, which is an important step in wastewater pretreatment and portable water purification for the removal of waterborne bacteria, suspended microparticles, algae etc. One of the ideal methods to construct nanofiber-based MF membrane with adjustable pore size and distribution of pore size is the electrospinning technique. The great potential of electrospun nanofiber in MF application, such as uniform fiber morphology with controllable pore size, interconnected open pore structure, high porosity, and membrane thickness, turn them to a superior substitute to replace the conventional MF membrane such as the Millipore GSWP MF membrane with an average pore size of 0.22 mm (Wang et al., 2012; Barhate and Ramakrishna, 2007).
Another novel application of electrospun nanofiber in water purification and bacterial rejection is thin-film nanocomposite membrane (TFNC), a major type of reverse osmosis (RO) and nanofiltration (NF) membranes, which compromises of three layers including the first barrier layer of interfacial polymerization, a polyacrylonitrile or poly (vinylidene fluoride) electrospun membrane as the second layer and nonwoven polyethylene terephthalate (PET) as the third layer. The third layer employed as a substructure layer to provide the whole membrane adequate mechanical strength (Subramanian and Seeram, 2013; Yin et al., 2012; Fig. 2 ). High water flux, great solute rejection, minimum membrane fouling, and perfect mechanical persistence are main properties of an ideal TFC membrane and turn it into an excellent candidate for microfiltration and ultrafiltration applications (Li and wang, 2010). Sato et al. (2011) fabricated a novel composite fibrous membranes, consisting of an ultra-fine cellulose nanofibrous infused into electrospun polyacrylonitrile (PAN, with an average diameter of 0.2 μm a mean diameter of about 30 μm as the barrier layer (40–100 μm in thickness) to provide filtration attributes) nanofibrous scaffold on a melt-blown polyethylene terephthalate (PET, with a mean diameter of 30 μm as the support layer (about 100 μm thick) to sustain mechanical strength) non-woven substrate for water purification. The nanostructure showed a retention rate of 99.9999% for E. coli filtering and the high percent of the MS2 virus, with 30 nm sizes, captured in the electrospun PAN scaffold infused with m-UFCNs (Sato et al., 2011). Recently, Taheran et al. (2019) fabricated a methodical portable water purification instrument using electrospun nanofiber. The device contained three distinct electrospun membranes. The first membrane was made by electrospinning of Polyacrylonitrile/chitosan solution at 85:15 mass ratio as an antibacterial membrane, the second membrane was produced from Laccase (10 unit g−1) immobilized onto PAN/biochar 95:05% electrospun mat for removal of micro-pollutants and the third layer was fabricated by electrospinning of PAN/biochar at 95:05 ratio as an adsorptive membrane. The applied technology led to approximately 99% removal of microorganisms, 83% of micro-pollutant removal, and more than 77% of turbidity decline during less than 5 min contact time (Taheran et al., 2019).
Fig. 2.
Schematic of thin film composite (TFC) membranes.
5. Factors involved in the function of electrospun nanofiber in water disinfection
The important characteristics of a nanofiber mat membrane for application as filters for the separation of contaminations and pathogens from a continuous fluid phase are wetting properties, permeability, porosity, fiber size distribution, and fiber structure.
5.1. Surface wetting properties
For water filtration, a membrane must be wet-table and surface wetting properties are generally specified by the contact angle. A surface with a low contact angle (below 90 degrees) is considered a hydrophilic surface, while a surface illustrating a high contact angle (over 90 degrees) is referred to as a hydrophobic surface. Sessile drop and the Captive bubble method are two common techniques used for measuring the nanofibers' contact angle (Nuraje et al., 2013).
5.2. Porosity
One of the key parameters in filter design and its performance is porosity. Generally, porosity is calculated from the apparent density and bulk density of the membrane. However, other alternative procedures inclusive of image analysis and mercury porosimeter are frequent methods applied for the evaluation of porosity in the nanofiber membrane (Ghasemi-Mobarakeh et al., 2007). Electrospun nanofibers are highly porous with interconnected pores in the size range of just a few times the fiber diameter. The small pore size of the nanofibrous membrane introduces a higher retention rate, the interconnected pores leads to better tolerance against fouling and the high porosity defined a higher permeability capability (Homaeigohar et al., 2010).
5.3. Water permeability
Clean water permeability (CWP (l/m2 •h•bar)) illustrates the highest amount of attainable flux dependent on the membrane condition. It can be assayed by calculating the flux at various trans membrane pressures (TMP). The slope of the eventuated curve is regarded as the CWP (Bjorge et al., 2009). The high CWP grants high flux operation to the membranes, introduces the nanofiber mat as an energy-saving membrane, and means that if fouling does not happen, enormous volumes can be treated (Daels et al., 2011; He et al., 2018).
5.4. Zeta potential
The surface charge on membranes is related to affinity corresponding interactions and considered as a significant parameter influencing the disinfection capabilities of the membrane. Surface charges can qualify the strength of biomolecular or even pathogen affinity on a material surface. In virus removal, surface charged nanofibers adsorb virus via electrostatic interactivity between the nanofibers and the counter-charges of virus and signify virus remediation improvement (Cho et al., 2012). A series of studies have confirmed that electrostatic attraction between the cationic membrane and the anionic surface of bacteria may lead to morphological defects in consequence of ROS generation and cell membrane destruction. Indeed, anionic membranes act as powerful non-adhesive site of bacteria attributable to electrostatic repulsion (Mukherjee and De, 2018; Kolewe et al., 2016).
5.5. Operating conditions
Operating conditions influence the antibacterial activity performance of the nanofibrous membrane. As proved by several experiments, bacterial cells are able to decline their size at higher operating pressure, hence resulting in enhancing permeation through the filter. Therefore, less trans membrane pressure (TMP) is usually desired, to retain antibacterial activity during long term application of the membrane. The TMP is described as the mean feed pressure minus the permeate pressure that is essential to push down water through a membrane (Mukherjee and De, 2017, Mukherjee and De, 2018).
6. Electrospun nanofiber strategies for water disinfection
Different factors including surface area, surface roughness, pore diameter, zeta potential, and inclusion of biocides or antibacterial agents determine the antimicrobial performance of membrane (Rahaman et al., 2014; Mukherjee and De, 2018). Accordingly, the employing of electrospun polymeric membranes in bacterial and virus removal from water is performed in two procedures including size exclusion and adsorption (Lee et al., 2016). In most cases, the diameter of water-borne bacteria is more than 0.2 μm. For example, the E. coli size is 0.5–2.0 μm and Brevundimonas dimimuta dimension is 0.3–0.9 μm. Previous studies have confirmed that using a 0.45 μm pore sized MF leads to a 2 log– 4 log bacteria reduction (Gómez et al., 2006; Ghayeni et al., 1999). Thus, based on the degree of exclusion, the electrospun membrane should have an average pore size of fewer than 0.2 μm. In addition, the narrow pore size distribution is requisite for achieving a high retention rate (Ma et al., 2014). There is a direct relationship between the pore size and the fiber diameter of a porous nonwoven structure. The relationship has been confirmed as the average pore size was approximately 3 ± 1 times the mean fiber diameter, and the greatest pore size was about 10 ± 2 times the mean fiber diameter. Thus pore size of electrospun fiber generally grows with increasing fiber diameters (Ma et al., 2011). Various conventionally employed membranes for the application as micro-filters have 0.2 μm theoretical pore sizes. The advantage of electrospun nanofiber membranes in comparison to conventionally used membranes are the simplicity of manufacture, adjustable size of the pores and high porosity (Saleem et al., 2020). In view of the fact that the membrane pore sizes can be controlled by adjusting the electrospinning parameters and besides the fact that the most aquatic bacteria dimensions are more than 0.2 mm, electrospun nanofibers can be designed efficiently with smaller pore dimensions suitable for MF applications (Wang and Hsiao, 2016). For instance, accelerating the flow rate raises the pore diameter by enhancing the fiber diameter. Moreover, increasing polymer solution concentration and using higher molecular weight polymer increases fiber diameter. Employing a secondary ring electrode circling the nozzle cause reducing the fiber deposition and consequently decrease the density of the membrane, the parameter which reduces the pore size. In addition, controlling fiber distribution, post electrospinning modification and using temporary spacers can be utilized for controlling pore size (Dong et al., 2015; Haider et al., 2018). As discussed above and based on the size exclusion process, microfiltration larger sized bacteria are substantially seized by the membrane but it is not efficient in separating small sized viruses within 0.01–0.1 μm range size (Mi and Heldt, 2014; Barhate and Ramakrishna, 2007). So, rejection of bacteria smaller than membrane pores or viruses needs the incorporation of antiviral or antibacterial agents into the membrane. Also, after size-exclusion microbial removal of the membrane, intercepted bacteria can be released and induce membrane biofouling during subsequent filtration. Therefore, antimicrobial agents are commonly used to prohibit bacterial growth and biofoul formation that would decline filter efficiencies (Botes and Eugene Cloete, 2010; Wen et al., 2017).
Various bioactive agents with different fundamental properties may have consequential impacts on bacteria removal. Plus, nanofiltration membranes or ultrafiltration membranes with a positive charge on the surface are able to remove viruses selectively (Mukherjee and De, 2017).
6.1. Biocide incorporation into electrospun nanofiber
Moreover, incorporating antimicrobial agents into electrospun nanofibers enhance the antimicrobial activity of fabricated nanofibrous membrane (Nasreen et al., 2013; Park and Kim, 2017). An ideal bioactive agent incorporated into the functionalized membrane should be non-toxic, water insoluble with no or slight leaching property. Also, the functionalization process should not cause adverse influences on the quality and overall performance of the membrane. Based on the majority of researches studied in this review, blending and post-modification strategies are two commonly used techniques to incorporate biocide agents into nanofibers aiming for water disinfection application (Shalaby et al., 2018; He et al., 2018; Makaremi et al., 2016).
6.1.1. Blending electrospinning
Blend electrospinning is an easy one-step procedure, mostly used for agents' incorporation into nanofibers (Shabafrooz et al., 2014). Using the same solvent, the bioactive agent is dissolved directly into the polymer solution and a homogeneous blended solution of the incorporating agents in the polymer solution is prepared for the electrospinning step (Pillay et al., 2013; Fahimirad and Ajalloueian, 2019).
6.1.2. Post- modification
The agents incorporation into electrospun fibers can be performed after the electrospinning process by physical or chemical treatments. Covalent and non-covalent immobilizations are fundamental methods for molecules attached to the fiber surface. Non-covalent immobilization is performed by immersion of electrospun mats in a solution compromising the bioactive molecules. By treating with plasma the surface gets activated for subsequent modification using specified ligands like active amine groups. The affinity of incorporated agents to the electrospun nanofiber surface improves by covalent immobilization (Wang and Windbergs, 2017; Kurusu and Demarquette, 2019).
6.2. Biocide incorporated into electrospun nanofiber for water disinfection
Some commonly used antimicrobial or antiviral agents in electrospun nanofibers are discussed in this section.
6.2.1. Metal and metal-oxide nanoparticles
6.2.1.1. Silver nanoparticles
Silver nanoparticles (AgNPs) are considered the most efficient nanoparticles for biological applications and the most extensively applied antibacterial agent for water disinfection (Fahimirad et al., 2019; Mukherjee and De, 2018). AgNPs are capable to puncture the microorganisms' cell walls, interact with their nucleic acids and attach to their enzymes, which cause the cell membrane destruction and finally growth inhibition. Different feasible interactions of Ag+ ions with various bacterial biomolecules are documented. Furthermore, the extended range of antibacterial activities and virulence effects of Ag+ ions toward several microorganisms (e.g. bacteria, viruses, and fungi) at only a few mg mL−1 are confirmed in previous studies. Thus, silver nanoparticles are recognized as potent disinfection agents (López-Heras et al., 2015). In water purification, nanosilver materials have been mainly applied to prevent the formation of bacterial biofilms on the surface or inside the pores of the membrane (Chou et al., 2005; Liu et al., 2014). Contrastingly, at high concentrations, AgNPs are settled onto the membranes, cause obstruction of the pores and subsequently reduce the water fluxes (Biswas and Bandyopadhyaya, 2017).
There are three main methods for AgNPs incorporation into electrospun nanofibers including 1) blending of prepared synthesized AgNPs solutions to the polymer solution, 2) AgNP synthesis in the polymer solution by employing a precursor, and 3) Post-treatments of the electrospun nanofibers for AgNP synthesis by reduction of the precursor that has been spun along with the electrospinning solution (Fahimirad and Ajalloueian, 2019).
6.2.1.2. Iron oxide nanoparticles (IONP)
There are three main approaches to produce iron oxide nanoparticle-nanofiber composites, including (1) electrospinning of solution containing prepared IONPs, (2) in-situ synthesizing of IONPs during the electrospinning process or in the solution to be electrospun and (3) post-treatment synthesized by post-modification processing technique to form IONPs from a precursor incorporated within the nanofiber (Mortimer and Wright, 2017).
Iron oxide nanoparticles have been reported to illustrate electrostatic interaction with the cell membrane of bacteria and viruses, stimulate toxic oxidative stress by the generation of the reactive oxygen species (ROS) (Ahmad et al., 2014). The significant toxicity of IONPs has been proved toward both gram-negative and gram-positive bacteria and a wide range of viruses (You et al., 2005; Kharisov et al., 2012).
6.2.1.3. Copper nanoparticles (CuNP)
It has been indicated that due to electrostatic interaction, CuNPs illustrate antibacterial functions on the bacterial cell through different mechanisms, such as adhesion to the bacterial cell wall, lead to detrimental impacts on protein structure within the cell membrane, denaturation of proteins in inertial parts of the cell, and adverse effects on phosphorus- and sulfur-containing compounds like DNA (Raffi et al., 2010).Recently, CuNPs have gained considerable interest because of their broad-spectrum and acutely effective antibacterial activity with comparatively low cost and high scalability (Taner et al., 2011).
6.2.1.4. Zinc oxide (ZnO)
Recently, zinc oxide (ZnO) has received much attention due to its non-toxic profile, effective antibacterial activity, adsorptive properties, mechanical, chemical, and thermal stability while encountering diverse environmental conditions (Tiwari et al., 2018). ZnO particles have illustrated antimicrobial activity against both Gram-positive, Gram-negative bacteria and even against spores (Guo et al., 2015; Wagner et al., 2016). ZnO NPs are considered bio-safe, non-toxic, and biocompatible (Hameed et al., 2016; Farrokhi et al., 2019). In comparison with bulk-sized particles, nanoparticles can pass through bacterial cell walls more simply. The release of Zn2+ ions from NPs destroy the cell membrane and subsequently enhance cellular internalization of the nanoparticles. It is also confirmed that the antimicrobial function of ZnO can be ascribed to photocatalytic activity. By receiving UV light which promotes its interaction with bacteria, ROS, which has a phototoxic effect on bacteria, will be produced (Dimapilis et al., 2018). Furthermore, the electrostatic force between positive sites of ZnO nanoparticles with the negatively charged bacteria cells results in cell membrane damage (Makaremi et al., 2016). The incorporation of ZnO NPs into electrospun nanofiber has been conducted through the fabrication of three kinds of nanofiber including (1) prepared synthesized ZnO NPs are blended with the polymeric spinning solution, (2) Zn precursor is incorporated into the polymeric spinning solution and ZnNps are synthesized de novo, (3) post-treatment of electrospun mat with ZnO precursor (Blachowicz and Ehrmann, 2020).
6.2.1.5. Titanium dioxide (TiO2)
TiO2 is a biocompatible chemical thermally stable compound with high photocatalytic activity and has shown good antimicrobial activities with wide spectrum function against microorganisms (Gram-negative and Gram-positive bacteria, fungi, and virus). The generation of reactive oxygen species (ROS) is the major mechanism of TiO2. Due to its photocatalytic nature, antimicrobial activity of TiO2 NPs enhances by exposing UV light on its surface (de Dicastillo et al., 2020; Levchuk et al., 2018b; Levchuk and Sillanpää, 2020).
6.2.1.6. Lanthanum oxide (La2O3)
It is proved that lanthanum compounds, such as lanthanum hydroxide (La(OH)3), lanthanum carbonate (La2CO3), and lanthanum hydroxide (La(OH)3) can attach to phosphate so firmly that they can generate LaPO4 and remove redundant phosphate in a bacterial cell. According to the very significant band to phosphate, Nano-Lanthanum (La) species represent high effectiveness adsorption and suppress microbial growth by inhibition of the microorganism growth (He et al., 2018; Liu et al., 2017).
6.2.2. Carbone- based antimicrobial compound
6.2.2.1. Graphene oxide
Carbon is the chemical element with atomic number 6 and six electrons situate 1 s2, 2 s2, and 2p2 atomic orbital. Graphene is a one-atom-thick hexagonal structure consisting of a 2-dimensional sp2 carbon bonded sheet organized in a honeycomb lattice structure (Power et al., 2018; Ramasamy et al., 2019). Graphene oxide is the oxidized form of graphene, with O functional groups on the edge and defective sites, includes carboxylic (–COOH), carbonyl (–C=O), and hydroxyl (OH) groups on both available sides. GO shows hydrophilic characteristics because of the O functional groups and is easily dispersed in aquatic solution (Perrozzi et al., 2014; Bhatnagar et al., 2013). Furthermore, the existence of these functional groups advances the interactions with biomolecules and leads to bacterial death with no intracellular process. GO nano-sheets with Sharpe edges hurt the bacterial cell membranes, lead to leakage of the intracellular matrix and eventually cause inactivation of bacteria. Plus, GO generate oxidative stress by producing ROS and lead to DNA damage and mitochondrial dysfunction (Kumar et al., 2019). In addition, the antiviral activity of GO is confirmed by several experiments (Ye et al., 2015).
6.2.2.2. Single-walled carbon nanotubes (SWNTs)
Single-walled carbon nanotubes (SWNTs) are nanometer diameter cylinders fabricated of rolled up graphene sheet in the form of a tube. Generally, SWCNT length is in the micrometer range and their diameters vary from 0.4 to 2 to 3 nm (Eatemadi et al., 2014). SWNTs have presented strong and board spectrum antimicrobial activities. The antimicrobial activity of SWCNTs has been confirmed to be varied by several factors. For instance, longer length nanotubes exhibited superior antimicrobial activity, SWCNTs having surface groups of –OH and –COOH illustrate more strong antimicrobial activity in comparison with SWCNTs-NH2, also the diameter of nanotubes is an important factor governing their antibacterial effects (Dong et al., 2012).
6.2.3. Chitosan hybrids
Chitosan [poly-(b-1/4)-2-amino-2-deoxy-d-glucopyranose] is a general name for a group of deacetylated chitin compounds. Chitosan is a biodegradable, non-toxic and biocompatible polymer and exhibits excellent biological potentials such as vast antimicrobial attributes against bacteria, viruses, and fungi (Kong et al., 2010; Zhao et al., 2020). Due to cationic surface charge of chitosan at physiological pH values, it can attach to the anionic cell wall of bacteria and subsequently alter biochemical activities, defect intracellular organelles and consequently lead to cell death (Cooper et al., 2013).
6.2.4. Quaternary ammonium compounds (QACs)
Quaternary ammonium cations are positively charged polyatomic ions. These ions contain a positively charged nitrogen “head” binding four bonds R including an alkyl group or an aryl group. Quaternary ammonium compounds are salts of quaternary ammonium cations (Tezel and Pavlostathis, 2012). Because of their positively charged sites, they are able to generate electrostatic bonds with the negatively charged sites on bacterial cell walls, resulting in disruption of a cell wall, defect cell membrane permeability and consequently sever leakage of intracellular low-molecular-weight materials (Chen et al., 2014). QACs target bacterial cell membranes. Therefore, they illustrate extended-spectrum antimicrobial activity and have been widely employed to construct an antibacterial surface (Jennings et al., 2015). Quaternized poly (2- (dimethylamino) ethyl methacrylate) (PDMAEMA), benzyl triethylammonium chloride (BTEAC), Cetyltrimethylammonium bromide (CTAB), Cetylpyridinium chloride (CPC), Poly[(dimethylimino)(2-hydroxy-1.3-propanedily)Chloride] (WSCP or busan 77) and benzalkonium chloride (BAC) are some important kinds of QACs (Zhu et al., 2018).
7. Leaching of incorporated biocide molecules from electrospun nanofiber
Easy release of biocides from the membrane improves their exposure rate to bacterial cell. There is a challenging point since the leaching profile of incorporated biocides determines long term bactericidal efficiency of the membrane. Leaching of bactericidal agents resulted in the diminution of the membrane antimicrobial performance over time. Gradual leaching of the blended biocides during the filtration process not only declines the antibacterial activity, but may also lead to secondary pollution (Fu et al., 2014). Besides chemical contamination and cytotoxicity issues, the continuous release of bactericidal agents causes the development of bacterial resistance due to being exposed to sub-inhibitory concentrations of biocides (Sile-Yuksel et al., 2014; Mukherjee and De, 2018). Thus, there is a challenge to provide process eluding leaching of toxic materials while illustrating rapid pathogens killing ability. Nanofiber coatings based methods which promote contact pathogen-killing capacity are promising and can be obtained by chemical modification with tethered biocides functionalities. These strategies may be successful by regarding the right control over the binding quality between the active agent and the underlying biomaterial surface (Zhang et al., 2016; Bazaka et al., 2015; Hilpert et al., 2009). Despite there are numerous researches on application of antibacterial electrospun nanofiber membrane in water filtration, the leaching pattern and durable bactericidal efficiency of the membranes have not been studied comprehensively.
8. Antibacterial performance evaluation of electrospun nanofiber for water filtration
E. coli as a gram-negative and S. aureus as a gram-positive bacterium are commonly used as samples to determine the antibacterial activity of the membranes. There are two main methods for evaluating the antibacterial performance of water purifying membrane filters, known as static and dynamic antibacterial filtration methods (Daels et al., 2011).
8.1. Static antibacterial assay
This method is generally used for testing the inherent antibacterial performance of fabricated electrospun nanofibers as a membrane. This assay generally consists of qualitative detection and quantitative measurement techniques (Zhu et al., 2018).
8.1.1. Qualitative detection of nanofiber antibacterial performance
8.1.1.1. Growth inhibition zone for bacteria
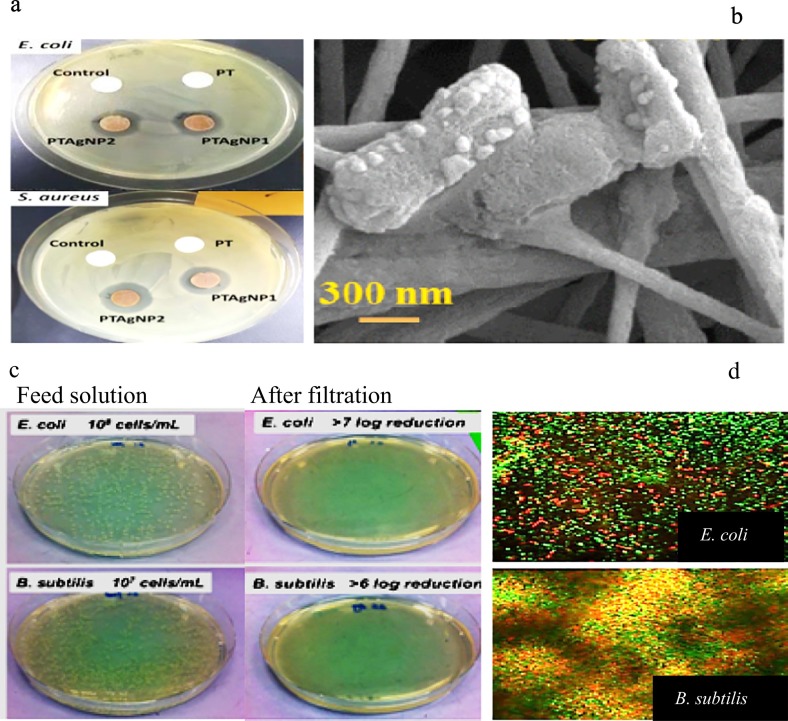
The inhibitory activity of electrospun nanofibers is assayed by the inhibition zone diameter or agar diffusion method toward the considered bacterial sample, based on the Clinical and Laboratory Standards Institute (CLSI Document M02-A12) (CLSI, 2015). For this reason, 100 μL overnight culture of the tested bacteria (106 CFU mL−1) is spread across the surface of an appropriate agar plate, the electrospun nanofiber is cut to disk with about 10 mm diameters, sterilized under UV light for 20 min and then incubated on the plates for 18–24 h at 37 °C. Then, the area of bacteria growth is detected, and the diameter of the inhibition zone around the electrospun nanofiber is measured. This procedure modifications are also used (Santos et al., 2016; Wahab and Al Mamun, 2020 Fig.3 a).
Fig. 3.
a. Agar disc diffusion assay showing the clear zone of inhibition around electrospun polyacrylonitrile nanofiber mats containing titania/AgNP composite nanoparticles (Wahab and Al Mamun, 2020); b. FESEM images of damaged E. coli on the single-walled carbon nanotubes-polyacrylonitrile/polyurethane/polyaniline electrospun nanofiber (Xie et al., 2020); c. Photographs of colonies formed by B. subtilis and E. coli in water samples before and after electrospun coated nanofbrous polyacrylonitrile with polydopamine and silver nanoparticles (cPAN-Ag1.5) nanofbrous membranes (Wang et al., 2017); d. Confocal Laser Scanning Microscopy (CLSM) images of cPAN-Ag1.5 nanofbrous membranes after filtration the bacteria suspension (green dots and red dots stands for live cells and dead cells, respectively) (Wang et al., 2017).
8.1.1.2. SEM and TEM assay
The antibacterial function of the membrane will lead to changes in the bacteria cell morphology. Scanning electron microscope (SEM) and transmission electron microscope (TEM) are utilized to determine the adverse effects of the antibacterial electrospun nanofiber membrane on the bacteria cell morphology (Prama Ekaputra et al., 2015; Fig. 3 b).
8.1.2. Quantitative measurement of nanofiber antibacterial performance
8.1.2.1. Standard plate counts
For this approach, the overnight culture of the selected bacteria is diluted with PBS buffer and adjusted to a density of 107 CFU mL−1 (colony forming units per milliliter). Then, 50 μL of bacterial suspension is placed on the sterilized electrospun nanofiber membranes (1 cm2), for the defined duration time (30 or 60 min). Then, the membrane is placed in 10 mL of 1 X PBS solution and vortexed for 2 min to transfer any remaining bacteria from the membrane to the 1 X PBS solution. Afterward, the quantity of surviving bacterial cells is evaluated by plating the extracted solution onto LB agar plates and subsequent colony counting after 12 h of incubation at 37 °C (Pant et al., 2011; Parekh et al., 2018; Fig. 3 c). The bacterial log reduction is calculated by following eq.
where A is the number of bacteria isolated from the electrospun nanofibers after incubation at the defined contact time, and B is the number of bacteria isolated at zero contact time (Park and Kim, 2017).
8.1.2.2. Bacterial growth inhibition rate
This method is a shaking flask method. Briefly, an appropriate amount of sample sterilized nanofiber is weighted, dipped into a flask containing PBS buffer with a cell concentration of 1–4 × 105 CFU mL−1. The flask is incubated with continuous shaking at 37 °C for a determined time. After serial dilutions by the phosphate buffer, the bacterial suspensions are plated in the agar plate. The inoculated plates were incubated at 37 °C for 24 h and the viable bacterial cells are counted by a colony counter (Kleyi et al., 2015). Also, the number of bacteria after incubation for a determined time can be indirectly measured by spectrometric optical density at 625 nm (Li et al., 2016). Then, the reduction rate is calculated with the following equation:
where R is the reduction rate, A is the number of bacteria isolated from the inoculated electrospun nanofibers after defined time contact time, and B is the number of bacteria isolated from the inoculated electrospun nanofibers at zero contact time (Yao et al., 2016).
8.1.2.3. Standard test method based on contact time
The AATCC 100 test method quantitatively evaluates the bacteriostatic (growth inhibition) or bactericidal (killing of bacteria) ability of textiles over a 24 h contact. For this test, firstly a defined weighted of nanofiber is cut (about 3 mg), get sterilized by UV light, then inoculated with 0.1 mL microbial suspension (1–1/5 × 105 CFU mL−1) and finally overnight incubated at 37 °C. Over determined contact period, 10 mL PBS buffer is added to the falcon tubes containing the inoculated treated electrospun nanofiber. After 1 min shaking, 10 μL of the solution is cultured on nutrient agar plates and incubated for 24 h (Ardekani et al., 2019). ASTM E2149 is another antimicrobial standard method used for evaluating the antibacterial function of immobilized antimicrobial nanofibers under dynamic contact conditions. The antibacterial efficiency is evaluated depending on the contact time from several mints to 24 h between the bacterial solution and the sample (Ungur and Hrůza, 2017). For both methods, the percentage of growth reduction is calculated with the R equation, mentioned above.
8.1.2.4. Dye-based antibacterial assay
In this method, some known dyes are been used to probe if the entrapped bacteria are inactivated by membranes and quantify surviving bacteria, representing an operative, visual and precise antibacterial assay (Zhu et al., 2018). For example, a common dye-based method is detecting the optimal analytical parameters for fluorescence measurements from the dyes SYTO and propidium iodide (PI). The basis of this approach is the attachment of SYTO to live-cell and propidium iodide (PI) to dead cells or cells with defected membranes. The optimal analytical parameters are used for measurement fluorescence by evaluating the intensity of emissions at 505–515 nm for SYTO and 600–610 nm for PI which interpret to quantify of the live cells (Robertson et al., 2019). On the other hand, resazurin as a non-fluorescent dye reduced to a pink and fluorescent dye by exposing to the metabolic activity of bacterial cells so employing a Setup of calibration curves for a known number of bacteria, it can be used for quantifying the survived cell. In this technique, electrospun nanofiber cuts with the side-length of about 1 cm, then placed in the bottom of well plates, 100 μL of bacterial suspension (1–3 × 105 CFU mL−1) is pipetted onto the surface of nanofibers and incubation is carried out for 4 h at 37 °C. Then the prepared solution of resazurin is added and the plate incubated under shaking situation for a defined time. Afterward, the membrane cuts are removed, and the fluorescence is measured at λex = 520 nm/λem = 590 nm in 30 min periods for 720 min. The number of viable bacteria in each well is calculated by the equations obtained of calibration curves (Travnickova et al., 2019; Fig. 3 d).
8.1.2.5. The minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (MIC) represents the minimum amount of antibacterial membrane, which could inhibit bacterial growth. In this method, the defined weighted of nanofiber is dissolved in water (or proper dissolvent), 100 μL of this solution is added to the first well and serially diluted by transferring 50 μL of the well pipetted content to the next well containing 50 μL media. Thereafter, 50 μL of bacterial cultures (1 × 106 CFU mL−1) is poured to each well and plate is incubated at 37 °C for 24 h. To detect the bacterial growth, resazurin or p-iodonitrotetrazolium chloride is added to wells. The wells that turned pink (if resazurin used) or purple (if p-iodonitrotetrazolium chloride used) represents the surviving of bacteria, hence no growth inhibition. The nanofiber concentration in the last growth inhibited well is considered as the MIC value (Nthunya et al., 2017).
8.2. Dynamic antibacterial filtration methods
8.2.1. Dead-end filtration
The bacteria retention test can also be performed with a dead-end filtration module using a vacuum filtration cell, a syringe filter holder 25 mm, Millipore and a dead-end filtration cell system (Jabur et al., 2016; Daels et al., 2011; Son et al., 2009). Before the experiments, the membrane cut diameter and sterilized. All pieces of filtration equipment are sterilized with an autoclave method for 20 min at 121 °C. The membrane is fitted into the device. After passing sterile water from the filter, the bacterial suspension is filtered through the membranes using a pressure.
In this step there are two different techniques for evaluation of bacterial retention:
-
1)
The filtrate is serially diluted with sterile distilled water and viable counts are assayed by plate counts. The colony count can be facilitated by staining bacterial cells with SYTO 9 fluorescent dye and using a fluorescence microscope (He et al., 2018). Then the bacterial retention ratio is calculated in terms of LRV (Log Reduction Value) by the equation:
where Cf and Cp show the concentrations of the feed solution and filtrate (CFU mL−1), respectively (Makaremi et al., 2016; Adibzadeh et al., 2014). An LRV of 1 show 90% removal of pathogens and an LRV of 3 represents 99.9% of the pathogens has been removed from the water (Panda and Sahoo, 2019).
-
2)
The filtrate is transformed into a falcon tube and kept in an incubator under 37 °C for 24 h. The concentration of bacteria suspension in feed and filtrate solutions is measured with spectrophotometry by the following calculation (Moslehi and Mahdavi, 2019):
8.2.2. Electrochemical filtration device
Electrochemical disinfection can destroy bacteria and viruses by electroporation and reactive oxygen species (ROS) during a short time. Electrochemical treatment devices electrochemical disinfection regarded as an effective portable water disinfectant. Fabrication of electrospun porous membrane filter using agents to provide a conducting bed and a strong electric field, facilitate electroporation and production of ROS, which signifies the disinfection process (Hong et al., 2016; Huo et al., 2018). For testing this ability, an electrochemical filtration device with electrospun nanofiber as a filtration membrane is used. Then, a saline solution containing bacterial suspension flows through the nanofiber filter using low voltages at a defined flow rate. The bacterial removal efficiency is calculated by the LVR equation (Wen et al., 2017; Tan et al., 2018; Xie et al., 2020).
8.2.3. Deposition of live bacteria on nanofiber membrane after filtration
In order to determine the deposition of bacteria on a filtrated membrane and the possibility of biofouling, instantly after filtration, the membrane is transferred to an autoclaved beaker filled with PBS buffer and sonicated. The bacteria in the suspended membranes are measured by counting the number of colony cells. Moreover, the morphology of trapped bacteria is investigated using SEM (Xie et al., 2020; Makaremi et al., 2016; Wen et al., 2017).
Different kinds of electrospun nanofibers have been recently fabricated for bacterial removal from water are illustrated in Table 1 .
Table 1.
A short list of electerospun nanofiber used for bacterial removal.
| Polymer | Other polymers | Incorporating strategy and Incorporating agents | Kind of electrospinning and Results of electrospinning | Tested Bacteria | Antibacterial evaluation method | Antibacterial function of the fibrous matrices | Reference |
|---|---|---|---|---|---|---|---|
| Polyacrylonitrile (PAN) | Polyethersulfone (PES) Polyvinylidene fluoride (PVDF) | Blending silica nanoparticles (1 wt% to solution), silver nanoparticles (1 wt% to solution) into 18 wt% solution |
The laboratory electrospinning (Needle electrospinning) flat sheet non–woven nanofiber membrane with a mean pore size of 0.4 μm a fiber diameter between 50 and 100 nm and a thickness of 120 μm |
E. coli | Dynamic antibacterial filtration (Vacuum filtration cell) | Nanofiber containing AgNPs illustrated better antibacterial activity | Mataram et al. (2015) |
| – | Post modification Soaking the PAN electrospun membrane in charged monomer layer solution containing 0.8 wt% 1-(1- vinylimidazolium) ethyl-3-vinylimdazolium dibromide (VEVIMIBr), 0.2 wt% ethoxylated trimethylolpropane triacrylate (EOTMTA), 0.04 wt% K2S2O8 |
The laboratory electrospinning (Needle electrospinning) The diameter of the PAN electrospun nanofibers was 150 nm; The surface of the PAN electrospun nanofiber was wrapped with a thin layer of monomers and thermal initiator in the aqueous solution; the mean pore size of the membrane was 0.21 μm–0.27 μm |
E. coli | Dynamic antibacterial filtration (Dead-end filtration using Millipore) | Electrospun nanofiber showed 99.9999% retention of bacteria and much more efficiency over commercial microfiltration membranes | Ma et al. (2014) | |
| – | Blending ZnO or CuO NPs 3 w% Post modification the exposure of the PAN homogenous solution containing 0.5 wt% of AgNO3 to UV irradiation for 3 and 6 h after electrospinning in order to complete silver ion reduction in the nanofibers to Ag NPs |
The laboratory electrospinning (Needle electrospinning) average diameters from 170 to 250 nm with no beads the FTIR results showed the Ag, ZnO and CuO NPs are successfully composited with PAN |
E. coli S. aureus |
Static antibacterial assay (Agar disk diffusion; Bacterial growth inhibition rate) | The inhibition of bacterial growth increased by employing nanofibers loaded with Ag, ZnO or CuO nanoparticles | Shalaby et al. (2018) | |
| – | Blending and post modification lanthanum nitrate (La(NO3)3· 6H2O) The mass ratio of PAN 5 wt% to La(NO3)3· 6H2O was 3:1 The obtained material were immerged in 0.1 M NaOH for 12 h at room temperature in order to convert La(NO3)3 in PAN nanofibers into La(OH)3. |
The laboratory electrospinning (Needle electrospinning) average diameter of around 110 nm the mean pore size of 0.14 μm |
E. coli | Dynamic antibacterial filtration (Dead-end filtration cell) | The positively charged membrane surface of PLNFs was more successful in entrapping the negatively charged bacteria in compare with the pristine PAN before modification | He et al. (2018) | |
| – | Post- modification Dopamine hydrochloride AgNO3 Immersion of nanofiber mat in bio-agents solutions |
The laboratory electrospinning (Needle electrospinning) |
E. coli B. subtilis |
Static antibacterial assay (Standard test method based on contact time,; Agar disk diffusion) Dynamic antibacterial filtration (Dead-end filtration; SEM image) |
The membrane illustrated >7 log reduction for E. coli and > 6 log reduction for B. subtilis | Wang et al. (2017) | |
| Polyurethane (TPU) Polyaniline (PANI) |
Blending Single-walled carbon nanotubes (SWNTs) 1 wt% |
The industrial Nanospider technique SWNTs were successfully embedded into nanofibers; diameter of fabricated nanofiber was about 190 nm; the pore size (~0.2 μm) |
E. coli S. aureus |
Dynamic antibacterial filtration (Electrochemical filtration device) | Thoroughly removal of bacteria by sieving mechanism; the immobilized SWNTs on nanofibers, making long-term antibacterial function | Xie et al. (2020) | |
| – | Postmodification AgNO3 The electrospun mat was immersed in salt buffers and AgNO3 solution 0.1 M followed by UV exposure |
The laboratory electrospinning (Needle electrospinning) The mean diameter was 2.36 μm |
E. coli | Static antibacterial assay (Standard plate counts) | The sample of the electrospun nonofiber as filter killed more than 99.99% of bacteria within 30 min contact time | Parekh et al. (2018) | |
| Chitosan | Post-modification zinc oxide (ZnO) 5 wt% |
The laboratory electrospinning (Needle electrospinning) PAN nanofibrous membranes were functionalized with zinc oxide (ZnO) nanoparticles and coated with a layer of electrospun chitosan (Cs) |
E. coli E. faecalis |
Static antibacterial assay (Standard test method based on contact time) Dynamic antibacterial filtration (Dead-end filtration using syringe filter holder; SEM image) |
The efficiency of the PAN/ ZnO–Cs membrane for bacteria removal has a log reduction value 2 times more than PAN membranes | Makaremi et al. (2016) | |
| PANI | Blending Silver nanowires with a diameter of approximately 50 nm and length of approximately 20 μm |
The laboratory electrospinning (Needle electrospinning) carbon fiber cloth with a thickness of 0.5 mm was used to cover the collector and deposition of nanofiber to form PAN/PANI/AgNWs-CC membrane; The characterizations results show that PAN/PANI/AgNWs with uniform diameters and without beads were successfully fabricated on CC. AgNWs were uniformly distributed in the PAN/PANI/AgNWs. |
E. coli S. aureus |
Dynamic antibacterial filtration (Electrochemical filtration device; SEM image) |
E. coli and S. aureus were thoroughly removed by the sieving mechanism. no bacteria colonies were detected on agar represent that PAN/PANI/AgNWs-CC has potent antimicrobial activity against E. coli and S. aureus; More than 99.999% deactivation of the sieved bacteria was gained during a few seconds by concurrent filtration |
Wen et al. (2017) | |
| Polyurethane (PU) | – | Blending Microparticles (700 nm to 1 μm) and nanoparticles (≈50 nm) of copper oxide (CuO) |
The industrial Nanospider technique SED-EDX results confirmed the presence of CuO for all of the modified samples; The range of 75–650 nm |
E. coli S. gallinarum |
Static antibacterial assay (ASTM E2149) | All of the produced composite layers including CuO particles in the concentration range from 7 to 12% illustrated significant antibacterial activity | Ungur and Hrůza (2017) |
| Poly(vinyl alcohol) (PVA) | – | Blending 2.6% Benzyl triethylammonium chloride (BTEAC) into the PVA solution (8 wt%) |
The laboratory electrospinning (Needle electrospinning) Diameter of nanofiber (nm) was 216.6 ± 50.9; percent of porosity 79%, the mean pore size 0.94 ± 0.56 μm |
E. coli S. aureus |
Dynamic antibacterial filtration (Dead-end filtration system) | The positively-charged BTEAC in BTEAC-PVA nanofibers can bind negatively-charged bacteria, resulting in the signifing of antimicrobial activity | Park and Kim (2017) |
| Polyethylene terephthalate (PET) | – | wiry needle-less electrospinning strategy nanofibrous mat modified by multi-step interfacial polymerization by immersion in the aqueous monomer solution The pore sizes of the modified PU/PET electrospun nanofibrous based membranes, ranged in 0.25 and 0.46 μm |
E. coli | Dynamic antibacterial filtration (Dead-end filtration using syringe filter holder) | The fabricated membrane could completely remove bacteria (~98–99%) | Moslehi and Mahdavi (2019) | |
| Chitosan | Poly(vinyl alcohol) (PVA) | Blending silver nanoparticles (AgNPs) 4 wt% AgNO3 blended to polymer concentration of 3 wt% |
The laboratory electrospinning (Needle electrospinning) mean diameters of final nanofiber was 59 ± 10 nm the formation of AgNPs into the blend solution and onto the surface of the nanofibers was confirmed |
E. coli | Static antibacterial assay (Standard plate counts) Dynamic antibacterial filtration (Dead-end filtration using syringe filter holder) |
Killed all bacteria within 30 min contact time. | Adibzadeh et al. (2014) |
| Polycaprolactone (PCL) | – | The laboratory electrospinning (Needle electrospinning) The diameter of nanofibres was 200–400 nm |
S. aureus | Static antibacterial assay (Standard plate counts) | The incorporation of 25% chitosan into the nanofibrous membrane declined S. aureus bacterial colonization by 50% compared to membranes fabricated of pure PCL fibers. | Cooper et al. (2013) | |
| Nylon-6 | – | Blending 1 wt% of TiO2 NPs average particle size of 21 nm into a 20 wt% nylon-6 solution Post modification TiO2/nylon-6 electrospun nanofiber mats (4 cm × 4 cm) were placed into 5 mL of 1 × 10−4 M AgNO3 solution. The photodeposition was conducted under UV light (at 254 nm) |
The laboratory electrospinning (Needle electrospinning) TEM images, UV–visible and XRD spectra confirmed that monodisperse Ag NPs (approximately 4 nm in size) were deposited selectively upon the TiO2 NPs of the prepared nanocomposite mat |
E. coli | Static antibacterial assay (Bacterial growth inhibition rate) | The nylon-6 and TiO2/nylon-6 mats demonstrated no antimicrobial effect, whereas the Ag–TiO2/nylon-6 mat represented an antimicrobial effect. | Pant et al. (2011) |
| Chitosan | – | The laboratory electrospinning (Needle electrospinning) The average diameter of nanofibres was 139 nm |
E. coli | Static antibacterial assay (Agar disk diffusion) Dynamic antibacterial filtration (Vacuum filtration cell; SEM image) |
The Chitosan concentration enhances the antibacterial activity to 96% at 30/70-Chitosan/Nylon ratio. SEM image showed the electrospun nanofiber entrapted and hindered the bacteria from penetrating into water | Jabur et al. (2016) | |
| Cellulose acetate (CA) | β-cyclodextrin (β-CD) | Blending and post modification AgNO3, FeCl3 The electrospun β-CD/CA nanofibres embedded with Ag+ and Ag+ /Fe3+ ions were irradiated with UV light in the presence of N2 gas to assist the eduction of the metal ions into NPs |
The laboratory electrospinning (Needle electrospinning) The average diameter of nanofibres was 382.12 nm |
B. cereus E. faecalis, E. coli K. pneumonia K. oxytoca, P. aeruginosa P. mirabilis S. boydii S. sonnei E. cloacae |
Static antibacterial assay (Agar disk diffusion; the minimum inhibitory concentration) |
antibacterial NPs did not leach out of The β-CD/CA nanofibres containing Ag/Fe as bacterial agents showed growth inhibition to all bacterial strains |
Nthunya et al. (2017) |
| Polyamide (PA) | – | Blending 5 wt% WSCP (Poly[(dimethylimino)(2-hydroxy-1,3-propanedily)Chloride) |
The laboratory electrospinning (Needle electrospinning) Resulted in a mean pore size of 0.4 μm, fiber diameter between 50 and 100 nm |
E. coli S. aureus |
Dynamic antibacterial filtration (Vacuum filtration cell) | 5.6 log10 CFU 100 mL−1 elimination for S. aureus and a 4.0 log10 CFU 100 mL−1 elimination for E. coli | Daels et al. (2011) |
9. Antiviral performance evaluation of electrospun nanofiber for water filtration
Usually, evaluation of the antiviral function of nano-filters is carried out using bacteriophage and E. coli as a host model. By performing the double agar method, a defined concentration plaque-forming unit (PFU mL−1) in PBS buffer is passed through the electrospun nanofiber membrane placed in a dead-ended device. The flow-through is collected in an autoclaved vial, and the bacteriophage concentration is determined by the plaque assay technique (Ma et al., 2014; Park and Kim, 2017). Virus retention by the membrane is calculated as the log reduction values defined as LRV (Mi et al., 2014; Zeytuncu et al., 2018). In virus cases, concentration is tested by titration with the MTT assay before and after contact with electrospun nanofibers. The antiviral activity of nanofiber can be evaluated using a static approach by shaking nanofiber cut into a tube containing a defined concentration of virus in PBS saline buffer (Bai et al., 2013). In the dynamic conditions, the defined PFU of viral cell solution passed through sterilized membranes produced and collected. Virus titers are then quantified by the MTT assay (Al-Attabi et al., 2019).
Table 2 illustrates a summary of recent water filtration applications of electrospun nanofibers for virus removal.
Table 2.
A short list of electerospun nanofiber used for bacterial removal.
| Polymer | Other polymers | Incorporating strategy and Incorporating agents | Technique of electrospinning and Result of electrospinning | Tested Viruses | Antiviral evaluation technique | Biocompatibility of the fibrous matrices | Reference |
|---|---|---|---|---|---|---|---|
| Polyacrylonitrile (PAN) | – | Post modification Soaking the PAN electrospun membrane in charged monomer layer solution containing 0.8 wt% 1-(1- vinylimidazolium)ethyl-3-vinylimdazolium dibromide (VEVIMIBr), 0.2 wt% ethoxylated trimethylolpropane triacrylate (EOTMTA), 0.04 wt% K2S2O8 The membrane was heated at 110 °C for 30 min and used after thoroughly being washed with water to remove un-reacted monomers |
The laboratory electrospinning (Needle electrospinning) The diameter of the PAN electrospun nanofibers was 150 nm; the mean pore size of the membrane was 0.21 μm–0.27 μm |
MS2 | Dynamic antibacterial filtration (Dead-end system) |
PAN electrospun and poly(EOTMTA)/PAN membranes whit no charged surface had zero retention for MS2. While, the positively charged membrane poly(VEVIMIBr)/PAN had complete retention up to 99.99% | Ma et al. (2014) |
| Blending Tetraethoxysilane (TEOS) Ammonium tetrathiomolybdate (ATTM) |
The laboratory electrospinning (Needle electrospinning) pore size distributions in the range of 0.8 to 3.1 μm |
Semliki Forest virus (SFV) | Dynamic antibacterial filtration (Dead-end system) |
The elimination efficiency of 12 wt% bare PAN membranes was 33.24%. The removal efficiency of 8 wt% ATTM/PAN membranes has improved to 97.2 and 98.9 for 8 wt% TEOS/ PAN membranes. | Al-Attabi et al. (2019) | ||
| poly(vinyl alcohol) (PVA) | – | Blending 2.6% Benzyl triethylammonium chloride (BTEAC) into the PVA solution (8 wt%) |
The laboratory electrospinning (Needle electrospinning) Diameter of nanofiber was 216.6 nm, percent of porosity 79%, the mean pore size 0.94 μm |
MS2 PhiX174 |
Dynamic antibacterial filtration (Dead-end system) |
BTEAC-PVA/GF was not effective in the removal of bacteriophage. The size of bacteriophage (23 nm) is considerably lesser than the pore size of BTEAC-PVA/GF (0.38 μm), | Park and Kim (2017) |
| Polyethyleneimine (PEI) | Blending Glycidyl methacrylate (GMA) |
The laboratory electrospinning (Needle electrospinning) First, PVA and PEI were acrylated with GMA to enable photopolymerization during the electrospinning process; The solution was spun while being irradiated by UV light (λmax = 365 nm; The fibers were deposited on the PET filter support paper The mean pore size was 0.48 μm’ |
MS2 | Dynamic antibacterial filtration (Dead-end system) |
The 99% retention of MS2 in flow-through virus clearance tests | Zeytuncu et al. (2018) | |
| Chitosan | polyvinyl alcohol (PVA) polyethylene oxide (PEO) | Blending Quaternary amine (HTCC) Graphene oxide (GO) |
The laboratory electrospinning (Needle electrospinning) | Porcine parvovirus (PPV) strain NADL-2 | Static antiviral assay | Graphene improved the ability to fabricate nanofibers with HTCC and enhanced the virus removal function. | Bai et al. (2013) |
| PVA | Blending Quaternary amine (HTCC) |
The laboratory electrospinning (Needle electrospinning) Crosslinking of HTCC into nanofibers Average nanofiber diameter was 119 nm |
Porcine parvovirus (PPV) strain NADL-2 Sindbis virus (heat resistant strain) |
Dynamic antibacterial filtration (Dead-end system) |
The water-stable nanofibers was able to bind to two different viruses and achieved a 3.3 LRV for PPV and a 4.2 LRV. | Mi et al. (2014) |
10. Commercially potential of electrospun nanofiber in water filtration
Despite board promising abilities of electrospun nanofiber membranes in laboratory experiments, their commercialization and large-scale production have been hindered since of some technical obstacles. The high cost of operating, potential toxicity toward human and environment, and compatibility with the presenting instruments are the main complexities. The future development of these nanotechnology-based membranes needed detailed deep investigation to overcome possible technical obstacles. However, currently, some of the electrospun nanofiber-based water membranes are available on the market. Large-scale production of some other laboratory proved electrospun membranes require much more research (Tlili and Alkanhal, 2019). Some examples of currently used electrospun membrane-based water filtration applicants which are present in the market are listed below. SpurTex MF produced by the SPUR Nanotechnologies company is a good example of commercial application of electrospun nanofiber in water purification. Using electrospun Polyurethane, Polyvinylidene fluoride and Cellulose acetate used as filter layer and Polypropylene nonwoven textile as support layer, the structure shows excellent retention of bacteria and fine solids, with 240–400 nm pore size and operating pressure < 2000 mbar. Naked filter is anther novel commercially application of nanofiber in household/bottled water filter with ability to remove 99.9999% of the micro-organic contaminants. Nanotrap is another commercial household water filter produced by Coway company. AstraPool, Fluidra has introduced nanofiber based product applied in filtration system for residential pools (http://electrospintech.com/products.html#.XvS_nm0zbIU).
Liquidity Nanotech Corporation has created electrospun nanofiber membrane made water purification cartridge with superior flow rate, about a cup per minute, good microbiological retention, 6-log bacteria reduction, 4-log virus reduction and 6-log cyst reduction and simple usage process (https://product.statnano.com/product/1981/liquidity-water-purification-cartridge).
PENTAIR company has produced polyethersulfone nanofiber-based cartridge for industrial water purification applications. The cartridge is an absolute barrier to bacteria and viruses: with more than 4-log reduction rate (https://www.directindustry.com/prod/pentair-x-flow/product-71363-1779744.html).
11. Conclusion and future perspective
The researcher's and industry's attention to research and development of electrospun nanofibrous membranes has been growing because of its simplicity, low-cost, scalable molecules incorporation process on the fabricated non-woven mats, production of membranes with the high surface area. High surface area to volume ratio, uniform pore size, and high pore interconnectivity and adequate antibacterial property improve the performance of the nanofibrous membrane in water disinfection application (Subramanian and Seeram, 2013). However, there are several major concerns to be noticed for the application of electrospun nanofiber in water disinfection. Although high surface area and porosity of the electrospun nanofiber are significant advantages, which enhance permeability and selectivity, they also lead to higher mechanical stresses. Consequently, the membrane might be compacted or deformed through the filtration process, which causes loss the porosity and subsequently decreases the permeability. Thus, some key measures should be taken for designing electrospinning conditions which lead to high porosity with favorite pore size proper for pathogen size exclusion and relatively narrow pore size distribution (Ma et al., 2014).
Another solution to overcome mechanical improprieties is the application of electrospun nanofiber as functionalized TFC membranes which have received significant attention in water disinfection (Nagandran et al., 2020). Moreover, the intended incorporation of electrospun nanofiber with functional molecules provides a great possibility for designing a membrane with specific activities, especially incorporation with biocides results in the fabrication of a membrane with significant antibacterial or antiviral activities. However, the selection of best methods of incorporation based on biomolecules and the nanofiber is crucial for retaining the activity of molecules, long time function and controllable leaching profile. Furthermore, additions of biocide molecules into nanofiber avoid biofouling, the main barriers to prolonged stability of membrane-based separation by providing substantial anti-adhesive characteristics on the membrane. It is important to consider that these incorporating molecules should represent strong antibacterial and antiviral function, high permanence, and perfect marketability availability (Zhu et al., 2018). So, more researches to introduce novel anti-biofouling molecular with proper biological properties, low cytotoxicity, and enhanced anti-pathogenic activities would improve the function of water disinfection. For example, in spite of significant antiviral and antibacterial activities of inorganic metallic nanoparticles but their leaching tendency and further risk of toxicity causes hydrophilic polymers like, PVA, PAA be preferred as biocide incorporating agents (Mukherjee and De, 2018; Fahimirad and Hatami, 2017). As it is shown in Table 1, Table 2, due to its chemical stability and excellent weatherability properties, Polyacrylonitrile (PAN) is the most used polymer for production of electrospun nanofiber in water filtration applications purposes. Blending is the most used incorporation strategy, metal nanoparticles and quaternary ammonium compounds (QACs) are the most used incorporating agents in electrospun nanofiber for bacterial and virus removal aims, respectively. E. coli and MS2 are the most common model bacteria and viruses used in filtration efficiency experiments, respectively. Chitosan with board antimicrobial properties is another common used as main polymer or blending agent in antimicrobial nanofibrous membrane production.
Also, further experimental studies needed to conduct proper control of biomolecules release rate from nanofiber, to ensure a balance between successfully deactivate the bacteria strains and lengthen the period of the function, and minimize contamination. Therefore, fabrication of membrane representing inherent self-cleaning, antiviral, and the antibacterial and anti-biofouling feature has gained immense attention for industrial application. Recently, focusing on the production of smart antibacterial surfaces has led to a promising “kill−release” strategy. This approach proposed the fabrication of dual-functional antibacterial surfaces by incorporating biocides into non-fouling materials. These membranes are able to maintain their long-term antibacterial activity by killing bacteria attached to their surface and subsequently are potent to release the dead bacteria to reveal a clean surface (Wei et al., 2017). Although these smart membranes are applied for biomedical applications, the strategy can be promising for further designing of novel electrospun nanofiber with these dual functions and strong long-term functional ability in water disinfection.
As it is illustrated in Table 1, Table 2, despite the significant results obtained from the application of electrospinning in water filtration membrane designing, there are some gaps in this research area. For instance, there are no unanimous standard methods for evaluating the antibacterial or antiviral potential of fabricated electrospun water disinfecting filters. Moreover, most of the researchers have used static antibacterial assay approaches that are unable to represent the membrane antibacterial performance under the dynamic water filtration process. Moreover, recent related studies have not investigated comprehensibly the long-term antibacterial or antiviral performance of produced nano-membrane in water disinfection. Due to extensive endeavors aiming to produce novel smart antibacterial and antiviral membranes and, electrospun nanofibers should be developed rapidly as great candidates for a high effective anti-biofouling membrane for water treatment.
Declaration of competing interest
The authors declare no conflict of interest.
Editor: Ewa Korzeniewska
References
- Adibzadeh S., Bazgir S., Katbab A.A. Fabrication and characterization of chitosan/poly (vinyl alcohol) electrospun nanofibrous membranes containing silver nanoparticles for antibacterial water filtration. Iran. Polym. J. 2014;23(8):645–654. Aug 1. [Google Scholar]
- Aghalari Z., Dahms H.U., Sillanpää M., Sosa-Hernandez J.E., Parra-Saldívar R. Effectiveness of wastewater treatment systems in removing microbial agents: a systematic review. Glob. Health. 2020;16(1):13. doi: 10.1186/s12992-020-0546-y. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Farrukh M.A., Khan M., Khaleeq-ur-Rahman M., Tahir M.A. Synthesis of iron oxide–tin oxide nanoparticles and evaluation of their activities against different bacterial strains. Canadian Chemical Transactions. 2014;2(2):122–133. [Google Scholar]
- Ahmed F.E., Lalia B.S., Hashaikeh R. A review on electrospinning for membrane fabrication: challenges and applications. Desalination. 2015;356:15–30. Jan 15. [Google Scholar]
- Ahmed M.B., Zhou J.L., Ngo H.H., Guo W., Thomaidis N.S., Xu J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: a critical review. J. Hazard. Mater. 2017;323:274–298. doi: 10.1016/j.jhazmat.2016.04.045. Feb 5. [DOI] [PubMed] [Google Scholar]
- Al-Attabi R., Rodriguez-Andres J., Schütz J.A., Bechelany M., Des Ligneris E., Chen X., Kong L., Morsi Y.S., Dumée L.F. Catalytic electrospun nano-composite membranes for virus capture and remediation. Sep. Purif. Technol. 2019;229:115806. Dec 15. [Google Scholar]
- Ardekani N.T., Khorram M., Zomorodian K., Yazdanpanah S., Veisi H., Veisi H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019;125:743–750. doi: 10.1016/j.ijbiomac.2018.12.085. Mar 15. [DOI] [PubMed] [Google Scholar]
- Aussawasathien D., Teerawattananon C., Vongachariya A. Separation of micron to sub-micron particles from water: electrospun nylon-6 nanofibrous membranes as pre-filters. J. Membr. Sci. 2008;315(1–2):11–19. May 1. [Google Scholar]
- Bai B., Mi X., Xiang X., Heiden P.A., Heldt C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr. Res. 2013;380:137–142. doi: 10.1016/j.carres.2013.08.020. Oct 18. [DOI] [PubMed] [Google Scholar]
- Barhate R.S., Ramakrishna S. Nanofibrous filtering media: filtration problems and solutions from tiny materials. J. Membr. Sci. 2007;296(1–2):1–8. Jun 15. [Google Scholar]
- Bazaka K., Jacob M.V., Chrzanowski W., Ostrikov K. Anti-bacterial surfaces: natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015;5(60):48739–48759. [Google Scholar]
- Bhatnagar A., Hogland W., Marques M., Sillanpää M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013;219:499–511. Mar 1. [Google Scholar]
- Biswas P., Bandyopadhyaya R. Biofouling prevention using silver nanoparticle impregnated polyethersulfone (PES) membrane: E. coli cell-killing in a continuous cross-flow membrane module. J. Colloid Interface Sci. 2017;491:13–26. doi: 10.1016/j.jcis.2016.11.060. Apr 1. [DOI] [PubMed] [Google Scholar]
- Bjorge D., Daels N., De Vrieze S., Dejans P., Van Camp T., Audenaert W., Hogie J., Westbroek P., De Clerck K., Van Hulle S.W. Performance assessment of electrospun nanofibers for filter applications. Desalination. 2009;249(3):942–948. (Dec 25) [Google Scholar]
- Blachowicz T., Ehrmann A. Recent developments in electrospun ZnO nanofibers: a short review. Journal of Engineered Fibers and Fabrics. 2020;15 Jan. (1558925019899682) [Google Scholar]
- Botes M., Eugene Cloete T. The potential of nanofibers and nanobiocides in water purification. Crit. Rev. Microbiol. 2010;36(1):68–81. doi: 10.3109/10408410903397332. Feb 1. [DOI] [PubMed] [Google Scholar]
- Bridge J.W., Oliver D.M., Chadwick D., Godfray H.C., Heathwaite A.L., Kay D., Maheswaran R., McGonigle D.F., Nichols G., Pickup R., Porter J. Engaging with the water sector for public health benefits: waterborne pathogens and diseases in developed countries. Bull. World Health Organ. 2010;88:873–875. doi: 10.2471/BLT.09.072512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins M., Oord H., Bloembergen P., Wolfhagen M., Casparie A., Degener J., Ruijs G. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. Eur J Clin Microbiol Infect Dis. 2005;24(5):305–313. doi: 10.1007/s10096-005-1309-7. [DOI] [PubMed] [Google Scholar]
- Cannon M.J., Hyde T.B., Schmid D.S. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol. 2011;21(4):240–255. doi: 10.1002/rmv.695. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang F., Liu Q., Du J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014;50(93):14482–14493. doi: 10.1039/c4cc03001j. [DOI] [PubMed] [Google Scholar]
- Chiam C.K., Sarbatly R. Purification of aquacultural water: conventional and new membrane-based techniques. Separation & Purification Reviews. 2011;40(2):126–160. Feb 18. [Google Scholar]
- Cho D., Lee S., Frey M.W. Characterizing zeta potential of functional nanofibers in a microfluidic device. J. Colloid Interface Sci. 2012;372(1):252–260. doi: 10.1016/j.jcis.2012.01.007. Apr 15. [DOI] [PubMed] [Google Scholar]
- Chou W.L., Yu D.G., Yang M.C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005;16(8):600–607. Aug. [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2015. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition. CLSI Document M02-A12. [Google Scholar]
- Cooley J.F. Improved methods of and apparatus for electrically separating the relatively volatile liquid component from the component of relatively fixed substances of composite fluids. United Kingdom Patent. 1900;6385:19. May. [Google Scholar]
- Cooper A., Oldinski R., Ma H., Bryers J.D., Zhang M. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohydr. Polym. 2013;92(1):254–259. doi: 10.1016/j.carbpol.2012.08.114. Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daels N., De Vrieze S., Sampers I., Decostere B., Westbroek P., Dumoulin A., Dejans P., De Clerck K., Van Hulle S.W. Potential of a functionalised nanofibre microfiltration membrane as an antibacterial water filter. Desalination. 2011;275(1–3):285–290. Jul 15. [Google Scholar]
- Dervin S., Dionysiou D.D., Pillai S.C. 2D nanostructures for water purification: graphene and beyond. Nanoscale. 2016;8(33):15115–15131. doi: 10.1039/c6nr04508a. [DOI] [PubMed] [Google Scholar]
- de Dicastillo C.L., Correa M.G., Martínez F.B., Streitt C., Galotto M.J. IntechOpen; 2020. Antimicrobial Effect of Titanium Dioxide Nanoparticles. InTitanium Dioxide. Jan 27. [Google Scholar]
- Dimapilis E.A., Hsu C.S., Mendoza R.M., Lu M.C. Zinc oxide nanoparticles for water disinfection. Sustainable Environment Research. 2018;28(2):47–56. Mar 1. [Google Scholar]
- Ditaranto N., Basoli F., Trombetta M., Cioffi N., Rainer A. Electrospun nanomaterials implementing antibacterial inorganic nanophases. Appl. Sci. 2018;8(9):1643. Sep. [Google Scholar]
- Dong L., Henderson A., Field C. Antimicrobial activity of single-walled carbon nanotubes suspended in different surfactants. Journal of Nanotechnology. 2012;2012 [Google Scholar]
- Dong Z.Q., Ma X.H., Xu Z.L., Gu Z.Y. Superhydrophobic modification of PVDF–SiO 2 electrospun nanofiber membranes for vacuum membrane distillation. RSC Adv. 2015;5(83):67962–67970. [Google Scholar]
- Eatemadi A., Daraee H., Karimkhanloo H., Kouhi M., Zarghami N., Akbarzadeh A., Abasi M., Hanifehpour Y., Joo S.W. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014;9(1):393. doi: 10.1186/1556-276X-9-393. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccini M., Borja G., Boerrigter M., Morillo Martín D., Martìnez Crespiera S., Vázquez-Campos S., Aubouy L., Amantia D. Electrospun carbon nanofiber membranes for filtration of nanoparticles from water. J. Nanomater. 2015;2015 [Google Scholar]
- Fahimirad S, Ajalloueian F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm.. 2019 May 21. [DOI] [PubMed]
- Fahimirad S., Hatami M. Medicinal Plants and Environmental Challenges. Springer; Cham: 2017. Heavy metal-mediated changes in growth and phytochemicals of edible and medicinal plants; pp. 189–214. [Google Scholar]
- Fahimirad S., Hatami M. Advances in Phytonanotechnology. Academic Press; 2019. Nanocarrier-based antimicrobial phytochemicals; pp. 299–314. Jan 1. [Google Scholar]
- Fahimirad S., Abtahi H., Razavi S.H., Alizadeh H., Ghorbanpour M. Production of recombinant antimicrobial polymeric protein beta casein-E 50-52 and its antimicrobial synergistic effects assessment with thymol. Molecules. 2017;22(6):822. doi: 10.3390/molecules22060822. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahimirad S., Razavi S.H., Abtahi H., Alizadeh H., Ghorbanpour M. Recombinant production and antimicrobial assessment of beta casein-IbAMP 4 as a novel antimicrobial polymeric protein and its synergistic effects with thymol. Int. J. Pept. Res. Ther. 2018;24(1):213–222. Mar 1. [Google Scholar]
- Fahimirad S., Ajalloueian F., Ghorbanpour M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019;168:260–278. doi: 10.1016/j.ecoenv.2018.10.017. Jan 30. [DOI] [PubMed] [Google Scholar]
- Farrokhi Z., Ayati A., Kanvisi M., Sillanpää M. Recent advance in antibacterial activity of nanoparticles contained polyurethane. J. Appl. Polym. Sci. 2019;136(4):46997. Jan 20. [Google Scholar]
- Fu Q., Wong E.H., Kim J., Scofield J.M., Gurr P.A., Kentish S.E., Qiao G.G. The effect of soft nanoparticles morphologies on thin film composite membrane performance. J. Mater. Chem. A. 2014;2(42):17751–17756. [Google Scholar]
- Gall A.M., Mariñas B.J., Lu Y., Shisler J.L. Waterborne viruses: a barrier to safe drinking water. PLoS Pathog. 2015;11(6) doi: 10.1371/journal.ppat.1004867. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geise G.M., Lee H.S., Miller D.J., Freeman B.D., McGrath J.E., Paul D.R. Water purification by membranes: the role of polymer science. J. Polym. Sci. B Polym. Phys. 2010;48(15):1685–1718. Aug 1. [Google Scholar]
- Ghasemi-Mobarakeh L., Semnani D., Morshed M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J. Appl. Polym. Sci. 2007;106(4):2536–2542. (Nov 15) [Google Scholar]
- Ghayeni S.S., Beatson P.J., Fane A.J., Schneider R.P. Bacterial passage through microfiltration membranes in wastewater applications. J. Membr. Sci. 1999;153(1):71–82. Feb 3. [Google Scholar]
- Gómez M., De la Rua A., Garralón G., Plaza F., Hontoria E., Gómez M.A. Urban wastewater disinfection by filtration technologies. Desalination. 2006;190(1–3):16–28. Apr 15. [Google Scholar]
- Gopal R., Kaur S., Ma Z., Chan C., Ramakrishna S., Matsuura T. Electrospun nanofibrous filtration membrane. J. Membr. Sci. 2006;281:581–586. [Google Scholar]
- Guo B.L., Han P., Guo L.C., Cao Y.Q., Li A.D., Kong J.Z., Zhai H.F., Wu D. The antibacterial activity of Ta-doped ZnO nanoparticles. Nanoscale Res. Lett. 2015;10(1):336. doi: 10.1186/s11671-015-1047-4. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W., Musiyiwa K., Mangori L. Sources, behaviour and health risks of antimicrobial resistance genes in wastewaters: a hotspot reservoir. Journal of Environmental Chemical Engineering. 2020;8(1) [Google Scholar]
- Haider A., Haider S., Kang I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018;11(8):1165–1188. Dec 1. [Google Scholar]
- Hameed A.S., Karthikeyan C., Ahamed A.P., Thajuddin N., Alharbi N.S., Alharbi S.A., Ravi G. In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep24312. (Apr 13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Wang W., Shi R., Zhang W., Yang X., Shi W., Cui F. High speed water purification and efficient phosphate rejection by active nanofibrous membrane for microbial contamination and regrowth control. Chem. Eng. J. 2018;337:428–435. Apr 1. [Google Scholar]
- Hilpert K., Elliott M., Jenssen H., Kindrachuk J., Fjell C.D., Körner J., Winkler D.F., Weaver L.L., Henklein P., Ulrich A.S., Chiang S.H. Screening and characterization of surface-tethered cationic peptides for antimicrobial activity. Chem. Biol. 2009;16(1):58–69. doi: 10.1016/j.chembiol.2008.11.006. Jan 30. [DOI] [PubMed] [Google Scholar]
- Homaeigohar S.S., Buhr K., Ebert K. Polyethersulfone electrospun nanofibrous composite membrane for liquid filtration. J. Membr. Sci. 2010;365(1–2):68–77. (Dec 1) [Google Scholar]
- Hong X., Wen J., Xiong X., Hu Y. Silver nanowire-carbon fiber cloth nanocomposites synthesized by UV curing adhesive for electrochemical point-of-use water disinfection. Chemosphere. 2016;154:537–545. doi: 10.1016/j.chemosphere.2016.04.013. Jul 1. [DOI] [PubMed] [Google Scholar]
- Hossain F., Perales-Perez O.J., Hwang S., Román F. Antimicrobial nanomaterials as water disinfectant: applications, limitations and future perspectives. Sci. Total Environ. 2014;466:1047–1059. doi: 10.1016/j.scitotenv.2013.08.009. Jan 1. [DOI] [PubMed] [Google Scholar]
- http://electrospintech.com/products.html#.XvS_nm0zbIU
- https://www.directindustry.com/prod/pentair-x-flow/product-71363-1779744.html
- Huo Z.Y., Li G.Q., Yu T., Lu Y., Sun H., Wu Y.H., Yu C., Xie X., Hu H.Y. Impact of water quality parameters on bacteria inactivation by low-voltage electroporation: mechanism and control. Environmental Science: Water Research & Technology. 2018;4(6):872–881. May 31. [Google Scholar]
- Jabur A.R., Abbas L.K., Moosa S.A. Fabrication of electrospun chitosan/nylon 6 nanofibrous membrane toward metal ions removal and antibacterial effect. Adv. Mater. Sci. Eng. 2016;2016 [Google Scholar]
- Jennings M.C., Minbiole K.P., Wuest W.M. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS infectious diseases. 2015;1(7):288–303. doi: 10.1021/acsinfecdis.5b00047. Jul 10. [DOI] [PubMed] [Google Scholar]
- Kharisov B.I., Dias H.R., Kharissova O.V., Jiménez-Pérez V.M., Pérez B.O., Flores B.M. Iron-containing nanomaterials: synthesis, properties, and environmental applications. RSC Adv. 2012;2(25):9325–9358. [Google Scholar]
- Kleyi P., Jacobs V., Na C.K., Frost C.L., Tshentu Z.R., Torto N. Fabrication and antibacterial activity of electrospun nylon 6 nanofibers grafted with 2-substituted vinylimidazoles. Int. J. Polym. Mater. Polym. Biomater. 2015;64(6):287–298. Aug 23. [Google Scholar]
- Kolewe K.W., Dobosz K.M., Rieger K.A., Chang C.C., Emrick T., Schiffman J.D. Antifouling electrospun nanofiber mats functionalized with polymer zwitterions. ACS Appl. Mater. Interfaces. 2016;8(41):27585–27593. doi: 10.1021/acsami.6b09839. Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. Nov 15. [DOI] [PubMed] [Google Scholar]
- Krasner S.W., Weinberg H.S., Richardson S.D., Pastor S.J., Chinn R., Sclimenti M.J., Onstad G.D., Thruston A.D. Occurrence of a new generation of disinfection byproducts. Environmental science & technology. 2006;40(23):7175–7185. doi: 10.1021/es060353j. Dec 1. [DOI] [PubMed] [Google Scholar]
- Kumar P., Huo P., Zhang R., Liu B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials. 2019;9(5):737. doi: 10.3390/nano9050737. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu R.S., Demarquette N.R. Surface modification to control the water wettability of electrospun mats. Int. Mater. Rev. 2019;64(5):249–287. Jul 4. [Google Scholar]
- Lee A., Elam J.W., Darling S.B. Membrane materials for water purification: design, development and application. Environ. Sci. Water Res. Technol. 2016;2:17–42. [Google Scholar]
- Levchuk I., Sillanpää M. Advanced Water Treatment. Elsevier; 2020. Titanium dioxide–based nanomaterials for photocatalytic water treatment; pp. 1–56. Jan 1. [Google Scholar]
- Levchuk I., Màrquez J.J., Sillanpää M. Removal of natural organic matter (NOM) from water by ion exchange–a review. Chemosphere. 2018;192:90–104. doi: 10.1016/j.chemosphere.2017.10.101. Feb 1. [DOI] [PubMed] [Google Scholar]
- Levchuk I., Kralova M., Rueda-Márquez J.J., Moreno-Andrés J., Gutiérrez-Alfaro S., Dzik P., Parola S., Sillanpää M., Vahala R., Manzano M.A. Antimicrobial activity of printed composite TiO2/SiO2 and TiO2/SiO2/Au thin films under UVA-LED and natural solar radiation. Appl. Catal. B Environ. 2018;239:609–618. Dec 30. [Google Scholar]
- Li D., Wang H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010;20(22):4551–4566. [Google Scholar]
- Li Q., Mahendra S., Lyon D.Y., Brunet L., Liga M.V., Li D., Alvarez P.J. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42(18):4591–4602. doi: 10.1016/j.watres.2008.08.015. Nov 1. [DOI] [PubMed] [Google Scholar]
- Li H., Wang M., Williams G.R., Wu J., Sun X., Lv Y., Zhu L.M. Electrospun gelatin nanofibers loaded with vitamins A and E as antibacterial wound dressing materials. RSC Adv. 2016;6(55):50267–50277. [Google Scholar]
- Lin T. Needleless electrospinning: a practical way to mass production of nanofibers. Journal of textile science and engineering. 2012;2(6):1–3. Jan 1. [Google Scholar]
- Liu H., Tang X., Liu Q. A novel point-of-use water treatment method by antimicrobial nanosilver textile material. J. Water Health. 2014;12(4):670–677. doi: 10.2166/wh.2014.197. Dec. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang G., Lu L., Guo Y., Yang L. Facile shape-controlled synthesis of lanthanum oxide with different hierarchical micro/nanostructures for antibacterial activity based on phosphate removal. RSC Adv. 2017;7(65):40965–40972. [Google Scholar]
- López-Heras M., Theodorou I.G., Leo B.F., Ryan M.P., Porter A.E. Towards understanding the antibacterial activity of Ag nanoparticles: electron microscopy in the analysis of the materials-biology interface in the lung. Environmental Science: Nano. 2015;2(4):312–326. [Google Scholar]
- Ma H., Burger C., Hsiao B.S., Chu B. Ultra-fine cellulose nanofibers: new nano-scale materials for water purification. J. Mater. Chem. 2011;21(21):7507–7510. [Google Scholar]
- Ma H., Hsiao B.S., Chu B. Functionalized electrospun nanofibrous microfiltration membranes for removal of bacteria and viruses. J. Membr. Sci. 2014;452:446–452. Feb 15. [Google Scholar]
- Makaremi M., Lim C.X., Pasbakhsh P., Lee S.M., Goh K.L., Chang H., Chan E.S. Electrospun functionalized polyacrylonitrile–chitosan Bi-layer membranes for water filtration applications. RSC Adv. 2016;6(59):53882–53893. [Google Scholar]
- Mataram A., Ismail A.F., Yuliwati E., Matsuura T., Zamheri A., Rizal S. Water treatment perfomance: application of electrospun nanofibers. Jurnal Teknologi. 2015;77(1) Oct 1. [Google Scholar]
- Matilainen A., Gjessing E.T., Lahtinen T., Hed L., Bhatnagar A., Sillanpää M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere. 2011;83(11):1431–1442. doi: 10.1016/j.chemosphere.2011.01.018. Jun 1. [DOI] [PubMed] [Google Scholar]
- Mi X., Heldt C.L. Adsorption of a non-enveloped mammalian virus to functionalized nanofibers. Colloids Surf. B: Biointerfaces. 2014;121:319–324. doi: 10.1016/j.colsurfb.2014.06.007. Sep 1. [DOI] [PubMed] [Google Scholar]
- Mi X., Vijayaragavan K.S., Heldt C.L. Virus adsorption of water-stable quaternized chitosan nanofibers. Carbohydr. Res. 2014;387:24–29. doi: 10.1016/j.carres.2014.01.017. (Mar 31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer C.J., Wright C.J. The fabrication of iron oxide nanoparticle-nanofiber composites by electrospinning and their applications in tissue engineering. Biotechnol. J. 2017;12(7):1600693. doi: 10.1002/biot.201600693. Jul. [DOI] [PubMed] [Google Scholar]
- Moslehi M., Mahdavi H. Controlled pore size nanofibrous microfiltration membrane via multi-step interfacial polymerization: preparation and characterization. Sep. Purif. Technol. 2019;223:96–106. Sep 15. [Google Scholar]
- Mukherjee M., De S. Investigation of antifouling and disinfection potential of chitosan coated iron oxide-PAN hollow fiber membrane using Gram-positive and Gram-negative bacteria. Mater. Sci. Eng. C. 2017;75:133–148. doi: 10.1016/j.msec.2017.02.039. Jun 1. [DOI] [PubMed] [Google Scholar]
- Mukherjee M., De S. Antibacterial polymeric membranes: a short review. Environmental Science: Water Research & Technology. 2018;4(8):1078–1104. [Google Scholar]
- Munir M.W., Ali U. Nanorods and Nanocomposites. IntechOpen; 2020. Classification of electrospinning methods. (Mar 11) [Google Scholar]
- Nadi Z.R., Salehi T.Z., Tamai I.A., Foroushani A.R., Sillanpää M., Dallal M.M. Evaluation of antibiotic resistance and prevalence of common salmonella enterica serovars isolated from foodborne outbreaks. Microchem. J. 2020;155:104660. Jun 1. [Google Scholar]
- Nagandran S., Goh P.S., Ismail A.F., Wong T.W., Dagang W.R. The recent progress in modification of polymeric membranes using organic macromolecules for water treatment. Symmetry. 2020;12(2):239. Feb. [Google Scholar]
- Nasreen S.A., Sundarrajan S., Nizar S.A., Balamurugan R., Ramakrishna S. Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes. 2013;3(4):266–284. doi: 10.3390/membranes3040266. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ncibi M.C., Mahjoub B., Mahjoub O., Sillanpää M. Remediation of emerging pollutants in contaminated wastewater and aquatic environments: biomass-based technologies. CLEAN–Soil, Air, Water. 2017;45(5):1700101. May. [Google Scholar]
- Nthunya L.N., Masheane M.L., Malinga S.P., Nxumalo E.N., Barnard T.G., Kao M., Tetana Z.N., Mhlanga S.D. Greener approach to prepare electrospun antibacterial β-cyclodextrin/cellulose acetate nanofibers for removal of bacteria from water. ACS Sustain. Chem. Eng. 2017;5(1):153–160. Jan 3. [Google Scholar]
- Nuraje N., Khan W.S., Lei Y., Ceylan M., Asmatulu R. Superhydrophobic electrospun nanofibers. J. Mater. Chem. A. 2013;1(6):1929–1946. [Google Scholar]
- Panda P.K., Sahoo B. Water purification by removal of pathogens using electrospun polymer nanofiber membranes: a review. Journal of Materials Sciences and Applications. 2019;5(1):1–8. [Google Scholar]
- Pant H.R., Pandeya D.R., Nam K.T., Baek W.I., Hong S.T., Kim H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard. Mater. 2011;189(1–2):465–471. doi: 10.1016/j.jhazmat.2011.02.062. May 15. [DOI] [PubMed] [Google Scholar]
- Parekh S.A., David R.N., Bannuru K.K., Krishnaswamy L., Baji A. Electrospun silver coated polyacrylonitrile membranes for water filtration applications. Membranes. 2018;8(3):59. doi: 10.3390/membranes8030059. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.A., Kim S.B. Antimicrobial filtration with electrospun poly (vinyl alcohol) nanofibers containing benzyl triethylammonium chloride: immersion, leaching, toxicity, and filtration tests. Chemosphere. 2017;167:469–477. doi: 10.1016/j.chemosphere.2016.10.030. Jan 1. [DOI] [PubMed] [Google Scholar]
- Perrozzi F., Prezioso S., Ottaviano L. Graphene oxide: from fundamentals to applications. J. Phys. Condens. Matter. 2014;27(1):013002. doi: 10.1088/0953-8984/27/1/013002. Nov 24. [DOI] [PubMed] [Google Scholar]
- Pillay V., Dott C., Choonara Y.E., Tyagi C., Tomar L., Kumar P., du Toit L.C., Ndesendo V.M. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013;2013 [Google Scholar]
- Power A., Chandra S., Chapman J. Graphene, electrospun membranes and granular activated carbon for eliminating heavy metals, pesticides and bacteria in water and wastewater treatment processes. Analyst. 2018;143(23):5629–5645. doi: 10.1039/c8an00922h. [DOI] [PubMed] [Google Scholar]
- Prabu G.T., Dhurai B. A novel profiled multi-pin electrospinning system for nanofiber production and encapsulation of nanoparticles into nanofibers. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-60752-6. (Mar 9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prama Ekaputra M., Munir M.M., Rajak A., Rahma A., Nuryantini A.Y. Synthesis of antibacterial nanofibrous membrane based on polyacrylonitrile (PAN)/chitosan by electrospinning technique for water purification application. InAdvanced Materials Research. 2015;Vol. 1112:76–79. (Trans Tech Publications Ltd) [Google Scholar]
- Raffi M., Mehrwan S., Bhatti T.M., Akhter J.I., Hameed A., Yawar W., ul Hasan M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010;60(1):75–80. Mar 1. [Google Scholar]
- Rahaman M.S., Thérien-Aubin H., Ben-Sasson M., Ober C.K., Nielsen M., Elimelech M. Control of biofouling on reverse osmosis polyamide membranes modified with biocidal nanoparticles and antifouling polymer brushes. J. Mater. Chem. B. 2014;2(12):1724–1732. doi: 10.1039/c3tb21681k. [DOI] [PubMed] [Google Scholar]
- Ramasamy D.L., Puhakka V., Doshi B., Iftekhar S., Sillanpää M. Fabrication of carbon nanotubes reinforced silica composites with improved rare earth elements adsorption performance. Chem. Eng. J. 2019;365:291–304. Jun 1. [Google Scholar]
- Ray S.S., Chen S.S., Li C.W., Nguyen N.C., Nguyen H.T. A comprehensive review: electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016;6(88):85495–85514. [Google Scholar]
- Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., DeMarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutation Research/Reviews in Mutation Research. 2007;636(1–3):178–242. doi: 10.1016/j.mrrev.2007.09.001. Nov 1. [DOI] [PubMed] [Google Scholar]
- Robertson J., McGoverin C., Vanholsbeeck F., Swift S. Optimisation of the protocol for the LIVE/DEAD BacLight bacterial viability kit for rapid determination of bacterial load. Front. Microbiol. 2019;10:801. doi: 10.3389/fmicb.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala W.A., Weber D.J. 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities. [Google Scholar]
- Saleem H., Trabzon L., Kilic A., Zaidi S.J. Recent advances in nanofibrous membranes: production and applications in water treatment and desalination. Desalination. 2020;478:114178. Mar 15. [Google Scholar]
- Santos M.R., Fonseca A.C., Mendonça P.V., Branco R., Serra A.C., Morais P.V., Coelho J.F. Recent developments in antimicrobial polymers: a review. Materials. 2016;9(7):599. doi: 10.3390/ma9070599. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Wang R., Ma H., Hsiao B.S., Chu B. Novel nanofibrous scaffolds for water filtration with bacteria and virus removal capability. J. Electron Microsc. 2011;60(3):201–209. doi: 10.1093/jmicro/dfr019. (May 11) [DOI] [PubMed] [Google Scholar]
- Shabafrooz V., Mozafari M., Vashaee D., Tayebi L. Electrospun nanofibers: from filtration membranes to highly specialized tissue engineering scaffolds. J. Nanosci. Nanotechnol. 2014;14(1):522–534. doi: 10.1166/jnn.2014.9195. Jan 1. [DOI] [PubMed] [Google Scholar]
- Shalaby T., Hamad H., Ibrahim E., Mahmoud O., Al-Oufy A. Electrospun nanofibers hybrid composites membranes for highly efficient antibacterial activity. Ecotoxicol. Environ. Saf. 2018;162:354–364. doi: 10.1016/j.ecoenv.2018.07.016. Oct 30. [DOI] [PubMed] [Google Scholar]
- Shamsollahi H.R., Ghoochani M., Sadeghi K., Jaafari J., Masinaei M., Sillanpää M., Yousefi M., Mirtalb S.T., Alimohammadi M. Evaluation of the physical and chemical characteristics of water on the removal efficiency of rotavirus in drinking water treatment plants and change in induced health risk. Process Saf. Environ. Prot. 2019;130:6–13. Oct 1. [Google Scholar]
- Shomar B., Al-Darwish K., Vincent A. Optimization of wastewater treatment processes using molecular bacteriology. Journal of Water Process Engineering. 2020;33 [Google Scholar]
- Sile-Yuksel M., Tas B., Koseoglu-Imer D.Y., Koyuncu I. Effect of silver nanoparticle (AgNP) location in nanocomposite membrane matrix fabricated with different polymer type on antibacterial mechanism. Desalination. 2014;347:120–130. Aug 15. [Google Scholar]
- Sillanpää M., Ncibi M.C., Matilainen A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: a comprehensive review. J. Environ. Manag. 2018;208:56–76. doi: 10.1016/j.jenvman.2017.12.009. Feb 15. [DOI] [PubMed] [Google Scholar]
- Son B., Yeom B.Y., Song S.H., Lee C.S., Hwang T.S. Antibacterial electrospun chitosan/poly (vinyl alcohol) nanofibers containing silver nitrate and titanium dioxide. J. Appl. Polym. Sci. 2009;111(6):2892–2899. Mar 15. [Google Scholar]
- Subramanian S., Seeram R. New directions in nanofiltration applications—are nanofibers the right materials as membranes in desalination? Desalination. 2013;308:198–208. Jan 2. [Google Scholar]
- Suja P.S., Reshmi C.R., Sagitha P., Sujith A. Electrospun nanofibrous membranes for water purification. Polym. Rev. 2017;57(3):467–504. Jul 3. [Google Scholar]
- Taheran M., Kumar P., Naghdi M., Brar S.K., Knystautas E.J., Verma M., Surampalli R.Y. Development of an advanced multifunctional portable water purifier. Nanotechnology for Environmental Engineering. 2019;4(1):7. Dec 1. [Google Scholar]
- Tan X., Chen C., Hu Y., Wen J., Qin Y., Cheng J., Chen Y. Novel AgNWs-PAN/TPU membrane for point-of-use drinking water electrochemical disinfection. Sci. Total Environ. 2018;637:408–417. doi: 10.1016/j.scitotenv.2018.05.012. Oct 1. [DOI] [PubMed] [Google Scholar]
- Taner M., Sayar N., Yulug I.G., Suzer S. Synthesis, characterization and antibacterial investigation of silver–copper nanoalloys. J. Mater. Chem. 2011;21(35):13150–13154. [Google Scholar]
- Tezel U., Pavlostathis S.G. Role of quaternary ammonium compounds on antimicrobial resistance in the environment. Antimicrobial resistance in the environment. 2012:349–387. Jan 24. [Google Scholar]
- Tiwari V., Mishra N., Gadani K., Solanki P.S., Shah N.A., Tiwari M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 2018;6(9):1218. doi: 10.3389/fmicb.2018.01218. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlili I., Alkanhal T.A. Nanotechnology for water purification: electrospun nanofibrous membrane in water and wastewater treatment. Journal of Water Reuse and Desalination. 2019;9(3):232–248. [Google Scholar]
- Travnickova E., Mikula P., Oprsal J., Bohacova M., Kubac L., Kimmer D., Soukupova J., Bittner M. Resazurin assay for assessment of antimicrobial properties of electrospun nanofiber filtration membranes. AMB Express. 2019;9(1):183. doi: 10.1186/s13568-019-0909-z. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungur G., Hrůza J. Modified polyurethane nanofibers as antibacterial filters for air and water purification. RSC Adv. 2017;7(78):49177–49187. [Google Scholar]
- Wagner G., Korenkov V., Judy J.D., Bertsch P.M. Nanoparticles composed of Zn and ZnO inhibit Peronospora tabacina spore germination in vitro and P. tabacina infectivity on tobacco leaves. Nanomaterials. 2016;6(3):50. doi: 10.3390/nano6030050. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab J.A., Al Mamun S. Polyacrylonitrile nanofiber mats containing titania/AgNP composite nanoparticles for antibacterial applications. Mater. Res. Express. 2020;7(1):015416. (Jan 27) [Google Scholar]
- Wang X., Hsiao B.S. Electrospun nanofiber membranes. Current opinion in chemical engineering. 2016;12:62–81. May 1. [Google Scholar]
- Wang J., Windbergs M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017;119:283–299. doi: 10.1016/j.ejpb.2017.07.001. Oct 1. [DOI] [PubMed] [Google Scholar]
- Wang R., Liu Y., Li B., Hsiao B.S., Chu B. Electrospun nanofibrous membranes for high flux microfiltration. J. Membr. Sci. 2012;392:167–174. Mar 1. [Google Scholar]
- Wang R., Guan S., Sato A., Wang X., Wang Z., Yang R., Hsiao B.S., Chu B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Membr. Sci. 2013;446:376–382. Nov 1. [Google Scholar]
- Wang J., Wu Y., Yang Z., Guo H., Cao B., Tang C.Y. A novel gravity-driven nanofibrous membrane for point-of-use water disinfection: polydopamine-induced in situ silver incorporation. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-02452-2. May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kjellberg I., Sikora P., Rydberg H., Lindh M., Bergstedt O., Norder H. Hepatitis E virus genotype 3 strains and a plethora of other viruses detected in raw and still in tap water. Water Res. 2020;168 doi: 10.1016/j.watres.2019.115141. [DOI] [PubMed] [Google Scholar]
- Warsinger D.M., Chakraborty S., Tow E.W., Plumlee M.H., Bellona C., Loutatidou S., Karimi L., Mikelonis A.M., Achilli A., Ghassemi A., Padhye L.P. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018;81:209–237. doi: 10.1016/j.progpolymsci.2018.01.004. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Tang Z., Yu Q., Chen H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl. Mater. Interfaces. 2017;9(43):37511–37523. doi: 10.1021/acsami.7b13565. Nov 1. [DOI] [PubMed] [Google Scholar]
- Wen J., Tan X., Hu Y., Guo Q., Hong X. Filtration and electrochemical disinfection performance of PAN/PANI/AgNWs-CC composite nanofiber membrane. Environmental science & technology. 2017;51(11):6395–6403. doi: 10.1021/acs.est.6b06290. Jun 6. [DOI] [PubMed] [Google Scholar]
- WHO . 4th ed. WHO Press; Geneva, Switzerland: 2011. Guidelines for Drinking-Water Quality. [Google Scholar]
- WHO . World Health Organization; Geneva: 2020. The World Health Report 2002. [Google Scholar]
- World Health Organization, 2019. Drinking-water, 01 May 2019. https://www.who.int /news-room/fact-sheets/detail/drinking-water.
- World Health Organization . vol. 23. World Health Organization; April 2020. Water, Sanitation, Hygiene, and Waste Management for the COVID-19 Virus: Interim Guidance. (2020) [Google Scholar]
- Xi C., Zhang Y., Marrs C.F., Ye W., Simon C., Foxman B., Nriagu J. Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl. Environ. Microbiol. 2009;75(17):5714–5718. doi: 10.1128/AEM.00382-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Shu Y., Hu Y., Cheng J., Chen Y. SWNTs-PAN/TPU/PANI composite electrospun nanofiber membrane for point-of-use efficient electrochemical disinfection: new strategy of CNT disinfection. Chemosphere. 2020;251:126286. doi: 10.1016/j.chemosphere.2020.126286. Jul 1. [DOI] [PubMed] [Google Scholar]
- Yao L.R., Song X.M., Zhang G.Y., Xu S.Q., Jiang Y.Q., Cheng D.H., Lu Y.H. Preparation of Ag/HBP/PAN nanofiber web and its antimicrobial and filtration property. J. Nanomater. 2016;2016 [Google Scholar]
- Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q., Lu Z., Chen L., He Q., Han H. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl. Mater. Interfaces. 2015;7(38):21571–21579. doi: 10.1021/acsami.5b06876. Sep 30. [DOI] [PubMed] [Google Scholar]
- Yin J., Kim E.S., Yang J., Deng B. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM-41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012;423:238–246. Dec 15. [Google Scholar]
- You Y., Han J., Chiu P.C., Jin Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environmental science & technology. 2005;39(23):9263–9269. doi: 10.1021/es050829j. Dec 1. [DOI] [PubMed] [Google Scholar]
- Zeytuncu B., Ürper M., Koyuncu İ., Tarabara V.V. Photo-crosslinked PVA/PEI electrospun nanofiber membranes: preparation and preliminary evaluation in virus clearance tests. Sep. Purif. Technol. 2018;197:432–438. May 31. [Google Scholar]
- Zhang X., Wang Z., Chen M., Liu M., Wu Z. Polyvinylidene fluoride membrane blended with quaternary ammonium compound for enhancing anti-biofouling properties: effects of dosage. J. Membr. Sci. 2016;520:66–75. Dec 15. [Google Scholar]
- Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne viruses: a review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. [Google Scholar]
- Zhao F., Yang Z., Wei Z., Spinney R., Sillanpää M., Tang J., Tam M., Xiao R. Polyethylenimine-modified chitosan materials for the recovery of La (III) from leachates of bauxite residue. Chem. Eng. J. 2020;388:124307. May 15. [Google Scholar]
- Zhu J., Hou J., Zhang Y., Tian M., He T., Liu J., Chen V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018;550:173–197. Mar 15. [Google Scholar]
- Zodrow K.R., Tousley M.E., Elimelech M. Mitigating biofouling on thin-film composite polyamide membranes using a controlled-release platform. J. Membr. Sci. 2014;453:84–91. Mar 1. [Google Scholar]