Abstract
Federally qualified health centers are on the frontlines of the coronavirus disease 2019 (COVID-19) pandemic in the United States. It is essential to develop the workflows necessary to evaluate patients, perform appropriate diagnostics, make clinical recommendations, and provide public health messaging. This brief report presents findings from our COVID-19 response and compares the characteristics between the 345 patients screened between March 16 and April 10, 2020. One hundred seventeen patients tested positive for COVID-19, an overall rate of 33.9%; and Black race, increased heart rate, elevated temperature, and the use of antipyretic agents were associated with positive results.
Abbreviation and Acronym: COVID-19, coronavirus disease 2019
The novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic continues to exert unprecedented challenges to the global health care system.1 New Orleans is among the cities bearing the highest burden.2,3 Given the high rate of community transmission and expected predominance of a mildly symptomatic disease course, an ambulatory-based approach to coronavirus disease 2019 (COVID-19) management and mitigation is essential.4,5 Evidence-based recommendations support patients receiving a clinical assessment, access to diagnostics, treatment guidance, health education on limiting transmissions, and triage for the utilization of higher levels of care.6,7,8 At CrescentCare, a federally qualified health center in New Orleans, Louisiana, we developed a community-based response with a walk-in COVID-19 clinic. Here, we present the initial findings of our intervention and compare positive cases with negative cases.
The objective of this study was to assess the rate of COVID-19 positivity in a community-based health center, evaluate the clinical symptoms, and follow patient outcomes.
Patients and Methods
The Advarra Institutional Review Board granted a full waiver of the Health Insurance Portability and Accountability Act authorization and deemed the study exempt. On March 16, 2020, our clinic developed a dedicated COVID-19 walk-in clinic. The clinic was open to all patients 17 years and older, new and existing, regardless of insurance coverage. It was intentionally not a drive-through testing site and centered on the patient/medical provider interaction. Clients entering the health center were screened for symptoms and triaged to the COVID-19 clinic. All 18,000 existing patients received text messages and e-mails directing them to the walk-in site if symptomatic. Radio service announcements, social media, and local press informed the community of our services.
Three tents were set up in the outdoor garage of our health center. Providers wore synthetic suits that were disinfected between patients, as well as N95 masks, gloves, and goggles. Only providers interacted within 6 ft of patients. Demographic information was obtained consistent with routine clinical procedures, a focused history including fever within the past 72 hours, cough, dyspnea, and pharyngitis as well as comorbidities were recorded. Testing was performed for patients with documented or subjective fever within the past 72 hours. They were swabbed for point-of-care influenza and streptococcal A, when appropriate. Coronavirus disease 2019 testing was performed using LabCorp’s nucleic acid amplification nasopharyngeal swab transported in viral culture medium.
Patients were informed of their results by a provider and asked follow-up questions about resolution or worsening of symptoms or hospitalization. Symptomatic patients had additional telephone encounters to continue to assess symptoms and limit unnecessary utilization of hospital-based services. Social distancing was reinforced during each contact.
Data were presented as means ± SDs and frequencies (percentages). Differences in distribution between those who tested positive and those who tested negative were assessed using the t test (Wilcoxon rank sum test for oxygen saturation) for continuous variables and the Pearson chi-square test for categorical variables. Logistic regression methods were used to assess combinations of predictors. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
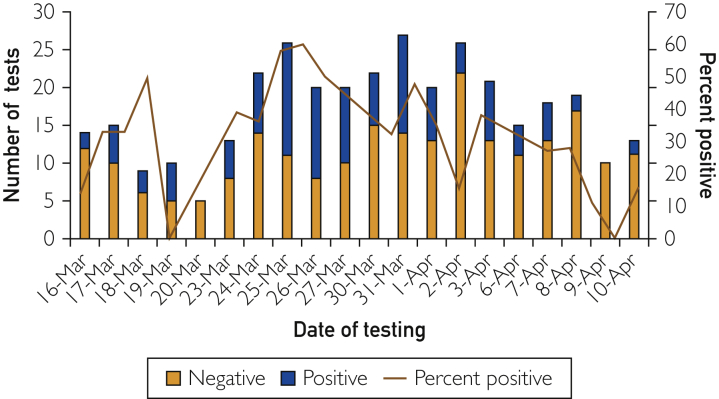
Our clinic tested 345 patients between March 16 and April 10, 2020. One hundred seventeen patients tested positive for COVID-19, a rate of 33.9% (Figure). Temperature reading at visit (mean, 99.4°F vs 98.6°F), heart rate (mean, 93.9 beats/min vs 86.8 beats/min), and the use of antipyretic agents were significantly associated with positive test results. Dyspnea and pharyngitis were not associated. Blacks had a significantly higher rate of test positivity (odds ratio, 2.1; 95% CI, 1.4 to 3.3) (Table).
Figure.
Severe acute respiratory syndrome coronavirus-2 positivity rate by day. The blue bar represents the number of positive cases; and yellow bar, the number of negative cases. The red line represents the percent positive cases per day.
Table.
SARS-CoV-2 Comparision of Positive and Negative Casesa
| Variable | N | Negative (n=228) |
Positive (n=117) |
P value | ||
|---|---|---|---|---|---|---|
| Mean | Range (min, max) | Mean | Range (min, max) | |||
| Age (y) | 228, 117 | 42.3±13.6 | (19, 76) | 44.4±15.0 | (17, 77) | .1875 |
| Temperature (°F) | 223, 115 | 98.6±0.7 | (97.3, 102.8) | 99.4±1.3 | (97.3, 102.9) | <.0001 |
| Heart rate (beats/min) | 219, 115 | 86.8±15.4 | (51, 130) | 93.9±16.9 | (58, 140) | <.0001 |
| Oxygen saturation (%) | 218, 115 | 97.8±1.8 | (88, 100) | 97.1±3.3 | (73, 100) | .1664 |
| n (%) | n (%) | ||
|---|---|---|---|
| Race | 90 (39.5) | 68 (58.1) | .0043 |
| Black | 101 (44.3) | 35 (29.9) | |
| White | 37 (16.2) | 14 (12) | |
| Other (Latinx) | 13 (5.7) | 7 (6.0) | |
| Gender | .5874 | ||
| Male | 106 (46.5) | 58 (49.6) | |
| Female | 115 (50.4) | 59 (50.4) | |
| T/GQ/non-binary | 7 (3.1) | 0 (0) | |
| Insurance | .4863 | ||
| None | 59 (25.9) | 30 (25.6) | |
| Medicaid | 55 (24.1) | 22 (18.8) | |
| Medicare/private | 114 (50.0) | 65 (55.6) | |
| New patient | 130 (57.3) | 72 (61.5) | .4460 |
| Cough | 181 (79.4) | 98 (83.8) | .3281 |
| Shortness of breath | 126 (55.3) | 53 (45.3) | .0795 |
| Sore throat | 112 (49.1) | 48 (41.0) | .1534 |
| Nasal congestion | 8 (3.5) | 6 (5.1) | .5659 |
| Taking antipyretic agents | 95 (41.7) | 63 (53.9) | .0316 |
| G symptoms | 80 (35.1) | 44 (37.6) | .6443 |
| Co-morbidities | |||
| Smoke | 64 (28.1) | 10 (8.6) | <.0001 |
| Diabetes | 21 (9.2) | 13 (11.1) | .5750 |
| Heart disease | 11 (4.8) | 14 (12.0) | .0154 |
| COPD | 16 (7.0) | 4 (3.4) | .1753 |
| HTN | 60 (26.3) | 49 (41.9) | .0032 |
| Recovered | 228 (100.0) | 112 (95.7) | .0042 |
| Positive responders | |||
| Anosmia/ageusia | 50 (43.1) | ||
| Requiring emergency department services | 9 (7.8) | ||
| Hospitalized | 6 (5.1) | ||
| Began taking HCQ | 5 (4.3) | ||
| Began taking azithromycin | 5 (4.3) |
COPD = chronic obstructive pulmonary disease; GI = gastrointestinal; HCQ = hydroxychloroquine; HTN = hypertension; SoB = shortness of breath; T/GQ/nonbinary = transgender, gender-queer.
Boldface indicates values that are statistically significant.
Of the 117 positive cases, 9 required emergency department services, with 6 hospitalized. The 6 patients hospitalized had a median age of 48.5 years (range, 28-67 years) and consisted of 5 women and 1 man; 3 were black, 2 were Latinx, and 1 was white. Two of these patients died after hospitalization in an intensive care unit. All other patients have recovered. Anosmia or ageusia was noted in 41.4% of COVID-19 cases.
The median time of the receipt of test results was 5 days, and our clinic was able to reach all patients to communicate their results.
Conclusion
The high rate of positive results underscores the widespread community transmission in New Orleans. Louisiana has experienced a considerable impact from COVID-19, especially in the Black community, with disparities in both hospitalization rates and mortality. Our study reports similar disparities in the outpatient setting in which Blacks are at higher risk of community transmission. Community health centers play a vital role in ensuring access to care during this pandemic, and this model prioritizes provider contact and patient education.
Overall, our patients recovered from COVID-19, with very few requiring referrals for immediate care. Over the course of the pandemic, our program noted an initial decline in percentage of those testing positive for COVID-19, supporting the public health benefit of social distancing initiatives. However over recent weeks, positive cases seen in our clinic have mirrored regional trends, further supporting the role of community health clinics through this pandemic.
Our findings elucidate the symptoms that are most prevalent in an outpatient setting during a community-wide outbreak. Elevated temperature, increased heart rate, and the use of antipyretic agents were most associated with positive test results. Subjective symptoms were similar between positive and negative cases. Reports have noted the wide range of initial symptoms in the outpatient setting.
A limitation of this study is that our federally qualified health center patient population is younger, with fewer comorbidities. In this study, fewer positive cases had a smoking history. This could explain our lower rate of hospitalization. Additionally, at the onset of testing, we encountered delays in the receipt of test results, limiting our ability to triage patients appropriately.
Community health centers play an essential role during this pandemic by evaluating symptoms, reinforcing public health messaging, and limiting unnecessary utilization of emergency services.
Acknowledgments
We thank both our incredible patients and dedicated staff at CrescentCare.
Footnotes
Grant Support: The work was supported in part by grant U54GM104940 (L.M.) from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical & Translational Science Center.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Coronavirus Disease 2019 (Covid-19) Situation Report 155. World Health Organization website. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200623-covid-19-sitrep-155.pdf?sfvrsn=ca01ebe_2 Accessed June 25, 2020.
- 2.COVID-19. Louisiana Department of Health website. http://ldh.la.gov/Coronavirus/
- 3.Prince-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parodi S.M., Liu V.X. From containment to mitigation of COVID-19 in the US. JAMA. 2020;323(15):1441–1442. doi: 10.1001/jama.2020.3882. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Fineberg H. Ten weeks to crush the curve. N Engl J Med. 2020;382(17):e37. doi: 10.1056/NEJMe2007263. [DOI] [PubMed] [Google Scholar]
- 7.Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html#Asymptomatic Accessed June 20, 2020.
- 8.Cohen P.A., Hall L.E., John J.N., Rapoport A.B. The early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic. Mayo Clin Proc. 2020;95(6):1124–1126. doi: 10.1016/j.mayocp.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]